Significance
Wood is a widely used renewable feedstock for industrial production and energy generation. The secondary cell wall (SCW) is the major component of wood. Two key transcription factor families, Vascular-Related NAC-Domain (VND) and Secondary Wall-Associated NAC Domain (SND), are master gene regulators for SCW biosynthesis. However, plants exhibit stunted growth or abnormal SCW development under excess VND or SND gene expression. In this study, we show that two splice variants, PtrVND6-C1IR and PtrSND1-A2IR, each from VND and SND families, act as negative regulators. We propose that PtrVND6-C1IR and PtrSND1-A2IR function together for reciprocal cross-regulation of VND and SND families to maintain homeostasis for xylem differentiation and plant development.
Keywords: reciprocal cross-regulation, NAC transcription factors, alternative splicing, wood formation, Populus trichocarpa
Abstract
Secondary cell wall (SCW) biosynthesis is the biological process that generates wood, an important renewable feedstock for materials and energy. NAC domain transcription factors, particularly Vascular-Related NAC-Domain (VND) and Secondary Wall-Associated NAC Domain (SND) proteins, are known to regulate SCW differentiation. The regulation of VND and SND is important to maintain homeostasis for plants to avoid abnormal growth and development. We previously identified a splice variant, PtrSND1-A2IR, derived from PtrSND1-A2 as a dominant-negative regulator, which suppresses the transactivation of all PtrSND1 family members. PtrSND1-A2IR also suppresses the self-activation of the PtrSND1 family members except for its cognate transcription factor, PtrSND1-A2, suggesting the existence of an unknown factor needed to regulate PtrSND1-A2. Here, a splice variant, PtrVND6-C1IR, derived from PtrVND6-C1 was discovered that suppresses the protein functions of all PtrVND6 family members. PtrVND6-C1IR also suppresses the expression of all PtrSND1 members, including PtrSND1-A2, demonstrating that PtrVND6-C1IR is the previously unidentified regulator of PtrSND1-A2. We also found that PtrVND6-C1IR cannot suppress the expression of its cognate transcription factor, PtrVND6-C1. PtrVND6-C1 is suppressed by PtrSND1-A2IR. Both PtrVND6-C1IR and PtrSND1-A2IR cannot suppress their cognate transcription factors but can suppress all members of the other family. The results indicate that the splice variants from the PtrVND6 and PtrSND1 family may exert reciprocal cross-regulation for complete transcriptional regulation of these two families in wood formation. This reciprocal cross-regulation between families suggests a general mechanism among NAC domain proteins and likely other transcription factors, where intron-retained splice variants provide an additional level of regulation.
Wood is an abundant and renewable raw material for energy, pulping, and solid wood products (1, 2). Wood is composed of secondary cell walls (SCWs), which in turn are made of three major polymers: cellulose, hemicelluloses, and lignin. SCW biosynthesis is a complex developmental process, which is regulated by control of transcription (3–5), mRNA splicing (6, 7), protein modification (8), and metabolic flux (9). Few studies of the transcriptional regulation of wood formation have been carried out in woody plants, although SCW biosynthesis has been studied extensively in Arabidopsis (3–5, 10–16). NAC (for NAM, ATAF1/2, and CUC2) and MYB transcription factors (TFs) regulate SCW differentiation (3–5). NAC domain proteins, in particular the VND6 and SND1 families, have been proposed as “master regulators” of SCW biosynthesis in Arabidopsis (10, 11). VND6 and SND1 activate downstream TFs, such as MYBs, to induce indirect expression of genes for cellulose, hemicelluloses, and lignin biosynthesis (3, 14, 17). In Arabidopsis, VND6 induces the differentiation of metaxylem and protoxylem vessel elements (3, 10, 12), while SND1 regulates deposition of SCWs in fibers (11, 12). Overexpression of VND6 or SND1 causes abnormal xylem or stunted growth (5, 10, 11). The regulation of these TFs is important for normal plant growth and development.
In Populus trichocarpa, the VND6 family has six members (PtrVND6-A1, -A2, -B1, -B2, -C1, -C2), and the SND1 family has four members (PtrSND1-A1, -A2, -B1, -B2) (6). We previously identified a high-level regulator, PtrSND1-A2IR, of the SND1 family (6). PtrSND1-A2IR is an intron-retained (IR) splice variant of PtrSND1-A2 that acts as a dominant negative to suppress the protein functions of PtrSND1 family members (6). PtrSND1-A2IR lacks the DNA binding and transcriptional activation domains but retains the protein dimerization domain. PtrSND1-A2IR is found exclusively in cytoplasmic foci. Through the formation of heterodimers with any of the PtrSND1 members, PtrSND1-A2IR can be translocated into the nucleus, where it suppresses the protein functions of the PtrSND1 family members (6). PtrSND1-A2IR also suppresses the self-activation of the PtrSND1 family members except for PtrSND1-A2, its cognate TF (6). How the expression of PtrSND1-A2 itself is regulated has remained unknown.
Many dominant negatives are derived from alternative splicing (18, 19). They usually lack DNA binding or transactivation domains but retain protein–protein interaction domains (20). In animals, dominant negatives derived from alternative splicing form heterodimers with their targets to disrupt the function of the target proteins (21, 22). In plants, this dominant-negative effect was first reported in Arabidopsis, where alternative splicing variants suppressed the protein function of their cognate TFs (23, 24). The dominant-negative effect suppressing members of a TF family was first reported in P. trichocarpa (6). There is no previous report on the regulation of one dominant negative on multiple TF families.
In this article, we report the discovery of an IR splice variant, PtrVND6-C1IR, derived from PtrVND6-C1 in the PtrVND6 family, which also acts as a dominant negative on its own family and on the PtrSND1 family. We performed laser capture microdissection (LCM) combined with RNA-sequencing (RNA-seq) and determined that all PtrVND6 and PtrSND1 family members, PtrSND1-A2IR, and PtrVND6-C1IR were expressed in the same cell types. A transactivation assay in stem-differentiating xylem (SDX) protoplasts was used to characterize their functions on transcriptional regulation. Combining these results with subcellular localization and bimolecular fluorescence complementation (BiFC), we have uncovered a reciprocal cross-regulation system of PtrVND6 and PtrSND1 families by their IR splice variants, PtrSND1-A2IR and PtrVND6-C1IR, in SCW biosynthesis.
Results
Identification of Six Xylem-Specific PtrVND6s.
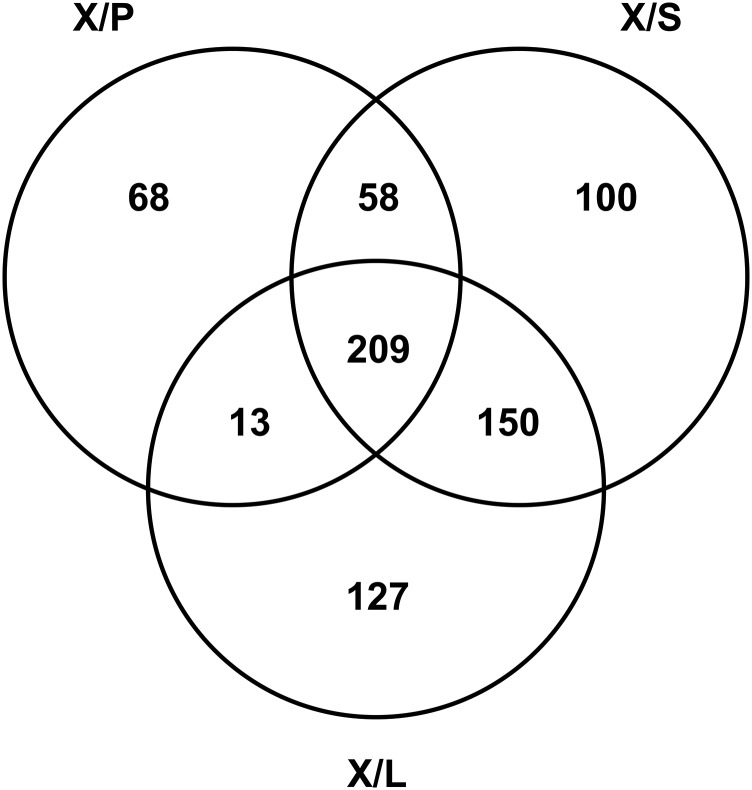
There are two VND families, VND6 and VND7, in the P. trichocarpa genome (6). We first identified which members of these VND families are preferentially expressed in the wood-forming tissue (SDX). To do this, we prepared total RNA from SDX (X), phloem (P), young shoots (S), and leaves (L) and carried out full transcriptome RNA-seq analysis. Two hundred and nine SDX differentially expressed TFs (FDR < 0.05) were identified by comparison of X/P, X/S, and X/L tissue pairs with all three transcript abundance ratios >1.5 (Fig. S1 and Dataset S1). These 209 TFs belong to 41 diverse TF families, including 21 in the NAC family. These 21 are as follows: four PtrSND1s, six PtrVND6s, six PtrSND2/3s (6), and five PNACs (Dataset S1). The six PtrVND6s are PtrVND6-A1 (POPTR_0015s14770, Potri.015G127400), PtrVND6-A2 (POPTR_0012s14660, Potri.012G126500), PtrVND6-B1 (POPTR_0003s11250, Potri.003G113000), PtrVND6-B2 (POPTR_0001s00220, Potri.001G120000), PtrVND6-C1 (POPTR_0007s13910, Potri.007G014400), and PtrVND6-C2 (POPTR_0005s11870, Potri.005G116800) (6). We then cloned the cDNAs of all six PtrVND6s.
Fig. S1.
Transcript abundance of the transcriptome in SDX, phloem (P), young shoot (S), and leaves (L) was analyzed by RNA-sEq. Two hundred and nine SDX (X) differentially expressed TFs were identified through the transcript abundance ratio >1.5 between (a) X/P: SDX and phloem (P), (b) X/S: SDX and young shoot (S), and (c) X/L: SDX and leaves.
Three Splice Variants of PtrVND6s Were Identified Through PCR Cloning and RNA-Seq.
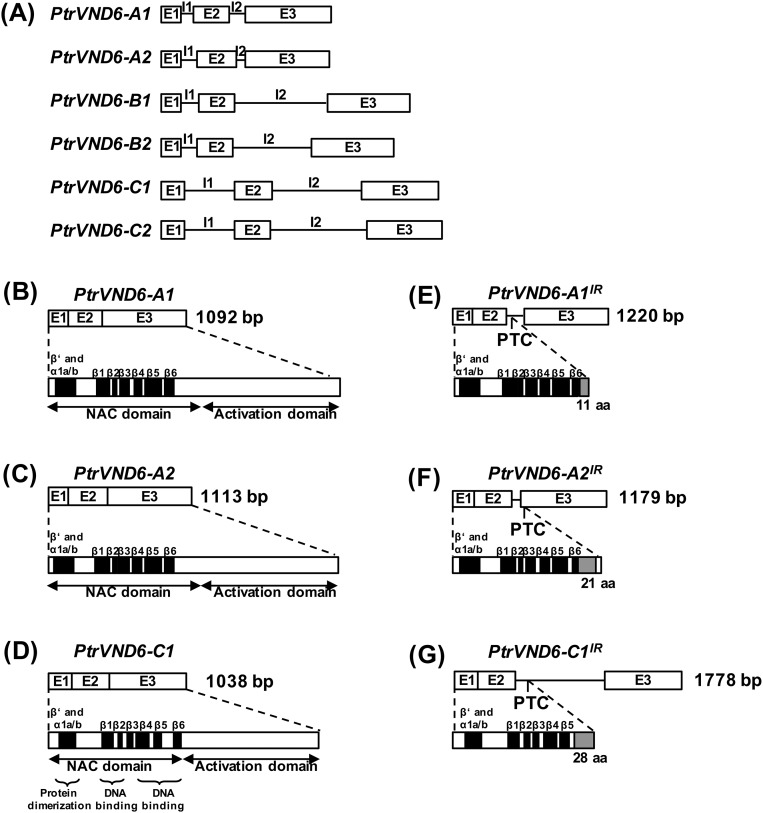
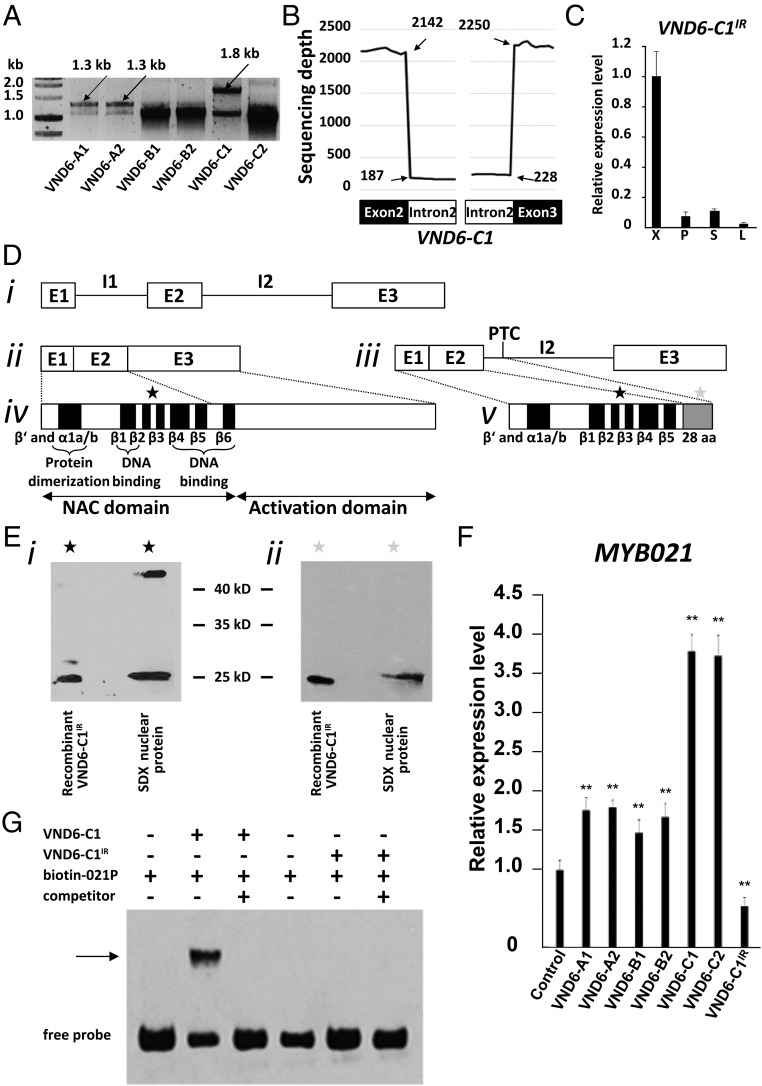
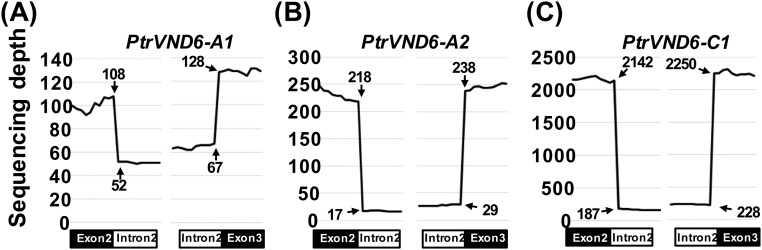
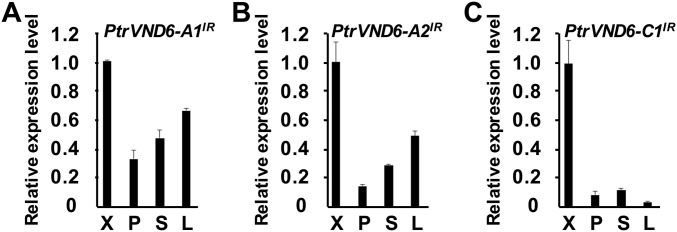
Each gene sequence of the PtrVND6s contains three exons and two introns (P. trichocarpa version JGI 2.2), indicating that all PtrVND6 cDNAs should be about 1.1 kb (Fig. S2 A–D). We PCR cloned all six PtrVND6 cDNAs, and all have the expected size and correct sequence (Fig. 1A). In addition to the expected PCR products of six PtrVND6s (∼1.1 kb), three larger fragments were detected for PtrVND6-A1 (∼1.3 kb), -A2 (∼1.3 kb), and -C1 (∼1.8 kb) (Fig. 1A). These fragments represent cDNAs that retain intron 2 (I2) of PtrVND6-A1 (Fig. S2E), PtrVND6-A2 (Fig. S2F), and PtrVND6-C1 (Fig. S2G). The retained introns are due to incomplete mRNA splicing. The inclusion of the retained introns was confirmed by sequence reads with the specific retained introns based on 18 independent RNA-seq analyses (Fig. 1B for PtrVND6-C1 and Fig. S3 for PtrVND6-A1, -A2, and -C1). We named these IR splice variants as PtrVND6-A1IR, -A2IR, and -C1IR. These three variants are also preferentially expressed in SDX compared with phloem, young shoots, and leaves (Fig. 1C for PtrVND6-C1IR and Fig. S4 for PtrVND6-A1IR, -A2IR, and -C1IR). We next investigated whether the mRNAs of the three splice variants are translated into proteins.
Fig. S2.
Genomic DNA, mRNA, and protein structure of PtrVND6-A1 and -A1IR, PtrVND6-A2 and -A2IR, and PtrVND6-C1 and -C1IR. (A) Genomic DNA of PtrVND6-A1, -A2, -B1, -B2, -C1, and -C2 with three exons (E1, E2, and E3) and two introns (I1 and I2). (B–D) PtrVND6-A1, -A2, and -C1 are each composed of a conserved N-terminal NAC domain and a C-terminal activation domain. (E) PtrVND6-A1IR mRNA has three exons and a retained intron (I2) that contains a PTC, and PtrVND6-A1IR has a complete NAC domain and 11 aa translated from the front part of I2 (before the PTC). (F) PtrVND6-A2IR mRNA has three exons and a retained intron (I2), and PtrVND6-A2IR has a complete NAC domain, 21 aa translated from I2, and 7 aa translated from the front part of exon 3 (before the PTC). (G) PtrVND6-C1IR mRNA has three exons (E1–3) and a retained intron (I2) with a PTC. PtrVND6-C1IR has an incomplete N-terminal NAC domain and 28 aa translated from the front part of intron 3 (before PTC).
Fig. 1.
Discovery of PtrVND6-C1IR and its functional analysis. (A) RT-PCR of six of the PtrVND6 family members. (B) Sequencing depth of 40 nt on the junctions of PtrVND6-C1 exon 2 (20 nt in the left black box) and I2 (20 nt in the left white box), and I2 (20 nt in the right white box) and exon 3 (20 nt in the right black box). We manually aligned the 5′ and 3′ junctions of each PtrVND6 gene and found no specific sequence for IR genes. (C) qRT-PCR analysis of the transcript abundance of PtrVND6-C1IR in xylem (X), phloem (P), young shoots (S), and leaves (L). The error bars represent SEs from three biological replicates. (D) Gene and protein structures of PtrVND6-C1 and PtrVND6-C1IR. (i) Genomic DNA of PtrVND6-C1 with three exons (E1–3) and two introns (I1–2). (ii) PtrVND6-C1 mRNA has three exons (E1–3). (iii) PtrVND6-C1IR mRNA has three exons (E1–3) and a retained intron (I2) that contains a PTC. We also manually aligned the I2 of each of the PtrVND6 genes and found no branch point-specific sequences for IR genes. (iv) PtrVND6-C1 is composed of a conserved N-terminal NAC domain containing β′, α1a/b, β1–6 subdomains, and a C-terminal activation domain. (v) PtrVND6-C1IR has an incomplete N-terminal NAC domain consisting of β′, α1a/b, β1–5 subdomains, and 28 aa translated from the front part of intron 3 (before PTC). (E) Western blot analysis using recombinant PtrVND6-C1IR and SDX nuclear proteins with the PtrVND6-C1 NAC domain antibody (black stars) (i) or PtrVND6-C1IR 28 aa-specific antibody (gray stars) (ii). (F) SDX protoplast transactivation assays overexpressing GFP (control) or PtrVND6-A1, -A2, -B1, -B2, -C1, -C2, or -C1IR. The transcript abundance of PtrMYB021 was detected using qRT-PCR. The error bars represent SEs from three biological replicates. Statistical significance was estimated using the Student t test (**P < 0.05; compared with control). (G) EMSA using PtrVND6-C1 or PtrVND6-C1IR recombinant proteins with PtrMYB021 promoter fragments labeled by biotin. PtrMYB021 promoter fragments without biotin labeling were used as competitors. The arrow shows the shifted band representing the protein–DNA complex.
Fig. S3.
The RNA-seq sequence reads of 40 nt on the junctions of (A) PtrVND6-A1, (B) PtrVND6-A2, and (C) PtrVND6-C1 exon 2 (20 nt in the left black box) and I2 (20 nt in the left white box) and I2 (20 nt in the right white box) and exon 3 (20 nt in the right black box).
Fig. S4.
qRT-PCR analysis was used to detect the transcript abundance of (A) PtrVND6-A1IR, (B) PtrVND6-A2IR, and (C) PtrVND6-C1IR in SDX (X), phloem (P), young shoot (S), and leaves (L). The error bars represent SEs from three biological replicates.
PtrVND6-C1IR Encodes an Incomplete NAC Domain Protein in P. trichocarpa SDX.
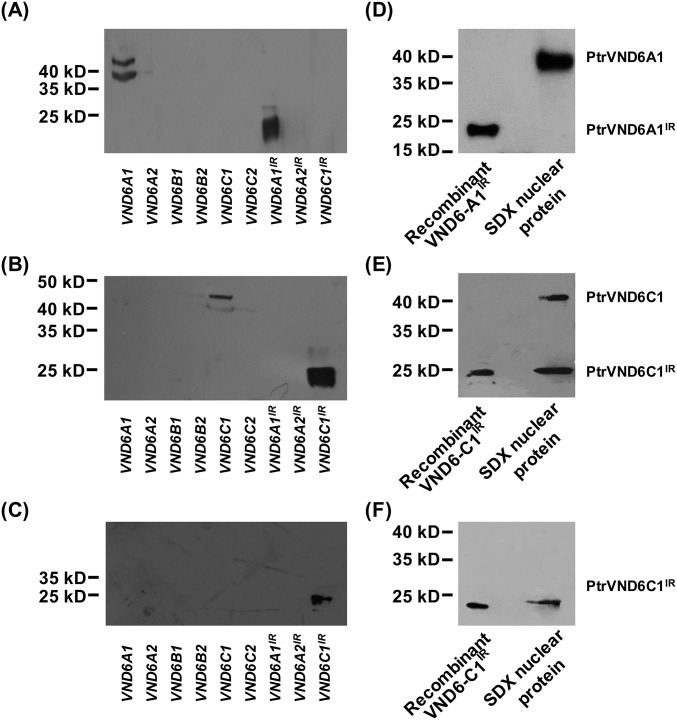
The inferred protein structures of PtrVND6-A1, -A2, and -C1 include an N-terminal NAC domain with β′, α1a/b, and β1–β6 subdomains and a C-terminal activation domain (Fig. 1 D, ii and iv for PtrVND6-C1 and Fig. S2 B–D for PtrVND6-A1, -A2, and -C1). A premature termination codon (PTC) in the retained introns of PtrVND6-A1IR, -A2IR, and -C1IR yields smaller proteins, which lack a C-terminal activation domain (Fig. 1 D, iii and v for PtrVND6-C1IR and Fig. S2 E–G for PtrVND6-A1IR, -A2IR, and -C1IR). Of these three variants, we focused on PtrVND6-A1IR and PtrVND6-C1IR (Fig. S3), both of which have the highest expression. We designed immunogens using polypeptides specific to the NAC domains of PtrVND6-A1 and -A1IR and the NAC domains of PtrVND6-C1 and -C1IR to produce their corresponding polyclonal antibodies. The antibodies were tested for specificity using recombinant proteins of the six full-size PtrVND6s and the variants PtrVND6-A1IR, -A2IR, and -C1IR produced by Escherichia coli (Fig. S5A for PtrVND6-A1 and -A1IR and Fig. S5B for PtrVND6-C1 and -C1IR). The antibodies were then used for Western blot analysis of nuclear proteins isolated from SDX. PtrVND6-A1 reacted with the antibody, showing a size of 42.3 kDa as predicted (Fig. S5D). No signal was detected for PtrVND6-A1IR (Fig. S5D), indicating that either the mRNA of PtrVND6-A1IR is not translated or its protein quantity is too low to detect. Strong signals were detected for PtrVND6-C1 and PtrVND6-C1IR around the predicted masses of 39.8 and 21.1 kDa, respectively (Fig. 1 E, i and Fig. S5E).
Fig. S5.
Western blot analysis using SDX nuclear protein shows that PtrVND6-C1IR can be detected but PtrVND6-A1IR cannot be detected. Antibody specificity test of (A) NAC domain of PtrVND6-A1 and PtrVND6-A1IR, (B) NAC domain of PtrVND6-C1 and PtrVND6-C1IR, and (C) 28 aa of PtrVND6-C1IR using recombinant proteins of PtrVND6-A1, -A2, -B1, -B2, -C1, -C2, -A1IR, -A2IR, and -C1IR. (D) Antibody specific to the NAC domain of PtrVND6-A1 and -A1IR detected only PtrVND6-A1 in SDX nuclear protein. Recombinant protein of PtrVND6-A1IR was used as the control. (E) Antibody specific to the NAC domain of PtrVND6-C1 and -C1IR detected both PtrVND6-C1 and -C1IR in SDX nuclear protein. (F) Antibody specific to the 28 aa of PtrVND6-C1IR detected PtrVND6-C1IR in SDX nuclear protein. Recombinant protein of PtrVND6-C1IR was used as the control in E and F.
To further confirm the presence of the PtrVND6-C1IR protein, we designed a polypeptide specific to its 28 unique amino acids upstream of the PTC (gray box in Fig. 1 D, v) and produced a polyclonal antibody. Antibody specificity was verified (Fig. S5E), and this antibody was able to discriminate PtrVND6-C1IR from PtrVND6-C1 and other PtrVND6s (Fig. S5C). Western blot analysis of the SDX nuclear protein shows a band around 21 kDa, confirming the presence and size of PtrVND6-C1IR (Fig. 1 E, ii and Fig. S5F). PtrVND6-C1IR is the only detected variant of PtrVND6; thus, we investigated the role of PtrVND6-C1IR in wood formation and its potential regulatory relationship with other full-size PtrVND6s.
PtrVND6-C1IR Inhibits the Transcription of PtrMYB021.
In Arabidopsis, AtVND6 can directly induce the expression of AtMYB46 (17). We used our P. trichocarpa SDX protoplast system (6, 25) to test for transregulation activity of PtrVND6-C1IR and the six full-size PtrVND6s. All six full-size PtrVND6s increased the expression of PtrMYB021 by 1.43–4.19-fold (Fig. 1F). PtrVND6-C1IR reduced PtrMYB021 gene expression by 54% (P < 0.02) (Fig. 1F). The inferred protein structure of PtrVND6-C1IR has a complete DNA binding domain (except for the β6 motif; Fig. 1 D, v) and has no activation domain (Fig. 1 D, v). This result suggests that PtrVND6-C1IR can compete with the direct binding of the six full-size PtrVND6s to the PtrMYB021 promoter and decrease the expression of PtrMYB021.
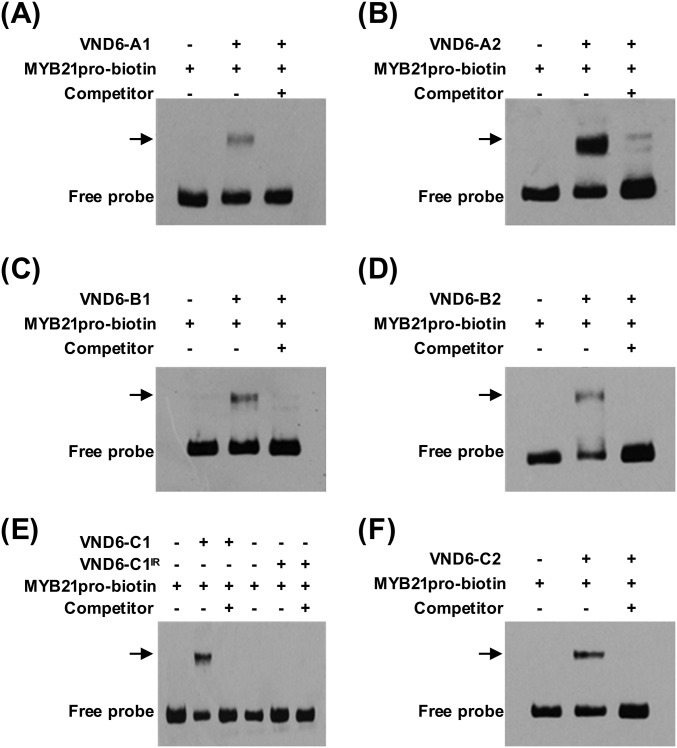
We then used an electrophoretic mobility shift assay (EMSA) (6) to test for the direct binding of full-size PtrVND6s and PtrVND6-C1IR to the PtrMYB021 promoter. Retardation of DNA probe mobility and probe competition showed that each of the six full-size PtrVND6s can bind to the PtrMYB021 promoter (PtrVND6-C1 and -C1IR in Fig. 1G and all PtrVND6s in Fig. S6). Contrary to expectation, PtrVND6-C1IR did not bind to the PtrMYB021 promoter (Fig. 1G). This result indicates that the absence of the β6 motif of PtrVND6-C1IR disrupts its DNA binding ability. The transactivation and EMSA assays show that PtrMYB021 is a common and direct target of all six full-size PtrVND6s and suggest that PtrVND6-C1IR represses the expression of PtrMYB021 by a mechanism that is not mediated by direct protein–DNA interaction.
Fig. S6.
EMSA of the recombinant proteins of PtrVND6-A1, -A2, -B1, -B2, -C1, -C1IR, and -C2 demonstrates that (A) PtrVND6-A1, (B) PtrVND6-A2, (C) PtrVND6-B1, (D) PtrVND6-B2, (E) PtrVND6-C1, and (F) PtrVND6-C2 can bind to the promoter of PtrMYB021 but (E) PtrVND6-C1IR cannot bind to the PtrMYB021 promoter. PtrMYB021 promoter fragments were labeled by biotin. PtrMYB021 promoter fragments without biotin labeling were used as competitors. Arrows represent the shifted bands that are the protein–DNA complexes.
PtrVND6-C1IR has characteristics of dominant negatives, because PtrVND6-C1IR suppresses its downstream genes (PtrMYB021), lacks an activation domain, lacks DNA binding ability, but retains the protein dimerization domain (6, 21, 23). Dominant negatives are also known to act as posttranslational regulators by forming protein heterodimers with their targets through protein dimerization domains, thereby suppressing the function of the targets (6, 23). NAC TFs contain a highly conserved protein dimerization domain (26). Therefore, we tested whether PtrVND6-C1IR can form heterodimers with each of the six full-size PtrVND6s to suppress their functions. To test for the formation of these heterodimers, we first determined the subcellular locations of PtrVND6-C1IR and the six full-size PtrVND6s.
Each of the Six Full-Size PtrVND6s Can Translocate PtrVND6-C1IR from the Cytoplasm into the Nucleus.
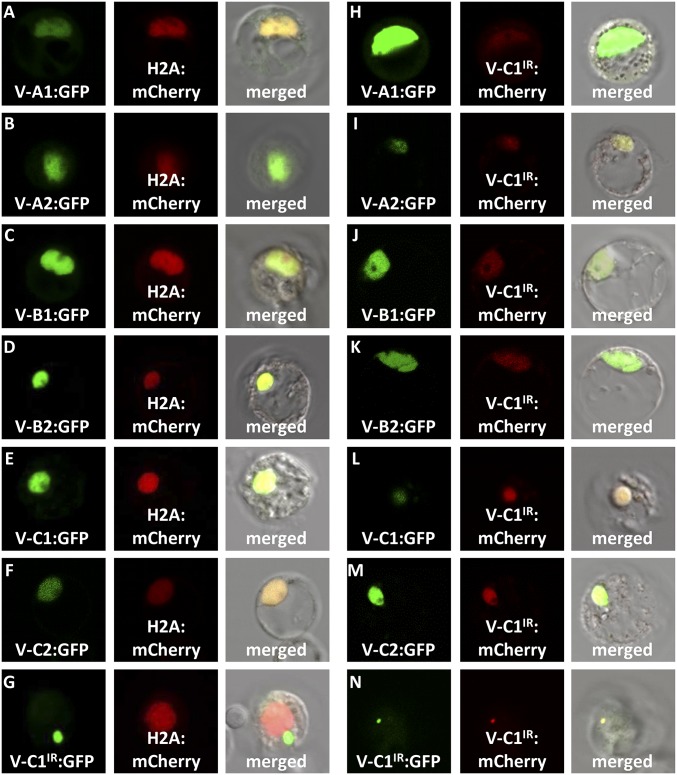
GFP was fused to each of the six full-size PtrVND6s and PtrVND6-C1IR and overexpressed in P. trichocarpa SDX protoplasts. H2A-fused mCherry was used as a nuclear marker (6). We detected both GFP and mCherry exclusively in the nucleus in ∼95% of the protoplasts transfected with each of the six full-size PtrVND6s, demonstrating that all of these VNDs are exclusively colocated with H2A in the nucleus (Fig. 2 A–F). In contrast, PtrVND6-C1IR is exclusively found in cytoplasmic foci in all transfected protoplasts (Fig. 2G). About 5% of the protoplasts showed the full-size PtrVND6s in both the nucleus and cytoplasmic foci. The sporadic location of the full-size PtrVND6s in cytoplasmic foci suggests that PtrVND6-C1IR may retain these PtrVND6s in the cytoplasm through protein–protein interactions. We then tested for such interactions by determining the subcellular location of the full-size PtrVND6s in the presence of PtrVND6-C1IR.
Fig. 2.
Subcellular colocalization demonstrates that PtrVND6-C1IR can be translocated from cytoplasmic foci into the nucleus. (A) PtrVND6-A1:GFP, (B) PtrVND6-A2:GFP, (C) PtrVND6-B1:GFP, (D) PtrVND6-B2:GFP, (E) PtrVND6-C1:GFP, and (F) PtrVND6-C2:GFP localized with H2A:mCherry in the nucleus, but (G) PtrVND6-C1IR:GFP localized in cytoplasmic foci. PtrVND6-C1IR:mCherry can be translocated into the nucleus by (H) PtrVND6-A1:GFP, (I) PtrVND6-A2:GFP, (J) PtrVND6-B1:GFP, (K) PtrVND6-B2:GFP, (L) PtrVND6-C1:GFP, and (M) PtrVND6-C2:GFP. (N) PtrVND6-C1IR:GFP and PtrVND6-C1IR:mCherry colocalized in cytoplasmic foci. The diameter of the SDX protoplasts is ∼30 μm.
Each of the six full-size PtrVND6s fused with GFP was cotransfected with mCherry-fused PtrVND6-C1IR. The full-size PtrVND6s were found in the nucleus, but PtrVND6-C1IR was translocated by the full-size PtrVND6s into the nucleus (Fig. 2 H–M). We also cotransfected GFP-fused PtrVND6-C1IR with mCherry-fused PtrVND6-C1IR and detected both GFP and mCherry only in cytoplasmic foci (Fig. 2N). Therefore, PtrVND6-C1IR can only be translocated into the nucleus by any of the six full-size PtrVND6s but not by itself. The translocation demonstrates that PtrVND6-C1IR interacts with each of the six full-size PtrVND6s. To verify these protein–protein interactions, we then carried out BiFC in P. trichocarpa SDX protoplasts.
PtrVND6-C1IR Dimerizes with Each of the Six Full-Size PtrVND6s in the Nucleus.
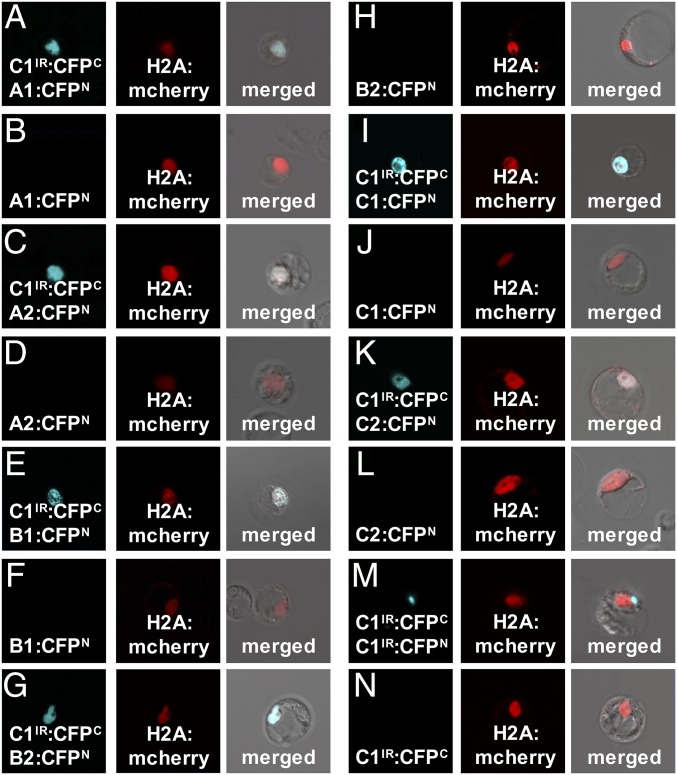
To perform BiFC, CFPC (amino acids 174–329) and CFPN (amino acids 1–173) (6) were fused to the C terminus of PtrVND6-C1IR and each of the six full-size PtrVND6s, resulting in PtrVND6-C1IR:CFPC and six PtrVND6s:CFPN. The positive CFP signal indicates an interaction of CFPC and CFPN due to the heterodimerization of PtrVND6-C1IR with a full-size PtrVND6. Each of the PtrVND6s:CFPN was cotransfected with PtrVND6-C1IR:CFPC and H2A:mCherry into SDX protoplasts. The signal of CFP was colocalized with mCherry (Fig. 3 A, C, E, G, I, and K), demonstrating that PtrVND6-C1IR formed a heterodimer with any of the full-size PtrVND6s and moved into the nucleus. We also cotransfected PtrVND6-C1IR:CFPC, PtrVND6-C1IR:CFPN, and H2A:mCherry and found CFP exclusively in cytoplasmic foci (Fig. 3M), showing that PtrVND6-C1IR forms homodimers only in cytoplasmic foci. PtrVND6-C1IR:CFPN, PtrVND6-C1IR:CFPC, and each of the PtrVND6s:CFPN were transfected individually as negative controls, and neither of the constructs showed a fluorescence signal (Fig. 3 B, D, F, H, J, L and N).
Fig. 3.
BiFC of PtrVND6-C1IR with each of the PtrVND6 family members. PtrVND6-C1IR was fused with CFPC, and each of the PtrVND6 family members was fused with CFPN. CFP signal was detected in the nucleus of the SDX protoplasts transfected by H2A:mCherry and PtrVND6-C1IR:CFPC with (A) PtrVND6-A1:CFPN, (C) PtrVND6-A2:CFPN, (E) PtrVND6-B1:CFPN, (G) PtrVND6-B2:CFPN, (I) PtrVND6-C1:CFPN, (K) PtrVND6-C2:CFPN, or (M) PtrVND6-C1IR:CFPN. SDX protoplasts transfected with only (B) PtrVND6-A1:CFPN, (D) PtrVND6-A2:CFPN, (F) PtrVND6-B1:CFPN, (H) PtrVND6-B2:CFPN, (J) PtrVND6-C1:CFPN, (L) PtrVND6-C2:CFPN, or (N) PtrVND6-C1IR:CFPC were used as negative controls. The diameter of the SDX protoplasts is ∼30 μm.
The results of BiFC and colocalization further support the characterization of PtrVND6-C1IR as a dominant negative based on the formation of heterodimers with the full-size PtrVND6s to suppress their function as direct activators. Many NAC TFs are known to self-activate their own genes (6, 27); therefore, we tested whether PtrVND6-C1IR can also suppress the self-activation function of the full-size PtrVND6s.
PtrVND6-C1IR Suppresses the Self-Activation of the Full-Size PtrVND6s.
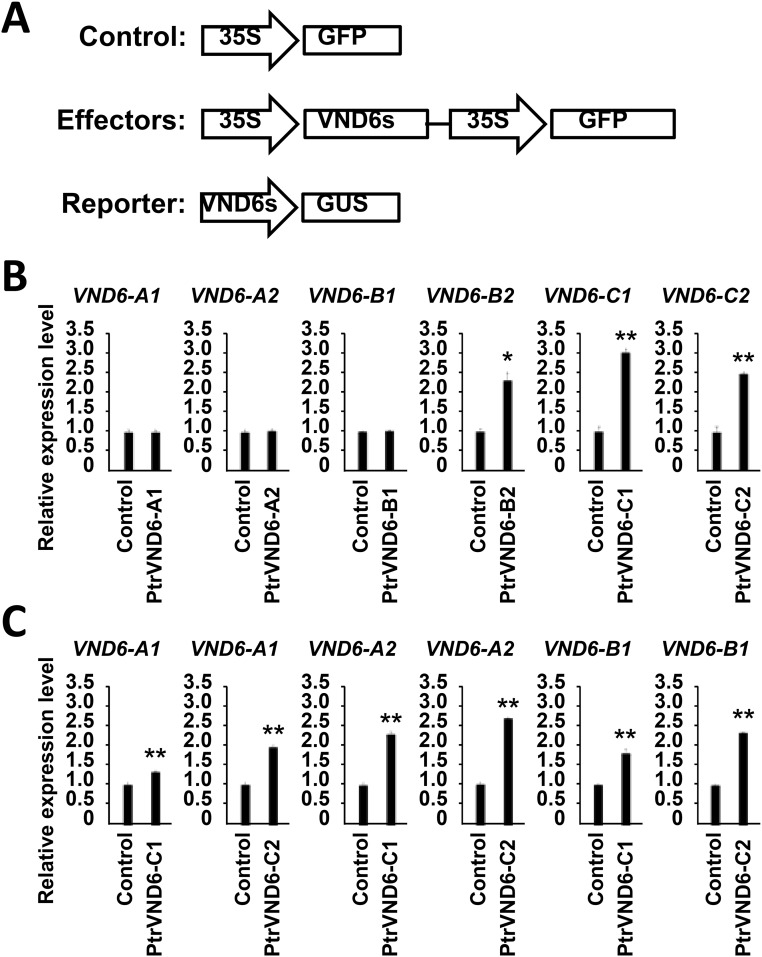
We first tested whether the six full-size PtrVND6s have a self-activation function using effector–reporter-based gene transactivation assays. PtrVND6-B2, PtrVND6-C1, and PtrVND6-C2 can activate their own gene expression (Fig. S7A), and PtrVND6-A1, PtrVND6-A2, and PtrVND6-B1 can be activated by PtrVND6-C1 or PtrVND6-C2 (Fig. S7B). In the other words, PtrVND6s family members are either self-activated or activated by other members. Overexpression of PtrVND6-C1IR in SDX protoplasts reduced the transcript abundance of PtrVND6-A1, -A2, -B1, -B2, and -C2 but did not affect the expression of its full-size isoform, PtrVND6-C1 (Fig. S8), suggesting that PtrVND6-C1 is also controlled by other TFs, which are not affected by PtrVND6-C1IR.
Fig. S7.
Effector–reporter-based gene transactivation assays were performed in Arabidopsis leaf protoplasts. (A) Each of the PtrVND6 family members driven by the 35S promoter was used as an effector, and GFP driven by the 35S promoter was used as the control. Approximately 2 kb of each of the PtrVND6 promoters driving GUS were used as reporters. (B) Each of the PtrVND6 member effectors was cotransfected with their own promoter reporters. (C) PtrVND6-C1 or PtrVND6-C2 effectors were cotransfected with PtrVND6-A1, PtrVND6-A2, or PtrVND6-B1 promoter reporters. The error bars represent SEs from three biological replicates. Statistical significance was estimated using the Student t test (*P < 0.1; **P < 0.05).
Fig. S8.
SDX protoplast transactivation assay shows that PtrVND6-C1IR suppresses the transcript abundance of PtrVND6 family members except PtrVND6-C1. Both GFP and PtrVND6-C1IR were overexpressed in SDX protoplasts, and qRT-PCR was used to quantify the transcript abundance of PtrVND6-A1, -A2, -B1, -B2, -C1, and -C2. The error bars represent SEs from three biological replicates. Statistical significance was estimated using the Student t test (**P < 0.05).
In our previous study, PtrSND1-A2IR, the splice variant of PtrSND1-A2, also suppressed the full-size PtrSND1 functions through the formation of heterodimers (6). Of the two major NAC families, SND1 and VND6, each has an IR splice variant suppressing the transcription of their own family members. It is unknown whether there are protein–protein interactions between PtrVND6 and PtrSND1 families. We then investigated whether PtrVND6-C1IR can also form heterodimers with each of the four full-size PtrSND1s (6) and whether heterodimerization occurs between PtrSND1-A2IR with the six full-size PtrVND6s. To test for the formation of cross-family heterodimers, we first investigated whether PtrVND6-C1IR, PtrSND1-A2IR, and the six full-size PtrVND6s and the four full-size PtrSND1s are expressed in the same cell type.
PtrVND6-C1IR, PtrSND1-A2IR, Full-Size PtrVND6, and PtrSND1 Members Are All Coexpressed in Fiber and Vessel Cells.
We used the stem cross-sections of P. trichocarpa (Fig. 4A) and our recently developed LCM to collect fibers (Fig. 4B), vessels (Fig. 4C), and a combination of three cell types (fibers + vessels + rays) (8, 9) (Fig. 4D). qRT-PCR demonstrated that PtrVND6-C1IR and PtrSND1-A2IR are expressed in fibers, vessels, and three cell types at roughly equivalent levels (Fig. 4 E and F). Similarly, all full-size PtrVND6s and PtrSND1s are expressed in fibers and vessels and three cell types (Fig. 4G). The presence of PtrVND6-C1IR, PtrSND1-A2IR, and all full-size PtrVND6s and PtrSND1s in the same cell types is essential for the proposed formation of cross-family heterodimers.
Fig. 4.
Fiber, vessel, and three cell types collected by LCM and the transcript abundance of the PtrVND6 and PtrSND1 families in these cell types. (A) Cross-section of the debarked stem from the 17th internode of P. trichocarpa. F, fiber cells; R, ray cells; V, vessel cells. LCM was used to collect (B) fiber cells, (C) vessel cells, and (D) three cell types. (Scale bars, 25 μm.) qRT-PCR was used to detect the transcript abundance of (E) PtrVND6-C1IR and (F) PtrSND1-A2IR in different cell types. (G) RNA-seq analysis was used to estimate the transcript abundance of full-length PtrVND6 and PtrSND1. The error bars represent SE from three biological replicates.
Full-Size PtrVND6s and PtrSND1s Cross-Interact with PtrVND6-C1IR and PtrSND1-A2IR to Translocate PtrVND6-C1IR and PtrSND1-A2IR from Cytoplasmic Foci into the Nucleus.
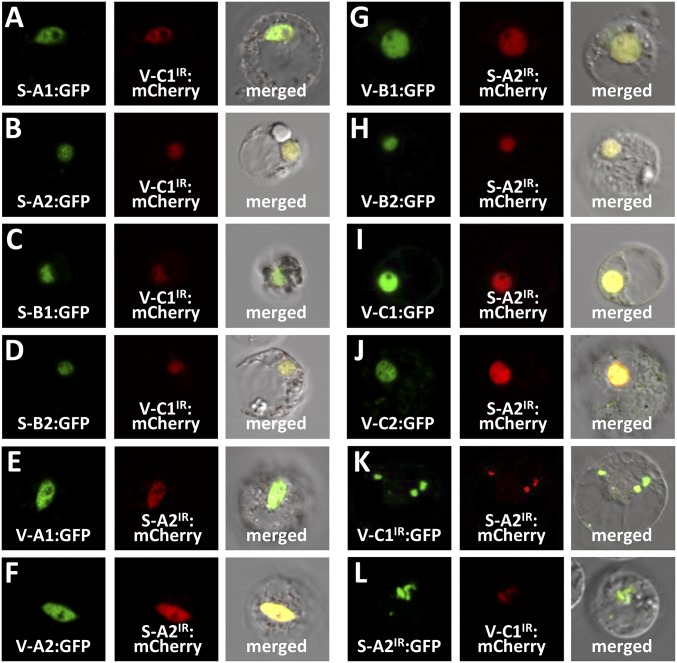
To test for cross-family heterodimerization, we first tested the subcellular localization of PtrVND6-C1IR with each of the four full-size PtrSND1s and PtrSND1-A2IR with each of the six full-size PtrVND6s. PtrVND6-C1IR was translocated from the cytosol (Fig. 2G) into the nucleus by any of the four full-size PtrSND1s (Fig. 5 A–D). Similarly, PtrSND1-A2IR was translocated from the cytosol (6) into the nucleus by each of the six full-size PtrVND6s (Fig. 5 E–J). The translocation of the splice variants can only be achieved by the full-size members, because PtrVND6-C1IR cannot translocate PtrSND1-A2IR into the nucleus and vice versa (Fig. 5 K and L).
Fig. 5.
Subcellular colocalization of PtrVND6-C1IR with each of the PtrSND1 family members and PtrSND1-A2IR with each of the PtrVND6 family members in SDX protoplasts. PtrVND6-C1IR and PtrSND1-A2IR were fused to either GFP or mCherry, and full-length PtrVND6 and PtrSND1 members were fused to GFP. PtrVND6-C1IR:mCherry colocalized with (A) PtrSND1-A1:GFP, (B) PtrSND1-A2:GFP, (C) PtrSND1-B1:GFP, or (D) PtrSND1-B2:GFP in the nucleus. PtrSND1-A2IR:mCherry colocalized with (E) PtrVND6-C1:GFP, (F) PtrVND6-A2:GFP, (G) PtrVND6-B1:GFP, (H) PtrVND6-B2:GFP, (I) PtrVND6-C1:GFP, or (J) PtrVND6-C2:GFP in the nucleus. (K) PtrVND6-C1IR:GFP colocalized with PtrSND1-A2IR:mCherry in the cytoplasmic foci. (L) PtrSND1-A2IR:GFP colocalized with PtrVND6-C1IR:mCherry in the cytoplasmic foci. The diameter of the SDX protoplasts is ∼30 µm.
PtrVND6-C1IR and PtrSND1-A2IR Form Cross-Family Heterodimers with PtrSND1s and PtrVND6s.
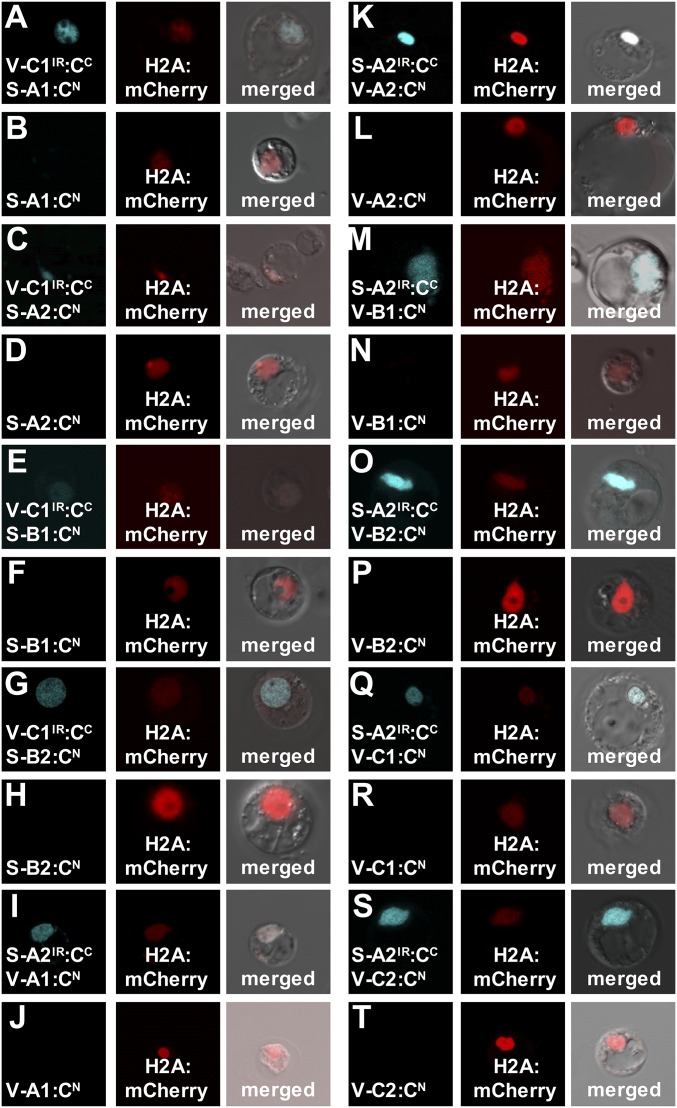
BiFC was used to test for protein–protein interactions between PtrVND6-C1IR and each of the four full-size PtrSND1s and between PtrSND1-A2IR and each of the six full-size PtrVND6s. We cotransfected PtrVND6-C1IR:CFPC with each of the four PtrSND1s:CFPN and also cotransfected PtrSND1-A2IR:CFPC with each of the six PtrVND6s:CFPN into SDX protoplasts. The CFP signal in all transfected protoplasts was colocalized with mCherry in the nucleus (Fig. 6 A, C, E, G, I, K, M, O, Q, and S), demonstrating cross-family heterodimerization. As negative controls, we transfected PtrVND6-C1IR:CFPC, PtrSND1-A2IR:CFPC, and each of the full-size PtrSND1s-CFPN and PtrVND6s-CFPN individually. CFP signal was not detected (Fig. 6 B, D, F, H, J, L, N, P, R, and T). We then tested whether cross-family heterodimerization suppresses the self-activation functions of full-size PtrSND1s and PtrVND6s.
Fig. 6.
BiFC of PtrVND6-C1IR with each of the PtrSND1 family members and PtrSND1-A2IR with each of the PtrVND6 family members in SDX protoplasts. PtrVND6-C1IR and PtrSND1-A2IR were fused with CFPC, and full-length PtrVND6 and PtrSND1 members were fused to CFPN. H2A was fused with mCherry as a nuclear marker. CFP signal was detected in the nucleus of the SDX protoplasts transfected by H2A:mCherry with the following combinations: (A) PtrSND1-A1:CFPN, (C) PtrSND1-A2:CFPN, (E) PtrSND1-B1:CFPN, or (G) PtrSND1-B2:CFPN each with PtrVND6-C1IR:CFPC; (I) PtrVND6-A1:CFPN, (K) PtrVND6-A2:CFPN, (M) PtrVND6-B1:CFPN, (O) PtrVND6-B2:CFPN, (Q) PtrVND6-C1:CFPN, or (S) PtrVND6-C2:CFPN each with PtrSND1-A2IR:CFPC. No CFP signal was detected from SDX transfected with only (B) PtrSND1-A1:CFPN, (D) PtrSND1-A2:CFPN, (F) PtrSND1-B1:CFPN, (H) PtrSND1-B2:CFPN, (J) PtrVND6-A1:CFPN, (L) PtrVND6-A2:CFPN, (N) PtrVND6-B1:CFPN, (P) PtrVND6-B2:CFPN, (R) PtrVND6-C1:CFPN, or (T) PtrVND6-C2:CFPN. The diameter of the SDX protoplasts is ∼30 µm.
PtrVND6-C1IR and PtrSND1-A2IR Inhibit the Self-Activation of Full-Size PtrVND6s and PtrSND1s.
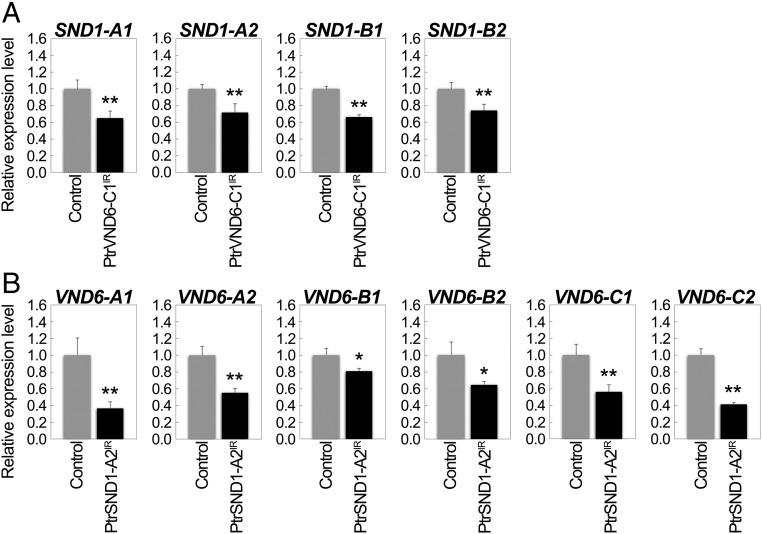
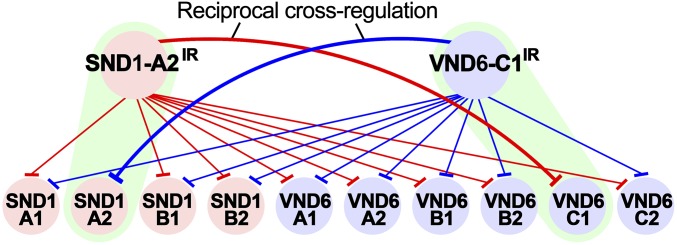
We overexpressed PtrVND6-C1IR in SDX protoplasts and found that the transcript abundance of each of the four full-size PtrSND1s was reduced (Fig. 7A), demonstrating that the function of each of the four full-size PtrSND1s was suppressed by PtrVND6-C1IR (Fig. 8). Similarly, overexpression of PtrSND1-A2IR reduced the transcript abundance of each of the six full-size PtrVND6s (Fig. 7B). The results demonstrate a plausible mechanism where cross-family heterodimerization may suppress PtrVND6 and PtrSND1 self-activation (Fig. 8).
Fig. 7.
Cross-regulation between PtrVND6 and PtrSND1 families through PtrVND6-C1IR and PtrSND1-A2IR. qRT-PCR was used to detect the transcript abundance of (A) PtrSND1-A1, -A2, - B1, or -B2 in SDX protoplasts overexpressing GFP (control) or PtrVND6-C1IR and (B) PtrVND6-A1, -A2, -B1, -B2, -C1, or -C2 in SDX protoplasts overexpressing GFP or PtrSND1-A2IR. The control values in A and B were set as 1, and the error bars represent SEs from two to three biological replicates. Statistical significance was estimated using the Student t test (*P < 0.1; **P < 0.05).
Fig. 8.
Cross-regulation between the PtrVND6 and PtrSND1 families through PtrVND6-C1IR and PtrSND1-A2IR. PtrSND1-A2IR suppresses the transcript abundance of the PtrSND1 and PtrVND6 families (red edges) except PtrSND1-A2 (green highlight). PtrVND6-C1IR suppresses PtrSND1 and PtrVND6 families (blue edges) except PtrVND6-C1 (green highlight).
These results combined with the previous study of PtrSND1-A2IR regulating the PtrSND1 family (6) demonstrate that PtrVND6 and PtrSND1 families can cross-regulate each other through their alternative splice variants (Fig. 8). The formation of these heterodimers suggests a general cross-regulation mechanism to maintain the homeostasis of the expression of PtrVND6 and PtrSND1 family members through their splice variants, PtrVND6-C1IR and PtrSND1-A2IR, providing xylem-specific NAC TF regulation in fibers and vessels in wood formation.
Discussion
VND6 and SND1 are distinct gene families based on their protein sequences in many plant species, including P. trichocarpa (6), Arabidopsis (10, 11), rice (28), maize (29), banana (30), and loquat (31). These two families act as transactivators with distinct functions in the regulation of SCW differentiation (3, 10–12). VND6 regulates the differentiation of vessels (3, 10, 12), while SND1 induces SCW thickening in fibers (11). Overexpression of VND6 and SND1 in Arabidopsis leads to abnormal xylem development or retarded growth (5, 10, 11). Regulation of VND6 and SND1 is necessary for normal growth and development (6). In P. trichocarpa, VND6 and SND1 (6) each has an IR splice variant. The existence of these unique regulatory variants in P. trichocarpa suggests a higher level of functional differentiation in xylogenesis or additional regulation to maintain homeostasis.
In both animals and plants, alternative splicing events regulate homeostasis in different states of development, differentiation, and metabolism (32, 33). More than 60% of intron-containing genes in plants have transcript variants derived from alternative splicing (34, 35). A major mode of alternative splicing is the retention of introns (36), which often results in a truncated protein due to premature termination (19, 23, 37, 38). If such truncated proteins are derived from TF genes, they may act as dominant negatives to suppress the function of the cognate TFs (6, 34, 35, 38). In P. trichocarpa, we previously identified a dominant negative, PtrSND1-A2IR, derived from the IR variant of PtrSND1-A2, which suppresses the self-activation of three out of the four PtrSND1 members (except PtrSND1-A2) and their protein functions (6). PtrSND1-A2IR was the first discovered dominant negative that suppresses multiple members within its own family (6). Here we demonstrated that PtrVND6-C1IR, another dominant negative derived from an IR variant of PtrVND6-C1, can also suppress multiple targets within its own family (Fig. S8). PtrVND6-C1IR and PtrSND1-A2IR both exert within-family regulation.
We now also found that PtrVND6-C1IR and PtrSND1-A2IR can exert cross-family (reciprocal) regulation. PtrVND6-C1IR suppresses all PtrSND1 members, while PtrSND1-A2IR suppresses all PtrVND6 members. The reciprocal regulation of the PtrVND6 and PtrSND1 families (Figs. 7 and 8) depends on the formation of heterodimers between IR variants and their full-size members. Cytoplasmic foci are the locations of the free IR variants (Fig. 5 K and L). In the presence of the full-size members, the IR variants form heterodimers in the cytosol and are subsequently translocated into the nucleus (Figs. 5 and 6). To carry out the reciprocal family regulation, both families have to be expressed in the same cells. Our LCM results showed that PtrVND6-C1IR, PtrSND1-A2IR, and their full-size family members are all expressed in both fibers and vessels (Fig. 4 E and F).
In our previous work, we showed that the PtrSND1-A2IR was able to suppress the expression of the PtrSND1 family except PtrSND1-A2, its cognate TF (6), raising the question of what regulates PtrSND1-A2 to maintain the down-regulation of this family. We have now identified PtrVND6-C1IR as this unknown regulator that can suppress PtrSND1-A2 expression. Similarly, PtrVND6-C1IR can suppress all members of the PtrVND6 family except PtrVND6-C1, and PtrVND6-C1 can be suppressed by PtrSND1-A2IR. This cross-family reciprocal regulation by these two splice variants provides a mechanism to maintain the homeostasis of the expression of both PtrVND6 and PtrSND1 families. This unique mechanism has not been reported for TF trans-regulation of distinct families with related functions, in this case wood formation. In Arabidopsis, the mRNA of VND6 and SND1 families do not have splice variants (10, 11), suggesting a relatively simple regulation of SCW differentiation in herbaceous plants. In contrast, perennial woody plants develop woody stems and undergo secondary growth, which requires more complex regulation. The discovery of PtrVND6-C1IR– and PtrSND1-A2IR–based reciprocal cross-regulation implicates a higher level of transcriptional control in perennial woody plants during wood formation.
The dimerization domain of a NAC TF is located in the highly conserved N-terminal NAC domain (26). The NAC domain protein sequence identities within the PtrVND6 and PtrSND1 family are at least 81% and 87%, respectively, and are at least 75% between PtrVND6 and PtrSND1 families (6). In our LCM results, 72 NAC TFs are expressed in both fibers and vessels. Due to the highly conserved NAC domain within the NAC family, splice variants may exert a broader interfamily regulation through the formation of heterodimers with these 72 NAC domain proteins. Members of many other TF families, such as MADS-box, bZIP, MYB, WRKY, and bHLH, form functional dimers (39, 40). If the mRNAs of these TFs have splice variants leading to truncated proteins with protein dimerization domains, then these splice variants may also act as dominant negatives to regulate their family members. These results suggest that reciprocal homeostatic mechanisms exist for other TF families, where splice variants may provide higher level transcriptional regulation of complex processes in adaptation, differentiation, development, and growth.
Materials and Methods
Plant materials, RNA extraction, qRT-PCR, PCR cloning, RNA-seq, Western blotting, SDX nuclear protein preparation, EMSA, effector–reporter-based gene transactivation assays, protoplast transfection, protein subcellular localization, and BiFC are described in detail in SI Materials and Methods. Primer sequences are listed in Table S1.
Table S1.
Primer sequence
| Usages | Primer name | Sequence, 5′ → 3′ |
| PCR clone | VND6-A1F | CACCTGGATTGCAGGACTCAAGGC |
| VND6-A1R | GCCATGAATCATAGATTTTGGCTGG | |
| VND6-A2F | CACCGGAGTATGCTGAATGGATTTCAGG | |
| VND6-A2R | AACTGGATATCATCATTTCCATAGATCA | |
| VND6-B1F | CACCGCTGCATTTCAGTTCTTGGAGGAG | |
| VND6-B1R | TAAGCCTTCACTTCCACAGATCA | |
| VND6-B2F | CACCAATCTTCAGTTCTTGGAGGAGATG | |
| VND6-B2R | GCGTTCACTTCCATAGATCAATTTGG | |
| VND6-C1F | CACCTATGCAGCATCATCAACGGTTGT | |
| VND6-C1R | TCCCCAGATTCATTTCTCAAATATGC | |
| VND6-C2F | CACCGGTGGTGGTGAATTTTATGCAGC | |
| VND6-C2R | CCTCTTCAAACCCCTCTCTTCAT | |
| VND6-A1IRR | ACCCTGCACACAACCCAACC | |
| VND6-A2IRR | ACCCTACACACGACCCAACCT | |
| VND6-C1IRR | CTAGGTCAATATGTAGAGCCTGTTC | |
| qRT-PCR | RTMYB021-F | GGACAAGGTTGCTGGAGTGATGTG |
| RTMYB021-R | GGCCTCAAGTAATTAATCCAACGAAGC | |
| RTSND1-A1-F | TAGGCTTGATGACAGCACCCATGAA | |
| RTSND1-A1-R | TCTAAATACCCGGCAAACCACCCAA | |
| RTSND1-A2-F | TCCGGGCAACTTAACGATTGGGTA | |
| RTSND1-A2-R | GCATTTGGGCCGGTAGTAAAGCA | |
| RTSND1-B1-F | AACTGGGCAACCCTTGATCGTCTA | |
| RTSND1-B1-R | GTAATGGTTGGGTCAATGCAGGGT | |
| RTVND6-A1-F | GGCCTAGACATAAACCAGACAAGCACTT | |
| RTVND6-A1-R | GACTTGATCAACTGCTTGCTCATGATTGC | |
| RTVND6-A2-F | CAAATAGAGCCACTGCTGCTGGG | |
| RTVND6-A2-R | AGGTCTTCCTCATTCCGATCAAGTCT | |
| RTVND6-B1-F | TCATGCAGCTGAGCAGATGCAT | |
| RTVND6-B1-R | ACTGGAAGTCGACGTTGAGGCATATTC | |
| RTVND6-B2-F | GCGAGTCCTTGACAAATTTGTTGCTTCC | |
| RTVND6-B2-R | TGCATTAAAGGTGGCTGTACTGGAGA | |
| RTVND6-C1-F | TCCTCAGTTAGAGAGCCCATCCCT | |
| RTVND6-C1-R | TCTGGGTGTTGTTGTTGGACAACATTCT | |
| RTVND6-C2-F | TGGTGATGGTGTTTCAAGCTTTGTTGAG | |
| RTVND6-C2-R | ACTTGTTCCCGTCATCTCTACCACTTTG | |
| RTSND1-A2IR-F | GATTTCTTCTATGTTCGGTTCTAGGC | |
| RTSND1-A2IR-R | CAAACCACCCACCCTTCTTCA | |
| RTVND6-C1IR-F | TTCACTGTGATGTTCTCTGGAAA | |
| RTVND6-C1IR-R | GCCCATACCAGAAAGAGAA | |
| RTLCMSND1-A2IR-F | TGTATTTTGACTTGAAGGAAACTACAGG | |
| RTLCMSND1-A2IR-R | GGCAAACCACCCACCCTT | |
| RTLCMVND6-C1IR-F | TGGTCCTCCACAGGCAAGC | |
| RTLCMVND6-C1IR-R | CAGCATACTAGGTCAATATGTAGAGCCT | |
| RT18S-F | CGAAGACGATCAGATACCGTCCTA | |
| RT18s-R | TTTCTCATAAGGTGCTGGCGGAGT | |
| Protein expression | pVND6-A1-F | ATCTAGACATGAATACTTTTACACATGTTCCTC |
| pVND6-A1NAC-R | CGTCGACCTCGAGGCAAACTGATTCATGCTCAC | |
| pVND6-A2-F | ATCTAGACATGAATTCTTTTACACACGTTCC | |
| pVND6-A2NAC-R | CGGATCCCTCGAGACAGACTGATTCATGCTCACTC | |
| pVND6-B1-F | ATCTAGACATGGTTGATATTGCTGCATTTCAGTT | |
| pVND6-B1NAC-R | CGTCGACCTCGAGCCAACATGGTGAGTCGTAGTCA | |
| pVND6-B2-F | ATCTAGACATGAATACCTTCTCGCATGTC | |
| pVND6-B2NAC-R | GCGTCGACCTCGAGCCAACATGGTGAGTCATAGTCA | |
| pVND6-C1-F | TCTAGACATGGAGTCAATGGAGTCG | |
| pVND6-C1NAC-R | CGTCGACCTCGAGTTCCTCATAGAAGTAGCTTGAG | |
| pVND6-C2-F | TCTAGACATGATGGAGTCAATGGAGTCT | |
| pVND6-C2NAC-R | CGTCGACCTCGAGGCTTGAGTCCCACCCTTCA | |
| pVND6-A1-R | ACTCGAGCTTCCATAGATCAATTTGACAACTG | |
| pVND6-A2-R | ACTCGAGTTTCCATAGATCAATTTGACAACT | |
| pVND6-B1-R | ACTCGAGCTTCCACAGATCAATTTGACAACT | |
| pVND6-B2-R | ACTCGAGCTTCCATAGATCAATTTGGCAACT | |
| pVND6-C1-R | ACTCGAGTTTCTCAAATATGCATATTCCGATA | |
| pVND6-C2-R | ACTCGAGTTTTTCAAATATGCATATTCCAATATC | |
| pVND6-C1IR-R | ACTCGAGGTATGTTGGAAGACAATGAAGA | |
| pVND6-A1IR-R | ACTCGAGTGCATAATCCAGTCCGATTTCTG | |
| pVND6-A2IR-R | ACTCGAGCACACGACCCAACCTTCTTCCTGA | |
| EMSA fragment amplification | 021EMSA-F | CACCAATTATGTGGTCCATTGA |
| 021EMSA-R | GGAGTTTGTTTCATAACTAAGCC | |
| Promoter cloning | VND6-A1prom-F | CTAGCTGCAGCTTGCTGATTTCTAGTTGGGGT |
| VND6-A1prom-R | CTAGTCTAGAAACAGCACACACTCTACAAG | |
| VND6-A2prom-F | CTCCTGCAGGCCTTGGGATCCATTCCGACA | |
| VND6-A2prom-R | CTAGTCTAGATCTGCTCAAAGACTCAACAT | |
| VND6-B1prom-F | CTAGCTGCAGAGCCATTATACGCCCCCTTT | |
| VND6-B1prom-R | CTAGGGATCCAATGACACAGCACGAGATTT | |
| VND6-B2prom-F | CTAGCTGCAGTTTTTCCCTTACATTGTGCATGA | |
| VND6-B2prom-R | CTAGGGATCCTATGTCTTGTTGACCAGAA | |
| VND6-C1prom-F | CTAGCTGCAGTAAGCTCCCGGTTCCGATTG | |
| VND6-C1prom-R | CTAGGGATCCTCAAGAAAGGGGTAAACGAC | |
| VND6-C2prom-F | CTAGCTGCAGTGGCAGGTGTGTCAGAAAGT | |
| VND6-C2prom-R | CTAGGGATCCTCGACAGAGAGGGAGAGATC | |
| Effector vector construction | VND6-A1E-F | CACCTGGATTGCAGGACTCAAGGC |
| VND6-A1E-R | GCCATGAATCATAGATTTTGGCTGG | |
| VND6-A2E-F | CACCGGAGTATGCTGAATGGATTTCAGG | |
| VND6-A2E-R | AACTGGATATCATCATTTCCATAGATCA | |
| VND6-B1E-F | CACCGCTGCATTTCAGTTCTTGGAGGAG | |
| VND6-B1E-R | TAAGCCTTCACTTCCACAGATCA | |
| VND6-B2E-F | CACCAATCTTCAGTTCTTGGAGGAGATG | |
| VND6-B2E-R | GCGTTCACTTCCATAGATCAATTTGG | |
| VND6-C1E-F | CACCTATGCAGCATCATCAACGGTTGT | |
| VND6-C1E-R | TCCCCAGATTCATTTCTCAAATATGC | |
| VND6-C2E-F | CACCGGTGGTGGTGAATTTTATGCAGC | |
| VND6-C2E-R | CCTCTTCAAACCCCTCTCTTCAT | |
| VND6-C1IRE-R | CTAGGTCAATATGTAGAGCCTGTTC | |
| Subcellular location | subVND6-A1-F | AGGATCCATGAATTCTTTTACACACGTTCCTC |
| subVND6-A1-R | ACTCGAGCTTCCATAGATCAATTTGACAACTG | |
| subVND6-A2-F | AGGATCCATGAATACTTTTACACATGTTCCTC | |
| subVND6-A2-R | ACTCGAGTTTCCATAGATCAATTTGACAACT | |
| subVND6-B1-F | AGGATCCATGGTTGATATTGCTGCATTTCAGTT | |
| subVND6-B1-R | ACTCGAGCTTCCACAGATCAATTTGACAACT | |
| subVND6-B2-F | AGGATCCATGAATACCTTCTCGCATGTC | |
| subVND6-B2-R | ACTCGAGCTTCCATAGATCAATTTGGCAACT | |
| subVND6-C1-F | AGGATCCATGATGGAGTCAATGGAGTCGTGTGT | |
| subVND6-C1-R | ACTCGAGTTTCTCAAATATGCATATTCCGATA | |
| subVND6-C2-R | ATCTAGAATGGAGTCAATGGAGTCTTGTG | |
| subVND6-C2-R | ACTCGAGTTTTTCAAATATGCATATTCCAATATC | |
| subVND6-C1IR-R | ACTCGAGGTATGTTGGAAGACAATGAAGA |
SI Materials and Methods
Plant Materials.
P. trichocarpa plants (genotype Nisqually-1) were grown and maintained in a greenhouse as described (4, 6). Stems of 5–9-mo-old plants were harvested for DNA, RNA, protein, and protoplast isolation.
RNA-Seq and qRT-PCR Analysis of Different Tissues and Cell Types.
Full transcriptome RNA-sEq. (3 replicate libraries each) was carried out for tissues SDX (X), leaf (L), phloem (P), and young shoot (S) and different cell types including fiber cells, vessel cells, and three cell types (fiber, vessel, and ray cells) collected by LCM (41). qRT-PCR was performed to quantify the transcript abundance of PtrSND1-A2IR and PtrVND6-C1IR using the primers listed in Table S1. The RNA-seq data were processed using our analysis pipeline (4, 41). We mapped the reads using TopHat, v2.0.9 (42) to the P. trichocarpa genome sequence (v3) and then extracted the reads mapped to whole sequences of the 41,336 annotated genes. The read counts mapped to these gene sequences were normalized using the Trimmed Mean of M values (43), Differentially expressed genes (DEGs) between tissue pairs were identified using EdgeR (4) with an FDR < 0.05. From a total of 2,455 annotated TFs based on PlantTFDB (44), we extracted the “SDX-specific” DEGs in all annotated TFs with transcript ratios of X/L, X/P, and X/S transcripts over 1.5 times. These SDX-specific TFs included 209 TFs from 41 diverse TF families.
Total RNA Extraction, RT-PCR, and Quantitative Real-Time PCR.
Total RNAs were isolated from SDX, phloem, young shoots, leaves, roots, or SDX protoplasts as described (6). RNA samples were treated with DNaseI and then reverse-transcribed using Omniscript RT kit (Qiagen) (4, 6) to generate the cDNAs. The cDNAs were used for qRT-PCR using the primers listed in Table S1. qRT-PCR was performed to detect the transcript abundances of PtrVND6s, PtrSND1s, and PtrMYB021 as described in ref. 6. The 18S ribosomal RNA was used as the internal control.
PCR Clone.
Full-length cDNAs of PtrVND6-A1, PtrVND6-A2, PtrVND6-B1, PtrVND6-B2, PtrVND6-C1, PtrVND6-C2, and PtrVND6-C1 IR were amplified using primer sets VND6-A1F/R, VND6-A2F/R, VND6-B1F/R, VND6-B2F/R, VND6-C1F/R, VND6-C2F/R, and VND6-C1F/C1IRR (Table S1) from the cDNA of P. trichocarpa SDX and then cloned in pENTR/d-TOPO vectors for sequencing. These primers were designed based on the genome sequence of P. trichocarpa version JGI 2.2.
RNA-Seq Analysis of Intron/Exon Sequence Depth of the Transcripts of PtrVND6-A1, PtrVND6-A2, and PtrVND6-C1 Genes.
RNA-seq was performed for 18 individual libraries, with each library generated from the SDX of three wild-type trees. Total RNAs of SDX were isolated using the RNeasy Plant RNA Isolation kit (Qiagen). RNA-seq libraries were prepared following the TruSeq RNA Sample Preparation Guide (45) with modifications. At least 10 μg of total RNAs were used for mRNA purification using Sera-Mag Magnetic Oligo (dT) beads. The mRNAs were fragmented and reverse-transcribed into double-strand cDNAs, followed by end repair and 3′-end adenylation. The generated cDNAs were ligated to multiplex adapters from the multiplex oligo only kit and collected. The RNA-seq libraries were generated from the modified cDNA amplification and validated using Agilent DNA 1000 kit. These RNA-seq libraries were sequenced using the Illumina GAIIx platform at the Genomic Science Laboratory, North Carolina State University. The resulting FASTAQ files from Illumina GAIIx were mapped to the genome of P. trichocarpa version 2.2 using Bowtie and Tophat. Multiple BAM (Binary sequence Alignment Map) files were merged using SAM tools, and the intron and exon junction of PtrVND6-A1, PtrVND6-A2, and PtrVND6-C1 was visualized in Fig. S3 using IGV (Integrative Genomics Viewer) (46–48). The intron and exon junction results that showed alternative splicing were averaged with the data of 18 libraries collected from 54 trees.
Antibody Production and Western Blotting.
Recombinant full-size protein production of PtrVND6 members in E. coli.
The coding sequences of PtrVND6-A1, PtrVND6-A2, PtrVND6-B1, PtrVND6-B2, PtrVND6-C1, and PtrVND6-C2 were amplified with primer sets pPtrVND6-A1-F/R, pPtrVND6-A2-F/R, pPtrVND6-B1-F/R, pPtrVND6-B2-F/R, pPtrVND6-C1-F/R, pPtrVND6-C2-F/R, pPtrVND6-A1IR-F/R, pPtrVND6-A2IR-F/R, and pPtrVND6-C1IR-F/R (Table S1). The cDNAs of PtrVND6-A1, PtrVND6-A2, PtrVND6-B1, PtrVND6-B2, PtrVND6-C1, PtrVND6-C2, PtrVND6-A1IR, PtrVND6-A2IR, and PtrVND6-C1IR were each digested and cloned into pGEXKG1 (GE Life Science) at the XbaI/XhoI sites. These constructs were transferred into E. coli BL21 (DE3) (Invitrogen). The transformed E. coli BL21s were cultured in 500 mL LB until the OD600 of the bacterial concentration reached 0.4–0.6 and then were incubated with the IPTG (final concentration of 4 mM in liquid medium) at 16 °C for 16 h to induce protein expression.
Purification of full-size proteins of PtrVND6 members for Western blotting.
The incubated E. coli were collected and suspended in 20 mL of PBST buffer (1× PBS, pH 7.4, 5 mM EDTA, 1% Triton X-100, 1 mM PMSF, 0.05% β-mercaptoethanol). Cells were disrupted by a Branson Digital Sonifier for 15 min (each pulse for 30 s:10 s on and 20 s off; 21% amplification). The cell lysates were centrifuged at 12,000 × g for 30 min at 4 °C. The supernatant was mixed with 1 mL of glutathione-S-agarose beads (Sigma) and held at 4 °C for 45 min with gentle shaking. The beads were washed eight times with PBST buffer without PMSF and then held at room temperature for 30 min in 3 mL of thrombin cleavage buffer (50 mM Tris, pH 8.0, 150 mM NaCl, 2.5 mM CaCl2, 0.05% β-mercaptoethanol) with 20 units of thrombin to remove GST. The proteins with no GST tag was concentrated and desalted with an Amicon Ultra centrifugal filter (Ultra-15, MWCO 10 kDa used for full-sizePtrVND6-A1IR, A2IR, C1IR proteins; Ultra-4, MWCO 30 kDa used for full-size PtrVND6-A1, -A2, -B1, -B2, -C1, and -C2 proteins) in 300 µL.
Antibody production and specificity test.
We synthesized the several peptide sequences from PtrVND6 members, including -WKATGRADKAIYSKHD from the PtrVND6-A1 N-terminal NAC domain sequence, -FWKATGRDKAIYSKQ from the PtrVND6-A2 N-terminal NAC domain sequence, -GFWKATGRDKSVYDKT from the PtrVND6-C1 N-terminal NAC domain sequence, and -TLPPSPPQLICHTIKVKA from the PtrVND6-C1IR intron-translated sequence. These peptide sequences were conjugated with keyhole limpet hemocyanin and used to immunize rabbits for polyclonal antibody production (Antagene). The purified recombinant VND proteins (full-size proteins PtrVND6-A1/-A2/-B1/-B2/-C1/-C2/-A1IR/-A2IR/-C1IR) were used in Western blotting to test the specificity of these polyclonal antibodies in Fig. S5.
Preparation of SDX nuclear protein.
For nuclear protein extraction from P. trichocarpa SDX, the fresh SDX tissues were collected to isolate nuclei using a CelyticTM PN Isolation kit (Sigma). The tissues were disrupted in 10 mL of 1× Nuclear Isolation Buffer (NIB; Sigma) containing 1 mM DTT, 10% (wt/wt) polyvinylpolypyrrolidone, 1 mM PMSF, 1 mg/mL pepstatin A, and 1 mg/mL leupeptin. The disrupted tissues were then homogenized with 0.5% Triton X-100 on ice for 10 min. The homogenized lysates (top layer) were loaded on freshly prepared density gradient [3 mL of 1× NIB buffer with 60% (vol/vol) percoll as the second layer and 3 mL of 2.3 M sucrose as the bottom layer] for centrifugation. After centrifugation at 3,200 × g for 30 min, most of the nuclei were banded between the percoll layer and the sucrose layer. The nuclei samples were collected and then washed with 8 mL of 1× NIB buffer. The washed nuclei were resuspended in 100 μL of working extraction buffer containing 5 mM DTT and then vortexed for 30 min at 4 °C. The mixtures were centrifuged at 12,000 × g for 10 min. The supernatants containing the soluble proteins were collected and stored for Western blotting. All reagents were prepared as described (49).
Western blotting assays for detecting PtrVND6-A1IR and PtrVND6-C1IR proteins in SDX nuclear proteins.
Each antibody for PtrVND6-A1 NAC domain, PtrVND6-C1 NAC domain, and PtrVND6-C1IR intron-translated sequences was used to detect PtrVND6-A1, PtrVND6-C1, and PtrVND6-C1IR in SDX nuclear proteins, respectively. Western blot analyses were carried out as described (4).
EMSA.
Recombinant NAC domain protein production of PtrVND6 members in E. coli.
The NAC domain coding regions of PtrVND6-A1, PtrVND6-A2, PtrVND6-B1, PtrVND6-B2, PtrVND6-C1, and PtrVND6-C2 were amplified with primer sets pPtrVND6-A1-F/NAC-R, pPtrVND6-A2-F/NAC-R, pPtrVND6-B1-F/NAC-R, pPtrVND6-B2-F/NAC-R, pPtrVND6-C1-F/NAC-R, and pPtrVND6-C2-F/NAC-R (Table S1), respectively. The NAC domain coding sequence of each PtrVND6-A1, PtrVND6-A2, PtrVND6-B1, PtrVND6-B2, PtrVND6-C1, and PtrVND6-C2 was digested and cloned into pGEXKG1 (GE Life Science) at the XbaI/XhoI sites. Each construct was transferred into E. coli BL21 (DE3) (Invitrogen). The transformed E. coli was cultured in 250 mL LB until the OD600 value of the bacterial concentration reached 0.5–0.8, and then protein expression (as GST fusion) was induced with 0.5 M IPTG at 28 °C for 8 h.
Purification of NAC domain proteins of PtrVND6 members for EMSA.
The incubated E. coli-expressing NAC domain proteins were collected and treated as described for the full-size PtrVND6 proteins. The GST-free protein was concentrated and desalted using an Amicon Ultra Centrifugal filter (Ultra-15, MWCO 10 kDa) in 300 µL. These proteins were quantified by Bradford assays and visualized by Commassie Brilliant Blue assays as described (6).
In vitro DNA binding assays.
The motif prediction tool Multiple EM for Motif Elicitation (MEME; meme-suite.org/) was used to analyze the SNBE (Secondary Wall NAC Binding Element) motifs in the ∼2-kb promoter sequence of PtrMYB021 (50). Promoter fragments, harboring the putative SNBE motifs, were amplified from PtrMYB021 promoters using primers 021EMSA-F/-R (Table S1). The PCR products were gel-extracted using Qiagen gel extraction kit. These fragments were biotin-labeled at the 3′ end (Biotin 3′ End DNA labeling kit; Thermo Scientific). The Lightshift Chemiluminescent EMSA kit was used to perform in vitro binding analyses (Thermo Scientific). The biotin-labeled PtrMYB021 DNA fragments were mixed with 100 ng of each purified NAC domain protein of PtrVND6-A1/-A2/-B1/-B2/-C1/-C2 and full-size PtrVND6-C1IR protein for 20 min in the binding buffer [10 mM Tris, pH 7.5, 50 mM KCl, 1 mM DTT, 2.5% (vol/vol) glycerol, 5 mM MgCl2, 0.05% Nonidet P-40, and 100 ng/μL poly (dI-dC)] at room temperature. Unlabeled promoter fragments in 20–100-fold molar excess relative to the labeled probes were used for performing the competition assays. The protein-bound biotinylated DNA fragments were separated by 6% native PAGE at 100 V, 4 °C, for 2–3 h. The DNA was electroblotted onto a positively charged nylon membrane (Hybond-N+; Amersham) and detected using the Lightshift Chemiluminescent EMSA Kit (Thermo Scientific).
Effector–Reporter-Based Gene Transactivation Assays.
The ∼2-kb promoters of PtrVND6-A1, PtrVND6-A2, PtrVND6-B1, PtrVND6-B2, PtrVND6-C1, and PtrVND6-C2 were identified based on Phytozome v9.1. These promoters were amplified using primer sets VND6-A1prom-F/-R, VND6-A2prom-F/-R, VND6-B1prom-F/-R, VND6-B2prom-F/-R, VND6-C1prom-F/-R, and VND6-C2prom-F/-R (Table S1). Each of the PCR products of PtrVND6-A1 and PtrVND6-A2 promoters were digested with PstI and XbaI and with SbfI and XbaI. PtrVND6-B1, PtrVND6-B2, PtrVND6-C1, and PtrVND6-C2 promoters were digested with PstI and BamHI. These digested fragments were then inserted into pUC19-35S-GUS (6), generating reporter constructs pUC19-35S-PtrVND6-A1P-GUS, pUC19-35S-PtrVND6-A2P-GUS, pUC19-35S-PtrVND6-B1P-GUS, pUC19-35S-PtrVND6-B2P-GUS, pUC19-35S-PtrVND6-C1P-GUS, and pUC19-35S-PtrVND6-C2P-GUS. The pENTR/D-TOPO vector of each PtrVND6 was used for LR recombination to replace the RfA in pUC19-35S-RfA-35S-sGFP (6) to generate the effector constructs pUC19-35S-PtrVND6-A1-35S-sGFP, pUC19-35S-PtrVND6-A2-35S-sGFP, pUC19-35S-PtrVND6-B1-35S-sGFP, pUC19-35S-PtrVND6-B2-35S-sGFP, pUC19-35S-PtrVND6-C1-35S-sGFP, pUC19-35S-PtrVND6-C2-35S-sGFP, and pUC19-35S-PtrVND6-C1 IR–35S-sGFP. Then, the plasmid DNAs for the reporter and effector constructs were prepared using CsCl density-gradient ultracentrifugation (4). Each combination of effector and reporter constructs was coexpressed (Fig. S7) into the mesophyll protoplasts isolated from Arabidopsis leaves as described (6, 51). After 12 h of incubation, the transfected protoplasts were collected, frozen in liquid nitrogen, and then lysed for GUS assays.
Overexpression of PtrVND6-A1, PtrVND6-A2, PtrVND6-B1, PtrVND6-B2, PtrVND6-C1, PtrVND6-C2, and PtrVND6-C1IR in SDX Protoplasts.
Each full-length PtrVND6 cDNA in pENTR/D-TOPO vectors was transferred to replace the RfA sequence in pUC19-35S-RfA-35S-sGFP described in ref. 6, generating pUC19-35S-PtrVND6-A1-35S-sGFP, pUC19-35S-PtrVND6-A2-35S-sGFP, pUC19-35S-PtrVND6-B1-35S-sGFP, pUC19-35S-PtrVND6-B2-35S-sGFP, pUC19-35S-PtrVND6-C1-35S-sGFP, pUC19-35S-PtrVND6-C2-35S-sGFP, and pUC19-35S-PtrVND6-C1IR-35S-sGFP for transient expression. Then, plasmid DNAs of the transient expression constructs were prepared using CsCl density-gradient ultracentrifugation (6, 25, 51) and then used to transform SDX protoplasts as described (25) and were incubated in a 100 × 15-mm2 Petri dish for 12–16 h. The protoplasts were collected by centrifugation at 300 × g for 3 min and frozen in liquid nitrogen. Total RNA of the protoplasts was isolated using an RNeasy Plant RNA Isolation kit (Qiagen) for qRT-PCR analyses.
Protein Subcellular Localization.
Constructs for full-size PtrVND6s and GFP fusion protein were prepared to examine subcellular localization of each of these proteins. The coding regions of these full-length PtrVND6s were amplified using primer pair sets subVND6-A1-F/R, subVND6-A2-F/R, subVND6-B1-F/R, subVND6-B2-F/R, subVND6-C1F/R, subVND6-C2-F/R, and subVND6-C1-F/C1IR-R with BamHI/XhoI restriction sites (Table S1). After being cloned into pGEM-T Easy vectors and sequenced, the coding regions of PtrVND6s were digested to generate the fragments. These fragments then were ligated into pUC19-35S-sGFP (4, 6), giving pUC19-35S-PtrVND6-A1:sGFP, pUC19-35S-PtrVND6-A2:sGFP, pUC19-35S-PtrVND6-B1:sGFP, pUC19-35S-PtrVND6-B2:sGFP, pUC19-35S-PtrVND6-C1:sGFP, pUC19-35S-PtrVND6-C2:sGFP, and pUC19-35S-PtrVND6-C1 IR:sGFP. The construct pUC19-35S-PtrH2A-1:mcherry was used to mark the nucleus. pUC19-35S-PtrSND1-A2IR: mCherry, pUC19-35S-PtrSND1-A1:sGFP, pUC19-35S-PtrSND1-A2:sGFP, pUC19-35S-PtrSND1-B1:sGFP, pUC19-35S-PtrSND1-B2:sGFP, and pUC19-35SPtrSND1-A2IR:sGFP were obtained from Li et al. (6), and pUC19-35S-PtrVND6-C1IR:mCherry was generated by replacing the H2A-1 sequence with PtrVND6-C1IR coding sequence in pUC19-35S-H2A:mCherry using BamHI/XhoI sites. Each of the GFP-fused PtrVND6s and PtrSND1s was coexpressed with H2A-1:mCherry, PtrVND6-C1IR:mCherry, or PtrSND1-A2IR:mCherry, as shown in Figs. 2 and 5, into SDX protoplasts. Fluorescence was observed under a Zeiss LSM 710 laser-scanning microscope. The excitation wavelength and the emission wavelength were 488 nm and 492–543 nm, respectively, for GFP and 561 nm and 582–662 nm, respectively, for mCherry.
BiFC.
The full-length PtrVND6 coding sequences with BamHI and XhoI sites were excised from the pGEM-T Easy constructs. Each of the coding sequences was cloned into pSCYNE(R) vectors (52), resulting in 35S-CFPN:VND6-A1, 35S-CFPN:VND6-A2, 35S-CFPN:VND6-B1, 35S-CFPN:VND6-B2, 35S-CFPN:VND6-C1, 35S-CFPN:VND6-C2, and 35S-CFPN:VND6-C1IR. Similarly, the VND6C1IR coding region was cloned into pSCYCE(R) (52), resulting in 35S-CFPC:VND6C1IR. BiFC constructs for PtrSND1s (35S- CFPN:PtrSND1-A1, 35S-CFPN:PtrSND1-A2, 35S-CFPN:PtrSND1-B1, 35S-CFPN:PtrSND1-B2, 35S-CFPN:PtrSND1-A2IR, and 35S-CFPC:PtrSND1-A2IR) were obtained from Li et al. (6).
Each of the CFPN:PtrSND1s/VND6s and CFPC:PtrSND1s-A2IR or VND6s-C1IR together with H2A-1:mCherry was cotransformed into SDX protoplasts (Figs. 3 and 6). As controls, each of the CFPN:PtrVND6s or CFPc:PtrVND6s-C1IR was cotransformed into SDX protoplasts (Figs. 3 and 6). After incubation for 12 h, SDX protoplasts were collected and examined under a Zeiss LSM 710 laser-scanning microscope. For fluorescence detection, the excitation wavelength and the emission wavelength were 458 nm and 462–531 nm, respectively, for CFP and 561 nm and 598–648 nm, respectively, for mCherry.
Supplementary Material
Acknowledgments
We thank the National Natural Science Foundation of China (NSFC) for financial support from Grants 31430093, 31522014, 31370593, and 31670674. This work was also supported by US Office of Science (Biological and Environmental Research), Department of Energy Grant DE-SC000691 and the North Carolina State University Jordan Family Distinguished Professor Endowment.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1714422114/-/DCSupplemental.
References
- 1.Sarkanen KV. Renewable resources for the production of fuels and chemicals. Science. 1976;191:773–776. doi: 10.1126/science.191.4228.773. [DOI] [PubMed] [Google Scholar]
- 2.Chiang VL. From rags to riches. Nat Biotechnol. 2002;20:557–558. doi: 10.1038/nbt0602-557. [DOI] [PubMed] [Google Scholar]
- 3.Ohtani M, et al. A NAC domain protein family contributing to the regulation of wood formation in poplar. Plant J. 2011;67:499–512. doi: 10.1111/j.1365-313X.2011.04614.x. [DOI] [PubMed] [Google Scholar]
- 4.Lin YC, et al. SND1 transcription factor-directed quantitative functional hierarchical genetic regulatory network in wood formation in Populus trichocarpa. Plant Cell. 2013;25:4324–4341. doi: 10.1105/tpc.113.117697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakano Y, Yamaguchi M, Endo H, Rejab NA, Ohtani M. NAC-MYB-based transcriptional regulation of secondary cell wall biosynthesis in land plants. Front Plant Sci. 2015;6:288. doi: 10.3389/fpls.2015.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Q, et al. Splice variant of the SND1 transcription factor is a dominant negative of SND1 members and their regulation in Populus trichocarpa. Proc Natl Acad Sci USA. 2012;109:14699–14704. doi: 10.1073/pnas.1212977109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao Y, Sun J, Xu P, Zhang R, Li L. Intron-mediated alternative splicing of WOOD-ASSOCIATED NAC TRANSCRIPTION FACTOR1B regulates cell wall thickening during fiber development in Populus species. Plant Physiol. 2014;164:765–776. doi: 10.1104/pp.113.231134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang JP, et al. Phosphorylation is an on/off switch for 5-hydroxyconiferaldehyde O-methyltransferase activity in poplar monolignol biosynthesis. Proc Natl Acad Sci USA. 2015;112:8481–8486. doi: 10.1073/pnas.1510473112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen HC, et al. Systems biology of lignin biosynthesis in Populus trichocarpa: Heteromeric 4-coumaric acid:coenzyme A ligase protein complex formation, regulation, and numerical modeling. Plant Cell. 2014;26:876–893. doi: 10.1105/tpc.113.119685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kubo M, et al. Transcription switches for protoxylem and metaxylem vessel formation. Genes Dev. 2005;19:1855–1860. doi: 10.1101/gad.1331305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhong R, Demura T, Ye ZH. SND1, a NAC domain transcription factor, is a key regulator of secondary wall synthesis in fibers of Arabidopsis. Plant Cell. 2006;18:3158–3170. doi: 10.1105/tpc.106.047399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ko JH, Yang SH, Park AH, Lerouxel O, Han KH. ANAC012, a member of the plant-specific NAC transcription factor family, negatively regulates xylary fiber development in Arabidopsis thaliana. Plant J. 2007;50:1035–1048. doi: 10.1111/j.1365-313X.2007.03109.x. [DOI] [PubMed] [Google Scholar]
- 13.Yamaguchi M, et al. VASCULAR-RELATED NAC-DOMAIN6 and VASCULAR-RELATED NAC-DOMAIN7 effectively induce transdifferentiation into xylem vessel elements under control of an induction system. Plant Physiol. 2010;153:906–914. doi: 10.1104/pp.110.154013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamaguchi M, et al. VASCULAR-RELATED NAC-DOMAIN7 directly regulates the expression of a broad range of genes for xylem vessel formation. Plant J. 2011;66:579–590. doi: 10.1111/j.1365-313X.2011.04514.x. [DOI] [PubMed] [Google Scholar]
- 15.Kim WC, et al. MYB46 directly regulates the gene expression of secondary wall-associated cellulose synthases in Arabidopsis. Plant J. 2013;73:26–36. doi: 10.1111/j.1365-313x.2012.05124.x. [DOI] [PubMed] [Google Scholar]
- 16.Kim WC, Kim JY, Ko JH, Kang H, Han KH. Identification of direct targets of transcription factor MYB46 provides insights into the transcriptional regulation of secondary wall biosynthesis. Plant Mol Biol. 2014;85:589–599. doi: 10.1007/s11103-014-0205-x. [DOI] [PubMed] [Google Scholar]
- 17.Ohashi-Ito K, Oda Y, Fukuda H. Arabidopsis VASCULAR-RELATED NAC-DOMAIN6 directly regulates the genes that govern programmed cell death and secondary wall formation during xylem differentiation. Plant Cell. 2010;22:3461–3473. doi: 10.1105/tpc.110.075036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaston K, Jayaraman P-S. Transcriptional repression in eukaryotes: Repressors and repression mechanisms. Cell Mol Life Sci. 2003;60:721–741. doi: 10.1007/s00018-003-2260-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seo PJ, Hong SY, Kim SG, Park CM. Competitive inhibition of transcription factors by small interfering peptides. Trends Plant Sci. 2011b;16:541–549. doi: 10.1016/j.tplants.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 20.Amoutzias GD, Robertson DL, Van de Peer Y, Oliver SG. Choose your partners: Dimerization in eukaryotic transcription factors. Trends Biochem Sci. 2008;33:220–229. doi: 10.1016/j.tibs.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 21.Staudt AC, Wenkel S. Regulation of protein function by ‘microProteins’. EMBO Rep. 2011;12:35–42. doi: 10.1038/embor.2010.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eguen T, Straub D, Graeff M, Wenkel S. MicroProteins: Small size-big impact. Trends Plant Sci. 2015;20:477–482. doi: 10.1016/j.tplants.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 23.Seo PJ, Kim MJ, Ryu JY, Jeong EY, Park CM. Two splice variants of the IDD14 transcription factor competitively form nonfunctional heterodimers which may regulate starch metabolism. Nat Commun. 2011a;2:303. doi: 10.1038/ncomms1303. [DOI] [PubMed] [Google Scholar]
- 24.Seo PJ, et al. A self-regulatory circuit of CIRCADIAN CLOCK-ASSOCIATED1 underlies the circadian clock regulation of temperature responses in Arabidopsis. Plant Cell. 2012;24:2427–2442. doi: 10.1105/tpc.112.098723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin YC, et al. A simple improved-throughput xylem protoplast system for studying wood formation. Nat Protoc. 2014;9:2194–2205. doi: 10.1038/nprot.2014.147. [DOI] [PubMed] [Google Scholar]
- 26.Ernst HA, Olsen AN, Larsen S, Lo Leggio L. Structure of the conserved domain of ANAC, a member of the NAC family of transcription factors. EMBO Rep. 2004;5:297–303. doi: 10.1038/sj.embor.7400093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang H, Zhao Q, Chen F, Wang M, Dixon RA. NAC domain function and transcriptional control of a secondary cell wall master switch. Plant J. 2011;68:1104–1114. doi: 10.1111/j.1365-313X.2011.04764.x. [DOI] [PubMed] [Google Scholar]
- 28.Ambavaram MMR, Krishnan A, Trijatmiko KR, Pereira A. Coordinated activation of cellulose and repression of lignin biosynthesis pathways in rice. Plant Physiol. 2011;155:916–931. doi: 10.1104/pp.110.168641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peng X, et al. Genomewide identification, classification and analysis of NAC type gene family in maize. J Genet. 2015;94:377–390. doi: 10.1007/s12041-015-0526-9. [DOI] [PubMed] [Google Scholar]
- 30.Negi S, Tak H, Ganapathi TR. In vitro xylem vessel elements formation from banana embryogenic cells and expression analysis of vessel development-related genes. Plant Biotechnol Rep. 2015;9:47–54. [Google Scholar]
- 31.Xu Q, et al. A NAC transcription factor, EjNAC1, affects lignification of loquat fruit by regulating lignin. Postharvest Biol Technol. 2015;102:25–31. [Google Scholar]
- 32.Yabas M, Elliott H, Hoyne GF. The role of alternative splicing in the control of immune homeostasis and cellular differentiation. Int J Mol Sci. 2015;17:E3. doi: 10.3390/ijms17010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bazin J, Bailey-Serres J. Emerging roles of long non-coding RNA in root developmental plasticity and regulation of phosphate homeostasis. Front Plant Sci. 2015;6:400. doi: 10.3389/fpls.2015.00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Syed NH, Kalyna M, Marquez Y, Barta A, Brown JW. Alternative splicing in plants—Coming of age. Trends Plant Sci. 2012;17:616–623. doi: 10.1016/j.tplants.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Filichkin S, Priest HD, Megraw M, Mockler TC. Alternative splicing in plants: Directing traffic at the crossroads of adaptation and environmental stress. Curr Opin Plant Biol. 2015;24:125–135. doi: 10.1016/j.pbi.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 36.Ner-Gaon H, et al. Intron retention is a major phenomenon in alternative splicing in Arabidopsis. Plant J. 2004;39:877–885. doi: 10.1111/j.1365-313X.2004.02172.x. [DOI] [PubMed] [Google Scholar]
- 37.Seo PJ, Park MJ, Park CM. Alternative splicing of transcription factors in plant responses to low temperature stress: Mechanisms and functions. Planta. 2013;237:1415–1424. doi: 10.1007/s00425-013-1882-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kelemen O, et al. Function of alternative splicing. Gene. 2013;514:1–30. doi: 10.1016/j.gene.2012.07.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eulgem T, Rushton PJ, Robatzek S, Somssich IE. The WRKY superfamily of plant transcription factors. Trends Plant Sci. 2000;5:199–206. doi: 10.1016/s1360-1385(00)01600-9. [DOI] [PubMed] [Google Scholar]
- 40.Ledent V, Vervoort M. The basic helix-loop-helix protein family: Comparative genomics and phylogenetic analysis. Genome Res. 2001;11:754–770. doi: 10.1101/gr.177001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shi R, et al. Tissue and cell-type co-expression networks of transcription factors and wood component genes in Populus trichocarpa. Planta. 2017;245:927–938. doi: 10.1007/s00425-016-2640-1. [DOI] [PubMed] [Google Scholar]
- 42.Trapnell C, Pachter L, Salzberg SL. TopHat: Discovering splice junctions with RNA-seq. Bioinformatics. 2009;25:1105–1111. doi: 10.1093/bioinformatics/btp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Robinson MD, Oshlack A. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol. 2010;11:R25. doi: 10.1186/gb-2010-11-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jin J, Zhang H, Kong L, Gao G, Luo J. PlantTFDB 3.0: A portal for the functional and evolutionary study of plant transcription factors. Nucleic Acids Res. 2014;42:D1182–D1187. doi: 10.1093/nar/gkt1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Illumine (2010) TruSeq RNA Sample Preparation Guide. Available at https://support.illumina.com/downloads/truseq_rna_sample_preparation_guide_15008136.html. Accessed October 13, 2017.
- 46.Tuskan GA, et al. The genome of black cottonwood, Populus trichocarpa (Torr. & Gray) Science. 2006;313:1596–1604. doi: 10.1126/science.1128691. [DOI] [PubMed] [Google Scholar]
- 47.Li H, et al. 1000 Genome Project Data Processing Subgroup The sequence alignment/map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thorvaldsdóttir H, Robinson JT, Mesirov JP. Integrative genomics viewer (IGV): High-performance genomics data visualization and exploration. Brief Bioinform. 2013;14:178–192. doi: 10.1093/bib/bbs017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li W, et al. A robust chromatin immunoprecipitation protocol for studying transcription factor-DNA interactions and histone modifications in wood-forming tissue. Nat Protoc. 2014;9:2180–2193. doi: 10.1038/nprot.2014.146. [DOI] [PubMed] [Google Scholar]
- 50.Zhong R, Lee C, Ye ZH. Global analysis of direct targets of secondary wall NAC master switches in Arabidopsis. Mol Plant. 2010;3:1087–1103. doi: 10.1093/mp/ssq062. [DOI] [PubMed] [Google Scholar]
- 51.Yoo SD, Cho YH, Sheen J. Arabidopsis mesophyll protoplasts: A versatile cell system for transient gene expression analysis. Nat Protoc. 2007;2:1565–1572. doi: 10.1038/nprot.2007.199. [DOI] [PubMed] [Google Scholar]
- 52.Waadt R, et al. Multicolor bimolecular fluorescence complementation reveals simultaneous formation of alternative CBL/CIPK complexes in planta. Plant J. 2008;56:505–516. doi: 10.1111/j.1365-313X.2008.03612.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.