Significance
The hallmark of remyelination in the CNS has been proposed to be the presence of thin myelin sheaths. This has been demonstrated in multiple experimental models and in multiple sclerosis. It is the only surrogate marker of remyelination, and therefore a crucial fingerprint of myelin repair. However, this has been challenged recently in separate studies, in which, by implication, the degree or presence of remyelinated axons has been underestimated. In this article, we provide evidence from two different models that thin myelin sheaths and short internodes persist almost indefinitely. Future attempts to promote myelin repair in models of multiple sclerosis will be crucially dependent on a definitive marker of repair. We propose that the thin myelin sheath remains the gold standard.
Keywords: axon, oligodendrocyte, remyelination
Abstract
The presence of thin myelin sheaths in the adult CNS is recognized as a marker of remyelination, although the reason there is not a recovery from demyelination to normal myelin sheath thickness remains unknown. Remyelination is the default pathway after myelin loss in all mammalian species, in both naturally occurring and experimental disease. However, there remains uncertainty about whether these thin sheaths thicken with time and whether they remain viable for extended periods. We provide two lines of evidence here that thin myelin sheaths may persist indefinitely in long-lived animal models. In the first, we have followed thin myelin sheaths in a model of delayed myelination during a period of 13 years that we propose results in the same myelin sheath deficiencies as seen in remyelination; that is, thin myelin sheaths and short internodes. We show that the myelin sheaths remain thin and stable on many axons throughout this period with no detrimental effects on axons. In a second model system, in which there is widespread demyelination of the spinal cord and optic nerves, we also show that thinly remyelinated axons with short internodes persist for over the course of 2 y. These studies confirm the persistence and longevity of thin myelin sheaths and the importance of remyelination to the long-term health and function of the CNS.
Remyelination of the CNS is the most robust endogenous form of repair of the brain and spinal cord and a major therapeutic target in demyelinating disease. It has been found to be the default pathway in a wide variety of experimental demyelinating disorders, with large and extensive areas of demyelination becoming totally remyelinated in the recovery phase of each disorder (1). In human demyelinating diseases, and in particular in multiple sclerosis (MS), remyelination can be extensive (2–4), but in MS, it diminishes with aging and disease course (5, 6). Persistently demyelinated axons are at risk for degeneration, and axon loss is likely the basis for patients developing secondary progressive MS and permanent neurological deficits (7–10). Remyelination in the CNS has been shown to restore nerve conduction (11), locomotor function (12, 13), and global neurologic recovery (1). Importantly, remyelination protects axons from ongoing degeneration (14–17), and is therefore a crucial repair strategy.
The morphologic hallmark of remyelination in both human and experimental disease is proposed to be the presence of axons with thin myelin sheaths that have a significant increase in the g ratio (axon diameter/total fiber diameter) (6, 18). Remyelinated axons also have shortened internodal lengths. These features of remyelinated axons have been described in many human and animal demyelinating disorders of any cause, in which there is subsequent remyelination (19–21). Most important, short internodes with thin myelin sheaths have also been reported in detail in MS tissue (22, 23). Although the prevailing view is that remyelination of the adult CNS results only in thin myelin sheaths (6), some recent studies have suggested that remyelination can lead to normal-thickness myelin with normal g ratios. These studies have examined remyelination in the corpus callosum after cuprizone-induced demyelination (24, 25) and in the spinal cord in experimental autoimmune encephalomyelitis (25). It was proposed that remyelinated myelin sheaths induced by neural precursor cell-derived oligodendrocytes (OLs) were thicker than those generated by oligodendrocyte progenitor cells (OPCs), and the g ratios of these fibers were normal (24). However, these observations were made in the corpus callosum, where it has been shown that g ratios can be unreliable measurements of remyelination (26), and this will require verification in other white matter tracts. In the study of Samanta et al. (25), remyelinated, small-diameter axons in the spinal cord were found to have normal g ratios. This, however, should be verified in all calibers of axons. We have previously shown that transplantation of OPCs into a mature myelin mutant, the shaking pup, at 8 mo of age, resulted in predominantly thin myelin sheaths (27). This may be equivalent to what is described in the present study; that is, delayed myelination of mature axons. In contrast, transplantation of OPCs into 2-wk-old recipients led to the production of normal-thickness myelin sheaths (27). Powers et al. (28) studied internode lengths and myelin sheath thickness of remyelinated axons after spinal cord injury. They showed that internode lengths of remyelinated axons were short early after injury, but elongated with time (6 mo), with myelin sheath thickness also increasing to normal. Whether this relates only to this model (spinal cord injury) is unknown, yet unlikely. Taken in context, these studies suggest that the estimation of remyelination using thin myelin sheaths as evidence may be underestimated, yet the proof of this does not appear overwhelming at present, and longer-term and more complete studies are needed.
The reason or reasons that remyelinated axons have thin myelin sheaths have not been determined experimentally, and questions remain about the long-term effectiveness of remyelination. For example, can thin myelin sheaths persist for long periods and continue to support normal conduction and function, preventing axons from degeneration (29, 30)? If thin myelin is less than optimum, is there a need to develop strategies that restore thin myelin sheaths to their normal thickness (31)? These are difficult questions to answer in MS, where it is impossible to determine how long axons have been remyelinated. In experimental rodent animal models, their short lifespan limits the length of time remyelinated axons can be followed. In an attempt to answer some of these questions, we studied two experimental models. In the first, for more than a decade, we followed the fate of thinly myelinated axons in the spinal cord of a canine mutant, resulting from a developmental delay in myelination, which we propose matches the changes seen in remyelinated fibers. Second, to test whether thinly remyelinated sheaths persist over time after demyelination, we turned to a model in which this was studied in animals over the course of 2 y after myelin repair.
Results
Genetic Disorder.
The disorder presented here is a unique developmental disorder of myelination of the superficial tracts of the cervical and thoracic spinal cord caused by a mutation in the FNIP2 gene (32). At 2 wk of age, affected dogs have a severe tremor, resulting in difficulty in ambulation. We studied the thoracic spinal cord from a 2-wk-old affected dog and demonstrated a zone of hypomyelination of the superficial tracts of the lateral and ventral columns with almost a total lack of myelin (Fig. 1 C and D) compared with the same tracts in controls that were completely myelinated (Fig. 1 A and B). The dorsolateral column appeared most deficient in myelin. On higher magnification of this area, the myelin deficiency was more obvious despite the presence of many glial cells and intact axons (Fig. 1D). Importantly, however, some larger-diameter axons in this zone were myelinated normally (Fig. 1D, Inset). In contrast, in both of the affected 13-y-old dogs, these areas appeared completely myelinated, but the outstanding feature was the presence of many thinly myelinated axons of all axonal calibers in the lateral and ventral columns of the cervical and thoracic spinal cord (Fig. 2 and Fig. S1). These thinly myelinated axons were seen predominantly in the subpial zones of the lateral and ventral columns, the site of original myelination delay (Fig. 1). This was also seen at both levels of the cord in the second dog, although less obviously than in the first case (Fig. S1). There were no other abnormalities in any of the spinal cord sections evaluated. There was no evidence of demyelination or myelin debris that would suggest myelin was not stable. Myelinated fiber density appeared identical to controls, and although rare degenerating axons were seen, this was similar to controls. There was no evidence of abnormal axoplasm, as we have previously identified in 1-μm sections in another canine myelin mutant, in which a wide array of axonal changes was documented (33). In addition, no swollen axons or spheroids were present in either case in the cervical and thoracic spinal cord.
Fig. 1.
Myelin deficiency in the subpial zone of the ventral and lateral tracts of the spinal cord in the mutant. Hemisections of the thoracic spinal cord from a 2-wk-old control dog (A and B) and age-matched affected dog (C and D). In the control, the spinal cord appears completely myelinated, and a higher-power image of the dorsolateral column (arrows) in B confirms this. In contrast, in the affected dog, there is a clear, pale subpial zone (C) that on higher power shows a marked myelin deficiency compared with the medial white matter (D). However, occasional large-diameter axons (arrow) are myelinated in this area (D, Inset). [Scale bars: 0.2 mm (A and C), 20 μm (B and D), and 10 μm (D, Inset).]
Fig. 2.
In the aged mutant, hypomyelination persists in the affected tracts, and internodes can be short. In the control dog, the spinal cord is normally myelinated (A), and this is confirmed on higher-power examination of subpial areas in the LCs (B) and VCs (C). The g ratio of myelinated axons in these areas was measured at two sites and the ratio noted A. In the first affected dog, the cord appears completely myelinated (D), yet on higher power, there are many thin myelin sheaths of all axon diameters in both the LC (E) and VC (F) in the subpial zone. This was confirmed by the g ratio measurements from these sites (D). High power of areas (*) in the control (G) and affected dog (H) show that these areas have a mix of normally myelinated (thick myelin sheaths) and hypomyelinated axons, as reflected by the g ratio range. (I) A short internode with a thin myelin sheath is seen between thicker internodes. (J) In the ventral column, a 7.6-µm-diameter axon has two short internodes, as marked by arrows. The node of Ranvier on the right has a paranode with a normal myelin sheath adjacent to the thin, short internode (g ratio, 0.83) (A). Arrows mark nodes of Ranvier. Internode lengths are detailed. [Scale bars: 0.5 mm (A and D), and 20 μm (B, C, E–J).]
Fig. 3 shows the distribution of g ratio measurements within each image, by subject and ventral column (VC) or lateral column (LC) locations. The number of axons measured at each position ranged from 249 to 505, and mean g ratio ranged from 0.62 to 0.84, with the variability having an SD of 0.07–0.10 (Table S1). We tested the difference between mean affected and WT g ratio adjusting for VC or LC location, using random effects for the subject and 1 μm image factors, to account for potentially higher correlation of measurements for axons within the same subject and/or the same 1-μm image (Table S2). g ratios measured in affected subjects were, on average, 0.126 (SE = 0.027; P < 0.0001) higher than in WT subjects. There was no difference in the g ratio between VC and LC locations of the cord.
Fig. 3.
Measurements of g ratios in the dorsolateral columns and VCs confirms the presence of abnormally thin myelin sheaths. Distribution of g ratios for randomly sampled axons within the 1-μm image of the cervical cord from affected and WT dogs. The suffix 1 and 2 in the LC and VC are two sections taken from those locations. The number of axons measured is shown below the image location. The box represents the median and 25th and 75th percentiles of the distribution of the values. Whiskers extend out no more than 1.5× the interquartile range away from the box. Values outside of this range are plotted as points.
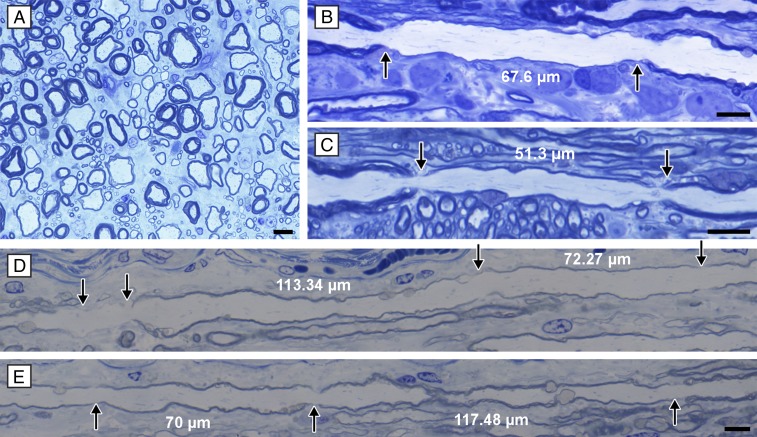
To determine whether these abnormally thin myelin sheaths also had short internodes, a hallmark of remyelination, tissue from the VC of the cervical and thoracic cord of the male dog was embedded for longitudinal sectioning. These sections enabled visualization of myelinated axons from the subpial surface to those in the more medial gray matter interface. Thinly myelinated short internodes were only seen in the subpial area and not more medially, as would be expected from the transverse sections (Fig. 2). In addition, some axons had adjacent, normal-thickness/thin myelin paranodes (Fig. 2J). This confirmed that this is a primary glial defect and not an abnormality of axons. Not all thinly myelinated internodes were short, however, and on occasion, they were more than 100 µm. It was also noted that nodal gaps were greater than normal at many nodes of the thinly myelinated axons (Fig. 4). In the more medial, normally myelinated areas, nodes of Ranvier with normal nodal gaps were seen on large-, medium-, and small-caliber axons, but it was impossible to measure internode length on these axons because of variation in fiber plane. However, those internodes that could be followed over some length were always longer than the thinly myelinated internodes seen in the subpial zone.
Fig. 4.
Widened nodal gaps seen on affected fibers. Although normal nodes were common on axons with normal thickness myelin sheaths (A) and those with thin myelin sheaths (B), many examples of nodal widening were seen at nodes where adjacent myelin sheaths were thin (C and D). (Scale bars: 20 μm.)
FIDID.
We have previously shown that irradiation of food at more than 30 Kgray results in a widespread and severe demyelinating and remyelinating disorder in cats (1). Here we studied this phenomenon in the spinal cord of three cats that were examined over the course of 16 mo to 2 y after neurologic recovery, and found thinly myelinated axons interspersed between mature myelin sheaths (Fig. 5A). These appeared to be stable lesions, as there was no myelin vacuolation and no foamy, lipid-filled macrophages, which are seen during and shortly after demyelination and remyelination. Longitudinal sections of the spinal cord showed frequent thinly myelinated axons with short but variable internode lengths (Fig. 5 B–E). In one of these animals, the optic nerves were dominated by thinly myelinated axons, and short internodes were also seen on longitudinal sections (Fig. 6). Two of the cats with feline irradiated diet-induced demyelination (FIDID) were part of a clinical trial in Madison, Wisconsin, and the other was a pet cat from Sydney, Australia, confirming the ubiquitous nature of the disease process and the long-term persistence of thinly myelinated axons.
Fig. 5.
Thin remyelinated sheaths persist in the spinal cord for more than a year after demyelination. (A) Area from the ventral column of the cervical spinal cord from a cat, 11/4 y after neurologic recovery from FIDID. Remyelinated axons are scattered between axons with normal myelin sheath thickness in an area of normal axon density. This is a nonactive lesion with no demyelinated axons and no lipid-filled macrophages. (B and C) Longitudinal sections from these areas show short internodes with thin myelin sheaths, all of which had g ratios >0.80. On some axons, multiple short internodes with thin myelin sheaths were seen (D). (D) The fiber seen continues in the panel below (E). Arrows mark nodes of Ranvier. (Scale bars: 20 μm.)
Fig. 6.
The optic nerve contains predominantly remyelinated axons. (A) In the second cat, 2 y after recovery, the optic nerve contains predominantly thin, remyelinated sheaths with only two mature, normal-thickness myelinated axons (arrows) that were not demyelinated. (B) The nerve also contained short, thin internodes. (Scale bars: 20 μm.)
Discussion
We provide here compelling evidence for this persistence of thin myelin sheaths over extended periods in two models, most notably in the canine genetic disorder. During the prolonged period of observation of the two affected dogs, from the time of recovery from their neurologic deficit at 6 mo, to their termination 13 y later, four possibilities were predicted regarding the fate of the thinly myelinated axons in the spinal cord. First, they could remain hypomyelinated, as we have previously demonstrated in mutant dogs up to 2 y of age (32). Second, the thin myelin sheaths thickened with time, eventually achieving normal thickness and g ratio as a result of oligodendrocyte compensation and plasticity. Third, thin myelin was not stable and eventually degenerated, leading to demyelination, or fourth, was not sufficient to maintain axonal viability with subsequent axon degeneration. We demonstrate here that the first outcome, that is, the persistence of hypomyelination with no other pathologic changes, was the outcome.
As thin myelin sheaths in the adult CNS are thought to indicate remyelination, do the results in this genetic model match the changes seen after myelin restoration? As detailed here, many thinly myelinated axons were seen in the LCs and VCs after 13 y. These differences were confirmed by the g ratio measurements in these sites in the cervical and thoracic cord of both dogs. g ratios were, on average, greater in the affected dogs at all sites measured, and although the number of test subjects was small, the myelin sheath abnormalities were consistent in both dogs. In addition to thin myelin sheaths, as seen on transverse section, thinly myelinated axons in the subpial zone frequently had short internode lengths (<100 µm). This is similar to that seen in remyelinated fibers in the CNS, as shown in study of single teased nerve fibers from the spinal cord of cats that had been demyelinated by focal lysolecithin injections (34). Some remyelinated axons can have longer internodes, close to the predicted diameter for the axon caliber (34), and this was also seen in some thinly myelinated axons in the present study. Axons in the medial aspect of the cord of varying diameters and normal-thickness myelin did not appear to have short internodes, although it was difficult to follow them for long distances.
Given that neurologic function was restored in the affected dogs by 4–6 mo of age, and this is correlated with the myelination of the spinal cord superficial tracts (32), it can be concluded that the thin myelin sheaths restored conduction. It has been known for some time that endogenous remyelination of CNS axons restores secure conduction from previous conduction block of axons demyelinated by lysolecithin injection (11). Despite the neurologic normality and likely normal conduction in the thinly myelinated axons, we saw numerous cases of nodal gap widening on the thinly myelinated axons that may also result in conduction abnormalities. Nodal gap widening is common and a nonspecific finding in many neurologic disorders (35). Despite these findings of thin myelin sheaths and nodal widening, they appear not to have any negative effect on neurologic function.
Thus, although we propose that the thin myelin sheaths that persist in this mutant are the same as those seen after remyelination of the adult CNS, the question remains, as with remyelination: Why do these myelin sheaths remain thin? Franklin and Hinks (36) proposed that thin myelin sheaths in remyelination result from OLs unsheathing mature axons of mature diameter and length. In contrast in normal development, early myelin internodes adapt to changes in axon caliber and length by becoming thicker in diameter, and longer. We propose that the Franklin and Hinks hypothesis applies in the developmental delay in myelination in these mutants. Myelin sheath thickness in the peripheral nervous system is controlled by neuregulin III signaling (37), whereas in the CNS, ERK1/ERK2 signaling, as found in both loss- and gain-of-function experiments, decreases or increases myelin sheath thickness, respectively (38, 39). The model proposed by Franklin and Hinks (36) to explain why remyelinated sheaths are thin could equally apply to the late-stage developmental myelination documented here. As oligodendrocytes are myelinating (or remyelinating) axons that are close to their maximum diameter and length, there is no need to undergo remodeling that is required in the early developing CNS. That is, the original association of the OL process with an axon is when the axon reaches a critical diameter, although it is still to undergo increases in its diameter and length. Indeed, Franklin and Hinks (36) suggest that remyelination in the adult CNS resulting from oligodendrocytes derived from adult progenitor cells represents “delayed” myelination, similar to what occurs in the Weimaraner mutant. The similarities between myelination and repair have led to the supposition that remyelination is simply a recapitulation of development, and although there are differences, these may be redundant (40). This has been confirmed recently in an investigation of the key transcription factor, myelin regulatory factor in experimental remyelination after lysolecithin-induced demyelination of the mouse corpus callosum, and in MS lesions (41). Myelin regulatory factor expression, which was essential for remyelination in both, broadly recapitulated the OL expression seen during development (41).
Although we propose that the thin myelin sheaths result from delayed myelination of the subpial axons, it could be suggested that the causative mutation in FNIP2 has a negative effect on myelination in addition to the proposed effect on migration and differentiation of OLs and their progenitors (32). However, we would argue against this, as some OLs that are found early (2 wk) in the subpial zone are able to myelinate axons normally (Fig. 1D), leading to the presence of scattered, thickly myelinated axons with a normal g ratio at 13 y, interspersed with the late-myelinated thin sheaths (Fig. 1H). There is no published evidence to indicate there is heterogeneity of OLs in the subpial zone of the spinal cord that could result in a difference in myelinating capacity of a subset of OLs in mutations of FNIP2.
The persistence of thin, remyelinated sheaths after demyelination was also clearly seen in the FIDID model. In all three cases in which tissue was collected over the course of 1–2 y after neurologic recovery, scattered thin myelin sheaths were seen in the spinal cord VCs and LCs, with these fibers being interspersed between mature myelin sheaths. This pattern of fiber mixing is seen during acute disease; that is, scattered myelinated axons (with mature myelin sheaths) escape demyelination and persist adjacent to demyelinated and remyelinated axons. In the three recovered cats recorded here, however, there was no evidence of active myelin breakdown, demyelinated axons, or macrophages; that is, these were nonactive areas of myelin damage and repair. The persistence of thin myelinated sheaths and short internodes confirms that these abnormalities had remained for more than 2 y. In one cat, this was even more dramatic in the optic nerves, where over 95% of axons had thin myelin sheaths with only occasional residual mature sheaths, primarily on large axons. The more extensive remyelination seen in the optic nerves reflects the global demyelination in the nerve compared with the spinal cord.
Are there alternative explanations for the finding of thinly myelinated axons with short internodes in the mature CNS? The concept that myelin sheaths remain static throughout life has been challenged by recent studies on oligodendrocyte dynamics in the normal adult CNS, which showed continuous division of OPCs in the brain and shortening of internode lengths in some tracts (42). This was not related to ongoing myelination of nonmyelinated axons; instead, it was suggested that there is continuous myelin remodeling that results in shortened internode lengths. Could this be the reason for this finding in the two dogs examined here? This would seem to be unlikely, however, as the only areas with thin myelin/short internodes were in the superficial tracts of the LCs and VCs. Myelinated axons medial to these zones had normal myelin sheath thickness and no evidence of shortened internodes as far as could be determined in longitudinal sections.
Despite the fact that many axons in the spinal cord were only ensheathed by thin myelin sheaths, there was no evidence that this compromised axon health or resulted in degeneration (29). The interactions between the axon and its myelin sheath/oligodendrocyte that are essential for axon survival are well recognized (43–46). Although the primary source of support for the axon has been shown to the metabolic coupling between the oligodendrocyte and axon, involving importing glucose and lactate, the myelin sheath itself is also likely to be involved. In Plp1 knockout mice, there is late-stage axonal degeneration (47), whereas mutation of the myelin gene Cnp1 likewise results in axon degeneration (48). In 19-mo-old rumpshaker mice which have a mutation in the Plp1 gene, thin myelin sheaths persist, yet the genetically deficient myelin results in a late-stage axonopathy (49). Here, however, we show that biochemically normal, yet thin, myelin sheaths appear to support the long-term integrity of axons as the myelin fiber density was qualitatively normal (Fig. 2).
The tissue fixation precluded immunolabeling axons for amyloid precursor protein or for demonstrating any increase in neurofilament phosphorylation (50), both of which may have been used to indicate axonal disturbance. However, from observations of axonal pathology in 1-μm sections from another canine myelin mutant (33), we believe we would have identified most potential changes in the axoplasm in the present model and certainly noted if axons were swollen. Absence of any such abnormalities leads us to conclude that thin myelin sheaths can support axon health and function for over the course of 13 y, and potentially indefinitely. Hence, the need to develop remyelinating strategies that restore myelin to its normal thickness to protect axons and sustain function (31) is not required.
Materials and Methods
All animals were handled and treated according to the guidelines and recommendations of the Research Animal Resources Center and the Animal Care and Use Committee at the University of Wisconsin–Madison. The genetic model studied here is a disorder in the Weimaraner breed of dog that has a unique developmental deficiency in myelination of the superficial tracts of the cervical and thoracic spinal cord (32, 51). Affected dogs develop a severe tremor and ataxia at 12–14 d of age, which gradually diminish and are lost in most cases by 3–4 mo. The disorder is inherited as an autosomal recessive trait, and the mutation is in the folliculin-interacting protein 2 (FNIP2) (32). We have proposed that the mutation results in a failure of migration or differentiation of a subpopulation of oligodendrocytes (32).
In the present study, we followed two homozygous affected littermates, a male and female, for more than 13 y. The male had a more severe and persistent tremor and ataxia than the female. In both dogs, however, no obvious tremor could be detected by 6 mo, and the male was no longer ataxic. Both dogs were neurologically normal for up to 13 y. Each was killed at around 13 1/2 y because of nonneurologic disease. Samples of the cervical and thoracic spinal cord were collected from the male immediately after death and immersion fixed in 2.5% glutaraldehyde. Tissue from the female dog was only available 3–4 h after death and immersion fixed, but the delay resulted in poorer fixation. For controls for the mature affected dogs, we used sections from the cervical and thoracic cord of 2-y-old normal dogs (WT; n = 2), as age-matched controls were not available. By 2 y of age, myelination is complete in the canine spinal cord, and myelin sheath thickness is representative of all mature canines (33). To illustrate the developmental defect on early myelination of the superficial tracts of the spinal cord and the change that occurs with time, we examined the thoracic spinal cord of a 2-wk-old affected Weimaraner with tremor and an age-matched control dog.
Blocks from the cervical and thoracic cord were trimmed and processed for plastic embedding and preparation of 1-μm toluidine blue-stained sections. One-micrometer sections were then used to measure the g ratios of ∼250–500 myelinated axons in the LCs and VCs of both animals from the cervical cord at two adjacent sites, using ImageJ software (NIH). The mean difference between case and control dogs was analyzed using a linear mixed-effects model (52), with two levels of random effects: one for measurements from the same dog and one for measurements taken within the same 1-μm slide. This model is considered a mixed-effect model with nested factors.
The second model used to study the persistence of thinly myelinated axons was a unique feline disorder in which feeding irradiated food resulted in profound and generalized demyelination and remyelination of the CNS (1). The disorder has been named FIDID. Two cats were studied as part of the original study at University of Wisconsin–Madison (1) and were killed over the course of 2 y after complete neurologic recovery. The third cat was part of an outbreak of clinical disease seen in Sydney, Australia (53). While on an irradiated diet, the cat developed severe neurologic disease (tetraparesis, ataxia) but recovered to almost normal ambulation and was studied 14 mo after recovery. In this animal, pieces of the cervical and thoracic spinal cord were immersion fixed in 2.5% glutaraldehyde after euthanasia, processed, and embedded in plastic. Plastic blocks from spinal cord and optic nerve of the first two cats were also studied.
Supplementary Material
Acknowledgments
We are grateful to Abigail Radcliff for her skilled preparation of this manuscript and to Dr. John Svaren and Dr. Ian Griffiths for our many discussions on this research and their review of the paper. We are also grateful to Dr. Sara Levén for her careful collection of the spinal cord of the first case and to Dr. Georgina Child for the second cat tissue from Sydney. These studies were supported in part by National Multiple Sclerosis Society Grant RG-1501-02876 and by a prior grant from the MS Hope for a Cure Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1714183114/-/DCSupplemental.
References
- 1.Duncan ID, Brower A, Kondo Y, Curlee JF, Jr, Schultz RD. Extensive remyelination of the CNS leads to functional recovery. Proc Natl Acad Sci USA. 2009;106:6832–6836. doi: 10.1073/pnas.0812500106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brück W, Kuhlmann T, Stadelmann C. Remyelination in multiple sclerosis. J Neurol Sci. 2003;206:181–185. doi: 10.1016/s0022-510x(02)00191-0. [DOI] [PubMed] [Google Scholar]
- 3.Patrikios P, et al. Remyelination is extensive in a subset of multiple sclerosis patients. Brain. 2006;129:3165–3172. doi: 10.1093/brain/awl217. [DOI] [PubMed] [Google Scholar]
- 4.Patani R, Balaratnam M, Vora A, Reynolds R. Remyelination can be extensive in multiple sclerosis despite a long disease course. Neuropathol Appl Neurobiol. 2007;33:277–287. doi: 10.1111/j.1365-2990.2007.00805.x. [DOI] [PubMed] [Google Scholar]
- 5.Goldschmidt T, Antel J, König FB, Brück W, Kuhlmann T. Remyelination capacity of the MS brain decreases with disease chronicity. Neurology. 2009;72:1914–1921. doi: 10.1212/WNL.0b013e3181a8260a. [DOI] [PubMed] [Google Scholar]
- 6.Franklin RJ, Goldman SA. Glia disease and repair-remyelination. Cold Spring Harb Perspect Biol. 2015;7:a020594. doi: 10.1101/cshperspect.a020594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hagemeier K, Brück W, Kuhlmann T. Multiple sclerosis–Remyelination failure as a cause of disease progression. Histol Histopathol. 2012;27:277–287. doi: 10.14670/HH-27.277. [DOI] [PubMed] [Google Scholar]
- 8.Bjartmar C, Kidd G, Mörk S, Rudick R, Trapp BD. Neurological disability correlates with spinal cord axonal loss and reduced N-acetyl aspartate in chronic multiple sclerosis patients. Ann Neurol. 2000;48:893–901. [PubMed] [Google Scholar]
- 9.Lovas G, Szilágyi N, Majtényi K, Palkovits M, Komoly S. Axonal changes in chronic demyelinated cervical spinal cord plaques. Brain. 2000;123:308–317. doi: 10.1093/brain/123.2.308. [DOI] [PubMed] [Google Scholar]
- 10.Medana IM, Esiri MM. Axonal damage: A key predictor of outcome in human CNS diseases. Brain. 2003;126:515–530. doi: 10.1093/brain/awg061. [DOI] [PubMed] [Google Scholar]
- 11.Smith KJ, Blakemore WF, McDonald WI. The restoration of conduction by central remyelination. Brain. 1981;104:383–404. doi: 10.1093/brain/104.2.383. [DOI] [PubMed] [Google Scholar]
- 12.Jeffery ND, Blakemore WF. Locomotor deficits induced by experimental spinal cord demyelination are abolished by spontaneous remyelination. Brain. 1997;120:27–37. doi: 10.1093/brain/120.1.27. [DOI] [PubMed] [Google Scholar]
- 13.Liebetanz D, Merkler D. Effects of commissural de- and remyelination on motor skill behaviour in the cuprizone mouse model of multiple sclerosis. Exp Neurol. 2006;202:217–224. doi: 10.1016/j.expneurol.2006.05.032. [DOI] [PubMed] [Google Scholar]
- 14.Kornek B, et al. Multiple sclerosis and chronic autoimmune encephalomyelitis: A comparative quantitative study of axonal injury in active, inactive, and remyelinated lesions. Am J Pathol. 2000;157:267–276. doi: 10.1016/S0002-9440(10)64537-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Irvine KA, Blakemore WF. Remyelination protects axons from demyelination-associated axon degeneration. Brain. 2008;131:1464–1477. doi: 10.1093/brain/awn080. [DOI] [PubMed] [Google Scholar]
- 16.Mei F, et al. Accelerated remyelination during inflammatory demyelination prevents axonal loss and improves functional recovery. Elife. 2016;5:e18246. doi: 10.7554/eLife.18246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schultz V, et al. Acutely damaged axons are remyelinated in multiple sclerosis and experimental models of demyelination. Glia. 2017;65:1350–1360. doi: 10.1002/glia.23167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Franklin RJM, Zhao C, Lubetzki C, Ffrench-Constant C. Endogenous remyelination in the CNS. In: Duncan ID, Franklin RJM, editors. Myelin Repair and Neuroprotection in Multiple Sclerosis. Springer; New York: 2013. pp. 71–92. [Google Scholar]
- 19.Gledhill RF, Harrison BM, McDonald WI. Pattern of remyelination in the CNS. Nature. 1973;244:443–444. doi: 10.1038/244443a0. [DOI] [PubMed] [Google Scholar]
- 20.Harrison BM, McDonald WI. Remyelination after transient experimental compression of the spinal cord. Ann Neurol. 1977;1:542–551. doi: 10.1002/ana.410010606. [DOI] [PubMed] [Google Scholar]
- 21.Clifford-Jones RE, Landon DN, McDonald WI. Remyelination during optic nerve compression. J Neurol Sci. 1980;46:239–243. doi: 10.1016/0022-510x(80)90082-9. [DOI] [PubMed] [Google Scholar]
- 22.Prineas JW, Connell F. The fine structure of chronically active multiple sclerosis plaques. Neurology. 1978;28:68–75. doi: 10.1212/wnl.28.9_part_2.68. [DOI] [PubMed] [Google Scholar]
- 23.Prineas JW, Connell F. Remyelination in multiple sclerosis. Ann Neurol. 1979;5:22–31. doi: 10.1002/ana.410050105. [DOI] [PubMed] [Google Scholar]
- 24.Xing YL, et al. Adult neural precursor cells from the subventricular zone contribute significantly to oligodendrocyte regeneration and remyelination. J Neurosci. 2014;34:14128–14146. doi: 10.1523/JNEUROSCI.3491-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Samanta J, et al. Inhibition of Gli1 mobilizes endogenous neural stem cells for remyelination. Nature. 2015;526:448–452. doi: 10.1038/nature14957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stidworthy MF, Genoud S, Suter U, Mantei N, Franklin RJM. Quantifying the early stages of remyelination following cuprizone-induced demyelination. Brain Pathol. 2003;13:329–339. doi: 10.1111/j.1750-3639.2003.tb00032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Archer DR, Cuddon PA, Lipsitz D, Duncan ID. Myelination of the canine central nervous system by glial cell transplantation: A model for repair of human myelin disease. Nat Med. 1997;3:54–59. doi: 10.1038/nm0197-54. [DOI] [PubMed] [Google Scholar]
- 28.Powers BE, et al. Remyelination reporter reveals prolonged refinement of spontaneously regenerated myelin. Proc Natl Acad Sci USA. 2013;110:4075–4080. doi: 10.1073/pnas.1210293110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dubois-Dalcq M, et al. From fish to man: Understanding endogenous remyelination in central nervous system demyelinating diseases. Brain. 2008;131:1686–1700. doi: 10.1093/brain/awn076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Münzel EJ, Williams A. Promoting remyelination in multiple sclerosis-recent advances. Drugs. 2013;73:2017–2029, and erratum (2014)74:157. doi: 10.1007/s40265-013-0146-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cole KLH, Early JJ, Lyons DA. Drug discovery for remyelination and treatment of MS. Glia. 2017;65:1565–1589. doi: 10.1002/glia.23166. [DOI] [PubMed] [Google Scholar]
- 32.Pemberton TJ, et al. A mutation in the canine gene encoding folliculin-interacting protein 2 (FNIP2) associated with a unique disruption in spinal cord myelination. Glia. 2014;62:39–51. doi: 10.1002/glia.22582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mayer JA, et al. Modeling the natural history of Pelizaeus-Merzbacher disease. Neurobiol Dis. 2015;75:115–130. doi: 10.1016/j.nbd.2014.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blakemore WF, Murray JA. Quantitative examination of internodal length of remyelinated nerve fibres in the central nervous system. J Neurol Sci. 1981;49:273–284. doi: 10.1016/0022-510x(81)90084-8. [DOI] [PubMed] [Google Scholar]
- 35.Arancibia-Carcamo IL, Attwell D. The node of Ranvier in CNS pathology. Acta Neuropathol. 2014;128:161–175. doi: 10.1007/s00401-014-1305-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Franklin RJM, Hinks GL. Understanding CNS remyelination: Clues from developmental and regeneration biology. J Neurosci Res. 1999;58:207–213. [PubMed] [Google Scholar]
- 37.Brinkmann BG, et al. Neuregulin-1/ErbB signaling serves distinct functions in myelination of the peripheral and central nervous system. Neuron. 2008;59:581–595. doi: 10.1016/j.neuron.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ishii A, Fyffe-Maricich SL, Furusho M, Miller RH, Bansal R. ERK1/ERK2 MAPK signaling is required to increase myelin thickness independent of oligodendrocyte differentiation and initiation of myelination. J Neurosci. 2012;32:8855–8864. doi: 10.1523/JNEUROSCI.0137-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ishii A, Furusho M, Bansal R. Sustained activation of ERK1/2 MAPK in oligodendrocytes and schwann cells enhances myelin growth and stimulates oligodendrocyte progenitor expansion. J Neurosci. 2013;33:175–186. doi: 10.1523/JNEUROSCI.4403-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fancy SP, Chan JR, Baranzini SE, Franklin RJ, Rowitch DH. Myelin regeneration: A recapitulation of development? Annu Rev Neurosci. 2011;34:21–43. doi: 10.1146/annurev-neuro-061010-113629. [DOI] [PubMed] [Google Scholar]
- 41.Duncan GJ, et al. Myelin regulatory factor drives remyelination in multiple sclerosis. Acta Neuropathol. 2017 doi: 10.1007/s00401-017-1741-7. [DOI] [PubMed] [Google Scholar]
- 42.Young KM, et al. Oligodendrocyte dynamics in the healthy adult CNS: Evidence for myelin remodeling. Neuron. 2013;77:873–885. doi: 10.1016/j.neuron.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee Y, et al. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature. 2012;487:443–448. doi: 10.1038/nature11314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nave KA. Myelination and support of axonal integrity by glia. Nature. 2010;468:244–252. doi: 10.1038/nature09614. [DOI] [PubMed] [Google Scholar]
- 45.Nave KA. Myelination and the trophic support of long axons. Nat Rev Neurosci. 2010;11:275–283. doi: 10.1038/nrn2797. [DOI] [PubMed] [Google Scholar]
- 46.Simons M, Nave KA. Oligodendrocytes: Myelination and axonal support. Cold Spring Harb Perspect Biol. 2015;8:a020479. doi: 10.1101/cshperspect.a020479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Griffiths I, et al. Axonal swellings and degeneration in mice lacking the major proteolipid of myelin. Science. 1998;280:1610–1613. doi: 10.1126/science.280.5369.1610. [DOI] [PubMed] [Google Scholar]
- 48.Lappe-Siefke C, et al. Disruption of Cnp1 uncouples oligodendroglial functions in axonal support and myelination. Nat Genet. 2003;33:366–374. doi: 10.1038/ng1095. [DOI] [PubMed] [Google Scholar]
- 49.Edgar JM, et al. Age-related axonal and myelin changes in the rumpshaker mutation of the Plp gene. Acta Neuropathol. 2004;107:331–335. doi: 10.1007/s00401-003-0808-9. [DOI] [PubMed] [Google Scholar]
- 50.Petzold A, et al. Phosphorylation and compactness of neurofilaments in multiple sclerosis: Indicators of axonal pathology. Exp Neurol. 2008;213:326–335. doi: 10.1016/j.expneurol.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kornegay JN, Goodwin MA, Spyridakis LK. Hypomyelination in Weimaraner dogs. Acta Neuropathol. 1987;72:394–401. doi: 10.1007/BF00687272. [DOI] [PubMed] [Google Scholar]
- 52.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- 53.Child G, Foster DJ, Fougere BJ, Milan JM, Rozmanec M. Ataxia and paralysis in cats in Australia associated with exposure to an imported gamma-irradiated commercial dry pet food. Aust Vet J. 2009;87:349–351. doi: 10.1111/j.1751-0813.2009.00475.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.