Significance
Sarcomeres, the contractile units of striated muscle, are composed of thick and thin/actin filaments. Thin filament length is closely associated with specific contractile properties of individual muscles, and it is tightly controlled by actin binding proteins. However, it is still unclear how these proteins work in concert to maintain proper thin filament length and whether there are additional factors involved. In this study, we found that deleting HSPB7 resulted in uncontrolled elongation of actin filaments and the formation of atypical actin filament bundles in cardiomyocytes. Biochemical studies revealed a previously unsuspected function of HSPB7 in interacting with and limiting actin monomer availability for actin filament polymerization, giving mechanistic insight into the etiology of aberrant sarcomeres observed in HSPB7 null heart.
Keywords: HSPB7, heart development, sarcomere, thin filament assembly, actin polymerization
Abstract
Small heat shock protein HSPB7 is highly expressed in the heart. Several mutations within HSPB7 are associated with dilated cardiomyopathy and heart failure in human patients. However, the precise role of HSPB7 in the heart is still unclear. In this study, we generated global as well as cardiac-specific HSPB7 KO mouse models and found that loss of HSPB7 globally or specifically in cardiomyocytes resulted in embryonic lethality before embryonic day 12.5. Using biochemical and cell culture assays, we identified HSPB7 as an actin filament length regulator that repressed actin polymerization by binding to monomeric actin. Consistent with HSPB7’s inhibitory effects on actin polymerization, HSPB7 KO mice had longer actin/thin filaments and developed abnormal actin filament bundles within sarcomeres that interconnected Z lines and were cross-linked by α-actinin. In addition, loss of HSPB7 resulted in up-regulation of Lmod2 expression and mislocalization of Tmod1. Furthermore, crossing HSPB7 null mice into an Lmod2 null background rescued the elongated thin filament phenotype of HSPB7 KOs, but double KO mice still exhibited formation of abnormal actin bundles and early embryonic lethality. These in vivo findings indicated that abnormal actin bundles, not elongated thin filament length, were the cause of embryonic lethality in HSPB7 KOs. Our findings showed an unsuspected and critical role for a specific small heat shock protein in directly modulating actin thin filament length in cardiac muscle by binding monomeric actin and limiting its availability for polymerization.
Several cardiac disease-causing mutations have been found within thin filament genes, such as ACTC1 (1), TPM1, and TNNT2 (2), reinforcing the realization that the proper function of cardiac muscle relies on precise regulation of thin filament contractile function. A critical characteristic of thin filaments is their tightly controlled length, which is closely related to specific contractile properties of distinct striated muscle types (3, 4). Several actin binding proteins regulate thin filament length. The pointed end capping protein tropomodulin 1 (Tmod1) (5) limits thin filament length by inhibiting addition of actin to the pointed end of actin filaments (6, 7). The actin nucleator, leiomodin2 (Lmod2) (8), is also required for regulating thin filament length in cardiac muscle, as mice lacking Lmod2 exhibit shorter filament lengths and reduced force-generating ability in cardiac myocytes (9). The importance of precise regulation of thin filament length is shown by the subsequent development of dilated cardiomyopathy in Lmod2 mutants (9) or in transgenic (Tg) mice overexpressing Tmod1 in their hearts (10). Moreover, loss of Lmod3, the Lmod gene expressed predominantly in skeletal muscle, results in severe nemaline myopathy characterized by sarcomere disorganization and shorter thin filaments in both humans and mice (11, 12). However, whether there are additional regulators that contribute to this process and how actin binding proteins work in concert to tightly regulate thin filament length remain largely unknown.
Small heat shock proteins (sHSPs) are a family of molecular chaperones that bind nonnative proteins to prevent their aggregation and assist in subsequent refolding by ATP-dependent chaperones, such as HSP70 (13), or in targeting unfolded proteins for degradation by proteasomal and/or autophagic pathways. While some sHSPs are ubiquitously expressed, others are relatively confined to heart and skeletal muscle, including HSPB6 and HSPB7 (14). sHSPs recognize a broad spectrum of substrates, ranging from cytoskeletal proteins to mitochondrial proteins (13). Interestingly, sHSPs also associate with the actin cytoskeleton (15). In addition, HSPB5/αB-crystallin is known to stabilize filamentous actin (16), while HSPB1 was originally purified from turkey smooth muscle as a fraction that inhibits actin polymerization (17). However, mechanisms by which sHSPs directly modulate actin dynamics await additional investigation.
HSPB7 (also known as cardiovascular HSP) is highly expressed in heart and skeletal muscle (18). Mutations found within the HSPB7 gene are associated with heart disease (19–22). In zebrafish, loss-of-function studies showed that HSPB7 is essential for left–right asymmetry and cardiac morphogenesis (23, 24). However, the role of HSPB7 in mammalian heart is still unclear. To further investigate biological functions of HSPB7, we generated mice with global KO of HPSB7 and found that they died from heart defects before embryonic day 12.5 (E12.5). Close examination of sarcomeres in KO cardiomyocytes revealed the presence of abnormal actin bundles (AABs), which were continuous throughout the length of the sarcomere and associated with α-actinin. Measurement of thin filament length revealed longer thin filaments, which coincided with up-regulation of the actin binding protein Lmod2. However, genetic ablation of Lmod2 in HSPB7 KO embryos did not prevent formation of AABs, although average thin filament lengths were reduced, consistent with deletion of Lmod2 alone (9). The foregoing suggests that up-regulation of Lmod2 was important for increased thin filament length but not for assembly of AABs in HSPB7 mutants. Interestingly, we found that HSPB7 bound to monomeric actin (G actin) and repressed actin polymerization, indicating that loss of HSPB7 could result in excessive actin polymerization and AAB formation. Our findings shed light on HSPB7’s role in thin filament length regulation and suggest a possible interplay between HSPB7 and regulators, such as Lmod2 and Tmod1.
Results
HSPB7 Is Essential for Fetal Heart Development.
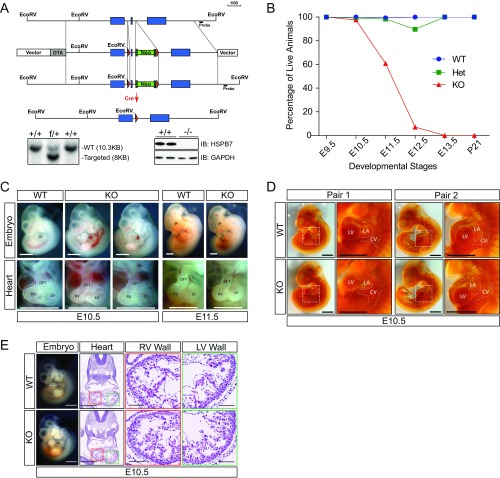
To investigate HSPB7’s function in vivo, we generated an HSPB7 KO mouse line utilizing homologous recombination. In brief, Exon 2 of the HSPB7 gene was flanked by two LoxP sites, and a neomycin cassette was inserted immediately downstream in Intron 2 to serve as a selection marker (Fig. S1A). Correctly targeted ES cells were used to generate living mice via germ-line transmission and bred with ubiquitously expressed Sox2-Cre (25) mice to generate an HSPB7 global KO allele. Western blot results from E11.5 hearts of KO animals indicated complete loss of HSPB7 protein (Fig. S1A). We were unable to recover viable KOs, suggesting that HSPB7 KO mice died in utero (Fig. S1B and Table S1). From E9.5 to E10.5, mutants were grossly indistinguishable from WT littermates (Fig. 1A). However, at E11.5, mutants had noticeably smaller left ventricles compared with WT littermates (Fig. 1A). Examination of embryonic hearts revealed that HSPB7 KO embryos had relatively smaller hearts, starting from E10.5 (Fig. S1C). We also observed enlargement of cardinal veins in E10.5 KO embryos by PECAM (platelet endothelial cell adhesion molecule) staining (Fig. S1D), an indication of congestive heart failure (26). At E11.5, nearly one-half of KO embryos had died and begun to be resorbed, and by E12.5, most HSPB7 KO embryos had died (Fig. S1B and Table S1).
Fig. S1.
General phenotype of HSPB7 KO mice. (A) Targeting strategy for the generation of HSPB7 KO mice. (Lower Left) Detection of WT (+) and f alleles by Southern blot analysis indicates correctly targeted ES cells. (Lower Right) Western blot analysis confirms complete depletion of HSPB7 in KO (−/−) animals. GAPDH served as a loading control. Blue box, HSPB7 exon; DTA, diphtheria toxin A chain gene; green box abutted to the to the Neo gene, FLP site; Neo, neomycin resistance gene; red triangle, LoxP site. IB, immunoblot. (B) Percentage of live WT, HSPB7 heterozygous KO (Het), and HSPB7 homozygous global KO embryos/mice at various developmental stages. The actual embryo/pup numbers of each genotype are presented in Table S1. (C) Whole-mount microscopic images of whole embryos (left lateral view) and embryonic hearts (anterior view) of WT and HSPB7 KO embryos from E10.5 to E11.5. LV, left ventricle; OFT, outflow tract; RV, right ventricle. (Scale bar: 1 mm.) (D) Microscopic left lateral views of two (pairs 1 and 2) PECAM-stained E10.5 WT and HSPB7 KO embryos. High magnification views correspond to the dotted box in the whole-embryo images. CV, cardinal vein; LA, left atria; LV, left ventricle. (Scale bar: 1 mm.) (E) H&E images of E10.5 WT and HSPB7 KO embryos acquired using NanoZoomer slide scanner. (Scale bar: 0.5 mm.) Estimated location of sectioning is indicated by a dotted line on the whole-mount embryo images to the left. (Scale bar: 1 mm.) Magnified right ventricle (RV; red box) and left ventricle (LV; green box) wall views are shown to the right. (Scale bars: 0.1 mm.)
Table S1.
Observed genotype distribution of embryos/pups recovered from mating between HSPB7 heterozygous (+/−) mice
| Stage | −/− | +/− | +/+ |
| E8.5 | 5 | 8 | 1 |
| E9.5 | 38 | 60 | 22 |
| E10.5 | 77 | 121 (1*) | 82 (2*) |
| E11.5 | 138 (54*) | 312 (5*) | 170 (1*) |
| E12.5 | 14 (13*) | 39 (4*) | 22 |
| E13.5 | 3 (3*) | 11 | 1 |
| E17.5 | 0 | 6 | 1 |
| P21 | 0 | 36 | 19 |
Dead. E, embryonic day; P, postnatal day.
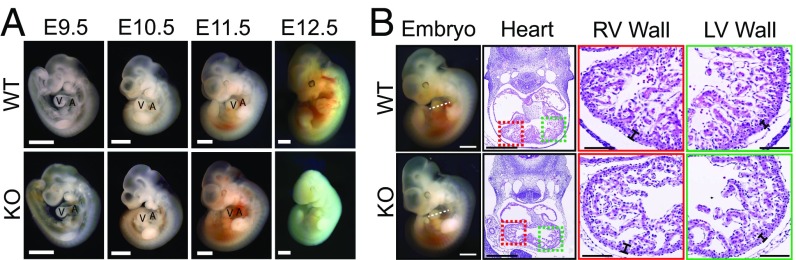
Fig. 1.
General phenotype of HSPB7 KO mice. (A) Images of WT and KO embryos from E9.5 to E12.5. A, left atria; V, left ventricle. (Scale bar: 1 mm.) (B) H&E images of E11.5 embryos acquired using NanoZoomer slide scanner. (Scale bar: 0.5 mm.) Estimated location of sectioning is indicated by a dotted line on the whole-mount embryos images, Left. (Scale bar: 1 mm.) Magnified right ventricle (RV; red box) and left ventricle (LV; green box) wall views are shown, Right. Left and right ventricle wall thicknesses are indicated by rulers. (Scale bar: 0.1 mm.)
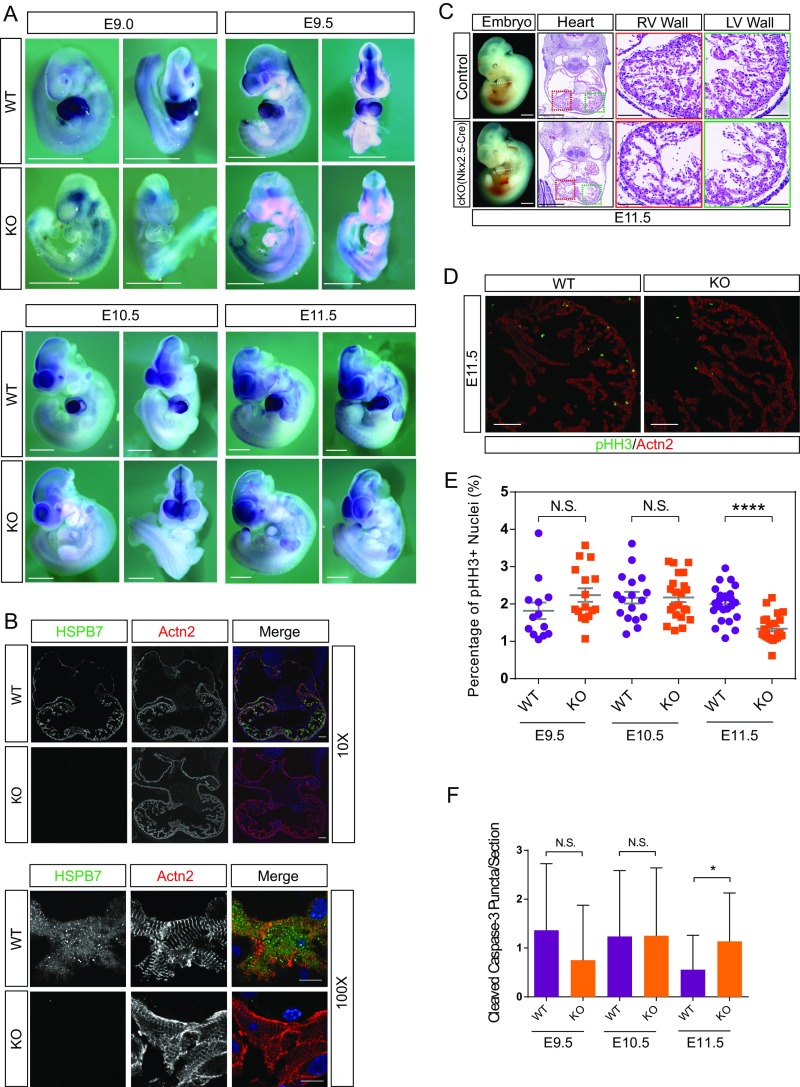
To uncover detailed morphological changes in mutant hearts, we performed H&E staining on embryonic heart sections. At E10.5, HSPB7 KO hearts were slightly smaller and more rounded in shape relative to controls (Fig. S1E). By E11.5, trabeculae were smaller and thinner, and the right ventricular wall was thinner in E11.5 mutant hearts relative to controls (Fig. 1B). To investigate whether the observed phenotypes were owing to loss of HSPB7 function in cardiomyocytes, we examined expression of HSPB7 by whole-mount RNA in situ hybridization of WT and mutant embryos. Results showed that HSPB7 was exclusively expressed in heart and undetectable in other parts of the embryo (Fig. S2A). Immunofluorescent staining using antibodies against HSPB7 and the cardiomyocyte marker α-actinin indicated that that HSPB7 expression was confined to cardiomyocytes (Fig. S2B). HSPB7 was expressed throughout the cytoplasm of cardiomyocytes in an irregular punctate pattern in contrast to the striated pattern observed for sarcomeric α-actinin (Fig. S2B). To further ensure that observed phenotypes resulted from a cardiomyocyte-specific requirement for HSPB7, we used Nkx2.5-Cre (27) and cTnT-Cre (28) mouse lines to delete HSPB7 specifically in cardiomyocytes. Timed pregnancy results showed that all HSPB7 cardiac-specific KO (cKO) embryos developed similar phenotypes as global KO embryos, similarly dying before E12.5 (Fig. S2C and Tables S2 and S3).
Fig. S2.
Expression pattern of HSPB7 in mouse embryos and general phenotype of HSPB7 cardiac-specific KO by Nkx2.5-Cre. (A) Whole-mount RNA in situ hybridization (ISH) analysis of HSPB7 expression in whole WT and HSPB7 KO embryos (left lateral and anterior views) at E9.0–E11.5 using an HSPB7-specific probe. The unspecific signal in the head/pharyngeal arches region of both WT and KO embryos is caused by ISH probes that were trapped in the cavities. (Scale bar: 1 mm.) (B) Immunofluorescence analysis of HSPB7 (green) and α-actinin (Actn2; red) in a traverse section of the heart of E11.5 WT and HSPB7 KO embryos. Colors are depicted in merged images. DNA was stained with DAPI. (Scale bar: 10× objective, 100 μm; 100× objective, 10 μm.) (C) H&E images of E11.5 control and HSPB7 cardiac-specific KO (cKO) using Nkx2.5-Cre embryos acquired using NanoZoomer slide scanner. (Scale bar: 0.5 mm.) Estimated location of sectioning is indicated by a dotted line on the whole-mount embryos images to the left. (Scale bar: 1 mm.) Magnified right ventricle (RV; red box) and left ventricle (LV; green box) wall views are shown to the right. Left and right ventricle wall thicknesses are indicated by rulers. (Scale bar: 0.1 mm.) (D) Representative immunofluorescence images of pHH3 (green) and Actn2 (red) in a transverse section of the heart of E11.5 WT and HSPB7 KO embryos. (Scale bar: 100 μm.) (E) Percentage of pHH3-positive cardiomyocytes in WT and HSPB7 KO embryos at various developmental stages. E9.5: four to seven sections per embryo, two to three embryos per group. E10.5: four to five sections per embryo, four to five embryos per group. E11.5: five to eight sections per embryo, four embryos per group. Error bars indicate mean ± SEM. Statistical significance was determined with two-tailed Student’s t test. Not significant (N.S.): E9.5, P = 0.1471; E10.5, P = 0.966. ****P < 0.0001. (F) Cleaved Caspase-3 puncta observed in WT and HSPB7 KO cardiomyocytes (highlighted by Actn2 staining) from E9.5 to E11.5. Two to three embryos, 11–34 sections per group. Error bars indicate mean ± SEM. Statistical significance was determined with two-tailed Student’s t test. *P = 0.0433.
Table S2.
Observed genotype distribution of embryos/pups recovered from mating between HSPB7+/−, Nkx2.5-Cre+ males and HSPB7 f/f females
| Stage | f/−, Cre+ | f/+, Cre+ | f/− | f/+ |
| E11.5 | 5 (1*) | 10 | 5 | 5 |
| E12.5 | 7 (6*) | 10 | 7 | 6 |
| E13.5 | 3 (3*) | 4 | 5 | 4 |
| P1–3 | 0 | 5 | 7 | 3 |
Dead. E, embryonic day; P, postnatal day.
Table S3.
Observed genotype distribution of embryos/pups recovered from mating between HSPB7+/−, cTnT-Cre+ males and HSPB7 f/f females
| Stage | f/−, Cre+ | f/+, Cre+ | f/− | f/+ |
| E9.5 | 3 | 2 | 4 | 1 |
| E10.5 | 1 | 4 | 2 | 2 |
| E11.5 | 3 (1*) | 2 | 3 | 3 |
| E12.5 | 9 (9*) | 7 | 1 | 9 |
| P21 | 0 | 9 | 12 | 18 |
Dead. E, embryonic day; P, postnatal day.
As expression of HSPB7 was confined to cardiomyocytes (Fig. S2 A and B) and the overall phenotype of cKOs resembled those of global KOs (Fig. 1B and Fig. S2C), we decided to use the global HSPB7 KO for the remainder of our experiments. To investigate whether a decrease in cardiomyocyte proliferation could account for smaller hearts and thinner trabeculae in mutant embryos, we used antibodies against the mitotic marker Phospho-Histone H3 (pHH3) and sarcomeric α-actinin to highlight proliferative cardiomyocytes (Fig. S2D). Quantification of the percentage of pHH3-positive cardiomyocytes indicated that proliferation in HSPB7 KO was not affected until E11.5 (Fig. S2E), when about 40% of mutant embryos had already died. Decreased proliferation observed at this stage just before death suggested that it might be a secondary event rather than causative for the observed phenotype. We barely observed apoptotic cardiomyocytes in both WT and HSPB7 KO E9.5–E11.5 embryos by Cleaved Caspase 3 staining (Fig. S2F).
AABs Form Within Sarcomeres of HSPB7 KO Cardiomyocytes.
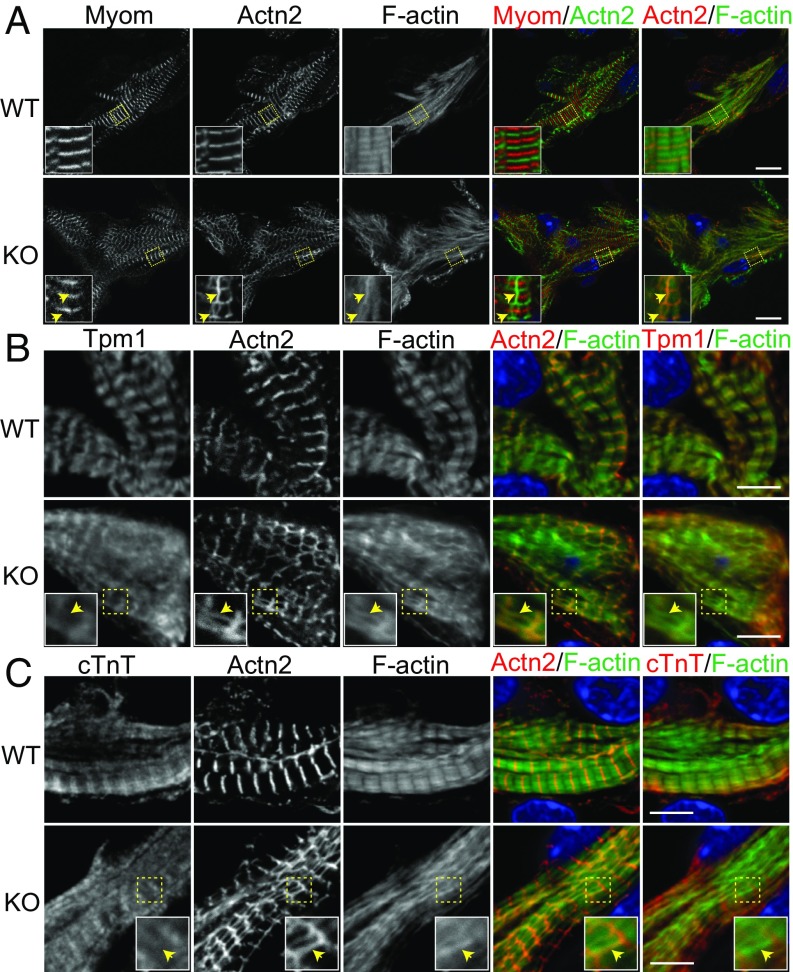
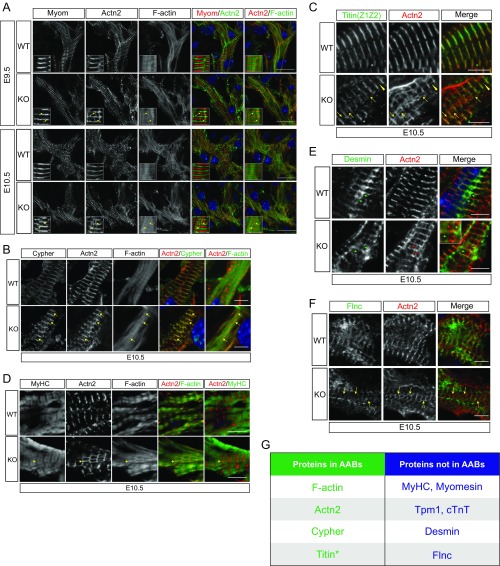
Sarcomeres are the fundamental unit of the contractile apparatus in cardiomyocytes. Interestingly, sHSPs translocate to and stabilize sarcomeres under stress conditions (29). We, therefore, asked whether there were defects in sarcomere assembly that might lead to insufficient cardiac function in HSPB7 KO embryos. To assess sarcomere integrity, we performed immunofluorescence staining of E11.5 embryonic heart sections using antibodies against two classic sarcomeric proteins: α-actinin at the Z line and myomesin at the M line. In WT cardiomyocytes, Z lines and M lines were prominent and discrete, indicating proper assembly of sarcomeres (Fig. 2A). On the contrary, sarcomeres in HSPB7 KO cardiomyocytes were poorly organized (Fig. 2A). Whereas M lines were relatively unchanged, Z lines were narrower and had a “checkerboard” appearance. Of note, there were atypical structures interconnecting Z lines that were stained by both α-actinin (Z line) and phalloidin (F actin) (yellow arrows in Fig. 2A), indicating that they were actin filaments associated with α-actinin. Similar abnormal structures were observed in E9.5 KO hearts and E10.5 KO hearts (yellow arrows in Fig. S3A), indicating that this phenomenon preceded emergence of an overt cardiac morphogenetic phenotype. As the abnormal structures could be stained by phalloidin and contained α-actinin, we designated them AABs. To further characterize AABs, we used antibodies against another Z-line protein, cypher (30, 31), to investigate whether cypher also might be a component of AABs. As shown in Fig. S3B, cypher staining was detected in most AABs marked by α-actinin (yellow arrows in Fig. S3B). We also used an antibody that specifically recognizes the Z-line portion of titin (Z1Z2) to examine whether titin was included in AABs. Although the Z-line portion of titin was largely overlapping with α-actinin in both WT and mutant sarcomeres, the great majority of AABs did not contain Z1Z2 of titin (yellow arrows in Fig. S3C). In very rare instances, Z1Z2 was observed within AABs (yellow arrowheads in Fig. S3C).
Fig. 2.
Characterization of AABs in HSPB7 KO embryos. (A–C) WT and KO heart cryosections were stained with phalloidin (F actin) and antibodies against α-actinin (Actn2) and (A) E11.5: Myomesin (Myom), (B) E10.5: Tpm1, or (C) E10.5: cTnT. The locations of AABs or their relative positions are indicated by yellow arrows. High magnification views are shown as Insets in corresponding images. Colors are depicted in merged images. DNA is stained with DAPI (blue). (Scale bar: A, 10 μm; B and C, 5 μm. Magnification: A, Insets, 3.3×; B and C, Insets, 2.2×.)
Fig. S3.
Characterization of AABs. (A) Representative immunofluorescence images of WT and HSPB7 KO E9.5–E10.5 heart sections stained with antibodies against α-actinin (Actn2), phalloidin (F actin), and (A) myomesin (Myom), (B) Cypher, and (D) Myosin heavy chain (MyHC). The locations of AABs or their relative locations are indicated by yellow arrows. High magnification views are shown as Insets in corresponding images. Colors are depicted in merged images. DNA is stained with DAPI (blue). (Scale bar: A, 10 μm; B and D, 5 μm.) (C) Representative immunofluorescence images of WT and HSPB7 KO E10.5 heart sections stained with antibodies against titin (Titin Z1Z2), Actn2, and phalloidin (F actin). Yellow arrowheads indicate AABs that contain titin; yellow arrows indicate AABs that do not contain titin. (Scale bar: 5 μm.) (E) Representative immunofluorescence images of WT and HSPB7 KO E10.5 heart sections stained with antibodies against desmin and Actn2. Red arrows indicate AABs; green arrows indicate desmin-containing structures that interconnect Z lines. Inset shows an enlarged view of the region marked by the yellow box in the HSPB7 KO merged image. (Scale bar: 5 μm.) (F) Representative immunofluorescence images of WT and HSPB7 KO E10.5 heart sections stained with antibodies against Flnc and Actn2. The locations of AABs or their relative locations are indicated by yellow arrows. (Scale bar: 5 μm.) (G) A table summarizing the protein components of AABs as discovered from our immunofluorescent analyses. *In rare cases. (Magnification: A, Insets, 3.3×; E, Inset, 1.7×.)
Troponin–tropomyosin complexes are pivotal to contractile function of sarcomeres (32). To investigate whether AABs possessed troponin–tropomyosin complexes and might have the potential to contract, we used two antibodies against Tpm1 (Fig. 2B) and cardiac troponin T (cTnT) (Fig. 2C). As expected, the staining pattern of Tpm1 or cTnT appeared closely flanking the Z lines, consistent with staining of the narrow cardiac I bands. However, neither Tpm1 nor cTnT were detected in AABs (yellow arrows in Fig. 2 B and C). This result indicated that AABs did not contain troponin–tropomyosin complexes and thus, would be unlikely to generate contractions. Moreover, neither myomesin nor myosin heavy chain were detected in the AABs, also indicating that they are noncontractile (Fig. 2A and Fig. S3D).
Desmin forms short filament-like structures interconnecting Z lines (33). We found similar desmin-containing structures in both WT and mutant cardiomyocytes (green arrows in Fig. S3E), but they did not overlap with AABs (red arrows in Fig. S3E), indicating that they are distinct from AABs. Because HSPB7 has been reported to interact with filamin C (Flnc) to prevent its aggregation and mislocalization (34), we also performed immunostaining with antibodies to Flnc. However, no changes in localization of Flnc and no evidence for Flnc aggregates were observed in mutant cardiomyocytes. Additionally, Flnc was not observed within AABs (yellow arrows in Fig. S3F).
Taken together, AABs found in HSPB7 KO cardiomyocytes appear to be an abnormal form of actin filaments bundles that lack a troponin–tropomyosin complex but include the Z-line components α-actinin and cypher (Fig. S3G). Formation of AABs appears to reflect defective actin filament/thin filament assembly, as no alterations were observed in thick filaments based on immunostaining for myomesin (Fig. 2A) and myosin heavy chain (Fig. S3D).
HSPB7 KO Hearts Have Increased Lmod2 and Longer Thin Filaments in Their Sarcomeres.
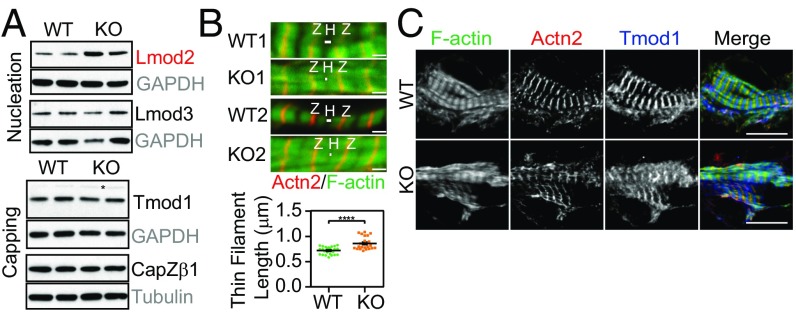
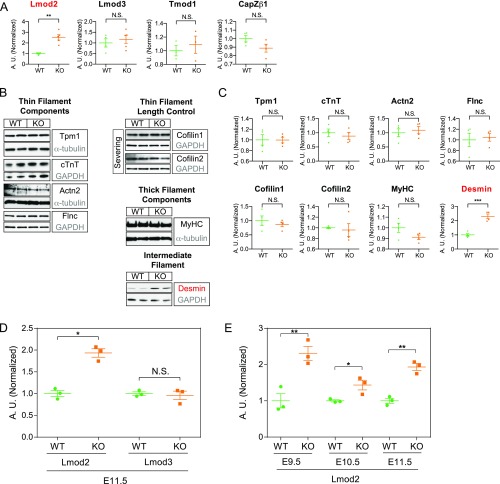
The foregoing observations suggested that thin filament assembly was dysregulated in HSPB7 KO hearts. As thin filament assembly is a highly regulated process involving a myriad of actin binding proteins (35), we examined the abundance of various key regulators of actin dynamics under the assumption that HSPB7 might stabilize one or some of these proteins acting as a molecular chaperone. While amounts of most candidate proteins remain unchanged, the actin nucleator Lmod2 was significantly up-regulated in HSPB7 KO hearts (Fig. 3A and Fig. S4 A–C). The intermediate filament protein desmin was also up-regulated (Fig. S4 B and C), although this might be a secondary effect, as its level increases in heart failure (36). Interestingly, qRT-PCR analyses showed that Lmod2 mRNA levels exhibited a similar fold increase to that of Lmod2 protein (two- to threefold) (Fig. S4 D and E). This observation suggested that HSPB7 might not directly regulate levels of Lmod2 through a protein–protein interaction.
Fig. 3.
Lmod2 is up-regulated, and thin filament length is increased in HSPB7 KO embryos. (A) Western blots of various thin filament length regulators in E11.5 hearts isolated from WT and KO. GAPDH or α-tubulin served as loading controls (gray). Proteins depicted in red were found to be significantly increased in HSPB7 KO embryos. Asterisk in Tmod1 indicates nonspecific bands. (B) Immunofluorescence images (Upper) of two (1, 2) WT and KO embryos from E10.5 heart sections. Z lines were stained using antibodies against α-actinin (Actn2; red), and actin (thin) filaments were stained using phalloidin (green). Quantification of WT vs. HSPB7 KO thin filament length (Lower). Two embryos and 12–20 sarcomeres per group. Error bars indicate mean ± SEM. Statistical significance was determined with two-tailed Student’s t test. H, actin-free H zone; Z, Z lines. ****P < 0.0001. (Scale bar, 1 μm.) (C) WT and HSPB7 KO E10.5 heart cryosections were stained with phalloidin (F actin) and antibodies against Actn2 and Tmod1. (Scale bar: 10 μm.)
Fig. S4.
Lmod2 transcript is up-regulated in HSPB7 KO embryos. (A) Quantitative densitometric analysis of Lmod2, Lmod3, Tmod1, and CapZβ1 in E11.5 hearts isolated from WT and HSPB7 KO. Proteins depicted in red were found to be significantly increased in HSPB7 KO embryos. Three to five embryos per group. Error bars indicate mean ± SEM. Statistical significance was determined with two-tailed Student’s t test. **P = 0.0012 (Lmod2). (B) Western blot and (C) corresponding quantitative densitometric analysis of various actin binding proteins in E11.5 hearts isolated from WT and HSPB7 KO. GAPDH or α-tubulin served as loading controls (gray). Flnc and cofilin1 share the same GAPDH loading control, as they were cut from the same membrane. Proteins depicted in red were found to be significantly increased in HSPB7 KO embryos. Four to five embryos per group. Error bars indicate mean ± SEM. Statistical significance was determined with two-tailed Student’s t test. ***P = 0.0005 (desmin). (D) qRT-PCR analysis of Lmod2 and Lmod3 in E11.5 WT and HSPB7 KO hearts. Data are normalized to corresponding 18S levels and expressed as arbitrary units (A.U.); n = 3 embryos per group with each reaction tested in triplicate. Data are represented as mean ± SEM. Statistical significance was determined with two-tailed Student’s t test. N.S. (P = 0.7251). *P = 0.0014. (E) qRT-PCR analysis of Lmod2 at different developmental stages (E9.5–E11.5) in WT and HSPB7 KO hearts. Data are normalized to corresponding 18S levels and expressed as A.U.; n = 3 embryos per group with each reaction tested in triplicate. Data are represented as mean ± SEM. Statistical significance was determined with two-tailed Student’s t test. N.S., not significant. *P = 0.0331 (E10.5); **P < 0.01 (E9.5, P = 0.0088; E11.5, P = 0.0014).
In a previous study, overexpression of Lmod2 in cultured cardiomyocytes reduced binding of the pointed end capping protein Tmod1 to pointed ends of actin filament, and was accompanied by thin filament elongation (37). As Lmod2 was significantly up-regulated in HSPB7 KO hearts, we assessed thin filament length in both WT and HSPB7 KO cardiomyocytes. Thin filament length was significantly increased in HSPB7 KO cardiomyocytes from E10.5 hearts (Fig. 3B), reminiscent of findings in Lmod2-overexpressing cardiomyocytes (37). Although overall amounts of the pointed end capping protein Tmod1 were unchanged (Fig. 3A and Fig. S4A), and some Tmod1 was associated with thin filament pointed ends in the middle of the sarcomere, another portion of Tmod1 appeared diffusely localized throughout the cytoplasm in the HSPB7 KO cardiomyocytes (Fig. 3C). This contrasts with WT sarcomeres, where all of the Tmod1 is located at the thin filament pointed ends and no cytoplasmic Tmod1 is detected (Fig. 3C). This suggests that not all Tmod1 had assembled at its normal sarcomeric location in the absence of HSPB7, consistent with defects in thin filament assembly. Reduced Tmod1 capping of thin filament pointed ends may contribute to their elongation (38).
Loss of HSPB7 Leads to Formation of AABs Independent of Lmod2 and Tmod1.
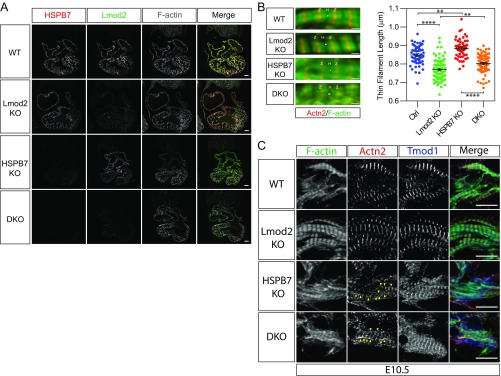
Lmod2 KO mouse cardiomyocytes have shorter thin filament lengths, and mice develop early-onset dilated cardiomyopathy (9). Thus, if abnormal elongation of thin filaments was caused by elevated expression of Lmod2, we hypothesized that depletion of Lmod2 in HSPB7 KO mice might ameliorate sarcomeric defects. To address this, we generated Lmod2/HSPB7 double-homozygous null mice. Immunofluorescent staining using antibodies against HSPB7 and Lmod2 indicated that both proteins were successfully eliminated in double-KO embryos (Fig. S5A), which died before E12.5, the same stage as HSPB7 KOs. As expected, thin filament length was reduced ∼9% in Lmod2 KO cardiomyocytes (9) and increased ∼4% in HSPB7 KOs (Fig. S5B). The average thin filament length observed in Lmod2 KOs was incrementally increased by ∼4% by loss of HSPB7, comparable with that observed in HSPB7 KOs alone. These observations suggested that each of these pathways regulates thin filament length in an opposite and independent fashion. Examination of thin filament assembly by performing immunostaining with sarcomeric α-actinin antibody and phalloidin showed that AABs were still present in double-KO embryos (yellow arrowheads in Fig. S5C). Together, these observations indicated that sarcomeric phenotypes observed in HSPB7 KOs could not be rescued by loss of Lmod2, and thus, were not consequent to observed up-regulation of Lmod2. These results indicate that HSPB7 directly limits thin filament length and that loss of HSPB7 leads to AAB formation by an Lmod2-independent pathway.
Fig. S5.
HSPB7 represses formation of AABs independent of Lmod2. (A) Immunofluorescence images of WT, Lmod2 KO, HSPB7 KO, and Lmod2/HSPB7 double KO (DKO) E10.5 heart sections stained with antibodies against HSPB7, Lmod2, and phalloidin (F actin). (Scale bar: 100 mm.) (B) Representative immunofluorescence images (Left) and thin filament length measurements (Right) of control (Ctrl; including WT and HSPB7 heterozygous KO), Lmod2 KO, HSPB7 KO, and Lmod2/HSPB7 DKO E10.5 embryos. Two embryos, 50–78 sarcomeres measured for each group. Error bars indicate mean ± SEM. Control, 0.849 ± 0.0069 mm; Lmod2 KO, 0.77 ± 0.0066 mm; HSPB7 KO, 0.885 ± 0.0074 mm; DKO, 0.802 ± 0.006 mm. Statistical significance was determined with one-way ANOVA. H, actin-free H zone; Z, Z lines. **P < 0.01 (Ctrl vs. HSPB7 KO, P = 0.0033; Lmod2 KO vs. DKO, P = 0.0021); ****P < 0.0001. (Scale bar: 1 μm.) (C) Representative immunofluorescence images of WT, Lmod2 KO (Lmod2 KO), HSPB7 KO, and Lmod2/HSPB7 DKO E10.5 heart cryosections stained with antibodies against Tmod1, α-actinin (Actn2), and phalloidin (F actin). AABs are indicated by yellow arrowheads. (Scale bar: 10 mm.)
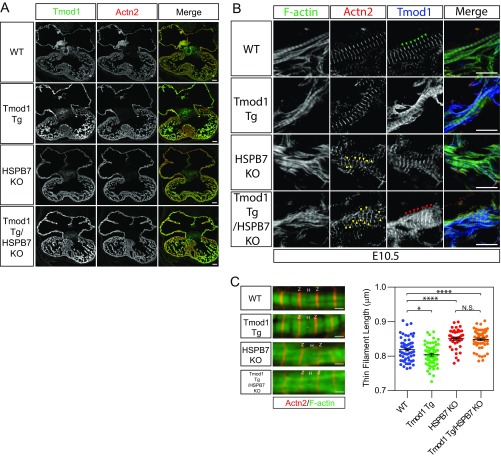
The presence of Tmod1 in the cytoplasm of HSPB7 KO cardiomyocytes could have resulted from the up-regulation of Lmod2, as Tmod1 and Lmod2 have been proposed to compete for binding to pointed ends in cultured cardiomyocytes (37). However, the presence of cytoplasmic Tmod1 was not corrected in Lmod2/HSPB7 double-KO embryos (Tmod1 staining in Fig. S5C), suggesting that alterations in Tmod1 localization were not caused by up-regulation of Lmod2. Previous studies in cultured cardiomyocytes have shown that inhibition of Tmod1 activity by antibody microinjection results in actin filament elongation from pointed ends, with increased overall length of thin filaments, and decreased beating of cardiomyocytes (38). These observations suggested that reduced Tmod1 association with thin filament pointed ends could potentially account for the increased length of sarcomeric actin filaments and/or formation of AABs in HSPB7 KO embryos. Therefore, to examine whether overexpression of Tmod1 could rescue HSPB7 phenotypes, we crossed Tmod1 Tg mice (10) into an HSPB7 KO background. Immunofluorescence images of whole embryonic hearts showed that expression of Tmod1 was substantially increased in Tmod1 Tg hearts and HSPB7 KO/Tmod1 Tg hearts (Fig. S6A). Despite the significant increase of Tmod1 at pointed ends (compare red arrowheads with green arrowheads in Fig. S6B), AABs were still present in HSPB7 KO/Tmod1 Tg embryonic hearts (yellow arrowheads in Fig. S6B). However, the thin filament length was not altered in HSPB7 KO/Tmod1 Tg compared with HSPB7 KO, although thin filaments were slightly shortened in Tmod1 Tg compared with WT (Fig. S6C). These results indicated that increasing Tmod1 at pointed ends could not rescue either the AAB or longer thin filament phenotype of the HSPB7 KOs.
Fig. S6.
HSPB7 represses formation of AABs independent of Tmod1. (A) Immunofluorescence images of WT, Tmod1 Tg, HSPB7 KO, and Tmod1 Tg/HSPB7 KO E11.5 heart sections stained with antibodies against Tmod1 and α-actinin (Actn2). (Scale bar: 100 mm.) (B) Representative immunofluorescence images of WT, Tmod1 Tg, HSPB7 KO, and Tmod1 Tg/HSPB7 KO E10.5 heart cryosections stained with antibodies against Tmod1, Actn2, and phalloidin (F actin). Yellow arrowheads indicate AABs marked by Actn2 staining. Green arrowheads indicate thin filament pointed ends of WT sarcomeres marked by Tmod1 staining. Red arrowheads indicate thin filament pointed ends of Tmod1 Tg/HSPB7 KO sarcomeres marked by Tmod1 staining. (Scale bar: 10 mm.) (C) Representative immunofluorescence images (Left) and thin filament length measurements (Right) of WT, Tmod1 Tg, HSPB7 KO, and Tmod1 Tg/HSPB7 KO E10.5 embryos. Two embryos, 41–63 sarcomeres measured for each group. Error bars indicate mean ± SEM. WT, 0.819 ± 0.0041 mm; Tmod1 Tg, 0.804 ± 0.004 mm; HSPB7 KO, 0.851 ± 0.0044 mm; HSPB7 KO/Tmod1 Tg, 0.849 ± 0.0038 mm. Statistical significance was determined with one-way ANOVA. Not significant (N.S.; P = 0.9936). H, actin-free H zone; Z, Z lines. *P < 0.05 (P = 0.0275); ****P < 0.0001. (Scale bar, 1 μm.)
HSPB7 Reduces Actin Polymerization by Directly Binding G Actin.
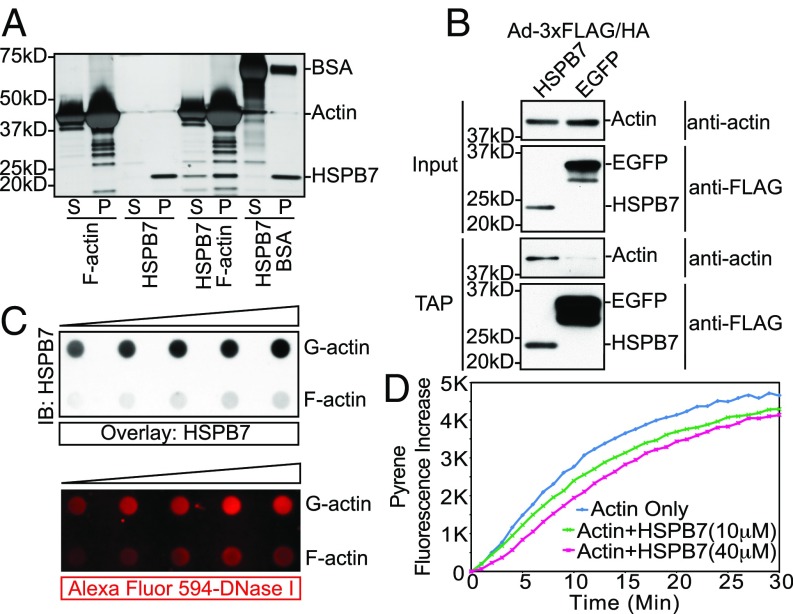
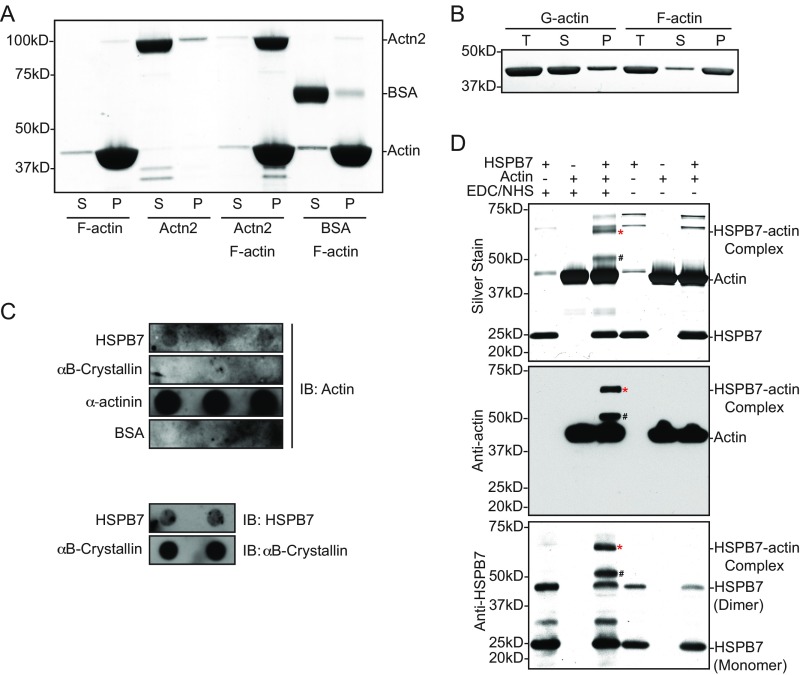
The foregoing findings suggested that HSPB7 might directly influence actin polymerization independent of Lmod2 and Tmod1. To investigate mechanisms by which HSPB7 could affect actin polymerization, we sought to determine whether there was a direct interaction between HSPB7 and actin. To this end, we investigated whether HSPB7 bound to F actin using a cosedimentation binding assay. We observed that HSPB7 alone pelleted after ultracentrifugation (Fig. 4A), probably because of its tendency to form high-molecular weight oligomers (39). Interestingly, we found that the portion of HSPB7 in the supernatant fraction containing G actin increased when coincubated with F actin but not with BSA (Fig. 4A), while F-actin binding protein α-actinin-2 was coprecipitated with F actin (Fig. S7A). This result suggested that HSPB7 might bind directly to G actin rather than to F actin. To examine whether HSPB7 interacted with actin in mouse cardiomyocytes, we performed tandem affinity purification (TAP) using isolated neonatal mouse cardiomyocytes infected with adenovirus-expressing 3xFLAG/HA-tagged HSPB7. Results confirmed that the interaction between HSPB7 and actin occurred in cardiomyocytes (Fig. 4B). To investigate a direct interaction between HSPB7 and G actin, we utilized a blot overlay assay by immobilizing either G actin or F actin on Nitrocellulose membranes and then, overlaying with HSPB7 protein. Western blotting with antibody to HSPB7 indicated that HSPB7 preferentially bound G actin over F actin in a manner comparable with preferential binding of DNase I to G actin over F actin (40) (Fig. 4C). The much weaker binding of HSPB7 observed in F-actin samples was likely owing to the presence of some unpolymerized G actin within F-actin preparations (Fig. S7B). Interestingly, the interaction with G actin was a unique feature of HSPB7, as another heart-enriched sHSP, HSPB5/αB-crystallin, did not bind G actin in reciprocal blot overlay assays (Fig. S7C). To further confirm the interaction between G actin and HSPB7, we performed zero-length covalent cross-linking experiments using EDC [N-(3-dimethylaminopropyl)-N'-ethylcarbodiimide hydrochloride] and NHS (N-hydroxysuccinimide) (41, 42). Purified HSPB7 and G actin could be covalently cross-linked to a complex with a molecular mass of ∼65 kDa, consistent with a 1:1 stoichiometry of the HSPB7–actin complex (asterisks in Fig. S7D). Furthermore, both HSPB7 and actin were found within the complex as shown by Western blot analysis (asterisks in Fig. S7D). Taken together, our results showed that HSPB7 bound G actin both in vitro and in cardiomyocytes.
Fig. 4.
HSPB7 binds G actin and inhibits actin polymerization. (A) Silver stain image of F-actin cosedimentation binding assay. S, supernatant; P, pellet. (B) TAP performed in isolated neonatal mouse cardiomyocytes infected by adenoviruses expressing either 3xFLAG/HA-tagged HSPB7 or EGFP. Samples were immunoblotted with actin or FLAG antibodies. The residual amount of actin in control EGFP sample is caused by unspecific binding of actin to EGFP, which was expressed at a much higher level than HSPB7. (C) Western blot image of blot overlay assay using an antibody against HSPB7 (Upper). Fluorescence image of Alexa Flour 594-conjugated DNase I incubated with the same membrane after treatment with stripping buffer (Lower). IB, immunoblot. (D) Actin polymerization assay with or without the presence of recombinant HSPB7 protein. Pyrene labeled G actin (2 μM) was preincubated with increasing amount of HSPB7 (10 μM, green; 40 μM, magenta) or buffer alone (blue).
Fig. S7.
HSPB7 binds G actin and inhibits actin polymerization. (A) InstantBlue-stained SDS/PAGE gel image of positive control [α-actinin (Actn2)] and negative control (BSA) for F-actin cosedimentation binding assay. (B) InstantBlue-stained SDS/PAGE gel image of G-actin and F-actin preparation for blot overlay assay after ultracentrifugation. Note that there was a small amount of unpolymerized G actin still present in F-actin preparation as well as a small amount of polymerized F actin present in G-actin preparation. P, pellet; S, supernatant; T, total actin. (C) Western blot images of blot overlay assay using an antibody against actin (Upper). HSPB7, αB-crystallin, Actn2 (positive control), and BSA (negative control) proteins were immobilized on Nitrocellulose membrane and incubated with G actin for 1 h at room temperature; then, the membrane was washed and immunoblotted with antibody against actin to reveal potential interactions between test proteins and G actin. The presence of HSPB7 or αB-crystallin on the Nitrocellulose membrane was confirmed by immunoblots using antibody raised against either HSPB7 or αB-crystallin (Lower). IB, immunoblot. (D) Silver stain and Western blot images of HSPB7 cross-linking with G actin by EDC/NHS. *HSPB7–actin complex; #band with molecular size consistent with a complex containing actin and HSPB7 degradation products (∼13 kDa) found during HSPB7 purification.
Next, we sought to determine whether HSPB7 was capable of inhibiting actin polymerization based on its ability to interact with G actin and thus, could have the potential to prevent excessive thin filament elongation in cells. To this end, we performed actin polymerization assays with or without purified recombinant HSPB7 protein. Interestingly, we found that HSPB7 seemed to reduce the rate of actin polymerization in a dose-dependent manner (Fig. 4D). Like other actin binding proteins [e.g., profilin (43) and LATS1], we had to use relatively high concentrations of HSPB7 to repress actin polymerization. Taken together, these results suggest that HSPB7 may transiently bind G actin and limit its ability to polymerize.
Discussion
In this study, we have identified a role for an sHSP in regulating thin filament length in cardiomyocytes in vivo and elucidated the mechanism by which it does so. HSPB7-deficient embryos died before E12.5 and exhibited increased thin filament length and AABs that extended across the normally actin-free H zone. Although defects in cardiomyocyte proliferation were observed at E11.5 in HSPB7 KOs (Fig. S2E), proliferative defects are likely to be secondary to cardiac dysfunction and not causal to cardiac dysfunction, as they occurred at a time when 40% of the HSPB7 KO embryos had died; also, defects in cardiac muscle structure were already evident by E9.5 (Fig. S3A). We found that expression of the actin nucleator Lmod2 was up-regulated, and the pointed end capping protein Tmod1 was mislocalized in HSPB7 KOs, suggesting that dysregulation of these proteins might account for sarcomeric phenotypes of HSPB7 KOs. However, genetic rescue studies indicated that sarcomeric phenotypes of HSPB7 KOs were independent of dysregulation of Lmod2 or Tmod1, suggesting a more direct role for HSPB7 in regulating thin filament length. Indeed, several biochemical analyses showed that HSPB7 directly binds to monomeric G actin, which could reduce its ability to polymerize into F actin and thus, limit thin filament growth.
We found that average thin filament length was increased in HSPB7 KO embryos. As overexpression of Lmod2 tends to increase thin filament length (37), we sought to determine if up-regulation of Lmod2 in HSPB7 KO could account for the phenotypes by creating Lmod2/HSPB7 double-KO mice. Like Lmod2 single-KO mice (9), the thin filament length was significantly reduced compared with HSPB7 single KO, underscoring the importance of Lmod2 in maintaining proper thin filament length. Although it seemed that up-regulation of Lmod2 could explain the average thin filament length increase in HSPB7 KO, we found that thin filaments in Lmod2/HSPB7 double KO were still significantly longer than Lmod2 single KO, indicating that HSPB7 may directly repress thin filament elongation via a distinct pathway from Lmod2. Interestingly, the percentage of thin filament length increase in Lmod2/HSPB7 double KO over Lmod2 KO was comparable with the increase in HSPB7 KO over control, indicating that the loss of HSPB7’s inhibitory effect, rather than increased expression of Lmod2, largely contributes to the longer thin filaments in the HSPB7 KO cardiomyocytes.
Overexpression of Lmod2 was thought to inhibit Tmod1’s binding to pointed end by direct competition (37); however, recently, a new model has been proposed that Lmod overexpression and enhanced actin nucleation would generate more nascent thin filaments that elongate from their barbed ends. An increased number of filaments with free pointed ends could compete with preexisting pointed ends for limiting Tmod, resulting in decreased capping frequency and increased actin addition at pointed ends and longer filaments (44). Similarly, the up-regulation of Lmod2 observed in HSPB7 KO cardiomyocytes could produce excess free pointed ends with reduced Tmod1 capping, possibly contributing to the longer thin filaments observed in HSPB7 KO. To test this possibility, we overexpressed Tmod1 by crossing Tmod1 Tg mice to the HSPB7 KO background. However, an increased amount of Tmod1 at the pointed end did not reduce the length of the elongated thin filaments in HSPB7 KO, although thin filament length was decreased in Tmod1 Tg mice compared with WT as expected (10). This observation implies that thin filaments may largely elongate from their barbed ends in HSPB7 KO, as Tmod1 only inhibits addition of actin to the pointed end (6). Additionally, HSPB7 reduced rates of spontaneous actin polymerization in vitro, consistent with an effect on barbed ends, as polymerization occurs predominantly at fast-growing barbed ends under the conditions tested (45).
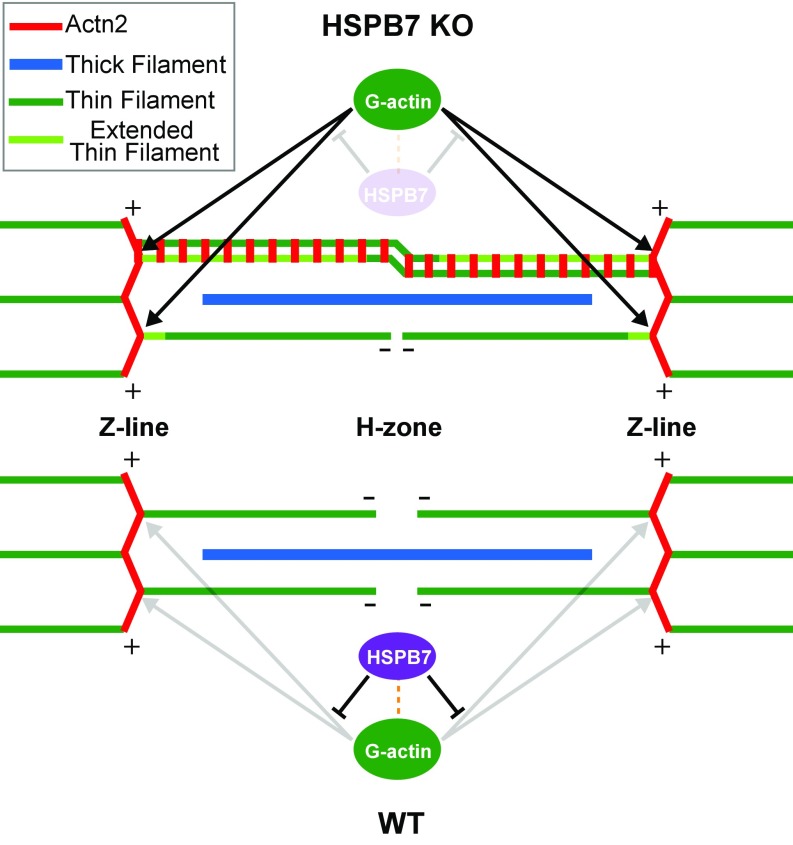
The AABs were continuous throughout the sarcomere. By probing with various antibodies raised against sarcomeric components, we discovered that these AABs bundles contain Z-line components, including α-actinin, but lack thin filament (Tpm1, cTnT) or thick filament components (myosin heavy chain, myomesin), which are critical for sarcomere contractile function (32). We concluded that these abnormal actin filament bundles are an aberrant Z-line structure and are unlikely to contract independently. We speculate that continued growth of thin filaments from their barbed ends through the H zone might result in confluence of antiparallel actin filaments, allowing binding of α-actinin and thus, mimicking the situation at the Z line (Fig. S8). Thus, α-actinin and other Z-line proteins (e.g., cypher) would recognize AABs as a pseudo-Z line. In addition, we found that expression of α-actinin remained constant in HSPB7 KO compared with WT (Fig. S4 B and C). The expansion of the abnormal Z-line structures containing α-actinin, F actin, cypher, and other Z-line components might decrease the amount of correctly localized α-actinin at the Z lines, which might further exacerbate defective thin filament assembly. The formation of these abnormal Z-line structures in HSPB7 KO cardiomyocytes resembles expanded Z-line pathologies referred to as streaming Z lines or nemaline bodies in skeletal muscle myopathies (46). To our knowledge, such structures have not been reported before in developing mouse cardiac muscles. Our data show that loss of the thin filament length regulator, Lmod2, in HSPB7 KOs does not rescue formation of AABs, implying that HSPB7 can act independently of Lmod2 in repressing excessive elongation of thin filaments and abnormal expansion of Z lines. Our data also suggest that the AABs, but not elongated thin filament length, may be the cause of cardiac defects and embryonic lethality in the HSPB7 KO mice. However, additional studies are needed to determine whether indeed AABs are sufficient to cause observed cardiac defects and lethality or whether the latter are caused by other as yet unidentified factors.
Fig. S8.
Model illustrating that HSPB7 represses excessive actin polymerization and formation of AABs. HSPB7 represses actin polymerization from barbed ends by binding actin monomers and limiting their availability to polymerize, thus maintaining normal Z-line width and proper thin filament length (WT). In HSPB7 KO mice, the inhibition of HSPB7 on actin polymerization from barbed ends is relieved, and as a result, actin filaments elongate from the barbed end, forming excessively wide Z structures extending across the sarcomere (light green portion). Some of the thin filaments extend beyond the actin-free H zone, overlapping with antiparallel actin filaments, and are cross-linked by α-actinin (an array of short red lines).
The mammalian sHSP family consists of 10 family members, HSPB1–10 (47). Although several sHSPs (e.g., HSPB5/αB-crystallin, HSPB6, HSPB7, and HSPB8) are relatively enriched in the heart (14), only ablating HSPB7 leads to an embryonic lethal phenotype in mice. In contrast, HSPB5/2 double-KO (48) and HSPB8-KO (49) mice are viable and do not have a basal cardiac phenotype. In addition, we recently showed that HSPB5, HSPB6, and HSPB8, which interact with BAG3, show a dramatic reduction in protein levels in Bag3 cardiac-specific KO mutant hearts (50). On the contrary, HSPB7, which does not interact with Bag3 (51), was unaltered (50). Together, these observations show that HSPB7 possesses an indispensable and unique function in the heart, which is distinct from other sHSPs. Our observation that, in contrast to HSPB7, αB-crystallin did not interact with G actin in blot overlay assays (Fig. S7C) also highlighted that the ability of HSPB7 to bind G actin and limit its availability for polymerization is not a common feature of sHSPs.
Recently, mice lacking HSPB7 specifically in skeletal muscle were reported to develop progressive myopathy (34). Flnc aggregation was found in HSPB7-deficient muscles, and the amount of Flnc aggregates was correlated with the severity of symptoms (34). However, we did not observe Flnc aggregation or any changes in Flnc localization in our HSPB7 KO embryos (Fig. S3F), indicating that HSPB7 may play different roles in cardiac and skeletal muscle. This notion was further supported by differences in HSPB7’s subcellular localization in embryonic heart and skeletal muscle. We observed that HSPB7 was evenly distributed in E11.5 cardiomyocytes. In contrast, HSPB7 was found to localize at the Z line in skeletal muscle cells (34) and adult cardiomyocytes (52), implying that HSPB7 might play distinct roles in embryonic and adult cardiomyocytes. These findings also indicate that HSPB7 could be involved in biological processes other than actin dynamics, which warrants additional investigation.
Materials and Methods
The sources of reagents and detailed methods are described in SI Materials and Methods. All animal studies were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals (53) and approved by the Institutional Animal Care and Use Committee of the University of California, San Diego. HSPB7 KO mice were generated as described (54). Immunostaining and in situ hybridization were performed as described (55, 56). qRT-PCR was performed as described (57). HSPB7 coding sequence was amplified from mouse adult heart cDNA and cloned into pET-trx1a (58).
SI Materials and Methods
Mice.
HSPB7 KO mice were generated by homologous recombination. The targeting vector was constructed using a plasmid containing a Neomycin cassette flanked by two Frt sites (54). A 446-bp PCR product containing exon 2 of the mouse HSPB7 gene was inserted into the BamHI site flanked by two loxP sites. A 4.3-kb upstream fragment and 4.5-kb downstream fragment were inserted into the vector to serve as 5′ and 3′ homologous arms, respectively. The completed targeting vector was linearized with NotI and electroporated into R1 mouse ES cells derived from 129-SV/J mice (University of California, San Diego Transgenic and Gene Targeting Core). After G418 selection, successfully targeted ES-cell clones were identified by Southern blot analysis after digestion with EcoRV. The WT allele generated a 10.3-kb band, whereas the correctly targeted mutant allele generated an 8-kb band. All PCRs were carried out using high-fidelity KOD Hot Start DNA polymerase (Novagen) and verified by Sanger sequencing. The 3′ probe (270 bp) used for Southern blot analysis was generated by PCR using primers Probe-F (5′-AAGCTCTCCGTACTCTGTAATGG-3′) and Probe-R (5′- AGTTTTAAGCTCGCAGACAGC-3′). A diphtheria toxin A expression cassette was placed at the 5′ end of the targeting construct to eliminate nonspecific homologous recombination. Two positive ES-cell clones with a floxed (f) HSPB7 exon 3 were microinjected into C57BL/6 blastocysts and transferred to pseudopregnant recipients. Male chimeras were mated with C57BL/6 females (Charles River), and agouti offspring were genotyped by PCR of tail DNA. After germ-line transmission of the targeted allele was confirmed, the Neomysin cassette was deleted by crossing with FLP mice (Jackson Labs). Heterozygous mice (HSPB7+/−) were obtained by crossing targeted mice (HSPB7 f/+) with Sox2-Cre (25) global deleter mice. Homozygous KO (HSPB7−/−) mice were generated by intercrossing heterozygous mice. Genotypes were determined by PCR using primers P1-2 (5′-CACTGGGAAAGGGCTGTAGT-3′) vs. P4 (5′-CCCTAGGGGTGAGATGTGAA-3′) for the KO allele and P3-2 (5′-TGACTCTCCCAGACAACAGC-3′) vs. P4-3 (5′-GTAGACACAGATTGAGGTGCGT-3′) for the WT allele. To generate cardiac-specific HSPB7 KO mice, Nkx2.5-Cre (27) or cTnT-Cre (28) heterozygous mice were crossed with HSPB7f/f mice to generate HSPB7 f/+, Cre+ mice. HSPB7 f/+, Cre+ male mice were backcrossed with HSPB7 f/f female mice to generate HSPB7 f/f, Cre+ offspring.
Lmod2 KO mice (9) and Tmod1 Tg mice (10) were generated as described previously. Lmod2/HSPB7 double-KO (Lmod2/HSPB7−/−) mice were obtained by intercrossing Lmod2/HSPB7 double-heterozygous mice. HSPB7 KO/Tmod1 Tg (HSPB7−/−, Tmod1 Tg) mice were generated by crossing HSPB7 heterozygous/Tmod1 Tg mice with HSPB7 heterozygous mice.
All procedures were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals (53) and approved by the Institutional Animal Care and Use Committee of the University of California, San Diego.
Antibodies.
Antibodies used for immunoblotting and immunofluorescence are listed as follows: HSPB7 (ab150390; Abcam); Phospho-Histone (Ser10, 9701; Cell Signaling); α-actinin (A7811; Genetex; GTX61894; Sigma-Aldrich); Myomesin (B4; DSHB); Cypher [1495 as previously described (59)]; Tpm1/Tpm1 (ab133292; Abcam); cTnT/cTnT (RV-C2; DSHB); pan-Myosin Heavy Chain/MyHC (A4.1025; DSHB); Titin Z1Z2 (gift from Siegfried Labeit, University of Heidelberg, Mannheim, Germany); Desmin (Y20, sc-7559; Santa Cruz Biotechnology); Flnc/Flnc (NBP1-89300; Novus); Lmod2 (E13, sc-135491; Santa Cruz Biotechnology); Lmod3 (14948–1-AP; Proteintech); Tmod1 [R1749 as previously described (60)]; CapZβ1 (1E5.25.4; DSHB); Cofilin1, 2 (gift from Walter Witke, University of Bonn, Bonn); GAPDH (6C5, sc-32233; Santa Cruz Biotechnology); α-tubulin (GTX27291; Genetex); CD31 (550274; BD Pharmingen); α-crystallin (ADI-SPA-223; Enzo); Panactin (C4, MAB1501; EMD Millipore); and HRP-conjugated FLAG antibody (M2, A8592; Sigma-Aldrich).
In Situ Hybridization.
Whole‐mount in situ hybridization was performed as previously described (61). Briefly, embryos isolated on E9.0–E11.5 were immediately fixed in ice-cold PBS with 4% paraformaldehyde (PFA) overnight at 4 °C and subsequently subjected to RNA in situ hybridization analyses. The HSPB7 RNA in situ probe was generated using the following primer set: HSPB7-ISH-Forward (5′-GGGGCACAGTACAAGAGGAG-3′) and HSPB7-ISH-Reverse (5′-ATGGAGAGGGATGCCCTAGT-3′). For histological analyses, after whole-mount in situ hybridization, embryos were embedded in paraffin, and 8-μm transverse sections were prepared using a microtome.
Whole-Mount PECAM/CD31 Staining.
Whole-mount PECAM staining was performed based on a previously described method (56). E10.5 WT and HSPB7 KO embryos were dissected and immediately fixed in PBS with 4% PFA overnight at 4 °C. Embryos were then washed in PBS, dehydrated in methanol (MeOH), and stored at −20 °C until antibody staining. Embryos were kept in Dent’s bleach (MeOH:DMSO:30% H2O2; 4:1:1) for 3 h at room temperature and washed with a series of descending MeOH/PBS + 0.1% Triton-X (PBST) concentrations (70% MeOH, 50% MeOH, PBST). Samples were blocked in 2% skimmed milk for 1 h and incubated with CD31 antibody overnight at 4 °C. Embryos were washed three times for 1 h with PBST before incubation with secondary antibodies overnight at 4 °C. Finally, embryos were washed three times for 1 h with PBST and applied with 3,3′-diaminobenzidine (Life Technologies) at room temperature.
Histology.
Histology was performed as previously described (62). Mouse embryos were dissected at various developmental stages and fixed in ice-cold PBS with 4% PFA overnight at 4 °C. After fixation, embryos were dehydrated with a series of 30–100% ethanol, embedded in paraffin, and cut into 8-μm sections by Microtome. Sections were stained with H&E using a standard protocol (57), mounted, and imaged using a Hamamatsu NanoZoomer 2.0HT Slide Scanning System.
Immunofluorescence.
Immunofluorescent histochemistry was performed as previously described (55, 62). Mouse embryos were dissected at various developmental stages and fixed in ice-cold PBS with 4% PFA overnight at 4 °C. Fixed embryos were then saturated in 5, 10, 15, and 20% sucrose in PBS, embedded in OCT Tissue-Tek (Thermo Fisher Scientific), and cut to 6-μm sections using a Leica CM 3050S cryostat (Leica Microsystems). Sections were blocked with PBST (1% BSA, 0.2% Tween-20 in PBS) for 1 h and then incubated with primary antibody solution (antibodies and 5% donkey serum in PBST) overnight in a humidified chamber at 4 °C. Subsequently, sections were washed three times with PBST and then incubated with secondary antibody solution (Jackson Immuno Research fluorescence-conjugated secondary antibodies, Life Technologies fluorescence-conjugated phalloidin, 5% donkey serum in PBST) for 2 h at room temperature. After washing with PBST another three times, sections were counterstained with DAPI (1:1,000) and mounted in DAKO fluorescence mounting medium (Agilent). Images were captured using an Olympus FluoView FV1000 Confocal Microscope. Images were taken from two to four embryos per group.
Immunoblotting.
E11.5 mouse hearts were dissected and snap-frozen in liquid nitrogen. Total protein extracts were prepared by homogenization of hearts in 100 μL RIPA buffer (50 mM Tris⋅Cl, pH 7.4, 150 mM sodium chloride, 1% Nonidet P-40, 0.1% SDS, 0.5% sodium deoxycholate, 1 mM EDTA) using a handheld pellet pestle (Sigma-Aldrich). Protein concentration was determined using a Micro BCA Protein Assay Kit (Thermo Fisher Scientific) before mixing with 4× LDS (lithium dodecyl sulfate) sample buffer (Life Technologies) and incubating for 10 min at 70 °C. Protein lysates were separated on 4–12% SDS/PAGE gels (Life Technologies) and transferred to PVDF membrane. Membranes were blocked in Tris Buffered Saline with 0.2% Tween-20 (TBST) supplemented with 5% BSA for 1 h at room temperature and incubated with indicated primary antibodies overnight at 4 °C. Blots were then washed with TBST and incubated with HRP-conjugated secondary antibodies for 1 h before immunoreactive protein bands were visualized using ECL reagent (Thermo). At least two independent experiments were performed for each antibody.
qRT-PCR.
qRT-PCR was performed as previously described (57). Briefly, E9.5–E11.5 hearts were isolated and snap-frozen in liquid nitrogen. Total RNA was extracted using TRIzol reagent per the manufacturer’s instructions (Life Technologies). cDNA was synthesized using SuperScript III reverse transcriptase (Life Technologies). qRT-PCR was performed on a CFX96 Real-Time System using SsoFast EvaGreen RT-PCR Supermix (Bio-Rad). Primer sequences for qRT-PCR are listed below.
Lmod2-F: 5′-CAGAGAAAACCCCAACAGGA-3′
Lmod2-R: 5′-cttcgctgttgctctctgtg-3′
Lmod3-F: 5′-TCACTCCCAGAGACCAGCTC-3′
Lmod3-R: 5′-ctgcactgttcgtgaaatgg-3′
Thin Filament Length Measurement.
Embryos were dissected in relaxing buffer (33) (150 mM KCl, 5 mM MgCl2, 10 mM MOPS [3-(N-morpholino)propanesulfonic acid], 1 mM EGTA, 4 mM ATP, pH 7.4 supplemented with 0.2% Triton X-100) and fixed in relaxing buffer with 4% PFA overnight at 4 °C. Embryos were then processed and sectioned as described in the immunofluorescence method. Thin filament length was measured using ImageJ software based on phalloidin-stained images [thin filament length = (distance from pointed end to pointed end)/2].
Bacterial Expression and Purification of Mouse HSPB7.
HSPB7 coding sequence was amplified from mouse adult heart cDNA and cloned into pET-trx1a (58). The plasmid was transformed into the Escherichia coli strain Rossetta (DE3) pLysS (Novagen). Cells where transferred to ampicillin (100 μg/mL)-supplemented LB agar plates. Colonies were inoculated to 10 mL Terrific Broth (Corning) with 100 μg/mL ampicillin and incubated at 37 °C overnight with shaking. The preculture was used to inoculate 500 mL Terrific Broth with 100 μg/mL ampicillin and grown at 37 °C until OD600 reached 1.0. The culture was cooled down to 18 °C, induced with IPTG (isopropyl β-D-1-thiogalactopyranoside) (final concentration 0.2 mM), and incubated at 18 °C overnight with shaking. Cells were harvested by centrifugation (4,000 rpm at 4 °C for 30 min, Sorvall RC5B-Plus with GS-3 rotor), resuspended in 50 mL ice cold PBS, and centrifuged again at 2,916 × g (Eppendorf Centrifuge 5810R with A-4-62 rotor) for 30 min at 4 °C. Cell pellets were snap-frozen in liquid nitrogen, thawed at room temperature, resuspended in T+ buffer (25 mM sodium phosphate, pH 8.0, 300 mM NaCl, 20 mM Imidazole, 0.2% Igepal 40, 2 mM β-mercaptoethanol), and supplemented with complete mini proteinase inhibitor mixture (Sigma-Aldrich), Lyzozyme (Biopioneer), and DNase I (Sigma-Aldrich). To facilitate cell lysis, the suspension was sonicated several times with 3,000 J, 60% power until appearance was clear. The insoluble material was removed by centrifugation (20,000 × g for 1 h at 4 °C). The supernatant was filtered through a 0.22-μm membrane directly to Ni-NTA Agarose (Qiagen) that was preequilibrated with T+ buffer in a gravity flow column (Bio-Rad). The supernatant was applied twice. The column was subsequently washed with T+, T− (T+ without detergents) buffers and eluted with buffer E (T− buffer supplemented with 300 mM Imidazole). The 6xHis-HSPB7 protein was exchanged to Actin assays-compatible Hepes buffer (20 mM Hepes, 20 mM KCl, pH 7.4) using a Slide-Alyzer Dialysis Cassette (Life Technologies) and concentrated with Amicon Ultra-15 Centrifugal Filter Units (EMD Millipore).
F-Actin Cosedimentation Binding Assay.
F-actin cosedimentation binding assay was performed using an Actin Binding Protein Biochem Kit (Muscle Actin, catalog no. BK001; Cytoskeleton, Inc.) following the manufacturer’s instructions. Briefly, HSPB7 protein, positive control α-actinin, and negative control BSA were incubated with F actin for 30 min followed by ultracentrifugation at 150,000 × g for 90 min at 24 °C. Supernatants were placed in separate tubes, and protein loading buffer (8 M urea, 2 M thiourea, 3% SDS, 75 mM DTT, 0.03% Bromophenol Blue, 0.05 M Tris⋅HCl, pH 6.8) was added. Pellets were resuspended in 30 μL Milli-Q water and mixed with protein loading buffer. Samples were heated at 95 °C for 5 min, separated on SDS/PAGE gels (Life Technologies), and analyzed by staining with InstantBlue (Expedeon) or Silver Stain Kit (Pierce).
Blot Overlay Assay.
F-actin stock and G-actin stock were prepared per the manufacturer’s instructions (Cytoskeleton, Inc.). A small aliquot of F-actin and G-actin stock underwent ultracentrifugation and analysis by SDS/PAGE to confirm identities. F actin and G actin in increasing amounts (1, 3, 5, and 7 μg) were transferred from solution to Nitrocellulose membrane by Bio-Dot apparatus (Bio-Rad), washed with TBST, and blocked with 5% BSA in TBST for 1 h. The membrane was then incubated with HSPB7 protein (1:50, final concentration 0.5 μM) in Tris Buffer Saline (pH 6.5) for 2 h at room temperature. After incubation with HSPB7, the membrane was washed three times with TBST and incubated with HSPB7 antibody (1:500, in TBST) overnight at 4 °C. The blot was then washed three times with TBST and incubated with HRP-conjugated secondary antibody for 1 h before immunoreactive protein bands were visualized using ECL reagent. To further confirm the interactions, additional blot overlay assay was performed similarly except immobilizing HSPB7, αΒ-crystallin, α-actinin (positive control) and BSA (negative control) on Nitrocellulose membrane and incubated with G actin, then performed immunoblots using antibody against actin to detect interactions between test proteins and G actin.
Actin Cross-Linking Assay.
Cross-linking of G actin and HSPB7 was performed according to a previously described protocol (41, 42). Briefly, G actin and HSPB7 were combined at a 1:1 molar ratio to a final concentration of 2 μM each in cross-linking buffer (5 mM Hepes, pH 7.5, 0.1 mM CaCl2) and incubated for 20 min with 1 mM EDC (Pierce) and 1 mM NHS (Pierce) at room temperature. Hydroxylamine was added at a final concentration of 10 mM to stop the reaction, and samples were separated by SDS/PAGE and stained using a Silver stain kit (Pierce).
TAP Assay.
TAP was performed as previously described (50). Neonatal mouse cardiomyocytes were infected with adenoviruses expressing 3xFlag/HA-HSPB7 or control 3xFlag/HA-EGFP. After 48 h of infection, cardiomyocytes were lysed and immunoprecipitated with anti-Flag M2 agarose (Sigma-Aldrich) overnight at 4 °C. The beads were washed and eluted. After removal of the Flag-agarose, the eluates were immunoprecipitated with anti-HA agarose (Sigma-Aldrich) at 4 °C overnight. HA agarose was washed and then eluted with 8 M urea at room temperature for 10 min. The immunoprecipitated products were separated on SDS/PAGE gels and analyzed with Western blots using antibodies against actin or FLAG tag.
Actin Polymerization Assay.
Assays were performed using an Actin Polymerization Biochem Kit (catalog no. BK003; Cytoskeleton, Inc.) according to the manufacturer’s instructions. To prepare pyrene-G actin, 5-μL frozen aliquots of pyrene actin were added to 1,035 μL ice-cold G buffer (final conc. 2 μM), pipetted to mix, and incubated on ice for 2 h to depolymerize actin oligomers. The pyrene actin was centrifuged at 14,000 × g for 30 min at 4 °C to remove seeds/aggregates, and the resulting supernatant was transferred to a new tube and labeled as G-actin stock; 100 μL G-actin stock was incubated with various amounts of recombinant HSPB7 as indicated in the figures for 10 min at room temperature. To initiate actin polymerization, 13 μL 10× Actin Polymerization Buffer was added to each reaction before analysis using a Synergy Plate Reader (BioTek) in kinetic mode (1-min intervals), with an excitation wavelength of 360 nm and an emission wavelength of 460 nm.
Statistical Analysis.
Data are presented as mean ± SEM. Statistical analysis was performed using GraphPad Prism 6.0 software, with two-tailed Student’s t test or one-way ANOVA used for comparisons among groups as indicated. P values less than 0.05 were considered significant.
Supplementary Material
Acknowledgments
We thank Dr. Siegfried Labeit (University of Heidelberg) and Dr. Walter Witke (University of Bonn) for providing titin Z1Z2 antibody and cofilin1, respectively. J.B. is supported by European Commission Marie Skłodowska-Curie Individual Fellowship Titin Signals 656636. R.B.N. and V.M.F. are funded by NIH Grant HL083464. C.T.P. and C.C.G. are funded by NIH Grant HL123078. S.M.E. is supported by grants from the National Heart, Lung, and Blood Institute (NHLBI). V.M.F. and J.C. are supported by NIH Grant P30 AR061303. J.C. was funded by grants from the NHLBI and Foundation Leducq.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1713763114/-/DCSupplemental.
References
- 1.Olson TM, et al. Inherited and de novo mutations in the cardiac actin gene cause hypertrophic cardiomyopathy. J Mol Cell Cardiol. 2000;32:1687–1694. doi: 10.1006/jmcc.2000.1204. [DOI] [PubMed] [Google Scholar]
- 2.Thierfelder L, et al. Alpha-tropomyosin and cardiac troponin T mutations cause familial hypertrophic cardiomyopathy: A disease of the sarcomere. Cell. 1994;77:701–712. doi: 10.1016/0092-8674(94)90054-x. [DOI] [PubMed] [Google Scholar]
- 3.Gokhin DS, Bang ML, Zhang J, Chen J, Lieber RL. Reduced thin filament length in nebulin-knockout skeletal muscle alters isometric contractile properties. Am J Physiol Cell Physiol. 2009;296:C1123–C1132. doi: 10.1152/ajpcell.00503.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gokhin DS, Fowler VM. A two-segment model for thin filament architecture in skeletal muscle. Nat Rev Mol Cell Biol. 2013;14:113–119. doi: 10.1038/nrm3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weber A, Pennise CR, Babcock GG, Fowler VM. Tropomodulin caps the pointed ends of actin filaments. J Cell Biol. 1994;127:1627–1635. doi: 10.1083/jcb.127.6.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gokhin DS, Fowler VM. Tropomodulin capping of actin filaments in striated muscle development and physiology. J Biomed Biotechnol. 2011;2011:103069. doi: 10.1155/2011/103069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Littlefield R, Almenar-Queralt A, Fowler VM. Actin dynamics at pointed ends regulates thin filament length in striated muscle. Nat Cell Biol. 2001;3:544–551. doi: 10.1038/35078517. [DOI] [PubMed] [Google Scholar]
- 8.Chereau D, et al. Leiomodin is an actin filament nucleator in muscle cells. Science. 2008;320:239–243. doi: 10.1126/science.1155313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pappas CT, et al. Knockout of Lmod2 results in shorter thin filaments followed by dilated cardiomyopathy and juvenile lethality. Proc Natl Acad Sci USA. 2015;112:13573–13578. doi: 10.1073/pnas.1508273112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sussman MA, et al. Myofibril degeneration caused by tropomodulin overexpression leads to dilated cardiomyopathy in juvenile mice. J Clin Invest. 1998;101:51–61. doi: 10.1172/JCI1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuen M, et al. Leiomodin-3 dysfunction results in thin filament disorganization and nemaline myopathy. J Clin Invest. 2014;124:4693–4708. doi: 10.1172/JCI75199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cenik BK, et al. Severe myopathy in mice lacking the MEF2/SRF-dependent gene leiomodin-3. J Clin Invest. 2015;125:1569–1578. doi: 10.1172/JCI80115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mymrikov EV, Seit-Nebi AS, Gusev NB. Large potentials of small heat shock proteins. Physiol Rev. 2011;91:1123–1159. doi: 10.1152/physrev.00023.2010. [DOI] [PubMed] [Google Scholar]
- 14.Vos MJ, Kanon B, Kampinga HH. HSPB7 is a SC35 speckle resident small heat shock protein. Biochim Biophys Acta. 2009;1793:1343–1353. doi: 10.1016/j.bbamcr.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 15.Mounier N, Arrigo AP. Actin cytoskeleton and small heat shock proteins: How do they interact? Cell Stress Chaperones. 2002;7:167–176. doi: 10.1379/1466-1268(2002)007<0167:acashs>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang K, Spector A. Alpha-crystallin stabilizes actin filaments and prevents cytochalasin-induced depolymerization in a phosphorylation-dependent manner. Eur J Biochem. 1996;242:56–66. doi: 10.1111/j.1432-1033.1996.0056r.x. [DOI] [PubMed] [Google Scholar]
- 17.Miron T, Wilchek M, Geiger B. Characterization of an inhibitor of actin polymerization in vinculin-rich fraction of turkey gizzard smooth muscle. Eur J Biochem. 1988;178:543–553. doi: 10.1111/j.1432-1033.1988.tb14481.x. [DOI] [PubMed] [Google Scholar]
- 18.Krief S, et al. Identification and characterization of cvHsp. A novel human small stress protein selectively expressed in cardiovascular and insulin-sensitive tissues. J Biol Chem. 1999;274:36592–36600. doi: 10.1074/jbc.274.51.36592. [DOI] [PubMed] [Google Scholar]
- 19.Cappola TP, et al. Common variants in HSPB7 and FRMD4B associated with advanced heart failure. Circ Cardiovasc Genet. 2010;3:147–154. doi: 10.1161/CIRCGENETICS.109.898395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matkovich SJ, et al. Cardiac signaling genes exhibit unexpected sequence diversity in sporadic cardiomyopathy, revealing HSPB7 polymorphisms associated with disease. J Clin Invest. 2010;120:280–289. doi: 10.1172/JCI39085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stark K, et al. Genetic association study identifies HSPB7 as a risk gene for idiopathic dilated cardiomyopathy. PLoS Genet. 2010;6:e1001167. doi: 10.1371/journal.pgen.1001167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Villard E, et al. Cardiogenics Consortium A genome-wide association study identifies two loci associated with heart failure due to dilated cardiomyopathy. Eur Heart J. 2011;32:1065–1076. doi: 10.1093/eurheartj/ehr105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lahvic JL, et al. Small heat shock proteins are necessary for heart migration and laterality determination in zebrafish. Dev Biol. 2013;384:166–180. doi: 10.1016/j.ydbio.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenfeld GE, Mercer EJ, Mason CE, Evans T. Small heat shock proteins Hspb7 and Hspb12 regulate early steps of cardiac morphogenesis. Dev Biol. 2013;381:389–400. doi: 10.1016/j.ydbio.2013.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hayashi S, Lewis P, Pevny L, McMahon AP. Efficient gene modulation in mouse epiblast using a Sox2Cre transgenic mouse strain. Gene Expr Patterns. 2002;2:93–97. doi: 10.1016/s0925-4773(02)00292-7. [DOI] [PubMed] [Google Scholar]
- 26.Chen JW, et al. Cardiomyocyte-specific deletion of the coxsackievirus and adenovirus receptor results in hyperplasia of the embryonic left ventricle and abnormalities of sinuatrial valves. Circ Res. 2006;98:923–930. doi: 10.1161/01.RES.0000218041.41932.e3. [DOI] [PubMed] [Google Scholar]
- 27.Moses KA, DeMayo F, Braun RM, Reecy JL, Schwartz RJ. Embryonic expression of an Nkx2-5/Cre gene using ROSA26 reporter mice. Genesis. 2001;31:176–180. doi: 10.1002/gene.10022. [DOI] [PubMed] [Google Scholar]
- 28.Jiao K, et al. An essential role of Bmp4 in the atrioventricular septation of the mouse heart. Genes Dev. 2003;17:2362–2367. doi: 10.1101/gad.1124803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Golenhofen N, Perng MD, Quinlan RA, Drenckhahn D. Comparison of the small heat shock proteins alphaB-crystallin, MKBP, HSP25, HSP20, and cvHSP in heart and skeletal muscle. Histochem Cell Biol. 2004;122:415–425. doi: 10.1007/s00418-004-0711-z. [DOI] [PubMed] [Google Scholar]
- 30.Zhou Q, Ruiz-Lozano P, Martone ME, Chen J. Cypher, a striated muscle-restricted PDZ and LIM domain-containing protein, binds to alpha-actinin-2 and protein kinase C. J Biol Chem. 1999;274:19807–19813. doi: 10.1074/jbc.274.28.19807. [DOI] [PubMed] [Google Scholar]
- 31.Zhou Q, et al. Ablation of cypher, a PDZ-LIM domain Z-line protein, causes a severe form of congenital myopathy. J Cell Biol. 2001;155:605–612. doi: 10.1083/jcb.200107092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stewart JM, Levy HM. The role of the calcium-troponin-tropomyosin complex in the activation of contraction. J Biol Chem. 1970;245:5764–5772. [PubMed] [Google Scholar]
- 33.Conover GM, Henderson SN, Gregorio CC. A myopathy-linked desmin mutation perturbs striated muscle actin filament architecture. Mol Biol Cell. 2009;20:834–845. doi: 10.1091/mbc.E08-07-0753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Juo LY, et al. HSPB7 interacts with dimerized FLNC and its absence results in progressive myopathy in skeletal muscles. J Cell Sci. 2016;129:1661–1670. doi: 10.1242/jcs.179887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ono S. Dynamic regulation of sarcomeric actin filaments in striated muscle. Cytoskeleton. 2010;67:677–692. doi: 10.1002/cm.20476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heling A, et al. Increased expression of cytoskeletal, linkage, and extracellular proteins in failing human myocardium. Circ Res. 2000;86:846–853. doi: 10.1161/01.res.86.8.846. [DOI] [PubMed] [Google Scholar]
- 37.Tsukada T, et al. Leiomodin-2 is an antagonist of tropomodulin-1 at the pointed end of the thin filaments in cardiac muscle. J Cell Sci. 2010;123:3136–3145. doi: 10.1242/jcs.071837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gregorio CC, Weber A, Bondad M, Pennise CR, Fowler VM. Requirement of pointed-end capping by tropomodulin to maintain actin filament length in embryonic chick cardiac myocytes. Nature. 1995;377:83–86. doi: 10.1038/377083a0. [DOI] [PubMed] [Google Scholar]
- 39.Lin W, Yang Z, Lu Y, Zhao X. Refined purification of large amounts of rat cvHsp/HspB7 and partial biological characterization in vitro. Protein Pept Lett. 2014;21:503–510. doi: 10.2174/092986652105140218121109. [DOI] [PubMed] [Google Scholar]
- 40.Heacock CS, Bamburg JR. The quantitation of G- and F-actin in cultured cells. Anal Biochem. 1983;135:22–36. doi: 10.1016/0003-2697(83)90725-x. [DOI] [PubMed] [Google Scholar]
- 41.Fischer RS, et al. Tropomodulin 3 binds to actin monomers. J Biol Chem. 2006;281:36454–36465. doi: 10.1074/jbc.M606315200. [DOI] [PubMed] [Google Scholar]
- 42.Yamashiro S, Speicher KD, Speicher DW, Fowler VM. Mammalian tropomodulins nucleate actin polymerization via their actin monomer binding and filament pointed end-capping activities. J Biol Chem. 2010;285:33265–33280. doi: 10.1074/jbc.M110.144873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lal AA, Korn ED. Reinvestigation of the inhibition of actin polymerization by profilin. J Biol Chem. 1985;260:10132–10138. [PubMed] [Google Scholar]
- 44.Fowler VM, Dominguez R. Tropomodulins and leiomodins: Actin pointed end caps and nucleators in muscles. Biophys J. 2017;112:1742–1760. doi: 10.1016/j.bpj.2017.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wanger M, Keiser T, Neuhaus JM, Wegner A. The actin treadmill. Can J Biochem Cell Biol. 1985;63:414–421. doi: 10.1139/o85-060. [DOI] [PubMed] [Google Scholar]
- 46.Luther PK. The vertebrate muscle Z-disc: Sarcomere anchor for structure and signalling. J Muscle Res Cell Motil. 2009;30:171–185. doi: 10.1007/s10974-009-9189-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kappé G, et al. The human genome encodes 10 alpha-crystallin-related small heat shock proteins: HspB1-10. Cell Stress Chaperones. 2003;8:53–61. doi: 10.1379/1466-1268(2003)8<53:thgecs>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morrison LE, Whittaker RJ, Klepper RE, Wawrousek EF, Glembotski CC. Roles for alphaB-crystallin and HSPB2 in protecting the myocardium from ischemia-reperfusion-induced damage in a KO mouse model. Am J Physiol Heart Circ Physiol. 2004;286:H847–H855. doi: 10.1152/ajpheart.00715.2003. [DOI] [PubMed] [Google Scholar]
- 49.Qiu H, et al. H11 kinase/heat shock protein 22 deletion impairs both nuclear and mitochondrial functions of STAT3 and accelerates the transition into heart failure on cardiac overload. Circulation. 2011;124:406–415. doi: 10.1161/CIRCULATIONAHA.110.013847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fang X, et al. Loss-of-function mutations in co-chaperone BAG3 destabilize small HSPs and cause cardiomyopathy. J Clin Invest. 2017;127:3189–3200. doi: 10.1172/JCI94310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vos MJ, et al. HSPB7 is the most potent polyQ aggregation suppressor within the HSPB family of molecular chaperones. Hum Mol Genet. 2010;19:4677–4693. doi: 10.1093/hmg/ddq398. [DOI] [PubMed] [Google Scholar]
- 52.Liao WC, Juo LY, Shih YL, Chen YH, Yan YT. HSPB7 prevents cardiac conduction system defect through maintaining intercalated disc integrity. PLoS Genet. 2017;13:e1006984. doi: 10.1371/journal.pgen.1006984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.National Research Council . Guide for the Care and Use of Laboratory Animals. 8th Ed National Academies Press; Washington, DC: 2011. [Google Scholar]
- 54.Liang X, et al. PINCH1 plays an essential role in early murine embryonic development but is dispensable in ventricular cardiomyocytes. Mol Cell Biol. 2005;25:3056–3062. doi: 10.1128/MCB.25.8.3056-3062.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stroud MJ, et al. Nesprin 1α2 is essential for mouse postnatal viability and nuclear positioning in skeletal muscle. J Cell Biol. 2017;216:1915–1924. doi: 10.1083/jcb.201612128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Arita Y, et al. Myocardium-derived angiopoietin-1 is essential for coronary vein formation in the developing heart. Nat Commun. 2014;5:4552. doi: 10.1038/ncomms5552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fang X, et al. Adipocyte-specific loss of PPARγ attenuates cardiac hypertrophy. JCI Insight. 2016;1:e89908. doi: 10.1172/jci.insight.89908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bogomolovas J, Simon B, Sattler M, Stier G. Screening of fusion partners for high yield expression and purification of bioactive viscotoxins. Protein Expr Purif. 2009;64:16–23. doi: 10.1016/j.pep.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 59.Huang C, et al. Characterization and in vivo functional analysis of splice variants of cypher. J Biol Chem. 2003;278:7360–7365. doi: 10.1074/jbc.M211875200. [DOI] [PubMed] [Google Scholar]
- 60.Gregorio CC, Fowler VM. Mechanisms of thin filament assembly in embryonic chick cardiac myocytes: Tropomodulin requires tropomyosin for assembly. J Cell Biol. 1995;129:683–695. doi: 10.1083/jcb.129.3.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhou W, et al. Modulation of morphogenesis by noncanonical Wnt signaling requires ATF/CREB family-mediated transcriptional activation of TGFbeta2. Nat Genet. 2007;39:1225–1234. doi: 10.1038/ng2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mu Y, et al. Cypher and Enigma homolog protein are essential for cardiac development and embryonic survival. J Am Heart Assoc. 2015;4:e001950. doi: 10.1161/JAHA.115.001950. [DOI] [PMC free article] [PubMed] [Google Scholar]