Abstract
Purpose
The primary purposes of eligibility criteria are to protect the safety of trial participants and define the trial population. Excessive or overly restrictive eligibility criteria can slow trial accrual, jeopardize the generalizability of results, and limit understanding of the intervention’s benefit-risk profile.
Methods
ASCO, Friends of Cancer Research, and the US Food and Drug Administration examined specific eligibility criteria (ie, brain metastases, minimum age, HIV infection, and organ dysfunction and prior and concurrent malignancies) to determine whether to modify definitions to extend trials to a broader population. Working groups developed consensus recommendations based on review of evidence, consideration of the patient population, and consultation with the research community.
Results
Patients with treated or clinically stable brain metastases should be routinely included in trials and only excluded if there is compelling rationale. In initial dose-finding trials, pediatric-specific cohorts should be included based on strong scientific rationale for benefit. Later phase trials in diseases that span adult and pediatric populations should include patients older than age 12 years. HIV-infected patients who are healthy and have low risk of AIDS-related outcomes should be included absent specific rationale for exclusion. Renal function criteria should enable liberal creatinine clearance, unless the investigational agent involves renal excretion. Patients with prior or concurrent malignancies should be included, especially when the risk of the malignancy interfering with either safety or efficacy endpoints is very low.
Conclusion
To maximize generalizability of results, trial enrollment criteria should strive for inclusiveness. Rationale for excluding patients should be clearly articulated and reflect expected toxicities associated with the therapy under investigation.
INTRODUCTION
Eligibility criteria are a foundational component of clinical trials and serve to define the patient population under study. They can be inclusionary, by, for example, specifying a tumor type or molecular alteration needed for study entry, or exclusionary, by specifying certain characteristics, such as laboratory test values, history of prior and concurrent malignancies, minimum age, or comorbidities, that would render a patient ineligible for enrollment. The primary purposes of eligibility criteria are to protect the safety of patients who participate in clinical trials and to define the characteristics of the study population. Excessive or overly restrictive eligibility criteria can impair clinical trial accrual and completion and prevent patients from accessing investigational interventions that may provide clinical benefit. Narrowly defined trial populations may also jeopardize the generalizability of trial results and limit the ability to understand the therapy’s benefit-risk profile across the broad patient population who ultimately may receive the intervention in the postmarket setting. The clinical generalizability of a study is directly connected to the degree to which trial participants reflect the range of characteristics of the patient population for whom the intervention has been devised.1
Common inclusion and exclusion criteria have developed over time, primarily through experience with cytotoxic chemotherapeutics. Eligibility criteria are often duplicated from previous trials as a start or template for the next study, but instead, they should be modified as appropriate to meet the objectives of each study in consideration of the anticipated safety of the investigational agent in the new study or the ability to recruit trial participants from the patient population. Given the increase in complexity of cancer treatments, the advent of novel therapeutic modalities with differing safety profiles, and the targeting of specific patient subpopulations, many have called for simplified, rational, modernized eligibility criteria that accurately reflect the population of patients with cancer who are the intended users of the investigational therapy once it reaches the market.2-5 Newer precision medicine agents are often studied in populations with specific genomic alterations because preclinical data indicate that the agent targets a specific molecular abnormality or pathway and is uniquely or preferentially effective in tumors that harbor the alteration. The fact that many of the alterations occur in low frequencies heightens the need to be maximally inclusive of patients whose tumors harbor the given alteration, as long as safety of the participants is considered.
Restrictive eligibility criteria may preclude enrollment of trial participants who represent the range of characteristics of the overall patient population with a given disease. For example, Kaiser Permanente conducted an analysis of 326 consecutively diagnosed patients with non–small-cell lung cancer (NSCLC) to determine how many would qualify for two trials involving chemotherapy and antiangiogenic therapy. The majority of patients (approximately 80%) were ineligible for the trials as a result of failure to meet eligibility criteria requirements and comorbidities.6 In addition, reviews of the National Cancer Institute clinical trials program concluded that exclusionary criteria arbitrarily eliminate patients and recommended that eligibility criteria be simplified and broadened.7,8
ELIGIBILITY CRITERIA INITIATIVE
Modernizing eligibility criteria was a key objective of the November 2011 ASCO Blueprint for Transforming Clinical and Translational Cancer Research.9 ASCO believed that an increasing number and complexity of eligibility criteria were compromising recruitment to clinical trials. A working group of the ASCO Cancer Research Committee conducted an analysis of clinical trials and survey of investigators and developed a recommended strategy to formulate inclusion and exclusion criteria, as well as encourage continuous reassessment of criteria throughout the research process.4 The resulting article provided a list of key questions to help focus trial designers on the relationship of criteria to the study objectives, generalizability of results, and risks to patients.
ASCO, in collaboration with Friends of Cancer Research (Friends), launched a collaborative initiative to reassess the approach for determining clinical trial eligibility. ASCO, Friends, and the US Food and Drug Administration (FDA) used the recommendations from ASCO’s original work to identify specific eligibility criteria that were most likely to restrict patients’ participation in trials and were least likely to impact the safety of trial participants. The project leadership initially selected the following four topics that commonly lead to exclusion of patients from clinical trials: brain metastases, minimum age for enrollment, HIV infection, and organ dysfunction and prior and concurrent malignancies. Each of these topics was explored by working groups composed of multiple stakeholders, including investigators, patient advocates, biostatisticians, pharmacologists, manufacturers, and regulators. The working groups reviewed the state of the science and existing studies in the literature and attempted to balance the needs of protecting patient safety, facilitating access to investigational therapies, and protecting trial integrity (including safety, efficacy, statistical, and operational considerations). The working groups engaged in multiple meetings to discuss their concerns and reached consensus on approaches that could be implemented to broaden eligibility criteria and enable recruitment of a trial population that is more representative of the population of patients with the given cancer who are the intended users of the intervention being studied. The draft recommendations were presented and vetted among all the working groups at a May 2016 workshop and were discussed at a public meeting in November 2016—the Friends Annual Meeting on Clinical Cancer Research.10 Representatives from the National Clinical Trials Network (NCTN) provided examples at the November meeting of ongoing efforts within the NCTN groups to appropriately expand eligibility criteria.
WORKING GROUP RECOMMENDATIONS
Detailed discussion of each of the working group recommendations is included in separate manuscripts that have been submitted for publication. This statement provides a high-level summary of each of the working group recommendations and discusses overarching principles to guide implementation. Recommended language for use in clinical trial protocols is included in Table 1.
Table 1.
Recommended Protocol Text
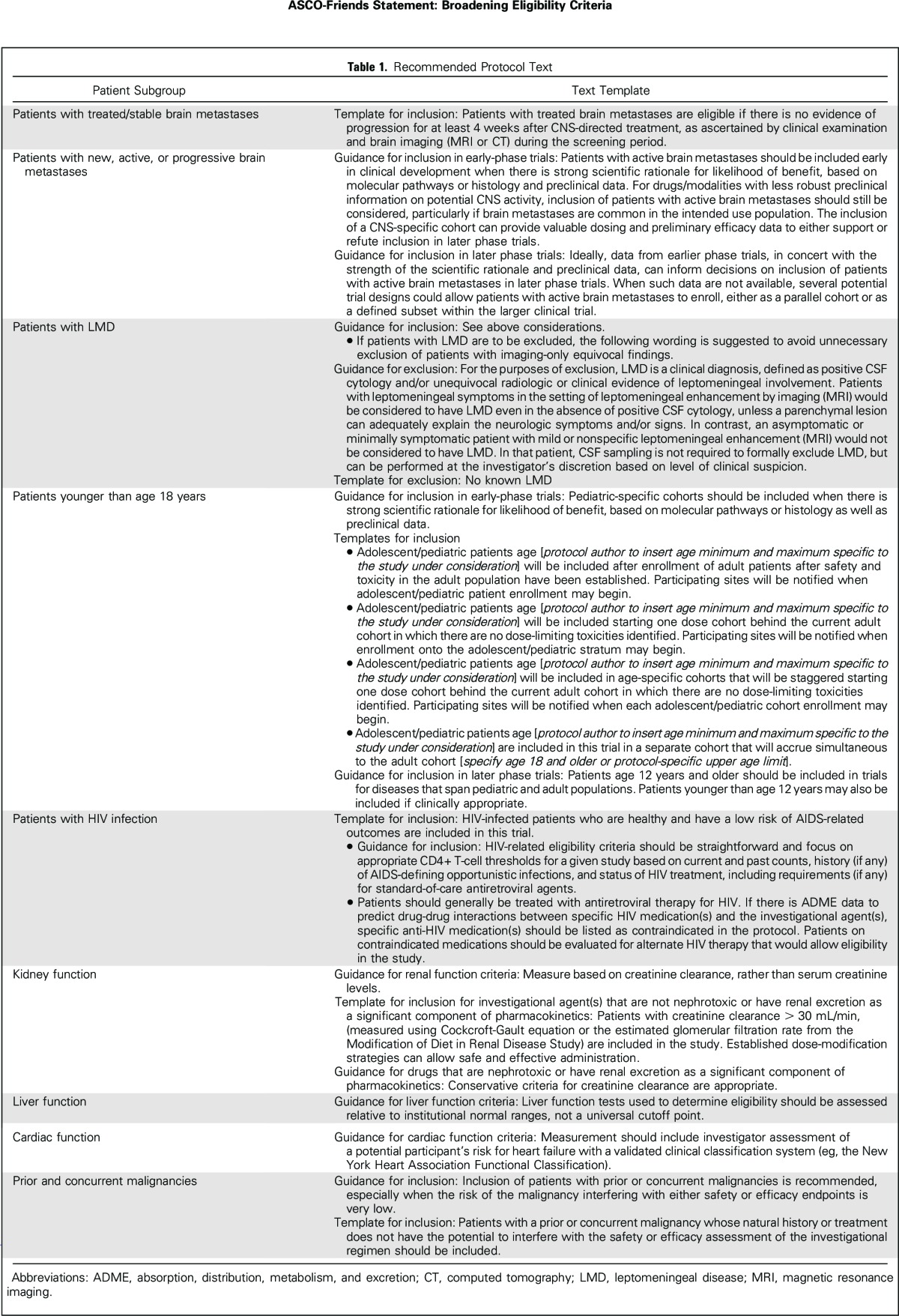
Brain Metastases
Broad or conditional exclusion of patients with brain metastases is common despite the high incidence of brain metastases in some tumor types.11 An FDA analysis of 250 Investigational New Drug applications for 2015 found that less than half permitted enrollment of patients with previously treated, inactive, and/or stable brain metastases (Jin et al, manuscript submitted for publication). Although life expectancy may be reduced for some patients with brain metastases and there have been concerns regarding a potentially greater risk of neurologic toxicity, existing literature does not indicate that these patients experience higher rates of serious adverse events.12 This working group developed recommendations specific to patients with treated or stable brain metastases; patients with new, active, or progressive brain metastases; and patients with leptomeningeal disease.13
Patients with treated and/or stable brain metastases (eg, no progression for at least 4 weeks after local prior therapy) should be routinely included in prospective clinical trials of all phases and only excluded if there is compelling rationale for exclusion. If there are specific safety concerns, then tailoring specific criteria to the concern is preferable to general exclusion of all patients with brain metastases.
For patients with active (eg, untreated or progressive) brain metastases, the working group recommends that such patients not be automatically excluded. However, a one-size-fits-all approach is not appropriate, and factors such as natural history of the disease, trial phase and design, and the drug’s mechanism of action, pharmaceutical properties, and potential for CNS penetration should determine whether such patients are included in a trial. If patients with active brain metastases are included, additional prospective planning may be required to better define safety and treatment response. Early stopping rules may be appropriate should excessive toxicity and/or lack of efficacy be observed.
In most trials, it remains appropriate to exclude patients with leptomeningeal disease as a result of their poor prognosis, although there may be situations that warrant a cohort of such patients in early-phase trials (eg, when CNS activity is anticipated), and these data could then support inclusion of such patients in later phase trials. If patients with leptomeningeal disease are excluded, justification for such exclusion should be provided alongside the exclusion criteria.
Minimum Age for Enrollment
Children and adolescents under the age of 18 years have traditionally been excluded from participating in clinical trials with novel agents until extensive data are available from studies of adults, often years after the introduction and approval of an agent. Because pediatric patients have historically been considered a vulnerable population, there is concern that a high-profile adverse event in a child could endanger the entire drug development program. However, a review of successful and failed development of oncology drugs over the past three decades yields no evidence to support this concern (G.H. Reaman, personal communication, March 2017). Drug exposure in adolescents (age 12 to 18 years) and adults is similar, supporting the enrollment of adolescents in adult trials that involve the same disease and/or therapeutic target.14,15 The Minimum Age Working Group developed recommendations for inclusion of pediatric patients in early- and late-phase trials.16
In initial dose-finding trials, pediatric-specific cohorts should be included when there is strong scientific rationale for likelihood of benefit, based on molecular pathways or histology or preclinical data. These cohorts would assess dose and pharmacokinetics separately in the pediatric population. Staggered enrollment starting with older children followed by younger children could be considered to address potential concerns specific to younger pediatric patients, including not only metabolic differences but also challenges related to the availability of appropriate formulations for young children.
Later phase trials in diseases and/or therapeutic targets that span adult and pediatric populations should include pediatric patients. Given the similarity in metabolism and excretion between adults and adolescents, patients age 12 years and older should be enrolled onto such trials. In some instances, it may also be appropriate to enroll patients younger than age 12 years with the proper clinical support and expertise.
HIV Infection
Many people infected with HIV now have a normal life expectancy as a result of substantial improvements in HIV treatment over the past 20 years.17,18 Cancer is now a leading cause of mortality in people with HIV; however, most oncology studies exclude this population, as confirmed by the FDA analysis of 2015 Investigational New Drug applications. Only five (1.7%) of 250 protocols allowed enrollment of HIV-positive patients with stable disease and/or adequate CD4+ T-cell counts (Jin et al, manuscript submitted for publication). A review of HIV eligibility criteria in recent industry-supported studies leading to successful new drug applications conducted by the working group found that zero of 46 studies contained inclusion criteria for patients with HIV, 30 studies contained exclusion criteria, and nine studies discussed general exclusion of patients with active infection but did not specify HIV infection. The HIV Working Group recommended the following eligibility considerations in cancer studies.19
Patients with cancer with HIV infection who are healthy and have a low risk of AIDS-related outcomes should be included in cancer clinical trials unless there is a specific rationale to exclude such patients.
Eligibility criteria should be straightforward and focus on current and past CD4 and T-cell counts, history (if any) of AIDS-defining conditions (eg, opportunistic infections), and status of HIV treatment. Healthy HIV-positive participants who are included in cancer clinical trials should be treated using the same standards as trial participants with other comorbidities. Antiretroviral therapy should be considered a concomitant medication.
Eligibility criteria for cancer clinical trials should allow for the patient to be treated concurrently with standard antiretroviral therapy (ART) following Department of Health and Human Services treatment guidelines.20 In cases where ART therapy may interact with cancer therapy, specific ART agents may be excluded.
Organ Dysfunction and Prior and Concurrent Malignancies
This working group first evaluated the types of organ dysfunction that were likely to drive most clinical trial exclusion criteria. The areas of focus included kidney, heart, and liver dysfunction, as well as exclusion based on a history of a previous malignancy. The group conducted analysis of these criteria from a large, representative data set that included a cohort of nearly 13,000 newly diagnosed patients with breast, colon, lung, and bladder cancers from 2013 to 2014. The analysis, as well as review of the literature, helped determine which of the organ dysfunction criteria to prioritize for development of recommendations.21
Renal function criteria should be based on creatinine clearance rather than serum creatinine levels. In situations where renal excretion is not a significant component of a drug’s clearance, liberal creatinine clearance criteria (eg, > 30 mL/min) should be used. Both the Cockcroft-Gault equation and the estimated glomerular filtration rate from the Modification of Diet in Renal Disease Study are reliable methods to estimate creatinine clearance.22 Trial sponsors should choose one of these methods and use it consistently across the research process. Established dose-modification strategies can allow safe and effective administration. Conservative criteria remain appropriate for nephrotoxic drugs.
Current clinically available tests of hepatic function (eg, tests of serum aminotransferases [ALT and AST] and bilirubin) inadequately describe liver function, particularly drug metabolism capability. In the absence of alternate testing methods, trials should continue to use standard clinical assessments of liver function relative to institutional normal ranges and avoid imposing a universal cutoff point that may be unnecessarily restrictive.
If an investigational therapy is not known to pose cardiac risks, arbitrary ejection fraction values should not be used to exclude patients from clinical trials. Trials should recommend investigator assessment of a potential participant’s risk for heart failure with a validated clinical classification system, such as the New York Heart Association Functional Classification.23 Concern about cardiac effects often leads to frequent ECG monitoring in early-phase trials to determine eligibility and ongoing risk for QT/QTc prolongation.24 Continued ECG monitoring should be eliminated in later phases if cardiac risk is not determined to be a concern.
Exclusions based on a history of prior malignancy or presence of concurrent malignancy should be liberalized, both in terms of when the malignancy occurred and was treated and types of prior malignancies. Inclusion of patients with prior or concurrent malignancies is recommended, especially when the risk of the malignancy interfering with either safety or efficacy endpoints is very low. Patients with a prior or concurrent malignancy whose natural history or treatment does not have the potential to interfere with the safety or efficacy assessment of the investigational regimen should be included.
DISCUSSION
Through the course of the working group discussions, potential benefits and risks of expanding eligibility criteria were identified (Table 2). As previously stated, the primary purpose of eligibility criteria is to protect the safety of clinical trial participants who may have characteristics that place them at increased risk for an adverse event from the intervention being studied. Thus, arguments against the use of broader eligibility criteria center on the concern that the development of an effective drug could be jeopardized if a serious adverse event occurs in a patient population that is inherently sicker or vulnerable. Inclusion of some patients may require additional screening or monitoring or the engagement of additional expertise to manage safety issues specific to that patient population. This would help to mitigate risk in these patients but could also increase trial cost and complexity.
Table 2.
Benefits and Risks of Expanded Eligibility Criteria
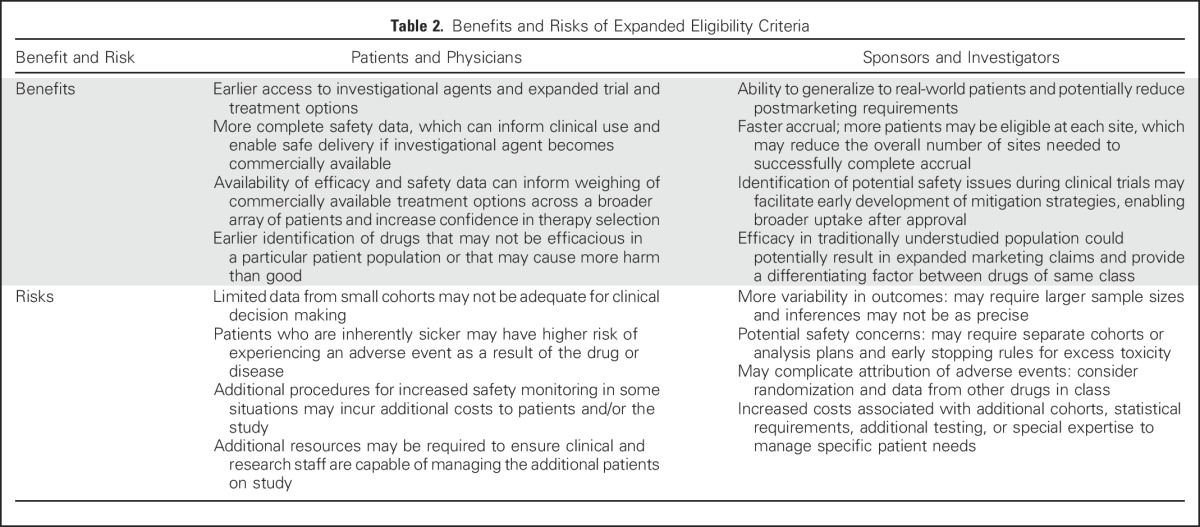
In some cases, the working groups concluded that eligibility criteria should be broadened for all trial participants, particularly when a drug’s known or expected safety profile does not pose inordinate risks to participants. In other cases, sponsors could consider enrolling an expanded, more heterogeneous population and exclude these patients from the primary efficacy analysis, so as not to compromise assessment of the drug’s efficacy, but include them in the safety analysis. Strategies could include enrolling restricted and expanded populations in the same clinical trial (Jin et al, manuscript submitted for publication), conducting simultaneous clinical trials and analyzing separately, or using an extended trial design to expand knowledge in particular populations, such as the elderly, by enriching the primary study population with such individuals.25 Additional potential study design options that can be considered to address these concerns and potentially mitigate risk are listed in Table 3.
Table 3.
Potential Trial Designs and Considerations
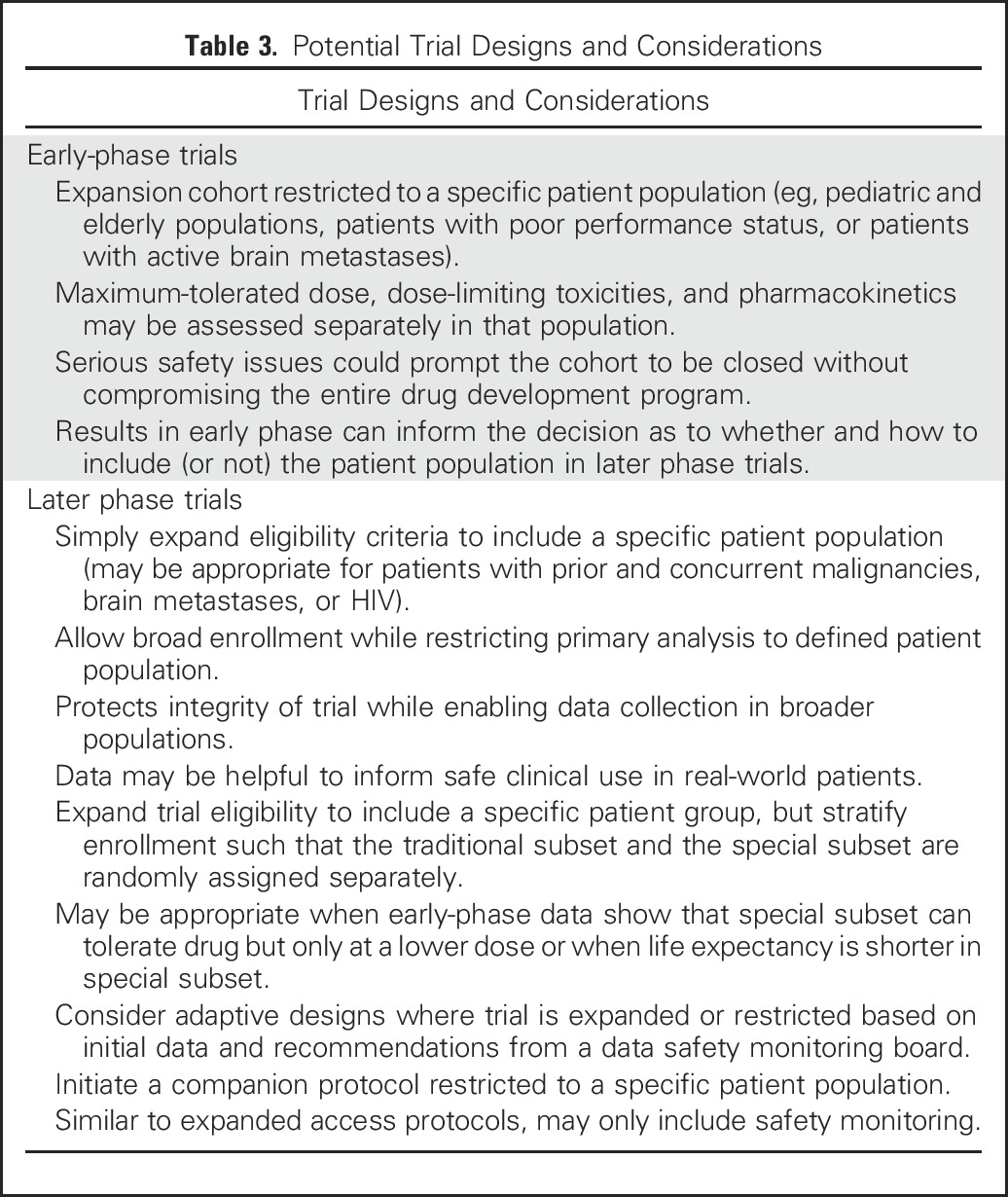
Although incorporation of an expanded trial population could present additional operational considerations, this practice could be accompanied by incentives such as the potential for expanded label indications resulting in competitive marketing claims. In addition, there is the potential for inclusion of additional information in the label’s prescribing information to help guide clinicians in adjusting administration and dosing in different populations. Adequate data generated in the clinical trial on under-represented populations, such as those with organ impairment, may obviate requirements for postmarketing studies. Discussion with regulators is encouraged to determine the best approach for each situation.
Cooperative groups have adapted eligibility criteria over the years. A review of Eastern Cooperative Oncology Group lung cancer trials determined that patients with prior malignancies were excluded from 94% of trials that used survival as a primary end point and 73% of trials that used other primary end points.26 Prior malignancies did not impact survival outcomes in patients with stage IV lung cancer or locally advanced lung cancer, suggesting that clinical trial outcomes would not be adversely impacted by inclusion of patients with a history of prior cancer.27,28 This analysis led the Alliance in Clinical Trials in Oncology Group to develop more inclusive criteria for patients with advanced lung cancer. The National Cancer Institute NCTN is also broadening eligibility criteria and changing clinical trial designs to address slow patient accrual. The Southwest Oncology Group revised the eligibility criteria of phase III trials of advanced NSCLC in a stepwise manner. From 1995 to 2014, the Southwest Oncology Group launched three NSCLC trials (S9509,29 S1400, and S1403) and progressively expanded its approach to inclusion of patients with brain metastases and prior malignancies.
ASCO’s Targeted Agent and Profiling Utilization Registry (TAPUR) Study has broad inclusion criteria of patients with prior and concurrent malignancies not requiring treatment, brain metastases, and HIV infection, and is in the process of lowering eligibility age from 18 to 12 years for drugs that have an established pediatric dose or drugs in which the pediatric dose can be derived from data from adult clinical trials.15 The TAPUR protocol enables patients with any prior or concurrent cancer to participate. Patients with brain metastases can participate, as long as the treatment of the brain metastases has been completed, the metastases are not progressive, and the patient has been off corticosteroids for at least 1 month. Patients also cannot have experienced a seizure or had a clinically significant change in neurologic status within 3 months of enrollment. Patients with HIV infection are allowed to enroll at the clinical investigator’s discretion, except for two study drugs with exclusions based on active HIV infection.
Fundamentally changing the approach to eligibility criteria requires a culture change across the entire clinical trials enterprise. At the design phase, investigators and trial sponsors should approach study development with an inclusive mindset, taking into consideration the safety profile of the investigational therapy, standard-of-care treatment, and the characteristics of the indicated population. A standard of inclusion, unless otherwise specified, would give investigators the responsibility to provide rationale and use their own clinical judgment and discretion as to why patients should be excluded from trial participation. Known or suspected risks of the investigational therapy should be the primary factors that warrant exclusion of patients. These risks should be outlined in a concise, easy-to-read format and provided to investigators, pharmacists, and the clinical research team for review. As information is gathered over the duration of a trial, eligibility criteria should be reconsidered at predefined time points or events and adjusted, if needed, during the clinical development plan to enable greater inclusion with an aim of having the study population in late-stage or registration trials reflect as closely as possible the indicated population. Discussions with regulatory officials can also stress the importance of gathering safety data and including data on a broader array of patients in prescribing information. Eligibility criteria that affirmatively state inclusion of patients will help to overcome potential investigator or research staff bias against inclusion of patients such as those with prior and concurrent malignancies and comorbidities.30 Outreach to institutional review boards and scientific review committees to educate them on the importance of being inclusive will also help to overcome concerns that may arise from these oversight bodies.
In conclusion, to maximize the generalizability of clinical trial results, eligibility criteria should strive for inclusiveness to enroll participants who are representative of the intended users of the intervention under study in a timely manner. Rationale for excluding patients with characteristics should be clearly articulated and reflect expected toxicities associated with the therapy under investigation based on existing data. In cases where the toxicity profile of the drug is unknown, eligibility criteria should be adjusted over the course of the research process as greater understanding of the agent’s pharmacokinetics and tolerability are developed. We anticipate that current efforts to expand eligibility in several ongoing and planned clinical trials will help to demonstrate the feasibility of expanding eligibility and that future FDA guidance will assist sponsors in designing more representative trials. ASCO and Friends plan to work with the clinical trial community to encourage incorporation of these recommendations in new and existing trials and identify opportunities to track progress.
ACKNOWLEDGMENT
This article was developed as part of a collaboration between ASCO, Friends of Cancer Research, and the US Food and Drug Administration. The contents of this document were presented on May 12, 2016, and November 16, 2016, as part of collaborative workshops.
Footnotes
Supported in part by Memorial Sloan-Kettering Cancer Center Support Grant No. P30 CA008748.
The opinions expressed in this article are those of the authors and do not necessarily reflect the views or policies of the authors’ affiliated institutions.
AUTHOR CONTRIBUTIONS
Administrative support: Suanna S. Bruinooge, Caroline Schenkel
Provision of study materials or patients: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Broadening Eligibility Criteria to Make Clinical Trials More Representative: American Society of Clinical Oncology and Friends of Cancer Research Joint Research Statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Edward S. Kim
Honoraria: Celgene, Eli Lilly, AstraZeneca, Boehringer Ingelheim
Consulting or Advisory Role: Eli Lilly, Celgene, AstraZeneca, Boehringer Ingelheim
Suanna S. Bruinooge
No relationship to disclose
Samantha Roberts
Employment: Genentech
Gwynn Ison
No relationship to disclose
Nancy U. Lin
Research Funding: Genentech, GlaxoSmithKline, Array BioPharma, Novartis, Kadmon, Oncothyreon, Cascadian
Lia Gore
Employment: ARIAD (I)
Leadership: ARIAD (I)
Stock or Other Ownership: ARIAD (I), Amgen, Sanofi, Celgene, ARIAD, Clovis Oncology, Agios (I)
Honoraria: Amgen
Consulting or Advisory Role: Celgene, MedImmune, Novartis, ProEd Communications, Genentech, Amgen, Medscape
Patents, Royalties, Other Intellectual Property: Patent held for diagnostic discovery and treatment response methodology tools in the use of magnetic resonance spectroscopy for leukemia.
Travel, Accommodations, Expenses: Amgen, Genentech
Thomas S. Uldrick
Research Funding: Celgene (Inst), Merck (Inst), Bayer (Inst), Genentech (Inst)
Patents, Royalties, Other Intellectual Property: As an employee of the US government, I have provisional patent application regarding methods for the treatment of Kaposi sarcoma and Kaposi sarcoma–associated herpesvirus–induced lymphoma using immunomodulatory compounds and uses of biomarkers (Inst)
Stuart M. Lichtman
Consulting or Advisory Role: Magellan Health
Nancy Roach
Travel, Accommodations, Expenses: Boehringer Ingelheim
Julia A. Beaver
No relationship to disclose
Rajeshwari Sridhara
No relationship to disclose
Paul J. Hesketh
No relationship to disclose
Andrea M. Denicoff
No relationship to disclose
Elizabeth Garrett-Mayer
Stock or Other Ownership: Abbott Laboratories, Abbvie
Consulting or Advisory Role: Tactical Therapeutics, Okava Pharmaceuticals
Eric Rubin
Employment: Merck
Pratik Multani
Employment: Ignyta
Leadership: Ignyta
Stock or Other Ownership: Ignyta
Tatiana M. Prowell
No relationship to disclose
Caroline Schenkel
No relationship to disclose
Marina Kozak
No relationship to disclose
Jeff Allen
No relationship to disclose
Ellen Sigal
Honoraria: AstraZeneca/MedImmune
Travel, Accommodations, Expenses: AstraZeneca/MedImmune, Alexandria Summit
Richard L. Schilsky
Research Funding: AstraZeneca (Inst), Bayer (Inst), Bristol-Myers Squibb (Inst), Genentech (Inst), Eli Lilly (Inst), Merck (Inst), Pfizer (Inst)
REFERENCES
- 1. Siu LL, Tannock IF: Problems in interpreting clinical trials, in Crowley J (ed): Handbook of Statistics in Clinical Oncology. New York, NY, Marcel Dekker, 2001, pp 473-491. [Google Scholar]
- 2.George SL: Reducing patient eligibility criteria in cancer clinical trials. J Clin Oncol 14:1364-1370, 1996 [DOI] [PubMed] [Google Scholar]
- 3.Fuks A, Weijer C, Freedman B, et al. : A study in contrasts: Eligibility criteria in a twenty-year sample of NSABP and POG clinical trials. J Clin Epidemiol 51:69-79, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Kim ES, Bernstein D, Hilsenbeck SG, et al. : Modernizing eligibility criteria for molecularly driven trials. J Clin Oncol 33:2815-2820, 2015 [DOI] [PubMed] [Google Scholar]
- 5.Beaver JA, Ison G, Pazdur R: Reevaluating eligibility criteria: Balancing patient protection and participation in oncology trials. N Engl J Med 376:1504-1505, 2017 [DOI] [PubMed] [Google Scholar]
- 6. Fehrenbacher L, Ackerson L, Somkin C: Randomized clinical trial eligibility rates for chemotherapy (CT) and antiangiogenic therapy (AAT) in a population-based cohort of newly diagnosed non-small cell lung cancer (NSCLC) patients. J Clin Oncol 27, 2009 (suppl 15s; abstr 6538) [Google Scholar]
- 7. National Cancer Institute: Report of the National Cancer Institute Clinical Trials Program Review Group. http://deainfo.nci.nih.gov/advisory/bsa/bsa_program/bsactprgmin.pdf.
- 8. Nass SJ, Moses HL, Mendelsohn J (eds): Institute of Medicine. A national cancer clinical trials system for the 21st century: Reinvigorating the NCI Cooperative Group program. Overview of conclusions and recommendations. http://www.nationalacademies.org/hmd/Reports/2010/A-National-Cancer-Clinical-Trials-System-for-the-21st-Century-Reinvigorating-the-NCI-Cooperative.aspx. [PubMed]
- 9.Meropol NJ, Kris MG, Winer EP: The American Society of Clinical Oncology’s blueprint for transforming clinical and translational cancer research. J Clin Oncol 30:690-691, 2012 [DOI] [PubMed] [Google Scholar]
- 10. Friends of Cancer Research: Friends Annual Meeting 2016. http://www.focr.org/events/friends-annual-meeting-2016.
- 11. doi: 10.1016/j.jtho.2015.10.024. McCoach CE, Berge EM, Lu X, et al: A brief report of the status of central nervous system metastasis enrollment criteria for advanced non-small cell lung cancer clinical trials: A review of the ClinicalTrials.gov trial registry. J Thorac Oncol 11:407-413, 2016. [DOI] [PubMed] [Google Scholar]
- 12.Lin NU: Targeted therapies in brain metastases. Curr Treat Options Neurol 16:276, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lin N, Prowell T, Tan A, et al: Modernizing Clinical Trial Eligibility Criteria: Recommendations of the American Society of Clinical Oncology-Friends of Cancer Research Brain Metastases Working Group. J Clin Oncol 35:3760-3773, 2017. [DOI] [PubMed]
- 14.Chuk MK, Mulugeta Y, Roth-Cline M, et al. : Enrolling adolescents in disease/target-appropriate adult oncology clinical trials of investigational agents. Clin Cancer Res 23:9-12, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Momper JD, Mulugeta Y, Green DJ, et al. : Adolescent dosing and labeling since the Food and Drug Administration Amendments Act of 2007. JAMA Pediatr 167:926-932, 2013 [DOI] [PubMed] [Google Scholar]
- 16. Gore L, Ivy P, Balis F, et al: Modernizing clinical trial eligibility: Recommendations of the American Society of Clinical Oncology–Friends of Cancer Research Minimum Age Working Group. J Clin Oncol 35:3781-3787, 2017. [DOI] [PMC free article] [PubMed]
- 17.Samji H, Cescon A, Hogg RS, et al. : Closing the gap: Increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One 8:e81355, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lewden C, Bouteloup V, De Wit S, et al. : All-cause mortality in treated HIV-infected adults with CD4 ≥500/mm3 compared with the general population: evidence from a large European observational cohort collaboration. Int J Epidemiol 41:433-445, 2012 [DOI] [PubMed] [Google Scholar]
- 19. Uldrick TS, Ison G, Rudek MA, et al: Modernizing clinical trial eligibility criteria: Recommendations of the American Society of Clinical Oncology–Friends of Cancer Research HIV Working Group. J Clin Oncol 35:3774-3780, 2017. [DOI] [PMC free article] [PubMed]
- 20. Department of Health and Human Services Panel on Antiretroviral Guidelines for Adults and Adolescents: Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. https://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf. [Google Scholar]
- 21. Lichtman SM, Harvey RD, Damiette Smit MA, et al: Modernizing clinical trial eligibility criteria: Recommendations of the American Society of Clinical Oncology–Friends of Cancer Research Organ Dysfunction, Prior or Concurrent Malignancy, and Comorbidities Working Group. J Clin Oncol 35:3753-3759, 2017. [DOI] [PubMed]
- 22. US Food and Drug Administration: Guidance for industry: Pharmacokinetics in patients with impaired renal function—Study design, data analysis, and impact on dosing and labeling. http://www.fda.gov/downloads/Drugs/Guidances/UCM204959.pdf.
- 23. Criteria Committee of the New York Heart Association: Diseases of the Heart and Blood Vessels: Nomenclature and Criteria for Diagnosis (ed 6). Boston, MA, Little, Brown and Co, 1964. [Google Scholar]
- 24.Naing A, Veasey-Rodrigues H, Hong DS, et al. : Electrocardiograms (ECGs) in phase I anticancer drug development: The MD Anderson Cancer Center experience with 8518 ECGs. Ann Oncol 23:2960-2963, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hurria A, Dale W, Mooney M, et al. : Designing therapeutic clinical trials for older and frail adults with cancer: U13 conference recommendations. J Clin Oncol 32:2587-2594, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gerber DE, Laccetti AL, Xuan L, et al. : Impact of prior cancer on eligibility for lung cancer clinical trials. J Natl Cancer Inst 106:dju302, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laccetti AL, Pruitt SL, Xuan L, et al. : Effect of prior cancer on outcomes in advanced lung cancer: Implications for clinical trial eligibility and accrual. J Natl Cancer Inst 107:djv002, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laccetti AL, Pruitt SL, Xuan L, et al. : Prior cancer does not adversely affect survival in locally advanced lung cancer: A national SEER-Medicare analysis. Lung Cancer 98:106-113, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kelly K, Crowley J, Bunn PA, Jr, et al. : Randomized phase III trial of paclitaxel plus carboplatin versus vinorelbine plus cisplatin in the treatment of patients with advanced non small-cell lung cancer: A Southwest Oncology Group trial. J Clin Oncol 19:3210-3218, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Townsley CA, Selby R, Siu LL: Systematic review of barriers to the recruitment of older patients with cancer onto clinical trials. J Clin Oncol 23:3112-3124, 2005 [DOI] [PubMed] [Google Scholar]


