Abstract
Clinical trial eligibility criteria are necessary to define the patient population under study and improve trial safety. However, there are concerns that eligibility criteria for cancer clinical trials are too restrictive and limit patient enrollment in clinical trials. Recently, there have been initiatives to re-examine and modernize eligibility criteria for oncology clinical trials. To assess current eligibility requirements for cancer clinical trials, we have conducted a comprehensive review of eligibility criteria for commercial investigational new drug clinical trial applications submitted to the US Food and Drug Administration Office of Hematology and Oncology Products in 2015. Our findings suggest that eligibility criteria for current cancer clinical trials tend to narrowly define the study population and limit the study to lower-risk patients, which may not be reflective of the greater patient population outside of the study. We discuss potential areas for expanding eligibility criteria to include more patients in clinical trials and design options for clinical trials incorporating expanded eligibility criteria. The broadening of clinical trial eligibility criteria can be considered to better reflect the real-world patient population, improve clinical trial participation, and increase patient access to new investigational treatments.
INTRODUCTION
An important consideration in clinical trial design is the choice of eligibility criteria, protocol requirements for inclusion and exclusion from a study that must be met for a patient to be eligible to participate. Eligibility criteria define the patient population for study by characteristics such as age, disease stage, performance status (PS), organ function, prior and concomitant treatments, and comorbidities. The purpose of eligibility criteria is to ensure that the study population is similar in baseline factors that may affect the potential benefits and risks from the intervention being studied. In addition, eligibility criteria exclude patients who may be at greater risk of adverse events from the trial and those who are not expected to benefit, improving trial safety. These features allow researchers to better detect efficacy and have greater confidence that trial results are due to the study treatment. For these reasons, the consideration of eligibility criteria is necessary to minimize confounding factors and ensure safety, while also maximizing the sample size and patient access to the clinical trial.1,2
However, there are many concerns regarding eligibility criteria in modern clinical trials, especially in oncology. There is long-standing concern that there are too many eligibility criteria in cancer clinical trials, causing enrollment to be too restrictive. Previous studies have argued that overly strict eligibility criteria result in lower patient accrual; increased length, complexity, and cost of trials; and decreased eventual generalizability of trials to the greater patient population outside of the trial.2 In the United States, patient participation in cancer clinical trials has always been low, with only approximately 3% of adult patients with cancer enrolling in trials.3 Low accrual to clinical trials has resulted in almost 20% of publically funded studies being unable to recruit enough participants.4 Greater restrictions to clinical trial enrollment may cause patient accrual to be even lower, and stringent eligibility criteria are believed to have turned away many patients from cancer clinical trials who would otherwise have been eligible.5 In addition, restrictive eligibility criteria can ultimately cause trial results to be less generalizable. Excluding patients with various comorbid conditions and risk factors limits the study population to healthier and lower-risk patients. This may not be reflective of the larger patient population, where comorbidities occur. Therefore, results of the clinical trial may not provide information about the safety and efficacy of treating higher-risk patients.1,2
Because of these concerns, it has previously been argued that eligibility criteria for cancer clinical trials should be reduced. A 1997 National Cancer Institute report stated that there were “too many exclusion criteria in the current clinical trials system” and “entry criteria for all studies need to be simplified and broadened.”5(p14,18) George2 also argued that, for most phase III cancer clinical trials, eligibility criteria could be reduced, improving patient accrual and trial generalizability and reducing cost and complexity of the trial, without compromising patient safety or the scientific validity of the trial.
However, concerns over restrictive eligibility criteria continue in modern oncology trials. In 2010, the Institute of Medicine reported that eligibility criteria are a barrier to clinical trial enrollment and recommended broadening eligibility criteria for greater patient participation.6 Patient surveys have also indicated that eligibility criteria, including strict prior treatment and disease stage restrictions, have excluded them from trials.3 In addition, eligibility criteria for cancer clinical trials seem to have increased and become more restrictive over time, especially with the advent of new molecular therapies and targeted drugs.1
The standardization and modernization of eligibility for cancer clinical trials has been identified as a primary concern by ASCO.7 In May 2016, ASCO and Friends of Cancer Research launched a joint initiative to modernize eligibility criteria for cancer clinical trials to promote greater patient access.8 Currently, four working groups have been established to consider expansion of clinical trial eligibility criteria in four areas: minimum age requirement, brain metastases, HIV/AIDS, and organ dysfunction.9
Because of concerns over the limitations of restrictive eligibility criteria, there is great interest in re-evaluating and modernizing eligibility criteria for oncology clinical trials. In response to these concerns, we have conducted a comprehensive review of eligibility criteria for cancer clinical trials submitted as investigational new drug (IND) applications to the US Food and Drug Administration (FDA) Office of Hematology and Oncology Drug Products (OHOP) in 2015. We examined all commercial INDs over this 1-year period to gain a better understanding of current eligibility criteria in oncology clinical trials. It is our hope that this will provide information about the current state of eligibility for recent trials and encourage consideration about the expansion of eligibility criteria.
EVALUATING ELIGIBILITY CRITERIA
We examined IND submissions to the US FDA OHOP in 2015. In total, 1,031 IND applications were received over the 1-year period. Of these, 704 (68.3%) were research INDs and 327 (31.7%) were commercial INDs. We assessed the eligibility criteria for commercial INDs, because these were larger studies.
Study protocols were obtained from the FDA electronic submission system. Wherever available, the full inclusion and exclusion criteria were compiled from the electronic record of the original protocol. For INDs where the original protocol was unavailable, eligibility criteria were obtained from the electronic clinical review if available.
Among 327 commercial IND application numbers, there were five IND numbers with multiple protocols, three single-patient INDs, one protocol for continued patient access to study drug, and three IND applications that had no electronic protocol or clinical review. Accounting for these, 326 commercial IND protocols were identified for oncology and hematology products in 2015. Of these, 29 (8.9%) were for noncancerous hematologic disorders and 297 (91.1%) were for oncology, including solid tumors and hematologic malignancies.
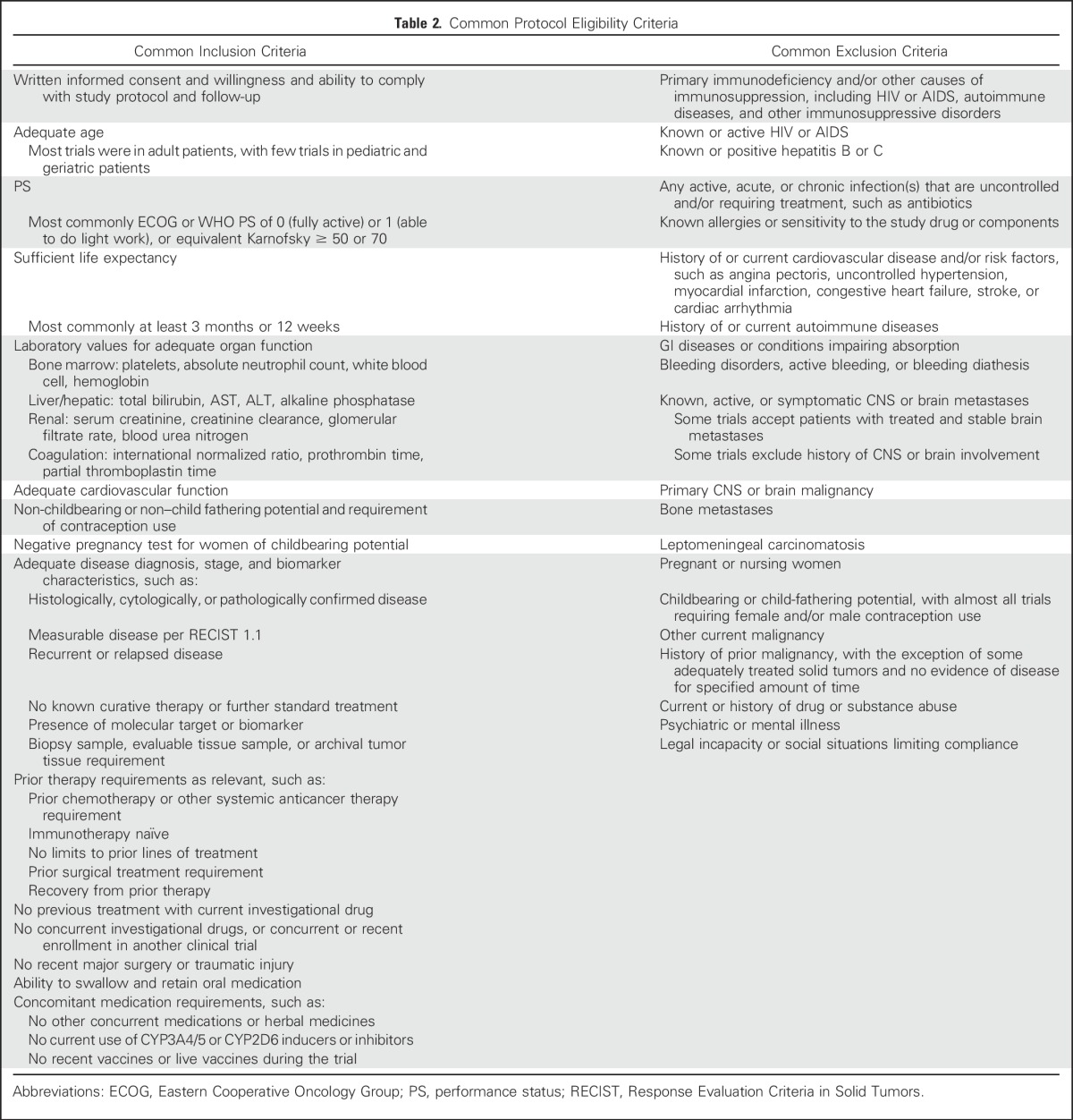
In total, 297 oncology protocols were evaluated for eligibility criteria. Of the 297 protocols, 293 were studies in patients with cancer and four were safety studies in healthy participants. By disease type, the largest number of protocols, 107 (36.0%), were for patients with various solid tumors or advanced malignancies. The distribution of protocols by disease type is displayed in Table 1. The common eligibility criteria observed are presented in Table 2.
Table 1.
Distribution of 2015 Commercial Oncology IND Protocols by Disease Type
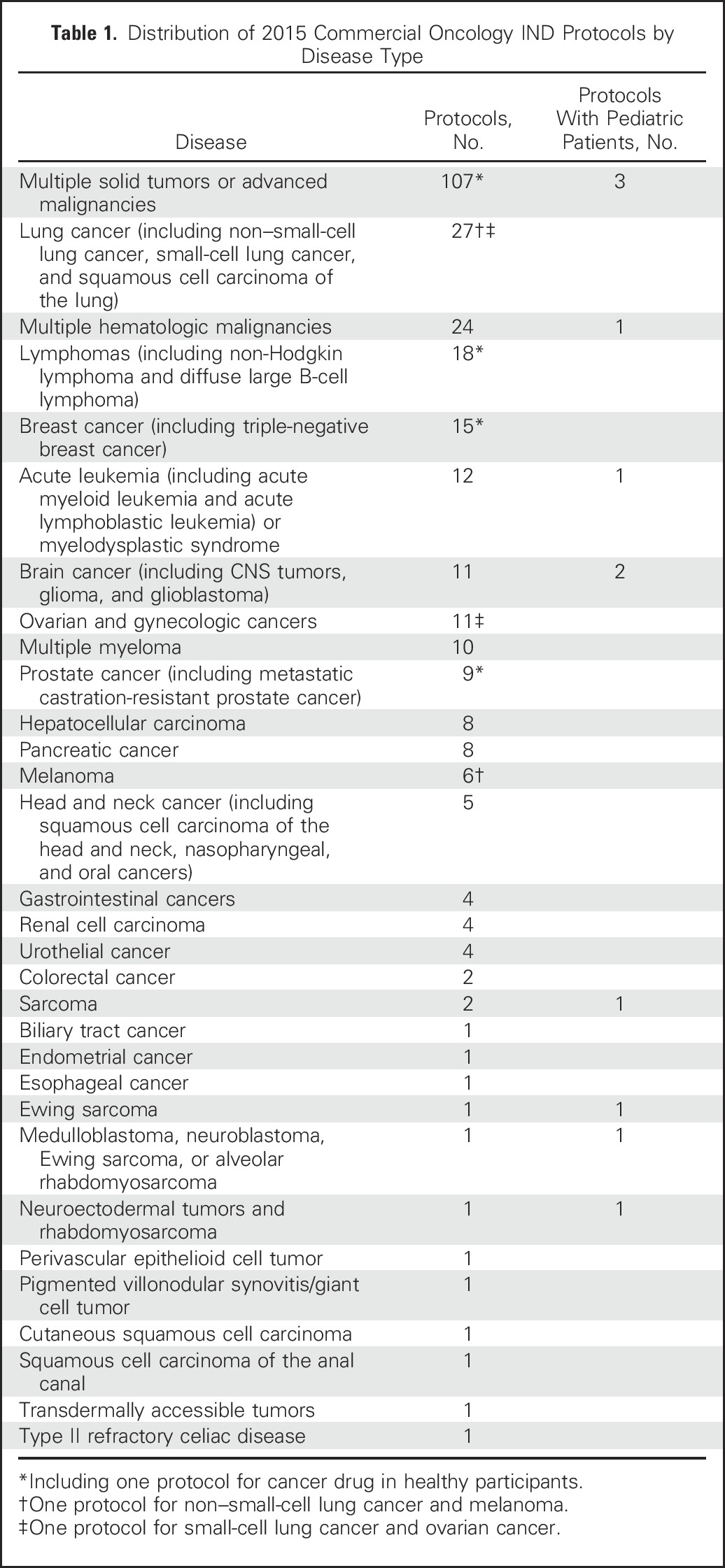
Table 2.
Common Protocol Eligibility Criteria
RESULTS AND OBSERVATIONS
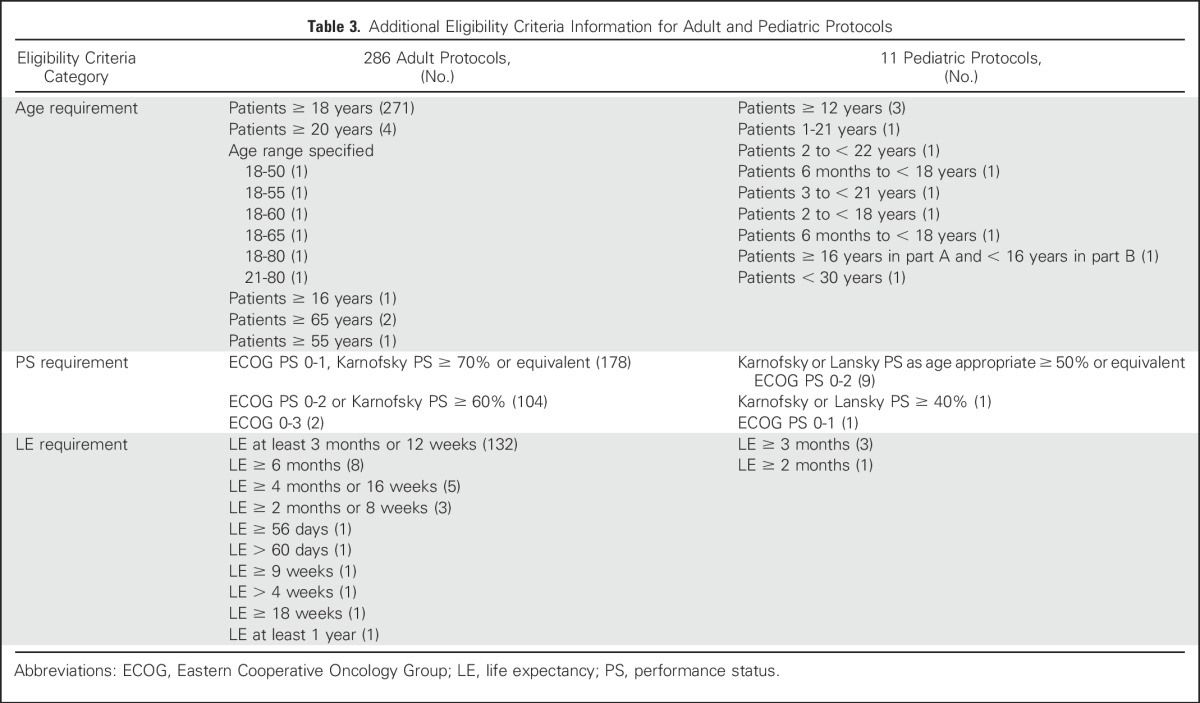
Of 297 oncology protocols, the majority were in adults, with 271 (91.2%) in patients ≥ 18 years of age. Some protocols had different minimum age requirements and age ranges (Table 3). There were three protocols in older patients only, two in patients ≥ 65 years and one in patients ≥ 55 years. Pediatric patients were included in or the focus of study for 11 protocols (3.7%). The majority of protocols, 266 (89.6%), enrolled both male and female participants. Twenty included females only (breast, ovarian, and other gynecologic cancers, and one study in healthy female patients) and 10 included males only (prostate cancer and two studies in healthy male patients).
Table 3.
Additional Eligibility Criteria Information for Adult and Pediatric Protocols
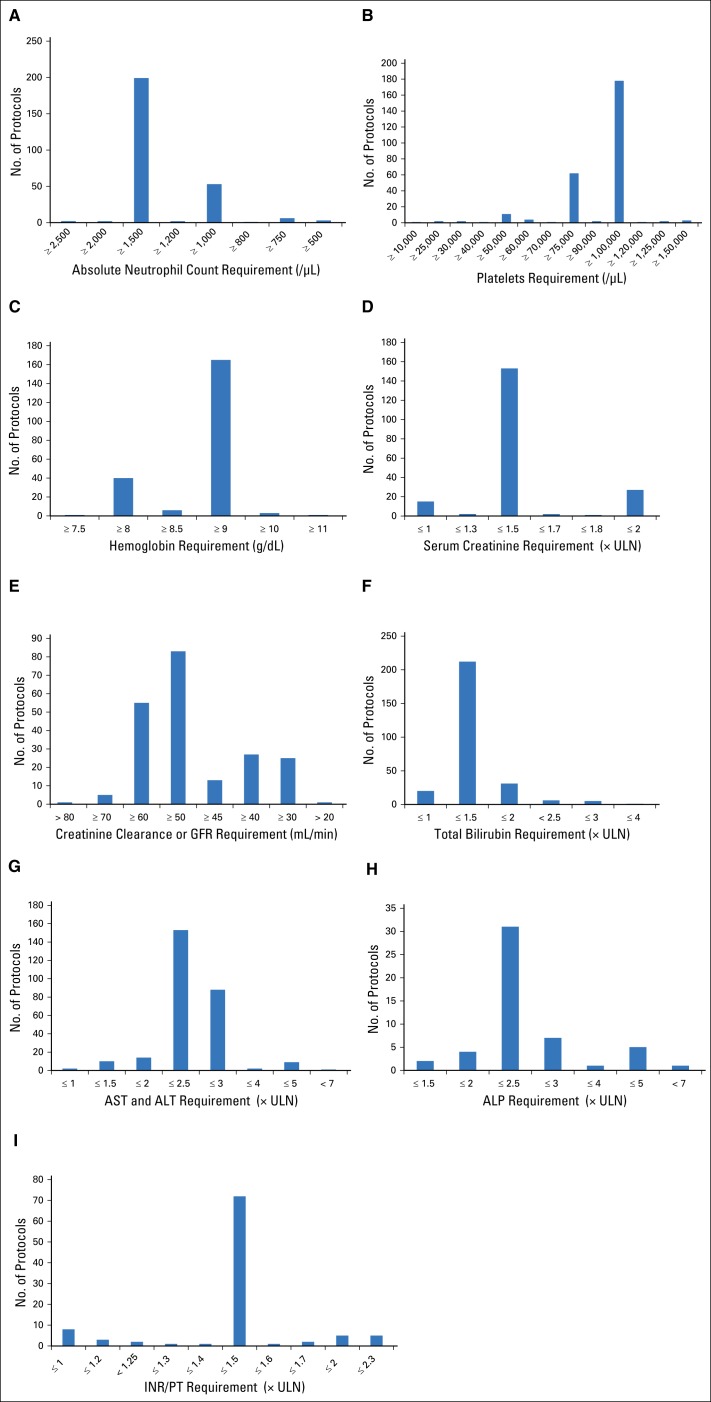
Almost all protocols required patients to have some laboratory tests of organ function as an eligibility requirement. Specific requirements for laboratory tests differed among protocols. The frequency of protocol-specified laboratory value requirements are displayed in Figures 1A-1I.
Fig 1.
Frequency of laboratory value requirements for 297 oncology investigational new drug protocols. Protocol-specified accepted laboratory test values and number of protocols with each requirement for (A) absolute neutrophil count (ANC), (B) platelet count, (C) hemoglobin, (D) serum creatinine, (E) creatinine clearance or glomerular filtration rate (GFR), (F) total bilirubin, (G) AST and ALT, (H) alkaline phosphatase (ALP), and (I) international normalized ratio (INR) or prothrombin time (PT). ULN, upper limit of normal.
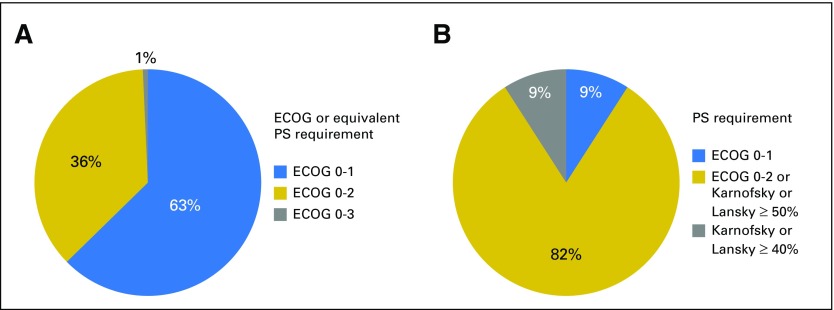
Of all adult oncology protocols assessed, 284 (95.6%) specified a patient PS requirement. One hundred seventy-eight protocols (60%) required an Eastern Cooperative Oncology Group (ECOG) PS of 0 or 1 or equivalent Karnofsky PS of ≥ 70% or other (Table 3). One hundred four protocols (35%) required an ECOG PS of 0 to 2 or equivalent Karnofsky PS of ≥ 60%. Two protocols included patients with an ECOG PS of 0 to 3 (Fig 2A). Of the 11 pediatric protocols, nine specified a Karnofsky or Lansky PS as age appropriate ≥ 50% or an equivalent ECOG PS of 0 to 2, one specified a Karnofsky or Lansky PS ≥ 40%, and one specified an ECOG PS of 0 or 1 (Fig 2B). Of all 297 oncology protocols, 158 (53.2%) specified sufficient life expectancy (LE) as an eligibility requirement, including 154 adult protocols and four pediatric protocols (Table 3).
Fig 2.
Performance status (PS) requirements for oncology investigational new drug (IND) protocols. (A) Proportion of PS requirements for 284 adult protocols with Eastern Cooperative Oncology Group (ECOG) or equivalent (Karnofsky or other) criteria. (B) Proportion of PS requirements for 11 pediatric protocols.
Two hundred thirty protocols (77.4%) excluded known, active, or symptomatic CNS or brain metastases. However, some of these trials allowed the enrollment of patients with previously treated CNS or brain metastases that were currently inactive, asymptomatic, or stable. In total, 140 (47.1%) protocols allowed treated or stable brain metastases. Eighteen protocols excluded any history of CNS or brain involvement, and 27 protocols excluded primary CNS or brain malignancies or tumors.
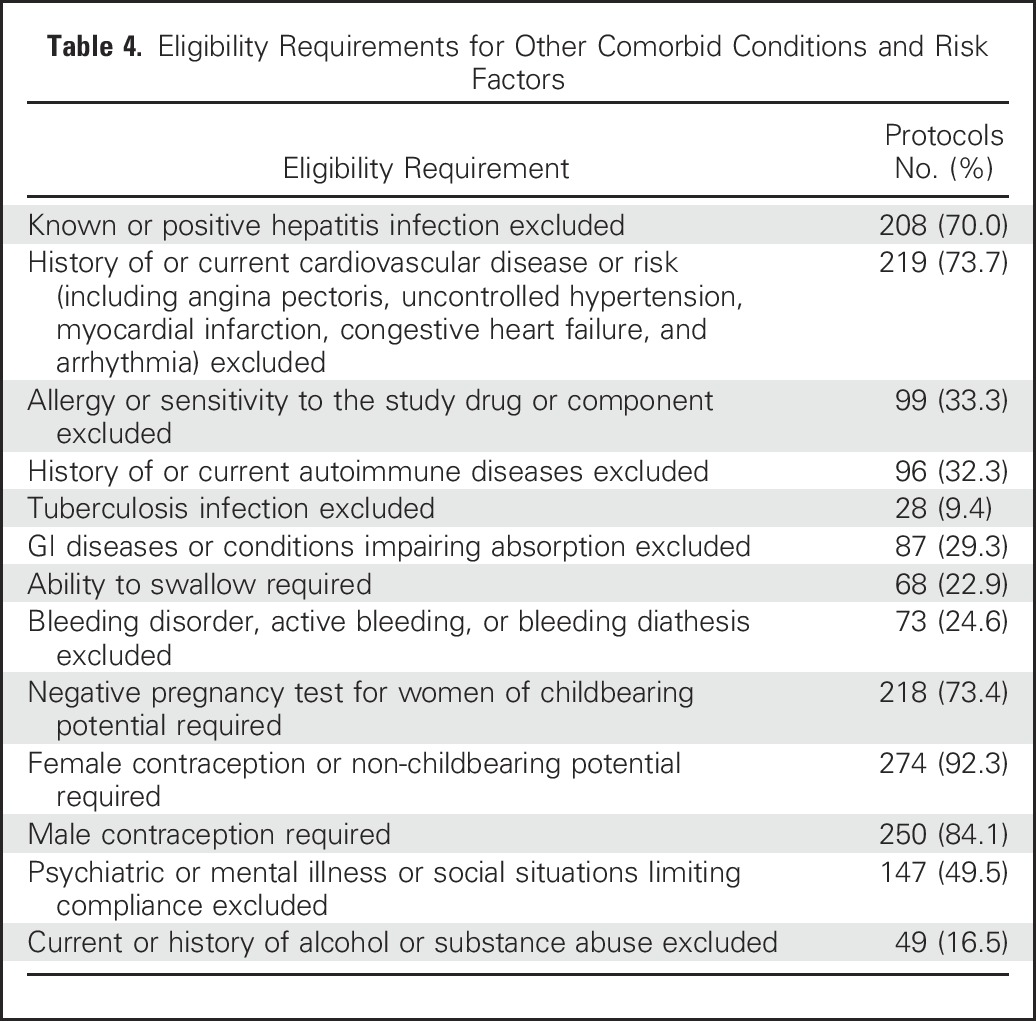
Of protocols that specified HIV or AIDS among the eligibility criteria, 250 (84.2%) excluded known or active HIV/AIDs. In five protocols (1.7%), HIV-positive patients were allowed given stable disease and/or adequate CD4 cell counts. Other comorbid conditions and risk factors were also excluded in many of the protocols (Table 4).
Table 4.
Eligibility Requirements for Other Comorbid Conditions and Risk Factors
DISCUSSION
We assessed the eligibility criteria from study protocols of IND applications submitted to the US FDA OHOP in 2015. Current cancer clinical trials contain many eligibility criteria that narrowly define the study population. Overall, patient enrollment is limited by various exclusions of comorbid conditions and risk factors. Variability is also observed in the definition of eligibility requirements, such as patient age ranges and differing definitions of adequate organ function. In general, the clinical trial population tends to be limited to healthier and lower-risk patients, and higher-risk patients are excluded from studies. Although some stringent eligibility criteria may be necessary for trial safety, others may be overly strict.
The majority of trials enrolled healthier patients, such as those with better PS and greater LE. Only 35% of the protocols included patients with ECOG PS of 2 and only two protocols included patients with ECOG PS of 3. In addition, more than half (53.2%) of the protocols specified an LE requirement. These areas of eligibility may be re-examined. In a survey of faculty and physicians, more than half believed that patients with an ECOG PS of 2 should not be excluded from molecularly targeted trials.1 Many of those in the medical community also believe that LE is an unnecessary and antiquated criterion, because a judgment of life expectancy is subjective.10
Age requirements in cancer clinical trials have commonly excluded elderly and pediatric patients.9 The majority of the 2015 IND protocols were in adults, with age requirements differing among some protocols. Some trials had an upper age limit, excluding elderly patients. There were only three protocols (1.0%) specifically in older patients, and only 11 protocols (3.7%) included pediatric patients younger than 16 years of age. Overall, the majority of protocols focused on younger adult patients, with few studies in elderly and pediatric patients. To better understand the safety and efficacy of treatments in these patients, we can consider expanding clinical trial eligibility to include them. The low enrollment of elderly patients in clinical trials is also unrepresentative of the general population of patients with cancer. More than 60% of cancer cases occur in people older than 65 years, but only approximately 22% of clinical trial participants are elderly.11 Minimum age requirement for clinical trials is also currently being examined by ASCO and Friends of Cancer Research.8,9
The development of CNS or brain metastases is a common reason for patients to be disqualified from cancer clinical trials, and this is another area of eligibility criteria under consideration by ASCO and Friends of Cancer Research.8,9 Among the IND protocols, most excluded patients with brain metastases, and 47% allowed patients with previously treated brain metastases. Excluding patients with brain metastases from clinical trials may not reflect the patient population outside of the trial. For instance, > 25% of patients with lung cancer develop brain metastases,12 and 10% to 16% of patients with stage IV breast cancer (BC) develop brain metastases.13 Despite the high proportion of patients with brain metastases, few trials of these cancers include patients with brain metastases. A meta-analysis of 413 non–small-cell lung cancer trials found that only 41% allowed the enrollment of patients with previously treated brain metastases, and 14% to 19% excluded patients with any history of brain metastases.14
The diagnosis of HIV or AIDS is another comorbidity that has traditionally excluded patients from cancer clinical trials. Among the 2015 IND protocols, 250 (84.2%) excluded patients with known or active HIV or AIDS. Only five protocols (1.7%) allowed the enrollment of HIV-positive patients. Although patients with HIV/AIDS have historically been excluded from clinical trials because of low expectation of survival, this may not need to be the case. The treatment of HIV has advanced greatly; antiretroviral therapy has dramatically improved the life expectancy and overall health of patients with HIV, and patients with access to treatment are expected to live for decades.15 ASCO and Friends of Cancer Research are developing recommendations on the eligibility of patients with HIV/AIDS.9
Eligibility for most cancer clinical trials requires laboratory tests of adequate organ function. Many patients with organ dysfunction are excluded from clinical trials, which is another area of eligibility under examination by ASCO and Friends of Cancer Research.8,9 From examination of the 2015 commercial IND protocols, most trials required adequate organ function. However, there was wide variability observed in the definitions of adequate organ function (Figs 1A-1I). Although certain values were the most common standard for adequate organ function, there were differing standards and a wide range of requirements. For example, the majority of protocols with an absolute neutrophil count (ANC) requirement specified an ANC of ≥ 1,500/μL, but ANC ≥ 1,000/μL was also a common requirement, and protocols specified anywhere from ≥ 500/μL to ≥ 2,500/μL. Although some requirements may differ based on disease type (ie some trials in hematologic malignancies may allow worse bone marrow function), differences were observed among trials of the same disease. Among protocols in lymphomas, some required ANC ≥ 750/μL and others ≥ 1,500/μL. There is variation in laboratory value eligibility requirements among studies, even of the same disease, suggesting that other trials can also loosen their criteria.
Another possible area of eligibility criteria expansion is the study of BC in men. Although rare, male BC accounts for approximately 0.7% of all diagnoses of BC per year.16,17 However, because of disease rarity, there are few studies of BC in men, and randomized trials in male patients only are not feasible.17 Furthermore, most BC studies include women only, and only approximately one third include men.18 The lack of data and clinical trial options for male patients with BC has been noted by researchers and ASCO.18,19 Of the 2015 IND protocols, 10 BC studies were in women only, and four included both men and women. To gain more knowledge about the treatment of male BC and increase accrual to BC trials, we can consider expanding the eligibility of all BC trials to include male patients if there is no reason to specifically exclude them.
There are concerns about expanding eligibility to patients with more risk factors, including safety and the perceived difficulty demonstrating safety and efficacy of a new drug treatment in higher-risk patients. To address questions about a heterogeneous trial population and the design and analysis of a clinical trial with expanded eligibility criteria, two possible trial design options incorporating expanded eligibility are discussed as follows.
The first is a randomized clinical trial (RCT) in which both patients defined by restricted eligibility criteria (denoted RElgPop) and higher-risk patients defined by expanded eligibility criteria (denoted ExpPop) are enrolled. The trial population consists of both groups and is stratified by RElgPop and ExpPop. Although the intent-to-treat (ITT) population consists of all enrolled patients, a modified ITT (MITT) population consists of only RElgPop. The trial would use hierarchical testing, and the primary analysis would be conducted in the MITT population, with subsequent analyses conducted in the ITT population. If the sample size is adequate and hypothesis driven, results in the expanded population can also be analyzed separately. This trial design addresses concerns about a heterogeneous population of patients, because the primary analysis is conducted in the restricted-eligibility patients, but the additional analyses provide safety and efficacy information about all patients. It is expected that the proportion of RElgPop patients would be greater than ExpPop patients (eg, 80%:20%), and the primary hypothesis, type I and type II errors, and number of events for the final analysis would be based on the restricted eligibility population of patients (MITT).
A second trial design option featuring expanded eligibility criteria is the simultaneous conduct of an RCT in patients enrolled under restricted criteria and enrollment of a single-arm cohort of higher-risk patients under expanded criteria. The ITT population would consist of the restricted criteria patients only, and the two populations would be analyzed separately. Descriptive statistics would be reported for the single-arm cohort of expanded eligibility patients. This method would provide safety and efficacy information for higher-risk patients, although not affecting the results of the RCT in restricted eligibility patients. However, the interpretation of toxic events, particularly deaths, may be difficult in the expanded eligibility population, because there is no control arm for comparison. Therefore, the first trial design option may be preferable, because both populations can be studied in an RCT.
In conclusion, cancer tends to be studied in a narrow population of patients who are often healthier and lower risk than the general population of patients with cancer. Current oncology clinical trials stipulate many inclusion and exclusion criteria that specifically define the patient population under study. Although eligibility criteria are needed to define the study population and improve safety, overly restrictive eligibility criteria limit participation in clinical trials, cause the study population to be unrepresentative of the general population of patients with cancer, and limit patient access to new treatments. There are various potential areas for the modification of cancer clinical trial eligibility criteria and design options for trials incorporating expanded eligibility. Broadening clinical trial eligibility where appropriate and without compromising patient safety can be considered to increase enrollment and better reflect the real-world patient population. The expansion of clinical trial eligibility can provide more information about new treatments for more patients and improve patient participation overall.
ACKNOWLEDGMENT
We thank Tamy Kim, Associate Director for Regulatory Affairs in the Office of Hematology and Oncology Products, Center for Drug Evaluation and Research, US Food and Drug Administration, for assistance in this project.
Footnotes
Supported by the Center for Drug Evaluation and Research, US Food and Drug Administration, Silver Spring, MD.
AUTHOR CONTRIBUTIONS
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Re-Evaluating Eligibility Criteria for Oncology Clinical Trials: Analysis of Investigational New Drug Applications in 2015
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Susan Jin
No relationship to disclose
Richard Pazdur
No relationship to disclose
Rajeshwari Sridhara
No relationship to disclose
REFERENCES
- 1.Kim ES, Bernstein D, Hilsenbeck SG, et al. : Modernizing eligibility criteria for molecularly driven trials. J Clin Oncol 33:2815-2820, 2015 [DOI] [PubMed] [Google Scholar]
- 2.George SL: Reducing patient eligibility criteria in cancer clinical trials. J Clin Oncol 14:1364-1370, 1996 [DOI] [PubMed] [Google Scholar]
- 3.Institute of Medicine Forum on Drug Discovery, Development, and Translation: Transforming clinical research in the United States: Challenges and opportunities: Workshop summary. https://www.ncbi.nlm.nih.gov/books/NBK50895/ [PubMed]
- 4.Bennette CS, Ramsey SD, McDermott CL, et al. : Predicting low accrual in the National Cancer Institute’s Cooperative Group clinical trials. J Natl Cancer Inst 108:djv324, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Cancer Institute. Report of the National Cancer Institute Clinical Trials Program Review Group : http://deainfo.nci.nih.gov/advisory/bsa/bsa_program/bsactprgmin.pdf
- 6.Nass SJ, Moses HL, Mendelsohn J (eds): Institute of Medicine. A National Cancer Clinical Trials system for the 21st century: Reinvigorating the NCI cooperative group program. http://www.nationalacademies.org/hmd/Reports/2010/A-National-Cancer-Clinical-Trials-System-for-the-21st-Century-Reinvigorating-the-NCI-Cooperative.aspx [PubMed]
- 7.Kris MG, Meropol NJ, Winer EP (eds): Accelerating progress against cancer: ASCO’s blueprint for transforming clinical and translational cancer research. http://www.asco.org/sites/new-www.asco.org/files/content-files/research-and-progress/documents/2011-blueprint-accelerating-progress-against-cancer.pdf [DOI] [PubMed]
- 8.American Society of Clinical Oncology: ASCO in Action: Initiative to modernize eligibility criteria for clinical trials launched. https://www.asco.org/advocacy-policy/asco-in-action/initiative-modernize-eligibility-criteria-clinical-trials-launched
- 9.Cavallo J: ASCO and Friends of Cancer Research launch initiative to modernize eligibility criteria for clinical trials. http://www.ascopost.com/issues/august-10-2016/asco-and-friends-of-cancer-research-launch-initiative-to-modernize-eligibility-criteria-for-clinical-trials/
- 10.Penel N, Clisant S, Lefebvre JL, et al. : “Sufficient life expectancy”: An amazing inclusion criterion in cancer phase II-III trials. J Clin Oncol 27:e105, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Aapro MS, Köhne CH, Cohen HJ, et al. : Never too old? Age should not be a barrier to enrollment in cancer clinical trials. Oncologist 10:198-204, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Langer CJ, Mehta MP: Current management of brain metastases, with a focus on systemic options. J Clin Oncol 23:6207-6219, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Lin NU, Bellon JR, Winer EP: CNS metastases in breast cancer. J Clin Oncol 22:3608-3617, 2004 [DOI] [PubMed] [Google Scholar]
- 14.McCoach CE, Berge EM, Lu X, et al. : A brief report of the status of central nervous system metastasis enrollment criteria for advanced non-small cell lung cancer clinical trials: A review of the ClinicalTrials.gov trial registry. J Thorac Oncol 11:407-413, 2016 [DOI] [PubMed] [Google Scholar]
- 15.Deeks SG, Lewin SR, Havlir DV: The end of AIDS: HIV infection as a chronic disease. Lancet 382:1525-1533, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giordano SH, Cohen DS, Buzdar AU, et al. : Breast carcinoma in men: A population-based study. Cancer 101:51-57, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Giordano SH: A review of the diagnosis and management of male breast cancer. Oncologist 10:471-479, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Helwick C: Is male breast cancer overlooked in clinical trials? http://www.ascopost.com/issues/may-25-2016/is-male-breast-cancer-overlooked-in-clinical-trials/
- 19.Goodman A: Male breast cancer differs from breast cancer in women, but little data informs treatment. http://www.ascopost.com/issues/december-15-2011/male-breast-cancer-differs-from-breast-cancer-in-women-but-little-data-informs-treatment/