Abstract
Misty mice (m/m) have a loss of function mutation in Dock7 gene, a guanine nucleotide exchange factor, resulting in low bone mineral density, uncoupled bone remodeling and reduced bone formation. Dock7 has been identified as a modulator of osteoblast number and in vitro osteogenic differentiation in calvarial osteoblast culture. In addition, m/m exhibit reduced preformed brown adipose tissue innervation and temperature as well as compensatory increase in beige adipocyte markers. While the low bone mineral density phenotype is in part due to higher sympathetic nervous system (SNS) drive in young mice, it is unclear what effect aging would have in mice homozygous for the mutation in the Dock7 gene. We hypothesized that age-related trabecular bone loss and periosteal envelope expansion would be altered in m/m. To test this hypothesis, we comprehensively characterized the skeletal phenotype of m/m at 16, 32, 52, and 78 wks of age. When compared to age-matched wild-type control mice (+/+), m/m had lower areal bone mineral density (aBMD) and areal bone mineral content (aBMC). Similarly, both femoral and vertebral BV/TV, Tb.N., and ConnD were decreased in m/m while there was also an increase in Tb.Sp. As low bone mineral density and decreased trabecular bone were already present at 16 wks of age in m/m and persisted throughout life, changes in age-related trabecular bone loss were not observed highlighting the role of Dock7 in controlling trabecular bone acquisition or bone loss prior to 16 wks of age. Cortical thickness was also lower in the m/m across all ages. Periosteal and endosteal circumferences were higher in m/m compared to +/+ at 16 wks. However, endosteal and periosteal expansion were attenuated in m/m, resulting in m/m having lower periosteal and endosteal circumferences by 78 wks of age compared to +/+, highlighting the critical role of Dock7 in appositional bone expansion. Histomorphometry revealed that osteoblasts were nearly undetectable in m/m and marrow adipocytes were elevated 3.5 fold over +/+ (p=0.014). Consistent with reduced bone formation, osteoblast gene expression of Alp, Col1a1, Runx-2, Sp7, and Bglap was significantly decreased in m/m whole bone. Furthermore, markers of osteoclasts were either unchanged or suppressed. Bone marrow stromal cell migration and motility were inhibited in culture and changes in senescence markers suggest that osteoblast function may also be inhibited with loss of Dock7 expression in m/m. Finally, increased Oil Red O staining in m/m ear mesenchymal stem cells during adipogenesis highlights a potential shift of cells from the osteogenic to adipogenic lineages. In summary, loss of Dock7 in the aging m/m resulted in an impairment of periosteal and endocortical envelope expansion, but did not alter age-related trabecular bone loss. These studies establish Dock7 as a critical regulator of both cortical and trabecular bone mass, and demonstrate for the first time a novel role of Dock7 in modulating compensatory changes in the periosteum with aging.
Keywords: Misty, aging, bone, Dock7, osteoporosis, periosteal
Introduction
Osteoporosis is characterized by enhanced skeletal fragility as a result of uncoupled bone remodeling, i.e. decreased bone formation, increased bone resorption, or both. Osteopenia predisposes to osteoporosis and affects approximately 54 million Americans [1]. Moreover, the two million osteoporosis-related fractures treated per year account for $19 billion in health care costs [2]. Age is a major and independent risk factor for bone loss leading to osteoporosis: half of women and a quarter of men over the age of 50 will experience an osteoporosis related fracture in their lifetime [1]. Aging mouse models have some of the same characteristics of age-related bone loss in humans, including decreased bone formation, increased bone resorption, increased marrow adiposity, decreased trabecular and cortical bone, and expansion of the periosteal envelope with age [3, 4]. Furthermore, bone is compromised with estrogen deficiency as in ovariectomy and menopause [5, 6].
We previously demonstrated that m/m have very low bone mineral density, as well as reduced trabecular and cortical bone volume fraction [4]. The low bone mass phenotype in the m/m results from a mutation in the Dock7 gene rendering the protein levels undetectable [4, 7–10]. DOCK7 is a guanine nucleotide exchange factor (GEF) and member of the Dock180 family of proteins [11, 12]. Dock7 interacts with Rac1, Cdc42, and RhoA catalyzing exchange of guanosine diphosphate (GDP) for guanosine triphosphate (GTP) [8, 9, 13–16]. Like other family members, DOCK7 contains a conserved dock homology region (DHR)-1 and DHR-2 domains. The DHR-1 domain interacts with phosphatidylinositol (3,5)–bisphosphate while DHR-2 domain contains the GTPase activity [12]. GTPase-dependent Dock7 activity has been implicated in ErbB2 and ErbB4 signaling [9, 17]. Further work has revealed that Dock7 also has GTPase independent activity interacting with TACC3 to control kinetic nuclear migration in radial glial cells [18].
Dock7 has been linked to developmental changes in the nervous system including primary axonal elongation, Schwann cell migrations, and the aforementioned radial glial cell migration [8, 9, 17, 18]. Humans homozygous for mutations in the Dock7 gene display signs of epilepsy, intellectual disability and cortical blindness [19]. Changes in bone metabolism have yet to be explored in this population.
The bone phenotype is more prominent in female m/m which have decreased bone formation, increased bone resorption and increased marrow adiposity. Furthermore, m/m have decreased innervation of brown adipose tissue and reduced interscapular temperature, suggesting a reduction of brown fat thermogenesis. In addition, thermogenic gene expression in response to cold was elevated in white fat, suggesting increased SNS signaling, which was in part responsible for bone loss in this model [4]. Studies have shown that the SNS signals to the osteoblast, via the β2-adrenergic receptor (β2AR), decreasing bone formation and increasing osteoclastogenesis through an upregulation of Rankl expression [20–22]. Consistent with a phenotype that was partially dependent upon the SNS, treatment with the β-adrenergic receptor antagonist, propranolol, partially rescued the bone loss in the m/m [4].
We have previously shown that the m/m have low bone mass at 8 and 16 wks of age [4]. To determine whether the m/m altered characteristics of aging bone, we aged +/+ and m/m to 78 wks of age and examined body composition, bone micro-architecture, and bone remodeling. Our current study demonstrated that female m/m have a persistent reduction in trabecular bone, a defect in cortical expansion both in the periosteal and endocortical envelopes, as well as increased marrow adiposity. Although the reduction in trabecular bone and increases in marrow adiposity recapitulate age-related bone loss, the absence of periosteal expansion in m/m suggests an important role for Dock7 in this particular characteristic of aging bone.
Methods
Mice
B6.D2(BKS)-Dock7m/J (Misty, m/m) mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA), backcrossed to C57BL/6J (Jackson Laboratory), and bred to produce m/m and wild-type littermate control mice (+/+). Mice were housed in polycarbonate cages on sterilized paper bedding and maintained under 14:10 hour light:dark cycles in the barrier, AAALAC-accredited animal facility at Maine Medical Center Research Institute. All studies were reviewed and approved by the Institutional Animal Care and Use Committee of Maine Medical Center, and followed the NIH guidelines for the Care and Use of Laboratory Animals. The experiments herein utilized only female m/m because female m/m have a more prominent low bone mass skeletal phenotype compared to male through 16 wks of age [4].
Dual-energy X-ray absorptiometry (DXA)
DXA for total and femoral areal bone mineral density (aBMD, g/cm2), areal bone mineral content (aBMC, g) and whole body composition exclusive of the head was performed using the PIXImus densitometer (GE-Lunar, Fairfield, CT, USA). The PIXImus was calibrated daily with a phantom provided by the manufacturer.
Micro-computed tomography (μCT)
Micro-architecture of trabecular bone in the distal femoral metaphysis, L5 vertebral body, and cortical bone at the femoral mid-diaphysis was analyzed by a high resolution μCT system (μCT40, Scanco Medical AG, Bruttisellen, Switzerland). Scans were acquired using a 10 μm3 isotropic voxel size, 70 kVP x-ray tube potential, 114 mA x-ray tube intensity, and 200 ms integration time. Trabecular bone in the distal femoral metaphysis was evaluated in a 1500 μm (150 transverse slices) long region beginning 200 μm superior to the distal growth plate and extending proximally. Trabecular bone in the L5 vertebral body was evaluated in a region beginning 100 μm inferior to the cranial growth plate and extending distally to 100 μm superior to the caudal growth plate. Trabecular bone in the femur and L5 vertebral body was segmented from soft-tissue using thresholds of 300 mgHA/cm3 and 386 mgHA/cm3, respectively. Measurements of trabecular regions included trabecular bone volume fraction (Tb.BV/TV, %), trabecular number (Tb.N., mm−1), trabecular thickness (Tb.Th., μm), trabecular separation (Tb.Sp., μm), connectivity density (ConnD, 1/mm3), and structure model index (SMI). For cortical bone, soft tissue was segmented using a threshold of 696 mgHA/cm3 and then was evaluated in a 500 μm (50 slice) long region at the femoral mid-diaphysis to measure total cross-sectional area (Tt.Ar, mm2), cortical bone area (Ct. Ar, mm2), medullary area (Ma.Ar, mm2), bone area fraction (Ct.Ar/Tt.Ar, %), cortical tissue mineral density (Ct.TMD, mgHA/cm3), cortical thickness (Ct.Th, mm), cortical porosity (%), and maximum, minimum, and polar moments of inertia (Imax, Imin, and pMOI, mm4. All scans were analyzed using manufacturer software (Scanco, version 4.05). Acquisition and analysis of μCT data were performed in accordance with published guidelines [23].
Histomorphometry
Static and dynamic histomorphometry measurements were performed in +/+ and m/m at 52 wks of age as previously described [4]. Mice were intraperitoneally injected with 20 mg/kg calcein and 40 mg/kg demeclocycline 9 days and 2 days, respectively, prior to sacrifice. Tibias were analyzed as described and standard nomenclature was used [24–26]. The calculations were performed using the Osteomeasure analysis software. Trabecular and cortex parameters were analyzed in 4 areas, total area was 1.3 mm width × 0.9 mm height (1 area was 0.65 mm width × 0.45 mm height). Mineral apposition rate (MAR) was calculated by the distance between 2 labels divided by number of days of interval. For the marrow adipocytes, the number and area of adipocyte ghosts were quantified in the same areas used to analyze trabecular structure and dynamic parameters. Osteomeasure (Osteometrics, Decatur, GA), was used to calculate an adipocyte number and volume and both values were divided by total area.
Serum Protein Parameters
For P1NP (AC-33F1) and CTx (AC-06F1), serum protein levels were measured using commercially available kits from IDS (Gaithersburg, MD) according to the manufacturer’s instructions. The assay sensitivities were 0.7 and 2 ng/mL for P1NP and CTx, respectively. The intra-assay variations were 6.3 and 6.9%, and the inter-assay variations were 8.5 and 12% respectively, for both assays. All measurements were performed in duplicate. For RANKL (MTR00), serum levels were measured using commercially available kit from R&D Systems (Minneapolis, MN) according to the manufacturer’s instructions. The assay sensitivity was less than 5 pg/mL. The intra- and inter-assay variations were 4.3 and 6.9 pg/mL respectively. All measurements were performed in duplicate.
Brown adipose tissue (BAT) and femurs histology
BAT was fixed in 10% neutral-buffered formalin immediately following sacrifice for 48 hours followed by storage in 70% ethanol. Samples were then paraffin embedded, sectioned, and stained with either an anti-uncoupling protein 1 (UCP-1; ab23841, Abcam, Cambridge, MA, USA) antibody at the Maine Medical Center Research Institute Histology Core or an anti-tyrosine hydroxylase (TH) antibody at the Michigan State University Histology Laboratory.
To view marrow adipocytes, femurs were fixed in 10% neutral-buffered formalin immediately following sacrifice for 48 hours and then decalcified in 14% EDTA, pH 7.3 until softened. Samples were then transferred to 70% ethanol before they were paraffin embedded, sectioned, and stained with hematoxylin and eosin (H&E).
RNA isolation and real-time PCR
Total RNA was isolated using a standard TRIzol extraction (15596018, ThermoFisher Scientific, Waltham, MA) method for tibias. cDNA was generated using the High Capacity cDNA Reverse Transcription kit (4368813, ThermoFisher Scientific, Waltham, MA) according to the manufacturer’s instructions. Quantification of mRNA expression was carried out using an iQ SYBR Green Supermix and an iQ™5 multicolor Real-Time PCR detection system (1708882, Bio-Rad, Hercules, CA). Hprt was used as an internal standard control gene. Primers were designed and tested to be 95–100% efficient by PrimerDesign (South Hampton, UK) or designed using NCBI: PrimerBlast and ordered from Integrative DNA Technologies (Bethesda, MD). Sequences are listed in Table S1.
Bone marrow stromal cell (BMSC) culture
BMSCs were isolated from the femurs and tibiae of 6–8 wks old age-matched female +/+ and m/m mice using previously established protocols [27]. Briefly, marrow was removed from the femurs and tibiae of 2–4 mice per genotype by centrifugation and resuspended in αMEM (12571, ThermoFisher Scientific, Waltham, MA) supplemented with 10% fetal bovine serum (Hyclone, Logan, UT), and 1% penicillin/streptomycin (15140, ThermoFisher Scientific, Waltham, MA) using a 25 gauge syringe. Cells were passed through a 70 μM strainer and plated at 10 million cells per well in a 6 well plate. Non-adherent cells were removed after 72 hrs and media was changed every 2–3 days of culture. BMSCs were grown to 80% confluence for transwell migration and adhesion assays.
Transwell Cell Migration Assay
Transwell cell migration assays were performed similarly to previously established protocols [28, 29]. Transwell inserts, 6.5 mm with 8 μm pore size, (29442-120, Corning, Corning, NY) were coated with 10 μg/mL collagen I (354236, Corning, Corning, NY) diluted in PBS overnight at 4°C. Collagen I solution was removed and inserts were blocked with BSA at 37°C, 5% CO2 for 1 hr. BMSCs were lifted with 0.25% Trypsin and resuspended in αMEM with 0.5% BSA, 1 mMMgCl2, and 0.2 mM MnCl2. 100,000 BMSCs were applied to the top of each transwell insert and cells were incubated at 37°C, 5% CO2. Following 1 or 2 hrs incubation, transwell inserts were removed and cells were stained with 0.1 mg/mL crystal violet, 5% methanol, in PBS for 10 minutes. Inserts were de-stained with 10% acetic acid in H20 and cells on the underside of the insert were counted.
Cell Adhesion Assay
Cell adhesion assays were performed similarly to previously established protocols [28, 29]. Flat bottom 48 well non-tissue culture treated plates (CC7672-7548, USA Scientific, Ocala, FL) were coated with collage I and blocked with BSA according to the protocol described for the transwell cell migration assay. BMSCs were lifted with 0.25% Trypsin and resuspended in αMEM with 0.5% BSA, 1 mM MgCl2, and 0.2 mM MnCl2. 50,000 BMSCs were added to each well and incubated at 37°C, 5% CO2 for approximately 20 to 40 minutes or until the cells began to attach. Once cells attached, they were then washed with PBS, stained, and de-stained according to protocol used for the transwell migration assay. Cells adhered to the bottom of the wells were counted.
Ear mesenchymal stem cells (eMSC) culture
eMSCs were isolated from the ears of 3–10 wks old age-matched female +/+ and m/m mice using previously established protocols [30, 31]. Briefly, the external ears were removed from 2–4 mice per genotype and digested in 2 mg/mL collagenase I (LS004196, Worthington Biochemical Corporation, Lakewood, NJ) in HBSS for 1 hr at 37°C while shaking. The digested tissue was filtered using a 70 μm cell strainer. Red blood cells were lysed and the remaining cells were plated at 2 ears per well (6-well plate) in base media containing DMEM/F-12 (11330, ThermoFisher Scientific), 15% fetal bovine serum (97068-085, VWR Life Science Seradigm, Radnor, PA), and 100 μg/mL primocin (ant-pm-1, InvivoGen, San Diego, CA). Cell culture media was refreshed every 2–3 days, and cells were passaged at 80% confluence.
Adipogenic differentiation of eMSCs and Oil Red O staining
eMSCs were differentiated into adipocytes using previous established protocols [30, 31]. Briefly, cells were plated at 200,000 cells/well of a 6-well plate. At 100% confluence, adipogenesis was initiated with the addition of 62.5 mM IBMX (I7018), 10 mg/mL insulin (I6634), and 1 mM dexamethasone (D4902) to the base media. All adipogenesis reagents were purchased from Sigma-Aldrich (St. Louis, MO) except rosiglitazone was purchased from Caymen Chemical (Ann Arbor, MI). At day 2 and 5 of adipogenic differentiation, cells were cultured in base media supplemented with 20 mM rosiglitazone (71740) and 2 mM insulin. Quantitative Oil Red O staining was used to assess lipid accumulation. Additionally, cells were de-stained with isopropanol and the absorbance was measured at 490 nm with a standard curve using a MRXTC Revelation by Dynex Technologies.
Proliferation of eMSC using BrdU incorporation
eMSCs were plated at 50,000 cells/well on glass cover slips in a 12 well plate in base media, incubating overnight at 37°C, 5% CO2. The next day, media was changed and replaced with base media containing 30 μg/mL BrdU (B5002, Sigma-Aldrich, St. Louis, MO). Plates were incubated at 37°C, 5% CO2 for 45 minutes. Cells were then washed and fixed with 100% EtOH and permeablized with 0.1% Triton in PBS for 5 min. DNA was denatured by treating cells with 1N HCl for 30 min at 55°C. Cover slips were blocked with 0.1% Triton and 2% BSA in PBS and stained for immunofluorescence with an anti-BrdU-Alexa488 antibody 1:200 (FCMAB101A4, Millipore, Billerica, MA) and DAPI prolong antifade (P363966, ThermoFisher Scientific, Waltham MA) for total nuclei. To assess proliferation, cells were counted on 3 cover slips and 4 images/cover slip. The ratio of proliferative (BrdU and DAPI positive) and non-proliferative cells (BrdU negative, DAPI positive) per total cell number was calculated.
Statistical Analyses
All data are expressed as the mean ± standard error of the mean (SEM) unless otherwise noted. Results were analyzed for statistically differences using Student’s t-test or ANOVA followed by Bonferroni’s multiple comparison post hoc test where appropriate. All statistics were performed with Prism 6 statistical software (GraphPad Software, Inc., La Jolla, CA, USA). A p-value of less than 0.05 was considered statistically significant.
Results
m/m show loss of fat mass and low bone mineral density with age
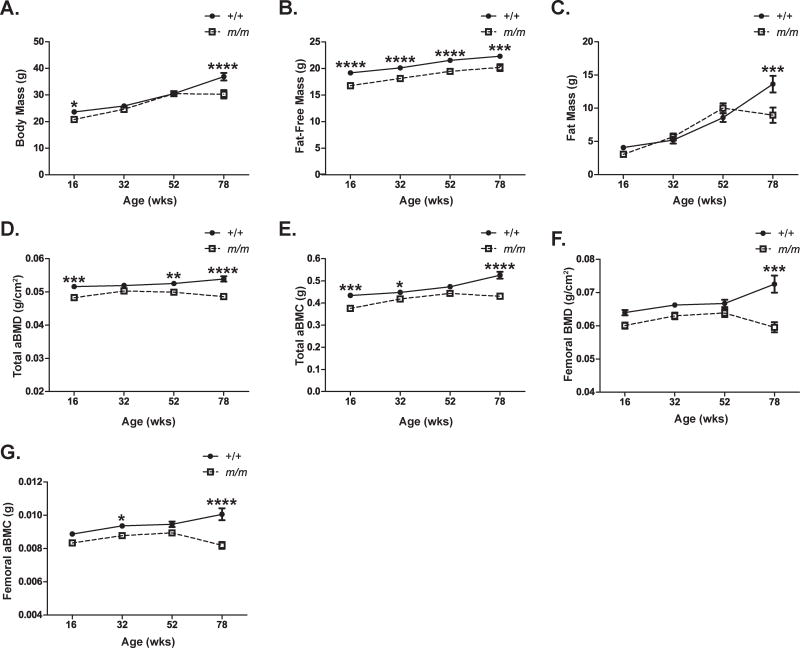
To examine the effect of aging on the bone phenotype of female m/m, m/m and the +/+ controls were aged to 78 wks and their body composition parameters were assessed by DXA analysis. The body weight of m/m was similar to +/+ through 52 wks of age, but decreased significantly at 78 wks. This decrease in body weight observed between 52 and 78 wks of age was primarily due to loss of fat mass since the +/+ cohort gained 60% fat mass and the m/m cohort lost 11% fat mass during this time. Lower fat-free mass in the m/m was previously reported, but remained constant with age [4]. Lower total and femoral aBMD and aBMC were observed at 78 wks of age in the m/m associated with a marked reduction in fat mass (Figure 1).
Figure 1. m/m show severe age-related bone loss.
Female m/m (open squares) and +/+ (closed circles) were weighted and screened using Lunar PIXImus Densitometer for body composition including (A) body mass, (B) fat-free mass, (C) fat mass, (D) total aBMD, (E) total aBMC, (F) femoral aBMD, and (G) femoral aBMC. Points represent mean ± SEM of n=13–15 per group. 2-way ANOVA: P <0.0001 for genotype in all measurements except fat mass (not significant). Post hoc: *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 compared to +/+.
Very low trabecular bone mass is characteristic of m/m
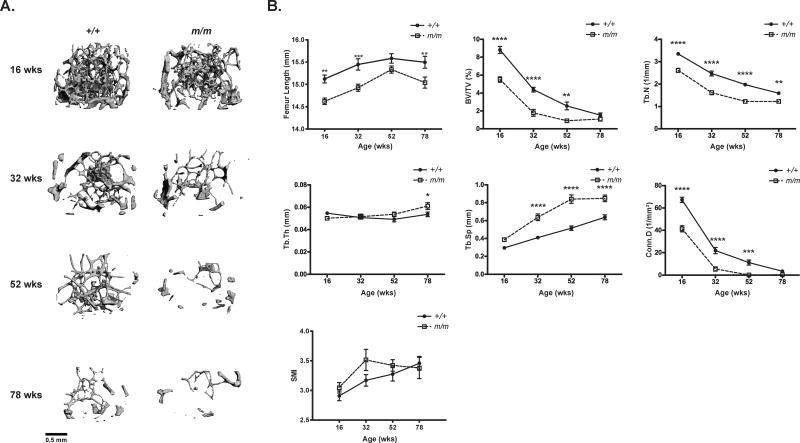
In order to further elucidate the effect of aging on the bone phenotype of m/m, femurs and L5 vertebrae were collected at 16, 32, 52, and 78 wks of age and assessed by micro-computed tomography (μCT). The m/m had reduced femur length and significantly lower trabecular bone volume fraction (BV/TV), trabecular number (Tb.N.), and connectivity density (Conn.D) at 16 through 52 wks in the distal femur. Consistent with this, trabecular separation (Tb.Sp.) increased significantly with age, but no difference in trabecular thickness (Tb.Th.) was observed (Figure 2, Table S2). No differences in Tb.BV/TV and Conn.D. were observed between m/m and +/+ at 78 wks of age due to the comprehensive loss of trabecular bone (Figure 2B, Table S2). Total relative trabecular bone loss from 16 to 78 wks was similar in +/+ and m/m (80% vs 83% respectively), when compared to genotype-matched 16 wk controls. However, +/+ did displayed a slightly higher rate of bone loss in femur when compared to m/m likely due to the higher starting Tb.BV/TV at 16 wks of age (Table S2, S4).
Figure 2. +/+ and m/m have similar trabecular bone loss with age.
(A) Representative μCT images of distal femur trabecular bone, and (B) femoral microarchitecture examined in +/+ and m/m from 16 to 78 wks of age. Trabecular microarchitecture was measured by μCT and femur length, trabecular bone volume/total volume (BV/TV), trabecular number (Tb.N.), trabecular thickness (Tb.Th), trabecular separation (Tb.Sp.), connectivity density (Conn.D.), and structural modeling index (SMI) were calculated. Closed circles (+/+) and open squares (m/m) represent mean ± SEM of n=9–10 per group. 2-way ANOVA: P <0.0001 for genotype in all measurements except Tb.Th. and SMI (not significant). Post hoc: *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 compared to +/+.
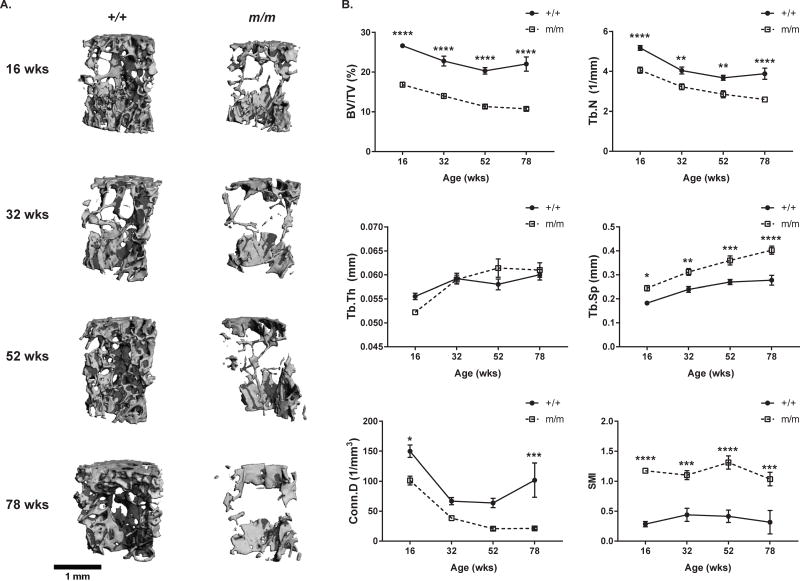
Similar to the microarchitecture of the distal femoral metaphysis, the microarchitecture of the L5 vertebrae showed a significant decrease in Tb.BV/TV, Tb.N., and Conn.D. with a significant increase in both Tb.Sp. and SMI score in m/m when compared +/+. This difference was present at 16 wks of age and persisted throughout aging resulting in significantly lower Tb.BV/TV and Tb.N. at 78 wks of age (Figure 3, Table S3). While relative vertebral Tb.BV/TV declined 36% from 16 to 78 wks in m/m and only 17% in the +/+ when compared to the 16 wks old genotype matched control, no difference in the actual rate of trabecular bone loss (BV/TV) was observed in the vertebrae between genotypes (Table S4).
Figure 3. +/+ and m/m have similar trabecular bone loss in vertebrae with age.
(A) Representative images of L5 vertebrae, and (B) vertebral microarchitecture examined in +/+ and m/m from 16 to 78 wks of age. Trabecular microarchitecture was measured by μCT and bone volume/total volume (BV/TV), trabecular number (Tb.N.), trabecular thickness (Tb.Th), trabecular separation (Tb.Sp.), connectivity density (Conn.D.), and structural modeling index (SMI) were calculated. Closed circles (+/+) and open squares (m/m) represent mean ± SEM of n=8–10 per group. 2-way ANOVA: P <0.0001 for genotype in all measurements except Tb.Th. (not significant). Post hoc: *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 compared to +/+.
m/m exhibit reduced cortical expansion
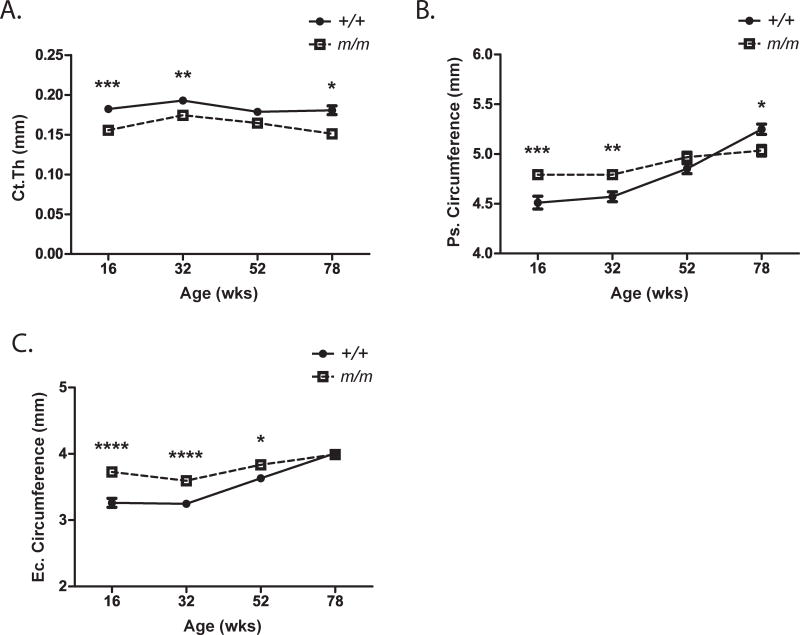
Cortical thickness (Ct.Th.) was significantly reduced in the femoral mid-shaft of m/m from 16 through 78 wks of age when compared to +/+ (Figure 4A). While periosteal (Ps.) and endocortical (Ec.) circumferences were higher in m/m at 16 wks of age, expansion of both surfaces was minimal with age (Figure 4, Table S5, S6). By 78 wks of age, +/+ exhibited a 16% increase in periosteal circumference and 23% increase in endosteal circumference over the 16 wks old +/+. However, m/m showed only a 5% and 7% increase in periosteal and endosteal circumferences, respectively, demonstrating a statistical reduction in cortical expansion (Figure 4B, C, Table S5). The lack of expansion on both the Ps. and Ec. surfaces resulted in no net change in cortical thickness by 78 wks of age. These data strongly suggest that m/m display defects in cortical expansion which could impact skeletal strength.
Figure 4. m/m have decreased cortical expansion.
(A) Cortical thickness, (B) periosteal (Ps) circumference, and (C) endosteal (Ec) circumference were measured by μCT. Closed circles (+/+) and open squares (m/m) represent mean ± SEM of n=9–10 per group. 2-way ANOVA: P <0.0001 for genotype in all measurements. Post hoc: *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 compared to +/+.
Osteoblast number is reduced and marrow adiposity is increased in the m/m
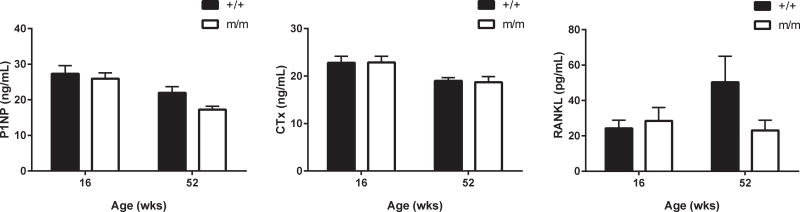
In order to determine how age affected bone cell activities in m/m, we performed static and dynamic histomorphometry on the proximal tibia in mice at 52 wks of age. Histormophometric analysis was performed at 52 wks rather than 78 wks of age because little trabecular bone was evident on μCT by 78 wks of age. Histomorphometric analysis showed BV/TV was reduced in m/m, similar to analysis of the microarchitecture by μCT (Table 1). Analysis of the cellular parameters revealed that osteoblasts number (N.Ob/B.Pm) was undetectable in m/m while no change in osteoclast number or surface was detected. Statistical changes in Ps. and Ec. mineral apposition rates (MAR) were not observed; although both MAR values showed a decreasing trend (Table 1). No change in either serum P1NP and CTx levels were detected at either 16 or 52 wks between +/+ and m/m (Figure 5). Furthermore, serum RANKL levels also remained unchanged providing further evidence and rationale for the lack of alternation in osteoclast numbers with age (Figure 5).
Table 1. m/m have decreased bone volume and increased marrow adipocytes.
Trabecular and cortical bone were assessed by static and dynamic histomorphometry in the tibia at 52 wks of age. Trabecular bone was analyzed 200 μm below the growth plate and cortical bone was analyzed 5 mm below the tibia-fibula junction.
| Parameters | +/+ | n | m/m | n | p value (t test) |
|---|---|---|---|---|---|
| BV/TV (%) | 0.78±0.96 | 9 | 0.12±0.28 | 10 | 0.05 |
| Ad.V/T.Ar (%) | 5.12±3.59 | 9 | 19.6±15.5* | 10 | 0.01 |
| N.Ad/T.Ar (/mm2) | 67.7±51.4 | 9 | 234±169* | 10 | 0.01 |
| Oc.S/B.Pm (%) | 9.94±6.55 | 8a | 10.5±12.1 | 4a | 0.92 |
| N.Oc/B.Pm (/mm) | 3.45±2.63 | 8a | 4.20±4.86 | 4a | 0.73 |
| Ob.S/B.Pm (%) | 0.87±1.79 | 8a | undetected | 4a | N.A. |
| N.Ob/B.Pm (/mm) | 0.96±2.04 | 8a | undetected | 4a | N.A. |
| Ps.MAR (μm/day) | 0.63±0.31 | 8a | 0.49±0.16 | 3a | 0.46 |
| Ec.MAR (μm/day) | 1.21±0.43 | 9 | 0.99±0.22 | 9a | 0.18 |
BV/TV = bone volume/total volume; Ad.V/T.Ar = adipocyte volume/total area; N.Ad/T.Ar = number of adipocytes/total area; Oc.S/B.Pm = osteoclast surface/bone perimeter; N.Oc/B.Pm = number of osteoclast/bone perimeter; Ob.S/B.Pm = osteoblast surface/bone perimeter; N.Ob/B.Pm = number of osteoblast/bone perimeter; Ps.MAR = periosteal mineral apposition rate; Ec.MAR = endocortical mineral apposition rate.
p<0.05 by Student’s t-test compared to +/+.
Sample numbers were reduced as not all sections displayed trabecular bone or labels.
Figure 5. Serum markers of bone formation and bone resorption were unchanged in m/m.
P1NP, CTx, and RANKL protein levels were measured by enzyme immunoassay in +/+ and m/m at 16 and 52 wks of age (n=10). 2-way ANOVA: All measures had non-significant P values for genotype. Post hoc: *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 compared to +/+.
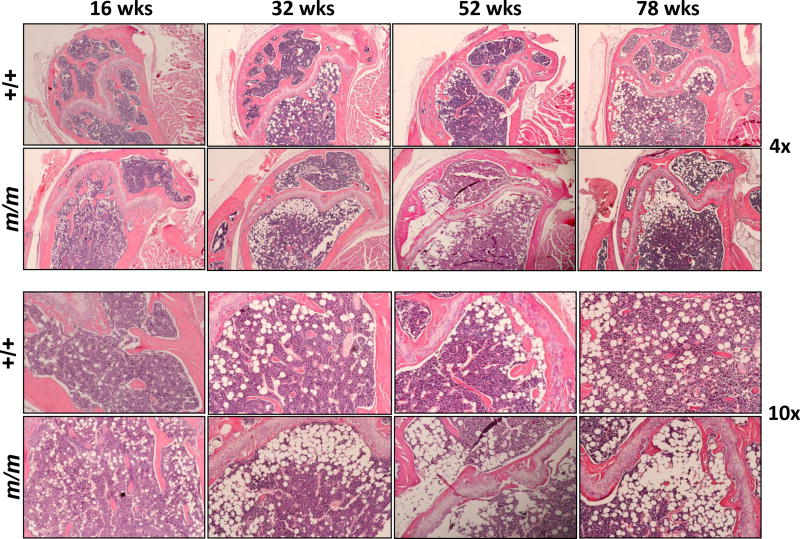
In terms of adipocytes, marrow adipocyte number (N.Ad/T.Ar) and marrow adipocyte volume (Ad.V/T.Ar) were increased over 3-fold in m/m compared to +/+ (Table 1). Representative images for dynamic labeling and marrow adipocyte quantification are shown in Figure S1. Furthermore, H& E staining of the m/m femurs showed a dramatic accumulation of adipocytes in the marrow space when compared to +/+ indicating that m/m mutation leads to enhanced in age-related marrow adiposity (Figure 6).
Figure 6. m/m have extreme age-related increase in marrow adiposity.
Femurs were isolated from 16, 32, 52, and 78 wks, decalcified, paraffin embedded, and stained with H & E. Images show accumulation of white marrow adipocyte ghosts in the distal femur epiphysis.
Reduced osteoblast gene expression and dysfunctional osteoblast motility point to a role for osteoblasts in the sustained low bone mass phenotype associated with aging in m/m
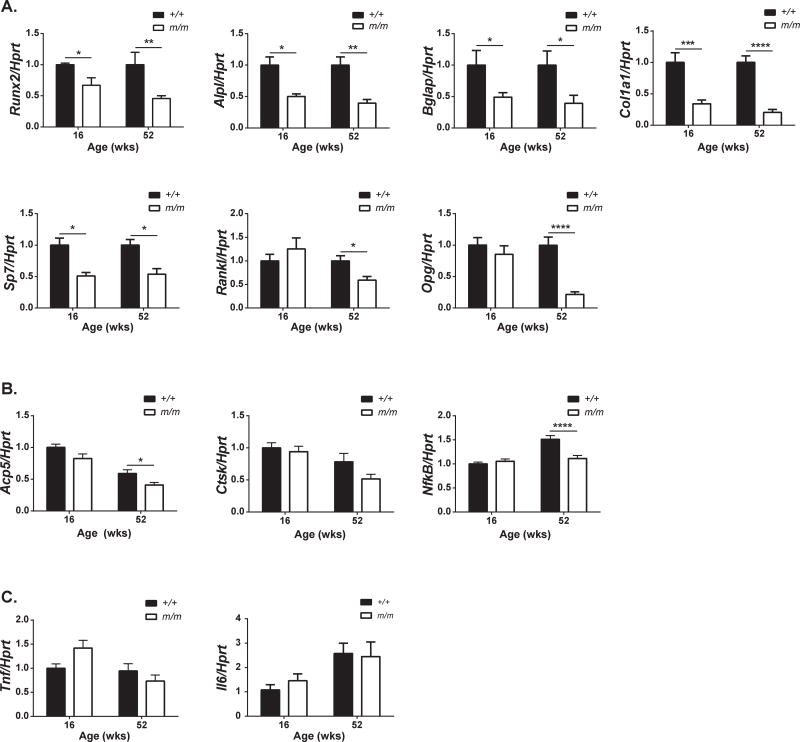
In order to gain some insight into the mechanism by which bone mass is reduced in m/m, expression of osteoblast, osteoclast, and inflammatory genes were analyzed in whole bone isolated from +/+ and m/m at 16 and 52 wks of age. Not surprisingly, osteoblast gene expression was reduced in both 16 and 52 wk old m/m consistent with reduced osteoblast number observed by this study and previous studies in younger m/m (Figure 7A) [4]. Trap5b and NfκB expression were also significantly reduced at 52 wks (Figure 7B). Both Rankl and Opg expression were lower in m/m at 52 wks, with Opg expression changes more pronounced than Rankl. Despite this, serum RANKL levels remained unaffected (Figure 5), and no change in osteoclast number per unit of bone surface was observed by histomorphometry (Table 1). Local inflammation was assessed with Tnf and Il6 expression and no changes were present in whole bone suggesting that the low bone mass phenotype was not a result of an age-associated inflammatory phenotype in the bone environment (Figure 7C). Taken together, these data suggest changes in osteoblast number are responsible, in part, for the reduced peak bone mass and reduced cortical expansion with aging in m/m.
Figure 7. Decreased osteoblast gene expression persists in the aged m/m.
Gene expression of whole tibia was analyzed by qRT-PCR for expression of (A) osteoblast genes, (B) osteoclast genes, and (C) inflammatory cytokines. n=8–10 per group. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 compared to +/+.
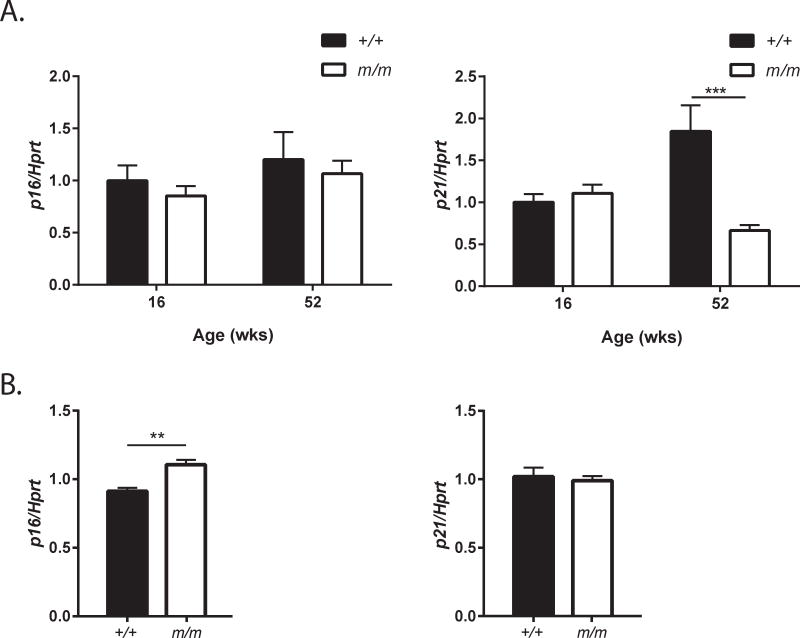
A reduction in osteogenic differentiation has previously been established in m/m highlighting the potential role of Dock7 in controlling bone formation directly in the osteoblast. We further investigated the cell autonomous role of Dock7 in bone marrow stromal cells (BMSCs). To determine if osteoblast number in m/m were reduced by increased senescence in bone cells, expression of senescence markers p16 and p21 were analyzed in whole bone. While no change in senescence markers was present in whole bone at 16 wks of age, a reduction in p21 expression was observed in m/m compared to +/+ at 52 wks of age (Figure 8A). In contrast, BMSCs from 8 wks old m/m demonstrated a modest increase in p16 indicating that loss of Dock7 in m/m may increase senescence specifically in the BMSC population (Figure 8B). However, the heterogeneity of cells in the whole bone RNA may be masking p16 changes observed in BMSCs.
Figure 8. Gene expression of senescence markers is modestly altered in m/m.
Gene expression of p16 and p21 senescence markers was analyzed by qRT-PCR in (A) whole tibia of 16 and 52 wks old mice and (B) BMSCs from +/+ and m/m mice. Values represent mean ± SEM of n=6–10 per group. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 compared to +/+.
As osteoblast numbers are reduced, we investigated osteoblast function, specifically migration and adhesion of BMSCs. Cell migration onto a collagen I matrix was decreased in BMSCs isolated from m/m compared to control +/+ in a transwell assay (Figure 9A). Conversely, cell adhesion on to a collagen I matrix was increased and may provide some rationale for the decreased migration (Figure 9B). In combination with previous data, these results suggest a reduction of osteogenic differentiation as well as provide additional evidence that the low bone mass in m/m may not only be influenced by reduced osteoblast numbers, but also by dysfunction in the osteoblasts themselves.
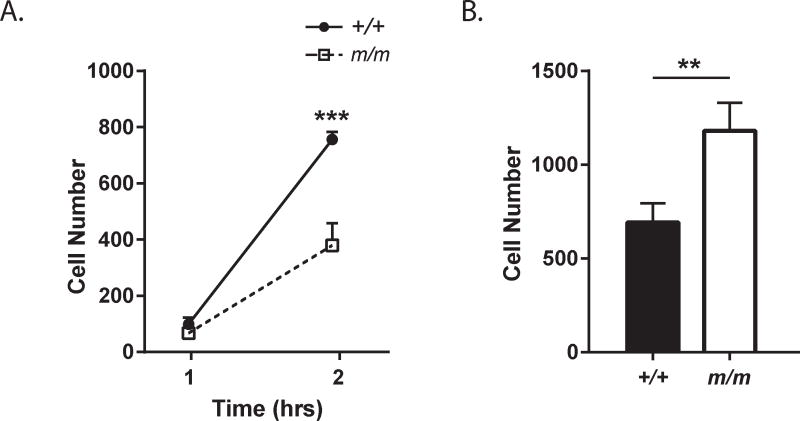
Figure 9. BMSCs motility is impaired in BMSCs on a Collagen I matrix.
(A) BMSC migration was assessed in a transwell assay on membranes coated with collagen I. (B) Adhesion of BMSCs was analyzed in collagen I coated wells. The number of cells on the membrane (A) or well (B) were quantified. Closed circles (+/+) and open squares (m/m) represent mean ± SEM. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 compared to +/+.
Potential role for adipose tissue and the SNS in regulating bone metabolism
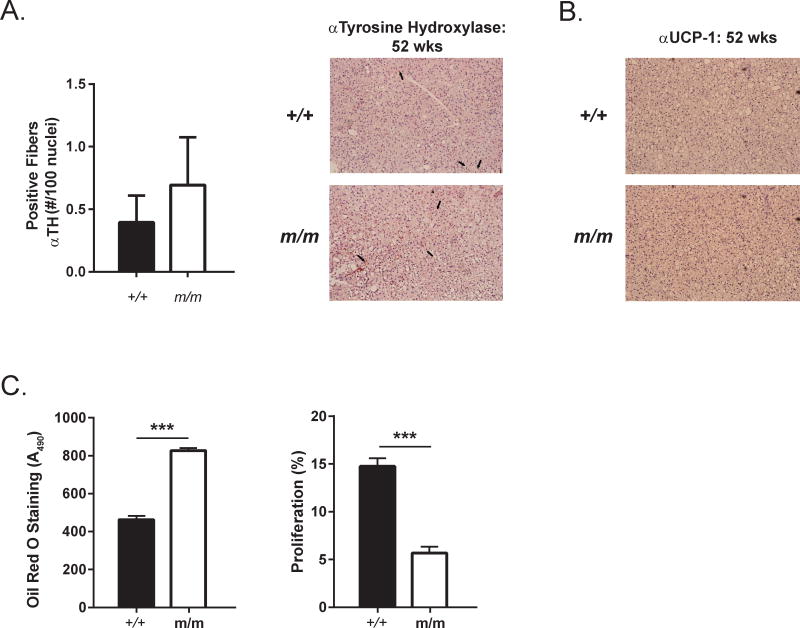
Previous studies have indicated that m/m have decreased innervation of BAT and reduced interscapular temperature and UCP-1 staining in BAT indicating BAT dysfunction. These data would suggest a potential upregulation of SNS activity in order to compensate for reduced BAT activity. As increased SNS activity is one marker of aging and has been linked to bone loss, we investigated BAT innervation in the aged m/m. At 52 wks of age, no changes in innervation of m/m BAT were present as measured by tyrosine hydroxylase (TH) staining (Figure 10A). Furthermore, no changes in UCP-1 protein levels were observed in m/m BAT at 52 wks (Figure 10B). These data suggest that BAT dysfuction may play a larger role in modulating bone metabolism in m/m prior to 16 wks of age, a time when BAT is thought to be most active.
Figure 10. Adipogenic differentiation is increased in m/m.
(A) Tyrosine hyroxylase (TH) positive sympathetic nerve fibers were quantified in histological BAT sections from 52 wks old +/+ and m/m. Representative images of staining are shown on right. Black arrows mark examples of TH positive nerve fibers. (B) UCP-1 protein expression was detected in histological BAT sections from 52 wks old +/+ and m/m with an anti-UCP-1 antibody. (C) Adipogenic differentiation was analyzed in eMSC by Oil Red O staining after 7 days in adipogenic media. Proliferation was measured in undifferentiated eMSCs with BrdU straining.
Finally, the role of marrow adipose tissue on bone metabolism is not completely understood. We have demonstrated that m/m has increased marrow adipose tissue at 52 wks, but it is unclear if this is a direct result of loss of Dock7 in adipocyte precursors. Ear mesenchymal stem cells (eMSCs) were used as a robust primary cell culture model to study adipogenic differentiation with the capacity to turn into adipocytes or osteoblast-like cells [30, 31]. eMSCs isolated from m/m demonstrated increased Oil Red O staining in adipogenic differentiation media. Proliferation was also decreased in m/m eMSCs. In combination with the decreased osteogenic differentiation previously observed and the increased marrow adipogenesis in m/m, these data suggest that loss of Dock7 in m/m may divert progenitors from the osteoblastic lineage toward the adipogenic lineage (Figure 10C).
Discussion
In this study, we found that the m/m mouse model, with a spontaneous mutation in the Dock7 gene, exhibits a low bone mineral density phenotype that is maintained throughout skeletal aging [4]. For example, by 52 weeks of age, there is almost a complete absence of femoral trabeculae. Cortical expansion was also significantly compromised on both periosteal and endocortical surfaces in m/m leading to a marked reduction in appositional growth. Furthermore, there was a striking increase in marrow adiposity in the m/m and a marked decrease in osteoblast number suggesting a possible shift in lineage toward adipogenesis. This tenet is further reinforced by previous studies from our group showing that calvarial osteoblasts from neonatal m/m mice differentiated at a much slower rate than +/+ [4].
Trabecular bone is integral for the mechanical integrity of bone in humans [32]. Analysis of bone mineral density in previous studies shows that m/m have statistically lower aBMD and aBMC as early as 4 wks of age by DXA analysis and decreased trabecular number as early as 8 wks of age measured by μCT analysis [4]. These studies revealed that peak trabecular and cortical bone acquisition is compromised with loss of Dock7 in these mice. Aging of these mice now highlights that the low bone mineral density and low trabecular bone phenotype persist through the 78 weeks without any sign of compensation. The almost complete absence of trabecular bone seen in m/m starting at 52 wks age is due to fewer trabeculae, greater trabecular separation, and reduced connectivity density. However, the rate of trabecular bone loss in m/m remains unchanged in vertebrae and even slightly lower in m/m femur compared to +/+. As there is no acceleration of trabecular bone loss with age, these studies suggest that low trabecular bone mass associated with loss of Dock7 in m/m primarily occurs during bone acquisition or with bone loss prior to 16 wks of age.
In contrast to trabecular bone, cortical bone provides much of the structural support for bone and expansion of the cortex. Appositional growth primarily involves mineralization on the periosteal surface by osteoblasts and resorption on the endosteal surface by osteoclasts [33, 34]. Interestingly, younger m/m showed greater periosteal and endosteal circumferences when compared to +/+ resulting in a larger bone medullary area at an early age. This undoubtedly resulted from the lower trabecular bone volume in m/m. On the other hand, m/m mice had minimal expansion of periosteal and endocortical envelopes with age. These data may reflect defects in either bone formation on the periosteal or endocortical surface and/or reduced resorption on the endosteal surface. In respect to the former, osteoblast number was negligible in the aged m/m mice by histomorphometry at 52 wks with no significant change in osteoclast number. These data suggest there is an important and persistent cell autonomous defect from absence of Dock7 in the endosteal osteoblast and possibly in the periosteal cells as well. Relative to changes in resorption, the number of osteoclasts did not differ between m/m and +/+ mice at 52 wks. However, it is worth noting that Rankl, Trap5, and Nfkb mRNA were significantly reduced in the aged but not the young m/m mice when compared to age-matched +/+, suggesting there may well be a modest reduction in endosteal resorption with age in the m/m vs. +/+. However, the reduction in Trap5 and Nfkb gene expression, with no changes in osteoclast number per unit bone surface, may in part be due to the reduced trabecular bone surface present in the m/m. Furthermore, the absence of increased osteoclastogenesis in the presence of suppressed Opg suggests a functional uncoupling of osteoblast-dependent osteoclast recruitment.
During human skeletal aging, resorption on the endosteal surface is compensated for through enhanced periosteal apposition to preserve bone strength [35–38]. Indeed, with estrogen deprivation after menopause, the marrow cavity expands and there is an increase in the periosteal circumference. However, some investigators believe that periosteal expansion is hampered in postmenopausal women with osteoporosis [33]. In the m/m, there is minimal periosteal expansion with age compared to +/+. The absence of age-related periosteal apposition may compromise skeletal integrity in the mutant mice. But it should be noted that the perimeter of the endosteal surface minimally expanded during aging in m/m. Hence it is conceivable that the reduction of periosteal expansion relates to a defect on the endocortical surface, either in the osteoblast, or possibly in the osteoclast signaling to the osteoblast. Either way, this model provides a unique opportunity for understanding age-related signaling between bone compartments.
Rac1 activity is important for normal bone development as well as adhesion, proliferation, and differentiation of osteoblasts in addition to controlling osteoclast number [39, 40]. Therefore, loss of Dock7, a modulator of Rac1 and Cdc42 [8, 9, 14, 41], may in fact be altering osteoblast and osteoclast differentiation and function. Previous studies have already suggested a cell autonomous role for Dock7 directly in controlling osteoblast differentiation and with an overall reduction of differentiation of calvarial osteoblasts in vitro [4]. Therefore, it follows that absence of Dock7 may affect the differentiation and proliferation of periosteal osteoblasts, as well as endocortical osteoclasts, which could result in the reduction of cortical expansion.
The SNS has emerged as a major regulator of bone metabolism with activation of the β2AR on osteoblasts as a primary SNS target leading to an increase in Rankl expression and increased osteoclastogenesis/bone resorption causing lower bone mass [20, 22, 42]. Increases in SNS activity with aging have been linked to age-related bone loss. Although, we did not find an increase in resorption at 52 wks dynamically or by bone turnover markers/gene expression in our study, it remains unclear whether higher SNS tone is responsible for the non-cell autonomous effects on bone with age in m/m [43]. In fact, Dock7 has been studied for its role in brain and nervous system [8, 9, 14, 18, 19, 44, 45], but it remains unclear if any of these alterations in the nervous system may affect bone development or remodeling. Propranolol treatment, a β2AR antagonist, has been shown to partially rescue m/m bone phenotype in young mice, suggesting that the SNS may indeed be contributing to bone loss in the m/m [4]. However, we found no change in innervation of BAT tissue or UCP-1 protein levels in BAT in aged mice, suggesting that the role of the SNS in bone metabolism in m/m may be limited to the time of bone development and early age-related trabecular bone loss.
While the m/m clearly demonstrated low trabecular bone mass and reduced periosteal expansion, there are some limitations with this global Dock7 deletion model. The skeletal phenotype of m/m is likely due to both a cell autonomous role for Dock7 in bone lineage cells as well as non-cell autonomous effects of Dock7 in other organ systems such as the SNS [4]. We currently cannot delineate which mechanism is dominant in controlling age-related bone loss in the aged m/m. However, we have developed a conditional deletion of Dock7 using CRISPR/Cas9 technology and are currently working toward selectively deleting Dock7 in osteoblasts, osteoclasts, and nervous system [46]. Additionally, we cannot be sure without mechanical testing whether the changes in periosteal apposition translate into a weaker bone. However, we did observe a substantial decrease in femoral moment of inertia at 78 wks of age, indicating m/m femur bones would likely have reduced load to fracture.
In conclusion, we found that the aged m/m mouse model had many of the same characteristics of the aging osteoporotic skeleton in humans. These hallmarks include low bone mineral density, reduced trabecular bone mass, decreased osteoblast number, and increased marrow adiposity. While trabecular bone loss was not accelerated with aging, absence of Dock7 resulted in the novel findings of impaired cortical expansion in the m/m, suggesting a critical role for Dock7 in appositional bone growth and possibly the function of periosteal osteoblasts. Since the m/m model displayed a low bone mass phenotype that is maintained during the aging process, this spontaneous mutant may become a useful model for further aging studies.
Supplementary Material
Highlights.
Loss of Dock7 in the Misty mice results in low bone mineral density. During the aging process, Misty mice show a similar rate of trabecular bone loss and reduced cortical expansion. Reduced cortical expansion may result from decreased osteoblast number and defects in osteoblast function. These data highlight the Misty mice as a model to study apposition bone growth and communication between the bone compartments.
Acknowledgments
The research reported in this publication was supported by the National Institute of Aging, grant number AG040217 to Clifford J. Rosen, MD, and the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under award number F32AR067071 to Kathleen A. Bishop, PhD. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This work was also supported, in part, by the services of the Physiology Core and the Molecular Phenotyping Core, part of NIH grant P30GM106391, “COBRE in Stem and Progenitor Cell Biology and Regenerative Medicine” as well as the Histology Core, part of NIH grant P30GM103392, “Phase III COBRE in Vascular Biology,” both funded through the National Institute of General Medical Sciences.
We thank members of the Rosen laboratory for the insight and suggestion as well as member of the Maine Medical Center Research Institute community. In particular, we thank Peter Brooks, Ph.D. and members of the Brooks lab for their help in developing the migration and adhesion assays.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wright NC, Looker AC, Saag KG, Curtis JR, Delzell ES, Randall S, Dawson-Hughes B. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. 2014;29:2520–6. doi: 10.1002/jbmr.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res. 2007;22:465–75. doi: 10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]
- 3.Adams DJ, Rowe DW, Ackert-Bicknell CL. Genetics of aging bone. Mamm Genome. 2016;27:367–80. doi: 10.1007/s00335-016-9650-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Motyl KJ, Bishop KA, DeMambro VE, Bornstein SA, Le P, Kawai M, Lotinun S, Horowitz MC, Baron R, Bouxsein ML, Rosen CJ. Altered thermogenesis and impaired bone remodeling in Misty mice. J Bone Miner Res. 2013;28:1885–97. doi: 10.1002/jbmr.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drake MT, Khosla S. Male osteoporosis. Endocrinol Metab Clin North Am. 2012;41:629–41. doi: 10.1016/j.ecl.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cauley JA. Estrogen and bone health in men and women. Steroids. 2015;99:11–5. doi: 10.1016/j.steroids.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 7.Sviderskaya EV, Novak EK, Swank RT, Bennett DC. The murine misty mutation: phenotypic effects on melanocytes, platelets and brown fat. Genetics. 1998;148:381–90. doi: 10.1093/genetics/148.1.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watabe-Uchida M, John KA, Janas JA, Newey SE, Van Aelst L. The Rac activator DOCK7 regulates neuronal polarity through local phosphorylation of stathmin/Op18. Neuron. 2006;51:727–39. doi: 10.1016/j.neuron.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 9.Yamauchi J, Miyamoto Y, Chan JR, Tanoue A. ErbB2 directly activates the exchange factor Dock7 to promote Schwann cell migration. J Cell Biol. 2008;181:351–65. doi: 10.1083/jcb.200709033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blasius AL, Brandl K, Crozat K, Xia Y, Khovananth K, Krebs P, Smart NG, Zampolli A, Ruggeri ZM, Beutler BA. Mice with mutations of Dock7 have generalized hypopigmentation and white-spotting but show normal neurological function. Proc Natl Acad Sci U S A. 2009;106:2706–11. doi: 10.1073/pnas.0813208106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gadea G, Blangy A. Dock-family exchange factors in cell migration and disease. Eur J Cell Biol. 2014 doi: 10.1016/j.ejcb.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 12.Nishikimi A, Kukimoto-Niino M, Yokoyama S, Fukui Y. Immune regulatory functions of DOCK family proteins in health and disease. Exp Cell Res. 2013;319:2343–9. doi: 10.1016/j.yexcr.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 13.Watabe-Uchida M, Govek EE, Van Aelst L. Regulators of Rho GTPases in neuronal development. J Neurosci. 2006;26:10633–5. doi: 10.1523/JNEUROSCI.4084-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamauchi J, Miyamoto Y, Hamasaki H, Sanbe A, Kusakawa S, Nakamura A, Tsumura H, Maeda M, Nemoto N, Kawahara K, Torii T, Tanoue A. The atypical Guanine-nucleotide exchange factor, dock7, negatively regulates schwann cell differentiation and myelination. J Neurosci. 2011;31:12579–92. doi: 10.1523/JNEUROSCI.2738-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamamoto K, Murata H, Putranto EW, Kataoka K, Motoyama A, Hibino T, Inoue Y, Sakaguchi M, Huh NH. DOCK7 is a critical regulator of the RAGE-Cdc42 signaling axis that induces formation of dendritic pseudopodia in human cancer cells. Oncol Rep. 2013;29:1073–9. doi: 10.3892/or.2012.2191. [DOI] [PubMed] [Google Scholar]
- 16.Zhou Y, Johnson JL, Cerione RA, Erickson JW. Prenylation and membrane localization of Cdc42 are essential for activation by DOCK7. Biochemistry. 2013;52:4354–63. doi: 10.1021/bi301688g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tai Y, Janas JA, Wang CL, Van Aelst L. Regulation of chandelier cell cartridge and bouton development via DOCK7-mediated ErbB4 activation. Cell Rep. 2014;6:254–63. doi: 10.1016/j.celrep.2013.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang YT, Wang CL, Van Aelst L. DOCK7 interacts with TACC3 to regulate interkinetic nuclear migration and cortical neurogenesis. Nat Neurosci. 2012;15:1201–10. doi: 10.1038/nn.3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perrault I, Hamdan FF, Rio M, Capo-Chichi JM, Boddaert N, Decarie JC, Maranda B, Nabbout R, Sylvain M, Lortie A, Roux PP, Rossignol E, Gerard X, Barcia G, Berquin P, Munnich A, Rouleau GA, Kaplan J, Rozet JM, Michaud JL. Mutations in DOCK7 in individuals with epileptic encephalopathy and cortical blindness. Am J Hum Genet. 2014;94:891–7. doi: 10.1016/j.ajhg.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kajimura D, Hinoi E, Ferron M, Kode A, Riley KJ, Zhou B, Guo XE, Karsenty G. Genetic determination of the cellular basis of the sympathetic regulation of bone mass accrual. J Exp Med. 2011;208:841–51. doi: 10.1084/jem.20102608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takeda S, Elefteriou F, Levasseur R, Liu X, Zhao L, Parker KL, Armstrong D, Ducy P, Karsenty G. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002;111:305–17. doi: 10.1016/s0092-8674(02)01049-8. [DOI] [PubMed] [Google Scholar]
- 22.Elefteriou F, Ahn JD, Takeda S, Starbuck M, Yang X, Liu X, Kondo H, Richards WG, Bannon TW, Noda M, Clement K, Vaisse C, Karsenty G. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature. 2005;434:514–20. doi: 10.1038/nature03398. [DOI] [PubMed] [Google Scholar]
- 23.Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Muller R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res. 2010;25:1468–86. doi: 10.1002/jbmr.141. [DOI] [PubMed] [Google Scholar]
- 24.Motyl KJ, DeMambro VE, Barlow D, Olshan D, Nagano K, Baron R, Rosen CJ, Houseknecht KL. Propranolol Attenuates Risperidone-Induced Trabecular Bone Loss in Female Mice. Endocrinology. 2015;156:2374–83. doi: 10.1210/en.2015-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dempster DW, Compston JE, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR, Parfitt AM. Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 2013;28:2–17. doi: 10.1002/jbmr.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeMambro VE, Le PT, Guntur AR, Maridas DE, Canalis E, Nagano K, Baron R, Clemmons DR, Rosen CJ. Igfbp2 Deletion in Ovariectomized Mice Enhances Energy Expenditure but Accelerates Bone Loss. Endocrinology. 2015;156:4129–40. doi: 10.1210/en.2014-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeMambro VE, Clemmons DR, Horton LG, Bouxsein ML, Wood TL, Beamer WG, Canalis E, Rosen CJ. Gender-specific changes in bone turnover and skeletal architecture in igfbp-2-null mice. Endocrinology. 2008;149:2051–61. doi: 10.1210/en.2007-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Favreau AJ, Vary CP, Brooks PC, Sathyanarayana P. Cryptic collagen IV promotes cell migration and adhesion in myeloid leukemia. Cancer Med. 2014;3:265–72. doi: 10.1002/cam4.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caron JM, Ames JJ, Contois L, Liebes L, Friesel R, Muggia F, Vary CP, Oxburgh L, Brooks PC. Inhibition of Ovarian Tumor Growth by Targeting the HU177 Cryptic Collagen Epitope. Am J Pathol. 2016;186:1649–61. doi: 10.1016/j.ajpath.2016.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gawronska-Kozak B. Preparation and differentiation of mesenchymal stem cells from ears of adult mice. Methods Enzymol. 2014;538:1–13. doi: 10.1016/B978-0-12-800280-3.00001-3. [DOI] [PubMed] [Google Scholar]
- 31.Gawronska-Kozak B. Regeneration in the ears of immunodeficient mice: identification and lineage analysis of mesenchymal stem cells. Tissue Eng. 2004;10:1251–65. doi: 10.1089/ten.2004.10.1251. [DOI] [PubMed] [Google Scholar]
- 32.Sornay-Rendu E, Boutroy S, Munoz F, Delmas PD. Alterations of cortical and trabecular architecture are associated with fractures in postmenopausal women, partially independent of decreased BMD measured by DXA: the OFELY study. J Bone Miner Res. 2007;22:425–33. doi: 10.1359/jbmr.061206. [DOI] [PubMed] [Google Scholar]
- 33.Szulc P, Seeman E, Duboeuf F, Sornay-Rendu E, Delmas PD. Bone fragility: failure of periosteal apposition to compensate for increased endocortical resorption in postmenopausal women. J Bone Miner Res. 2006;21:1856–63. doi: 10.1359/jbmr.060904. [DOI] [PubMed] [Google Scholar]
- 34.Seeman E. Structural basis of growth-related gain and age-related loss of bone strength. Rheumatology (Oxford) 2008;47(Suppl 4):iv2–8. doi: 10.1093/rheumatology/ken177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jepsen KJ, Andarawis-Puri N. The amount of periosteal apposition required to maintain bone strength during aging depends on adult bone morphology and tissue-modulus degradation rate. J Bone Miner Res. 2012;27:1916–26. doi: 10.1002/jbmr.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yakar S, Canalis E, Sun H, Mejia W, Kawashima Y, Nasser P, Courtland HW, Williams V, Bouxsein M, Rosen C, Jepsen KJ. Serum IGF-1 determines skeletal strength by regulating subperiosteal expansion and trait interactions. J Bone Miner Res. 2009;24:1481–92. doi: 10.1359/JBMR.090226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lauretani F, Bandinelli S, Griswold ME, Maggio M, Semba R, Guralnik JM, Ferrucci L. Longitudinal changes in BMD and bone geometry in a population-based study. J Bone Miner Res. 2008;23:400–8. doi: 10.1359/JBMR.071103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yukata K, Xie C, Li TF, Takahata M, Hoak D, Kondabolu S, Zhang X, Awad HA, Schwarz EM, Beck CA, Jonason JH, O’Keefe RJ. Aging periosteal progenitor cells have reduced regenerative responsiveness to bone injury and to the anabolic actions of PTH 1–34 treatment. Bone. 2014;62:79–89. doi: 10.1016/j.bone.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lane SW, De Vita S, Alexander KA, Karaman R, Milsom MD, Dorrance AM, Purdon A, Louis L, Bouxsein ML, Williams DA. Rac signaling in osteoblastic cells is required for normal bone development but is dispensable for hematopoietic development. Blood. 2012;119:736–44. doi: 10.1182/blood-2011-07-368753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu M, Sun BH, Saar K, Simpson C, Troiano N, Dallas SL, Tiede-Lewis LM, Nevius E, Pereira JP, Weinstein RS, Tommasini SM, Insogna KL. Deletion of Rac in Mature Osteoclasts Causes Osteopetrosis, an Age-Dependent Change in Osteoclast Number, and a Reduced Number of Osteoblasts In Vivo. J Bone Miner Res. 2016;31:864–73. doi: 10.1002/jbmr.2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pinheiro EM, Gertler FB. Nervous Rac: DOCK7 regulation of axon formation. Neuron. 2006;51:674–6. doi: 10.1016/j.neuron.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 42.Motyl KJ, Rosen CJ. The skeleton and the sympathetic nervous system: it’s about time! J Clin Endocrinol Metab. 2012;97:3908–11. doi: 10.1210/jc.2012-3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Farr JN, Charkoudian N, Barnes JN, Monroe DG, McCready LK, Atkinson EJ, Amin S, Melton LJ, 3rd, Joyner MJ, Khosla S. Relationship of sympathetic activity to bone microstructure, turnover, and plasma osteopontin levels in women. J Clin Endocrinol Metab. 2012;97:4219–27. doi: 10.1210/jc.2012-2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Majewski L, Sobczak M, Havrylov S, Jozwiak J, Redowicz MJ. Dock7: a GEF for Rho-family GTPases and a novel myosin VI-binding partner in neuronal PC12 cells. Biochem Cell Biol. 2012;90:565–74. doi: 10.1139/o2012-009. [DOI] [PubMed] [Google Scholar]
- 45.Murray DW, Didier S, Chan A, Paulino V, Van Aelst L, Ruggieri R, Tran NL, Byrne AT, Symons M. Guanine nucleotide exchange factor Dock7 mediates HGF-induced glioblastoma cell invasion via Rac activation. Br J Cancer. 2014;110:1307–15. doi: 10.1038/bjc.2014.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bishop KA, Harrington A, Kouranova E, Weinstein EJ, Rosen CJ, Cui X, Liaw L. CRISPR/Cas9 Mediated Insertion of loxP Sites in the Mouse Dock7 Gene Provides an Effective Alternative to Use of Targeted Embryonic Stem Cells. G3 (Bethesda) 2016 doi: 10.1534/g3.116.030601. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.