Among the multitude of biological machines that nature employs to keep the cell operational, molecular motor proteins are certainly among the most captivating. These proteins convert chemical energy into mechanical work and drive most forms of motion (1). Cytoplasmic motors, for example, are proteins that move along a track and can transport cargo or induce muscle contraction. Beside these types of linear motion, rotary motion is ubiquitous. It occurs, for example, in flagella, which propel bacteria, or in ATP synthase, the protein that creates ATP. Alternatively, polymerization motors, such as actin filaments or microtubules, generate force by their assembly or disassembly. To understand the dynamics of the living cell, as well as to create increasingly complex artificial systems, chemists strive to construct artificial molecular motors and machines. In PNAS, Fredy et al. (2) present an innovative design that combines molecular motion with supramolecular chemistry to build a light-powered self-assembled machine in which energy is accumulated and released. This induces a mechanical effect that mimics the pulling force of microtubule disassembly.
Synthetic molecular machines of increasing sophistication have been built and studied for several decades (3). Analogous to molecular motors found in nature, they are defined as molecules that can convert an energy input, typically in the form of chemical fuel or light, into translational or rotational motion. To harvest this output for mechanical work remains a fundamental challenge as molecular machines usually operate in solution. At the molecular scale, Brownian movement and viscous forces dominate, while the influence of gravity and inertia is negligible. Any force generated by a single molecular machine is negated by the devastating Brownian motion, while collective output is nearly impossible when all individual molecules are randomly oriented. To utilize molecular machines in soft nanotechnology, motion needs to be amplified across length scales. Considerable successes toward this goal have been achieved though covalent linking of motors or switches, for example, in polymers (4, 5) or through surface functionalization (6, 7).
A promising alternative is to look at “chemistry beyond the molecule,” into supramolecular functional systems consisting of noncovalently bonded molecular subunits (8, 9). For instance, over a decade ago, doping of a liquid crystalline film with a light-driven molecular motor was already used to induce directional rotation of a microscale glass rod (10). Self-assembled systems, in particular, provide an opportunity to organize matter on a larger length scale (9). In the process of self-assembly, individual building blocks organize themselves into larger architectures without human intervention. The prime example of a dynamic functional self-assembled system is the living cell, which consists of thousands of different molecular components. Self-assembly not only offers a novel route toward creating larger structures for nanotechnology but also allows them to work out of equilibrium, which is of fundamental importance in studies concerning the origin of life (11). However, our understanding of dynamic and out-of-equilibrium self-assembly is still scarce and artificial self-assembled supramolecular structures that act like machines largely remain a future vision. Nevertheless, promising steps in that direction have been taken through the development of functional supramolecular polymers, polymers in which the monomers are connected by noncovalent interactions, and responsive nanotubes (12, 13). For example, a self-assembled supramolecular polymer fiber was recently reported, which could contract and expand in a manner reminiscent of muscle fibers (14). Alternatively, these materials resemble the filaments of the cytoskeleton and, as such, may find application as polymerization motors.
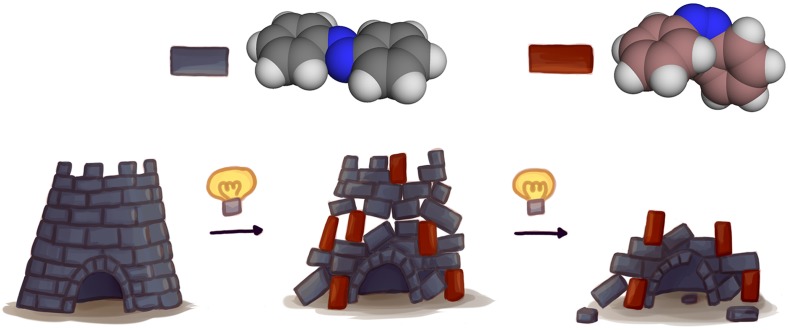
In PNAS, Fredy et al. (2) describe a novel self-assembled supramolecular system that has the potential to harvest work from molecular photoswitches (Fig. 1). Their system is modeled after microtubules, dynamic filaments that make up a large part of the cytoskeleton and can be considered supramolecular machines. Microtubules self-assemble through a ratcheting growth mechanism which generates pushing forces in the cell, while they disassemble in small abrupt bursts following a slow build-up of strain (15). It has been demonstrated previously that the force generated in these disassembly events may be harvested to generate work (16). Fredy et al. (2) designed a synthetic supramolecular tubule that incorporates photoswitchable building blocks. Their photoswitch of choice is azobenzene, a molecule that is planar in its thermally stable trans configuration. Interestingly, the trans-azobenzene can contribute to self-assembly through π-π stacking, a favorable electronic interaction that also contributes to the “spiral staircase”-like arrangement of nucleobases in the DNA double helix. Irradiation with UV light causes the molecule to switch to its cis form, a process that can be reversed either by irradiation with visible light or by prolonged heating. In contrast to the trans form, the cis form is not planar, causing a simultaneous loss of stacking interactions and a disturbance of the ordered structure. Therefore, upon irradiation, the microtubules are destabilized and ultimately break up into shorter tubes. Supramolecular polymers are often dynamic assemblies which continuously exchange building blocks with their environment. Storing energy in the form of strain build-up is difficult to achieve because in an equilibrium state, a locally high concentration of the disrupting cis form would be exchanged by trans building block from the solvent. By operating in water, a medium in which the cis building blocks are very poorly soluble, Fredy et al. (2) were able to prevent such exchange, allowing the system to operate out of equilibrium. Notably, this process reflects the natural disassembly of cytoskeletal microtubules because irradiation does not immediately affect microtubule length while strain is accumulating, as was supported by simulations. After a critical percentage of cis isomer is reached, however, the tubules suddenly start to break up. Atomistic modeling confirms that above 15% of photoconversion to the cis isomer, holes start to appear in the tubules. Depending on the concentration, prolonged irradiation can result in either in a higher concentration of smaller tubules or the complete disappearance of the self-assembled structures.
Fig. 1.
Light-induced strain-driven disassembly of supramolecular tubes. (Left) In the initial state, the photoswitchable azobenzene moieties integrated in the building blocks are in the trans configuration (gray), forming a stable, self-assembled tubular structure. (Middle) During irradiation, the azobenzene moieties switch to the nonplanar cis configuration (red). As a result, the tubules build up strain but remain structurally intact. (Right) Once a critical amount of azobenzene building blocks have switched, the tubes suddenly disintegrate.
With their self-assembled tubular system, Fredy et al. (2) present an intriguing new approach toward the construction of supramolecular machines. The conversion of light into tubular strain offers the opportunity to translate molecular motion into energy storage and macroscopic work. Although the produced
In PNAS, Fredy et al. present an innovative design that combines molecular motion with supramolecular chemistry to build a light-powered self-assembled machine in which energy is accumulated and released.
force is currently not converted into work, cytoskeletal microtubule disassembly has previously been employed to move cargo (16); a similar application for the system designed by Fredy et al. (2) can easily be envisioned. Furthermore, the out-of-equilibrium state of these assemblies might be utilized in the development of synthetic systems with life-like properties. It is inspiring to see that the simple action of azobenzene switching, a chemical transformation that has been known for 80 y (17), can be converted into controlled motion and potential (stored) energy in a giant conglomerate of molecules, in a compelling imitation of microtubule assembly.
Supplementary Material
Acknowledgments
We thank Kaja Sitkowska for Fig. 1. Our research is funded by NanoNed, The Netherlands Organization for Scientific Research (Top Grant to B.L.F., Veni Grant No. 722.014.006 to S.J.W.); the Royal Netherlands Academy of Arts and Sciences; the Ministry of Education, Culture and Science (Gravitation Program 024.001.035); and the European Research Council (Advanced Investigator Grant 694345 to B.L.F.).
Footnotes
The authors declare no conflict of interest.
See companion article on page 11850.
References
- 1.Schliwa M, Woehlke G. Molecular motors. Nature. 2003;422:759–765. doi: 10.1038/nature01601. [DOI] [PubMed] [Google Scholar]
- 2.Fredy JW, et al. Molecular photoswitches mediating the strain-driven disassembly of supramolecular tubules. Proc Natl Acad Sci USA. 2017;114:11850–11855. doi: 10.1073/pnas.1711184114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kassem S, et al. Artificial molecular motors. Chem Soc Rev. 2017;46:2592–2621. doi: 10.1039/c7cs00245a. [DOI] [PubMed] [Google Scholar]
- 4.Iamsaard S, et al. Conversion of light into macroscopic helical motion. Nat Chem. 2014;6:229–235. doi: 10.1038/nchem.1859. [DOI] [PubMed] [Google Scholar]
- 5.Li Q, et al. Macroscopic contraction of a gel induced by the integrated motion of light-driven molecular motors. Nat Nanotechnol. 2015;10:161–165. doi: 10.1038/nnano.2014.315. [DOI] [PubMed] [Google Scholar]
- 6.Berná J, et al. Macroscopic transport by synthetic molecular machines. Nat Mater. 2005;4:704–710. doi: 10.1038/nmat1455. [DOI] [PubMed] [Google Scholar]
- 7.Chen K-Y, et al. Control of surface wettability using tripodal light-activated molecular motors. J Am Chem Soc. 2014;136:3219–3224. doi: 10.1021/ja412110t. [DOI] [PubMed] [Google Scholar]
- 8.Lehn J-M. Supramolecular chemistry: Where from? Where to? Chem Soc Rev. 2017;46:2378–2379. doi: 10.1039/c7cs00115k. [DOI] [PubMed] [Google Scholar]
- 9.Whitesides GM, Grzybowski B. Self-assembly at all scales. Science. 2002;295:2418–2421. doi: 10.1126/science.1070821. [DOI] [PubMed] [Google Scholar]
- 10.Eelkema R, et al. Molecular machines: Nanomotor rotates microscale objects. Nature. 2006;440:163. doi: 10.1038/440163a. [DOI] [PubMed] [Google Scholar]
- 11.van Rossum SAP, Tena-Solsona M, van Esch JH, Eelkema R, Boekhoven J. Dissipative out-of-equilibrium assembly of man-made supramolecular materials. Chem Soc Rev. 2017;46:5519–5535. doi: 10.1039/c7cs00246g. [DOI] [PubMed] [Google Scholar]
- 12.Aida T, Meijer EW, Stupp SI. Functional supramolecular polymers. Science. 2012;335:813–817. doi: 10.1126/science.1205962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coleman AC, et al. Light-induced disassembly of self-assembled vesicle-capped nanotubes observed in real time. Nat Nanotechnol. 2011;6:547–552. doi: 10.1038/nnano.2011.120. [DOI] [PubMed] [Google Scholar]
- 14.Goujon A, et al. Hierarchical self-assembly of supramolecular muscle-like fibers. Angew Chem Int Ed Engl. 2016;55:703–707. doi: 10.1002/anie.201509813. [DOI] [PubMed] [Google Scholar]
- 15.Akhmanova A, Steinmetz MO. Control of microtubule organization and dynamics: Two ends in the limelight. Nat Rev Mol Cell Biol. 2015;16:711–726. doi: 10.1038/nrm4084. [DOI] [PubMed] [Google Scholar]
- 16.Grishchuk EL, Molodtsov MI, Ataullakhanov FI, McIntosh JR. Force production by disassembling microtubules. Nature. 2005;438:384–388. doi: 10.1038/nature04132. [DOI] [PubMed] [Google Scholar]
- 17.Hartley GS. The cis-form of azobenzene. Nature. 1937;140:281. [Google Scholar]