Abstract
Serine/arginine-rich proteins (SR proteins) are a family of nuclear factors that play important roles in both constitutive and regulated precursor mRNA splicing. The domain rich in arginine/serine (RS) repeats (RS domain) serves as both a nuclear and subnuclear localization signal. We previously identified an importin β family protein, transportin-SR2 (TRN-SR2), that specifically interacts with phosphorylated RS domains. A TRN-SR2 mutant deficient in Ran binding colocalizes with SR proteins in nuclear speckles, suggesting a role of TRN-SR2 in nuclear targeting of SR proteins. Using in vitro import assays, we here show that nuclear import of SR protein fusions requires cytosolic factors, and that the RS domain becomes phosphorylated in the import reaction. Reconstitution of SR protein import by using recombinant transport factors clearly demonstrates that TRN-SR2 is capable of targeting phosphorylated, but not unphosphorylated, SR proteins to the nucleus. Therefore, RS domain phosphorylation is critical for TRN-SR2-mediated nuclear import. Interestingly, we found that the RNA-binding activity of SR proteins confers temperature sensitivity to their nuclear import. Finally, we show that TRN-SR2 interacts with a nucleoporin and is targeted not only to the nuclear envelope but also to nuclear speckles in vitro. Thus, TRN-SR2 may perhaps escort SR protein cargoes to nuclear subdomains.
Serine/arginine-rich proteins (SR proteins) comprise a large family of eukaryotic pre-mRNA splicing factors that contain a discrete domain rich in arginine/serine (RS) dipeptide repeats (RS domain). SR proteins are essential for pre-mRNA splicing and also play a pivotal role in determining alternative splice site selection (1–3). Cytological studies reveal that SR proteins are primarily localized in nuclear speckles—structures that serve as the sites for storage and/or reassembly of splicing factors. However, some, but not all, SR proteins continuously shuttle between the nucleus and the cytoplasm (4). Subcellular localization of SR proteins is influenced by their phosphorylation state and by the level of transcription activity within the cells (4–8). Hyperphosphorylation of the RS domain causes relocalization of SR proteins from nuclear speckles to a more diffuse distribution in the nucleoplasm or causes their cytoplasmic accumulation (4–8). When mRNA synthesis is blocked, the speckled pattern of some SR proteins becomes more prominent and, nevertheless, a subset of shuttling SR proteins is retained in the cytoplasm (4). Therefore, both the nucleocytoplasmic transport and subnuclear localization of SR proteins are likely to be complex and regulated processes, the underlying mechanisms for which remain to be delineated.
Nucleocytoplasmic transport of macromolecules via the nuclear pore complex (NPC) is generally mediated by saturable transport receptors that recognize specific signals within proteins (9–12). Nuclear import of proteins with a classical basic nuclear localization signal (NLS) is mediated by the dimeric complex of importin α/β, of which the α subunit binds the NLS directly and serves as the adaptor to importin β (impβ) (9–12). To date, many nonprototypical NLSs have been reported, including the M9 domain of the heterogeneous nuclear ribonucleoprotein (hnRNP) A1 and the arginine-rich segments of ribosomal proteins and the HIV Rev and Tat proteins (10, 12). Unlike the NLS prototype, these NLSs are recognized directly by the corresponding importin β family of transport receptors (9–12). Importin β proteins associate with particular nucleoporins in the NPC, thus facilitating the translocation of importin–cargo complexes (9–12). After translocation, high levels of RanGTP in the nucleoplasm promotes cargo release from the import complex by directly binding to the importin β proteins (9–12).
The RS domain of SR proteins serves as both a NLS and subnuclear localization signal (13, 14). A human importin β family protein, transportin-SR1 (TRN-SR1), is shown to mediate in vitro nuclear import of recombinant SR proteins via the RS domain (15). We recently identified another importin β protein, TRN-SR2, that is almost identical to TRN-SR1 except that the latter contains an additional unique internal fragment (16). Our data indicated that phosphorylation of the RS domain by the SR protein kinase SRPK1 greatly enhanced the interaction of SR proteins with TRN-SR2 (16). A truncated TRN-SR2, defective in Ran binding, colocalizes with SR proteins in nuclear speckles, suggesting that TRN-SR2 plays a role in nuclear targeting of SR proteins (16). TRN-SRs are homologues of the yeast Mtr10p, an importin β family protein that mediates the nuclear import of the yeast SR-like RNA-binding protein Npl3p (17, 18). The composite NLS of Npl3p consists of a repetitive RGG motif and several nonconsecutive RS/SR dipeptides. The yeast SR protein kinase Sky1p phosphorylates Npl3p only at the most C-terminal RS dipeptide (19, 20). Sky1p-mediated phosphorylation of Npl3p promotes its nuclear import by facilitating the interaction of the RGG motif with Mtr10p in vivo (19, 20). Studies of mammalian SR proteins have shown that overexpression of kinase-inactive SRPK2 or substitution of RS with KS in the RS domain resulted in cytoplasmic accumulation of ASF/SF2 (21, 22). Thus, SRPK-mediated phosphorylation likely plays an important role in nuclear import of SR proteins in mammalian cells.
Here, we show directly that phosphorylation of the RS domain is required for TRN-SR2-mediated SR protein nuclear import in a permeabilized cell system. Moreover, we describe the interactions of TRN-SR2 with nucleoporins and the association of TRN-SR2 with the nuclear envelope and nuclear speckles.
Materials and Methods
Plasmid Construction.
Plasmid pGST-RS was constructed by insertion of a PCR fragment corresponding to the RS domain of ASF (amino acids 198–248) into the Escherichia coli expression vector pGEX-5X-1 (Amersham Pharmacia Biotech). The vectors for expression of glutathione S-transferase (GST)-ASF and GST-SRPK1 were used as previously described (16). The pGST-ΔRRM1 plasmid was constructed from pGST-ASF by removing the coding region for the N-terminal 73 amino acids of ASF. For the FF-DD mutant of ASF, site-directed mutagenesis was performed with oligonucleotides gccgcggggaccgcccgacgccgacgttgagttcgaggaccc and gggtcctcgaactcaacgtcggcgtcgggcggtcccccgcggc, according to the method recommended by Stratagene. Plasmid pGST-TRN-SR2 was obtained by subcloning the DNA fragment corresponding to the full-length TRN-SR2 from pBS-TRN-SR2 (16) into pGEX-2T (Amersham Pharmacia Biotech). The resulting plasmid was used to produce recombinant GST-TRN-SR2 protein in bacteria. The vector for expression of GST-impβ was a kind gift from Y. Yoneda (Osaka University, Osaka). To construct the pGST-TRN plasmid, a DNA fragment encoding full-length TRN was excised from pVP16-HA-MIP (a gift from B. R. Cullen, Duke University, Durham, NC) and then inserted into pGEX-2T. The sequences of the above constructed plasmids were verified by automatic sequencing.
Expression and Purification of Recombinant Proteins.
Plasmids for expression of wild-type Ran, RanQ69L, and NTF2 were kindly provided by I. W. Mattaj (European Molecular Biology Laboratory, Heidelberg). Expression and purification were performed essentially as in Gorlich et al. (23). Before use, wild-type Ran and RanQ69L were charged with 1 mM GDP or GTP, as described previously (16). All of the GST-fusion proteins were overexpressed in E. coli strain XA90 by overnight incubation at 15°C on induction with 0.5 mM isopropyl β-d-thiogalactoside. The GST-fusion proteins were purified by using glutathione-Sepharose and then dialyzed against transport buffer containing 20 mM Hepes (pH 7.3), 110 mM potassium acetate, 2 mM magnesium acetate, 5 mM sodium acetate, 2 mM DTT, 1 mM EGTA, 8.7% glycerol, and 1 mM phenylmethylsulfonyl fluoride.
Preparation of HeLa Cell Cytoplasmic Extract and Bacterial Extracts.
The method for preparation of the HeLa cell cytoplasmic extract was described previously (16). Bacterial extract containing TRN-SR2 was also prepared as previously described (16); the total protein concentration of the lysate was ≈9 mg/ml, and recombinant TRN-SR2 was ≈0.3 mg/ml as measured by Western blotting. Mock extract used as the control in the reconstituted import assays was prepared from untransformed E. coli.
In Vitro Import Assay.
Fluorescent BSA-NLS conjugate was prepared essentially according to Adam et al. (24) by using the synthetic simian virus (SV)40 NLS peptide (CGGGPKKKRKVED). Permeabilization of HeLa cells was performed essentially as in Jakel and Gorlich (25), except that 30 μg/ml of digitonin (Calbiochem) was used for permeabilization. Permeabilized cells on coverslips were incubated with a 20-μl import reaction mixture at 30°C for 30 min. The mixture contained an energy regeneration system (25), protease inhibitor mixture (Roche Molecular Biochemicals), import substrate (1 μg of FITC-BSA-NLS or 1.2 μg of GST-SR protein fusions), and 5 μl of HeLa cell cytoplasmic extract. In reconstituted import assays, bacterial lysate containing TRN-SR2 (8 μl) was used together with 1.5 μg of purified RanGDP and 0.2 μg of NTF2. Before reconstituted import assays, in vitro phosphorylation of GST-SR protein fusions was performed as described previously (16). For RNase treatment, RNase A (10 μg/ml) was added to the import reaction mixture at 30°C for 30 min before incubation with permeabilized cells. When fluorescent BSA-NLS was used, cells were fixed with 2% formaldehyde at room temperature for 20 min and mounted with 50% glycerol in transport buffer. To monitor nuclear import of GST fusion proteins, indirect immunofluorescence was performed according to Lai et al. (16) by using anti-GST polyclonal antibodies (3 μg/ml; Upstate Biotechnology). To examine nuclear import of GST-impβ and GST-TRN-SR2, the import reaction mixture containing 1.5 μg each of purified GST-transport receptor was incubated with permeabilized cells as above. Double-label immunofluorescence was then performed as in Lai et al. (16).
UV Crosslinking.
Phosphorylated GST-SR protein fusions (20 pmol) were each incubated at 30°C for 20 min with 24 fmol of yeast actin pre-mRNA (labeled with [α-32P]UTP to a specific activity of 2.8 × 107 cpm/μg) in 20 μl of reaction containing 0.5 mM ATP, 20 mM creatine phosphate, 2.4 mM MgCl2, and 0.5 units/μl RNasin (Promega). UV crosslinking was performed essentially according to Caceres and Krainer (26). Crosslinked products were fractionated on SDS/PAGE and quantitated by using InstantImager (Packard).
Glycerol Gradient.
Phosphorylated GST-ASF was incubated with E. coli lysate under the nuclear import condition, except that TRN-SR2 was omitted. The reaction mixture (50 μl) was sedimented on a 10–30% glycerol gradient containing transport buffer; centrifugation was performed at 45,000 rpm in an SW41 rotor (Beckman Instruments, Palo Alto, CA) for 15 h at 4°C. Gradients were fractionated into 22 samples, followed by Western blot analysis by using anti-GST antibodies.
Native Gel Analysis.
To radiolabel and phosphorylate SR peptide [CGGGRKRRQ(RS)x8RRRR], a 10-μl reaction mixture containing 2 μM peptide, 0.33 μM [γ-32P]ATP (3,000 Ci/mmol), 10 μM nonradiolabeled ATP, and 0.1 μM GST-SRPK1 was incubated at 30°C for 45 min. For peptide-TRN-SR2 binding, the reaction mixture containing 0.4 μM GST-TRN-SR2 and 0.04 μM phosphorylated SR peptide (2 × 105 cpm) was incubated at 30°C for 30 min. For Ran competition, 0.8 or 2.4 μM RanGTP or RanGDP was added to the reaction mixture. The reaction mixture was then analyzed by electrophoresis on a nondenaturing 5.5% polyacrylamide gel in 0.5× TBE buffer (90 mM Tris/64.6 mM boric acid/2.5 mM EDTA, pH 8.3). Electrophoresis was performed at 250 V for 2.5 h at 4°C.
In Vitro Pull-Down Assay.
To pull down nucleoporins in vitro, 2 μg each of GST-transport receptor protein was incubated with 15 μl of HeLa cell cytoplasmic extract (≈450 μg of protein) in a 60-μl mixture containing NET-2 buffer [50 mM Tris⋅HCl (pH 7.4)/150 mM NaCl/0.05% Nonidet P-40] at 30°C for 30 min. Proteins that interacted with the GST-transport receptors were selected by glutathione-Sepharose and detected by immunoblotting with mAb414 (Babco, Richmond, CA) or anti-p62 antibody (Transduction Laboratories, Lexington, KY).
Results
Nuclear Import of Phosphorylated SR Proteins Requires Cytosolic Factors.
To examine the role of RS domain phosphorylation during nuclear import of SR proteins, we performed in vitro nuclear import assays by using digitonin-permeabilized HeLa cells. We initially attempted to use SR peptide-conjugated BSA as a substrate in our import assay. However, fluorescein-labeled BSA-SR formed aggregates in HeLa cell cytoplasmic extract, presumably because of phosphorylation of the SR peptide moiety (data not shown). Therefore, two GST fusion proteins were used as import substrates, one containing full-length ASF (GST-ASF) and the other containing the RS domain from ASF only (GST-RS). Immunofluorescence by using antibodies to GST was then performed to monitor the import of the fusion proteins. Nuclear import of the two SR protein fusions was observed in the presence of the HeLa cell cytoplasmic extract (hereafter referred to as cytosol) and an ATP-regenerating system (Fig. 1 A e and h), whereas no import occurred in the absence of the cytosol (Fig. 1 A d and g). SV40 NLS-conjugated BSA, a positive control substrate, also accumulated efficiently in nuclei in the presence of the cytosol (Fig. 1Ab). Next, to examine whether SR fusion proteins become phosphorylated, an aliquot of the reaction mixture was subjected to Western blot analysis. After incubation with the cytosol in the presence of ATP, both of the SR protein fusions showed altered electrophoretic mobility and became detectable by mAb 104 (Fig. 1B), which specifically reacts with phosphorylated RS domains (27). The results therefore indicate that SR proteins are likely phosphorylated by cytosolic SR protein kinases during the import reaction, and that RS domain phosphorylation correlates with the nuclear import.
Figure 1.
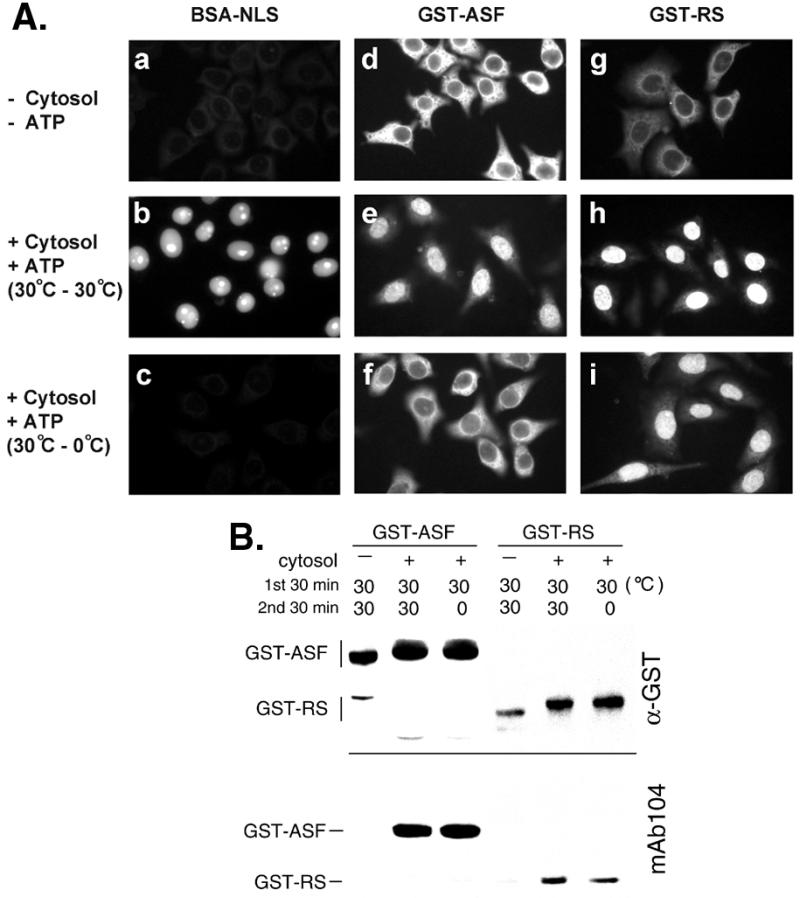
Nuclear import of SR proteins in permeabilized HeLa cells. (A) Nuclear import of BSA-NLS and two GST-SR protein fusions was performed by using permeabilized HeLa cells. Fluorescein-labeled BSA-NLS or GST-fusion proteins were incubated with the HeLa cell cytoplasmic extract (cytosol) at 30°C for 30 min in the presence of an ATP-regeneration system. Reaction mixtures were then added to the permeabilized HeLa cells and the incubation continued for another 30 min at 30°C (b, e, and h) or on ice (c, f, and i). In a, d, and g, the cytosol and ATP were not added to the reaction mixtures, although the incubations with permeabilized cells were performed at 30°C. (B) Reaction mixtures containing GST-SR protein fusions were subjected to Western blot analysis by using anti-GST antibodies (Upper) and mAb 104 (Lower). Temperatures used for the two-step incubation with the cytosol as above are indicated.
Although SR protein fusions failed to import in the absence of the cytosol, a possibility still remained whether they could diffuse into the nucleus after phosphorylation by cytosolic kinases. To test this possibility, SR proteins were previously phosphorylated by using cytosol, and the subsequent import reaction was carried out on ice. Accumulation of GST-ASF in the nuclei was markedly reduced under this circumstance (Fig. 1 Af), suggesting that nuclear import of GST-ASF is mediated by factors in the HeLa cytosol. In contrast, nuclear uptake of GST-RS still occurred efficiently on ice (Fig. 1Ai). Therefore, phosphorylated GST-RS may diffuse into nuclei without the aid of cytosolic factors, or such factors are required for GST-RS import but the import is temperature independent; the two possibilities would be distinguished in the following experiments. Moreover, one might argue that full-length ASF, but not GST-RS, is capable of shuttling between nucleus and cytoplasm (4). However, nuclear export of ASF is an active process (4); it is thus unlikely that GST-ASF exited from the nucleus after import at low temperature.
Phosphorylation of the RS Domain Is Required for Nuclear Import of SR Proteins Mediated by TRN-SR2.
Because phosphorylation of SR proteins occurred in conjunction with their interactions with transport receptors in HeLa cytosol, the above experiments did not clearly address whether phosphorylation of the RS domain is important for SR protein import. Therefore, the reconstituted import by using recombinant transport factors was then carried out. We previously showed that TRN-SR2 specifically recognizes phosphorylated SR proteins and is likely involved in nuclear import of SR proteins (16). Therefore, the import assay used cell lysate from E. coli that overexpressed TRN-SR2. The assay was also supplemented with purified Ran and NTF2 (p10) (23). Recombinant TRN-SR2 specifically binds to phosphorylated GST-ASF and GST-RS, and these interactions can be disrupted by RanGTP, but not by RanGDP (ref. 16 and data not shown). Fig. 2 shows that phosphorylated, but not unphosphorylated, GST-fusion SR proteins accumulate in the nuclei of permeabilized cells in the presence of TRN-SR2 (Fig. 2 a, c, f, and h). E. coli lysate lacking TRN-SR2 failed to promote SR protein entry into the nuclei (Fig. 2 b and g). We found that Ran also plays an important role, because nuclear accumulation of SR protein fusions was greatly reduced in its absence (data not shown). In addition, we added the mutant RanQ69L, which is locked in the GTP-bound form, to the import reaction and observed impaired nuclear uptake of both GST-fusion SR proteins (Fig. 2 e and j). This result indicates that RanQ69L-GTP likely interferes with the interaction of TRN-SR2 with its import cargo, thereby blocking nuclear import. All above results demonstrate that SR proteins import into the nucleus in a transport factor-assisted manner and, more importantly, that phosphorylation of the RS domain is essential for TRN-SR2-mediated import.
Figure 2.
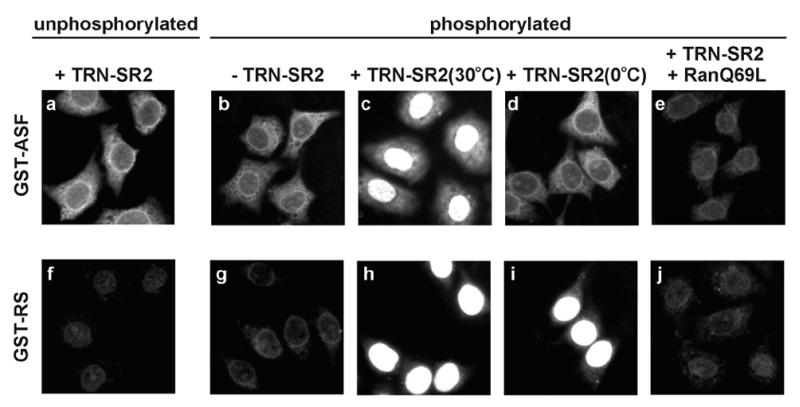
TNR-SR2 mediates nuclear import of phosphorylated SR proteins. GST-ASF and -RS were in vitro phosphorylated or mock-treated by SRPK1. The nuclear import assay for these two GST-SR protein fusions was carried out in a bacterial extract containing TRN-SR2 (+TRN-SR2) or in mock extract (−TRN-SR2). Effects of low temperature (d and i) or of RanQ69L-GTP (0.1 mg/ml) (e and j) on import were examined.
The RNA-Binding Activity of SR Proteins Confers Temperature Sensitivity to Nuclear Import.
In the reconstituted system, we found that nuclear import of phosphorylated GST-RS still occurred at 0°C (Fig. 2i), consistent with the observation above by using the HeLa cytosol (Fig. 1Ai). Thus, the nuclear import of GST-RS appears to be insensitive to low temperature, in sharp contrast to that of GST-ASF (Figs. 1Af and 2d). We further investigated the factors responsible for this difference in temperature sensitivity. In addition to the RS domain, ASF contains two RNA recognition motifs (RRMs). Therefore, we hypothesized that RRM-mediated RNA binding might confer temperature dependence on the import of full-length ASF. To inactivate the RRM of ASF, a large portion of RRM1 was deleted from ASF (GST-ΔRRM1), or the conserved phenylalanines at positions 56 and 58 in RRM1 were substituted with aspartic acids (26) (GST-FFDD, Fig. 3A). Consistent with a previous report (26), the UV-crosslinking efficiency of phosphorylated SR protein fusions to yeast actin pre-mRNA was greatly decreased in the case of GST-ΔRRM1 and -RS and moderately impaired in the case of the FF-DD mutant (Fig. 3A). Subsequently, nuclear import assays showed that, in the presence of TRN-SR2, both GST-ΔRRM1 and -FFDD accumulated in the nucleus (Fig. 3 B a–d). Moreover, nuclear accumulation of these two GST-ASF mutants was detectable at 0°C, albeit GST-FFDD with relatively low efficiency of import (Fig. 3 B g and h). These data thus indicate that the loss of RNA-binding capacity in SR proteins correlates well with temperature insensitivity in nuclear import assays.
Figure 3.
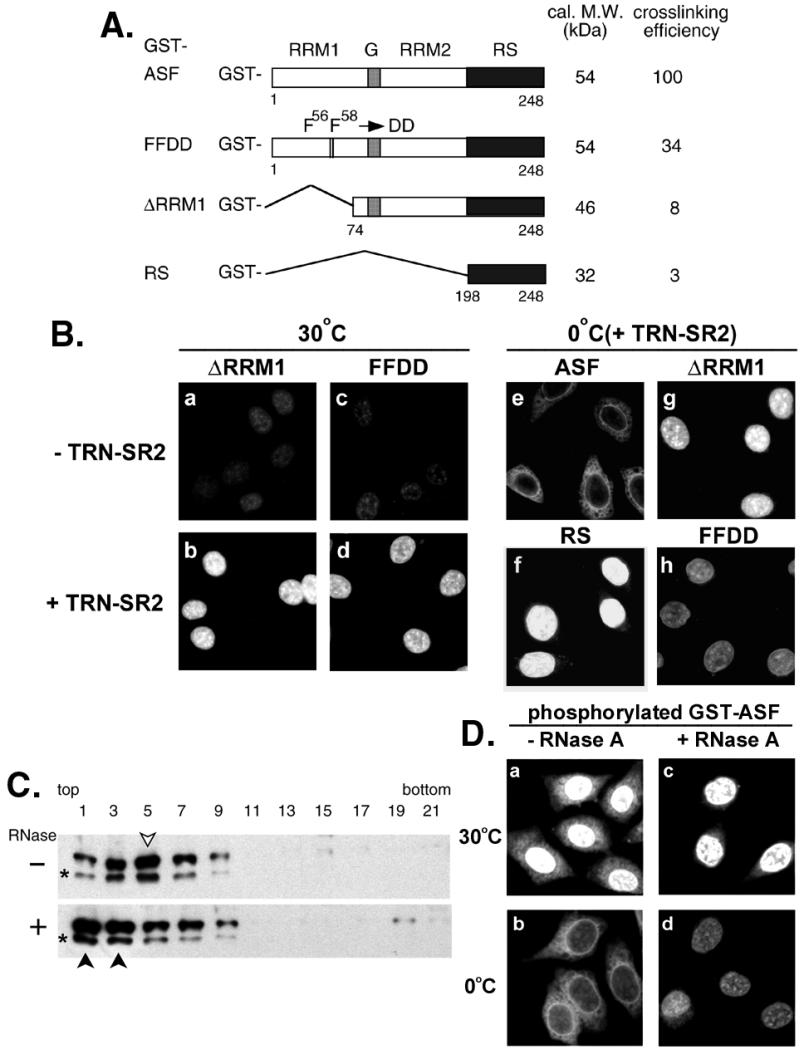
RNA-binding capacity of SR proteins correlates with their temperature sensitivity to nuclear import. (A) Schematic representation of the GST fusions with ASF, FFDD, ΔRRM1, and RS domain only. Calculated molecular mass (in kDa) of unphosphorylated fusion proteins and relative UV-crosslinking efficiency of phosphorylated proteins are indicated, respectively (Right). G represents the glycine-rich hinge of ASF. (B) (a–d) Nuclear import of phosphorylated GST-ΔRRM1 and GST-FFDD was assayed at 30°C in the mock E. coli lysate or in lysate containing TRN-SR2. (e–h) Phosphorylated GST fusion SR proteins (as indicated) were subjected to nuclear import assay in the TRN-SR2-containing lysate on ice. (C) Phosphorylated GST-ASF was incubated with E. coli lysate in the absence (−) or presence (+) of RNase A under the import conditions. The reaction mixtures were sedimented on 10–30% glycerol gradient, followed by Western blot analysis by using anti-GST antibodies. GST-ASF peak fractions are indicated by arrowheads (open for mock-treated and closed for RNase-treated). Asterisks represent partially degraded GST-ASF. (D) Nuclear import of phosphorylated GST-ASF was assayed in the RNase A-treated (+RNase A) or mock-treated (−RNase A) TRN-SR2-containing extract at 30°C or on ice.
To confirm that the blockage of ASF import into the nucleus at 0°C results from its RNA binding, the import reaction was treated with RNase A. RNase treatment dissociated the majority of ASF from larger complexes, as evident by glycerol gradient sedimentation (Fig. 3C), suggesting that ASF forms complexes with RNA in the import reaction. Such treatment apparently had no effect on ASF binding to TRN-SR2 in a pull-down assay (data not shown). Nevertheless, after RNase treatment, nuclear import of GST-ASF was detected at 0°C, albeit less efficiently than that at 30°C (Fig. 3D), perhaps because of incomplete disruption of RNP complexes. TRN-SR2 was still required for ASF import under this condition, arguing against the possibility that phosphorylated ASF diffused into the nuclei after removal of bound RNA (data not shown). Therefore, this result is in good agreement with our supposition that the temperature dependence of nuclear import of SR proteins is closely related to their ability to bind RNA.
SR Peptide Blocks Nuclear Import of ASF via Interaction with TRN-SR2.
SR proteins often contain at least one RRM as well as an RS domain. We therefore went on to test whether the RS domain serves as the only NLS for SR proteins by using a SR peptide as the competitor in the nuclear import assay. The SR peptide contains eight consecutive RS repeats flanked by two short arginine-rich stretches derived from an NLS of the Drosophila SR protein Tra (13) (see Materials and Methods). Initially, we tested the binding of the SR peptide to TRN-SR2. In pull-down experiments as described previously (16), only phosphorylated, but not unphosphorylated, peptide can disrupt GST-ASF binding to TRN-SR2 (data not shown). Next, to examine the direct and specific binding of phosphorylated SR peptide to TRN-SR2, we exploited a gel mobility-shift assay. As shown in Fig. 4A, the 32P-labeled phosphorylated SR peptide binds directly to TRN-SR2 to form a complex (lane 2). This complex was disturbed by the presence of RanGTP but not by RanGDP (Fig. 4A, lanes 3–6), in a concentration-dependent manner, as expected for specific transport receptor–cargo interactions. Moreover, neither importin β nor TRN formed a complex with the SR peptide (Fig. 4A, lanes 7 and 8), demonstrating the specificity of this peptide to TRN-SR2.
Figure 4.
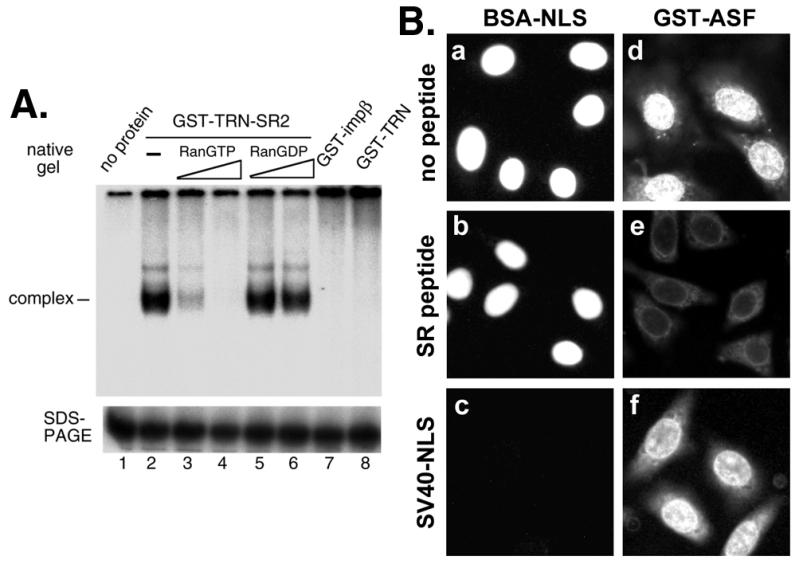
SR peptide interacts directly with TRN-SR2 and blocks nuclear import of GST-ASF. (A) Radiolabeled phosphorylated SR peptide (0.04 μM) was incubated with buffer alone or with 0.4 μM GST-TRN-SR2, GST-impβ, or GST-TRN. For competition experiments by using Ran, 0.8 or 2.4 μM RanGTP or RanGDP was added to appropriate reaction mixtures. One-half of each reaction mixture was analyzed by electrophoresis on a 5.5% nondenaturing gel (native gel, Upper), and the other half was fractionated by SDS/PAGE on a 20% gel (Lower) for monitoring the peptide level in the reactions, because free peptide was not detectable in the nondenaturing gel. (B) Nuclear import of GST-ASF (0.5 μM) was compared with that of BSA-NLS (0.5 μM) under control conditions (no peptide) or in the presence of phosphorylated SR peptide at a 45× molar excess over import substrate or SV40 NLS peptide at a 120× molar excess.
Next, nuclear import assays by using full-length ASF as the import substrate were carried out in the HeLa cytosol, which contains a variety of transport receptors. SR peptide completely inhibited nuclear import of GST-ASF (Fig. 4Be), most likely by blocking the function of TRN-SR2, given that the peptide did not block the import of BSA-NLS (Fig. 4Bb). In contrast, a SV40 NLS control peptide did not affect GST-ASF nuclear import (Fig. 4Bf). The possibility that SR peptide titrated out SRPKs can be excluded, because GST-ASF was preincubated with the cytosol to become phosphorylated before addition of the peptide. These data thus suggest that none of the transport receptors present in HeLa cytosol could target SR proteins to the nucleus via recognition of their RRM(s), at least in the presence of the RS domain NLS. However, this result appears to be contradictory to data observed from previous transient transfection experiments, which showed that a mutant ASF lacking its RS domain localizes mainly to the nucleus (14). Nevertheless, one must consider the possibility that SR proteins may be transported to the nucleus via RNP complexes in vivo (see Discussion), even if the RS domain is lacking. Therefore, the most straightforward interpretation of our data is that the RS domain is the only functional NLS of SR proteins.
TRN-SR2 Interacts with a Nucleoporin and Accumulates in Nuclear Speckles in Vitro.
The binding of transport receptors to nucleoporins is central to their transfer through the NPC. Therefore, we next examined the binding capacity of TRN-SR2 for a subset of nucleoporins. GST-tagged transporters were each incubated with the HeLa cytosol, and the reaction mixtures were then subjected to selection with glutathione-Sepharose. The bound proteins were detected by mAb 414 that recognizes FXFG repeats within nucleoporins from a variety of species (28, 29). Of all of the mAb 414-reactive nucleoporins in the HeLa cytosol (Fig. 5A, lane 1), GST-TRN-SR2 brought down only p62 (lane 4), suggesting that TRN-SR2 interacts with p62 or its associated complex (30). In contrast, GST-impβ and GST-TRN interacted not only with p62 but also with Nup153 (Fig. 5A, lanes 3 and 5), as previously reported (31–33). Although the binding of p62 to TRN-SR2 was comparable to that of importin β in several independent experiments (Fig. 5A, lanes 6 and 7), no binding of Nup153 to TRN-SR2 was observed (lane 4 and data not shown). mAb RL1 that reacts with a group of mammalian NPC glycoproteins was also used. Still, in contrast to importin β, no significant band was detected with GST-TRN-SR2 selected protein (data not shown). Moreover, the interaction of p62 with TRN-SR2 was substantially disturbed by RanQ69L-GTP (Fig. 5A, lane 9) but not by RanGDP (data not shown) or by excess SR peptide (lane 8). Whether p62 functions as a docking site for TRN-SR2–cargo complexes at the NPC remains to be studied, our present data suggest that the import route used by TRN-SR2 is in part distinct from that of two other nuclear transport receptors, importin β and transportin.
Figure 5.
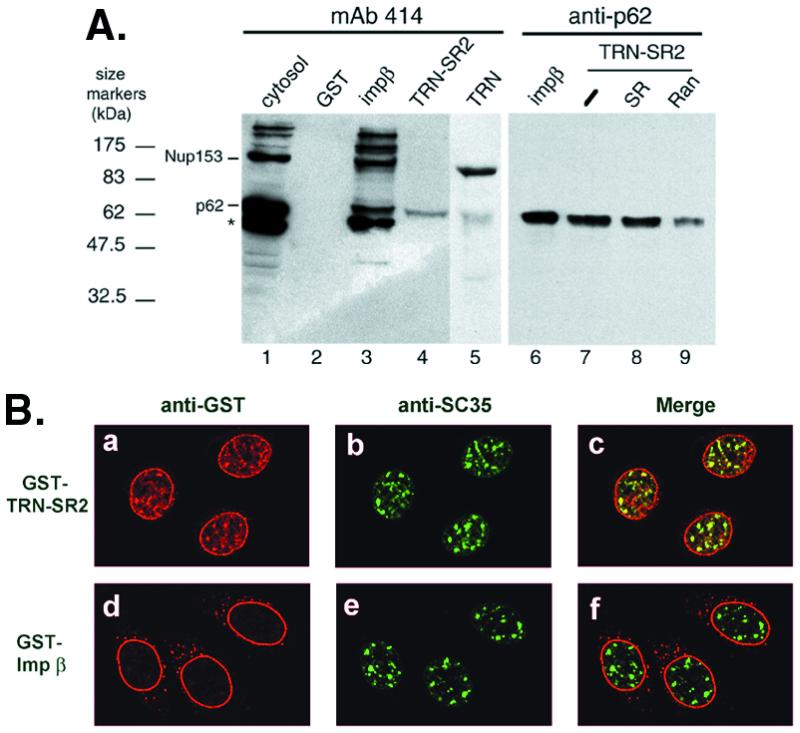
TRN-SR2 interacts with nucleoporin p62 and is targeted to nuclear speckles. (A) GST or GST-transport receptor fusions (2 μg each) were incubated with HeLa cytosol, as described in Materials and Methods. Proteins interacting with GST or GST-fusion transporters were selected by glutathione-Sepharose and detected by immunoblotting with mAb 414 (lanes 1–5) or an antibody to p62 (lanes 6–9). For competition, binding reaction mixtures contained transport buffer alone (−; lane 7), 10 μM phosphorylated SR peptide (SR; lane 8), or 5 μM RanQ69L-GTP (Ran; lane 9). Lane 1 shows 6.6% of the input into the pull-down assay. The asterisk represents a band that was detected in some but not all batches of the cytosol used. (B) Permeabilized HeLa cells were incubated with 0.6 μM GST-TRN-SR2 or GST-impβ in the presence of an ATP regeneration system. Double-label immunofluorescence was performed by using polyclonal anti-GST (a and d) and monoclonal anti-SC35 (b and e) antibodies. Merged images are shown in c and f.
The above result indicated that TRN-SR2 interacts with a nucleoporin protein, p62; we therefore went on to examine whether TRN-SR2 is targeted to NPCs in vitro. A GST-TRN-SR2 fusion protein was incubated with permeabilized HeLa cells under the import condition and then visualized by immunofluorescence by using anti-GST antibodies. Surprisingly, TRN-SR2 was localized not only at the rim of the nuclear envelope but also in speckled domains within the nucleus (Fig. 5Ba). The nuclear rim staining suggests that TRN-SR2 binds to NPCs, as expected for nuclear transport receptors. Furthermore, double immunofluorescence showed that the sites where TRN-SR2 accumulated within the nucleus mostly coincided with those stained by anti-SC35 antibody (Fig. 5 B a–c), suggesting colocalization of TRN-SR2 with SR proteins. In contrast, GST-impβ staining was mainly at the rim of the nucleus (Fig. 5Bd), as reported previously (34). This result indicates that TRN-SR2 is capable of targeting not only to the NPCs but also to the nuclear speckles.
Discussion
Using in vitro import assays, we showed that cytosolic factors are required for nuclear import of SR proteins (as GST fusions), and that RS domains undergo phosphorylation probably by cytosolic SR kinases in the import reaction. Furthermore, the data of reconstituted import clearly demonstrated that only phosphorylated SR proteins accumulate efficiently in the nucleus, through their association with TRN-SR2. Previously, it was shown by Kataoka et al. that TRN-SR1 mediates nuclear import of recombinant RS domain-containing proteins in permeabilized cells (15). Although the effects of phosphorylation were not addressed in this article, recombinant SR proteins were unlikely to be phosphorylated particularly in a reconstituted import system. Given that TRN-SR1 contains a central domain not present in TRN-SR2, the questions thus remain whether this domain is actually responsible for discriminating phosphorylated vs. nonphosphorylated cargo, and whether nuclear import of SR proteins in mammalian cells is differentially regulated by phosphorylation and mediated by distinct but related transport receptors. Moreover, the possibility that the two highly related TRN-SRs recognize different sets of SR proteins still remains open. Nevertheless, our data demonstrated that phosphorylation of the ASF RS domain by SRPK1 is critical for efficient nuclear import of SR fusion proteins via TRN-SR2. This result echoes recent reports that Sky1p-mediated phosphorylation at a RS dipeptide of Npl3p triggers nuclear import of this protein in yeast (19, 20) and supports the observations that phosphorylation by SRPKs is important for mammalian SR protein nuclear localization (21, 22). However, it must be noted that overexpression of SR protein kinases causes cytoplasmic accumulation of SR proteins (14, 16, 22). If this observation results from impaired import of hyperphosphorylated SR proteins, it will be interesting to analyze the phosphorylation sites of an RS domain at different levels of SR kinases and to examine whether differentially phosphorylated RS domains exhibit different affinity to TRN-SR2.
In the present study, we found that nuclear import of GST-ASF, but not GST-RS, was completely blocked at 0°C. This observation was consistent between the HeLa cytosol-based and the reconstituted import assays (Figs. 1 and 2). ASF lacking its RRM1 or with point mutations in RRM1 behaved similarly to GST-RS in this regard, perhaps because of the loss of RNA-binding activity. Moreover, our data showed that RNase treatment facilitated the nuclear import of ASF at low temperature, probably as a consequence of dissociation of RNA from ASF. We thus presumed that unwinding of RNA or unfolding of RNP complexes, a process that may require NTP hydrolysis at physiological temperature, is necessary for translocation of ASF through the NPC in our assay. Recently, the DEAD box protein Dbp5 was found to be associated with the cytoplasmic face of the NPC (35, 36). Dbp5p has an ATP-dependent RNA-unwinding activity that is likely to be involved in mRNP reorganization during mRNA export. Nevertheless, our result provokes questions such as whether SR proteins are bound to RNA during nuclear import in vivo and whether bound RNA, if any, undergoes unfolding with the aid of Dbp5p or related RNA helicase. Although whether TRN-SR2 can bind ASF while ASF associates with RNA is apparently uncertain, it is worth noting that transportin could form a complex with RNA-bound hnRNP A1 (37). However, transportin cannot bind hnRNP A1 that is associated with hnRNP complexes, indicating that the M9 NLS is sequestered in such complexes (37). Thus, further studies are also needed to address the question whether the RS domain also becomes inaccessible to TRN-SR2 while SR proteins interact each other via the RS domain.
We previously observed that overexpressed TRN-SR2 distributes homogeneously throughout the whole cell, whereas the Ran-binding deficient TRN-SR2 mutant is restricted in the nucleus, particularly within nuclear speckles (16). We reason that the mutant TRN-SR2 is capable of translocating SR proteins to the nucleus, but that its cargo is not dissociated by nuclear RanGTP. Surprisingly, ligand-free full-length TRN-SR2, unlike importin β, is targeted to nuclear speckles in permeabilized cells. Ran-GTP or a combination of Ran-GTP plus RNA is expected to release nuclear SR proteins from TRN-SR2. However, addition of HeLa cytosol or recombinant RanQ69L-GTP alone or each together with yeast total RNA did not significantly alter the speckled distribution of TRN-SR2 in vitro (data not shown). This observation implied that TRN-SR2 is unlikely to be dragged by endogenous SR proteins to nuclear speckles. At this juncture, the mechanism by which TRN-SR2 is destined for nuclear speckles remains to be delineated. Apparently, it is also not clear why full-length TRN-SR2 was not observed in nuclear speckles in vivo (16). We presumed that TRN-SR2 reaches speckles shortly and returns rapidly back to the nucleoplasm or the cytoplasm in living cells but not in permeabilized cells. Alternatively, an additional activity, absent in the in vitro system, may be required to promote the dissociation of TRN-SR2 from SR proteins. Notably, transportin was observed in the nucleoplasm where hnRNP A1 mainly locates (37). It was suggested that transportin plays roles in the nucleus in addition to its role in nuclear import of hnRNP proteins (37). Thus, there remains the interesting question whether TRN-SR2 or other nuclear import receptors, such as transportin, are capable of escorting their cargoes to specific nuclear subdomains.
Acknowledgments
We are grateful to Drs. Iain W. Mattaj, Bryan R. Cullen, and Yoshihiro Yoneda for kindly providing plasmids. We thank Chi-Hong Lou for technical assistance, Kuan-Yu Chou for confocal microscopy assistance, Chen-Chien Chang for peptide analysis, and Dr. Miki Hieda for technical suggestions. Our thanks go also to Drs. Soo-Chen Cheng, Young-Sun Lin, and Tim C. Taylor for critical reading of the manuscript. This work was supported by Academia Sinica and Grant NHRI-EX90–8812BC from the National Health Research Institutes of Taiwan.
Abbreviations
- SR protein
serine/arginine-rich protein
- NPC
nuclear pore complex
- NLS
nuclear localization signal
- hnRNP
heterogeneous nuclear ribonucleoprotein
- RRM
RNA recognition motif
- SV40
simian virus 40
- GST
glutathione S-transferase
References
- 1.Fu X-D. RNA. 1995;1:663–680. [PMC free article] [PubMed] [Google Scholar]
- 2.Manley J L, Tacke R. Genes Dev. 1996;10:1569–1579. doi: 10.1101/gad.10.13.1569. [DOI] [PubMed] [Google Scholar]
- 3.Valcarcel J, Green M R. Trends Biochem Sci. 1996;21:296–301. [PubMed] [Google Scholar]
- 4.Caceres J F, Screaton G R, Krainer A R. Genes Dev. 1998;12:55–66. doi: 10.1101/gad.12.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gui J F, Lane W S, Fu X-D. Nature (London) 1994;369:678–682. doi: 10.1038/369678a0. [DOI] [PubMed] [Google Scholar]
- 6.Colwill K, Pawson T, Andrews B, Prasad J, Manley J L, Bell J C, Duncan P I. EMBO J. 1996;15:265–275. [PMC free article] [PubMed] [Google Scholar]
- 7.Misteli T, Spector D L. Mol Biol Cell. 1996;7:1559–1572. doi: 10.1091/mbc.7.10.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang H Y, Lin W, Dyck J A, Yeakley J M, Songyang Z, Cantley L C, Fu X-D. J Cell Biol. 1998;140:737–750. doi: 10.1083/jcb.140.4.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Izaurralde E, Adam S. RNA. 1998;4:351–364. [PMC free article] [PubMed] [Google Scholar]
- 10.Mattaj I W, Englmeier L. Annu Rev Biochem. 1998;67:265–306. doi: 10.1146/annurev.biochem.67.1.265. [DOI] [PubMed] [Google Scholar]
- 11.Gorlich D, Kutay U. Annu Rev Cell Dev Biol. 1999;15:607–660. doi: 10.1146/annurev.cellbio.15.1.607. [DOI] [PubMed] [Google Scholar]
- 12.Nakielny S, Dreyfuss G. Cell. 1999;99:677–690. doi: 10.1016/s0092-8674(00)81666-9. [DOI] [PubMed] [Google Scholar]
- 13.Hedley M L, Amrein H, Maniatis T. Proc Natl Acad Sci USA. 1995;92:11524–11528. doi: 10.1073/pnas.92.25.11524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caceres J F, Misteli T, Screaton G R, Spector D L, Krainer A R. J Cell Biol. 1997;138:225–238. doi: 10.1083/jcb.138.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kataoka N, Bachorik J L, Dreyfuss G. J Cell Biol. 1999;145:1145–1152. doi: 10.1083/jcb.145.6.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lai M C, Lin R I, Huang S Y, Tsai C W, Tarn W Y. J Biol Chem. 2000;275:7950–7957. doi: 10.1074/jbc.275.11.7950. [DOI] [PubMed] [Google Scholar]
- 17.Pemberton L F, Rosenblum J S, Blobel G. J Cell Biol. 1997;139:1645–1653. doi: 10.1083/jcb.139.7.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Senger B, Simos G, Bischoff F R, Podtelejnikov A, Mann M, Hurt E. EMBO J. 1998;17:2196–2207. doi: 10.1093/emboj/17.8.2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yun C Y, Fu X-D. J Cell Biol. 2000;150:707–718. doi: 10.1083/jcb.150.4.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gilbert W, Siebel C W, Guthrie C. RNA. 2001;7:302–313. doi: 10.1017/s1355838201002369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yeakley J M, Tronchere H, Olesen J, Dyck J A, Wang H Y, Fu X-D. J Cell Biol. 1999;145:447–455. doi: 10.1083/jcb.145.3.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koizumi J, Okamoto Y, Onogi H, Mayeda A, Krainer A R, Hagiwara M. J Biol Chem. 1999;274:11125–11131. doi: 10.1074/jbc.274.16.11125. [DOI] [PubMed] [Google Scholar]
- 23.Gorlich D, Pante N, Kutay U, Aebi U, Bischoff F R. EMBO J. 1996;15:5584–5594. [PMC free article] [PubMed] [Google Scholar]
- 24.Adam S A, Marr R S, Gerace L. J Cell Biol. 1990;111:807–816. doi: 10.1083/jcb.111.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jakel S, Gorlich D. EMBO J. 1998;17:4491–4502. doi: 10.1093/emboj/17.15.4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caceres J F, Krainer A R. EMBO J. 1993;12:4715–4726. doi: 10.1002/j.1460-2075.1993.tb06160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zahler A M, Lane W S, Stolk J A, Roth M B. Genes Dev. 1992;6:837–847. doi: 10.1101/gad.6.5.837. [DOI] [PubMed] [Google Scholar]
- 28.Davis L I, Blobel G. Cell. 1986;45:699–709. doi: 10.1016/0092-8674(86)90784-1. [DOI] [PubMed] [Google Scholar]
- 29.Sukegawa J, Blobel G. Cell. 1993;72:29–38. doi: 10.1016/0092-8674(93)90047-t. [DOI] [PubMed] [Google Scholar]
- 30.Finlay D R, Bradley M E, Horecka J, Forbes D J. J Cell Biol. 1991;114:169–183. doi: 10.1083/jcb.114.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Radu A, Blobel G, Moore M S. Proc Natl Acad Sci USA. 1995;92:1769–1773. doi: 10.1073/pnas.92.5.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shah S, Tugendreich S, Forbes D. J Cell Biol. 1998;141:31–49. doi: 10.1083/jcb.141.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakielny S, Shaikh S, Burke B, Dreyfuss G. EMBO J. 1999;18:1982–1995. doi: 10.1093/emboj/18.7.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakielny S, Dreyfuss G. Curr Biol. 1997;8:89–95. doi: 10.1016/s0960-9822(98)70039-9. [DOI] [PubMed] [Google Scholar]
- 35.Hodge C A, Colot H V, Stafford P, Cole C N. EMBO J. 1999;18:5778–5788. doi: 10.1093/emboj/18.20.5778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmitt C, von Kobbe C, Bachi A, Pante N, Rodrigues J P, Boscheron C, Rigaut G, Wilm M, Seraphin B, Carmo-Fonseca M, Izaurralde E. EMBO J. 1999;18:4332–4347. doi: 10.1093/emboj/18.15.4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Siomi M C, Eder P S, Kataoka N, Wan L, Liu Q, Dreyfuss G. J Cell Biol. 1997;138:1182–1192. doi: 10.1083/jcb.138.6.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]


