Visual Abstract
Key Words: advanced cardiopulmonary life support, cardiac arrest, cardiopulmonary resuscitation, ECMO, extracorporeal membrane oxygenation, ischemic refractory ventricular fibrillation, ST-segment elevation myocardial infarction, ventricular fibrillation
Abbreviations and Acronyms: ACLS, advanced cardiac life support; BLS, basic life support; CA, cardiac arrest; CCL, cardiac catheterization laboratory; CPP, coronary perfusion pressure; CPR, cardiopulmonary resuscitation; ECMO, extracorporeal membrane oxygenation; EPI, epinephrine; LAD, left anterior descending artery; PCI, percutaneous coronary intervention; ROSC, return of spontaneous circulation; VF, ventricular fibrillation
Highlights
-
•
The Minnesota Resuscitation Consortium has established a protocol for rapid transport of patients with refractory out-of-hospital VF cardiac arrest to the cardiac catheterization laboratory for rapid evaluation and stabilization often requiring ECMO. This protocol provides new challenges to treatment paradigms that were created to rapidly achieve return of spontaneous circulation in the field.
-
•
A porcine model of refractory VF cardiac arrest was developed, including initiation of VF using endovascular occlusion of the proximal LAD followed by 5 min of untreated VF. Resuscitation begins with 10 min of high-quality CPR followed by 35 min of ACLS and reconstitution of coronary flow.
-
•
A 2 × 2 study design was used with animals randomized to use of epinephrine or placebo during ACLS and then again randomized to ECMO or no ECMO at the time of reinitiation of coronary flow.
-
•
ECMO-facilitated coronary reperfusion and hemodynamic stabilization improved 4-h survival compared with CPR-facilitated reperfusion and standard ACLS in a porcine model of refractory VF cardiac arrest.
-
•
Repeated epinephrine boluses provided in accordance with standard ACLS protocols increased systemic blood pressure and coronary perfusion pressure but provided no benefit in survival compared with placebo.
-
•
Over 50% of the animals receiving ECMO met criteria for decannulation at 4 h, suggesting that rapid cardiac and hemodynamic recovery is possible in severely injured animals treated with ECMO.
Summary
Extracorporeal membrane oxygenation (ECMO) is used in cardiopulmonary resuscitation (CPR) of refractory cardiac arrest. The authors used a 2 × 2 study design to compare ECMO versus CPR and epinephrine versus placebo in a porcine model of ischemic refractory ventricular fibrillation (VF). Pigs underwent 5 min of untreated VF and 10 min of CPR, and were randomized to receive epinephrine versus placebo for another 35 min. Animals were further randomized to left anterior descending artery (LAD) reperfusion at minute 45 with ongoing CPR versus venoarterial ECMO cannulation at minute 45 of CPR and subsequent LAD reperfusion. Four-hour survival was improved with ECMO whereas epinephrine showed no effect.
The Minnesota Resuscitation Consortium (MRC) has modified the advanced cardiac life support (ACLS) treatment algorithm (1) by establishing the first reported protocol in the United States to treat patients with out-of-hospital refractory ventricular fibrillation (VF) (2). The protocol includes early transport by emergency medical services to a cardiac catheterization laboratory (CCL) with intent to receive extracorporeal membrane oxygenation (ECMO)–facilitated coronary revascularization (angioplasty) (2). Initial treatment and transport of out-of-hospital VF arrest patients to the CCL often requires 45 to 60 min.
There are currently no controlled clinical trials or published animal models of ischemic VF demonstrating improved survival when ECMO is utilized to facilitate percutaneous coronary intervention (PCI) instead of cardiopulmonary resuscitation (CPR). The effect of epinephrine (EPI) use in this setting is also unknown.
Therefore, we developed a porcine model of prolonged CPR with refractory ischemic VF that simulated our MRC clinical protocol (3) and implemented a randomized 2 × 2 factorial design to test 2 hypotheses: 1) use of EPI in prolonged resuscitation improves short-term 4-h survival; and 2) ECMO-facilitated reperfusion improves short-term 4-h survival and cardiac function compared with CPR-facilitated reperfusion with no ECMO support.
Methods
This 2 × 2 randomized experimental study in pigs was performed at the University of Minnesota and approved by the Institutional Animal Care and Use Committee. All studies and animal care complied with the National Research Council's 1996 Guidelines for the Care and Use of Laboratory Animals (protocol number: 12-11). All studies and procedures were performed by a qualified, experienced research team in farm-bred female Yorkshire pigs. A certified and licensed veterinarian assured the studies were performed in accordance with the National Research Council's Guidelines.
Preparatory phase
We used 33 pigs with an average weight of 44 ± 3 kg. The surgical preparation under aseptic conditions, anesthesia, data monitoring, and recording procedures used in the present study have been described elsewhere 3, 4, 5. Briefly, pigs were anesthetized with intramuscular ketamine (1,000 mg) followed by inhaled isoflurane at a dose of 0.8% to 1.2%. Endotracheal intubation was secured with a 7.0-mm endotracheal tube. Animals were ventilated with room air using volume-controlled ventilation with a tidal volume of 10 ml/kg (Narkomed, Telford, Pennsylvania). The respiratory rate was adjusted to maintain a partial pressure of carbon dioxide in arterial blood (PaCO2) of 36 to 42 mm Hg. A partial pressure of oxygen in arterial blood (PaO2) of at least 80 mm Hg (blood oxygen saturation >95%) was maintained prior to VF initiation. Core temperature was measured with an esophageal temperature probe with normothermia (37° ± 0.5°C) maintained prior to VF initiation using a warming blanket (Bair Hugger, Augustine Medical, Eden Prairie, Minnesota). Therapeutic hypothermia was initiated after the resuscitation with a target temperature of 34°C using a Blanketrol cooling blanket (Cincinnati Sub Zero, Cincinnati, Ohio).
The aortic blood pressure was recorded continuously with a Millar catheter (Mikro-Tip Transducer, Millar Instruments, Houston, Texas) placed through an 8-F left femoral arterial sheath into the descending thoracic aorta. A second Millar catheter was inserted into the right atrium through an 8-F sheath placed into the right external jugular vein to record central venous pressure. All sheaths were placed percutaneously using ultrasound guidance.
To prevent thrombosis due to endovascular instrumentation, an intravenous heparin bolus of 100 U/kg was administered every 60 min. Hemodynamic data were continuously monitored and recorded (LabVIEW 2015, National Instruments, Austin, Texas). Coronary perfusion pressure (CPP) was calculated as the difference between diastolic blood pressure and right atrial pressure. During CPR, CPP was calculated during the decompression phase. End-tidal carbon dioxide, tidal volume, minute ventilation, and blood oxygen saturation were continuously measured (COSMO Plus, Novametrix Medical Systems, Wallingford, Connecticut). Electrocardiograms (ECGs) were continuously recorded.
Experimental protocol
After anesthesia and instrumentation, 6-F arterial and venous sheaths were placed in the right femoral artery and vein. A second 8-F left femoral arterial sheath was placed for coronary procedures. The timeline of the experimental protocol is shown in Figure 1.
Figure 1.
Schematic Representation of the Randomized 2 × 2 Protocol Including Epi and ECMO Randomization
Each experiment began with endovascular balloon occlusion of the ostial left anterior descending artery (LAD). If ventricular fibrillation (VF) did not occur spontaneously, it was induced electrically after 5 min. Ventilations were then halted and no treatment was provided for 5 min. High-quality cardiopulmonary resuscitation (CPR) was then initiated for 10 min of basic life support (BLS), representing first-responder CPR. Animals were then randomized to receive epinephrine (Epi) 0.5 mg intravenous or saline placebo every 5 min for the 35 min of advanced life support (ACLS). At minute 45 of CPR, the animals were again randomized into the CPR-facilitated group (no extracorporeal membrane oxygenation [NO ECMO]) or the ECMO+ group. In the NO ECMO group, the LAD balloon was deflated and resuscitation continued for up to 15 more min. If no return of spontaneous circulation (ROSC) was achieved, the animal was declared dead. If ROSC was achieved, the animal was maintained for 4 h. ECMO+ animals were cannulated for venoarterial ECMO followed by LAD balloon deflation and resuscitation continued. If ROSC was not achieved within 15 min, the animal was declared dead. If ROSC was achieved, the animal was maintained for 4 h, at which time it was assessed for suitability for decannulation. ECMO+ pigs meeting criteria for decannulation were maintained for 1 more hour, assessed again for decannulation criteria, and then sacrificed at hour 5.
Coronary occlusion protocol
The coronary occlusion protocol has been described previously 3, 6, 7. A 6-F Amplatz Left 0.75 short-tipped guide catheter was engaged into the left main coronary artery under fluoroscopic guidance. A Balance Middleweight coronary guidewire (Abbott Vascular, Santa Clara, California) was advanced into the distal part of the left anterior descending artery (LAD). Acute coronary occlusion was induced using a 4.0 mm × 16 mm balloon inflated at 6 to 8 atm in the ostial LAD proximal to the first diagonal with angiographic evidence of complete coronary flow obstruction.
Induction of VF and initiation of CPR
If spontaneous VF did not occur within 5 min of balloon occlusion, VF was electrically induced at 5 min using a pacing wire inserted through the right jugular vein sheath into the right ventricle with stimulation of the myocardium at 300 cycles/min. Once VF was initiated, ventilation ceased and no treatment was provided for 5 min. This period represents the average time from an emergency call to first-aid arrival in the state of Minnesota for patients suffering cardiac arrest (CA). After 5 min of untreated VF, all animals received high-quality CPR with active chest decompression and an impedance threshold device (8). Chest compressions were performed using a specially designed automated device enabling control of compression and decompression amplitude. Compressions were supplied at a rate of 100 compressions/min with a 50% compression–decompression duty cycle using a servomotor-driven piston with a neoprene suction cup that provides adequate area for compressions. Compression depth was set to 20% of the anteroposterior diameter or 2 inches, whichever was greater. The animals were treated with active compression–decompression and impedance threshold device to optimize perfusion for prolonged CA (8). Chest compressions were paused only for defibrillation in the CPR-facilitated group until return of spontaneous circulation (ROSC) was achieved. In the ECMO-facilitated group, chest compressions were continuous until ECMO was placed. Ventilation during CPR was performed using zero positive end-expiratory pressure and a tidal volume of 6 ml/kg at a rate of 10 breaths/min.
Randomization
At the start of ACLS CPR, animals were randomized to receive either EPI or no EPI. At the end of ACLS CPR, they were further randomized to receive either ECMO-facilitated reperfusion or CPR-facilitated reperfusion (no ECMO). These randomizations resulted in 4 groups: EPI−, ECMO− (n = 8); EPI+, ECMO− (n = 8); EPI−, ECMO+ (n = 8); and EPI+, ECMO+ (n = 9).
Duration of CPR and resuscitation efforts
Resuscitation efforts were performed to closely simulate the out-of-hospital and in-hospital phases of the MRC clinical protocol. We describe each phase in detail subsequently.
Out-of-hospital CPR
The initial phase of CPR was termed basic life support (BLS), incorporating high-quality CPR alone. After 10 min of BLS, ACLS was initiated, which included continued high-quality CPR in addition to EPI, as determined by the experimental group. All animals randomized to EPI+ received 0.5 mg of intravenous EPI diluted in 5 ml of normal saline flushed in with an additional 5 ml of normal saline. EPI was given at the initiation of the ACLS phase and every 5 min thereafter. Animals randomized to EPI− received 10 ml of normal saline at the same time intervals. Investigators were blinded to drug therapy allocation. ACLS was continued for 35 min (45 min of total CPR). The duration of BLS and ACLS CPR corresponded to the care received by refractory VF CA patients from initial treatment by BLS providers, subsequent ACLS treatment, first EPI dose, emergency medical services transport, and admission to the CCL (2).
Cardiac catheterization–based resuscitation
After 45 min of CPR, pigs randomized to ECMO-facilitated reperfusion had ECMO cannulas inserted with ongoing CPR similar to the MRC clinical protocol. A 15-F arterial cannula was inserted in the right common femoral artery and a 21-F venous cannula was inserted in the right femoral vein and advanced as close to the right atrium as the length of the torso of the pigs allowed under direct fluoroscopy. Once on ECMO support, the LAD balloon was deflated and vessel patency was verified by angiography. Animals with CPR-facilitated reperfusion had their LAD balloon deflated at minute 45. On average, that occurred 5 min earlier than in the ECMO+ group.
Post–coronary reperfusion management
Animals without ECMO support
After LAD reperfusion (balloon deflation) in the ECMO− group, intravenous amiodarone 40 mg was given and CPR was continued up to 15 min with defibrillations every 3 min until ROSC. Animals in the EPI+ group continued to receive intravenous EPI 0.5 mg every 5 min during this phase. EPI− animals continued to receive saline as a control instead of EPI. ROSC was defined as an organized ECG rhythm with mean arterial pressure >60 mm Hg (9). If ROSC could not be achieved after 15 min, CPR was terminated and the pig was declared dead (total resuscitation duration of 60 min). Animals who had ROSC were maintained using fluid resuscitation and intravenous EPI to maintain mean arterial pressure >60 mm Hg. EPI− animals received intravenous EPI as needed after ROSC. Sodium bicarbonate infusion was used for metabolic acidosis as needed.
Animals with ECMO support
For ECMO+ animals the same strategy was used. ROSC was defined as an organized ECG rhythm with mean arterial pressure >60 mm Hg. EPI+ animals continued to receive intravenous EPI 0.5 mg every 5 min during this phase whereas EPI− animals continued to receive saline as a control. If defibrillation attempts were unsuccessful after 15 min while on full ECMO support following LAD reperfusion, the animals were declared dead. If defibrillation was successful, animals were maintained using fluid resuscitation and intravenous EPI to maintain mean arterial pressure >60 mm Hg. Sodium bicarbonate infusion was used for metabolic acidosis as needed.
Management of ECMO
The ECMO+ animals received ECMO support similar to MRC patients (2) using the CARDIOHELP System (Maquet Cardiovascular, Wayne, New Jersey) combining an oxygenator and an integrated centrifugal pump. The number of pump rotations per minute was adjusted to obtain maximum stable ECMO flow without venous collapse. Target flow was 2.8 to 3.5 l/min with venous pressure not exceeding −100 mm Hg. Fluid resuscitation and continuous intravenous EPI were administered to maintain mean arterial pressure >60 mm Hg as necessary. Gas exchange and blood gases were managed via the ECMO circuit.
Echocardiographic evaluation
Transthoracic echocardiograms were acquired at baseline, 1 h, and 4 h in surviving animals. Parasternal long- and short-axis views were obtained and analyzed by clinical cardiologists blinded to the intervention (4).
Arterial blood gases and lactic acid assessment
Arterial blood gas measurements (Gem 3000, Instrumentation Laboratory, Bedford, Massachusetts) were obtained at baseline, and every 5 min after initiation of CPR until 45 min of CPR were complete. Arterial blood gases were then collected every hour starting at 1-h post-LAD revascularization until death or sacrifice at 4 h. Lactic acid was quantified using the same samples at the same intervals.
Study endpoints
The primary study endpoint was survival at 4 h after completion of the ACLS phase of CPR (Figure 1). This time began with LAD reperfusion in the ECMO− animals and ECMO cannulation in the ECMO+ animals. ECMO− animals were considered survivors if they maintained a mean arterial pressure >60 mm Hg with an organized ECG at the end of the 4 h. In ECMO+ groups, survival was defined as an organized ECG rhythm, a mean arterial pressure >60 mm Hg, ECMO flow at least 60 ml/kg, and improving lactic acid blood levels with or without evidence of pulsatility (9).
The secondary endpoints included ROSC (as discussed previously), suitability for ECMO decannulation, hemodynamics, left ventricular ejection fraction (LVEF), and arterial blood lactic acid levels. ECMO+ animals that survived were further evaluated for ECMO decannulation with an ECMO turn down. Animals were deemed suitable for decannulation if they met 2 criteria: 1) sustained mean pulsatile aortic pressure of more than 60 mm Hg with a pulse pressure of >20 mm Hg on <1.5 l/min of ECMO support; and 2) LVEF >30% by echocardiographic evaluation. Animals meeting these criteria were left at the lowest flow rate for another hour with continuous observation. The decannulation criteria were again assessed at hour 5 to ensure stable hemodynamics. At the end of 5 h, the ECMO was turned off and surviving animals were euthanized. Animals surviving to 4 h but failing to meet decannulation criteria were considered meeting the primary endpoint of 4-h survival.
Statistical analysis
Values were expressed as mean ± SEM and percentages. The statistical analysis was performed with GraphPad Prism 7 (GraphPad Software, La Jolla, California). A 2-way analysis of variance was used to evaluate lactic acid curves. Unpaired t test was used to compare numerical values and chi-square test was used to compare categorical values from the 2 different groups of animals. The effect of EPI (EPI+) and ECMO (ECMO+) on 4-h survival was assessed with Fisher’s exact test. The interaction of EPI and ECMO was tested with a logistic model including an interaction term. The survival of each group over time was assessed using Kaplan-Meier survival curves analyzed with a Mantel-Cox test. A p value of <0.05 was considered significant.
Results
Survival to 4 h
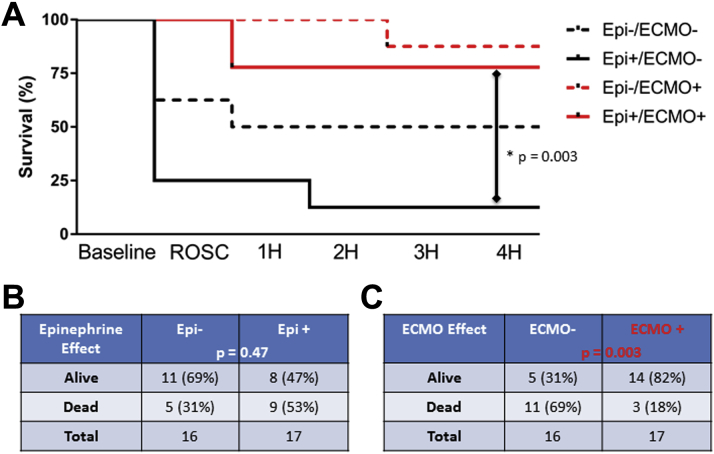
Survival curves for all groups are shown in Figure 2A, with group analysis in Figures 2B and 2C.
Figure 2.
Clinical Outcomes by Group
(A) Kaplan-Meier survival curves demonstrating survival at each time point noted. Survival tables showing alive and dead animals at 4 h based on the presence or absence of (B) Epi or (C) ECMO. The p values are noted. There was no interaction between Epi and ECMO treatments (p = 0.77). Abbreviations as in Figure 1.
EPI effect
There was no significant difference in 4-h survival between animals receiving EPI versus placebo (EPI+ 47% vs. EPI− 69%; p = 0.47) (Figure 2B).
ECMO effect
Animals randomized to ECMO support with ECMO-facilitated reperfusion had significantly improved 4-h survival (ECMO+ 82% vs. ECMO− 31%; p = 0.003) (Figure 2C). The primary ECMO effect appeared to be achievement and maintenance of ROSC, accomplished in 100% of ECMO+ animals versus 44% of ECMO− animals (p = 0.001). There was no interaction between EPI and ECMO (p = 0.77 for interaction). However, a significant difference was observed between the survival curves of the EPI+, ECMO− and EPI+, ECMO+ groups, suggesting that ECMO may be particularly beneficial when EPI is given during resuscitation (p = 0.003).
Hemodynamics
There were no significant differences in hemodynamics between any of the groups during the initial 10 min of CPR (BLS phase) (Figures 3A to 3D). However, after 30 min of CPR, EPI+ animals had significantly higher aortic pressures (systolic blood pressure 83 mm Hg vs. 65 mm Hg, p = 0.0013; diastolic blood pressure 35 mm Hg vs. 18 mm Hg, p = 0.0004) compared with EPI− animals (Figures 3E and 3F). These differences continued through completion of the ACLS phase at 45 min of CPR. At 45 min of CPR, the CPP had also become significantly higher in EPI+ compared with EPI− animals (25 mm Hg vs. 15 mm Hg; p = 0.04). Initiating ECMO led to physiologic CPP and circulating blood flow. The effect was sustained from LAD balloon deflation to 4 h.
Figure 3.
Hemodynamic Monitoring at Baseline, During Prolonged CPR, and After ROSC for Each Study Group
The (A) aortic systolic blood pressure, (B) aortic diastolic blood pressure, (C) right atrial pressure, and (D) coronary perfusion pressure (CPP) were measured throughout prolonged CPR and after ROSC was achieved. All pressures were measured in mm Hg. The mean pressures for no Epi (Epi–) and Epi (Epi+) animals at (E) 30 min and (F) 45 min are shown in table form with p values as shown. There was no significant difference between the ECMO– and ECMO+ groups. Error bars and error values in the tables represent SEM. BL = baseline; DBP = diastolic blood pressure; SBP = systolic blood pressure; other abbreviations as in Figure 1.
Arterial blood gasses and lactic acid levels
There were no significant differences between groups in any arterial blood gas component (Table 1). EPI led to significantly higher lactic acid levels at the end of the out-of-hospital ACLS phase of CPR and around 45 min of resuscitation (Figure 4). Lactic acid levels recovered in all groups during the 4-h ROSC phase. Although this decrease was similar between groups, the CPR-only (ECMO−) groups had substantial attrition of the sickest animals at the ROSC and 1 h time points, which may reduce the observed lactic acid levels. The low numbers of surviving ECMO− animals did not allow for statistical comparisons.
Table 1.
Arterial Blood Gas Results at Baseline, During Prolonged CPR, and After ROSC
| Baseline | 15 min | 30 min | 45 min | 60 min | 2 h | 4 h | |
|---|---|---|---|---|---|---|---|
| EPI−, ECMO− | CPR | CPR | CPR | ROSC | ROSC | ROSC | |
| pH | 7.47 ± 0.07 | 7.33 ± 0.18 | 7.25 ± 0.21 | 7.17 ± 0.29 | 7.34 ± 0.05 | 7.39 ± 0.10 | 7.40 ± 0.15 |
| PaCO2 | 44 ± 2.5 | 33 ± 3.9 | 36 ± 5.7 | 38 ± 4.3 | 42 ± 7.5 | 39 ± 4 | 43 ± 4.5 |
| PaO2 | 97 ± 6.1 | 99 ± 4.6 | 127 ± 14.3 | 72 ± 23.2 | 107 ± 36 | 99 ± 29 | 95 ± 46 |
| HCO2 | 31 ± 2.1 | 18 ± 2.9 | 15 ± 1.4 | 12 ± 1.79 | 23 ± 3.5 | 23 ± 2.5 | 27 ± 3 |
| %SaO2 | 98 ± 6.1 | 94 ± 6.1 | 95 ± 2.5 | 90 ± 2.9 | 97 ± 2.5 | 98 ± 9 | 96 ± 5.5 |
| EPI+, ECMO− | CPR | CPR | CPR | ROSC | ROSC | ROSC | |
| pH | 7.48 ± 0.04 | 7.36 ± 0.04 | 7.31 ± 0.04 | 7.25 ± 0.04 | 7.28 ± 0.07 | 7.24 | 7.21 |
| PaCO2 | 41 ± 3.2 | 31 ± 2.5 | 27 ± 3.2 | 36 ± 2.5 | 30 ± 8.6 | 29 | 32 |
| PaO2 | 105 ± 9.6 | 241 ± 30.7 | 189 ± 22.5 | 151 ± 29 | 189 ± 64 | 135 | 152 |
| HCO2 | 30 ± 1.8 | 18 ± 2.1 | 13 ± 1.4 | 14 ± 1.4 | 13 ± 2.1 | 11 | 10 |
| %SaO2 | 98 ± 2.5 | 99 ± 2.5 | 99 ± 1.8 | 94 ± 3.2 | 97 ± 5 | 96 | 99 |
| EPI−, ECMO+ | CPR | CPR | CPR | ECMO | ECMO | ECMO | |
| pH | 7.48 ± 0.04 | 7.36 ± 0.004 | 7.32 ± 0.07 | 7.28 ± 0.01 | 7.28 ± 0.07 | 7.29 ± 0.04 | 7.27 ± 0.12 |
| PaCO2 | 39 ± 1.4 | 30 ± 3.2 | 30 ± 5.4 | 34 ± 3.2 | 32 ± 1.4 | 30 ± 1.8 | 30 ± 2.7 |
| PaO2 | 107 ± 8.9 | 103 ± 4.3 | 135 ± 12.5 | 138 ± 17 | 290 ± 35 | 291 ± 29 | 292 ± 42 |
| HCO2 | 29 ± 0.71 | 16 ± 2.5 | 14 ± 1.1 | 14 ± 2.5 | 15 ± 1.4 | 15 ± 2.5 | 14 ± 2.3 |
| %SaO2 | 98 ± 2.1 | 96 ± 2.9 | 95 ± 3.2 | 88 ± 5 | 100 ± 0 | 100 ± 0 | 100 ± 0 |
| EPI+, ECMO+ | CPR | CPR | CPR | ECMO | ECMO | ECMO | |
| pH | 7.47 ± 0.03 | 7.32 ± 0.07 | 7.27 ± 0.03 | 7.26 ± 0.07 | 7.29 ± 0.04 | 7.32 ± 0.04 | 7.36 ± 0.08 |
| PaCO2 | 38 ± 1.3 | 38 ± 2.3 | 44 ± 4.3 | 43 ± 2.7 | 42 ± 1.2 | 46 ± 1.9 | 44 ± 1.2 |
| PaO2 | 103 ± 7.7 | 206 ± 25 | 163 ± 13.3 | 176 ± 37.7 | 227 ± 31 | 322 ± 36 | 272 ± 36 |
| HCO2 | 31 ± 0.67 | 23 ± 1.67 | 20 ± 0.67 | 19 ± 1 | 22 ± 1.9 | 22 ± 1.9 | 22 ± 1.9 |
| %SaO2 | 98 ± 0.33 | 99 ± 1 | 91 ± 3.3 | 99 ± 3.3 | 100 ± 0 | 100 ± 0 | 100 ± 0 |
Values are mean ± SEM. Data are shown per group assigned by the 2 × 2 study design. All partial pressures are shown in mm Hg. There are no error values noted for the EPI+, ECMO− group at 2 h and 4 h because only 1 animal survived to those time points in that group.
%SaO2 = oxygen saturation in arterial blood; CPR = cardiopulmonary resuscitation; ECMO = extracorporeal membrane oxygenation; EPI = epinephrine; PaCO2 = partial pressure of carbon dioxide in arterial blood; PaO2 = partial pressure of oxygen in arterial blood; ROSC = return of spontaneous circulation.
Figure 4.
Arterial Blood Lactic Acid Levels in Each Group at BL, During Prolonged CPR, and After ROSC
Arterial lactic acid levels are shown for (A) each individual group and (B) animals grouped into those who received either epinephrine or placebo. Error bars represent SEM. Statistical significance (p < 0.05). BL = baseline; other abbreviations as in Figure 1.
ROSC
All animals that achieved ROSC were successfully defibrillated after LAD reperfusion, well after 45 min of CPR. Animals receiving ECMO+ had higher rates of ROSC versus ECMO− pigs (100% vs. 44%; p = 0.0003). There were no significant ROSC differences in EPI+ versus EPI− animals (65% vs. 81%, respectively; p = 0.438).
Left ventricular function
In all surviving animals, LVEF decreased from 62 ± 7% at baseline to 25 ± 12% at 1 h after LAD reperfusion. The LVEF recovered to 37 ± 8% at hour 4. LVEF was independent of treatment groups. No differences were detected between treatment groups due to the limited number of survivors in the ECMO− groups.
Decannulation
Of the 7 animals that survived to 4 h in the EPI+, ECMO+ group, 4 met criteria for decannulation (44% of the overall group), whereas 5 of 7 animals in the EPI−, ECMO+ group (63% of the overall group) were suitable for decannulation (p = NS). All animals meeting criteria for decannulation at hour 4 also met criteria at hour 5.
Discussion
This study demonstrates that ECMO-facilitated reperfusion significantly improves short-term survival compared with CPR-facilitated reperfusion without ECMO support in the setting of refractory VF caused by coronary ischemia. Our study helps substantiate the scientific hypothesis providing the foundation for the MRC protocol (2).
In addition, this study shows that the use of EPI in the setting of ischemic refractory VF offered no advantage, as it failed to improve survival or ROSC rates compared with placebo. Further, EPI-treated animals had higher arterial blood lactic acid levels at the completion of the ACLS period before ECMO initiation or LAD reperfusion. Higher arterial lactic acid level was associated with worse prognosis in our clinical MRC protocol (2) and could be considered a marker for poor outcomes.
Cardiac function was similar in surviving animals of all groups. Even animals that did not have ECMO support had some degree of acute left ventricular function recovery within 4 h. The number of surviving ECMO− animals was small (1 for EPI+ and 4 for EPI− treated animals), preventing statistical comparisons. Cardiac functional recovery as assessed by LVEF was significantly faster (4 h) than that observed for humans encountering a similar injury in our MRC protocol (≥72 h) (2). This significant difference in our porcine model may limit the translation of cardiac function recovery results to humans.
Several previously published studies have evaluated ECMO as a resuscitation tool in different animal models of CA 10, 11, 12, 13, 14, 15, 16. They found improved clinical outcomes using ECMO, but the experimental models did not attempt to reproduce refractory CA triggered by acute myocardial infarction. Coronary ischemia is an important aspect of our model because it is the main cause of VF CA 17, 18. Further, presence of arterial occlusion influences ROSC rate (19). Timing of coronary reperfusion and resulting degree of myocardial damage also potentially influence subsequent left ventricular functional recovery 19, 20.
Prolonged CPR (45 min) was associated with a very low ROSC rate in the ECMO− group. Prolonged CPR combined with coronary ischemia decreases the probability of ROSC in humans 20, 21. The significant ROSC rate and subsequent survival advantage in the ECMO+ group seen in our study supports the use of ECMO-facilitated PCI compared with CPR-facilitated PCI. This is an important finding because the role of ECMO in human CA is debated, with no randomized studies, and retrospective studies yielding conflicting results (22). Further, this experimental model provides an avenue for additional refinement and experimentation with regard to the therapies provided.
Lactic acid level on arrival to the CCL has been associated with poor outcomes in our MRC protocol experience (2). In this animal study, all surviving animals demonstrated improving lactic acid levels. However, the significant attrition of animals in the ECMO− groups over time likely selects for lower lactic acid levels, thus producing falsely normalizing values. Despite having higher CPP and aortic pressures, animals that received intravenous EPI during the ACLS phase had higher lactic acid levels at 45 min of CPR. This may represent reduced overall perfusion during CPR, reduced local perfusion due to vasoconstriction, increased transport of lactic acid out of tissues (23), or enhanced glycolysis induced by EPI (24). ECMO+ animals exhibited improving lactic acid levels during the 4 h post-ECMO. However, the rate of decline in arterial lactic acid levels was reduced compared to the surviving animals without ECMO. This may be due to the ability of ECMO to salvage animals that have sustained a more severe injury, whereas those animals do not survive in the ECMO− group. Alternatively, the arterial ECMO cannula may be partially or totally occluding the common femoral artery in ECMO+ animals, leading to leg ischemia and lactic acid production. Whereas in humans a distal perfusion cannula would be placed in the superficial femoral artery distal to the access for the large arterial cannula, the distal vasculature is too small to accommodate this in pigs, leaving their limb potentially ischemic.
Study limitations
First, our animal model may have limited translatability to humans, despite remarkable similarities to our MRC clinical population.
The model of coronary ischemia imperfectly represents real-life conditions, with simulated revascularization consisting only of balloon deflation. To our knowledge, no model incorporating atherosclerosis, calcification, or thrombus associated with coronary occlusion is currently available in pigs 25, 26. In addition, no defibrillations were provided during the ACLS phase, resulting in prolonged resuscitation for all animals. This may cause inclusion of animals that would otherwise have achieved ROSC, which may reduce the observed injury and minimize the differences between groups. This would likely limit the observed effectiveness of ECMO. Prior studies performing standard ACLS in this same model of ischemic VF have demonstrated a low ROSC rate, suggesting a minimal impact in this study (3). This may also eliminate the injury typically caused by repeated defibrillation in clinical situations. Although this injury is not thought to be the predominant factor in cardiac dysfunction after prolonged CA, its contribution cannot be assessed in this study. All animals were treated the same with regard to defibrillation, independent of their treatment group, such that comparisons represent the effects of the experimental treatments. The use of anesthesia, required for animal studies, may also limit the differences observed in the study. Inhaled anesthetic agents, including isoflurane, which was used in this study, can provide cardioprotective effects (27). As this was used consistently in all animals, it may negate differences in cardiac outcomes observed between the 2 groups. In addition, this study did not include neurologic assessment. Potential leg ischemia caused by ECMO cannula femoral artery occlusion complicates assessment of long-term survival. However, the size of the pigs used for the study was limited, as larger pigs have very long torsos, such that the venous ECMO cannula cannot drain the right atrium reliably, and smaller animals have prohibitive femoral artery sizes for cannulation. Finally, this study might have been underpowered for an interaction between EPI and ECMO in survival given the minimal effect and trend toward worse outcomes observed with EPI.
Conclusions
ECMO-facilitated coronary reperfusion significantly improved 4-h survival compared with CPR-facilitated reperfusion in a pig model of ischemia-induced refractory VF CA and prolonged CPR. ECMO support enabled cardiac recovery and hemodynamic stability within 4 h. EPI use during prolonged CPR did not affect survival.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: CA causes severe systemic injury, which worsens as the duration of resuscitation efforts increases. ECMO has been used to provide hemodynamic support to halt progressive injury and bridge the patient to recovery after prolonged refractory CA. Stabilization with ECMO also allows further diagnostic and therapeutic interventions including coronary angiography to proceed to address the underlying etiology of the CA. EPI, commonly used during resuscitation efforts, may not provide benefit during prolonged arrest when the effects of repeated EPI doses may cause additional injury.
TRANSLATIONAL OUTLOOK: ECMO significantly improves short-term survival with hemodynamic stabilization after refractory VF CA and prolonged CPR. Although further studies are necessary to assess the potential long-term benefits, 53% of animals treated with ECMO were stable for decannulation at 4 h supporting the use of ECMO as a temporary bridge to recovery. EPI use did not provide any benefit to survival in this model of prolonged CA. As ECMO is more widely incorporated into clinical care and treatment of prolonged CA becomes feasible, reassessment of commonly used therapies will be necessary to evaluate their effects in prolonged arrest.
Acknowledgments
The authors would like to thank Maquet Cardiopulmonary GmbH; Rastatt, Germany, part of the GETINGE GROUP for donating all the ECMO circuits that made this important and timely investigation possible.
Footnotes
This study was funded by a grant (R01HL108926) to Dr. Yannopoulos from the National Institutes of Health National Heart, Lung, and Blood Institute. The authors have reported that they have no relationships relevant to the contents of this paper to disclose. Drs. Bartos and Voicu contributed equally to this work.
All authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
References
- 1.Link M.S., Berkow L.C., Kudenchuk P.J. Part 7: Adult Advanced Cardiovascular Life Support: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015;132:S444–S464. doi: 10.1161/CIR.0000000000000261. [DOI] [PubMed] [Google Scholar]
- 2.Yannopoulos D., Bartos J.A., Martin C. Minnesota Resuscitation Consortium's Advanced Perfusion and Reperfusion Cardiac Life Support Strategy for Out-of-Hospital Refractory Ventricular Fibrillation. J Am Heart Assoc. 2016;5:e003732. doi: 10.1161/JAHA.116.003732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yannopoulos D., Bartos J.A., George S.A. Sodium nitroprusside enhanced cardiopulmonary resuscitation improves short term survival in a porcine model of ischemic refractory ventricular fibrillation. Resuscitation. 2017;110:6–11. doi: 10.1016/j.resuscitation.2016.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Segal N., Matsuura T., Caldwell E. Ischemic postconditioning at the initiation of cardiopulmonary resuscitation facilitates functional cardiac and cerebral recovery after prolonged untreated ventricular fibrillation. Resuscitation. 2012;83:1397–1403. doi: 10.1016/j.resuscitation.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartos J.A., Matsuura T.R., Sarraf M. Bundled postconditioning therapies improve hemodynamics and neurologic recovery after 17 min of untreated cardiac arrest. Resuscitation. 2015;87:7–13. doi: 10.1016/j.resuscitation.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartos J.A., Matsuura T.R., Tsangaris A. Intracoronary poloxamer-188 prevents reperfusion injury in a porcine model of ST-segment elevation myocardial infarction. J Am Coll Cardiol Basic Trans Science. 2016;1:224–234. doi: 10.1016/j.jacbts.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yannopoulos D., Zviman M., Castro V. Intra-cardiopulmonary resuscitation hypothermia with and without volume loading in an ischemic model of cardiac arrest. Circulation. 2009;120:1426–1435. doi: 10.1161/CIRCULATIONAHA.109.848424. [DOI] [PubMed] [Google Scholar]
- 8.Aufderheide T.P., Frascone R.J., Wayne M.A. Standard cardiopulmonary resuscitation versus active compression-decompression cardiopulmonary resuscitation with augmentation of negative intrathoracic pressure for out-of-hospital cardiac arrest: a randomised trial. Lancet. 2011;377:301–311. doi: 10.1016/S0140-6736(10)62103-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reynolds J.C., Salcido D.D., Sundermann M.L., Koller A.C., Menegazzi J.J. Extracorporeal life support during cardiac arrest resuscitation in a porcine model of ventricular fibrillation. J Extra Corpor Technol. 2013;45:33–39. [PMC free article] [PubMed] [Google Scholar]
- 10.Trummer G., Foerster K., Buckberg G.D. Superior neurologic recovery after 15 minutes of normothermic cardiac arrest using an extracorporeal life support system for optimized blood pressure and flow. Perfusion. 2014;29:130–138. doi: 10.1177/0267659113497776. [DOI] [PubMed] [Google Scholar]
- 11.Foerster K., D'Inka M., Beyersdorf F. Prolonged cardiac arrest and resuscitation by extracorporeal life support: favourable outcome without preceding anticoagulation in an experimental setting. Perfusion. 2013;28:520–528. doi: 10.1177/0267659113495081. [DOI] [PubMed] [Google Scholar]
- 12.Liakopoulos O.J., Allen B.S., Buckberg G.D. Resuscitation after prolonged cardiac arrest: role of cardiopulmonary bypass and systemic hyperkalemia. Ann Thorac Surg. 2010;89:1972–1979. doi: 10.1016/j.athoracsur.2010.02.052. [DOI] [PubMed] [Google Scholar]
- 13.Reynolds J.C., Salcido D., Koller A.C. Tissue oximetry by near-infrared spectroscopy in a porcine model of out-of-hospital cardiac arrest and resuscitation. Resuscitation. 2013;84:843–847. doi: 10.1016/j.resuscitation.2012.11.031. [DOI] [PubMed] [Google Scholar]
- 14.Menegazzi J.J., Salcido D.D., Housler G.J., Logue E.S. Feasibility of initiating extracorporeal life support during mechanical chest compression CPR: a porcine pilot study. Resuscitation. 2012;83:130–133. doi: 10.1016/j.resuscitation.2011.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mlcek M., Ostadal P., Belohlavek J. Hemodynamic and metabolic parameters during prolonged cardiac arrest and reperfusion by extracorporeal circulation. Physiol Res. 2012;61(Suppl 2):S57–S65. doi: 10.33549/physiolres.932454. [DOI] [PubMed] [Google Scholar]
- 16.Belohlavek J., Mlcek M., Huptych M. Coronary versus carotid blood flow and coronary perfusion pressure in a pig model of prolonged cardiac arrest treated by different modes of venoarterial ECMO and intraaortic balloon counterpulsation. Crit Care. 2012;16:R50. doi: 10.1186/cc11254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spaulding C.M., Joly L.M., Rosenberg A. Immediate coronary angiography in survivors of out-of-hospital cardiac arrest. N Engl J Med. 1997;336:1629–1633. doi: 10.1056/NEJM199706053362302. [DOI] [PubMed] [Google Scholar]
- 18.Garcia S., Drexel T., Bekwelem W. Early access to the cardiac catheterization laboratory for patients resuscitated from cardiac arrest due to a shockable rhythm: the Minnesota Resuscitation Consortium Twin Cities Unified Protocol. J Am Heart Assoc. 2016;5:e0002670. doi: 10.1161/JAHA.115.002670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu M.Y., Tseng Y.H., Chang Y.S., Tsai F.C., Lin P.J. Using extracorporeal membrane oxygenation to rescue acute myocardial infarction with cardiopulmonary collapse: the impact of early coronary revascularization. Resuscitation. 2013;84:940–945. doi: 10.1016/j.resuscitation.2012.12.019. [DOI] [PubMed] [Google Scholar]
- 20.Sideris G., Magkoutis N., Sharma A. Early coronary revascularization improves 24h survival and neurological function after ischemic cardiac arrest. A randomized animal study. Resuscitation. 2014;85:292–298. doi: 10.1016/j.resuscitation.2013.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grunau B., Reynolds J.C., Scheuermeyer F.X. Comparing the prognosis of those with initial shockable and non-shockable rhythms with increasing durations of CPR: Informing minimum durations of resuscitation. Resuscitation. 2016;101:50–56. doi: 10.1016/j.resuscitation.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 22.Maekawa K., Tanno K., Hase M., Mori K., Asai Y. Extracorporeal cardiopulmonary resuscitation for patients with out-of-hospital cardiac arrest of cardiac origin: a propensity-matched study and predictor analysis. Crit Care Med. 2013;41:1186–1196. doi: 10.1097/CCM.0b013e31827ca4c8. [DOI] [PubMed] [Google Scholar]
- 23.Grip J., Jakobsson T., Hebert C. Lactate kinetics and mitochondrial respiration in skeletal muscle of healthy humans under influence of adrenaline. Clin Sci (Lond) 2015;129:375–384. doi: 10.1042/CS20140448. [DOI] [PubMed] [Google Scholar]
- 24.Levy B. Lactate and shock state: the metabolic view. Curr Opin Crit Care. 2006;12:315–321. doi: 10.1097/01.ccx.0000235208.77450.15. [DOI] [PubMed] [Google Scholar]
- 25.Bellera N., Barba I., Rodriguez-Sinovas A. Single intracoronary injection of encapsulated antagomir-92a promotes angiogenesis and prevents adverse infarct remodeling. J Am Heart Assoc. 2014;3:e000946. doi: 10.1161/JAHA.114.000946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hanes D.W., Wong M.L., Jenny Chang C.W. Embolization of the first diagonal branch of the left anterior descending coronary artery as a porcine model of chronic trans-mural myocardial infarction. J Transl Med. 2015;13:187. doi: 10.1186/s12967-015-0547-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bartos J.A., Debaty G., Matsuura T., Yannopoulos D. Post-conditioning to improve cardiopulmonary resuscitation. Curr Opin Crit Care. 2014;20:242–249. doi: 10.1097/MCC.0000000000000087. [DOI] [PubMed] [Google Scholar]