Abstract
Purpose
To investigate the efficacy of α-adrenergic agonist brimonidine either alone or combined with pirenzepine for inhibiting progressing myopia in guinea pig lens–myopia-induced models.
Methods
Thirty-six guinea pigs were randomly divided into six groups: Group A received 2% pirenzepine, Group B received 0.2% brimonidine, Group C received 0.1% brimonidine, Group D received 2% pirenzepine + 0.2% brimonidine, Group E received 2% pirenzepine + 0.1% brimonidine, and Group F received the medium. Myopia was induced in the right eyes of all guinea pigs using polymethyl methacrylate (PMMA) lenses for 3 weeks. Eye drops were administered accordingly. Intraocular pressure was measured every day. Refractive error and axial length measurements were performed once a week. The enucleated eyeballs were removed for hematoxylin and eosin (H&E) and Van Gieson (VG) staining at the end of the study.
Results
The lens-induced myopia model was established after 3 weeks. Treatment with 0.1% brimonidine alone and 0.2% brimonidine alone was capable of inhibiting progressing myopia, as shown by the better refractive error (p=0.024; p=0.006) and shorter axial length (p=0.005; p=0.0017). Treatment with 0.1% brimonidine and 0.2% brimonidine combined with 2% pirenzepine was also effective in suppressing progressing refractive error (p=0.016; p=0.0006) and axial length (p=0.017; p=0.0004). The thickness of the sclera was kept stable in all groups except group F; the sclera was much thinner in the lens-induced myopia eyes compared to the control eyes.
Conclusions
Treatment with 0.1% brimonidine alone and 0.2% brimonidine alone, as well as combined with 2% pirenzepine, was effective in inhibiting progressing myopia. The result indicates that intraocular pressure elevation is possibly a promising mechanism and potential treatment for progressing myopia.
Introduction
Myopia is a highly prevalent ocular condition, induced by a combination of environmental and genetic factors [1-3], that causes great economic burden [4-6]. The prevalence of myopia has increased by 23% in East Asia in recent decades, and a recent meta-analysis showed that 79.6% of that population by 18 years old has myopia [7]. The continuous elongation of the axis oculi and degenerative changes in pathologic myopia cause a series of complications, including posterior staphyloma, chorioretinal atrophy, and maculopathy which are all serious threats to visual acuity and quality of life [8].
The mechanism leading to the occurrence of myopia has been widely investigated, such as the remodeling of the sclera extracellular matrix [9,10], dysfunction of RPE cells [11], and retinal dopamine secretion [12]. Among different mechanisms, accommodation is considered a crucial factor for myopia development. Research has indicated that continuous near work induces transient myopia shift, and this near work–induced transient myopia shift lasts longer for patients who are developing myopia [13,14]. However, the mechanism linking accommodation to myopia progression has not been clarified. Intraocular pressure (IOP) elevation has been suggested as a possible factor contributing to accommodation inducing myopia. IOP is associated with the elongation of the axis oculi, and IOP has been reported as an independent plausible factor for myopia [15-17]. It has been indicated that IOP is significantly higher in the severely myopic group compared with the emmetrope group in observational studies [18-20]. A prospective study found no statistical difference in IOP between myopic and non-myopic children; however, IOP was higher in the myopic group following the onset of myopia than before its onset [21]. Another experiment showed that axial elongation and myopia were able to be caused by high IOP induced by intravitreal fluid injection [22]. Consistently, the axial length decreased significantly with the IOP lowering after a trabeculectomy compared with the axial length before the surgery, which provided direct evidence for the connection between IOP and axial length [23]. Our previous study indicated that IOP elevation with accommodation might be related to myopia progression [24]. Thus, the present study aimed to further explore whether IOP serves as an intermediate factor between accommodation and myopia development, and tests the efficacy of IOP-lowering drug administration for stabilizing progressing myopia.
The results of previous research on the efficacy of IOP-lowering drugs arresting myopia development were controversial [25,26]. Unfortunately, most of the supporting data suggesting that IOP-lowering drugs were effective in improving myopia development lacked a control group. A randomized clinical trial in which timolol was applied to Danish children indicated no significant difference in the development of myopia between the timolol group and the single vision group. Although well designed, the research was the only clinical trial with a limited sample and only one IOP-lowering drug, and the experiment failed to directly illustrate the role of IOP in accommodation-inducing myopia development. Brimonidine is an α2-adrenoceptor agonist that can effectively decrease IOP in patients with glaucoma via inhibition of cAMP-dependent formation of aqueous humor and promotion of aqueous outflow, which is a relatively new IOP-lowering medicine. The mechanism is different from timolol [27,28]. The guinea pig is an ideal animal model for myopia research because of its low cost and similar ocular structure to humans [29,30]. Deprivation and lens induction are two commonly applied methods used to induce myopia in guinea pigs [31,32], and they are effective in inducing myopia in guinea pigs [33]. Previous researchers investigated the efficacy of four distinct IOP-lowering drugs in guinea pigs. Among these four drugs, carteolol showed no statistically significant IOP-reducing effect, while brimonidine had the maximum treatment effect [34]. Both 0.1% brimonidine and 0.2% brimonidine are commonly used concentrations in clinical practice, and both concentrations are strong enough to activate the receptor [35,36]. Thus, 0.1% brimonidine and 0.2% brimonidine were selected for the present study. Pirenzepine is a selective M1 receptor antagonist that has been indicated to inhibit myopia development in animal experiments and in clinical trials [37-40]. In the present study, pirenzepine was applied as a positive control to examine whether brimonidine can have the same efficacy in inhibiting progressing myopia as pirenzepine does, and whether a combination of brimonidine and pirenzepine performs better than a single drug administration. In the present study, a lens-induced myopia model was performed on guinea pigs to investigate whether brimonidine alone or in combination with pirenzepine is effective in stabilizing progressing myopia.
Methods
Animals
Thirty-six 4-week-old pigmented guinea pigs (Cavia porcellus) were obtained from a local provider (Department of Laboratory Animal Science in Peking University Health Science Center, Beijing, China). The guinea pigs were raised in a temperature-controlled environment (25 °C) and a 12 h:12-h light-dark cycle. Regular chow and water were available ad libitum. The animals used in this study were handled in accordance with the ARVO Animal Statement for Use of Animals in Research, and the study was conducted under the regulation of the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1978). All experimental protocols were approved by the Animal Care and Use Committee of Peking University Health Science Center.
Method
The 36 guinea pigs were randomly divided into six groups: Group A received 2% pirenzepine group (n=6), Group B received 0.2% brimonidine (n=6), Group C received 0.1% brimonidine (n=6), Group D received 2% pirenzepine + 0.2% brimonidine (n=6), Group E received 2% pirenzepine + 0.1% brimonidine (n=6), and Group F received medium (n=6). All guinea pigs were treated with ophthalmic solutions for both eyes. Group A served as a positive control group, and Group F served as a negative control.
A lens-induced myopia (LIM) model was built in the right eyes of guinea pigs in all groups. A Velcro belt was shaped into a ring with a 0.8-cm diameter hole in the middle, and a −4 D polymethyl methacrylate (PMMA) lens (lens diameter 15 mm, optical diameter 10.5 mm, and base curve 9.64 mm) was inserted into the hole with the convex plane facing outward as described in many previous research studies [31,41]. A Velcro belt with a PMMA contact lens was glued in front of the right eyes of the guinea pigs with adhesive tape. The left eyes were used as self-controls without any lens. One drop of brimonidine (ALPHAGAN, Allergan, Dublin, Ireland) or pirenzepine (Sigma, Billerica, MA) was administered according to the grouping twice a day at 9 AM and 4 PM in each eye. The medium in Group F was the 0.9% normal saline. All eye drops were administered from the first day the model was established to the end of the experiment.
Ocular measurement
Ocular measurements were performed before and after myopia was induced. A solution of 1% tropicamide phenylephrine ophthalmic solution (Saten, Osaka, Japan) was administered on the conjunctiva sac of both eyes three times in 10 min intervals before refractive error was measured with retinoscopy optometry. The refractive error was measured and recorded as a spherical equivalent.
Before the axial length was measured, the guinea pigs were anaesthetized with a 2% pentobarbital sodium (30 mg/kg) intraperitoneal injection and 0.4% tetracaine topical anesthesia. The axial length from the corneal surface to the retina was manually measured ten times with A-scan ultrasonography (Maida Corporation, Tianjin, China) with accuracy at 0.01 mm. These measurements were performed on days 0, 7, 14, and 21 before and after the lenses were fixed.
Intraocular pressure was measured with rebound tonometry (Tono-Pen, Medtronic Solan, Minneapolis, MN) every day at 12 PM during the experiment.
H&E and VG staining
At the end of 3 weeks, the guinea pigs’ eyeballs were quickly enucleated after euthanasia was induced with an overdose of anesthesia (100 mg/kg pentobarbital sodium intraperitoneal injection). The tissue was fixed using 4% paraformaldehyde. Afterward, 7-μm-thin frozen sections were cut from the posterior pole regions. Hematoxylin and eosin (H&E) and Van Gieson (VG) staining was administered for light microscopy observation.
Statistical analysis
All values in Figure 1 were calculated as the mean ± standard error of the mean (SEM) for each week in Group F. All values in Figure 2 and Figure 3 were calculated as the difference between the right eye and the left eye for a single guinea pig and presented as the mean ± SEM for each group to eliminate the baseline difference. Image J (Bethesda, MD) was applied to analyze the H&E and VG staining results. Thickness was measured in the same three places (right, middle, and left sides) in each picture and calculated as the mean ± SEM.
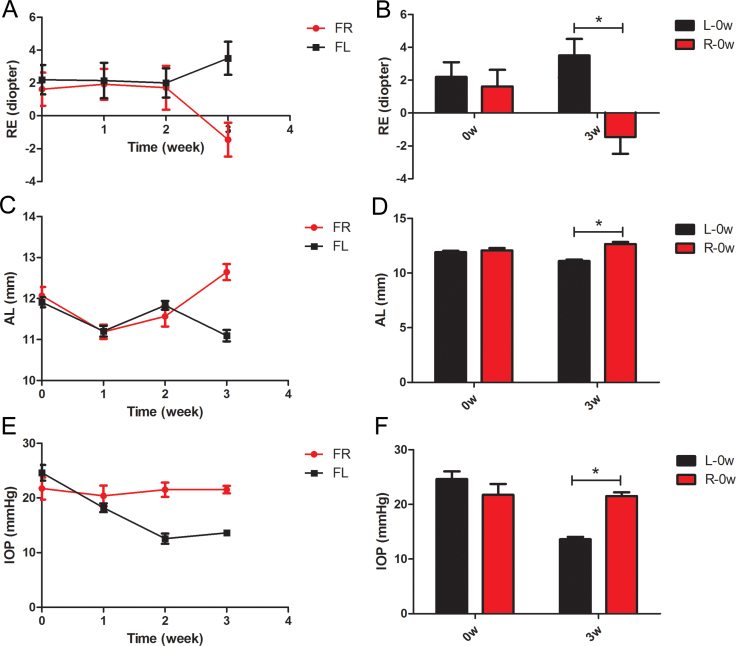
Figure 1.
The alternation of refractive error, axial length, and intraocular pressure in Group F during the experiment. A: Refractive error (RE) changing course in 3 weeks. B: Axial length (AL) changing course in 3 weeks. C: Intraocular pressure (IOP) changing course in 3 weeks. D: Histogram analysis the difference in the RE, AL, and IOP between the right and left eyes before and after the experiment. *p<0.05 (n=6). (L: left; R: right).
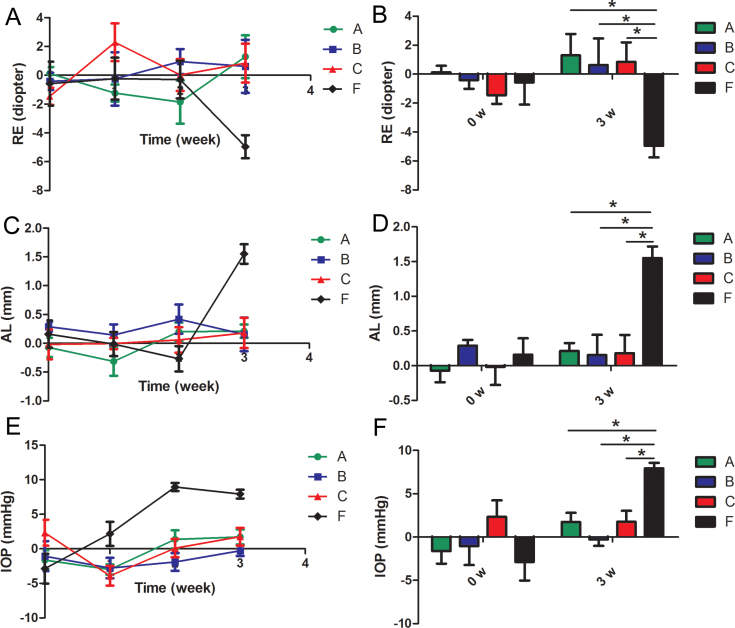
Figure 2.
Efficacy of brimonidine on inhibiting progressing myopia. The alternation of refractive error (RE), axial length (AL), and intraocular pressure (IOP) during experiment in groups A, B, C, and F is shown. All values were calculated as the difference between the right eye and the left eye. A: RE changing course in 3 weeks. B: Histogram analysis of the difference in RE before and after the experiment. C: AL changing course in 3 weeks. D: Histogram analysis of the difference in AL before and after the experiment. E: IOP changing course in 3 weeks. F: Histogram analysis of the difference in the IOP before and after the experiment. *p<0.05 (n=6). (Group A: 2% pirenzepine group; Group B: 0.2% brimonidine group; Group C: 0.1% brimonidine group; Group F: medium group).
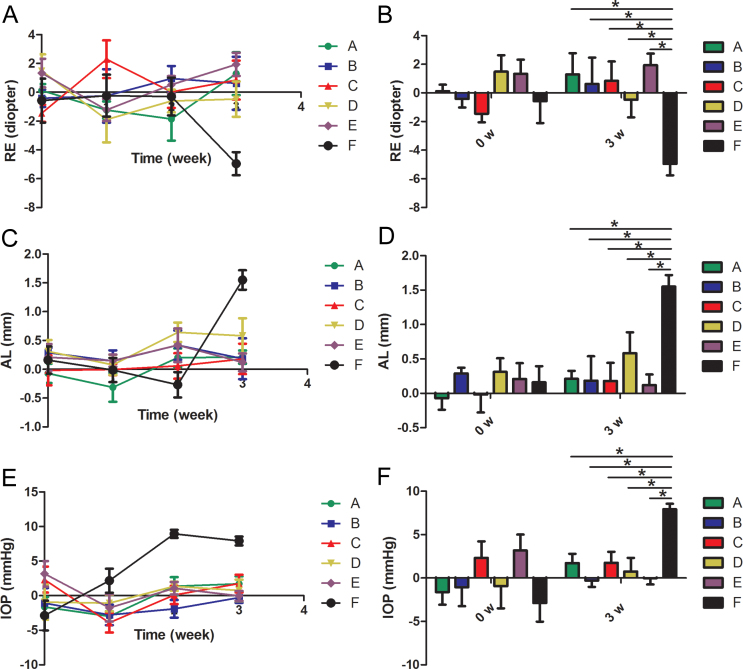
Figure 3.
Efficacy of brimonidine combined with pirenzepine on inhibiting progressing myopia. The difference in the alternation of refractive error (RE), axial length (AL), and intraocular pressure (IOP) during the experiment in groups A through F is shown. All values were calculated as the difference between the right eye and the left eye. A: RE changing course in 3 weeks. B: Histogram analysis of the difference in RE before and after the experiment. C: AL changing course in 3 weeks. D: Histogram analysis of the difference in AL before and after the experiment. E: IOP changing course in 3 weeks. F: Histogram analysis of the difference in the IOP before and after the experiment. *p<0.05 (n=6). (Group A: 2% pirenzepine group; Group B: 0.2% brimonidine group; Group C: 0.1% brimonidine group; Group D: 2% pirenzepine + 0.2% brimonidine group; Group E: 2% pirenzepine + 0.1% brimonidine group; Group F: medium group).
SPSS 20 (IBM) software was used for the data analysis. The Student t test was used to compare the data between two groups. One-way ANOVA (ANOVA) was used to compare the data between multiple groups, and least significant difference (LSD) was used for the post hoc comparison. Linear regression was performed to explore the IOP alternation tendency in different groups. The line charts and histograms in Figure 1, Figure 2, Figure 3, and Figure 4 were composed in GraphPad Prism (La Jolla, CA) software according to the Student t test and one-way ANOVA, and are shown as mean ± SEM for each column.
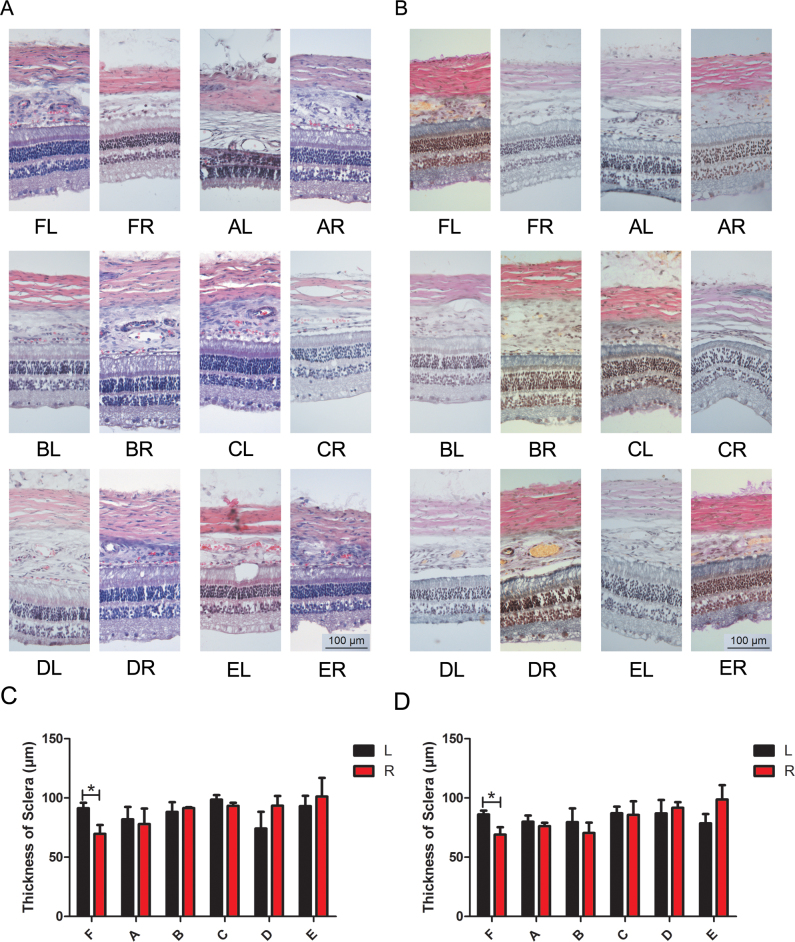
Figure 4.
Efficacy of brimonidine alone or combined with pirenzepine on inhibiting the thinning of the sclera. The hematoxylin and eosin (H&E) stains, the Van Gieson (VG) stains, and the thickness of the sclera of each group are shown. A: H&E stains of each group. B: VG stains of each group. C: Thickness of the sclera in each group according to the H&E stains. D: Thickness of the sclera in each group according to the VG stain. *p<0.05 (n=6). (A = Group A 2% pirenzepine group; B = Group B 0.2% brimonidine group; C = Group C 0.1% brimonidine group; D = Group D 2% pirenzepine + 0.2% brimonidine group; E = Group E 2% pirenzepine + 0.1% brimonidine group; F = Group F medium group; L: left; R: right).
Results
Establishment of the lens-induced myopia model
The refractive error (RE), axial length (AL), and IOP measurements before and after myopia was induced at 1, 2, and 3 weeks for Group F are shown in Figure 1 and summarized in Table 1. As shown in Figure 1A and 1C, the RE gradually decreased, with the AL increasing, in the lens-induced myopia eyes compared with the control eyes in Group F. As myopia progressed, the IOP in the lens-induced myopia eyes gradually became higher than in the control eyes, as shown in Figure 1E. After the induction, −1.45±2.3 D myopia was induced, the axial length increased by 1.55±0.41 mm, and the intraocular pressure was 7.92±1.43 mmHg higher in the lens-induced myopia eyes compared to the control eyes. Statistical significance was found in refractive error (p=0.0088), axial length (p<0.0001), and IOP (p<0.0001) at the 21st day, but not at the beginning of the experiment in refractive error (p=0.68), axial length (p=0.53), or IOP (p=0.27) between the lens-induced eyes and the control eyes, as shown in the summarized histogram in Figure 1B,D,F.
Table 1. Axial length (AL), refractive error (RE) and intraocular pressure (IOP) before and after myopia induction at the time of 1 week, 2 weeks and 3 weeks.
| Group | Eye | Baseline (mm) | 1 week (mm) | 2 weeks (mm) | 3 weeks (mm) |
|---|---|---|---|---|---|
|
AL |
|
|
|
|
|
| A |
Right |
10.63±0.34 |
11.41±0.65 |
11.82±0.51* |
12.01±0.56* |
| |
left |
10.70±0.60 |
11.73±0.28* |
11.62±0.41 |
11.80±0.48* |
| B |
Right |
11.33±0.42 |
11.57±0.30 |
11.63±0.68 |
11.81±0.72 |
| |
left |
11.05±0.58 |
11.43±0.46 |
11.21±0.39 |
11.62±0.2 |
| C |
Right |
11.28±0.31 |
11.46±0.44 |
11.54±0.30 |
11.57±0.24 |
| |
left |
11.30±0.60 |
11.47±0.30 |
11.48±0.51 |
11.39±0.37 |
| D |
Right |
11.79±0.57 |
11.32±0.40 |
12.09±0.72# |
11.99±0.39# |
| |
left |
11.48±0.49 |
11.24±0.39 |
11.45±0.34 |
11.41±0.38 |
| E |
Right |
12.17±0.84 |
11.51±0.48 |
12.12±0.72 |
12.19±0.33 |
| |
left |
11.96±0.59 |
11.36±0.37 |
11.71±0.42 |
12.07±0.18 |
| F |
Right |
12.07±0.52 |
11.19±0.43 |
11.57±0.61 |
12.65±0.48# |
| |
left |
11.91±0.30 |
11.20±0.33 |
11.70±0.46 |
11.10±0.34 |
|
RE |
|
|
|
|
|
| A |
Right |
2.29±2.59 |
2.40±2.58 |
0.77±4.02 |
1.29±2.81 |
| |
left |
2.17±2.01 |
3.63±2.28 |
2.63±0.46 |
−0.01±1.88 |
| B |
Right |
2.67±1.57 |
3.13±2.45 |
4.11±2.03 |
1.55±2.34 |
| |
left |
3.08±2.54 |
3.38±2.60 |
3.15±1.64 |
0.93±2.47 |
| C |
Right |
1.69±2.33 |
2.86±5.00 |
3.09±2.50 |
2.69±0.74 |
| |
left |
3.15±2.55 |
0.52±2.21 |
3.07±2.06 |
1.85±3.18 |
| D |
Right |
3.37±1.78 |
1.59±1.36* |
2.75±2.33 |
1.35±2.04 |
| |
left |
1.88±2.69 |
3.48±3.79 |
2.48±1.40 |
1.83±0.84 |
| E |
Right |
1.38±2.34 |
1.84±1.83 |
2.75±1.88 |
2.63±1.20 |
| |
left |
0.04±1.49 |
3.07±1.47 |
2.49±1.59* |
1.66±1.53 |
| F |
Right |
1.62±2.47 |
1.92±2.31 |
1.71±3.27 |
−1.45±2.30 |
| |
left |
2.20±2.20 |
2.15±2.64 |
2.00±2.19 |
3.23±2.12 |
|
IOP |
|
|
|
|
|
| A |
Right |
15.38±5.24 |
15.72±3.01 |
14.15±1.76 |
13.60±2.56 |
| |
left |
17.02±5.81 |
18.72±2.18 |
12.78±2.68 |
11.88±2.94# |
| B |
Right |
17.28±4.31 |
16.65±0.74 |
13.05±1.78# |
13.43±1.54# |
| |
left |
18.35±3.56 |
19.43±3.98 |
12.1±2.45 |
13.72±2.13 |
| C |
Right |
17.03±4.48 |
14.15±1.59 |
13.05±2.43 |
13.18±1.43 |
| |
left |
14.7±2.12 |
18.05±2.81 |
12.95±1.91 |
11.43±1.28 |
| D |
Right |
16.57±3.77 |
18.52±2.69 |
14.2±2.06# |
13.33±2.48 |
| |
left |
17.50±3.83 |
19.60±4.66 |
12.83±1.44 |
12.57±1.55 |
| E |
Right |
24.6±3.94 |
20.07±2.00 |
12.95±0.92*# |
13.18±2.47* |
| |
left |
21.43±4.77 |
21.83±3.50 |
11.88±1.79 |
13.20±1.91 |
| F |
Right |
21.73±4.93 |
20.38±4.62 |
21.5±3.22 |
21.52±1.55 |
| left | 24.60±3.84 | 19.3±3.59 | 12.55±2.33*# | 13.60±1.02*#ψ |
The axial length (AL), refractive error (RE) and intraocular pressure (IOP) at baseline and after myopia induction at the time of 1 week, 2 weeks and 3 weeks shown in Mean±SEM for right and left eye separately for all groups. *p<0.05 versus baseline for the same eye and group; #p<0.05 versus 1 week for the same eye and group. ψp<0.05 versus the other eye in the same group (n=6).
Brimonidine alone attenuated myopia effectively
To evaluate the efficacy of brimonidine in attenuating progressing myopia, 0.2% or 0.1% brimonidine was administered to the guinea pigs in groups B and C twice a day. The administration of 2% pirenzepine was used as a positive control. The RE, AL, and IOP measurement data before and after myopia was induced at 1, 2, and 3 weeks for groups A, B, C, and F are shown in Figure 2 in the form of the difference between two eyes. The data for the RE, AL, and IOP measurements for the right and left eyes, respectively, for each group are summarized in Table 1.
As shown in Figure 2A,C, the RE and AL were stable in groups B and C compared with Group F during the experiment. A summarizing histogram in Figure 2B,D shows statistically significantly more desired outcomes in the RE (p=0.024; p=0.006) and AL (p=0.005; p=0.0017) in groups B and C compared with Group F at the end, without any difference in the RE (p=0.92; p=0.6) and AL (p=0.65; p=0.62) from the beginning of the experiment. No statistically significant difference was found between groups B and C and the positive control Group A in the RE (p=0.78; p=0.84) or AL (p=0.94; p=0.9), respectively. Consistent with the above, the IOP was stable and statistically significantly lower in groups B and C compared with Group F at the end (p<0.0001; p=0.0024), which showed no difference from the beginning of the experiment (p=0.27; p=0.1). Different concentrations of brimonidine manifested no difference in its efficacy in inhibiting myopia progression in the RE (p=0.93), AL (p=0.99), and IOP (p=0.18).
Brimonidine with pirenzepine attenuated myopia effectively
To investigate whether a combination of brimonidine and pirenzepine can effectively control progressing myopia, 0.2% or 0.1% brimonidine with 2% pirenzepine was administered to the guinea pigs in groups D and E twice a day. The RE, AL, and IOP measurements before and after myopia was induced at 1, 2, and 3 weeks for all groups are shown in Figure 3 in the form of the difference between two eyes. The RE, AL, and IOP measurement data for the right and left eyes, respectively, for each group are summarized in Table 1.
The combination of brimonidine and pirenzepine was capable of stabilizing the RE and AL compared with Group F during the lens-induced process, as shown in Figure 3A,C. A histogram summarizing the results in Figure 3B,D shows statistically significant improvement in the RE (p=0.016; p=0.0006) and AL (p=0.017; p=0.0004) in groups D and E compared with Group F after lens-induced myopia, which did not show much difference from the starting point of the experiment in the RE (p=0.31; p=0.32) and AL (p=0.63; p=0.89), respectively. The IOP was also kept stable and statistically significantly lower in groups D and E compared with Group F at the end of experiment (p=0.0035; p<0.0001), which did not show a difference from the beginning of the experiment (p=0.058; p=0.59).
Additionally, a combination of 0.2% or 0.1% brimonidine and 2% pirenzepine showed no statistical difference compared with 0.2% or 0.1% brimonidine administered alone in RE (p=0.32; p=0.51), AL (p=0.42; p=0.85), or IOP (p=0.56; p=0.27); there was also no statistical difference using 2% pirenzepine alone in RE (p=0.4; p=0.75), AL (p=0.25; p=0.64) or IOP (p=0.63; p=0.27), respectively.
Linear regression for IOP alternation tendency
To explore the changing cascade of IOP in different groups during lens-induced myopia establishment, linear regression was done including IOP data for 21 days for each eye independently. As shown in Table 2, both eyes in all groups were capable of linear fitting except the right eye of Group F. The regression coefficient was below zero for each line, and the absolute value of the regression coefficient for the left eye was larger than that for the right eye for all groups.
Table 2. The result of linear regression between intraocular pressure (IOP) and time.
| Group | Eye | R2 | Regression coefficient | Constant | P |
|---|---|---|---|---|---|
| A |
Right |
0.239 |
−0.369 |
20.639 |
<0.0001 |
| |
Left |
0.366 |
−0.53 |
22.237 |
<0.0001 |
| B |
Right |
0.344 |
−0.373 |
19.806 |
<0.0001 |
| |
Left |
0.396 |
−0.484 |
21.229 |
<0.0001 |
| C |
Right |
0.324 |
−0.398 |
19.685 |
<0.0001 |
| |
Left |
0.479 |
−0.567 |
21.539 |
<0.0001 |
| D |
Right |
0.431 |
−0.469 |
20.981 |
<0.0001 |
| |
Left |
0.425 |
−0.553 |
22.145 |
<0.0001 |
| E |
Right |
0.462 |
−0.574 |
22.27 |
<0.0001 |
| |
Left |
0.541 |
−0.679 |
22.96 |
<0.0001 |
| F |
Right |
0.01 |
−0.025 |
19.995 |
0.682 |
| Left | 0.506 | −0.644 | 22.704 | <0.0001 |
The result of linear regression between intraocular pressure (IOP) and time for right and left eye separately for all groups was shown. The square of correlation coefficient (R2), regression coefficient, constant for the regression formula and p value was demonstrated in the table.
Brimonidine alone or with pirenzepine attenuated the thinning of the sclera
The characteristic structural alternation in myopia is the elongation of the AL associated with the thinning of the sclera. To assess whether brimonidine administered alone or combined with pirenzepine was effective in blocking the thinning of the sclera, H&E and VG staining was performed on frozen sections from the posterior pole of the enucleated eyeballs. The images of H&E and VG staining are shown in Figure 4A,B. The statistical results are shown in Figure 4C,D, respectively, and summarized in Table 3.
Table 3. The thickness of sclera from HE (Haematoxylin Eosin) and VG (Van Gieson) stain.
| Group | Eye | HE stain (μm) | VG stain (μm) |
|---|---|---|---|
| A |
Right |
77.99±13.01 |
76.20±2.87 |
| |
Left |
81.93±10.52 |
79.89±5.32 |
| B |
Right |
91.41±1.6 |
70.47±8.71 |
| |
Left |
88.22±8.16 |
79.53±11.66 |
| C |
Right |
93.35±2.59 |
85.69±11.51 |
| |
Left |
98.59±3.89 |
87.07±5.58 |
| D |
Right |
93.48±8.15 |
91.67±4.72 |
| |
Left |
74.13±14.23 |
86.96±11.32 |
| E |
Right |
101.22±15.66 |
98.70±12.14 |
| |
Left |
93.12±8.64 |
78.53±7.86 |
| F |
Right |
69.75±7.45 |
69.02±6.35 |
| Left | 91.31±4.68ψ | 86.00±3.32ψ |
The thickness of sclera from HE and VG stain for right and left eye separately for all groups was shown Mean±SEM ψp<0.05 versus the other eye in the same group (n=6).
The sclera of the lens-induced myopia right eyes was statistically significantly thinner than the sclera in left eyes without treatment in Group F (administered medium only), as presented in the H&E (p=0.049) and VG (p=0.049) stains. However, the H&E and VG stains indicated that the thickness of the sclera remained relatively stable for comparing the two eyes in a certain guinea pig to other groups.
Discussion
The main finding of the research is that lens-induced myopia was established by the elongation of the AL and the increase in IOP. Additionally, administering brimonidine alone or combined with pirenzepine was effective in stabilizing progressing myopia.
The occurrence of myopia involves complex mechanisms that have not yet been clarified. IOP was discovered to be relevant to myopia regardless of family history, age, and other factors in a cross-sectional survey decades ago [42]. However, the exact relationship between IOP and myopia is controversial. Several studies found no statistical difference in IOP between groups of persons with emmetropia and persons with myopia, or between groups with different levels of myopia [43,44]. On the contrary, other studies showed that IOP was statistically significantly higher in the severe myopia group compared with the emmetrope group and associated with AL [15,18,20]. Despite contradictory results of different research, a prospective study indicated that IOP was higher in the myopia group following the onset of myopia than it was before onset; nevertheless, no differences in IOP between myopic and non-myopic children were found [21]. The results indicate that IOP is probably relevant to myopia progressing. Following onset, children with higher IOP had a higher progressing rate of myopia [45]. Our previous study demonstrated that accommodation could induce a transient IOP elevation in the group with progressing myopia, with the anterior chamber depth decreasing and the anterior chamber angle narrowing simultaneously, but not in the group with emmetropia [24]. The consequences suggested that IOP might serve as an intermediate factor for myopia establishment and development. Our study was designed and conducted accordingly. A lens-induced myopia model in guinea pigs was established in 3 weeks. The decrement in the RE and the increase in the AL were progressing, accompanied by higher IOP, in the experimental eyes compared with the control eyes. The AL in Group F at 1 week was seemingly lower than the AL at the beginning of the experiment, but no statistically significant differences were observed between the baseline and 1 week for both eyes respectively (p=0.062; p=0.14). Therefore, it might be attributed to errors in measuring AL using ocular A-scan ultrasound. We found the IOP levels remained at a relatively high level during the experiment in the myopia-induced eyes (the right eyes in Group F), while it decreased statistically significantly in the other eyes due to the use of IOP-lowering medication. Additionally, after linear regression, the absolute value of the regression coefficient for the left eyes was larger than that for the right eyes for all groups, which further manifests that accommodation-induced myopia development is associated with elevation in IOP. Interestingly, except the right eyes of Group F, the IOP showed a decreasing tendency during the experiment, even for the left eyes of Group F. The IOP changing mode in this experiment is different from that in other research, which showed a slight increase in IOP during first few months of the guinea pigs’ lifespan [46]. However, the IOP measurement in the previous research was performed by rebound tonometry calibrated for rats based on the comparatively thin cornea thickness for rodents, which is about 20 mmHg. Another study measured the IOP of 12-week-old guinea pigs and obtained similar IOP values to ours after 1 week [34]. Combined with the present analysis, this indicates that the linear decrease alternation of IOP might be due to extra stimulus at the beginning of this experiment, ocular measurements, and wear on lenses, which resulted in relatively high IOP levels in all groups. The extraordinary high IOP rapidly returned to normal levels after 1-week adaptation for the left eyes of Group F. The IOP decrease in other groups might be due to a combination of stimulus adaptation and IOP-lowering drug effects. Taken together, the research possibly revealed that IOP elevation is a promising mechanism in myopia development and progression, which indicated a potential pathway stabilizing myopia progressing through IOP stabilizing.
Previous research indicated that high IOP induced by fluid injection is able to cause axial elongation and myopia in chicks [22]. Additionally, axial length decreased statistically significantly after IOP-lowering trabeculectomy compared with the axial length before the surgery [23]. These findings provided direct evidence for the positive relationship between IOP and AL. Thus, blocking progressing IOP elevation is probably effective in stabilizing the development of myopia and elongation of AL. The present study identified that brimonidine administration alone was capable of inhibiting lens-induced myopia, which was indicated by the stabilized refractive error and axial length compared to the control group with medium eye drops. It was also demonstrated that brimonidine administered alone achieved a similar efficacy with pirenzepine in attenuating progressing myopia. Pirenzepine is a selective M1 receptor antagonist [47]. It was reported that pirenzepine inhibits the progression of myopia and AL elongation in animal experiments and clinical trials [38-40]. Pirenzepine administration was used as a positive control compared to other groups in the current study, demonstrating the effect of this drug in restraining progressing myopia. Aside from the refractive error, pirenzepine demonstrated efficacy in preventing an increase in IOP in Group A. Guinea pigs have thick lenses compared with humans and other primates [48]. Pirenzepine partly shares the mydriasis effect with other muscarinic antagonists. Research has indicated that 2% pirenzepine induces an increase in pupil size and nearly complete cycloplegia in rhesus monkeys [49]. Thus, the effect of pirenzepine in stabilizing IOP was probably due to the mydriasis effect on thick lenses, eliminating pupil block and promoting flowing aqueous fluid.
Brimonidine is an effective α2-adrenoceptor agonist that is able to decrease IOP, inhibiting the formation of aqueous humor and promotion of aqueous outflow [27]. The effect of brimonidine on inhibiting myopia is probably achieved by precluding the progressing elongation of AL associated with IOP elevation. Previous investigation showed that the dynamic alternation of AL was associated with the corresponding change in IOP [16], and elevation of IOP induced by suction cup was associated with the elongation of the AL statistically significantly in humans [17]. These findings indicate that the stabilization of IOP is probably a key factor in inhibiting the efficacy of brimonidine in myopia.
It has been reported that myopia and AL elongation are closely associated with scleral extracellular matrix remodeling for many years [9]. A decrease in glycosaminoglycan and collagen content was found in human samples of patients with myopia [50]. In the present study, the thinning of the sclera was observed after 3 weeks of lens-induced myopia in the group administered medium alone, which demonstrated extracellular matrix remodeling during progressing myopia; however, the sclera was not thinner in the other groups after myopia induction. The results indicate that brimonidine administration alone or combined with pirenzepine exerts a preventive effect on the thinning sclera contrastively, which inhibits the extracellular matrix remodeling process. As commonly known, pathological myopia involves the continuous elongation of axis length and the thinning of choroid and sclera, leading to a series of complications including posterior staphyloma, chorioretinal atrophy, and maculopathy. According to the present research, brimonidine prevented the thinning of sclera, which was possibly effective in preventing complications of pathological myopia.
The exact mechanism of how brimonidine inhibits extracellular matrix remodeling observed in the present study is unknown; however, some indication may lie in newly found neuroprotective mechanisms [51]. Brimonidine might restrain lens-induced myopia in IOP-independent mechanisms mediating the sclera’s extracellular matrix. Recent studies showed that brimonidine could provide neuroprotective effects in a series of different neuropathies in different animal models [51,52]. Further investigations indicated that brimonidine conducts its neuroprotective efficacy via enhancing the expression of neurotropic factors, such as basic fibroblast growth factor (bFGF) [53]. Interestingly, bFGF activity was reported statistically significantly downregulated in scleral desmocytes in the anterior and posterior poles of the sclera after myopia was induced in guinea pig models [54]. Additionally, peribulbar bFGF injection precluded the formation and development of myopia compared with the control eyes in lens-induced myopia in guinea pigs [55]. Together, brimonidine might inhibit lens-induced myopia via promoting the expression of bFGF, and thus affect the remodeling of the sclera. Future studies need to reveal the effect of adrenergic receptors on sclera and whether brimonidine is effective in affecting the sclera extracellular matrix metabolism through bFGF or other cytokines to confirm the present speculation.
Additionally, various concentrations of brimonidine were applied in the present study. The concentrations were chosen according to the clinical practice as stated in the introduction. No statistically significant difference was shown between the two brimonidine concentrations for changes in the AL, RE, and IOP. Previous research revealed that the vitreous concentration of brimonidine when 0.2% brimonidine was used was higher than when 0.1% brimonidine was used [35,36]. However, the vitreous concentration when 0.1% brimonidine was used was 2 times higher than 2 nM, which was also demonstrated to be enough to activate neuroprotective a-2 receptors in animal retinas [36]. This may partially explain why different concentrations of brimonidine exert the same effect on myopia progressing inhibition. Lower concentrations of brimonidine should be examined in future investigations.
Brimonidine combined with pirenzepine also showed statistically significant efficacy stabilizing progressing myopia and axial length elongation in the present study. No statistically significant difference was indicated among brimonidine administration alone, pirenzepine administration alone, or a combination of the two. Previous research on the mechanism revealed that pirenzepine regulated the balance of matrix metalloproteinase-2 (MMP-2) and tissue inhibitor of metalloproteinases 2 (TIMP2) expression through the M receptor on the sclera directly, or through mediated neurotrophic factors, such as transforming growth factor beta (TGFβ), and bFGF, to affect scleral remodeling [37,38,56,57]. The speculated mechanism for brimonidine inhibiting myopia was discussed above, which might involve IOP-dependent and -independent pathways. Previous research demonstrated that brimonidine also affects the function of TGFβ on fibroblasts [58]. Thus, both drugs are associated with neurotrophic factors, which means that there might be an intersection between the two drugs to inhibit progressing myopia. In other words, the pathways of the two drugs might overlap; thus, no obvious synergistic effect was observed in the present study. As single-drug administration was enough to stabilize the formation and development of myopia, the combination of drug administration showed no preponderance taking side effects into consideration. Future study is needed on the connection between these two drugs.
In summary, high IOP was observed in lens-induced guinea myopia models, and IOP-lowering drug brimonidine alone and combined with pirenzepine was effective in inhibiting progressing myopia, axial elongation, and thinning of sclera in lens-induced guinea pig experimental myopia. Consequently, transient or continuous IOP elevation is probably a promising mechanism for myopia progressing and a potential key in myopia treatment. The IOP-lowering drug brimonidine apparently inhibits progressing myopia through IOP-dependent or -independent pathways. Future research should focus more on the specific mechanisms of IOP-lowering drugs in myopia inhibition.
Acknowledgments
This research was supported by Beijing Municipal Science and Technology Commission (Z141107002514042).
References
- 1.Ramamurthy D, Lin Chua SY, Saw SM. A review of environmental risk factors for myopia during early life, childhood and adolescence. Clin Exp Optom. 2015;98:497–506. doi: 10.1111/cxo.12346. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Q. Genetics of Refraction and Myopia. Prog Mol Biol Transl Sci. 2015;134:269–79. doi: 10.1016/bs.pmbts.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 3.Hysi PG, Wojciechowski R, Rahi JS, Hammond CJ. Genome-wide association studies of refractive error and myopia, lessons learned, and implications for the future. Invest Ophthalmol Vis Sci. 2014;55:3344–51. doi: 10.1167/iovs.14-14149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foster PJ, Jiang Y. Epidemiology of myopia. Eye (Lond) 2014;28:202–8. doi: 10.1038/eye.2013.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rein DB, Zhang P, Wirth KE, Lee PP, Hoerger TJ, McCall N, Klein R, Tielsch JM, Vijan S, Saaddine J. The economic burden of major adult visual disorders in the United States. Arch Ophthalmol. 2006;124:1754–60. doi: 10.1001/archopht.124.12.1754. [DOI] [PubMed] [Google Scholar]
- 6.Vitale S, Cotch MF, Sperduto R, Ellwein L. Costs of refractive correction of distance vision impairment in the United States, 1999–2002. Ophthalmology. 2006;113:2163–70. doi: 10.1016/j.ophtha.2006.06.033. [DOI] [PubMed] [Google Scholar]
- 7.Rudnicka AR, Kapetanakis VV, Wathern AK, Logan NS, Gilmartin B, Whincup PH, Cook DG, Owen CG. Global variations and time trends in the prevalence of childhood myopia, a systematic review and quantitative meta-analysis: implications for aetiology and early prevention. Br J Ophthalmol. 2016 doi: 10.1136/bjophthalmol-2015-307724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verkicharla PK, Ohno-Matsui K, Saw SM. Current and predicted demographics of high myopia and an update of its associated pathological changes. Ophthalmic Physiol Opt. 2015;35:465–75. doi: 10.1111/opo.12238. [DOI] [PubMed] [Google Scholar]
- 9.Harper AR, Summers JA. The dynamic sclera: extracellular matrix remodeling in normal ocular growth and myopia development. Exp Eye Res. 2015;133:100–11. doi: 10.1016/j.exer.2014.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Metlapally R, Wildsoet CF. Scleral Mechanisms Underlying Ocular Growth and Myopia. Prog Mol Biol Transl Sci. 2015;134:241–8. doi: 10.1016/bs.pmbts.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y, Wildsoet CF. RPE and Choroid Mechanisms Underlying Ocular Growth and Myopia. Prog Mol Biol Transl Sci. 2015;134:221–40. doi: 10.1016/bs.pmbts.2015.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feldkaemper M, Schaeffel F. An updated view on the role of dopamine in myopia. Exp Eye Res. 2013;114:106–19. doi: 10.1016/j.exer.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 13.Ehrlich DL. Near vision stress: vergence adaptation and accommodative fatigue. Ophthalmic Physiol Opt. 1987;7:353–7. [PubMed] [Google Scholar]
- 14.Vera-Diaz FA, Strang NC, Winn B. Nearwork induced transient myopia during myopia progression. Curr Eye Res. 2002;24:289–95. doi: 10.1076/ceyr.24.4.289.8418. [DOI] [PubMed] [Google Scholar]
- 15.Tokoro T, Funata M, Akazawa Y. Influence of intraocular pressure on axial elongation. J Ocul Pharmacol. 1990;6:285–91. doi: 10.1089/jop.1990.6.285. [DOI] [PubMed] [Google Scholar]
- 16.Read SA, Collins MJ, Iskander DR. Diurnal variation of axial length, intraocular pressure, and anterior eye biometrics. Invest Ophthalmol Vis Sci. 2008;49:2911–8. doi: 10.1167/iovs.08-1833. [DOI] [PubMed] [Google Scholar]
- 17.Leydolt C, Findl O, Drexler W. Effects of change in intraocular pressure on axial eye length and lens position. Eye (Lond) 2008;22:657–61. doi: 10.1038/sj.eye.6702709. [DOI] [PubMed] [Google Scholar]
- 18.Shen M, Fan F, Xue A, Wang J, Zhou X, Lu F. Biomechanical properties of the cornea in high myopia. Vision Res. 2008;48:2167–71. doi: 10.1016/j.visres.2008.06.020. [DOI] [PubMed] [Google Scholar]
- 19.Oner V, Tas M, Ozkaya E, Oruc Y. Effect of pathological myopia on biomechanical properties: a study by ocular response analyzer. Int J Ophthalmol. 2015;8:365–8. doi: 10.3980/J.ISSN.2222-3959.2015.02.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nomura H, Ando F, Niino N, Shimokata H, Miyake Y. The relationship between age and intraocular pressure in a Japanese population: the influence of central corneal thickness. Curr Eye Res. 2002;24:81–5. doi: 10.1076/ceyr.24.2.81.8161. [DOI] [PubMed] [Google Scholar]
- 21.Edwards MH, Brown B.IOP in myopic children: the relationship between increases in IOP and the development of myopiaOphthalmic Physiol Opt 199616243–6. [PubMed] [Google Scholar]
- 22.Genest R, Chandrashekar N, Irving E. The effect of intraocular pressure on chick eye geometry and its application to myopia. Acta Bioeng Biomech. 2012;14:3–8. [PubMed] [Google Scholar]
- 23.Saeedi O, Pillar A, Jefferys J, Arora K, Friedman D, Quigley H. Change in choroidal thickness and axial length with change in intraocular pressure after trabeculectomy. Br J Ophthalmol. 2014;98:976–9. doi: 10.1136/bjophthalmol-2013-304433. [DOI] [PubMed] [Google Scholar]
- 24.Yan L, Huibin L, Xuemin L. Accommodation-induced intraocular pressure changes in progressing myopes and emmetropes. Eye (Lond) 2014;28:1334–40. doi: 10.1038/eye.2014.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saw SM, Gazzard G, Au Eong KG, Tan DT. Myopia: attempts to arrest progression. Br J Ophthalmol. 2002;86:1306–11. doi: 10.1136/bjo.86.11.1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ganesan P, Wildsoet CF. Pharmaceutical intervention for myopia control. Expert Rev Ophthalmol. 2010;5:759–87. doi: 10.1586/eop.10.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adkins JC, Balfour JA. Brimonidine. A review of its pharmacological properties and clinical potential in the management of open-angle glaucoma and ocular hypertension. Drugs Aging. 1998;12:225–41. doi: 10.2165/00002512-199812030-00005. [DOI] [PubMed] [Google Scholar]
- 28.Akman A, Cetinkaya A, Akova YA, Ertan A. Comparison of additional intraocular pressure-lowering effects of latanoprost vs brimonidine in primary open-angle glaucoma patients with intraocular pressure uncontrolled by timolol-dorzolamide combination. Eye (Lond) 2005;19:145–51. doi: 10.1038/sj.eye.6701428. [DOI] [PubMed] [Google Scholar]
- 29.Schaeffel F, Feldkaemper M. Animal models in myopia research. Clin Exp Optom. 2015;98:507–17. doi: 10.1111/cxo.12312. [DOI] [PubMed] [Google Scholar]
- 30.Xiao H, Fan ZY, Tian XD, Xu YC. Comparison of form-deprived myopia and lens-induced myopia in guinea pigs. Int J Ophthalmol. 2014;7:245–50. doi: 10.3980/j.issn.2222-3959.2014.02.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dong F, Zhi Z, Pan M, Xie R, Qin X, Lu R, Mao X, Chen JF, Willcox MD, Qu J, Zhou X. Inhibition of experimental myopia by a dopamine agonist: different effectiveness between form deprivation and hyperopic defocus in guinea pigs. Mol Vis. 2011;17:2824–34. [PMC free article] [PubMed] [Google Scholar]
- 32.Howlett MH, McFadden SA. Spectacle lens compensation in the pigmented guinea pig. Vision Res. 2009;49:219–27. doi: 10.1016/j.visres.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 33.Jiang L, Long K, Schaeffel F, Zhang S, Zhou X, Lu F, Qu J. Disruption of emmetropization and high susceptibility to deprivation myopia in albino guinea pigs. Invest Ophthalmol Vis Sci. 2011;52:6124–32. doi: 10.1167/iovs.10-7088. [DOI] [PubMed] [Google Scholar]
- 34.Di Y, Luo XM, Qiao T, Lu N. Intraocular pressure with rebound tonometry and effects of topical intraocular pressure reducing medications in guinea pigs. Int J Ophthalmol. 2017;10:186–90. doi: 10.18240/ijo.2017.02.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Acheampong AA, Shackleton M, John B, Burke J, Wheeler L, Tang-Liu D. Distribution of brimonidine into anterior and posterior tissues of monkey, rabbit, and rat eyes. Drug Metab Dispos. 2002;30:421–9. doi: 10.1124/dmd.30.4.421. [DOI] [PubMed] [Google Scholar]
- 36.Takamura Y, Tomomatsu T, Matsumura T, Takihara Y, Kozai S, Arimura S, Yokota S, Inatani M. Vitreous and aqueous concentrations of brimonidine following topical application of brimonidine tartrate 0.1% ophthalmic solution in humans. J Ocul Pharmacol Ther. 2015;31:282–5. doi: 10.1089/jop.2015.0003. [DOI] [PubMed] [Google Scholar]
- 37.Ji X, Zhang J, Wang Y, Sun H, Jia P. Mechanism of Smad 3 signaling pathway and connective tissue growth factor in the inhibition of form deprivation myopia by pirenzepine. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2009;34:349–55. [PubMed] [Google Scholar]
- 38.Qian L, Zhao H, Li X, Yin J, Tang W, Chen P, Wang Q, Zhang J. Pirenzepine Inhibits Myopia in Guinea Pig Model by Regulating the Balance of MMP-2 and TIMP-2 Expression and Increased Tyrosine Hydroxylase Levels. Cell Biochem Biophys. 2015;71:1373–8. doi: 10.1007/s12013-014-0359-9. [DOI] [PubMed] [Google Scholar]
- 39.Tan DT, Lam DS, Chua WH, Shu-Ping DF, Crockett RS. One-year multicenter, double-masked, placebo-controlled, parallel safety and efficacy study of 2% pirenzepine ophthalmic gel in children with myopia. Ophthalmology. 2005;112:84–91. doi: 10.1016/j.ophtha.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 40.Siatkowski RM, Cotter SA, Crockett RS, Miller JM, Novack GD, Zadnik K, U.S. Pirenzepine Study Group Two-year multicenter, randomized, double-masked, placebo-controlled, parallel safety and efficacy study of 2% pirenzepine ophthalmic gel in children with myopia. J AAPOS. 2008;12:332–9. doi: 10.1016/j.jaapos.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 41.Li W, Lan W, Yang S, Liao Y, Xu Q, Lin L, Yang Z. The effect of spectral property and intensity of light on natural refractive development and compensation to negative lenses in guinea pigs. Invest Ophthalmol Vis Sci. 2014;55:6324–32. doi: 10.1167/iovs.13-13802. [DOI] [PubMed] [Google Scholar]
- 42.Quinn GE, Berlin JA, Young TL, Ziylan S, Stone RA. Association of intraocular pressure and myopia in children. Ophthalmology. 1995;102:180–5. doi: 10.1016/s0161-6420(95)31038-x. [DOI] [PubMed] [Google Scholar]
- 43.Lee AJ, Saw SM, Gazzard G, Cheng A, Tan DT. Intraocular pressure associations with refractive error and axial length in children. Br J Ophthalmol. 2004;88:5–7. doi: 10.1136/bjo.88.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Urban B, Bakunowicz-Lazarczyk A. Intraocular pressure in children and adolescents with myopia. Klin Oczna. 2010;112:304–6. [PubMed] [Google Scholar]
- 45.Jensen H. Myopia progression in young school children and intraocular pressure. Doc Ophthalmol. 1992;82:249–55. doi: 10.1007/BF00160772. [DOI] [PubMed] [Google Scholar]
- 46.Ostrin LA, Wildsoet CF. Optic nerve head and intraocular pressure in the guinea pig eye. Exp Eye Res. 2016;146:7–16. doi: 10.1016/j.exer.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cooper J, Schulman E, Jamal N. Current status on the development and treatment of myopia. Optometry. 2012;83:179–99. [PubMed] [Google Scholar]
- 48.Ostrin LA, Garcia MB, Choh V, Wildsoet CF. Pharmacologically stimulated pupil and accommodative changes in Guinea pigs. Invest Ophthalmol Vis Sci. 2014;55:5456–65. doi: 10.1167/iovs.14-14096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ostrin LA, Frishman LJ, Glasser A. Effects of pirenzepine on pupil size and accommodation in rhesus monkeys. Invest Ophthalmol Vis Sci. 2004;45:3620–8. doi: 10.1167/iovs.04-0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Avetisov ES, Savitskaya NF, Vinetskaya MI, Iomdina EN. A study of biochemical and biomechanical qualities of normal and myopic eye sclera in humans of different age groups. Metab Pediatr Syst Ophthalmol. 1983;7:183–8. [PubMed] [Google Scholar]
- 51.Dong CJ, Guo Y, Agey P, Wheeler L, Hare WA. Alpha2 adrenergic modulation of NMDA receptor function as a major mechanism of RGC protection in experimental glaucoma and retinal excitotoxicity. Invest Ophthalmol Vis Sci. 2008;49:4515–22. doi: 10.1167/iovs.08-2078. [DOI] [PubMed] [Google Scholar]
- 52.Lee D, Kim KY, Noh YH, Chai S, Lindsey JD, Ellisman MH, Weinreb RN, Ju WK. Brimonidine blocks glutamate excitotoxicity-induced oxidative stress and preserves mitochondrial transcription factor a in ischemic retinal injury. PLoS One. 2012;7:e47098. doi: 10.1371/journal.pone.0047098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lai RK, Chun T, Hasson D, Lee S, Mehrbod F, Wheeler L. Alpha-2 adrenoceptor agonist protects retinal function after acute retinal ischemic injury in the rat. Vis Neurosci. 2002;19:175–85. doi: 10.1017/s0952523802191152. [DOI] [PubMed] [Google Scholar]
- 54.Chen BY, Wang CY, Chen WY, Ma JX. Altered TGF-beta2 and bFGF expression in scleral desmocytes from an experimentally-induced myopia guinea pig model. Graefe’s archive for clinical and experimental ophthalmology = Albrecht Von Graefes Arch Klin Exp Ophthalmol. 2013;251:1133–44. doi: 10.1007/s00417-013-2269-8. [DOI] [PubMed] [Google Scholar]
- 55.Tian XD, Cheng YX, Liu GB, Guo SF, Fan CL, Zhan LH, Xu YC. Expressions of type I collagen, alpha2 integrin and beta1 integrin in sclera of guinea pig with defocus myopia and inhibitory effects of bFGF on the formation of myopia. Int J Ophthalmol. 2013;6:54–8. doi: 10.3980/j.issn.2222-3959.2013.01.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu Q, Yu J, Zeng JW. Effect on of pirenzepine on expression of mAChRs in the ocular tissues of guinea pig with form-deprived myopia. Zhonghua Yan Ke Za Zhi. 2010;46:221–6. Zhonghua yan ke za zhi. [PubMed] [Google Scholar]
- 57.Dai SZ, Zeng JW, Wang LY. Effect of pirenzepine on form deprivation myopia in chicks and its possible mechanism. Zhonghua Yan Ke Za Zhi. 2006;42:42–7. Zhonghua yan ke za zhi. [PubMed] [Google Scholar]
- 58.Hong S, Han SH, Kim CY, Kim KY, Song YK, Seong GJ. Brimonidine reduces TGF-beta-induced extracellular matrix synthesis in human Tenon’s fibroblasts. BMC Ophthalmol. 2015;15:54. doi: 10.1186/s12886-015-0045-8. [DOI] [PMC free article] [PubMed] [Google Scholar]