Abstract
Circadian oscillators play an indispensable role in the coordination of physiological processes with the cyclic changes in the physical environment. A significant number of recent clinical and molecular studies suggest that circadian biology may play an important role in the regulation of adipose and other metabolic tissue functions. In this discussion, we present the hypothesis that circadian dysfunction may be involved in the pathogenesis of obesity, type 2 diabetes, and the metabolic syndrome.
Introduction
Most modern organisms have developed internal time-keeping mechanisms to help them respond to the challenges imposed by the daily cyclic changes in the environment. Termed circadian, these molecular clocks respond to environmental cues (zeitgebers) by synchronizing (entraining) the organism’s physiological processes with their environment (1).
Mammalian circadian clocks are comprised of two transcriptional feedback loops. Transcriptional regulatory proteins encoded by Clock (or its paralog Npas2) and Bmal1 form heterodimers that activate the expression of Period (Per) and Cryptochrome (Cry) genes; once PER and CRY proteins accumulate in the cytoplasm, they heterodimerize and translocate to the nucleus to inhibit the activity/expression of CLOCK (NPAS2) and BMAL1 (2, 3). This, in turn, leads to the down-regulation of Per/Cry expression, alleviating the CLOCK-BMAL1 suppression, and results in the re-initiation of the regulatory loop. The end product of these feedback cycles is the cyclic expression of CLOCK-BMAL1 and PER-CRY, occurring in relative antiphase of one another (4, 5). Apart from regulating the expression of Per and Cry, CLOCK-BMAL1 can drive the expression of many “output” genes including Dbp and the nuclear hormone receptors Rev-erbα and β (6, 7). Presumably, CLOCK-BMAL1 heterodimers act through the recognition and binding of E-box cis-acting elements found within promoters and/or enhancers of target genes (3, 8, 9). Because of the rhythmic nature of their own transcriptional activity, CLOCK-BMAL1 heterodimers generate distinct oscillatory patterns in the expression of their target genes, thus propagating the circadian rhythms from the oscillator to the downstream genes.
Early circadian studies have described the oscillator within the suprachiasmatic nucleus (SCN)1 of the hypothalamus as the body’s “master” clock, whose function is to entrain the oscillators in peripheral tissues to the daily light/dark cycles. This notion was supported by the fact that the ablation of the SCN led to the complete loss of activity/behavioral rhythms (10), as well as the fact that the SCN explants showed more robust and sustained oscillatory behavior ex vivo compared with other tissues (11). More recently, a body of evidence emerged supporting the notion that, at least some, peripheral tissue oscillators can function independently, and even become uncoupled from the SCN. Several studies have shown that temporally restricted feeding (RF) regimens (12, 13), as well as glucocorticoid (14) and amphetamine injections (15), have the ability to shift the oscillatory phase within peripheral clocks without affecting the phase of SCN. Furthermore, the peripheral oscillators in animals with fully ablated SCN can be entrained through RF and stimulation of physical activity (16), whereas the liver and muscle explants of mPer2-Luc mice show persistent rhythmic reporter output for >20 days ex vivo (17). These findings have raised the question of whether the actual role of the SCN is to influence feeding and physical activity rhythms, which, in turn, act to coordinate the phase of peripheral tissue oscillators with the organism’s metabolic demands (18).
Adipose Physiology “Round the Clock”
A variety of clinical conditions associated with adipose tissue function also display perturbations of circadian rhythms. Night-shift workers, whose activity period is reversed in relation to the day/night cycle, are much more likely to develop the metabolic syndrome (19). People who habitually sleep <6 or >9 hours per night have increased risk of developing type 2 diabetes and impaired glucose tolerance (20). One of the major symptoms of the bipolar disorder is an abnormal sleep pattern. Lithium therapy alleviates this problem but also results in significant weight gain, often accompanied by the development of obesity (21). Several studies looking into the molecular effects of lithium administration have found that it regulates glycogen synthase kinase-3β, a kinase whose actions have been studied in the context of adipocyte differentiation and insulin action (22, 23), as well as the regulation of intracellular circadian oscillators (24, 25). Myocardial infarction, sudden death, and heart failure tend to occur most frequently in the morning hours (26), except in patients who also suffer from obesity and type 2 diabetes; they experience these events evenly throughout the day (26). Interestingly, the morning peak in the incidence of myocardial infarctions coincides with an increase in the circulating levels of plasminogen activator inhibitor 1 (PAI-1), a prothrombic factor associated with a number of atherosclerotic risk factors (27). In fact, the PAI-1 promoter contains DNA response elements recognized by the CLOCK-BMAL1 heterodimers (27). Adipose tissue secretes PAI-1 and other metabolic mediators such as tumor necrosis factor-α, interleukin-6, adiponectin, resistin, visfatin, leptin, and ghrelin. The circulating levels of the majority of these mediators exhibit distinct circadian rhythms, which can be entrained by meal timing but are blunted in obese patients (28, 29, 30).
Recent studies using both in vivo and in vitro approaches have provided additional insights into the relationship between circadian rhythms and adipose tissue. Homozygous Clock mutant mice are overweight, hyperphagic, and develop symptoms of metabolic syndrome, such as hypoinsulinemia, hyperglycemia, hyperleptinemia, hyperlipidemia, and hepatic steatosis (31). Mutations of Clock and deletion of Bmal1 have also been shown to alter the daily fluctuations of plasma glucose and triglycerides. A high-fat diet amplifies the daily oscillations in glucose tolerance and insulin sensitivity, but the Clock mutation seems to protect against this effect (32). Novel transcriptomic studies have revealed rhythmic expression of circadian oscillator genes and adipokine genes resistin, adiponectin, and visfatin in visceral fat tissue (33). Interestingly, the oscillations of adipokine mRNA are attenuated in the obese mouse strain and even further reduced in the more obese/diabetic strain. Work from our laboratory has shown the presence of active circadian oscillators in inguinal white adipose tissue, epididymal white adipose tissue, and brown adipose tissue, responsive to entrainment by RF (34). Together, these studies not only provide evidence for the regulation of lipid and glucose metabolism by the components of the circadian system, but also suggest that feeding or development of obesity/type 2 diabetes can influence the activity of adipose circadian clocks.
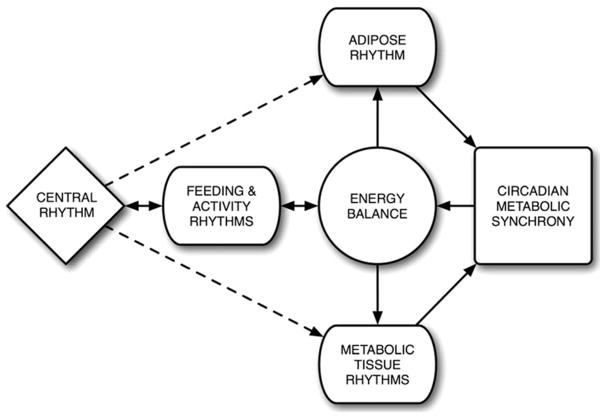
Considering the nature of the above-mentioned genotypes, it is logical to conclude that the effects of circadian rhythms on adipose function are more likely regulatory than essential. Because the organism’s homeostatic processes and energy demands fluctuate significantly throughout the day, adipose functions need to be coordinated with other metabolic peripheral tissues, in order to anticipate the upcoming changes in energy needs and properly handle the task of maintaining energy and metabolic homeostasis. Evidence to support this notion has already been observed: plasma glucose levels, peripheral tissue glucose tolerance, insulin sensitivity, and glucose uptake all follow a diurnal rhythm, with a peak immediately preceding feeding and the onset of the physical activity period (35, 36). Thus, the organism is able to generate and properly channel the flux of energy necessary to increase physical activity in anticipation of feeding. Furthermore, by accommodating a timely onset of physical activity, the organism ensures that the subsequent behaviors, such as feeding, will occur in synchrony with the specific tissues prepared to handle the stress those behaviors will place on the maintenance of energy homeostasis (Figure 1).
Figure 1.
Circadian regulation of energy homeostasis. Feeding and activity rhythms, generated by the SCN, regulate energy balance and the temporal/functional synchronization among peripheral metabolic tissues.
When Good Clocks Go Bad
Studies of cardiac metabolism have shown that the metabolic flux and contractile function of the heart show significant diurnal oscillations, indicating that the healthy heart anticipates and responds to the changing demands of an active organism (37). However, the loss of this anticipatory cyclic behavior, brought on by cardiac hypertrophy or streptozotocin-induced diabetes, leads to the loss of plasticity that may contribute to the development of contractile dysfunction (38, 39). Could the same paradigm exist in adipose and other metabolic tissues? Can circadian rhythms regulate metabolic activity and energy homeostasis and vice versa? The clinical associations between circadian and adipose biology definitely point in that direction. Therefore, what could be the possible mechanism(s) for this interaction?
In vitro studies with cultured Rat-1 fibroblasts revealed that fresh serum, added during media exchange, triggers circadian gene expression in these rhythmically quiescent cells (40). Further studies pinpointed glucose, a major food metabolite, as the factor responsible for the activation of the circadian oscillator: within this system, glucose induced down-regulation of Per1 and 2 expression, as well as the genes involved in cell cycle and cholesterol biosynthesis. Fluctuations in the cell redox potential also have the ability to entrain the circadian clock. DNA-binding activities of both CLOCK-BMAL1 and NPAS2-BMAL1 are greatly enhanced by the abundance of reduced cofactors NADH and NADPH and are speculated to represent the mechanism of clock entrainment by both RF and neuronal activity (41). As a tissue essential for energy homeostasis, adipose would without a doubt be similarly affected by chronic changes in glucose levels and redox potential. Therefore, one can easily picture how these factors could have a strong regulatory effect on the oscillatory and transcriptional activity of circadian genes in fat depots.
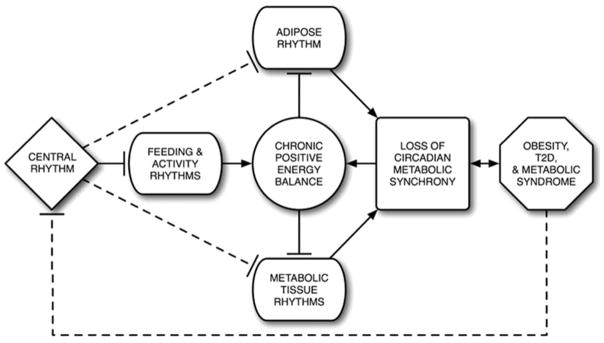
The onset of obesity is preceded by long-term positive energy balance, caused by excessive calorie intake and/or lack of physical activity. Chronic obesity often leads to ectopic fat deposition, insulin resistance, hyperglycemia, hyperlipidemia, and type 2 diabetes. Presumably, a mechanism must exist by which excess energy corrupts the proper function of the same tissues that normally regulate energy homeostasis. Recent molecular studies have shown that BMAL1 plays an important role in adipocyte differentiation and lipogenesis in mature adipocytes: loss of BMAL1 expression in the experimental system led to a significant decrease in adipogenesis and the gene expression of several key adipogenic/lipogenic factors (PPARγ2, aP2, C/EBPα, C/EBPδ, SREBP-1a, PEPCK, FAS) (42). EPAS1 (HIF-2α), a basic helix-loop-helix PAS domain (bHLH-PAS) transcription factor related to CLOCK and BMAL1, has also been implicated in the regulation of adipose function. 3T3-L1 adipocytes expressing the loss-of-function form of this protein failed to differentiate as robustly as the control lines (43). Furthermore, the loss of EPAS1 resulted in impaired lipid storage and decreased expression of glucose uptake-regulators GLUT1, GLUT4, and IRS3. Similarly, the loss of bHLH-PAS transcription factor ARNT (HIF-1β) in pancreatic β cells resulted in the alterations of gene expression similar to those in patients with type 2 diabetes, especially the genes involved in glucose-stimulated insulin release (44). These studies showed potential mechanisms by which circadian mechanisms can regulate, as well as disrupt, the normal activity of adipose and other metabolic tissues. If the signals of positive energy balance, such as increased circulating food metabolites (glucose) and changes in redox potential, can alter the transcriptional activity of key circadian transcriptional factors, these changes can be postulated as biochemically causal factors for increased adipogenesis, abnormal lipogenesis, and impaired glucose metabolism. Furthermore, because individual metabolic tissues handle specific aspects of energy homeostasis, their circadian oscillators may not be affected by chronic positive energy balance in an identical manner, thus creating a basis for the loss of temporal/functional synchronization among metabolic tissues. Together, the physiological and functional changes in these organs, as well as their inability to handle the body’s metabolic demands at appropriate times, can likely lead to the complications of the metabolic syndrome, often seen in chronic obesity (Figure 2).
Figure 2.
Loss of circadian regulation of energy homeostasis in chronic obesity. Chronic positive energy balance may disrupt the circadian transcriptional activity within adipose and other metabolic tissues, resulting in the loss of temporal/functional synchronization among those tissues and setting the stage for the development of obesity, type 2 diabetes (T2D), and the metabolic syndrome.
Conclusion
Obesity and type 2 diabetes have emerged as significant health problems in the industrialized world. Although current therapeutics alleviate some of the obesity-related health problems, our further understanding of the underlying mechanisms leading to the development of these complications is essential for the success of continued medical and pharmacological efforts to counteract this epidemic. An emerging body of evidence implicates circadian transcriptional machinery in the control of metabolism and adipose function, thus offering a novel focus for further research into the pathogenesis of obesity and its associated comorbidities.
Acknowledgments
This project was supported in part by funding from the Pennington Biomedical Research Foundation and the Pennington Biomedical Research Center Clinical Nutrition Research Unit (National Institute of Diabetes and Digestive and Kidney Diseases DK072476).
Nonstandard abbreviations
- SCN
suprachiasmatic nucleus
- RF
temporally restricted feeding
- PAI-1
plasminogen activator inhibitor 1
References
- 1.Allada R, Emery P, Takahashi JS, Rosbash M. Stopping time: the genetics of fly and mouse circadian clocks. Annu Rev Neurosci. 2001;24:1091–1119. doi: 10.1146/annurev.neuro.24.1.1091. [DOI] [PubMed] [Google Scholar]
- 2.Darlington TK, Wager-Smith K, Ceriani MF, et al. Closing the circadian loop: CLOCK-induced transcription of its own inhibitors per and tim. Science. 1998;280:1599–1603. doi: 10.1126/science.280.5369.1599. [DOI] [PubMed] [Google Scholar]
- 3.Gekakis N, Staknis D, Nguyen HB, et al. Role of the CLOCK protein in the mammalian circadian mechanism. Science. 1998;280:1564–1569. doi: 10.1126/science.280.5369.1564. [DOI] [PubMed] [Google Scholar]
- 4.Griffin EA, Jr, Staknis D, Weitz CJ. Light-independent role of CRY1 and CRY2 in the mammalian circadian clock. Science. 1999;286:768–771. doi: 10.1126/science.286.5440.768. [DOI] [PubMed] [Google Scholar]
- 5.Shearman LP, Sriram S, Weaver DR, et al. Interacting molecular loops in the mammalian circadian clock. Science. 2000;288:1013–1019. doi: 10.1126/science.288.5468.1013. [DOI] [PubMed] [Google Scholar]
- 6.Balsalobre A, Damiola F, Schibler U. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell. 1998;93:929–937. doi: 10.1016/s0092-8674(00)81199-x. [DOI] [PubMed] [Google Scholar]
- 7.Ripperger JA, Shearman LP, Reppert SM, Schibler U. CLOCK, an essential pacemaker component, controls expression of the circadian transcription factor DBP. Genes Dev. 2000;14:679–689. [PMC free article] [PubMed] [Google Scholar]
- 8.Hogenesch JB, Gu YZ, Jain S, Bradfield CA. The basic-helix-loop-helix-PAS orphan MOP3 forms transcriptionally active complexes with circadian and hypoxia factors. Proc Natl Acad Sci U S A. 1998;95:5474–5479. doi: 10.1073/pnas.95.10.5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jin X, Shearman LP, Weaver DR, Zylka MJ, de Vries GJ, Reppert SM. A molecular mechanism regulating rhythmic output from the suprachiasmatic circadian clock. Cell. 1999;96:57–68. doi: 10.1016/s0092-8674(00)80959-9. [DOI] [PubMed] [Google Scholar]
- 10.Sakamoto K, Nagase T, Fukui H, et al. Multitissue circadian expression of rat period homolog (rPer2) mRNA is governed by the mammalian circadian clock, the suprachiasmatic nucleus in the brain. J Biol Chem. 1998;273:27039–27042. doi: 10.1074/jbc.273.42.27039. [DOI] [PubMed] [Google Scholar]
- 11.Yamazaki S, Numano R, Abe M, et al. Resetting central and peripheral circadian oscillators in transgenic rats. Science. 2000;288:682–685. doi: 10.1126/science.288.5466.682. [DOI] [PubMed] [Google Scholar]
- 12.Stokkan KA, Yamazaki S, Tei H, Sakaki Y, Menaker M. Entrainment of the circadian clock in the liver by feeding. Science. 2001;291:490–493. doi: 10.1126/science.291.5503.490. [DOI] [PubMed] [Google Scholar]
- 13.Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000;14:2950–2961. doi: 10.1101/gad.183500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balsalobre A, Brown SA, Marcacci L, et al. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science. 2000;289:2344–2347. doi: 10.1126/science.289.5488.2344. [DOI] [PubMed] [Google Scholar]
- 15.Honma K, Honma S, Hiroshige T. Activity rhythms in the circadian domain appear in suprachiasmatic nuclei lesioned rats given methamphetamine. Physiol Behav. 1987;40:767–774. doi: 10.1016/0031-9384(87)90281-2. [DOI] [PubMed] [Google Scholar]
- 16.Wakamatsu H, Yoshinobu Y, Aida R, Moriya T, Akiyama M, Shibata S. Restricted-feeding-induced anticipatory activity rhythm is associated with a phase-shift of the expression of mPer1 and mPer2 mRNA in the cerebral cortex and hippocampus but not in the suprachiasmatic nucleus of mice. Eur J Neurosci. 2001;13:1190–1196. doi: 10.1046/j.0953-816x.2001.01483.x. [DOI] [PubMed] [Google Scholar]
- 17.Yoo SH, Yamazaki S, Lowrey PL, et al. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci U S A. 2004;101:5339–5346. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rutter J, Reick M, McKnight SL. Metabolism and the control of circadian rhythms. Annu Rev Biochem. 2002;71:307–331. doi: 10.1146/annurev.biochem.71.090501.142857. [DOI] [PubMed] [Google Scholar]
- 19.Holmback U, Forslund A, Lowden A, et al. Endocrine responses to nocturnal eating—possible implications for night work. Eur J Nutr. 2003;42:75–83. doi: 10.1007/s00394-003-0386-6. [DOI] [PubMed] [Google Scholar]
- 20.Gottlieb DJ, Punjabi NM, Newman AB, et al. Association of sleep time with diabetes mellitus and impaired glucose tolerance. Arch Intern Med. 2005;165:863–867. doi: 10.1001/archinte.165.8.863. [DOI] [PubMed] [Google Scholar]
- 21.Fagiolini A, Frank E, Houck PR, et al. Prevalence of obesity and weight change during treatment in patients with bipolar I disorder. J Clin Psychiatry. 2002;63:528–533. doi: 10.4088/jcp.v63n0611. [DOI] [PubMed] [Google Scholar]
- 22.Eldar-Finkelman H, Krebs EG. Phosphorylation of insulin receptor substrate 1 by glycogen synthase kinase 3 impairs insulin action. Proc Natl Acad Sci U S A. 1997;94:9660–9664. doi: 10.1073/pnas.94.18.9660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eldar-Finkelman H, Schreyer SA, Shinohara MM, LeBoeuf RC, Krebs EG. Increased glycogen synthase kinase-3 activity in diabetes- and obesity-prone C57BL/6J mice. Diabetes. 1999;48:1662–1666. doi: 10.2337/diabetes.48.8.1662. [DOI] [PubMed] [Google Scholar]
- 24.Iitaka C, Miyazaki K, Akaike T, Ishida N. A role for glycogen synthase kinase-3beta in the mammalian circadian clock. J Biol Chem. 2005;280:29397–29402. doi: 10.1074/jbc.M503526200. [DOI] [PubMed] [Google Scholar]
- 25.Yin L, Wang J, Klein PS, Lazar MA. Nuclear receptor Rev-erbalpha is a critical lithium-sensitive component of the circadian clock. Science. 2006;311:1002–1005. doi: 10.1126/science.1121613. [DOI] [PubMed] [Google Scholar]
- 26.Rana JS, Mukamal KJ, Morgan JP, Muller JE, Mittleman MA. Circadian variation in the onset of myocardial infarction: effect of duration of diabetes. Diabetes. 2003;52:1464–1468. doi: 10.2337/diabetes.52.6.1464. [DOI] [PubMed] [Google Scholar]
- 27.van der Bom JG, Bots ML, Haverkate F, Kluft C, Grobbee DE. The 4G5G polymorphism in the gene for PAI-1 and the circadian oscillation of plasma PAI-1. Blood. 2003;101:1841–1844. doi: 10.1182/blood-2002-07-2181. [DOI] [PubMed] [Google Scholar]
- 28.Gavrila A, Peng CK, Chan JL, Mietus JE, Goldberger AL, Mantzoros CS. Diurnal and ultradian dynamics of serum adiponectin in healthy men: comparison with leptin, circulating soluble leptin receptor, and cortisol patterns. J Clin Endocrinol Metab. 2003;88:2838–2843. doi: 10.1210/jc.2002-021721. [DOI] [PubMed] [Google Scholar]
- 29.Kalsbeek A, Fliers E, Romijn JA, et al. The suprachiasmatic nucleus generates the diurnal changes in plasma leptin levels. Endocrinology. 2001;142:2677–2685. doi: 10.1210/endo.142.6.8197. [DOI] [PubMed] [Google Scholar]
- 30.Saad MF, Riad-Gabriel MG, Khan A, et al. Diurnal and ultradian rhythmicity of plasma leptin: effects of gender and adiposity. J Clin Endocrinol Metab. 1998;83:453–459. doi: 10.1210/jcem.83.2.4532. [DOI] [PubMed] [Google Scholar]
- 31.Turek FW, Joshu C, Kohsaka A, et al. Obesity and metabolic syndrome in circadian clock mutant mice. Science. 2005;308:1043–1045. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rudic RD, McNamara P, Curtis AM, et al. BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol. 2004;2:e377. doi: 10.1371/journal.pbio.0020377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ando H, Yanagihara H, Hayashi Y, et al. Rhythmic messenger ribonucleic acid expression of clock genes and adipocytokines in mouse visceral adipose tissue. Endocrinology. 2005;146:5631–5636. doi: 10.1210/en.2005-0771. [DOI] [PubMed] [Google Scholar]
- 34.Zvonic S, Ptitsyn AA, Conrad SA, et al. Characterization of peripheral circadian clocks in adipose tissues. Diabetes. 2006;55:962–970. doi: 10.2337/diabetes.55.04.06.db05-0873. [DOI] [PubMed] [Google Scholar]
- 35.la Fleur SE. Daily rhythms in glucose metabolism: suprachiasmatic nucleus output to peripheral tissue. J Neuroendocrinol. 2003;15:315–322. doi: 10.1046/j.1365-2826.2003.01019.x. [DOI] [PubMed] [Google Scholar]
- 36.la Fleur SE, Kalsbeek A, Wortel J, Fekkes ML, Buijs RM. A daily rhythm in glucose tolerance: a role for the suprachiasmatic nucleus. Diabetes. 2001;50:1237–1243. doi: 10.2337/diabetes.50.6.1237. [DOI] [PubMed] [Google Scholar]
- 37.Young ME, Razeghi P, Cedars AM, Guthrie PH, Taegtmeyer H. Intrinsic diurnal variations in cardiac metabolism and contractile function. Circ Res. 2001;89:1199–1208. doi: 10.1161/hh2401.100741. [DOI] [PubMed] [Google Scholar]
- 38.Young ME, Razeghi P, Taegtmeyer H. Clock genes in the heart: characterization and attenuation with hypertrophy. Circ Res. 2001;88:1142–1150. doi: 10.1161/hh1101.091190. [DOI] [PubMed] [Google Scholar]
- 39.Young ME, Wilson CR, Razeghi P, Guthrie PH, Taegtmeyer H. Alterations of the circadian clock in the heart by streptozotocin-induced diabetes. J Mol Cell Cardiol. 2002;34:223–231. doi: 10.1006/jmcc.2001.1504. [DOI] [PubMed] [Google Scholar]
- 40.Hirota T, Okano T, Kokame K, Shirotani-Ikejima H, Miyata T, Fukada Y. Glucose down-regulates Per1 and Per2 mRNA levels and induces circadian gene expression in cultured Rat-1 fibroblasts. J Biol Chem. 2002;277:44244–44251. doi: 10.1074/jbc.M206233200. [DOI] [PubMed] [Google Scholar]
- 41.Rutter J, Reick M, Wu LC, McKnight SL. Regulation of clock and NPAS2 DNA binding by the redox state of NAD cofactors. Science. 2001;293:510–514. doi: 10.1126/science.1060698. [DOI] [PubMed] [Google Scholar]
- 42.Shimba S, Ishii N, Ohta Y, et al. Brain and muscle Arnt-like protein-1 (BMAL1), a component of the molecular clock, regulates adipogenesis. Proc Natl Acad Sci U S A. 2005;102:12071–12076. doi: 10.1073/pnas.0502383102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shimba S, Wada T, Hara S, Tezuka M. EPAS1 promotes adipose differentiation in 3T3–L1 cells. J Biol Chem. 2004;279:40946–40953. doi: 10.1074/jbc.M400840200. [DOI] [PubMed] [Google Scholar]
- 44.Gunton JE, Kulkarni RN, Yim S, et al. Loss of ARNT/HIF1beta mediates altered gene expression and pancreatic-islet dysfunction in human type 2 diabetes. Cell. 2005;122:337–349. doi: 10.1016/j.cell.2005.05.027. [DOI] [PubMed] [Google Scholar]