Abstract
Objective
To determine the effect of age on completion of and toxicities following treatment of local regionally advanced cervical cancer (LACC) on Gynecologic Oncology Group (GOG) Phase I–III trials.
Methods
An ancillary data analysis of GOG protocols 113, 120, 165, 219 data was performed. Wilcoxon, Pearson, and Kruskal-Wallis tests were used for univariate and multivariate analysis. Log rank tests were used to compare survival lengths.
Results
One-thousand-three-hundred-nineteen women were included; 60.7% were Caucasian, 15% were age 60–70 years and an additional 5% were >70; 87% had squamous histology, 55% had stage IIB disease and 34% had IIIB disease. Performance status declined with age (p = 0.006). Histology and tumor stage did not significantly differ.
Number of cycles of chemotherapy received, radiation treatment time, nor dose modifications varied with age. Notably, radiation protocol deviations and failure to complete brachytherapy (BT) did increase with age (p = 0.022 and p < 0.001 respectively).
Only all grade lymphatic (p = 0.006) and grade ≥3 cardiovascular toxicities (p= 0.019) were found to vary with age.
A 2% increase in the risk of death for every year increase >50 for all-cause mortality (HR 1.02; 95% CI, 1.01–1.04) was found, but no association between age and disease specific mortality was found.
Conclusion
This represents a large analysis of patients treated for LACC with chemo/radiation, approximately 20% of whom were >60 years of age. Older patients, had higher rates of incomplete brachytherapy which is not explained by collected toxicity data. Age did not adversely impact completion of chemotherapy and radiation or toxicities.
Keywords: Biomarkers, Cervical cancer, Chemoradiation
1. Introduction
The older population, typically defined as persons 65 years or older, numbered 39.6 million in 2009 and represented 13% of the U.S. population [1]. It is estimated that by 2030 there will be approximately 72 million older persons, more than twice their number in 2000, and persons age 65 and older are expected to represent 19% of the population [1]. Along with the rapid growth in the aging population comes an increased prevalence of cancer diagnosis within this group. Cancer of the uterine cervix is one of these malignancies with potential for increased incidence in this increasingly aged population.
According to the American Cancer Society, it is estimated that 12,990 women will be diagnosed with cervical cancer and 4120 women will die from their disease in 2016 [2]. Although the overall incidence of cervical cancer has declined since the introduction of regular cervical screening guidelines, the proportion of older women being diagnosed with the disease has increased. In 2007, the incidence rate of cervical cancer for women greater than age 50 was 10.3 per 100,000 compared to 5.1 per 100,000 for those younger than 50 [3]. This is not unexpected given that cervical cancer is bimodal in its age related distribution with peaks between the ages of 30–39 and a second peak from 60 to 69 [4].
While the increase in incidence of cervical cancer with age is anticipated, the role of age in prognosis has not yet been determined. There are very contradictory results regarding the effect of age on survival. The standard of care for local advanced cervical cancer is concurrent chemoradiation (CCRT) plus brachytherapy. CCRT is also applicable in early-stage cervical cancer patients not suitable for radical surgery, such as in the case of some older persons with multiple medical comorbidities [5].
Studies have suggested that both overall and disease specific outcomes are poorer among older populations usually defined as >65. This finding, however, is likely due in large part to disparities in treatment rendered. Several studies demonstrate that when older patients are treated with standard of care therapies their outcomes are comparable to younger cohorts and without unacceptable increase in toxicity [4, 6–8]. Previous studies among endometrial cancer patients have not shown differences in complications of radiation therapy for older compared with younger patients [7, 9, 10]. However, cervical cancer patients differ greatly from those with endometrial cancer with intrinsic differences in tumor biology as well as significant differences in the radiation dose delivered. Cervix cancer patients are also more commonly of lower socioeconomic status with attendant health related challenges such as tobacco and substance abuse, poor nutrition, and poor social support.
It is important to evaluate age as a factor for tolerance and completion of CCRT as well as survival in the treatment of cervical cancer. Currently there is a paucity of prospective data investigating this unique topic. Kunos et al. evaluated patients treated on GOG protocol 120 and 165 and found no difference in seriousness or frequency of treatment related sequelae with a cut point of 55 years of age [4]. Whether 55 is a good representation of the older population for cervix cancer is an outstanding question given that standard definitions start at age 65 or greater.
This current work is an ancillary analysis of cervical cancer patients treated with primary CCRT on GOG protocols 113, 120, 165 and 219 with data stratified by decade of age with a goal of evaluating patients ≥65. The age groups would be compared for tolerance and completion of CCRT including prescribed BT [11–14].
The Alliance currently incorporates specific assessments for older persons in their study designs. This is not routinely done for NRG and other cooperative studies, but there is an increasing mandate that we do so. Data gathered from this analysis will provide information about elderly cervical cancer patients, a vulnerable population, and may demonstrate the need for tailored assessments to this group including dose modifications and toxicity considerations.
2. Methods and statistics
The data set comprised patients from GOG-0113, 120, 165, and 219 who were evaluated and treated for carcinoma of the cervix.
Inclusion criteria and treatment details for each of these trials has been published previously. In summary, each of these trials enrolled patients with stage IIB–IVA (GOG 219 allowed IB2) invasive squamous, adenocarcinoma and adenosquamous cervical carcinoma treated with radiation with or without cisplatin containing regimens. This analysis includes those patients treated with cisplatin containing regimens concurrent with radiation (CCRT) [11–14].
Stage was established by physical examination in all studies and the pathology was centrally reviewed although this was not a prerequisite for study initiation. In all studies, patients with stage IIIA disease or those with distal vaginal involvement were excluded due to the complexity and required individualization of brachytherapy.
Evidence of para-aortic lymph node positivity (either pathologic or radiographic) or disease outside of the pelvis was exclusionary on all studies. GOG 113 and 120 required extra-peritoneal lymph node dissection with confirmation of negative nodes for study entry [11, 12]. GOG 165 and 219 required negative computed tomography or lymphoscintigraphy but did not require histologic confirmation [14]. All participating institutions obtained Institutional Review Board approval prior to enrolling patients and all patients had to sign informed consent prior to study participation.
3. Treatment received
3.1. Chemotherapy
GOG 113 was anon-randomized Phase I–II trial that treated patients with continuous infusion 5-FU, hydroxyurea and cisplatin. Patients received continuous infusion 5-FU over 96 h on days 2–5 and days 30– 33 at an initial dose of 800 mg/m2/day. Cisplatin was given 4 h before radiation at a dose of 50 mg/m2 on day 1 and 29. Patients took hydroxyurea orally twice weekly during external radiation. Chemotherapy was held for white blood cell (WBC) < 3000/µl or platelets <100,000/µl [11]. All 75 patients enrolled in GOG 113 were included in this ancillary analysis.
GOG 120, 165 and 219 all included patients randomly assigned to receive weekly cisplatin at a dose of 40 mg/m2 on day 1, 8, 15, 22, 29 and 36 during external radiation therapy. GOG 120 also had an arm identical to the chemotherapy prescribed in GOG 113. Doses of cisplatin were modified for leucopenia <2500/mm3 on GOG 120, <3000 on GOG 165 and <1000/mm3 on GOG 219 as well as for thrombocytopenia b50,000/mm3 on GOG 120 and GOG 219 and b100,000/mm3 on GOG 165. Once toxicities had recovered, doses were resumed with pre specified dose modification [11, 13, 14]. All patients who were randomized to receive chemoradiation on one of these protocols were included in this analysis.
3.2. Radiation
GOG 113
External beam radiation was given in two field AP/PA at a dose of 4080 cGy for stage IIB and 5100 cGy for stage III and IV in 30 fractions. A 4-field box technique was permitted. The radiation source was required to be 4 MV or higher or 60Co irradiators. Following external beam radiation, 1 or 2 applications of LDR were given to a dose of 40 Gy at point A for stage IIB and 30 Gy to point A for IIIB–IVA disease. HDR was not included in this protocol [11].
GOG 120
External beam radiation was given as a 4 field box delivered in 1.7 Gy/day fractions using 4 MV photos or higher. Doses ranged from 40.8 Gy for stage II disease to 51.0 Gy for stage III/IVA disease. External beam therapy was followed by 1 or 2 applications of intra-cavitary BT delivered via LDR. HDR was not allowed on this protocol [12].
GOG 165
External beam radiation was given as a 4 field box delivered in 1.8 Gy fractions using 4 MV photons or higher to a dose of 45.0 Gy. Intra-cavitary radiation followed external beam and could include either 40 Gy to point A in 1 or 2 fractions via LDR or 30 Gy to point A in 5 fractions if using HDR [13].
GOG 219
Patients were prescribed 41.4 to 45 Gy external beam radiation therapy delivered in 23 to 25 fractions of 1.8 Gy. After completion of external beam, patients received 35 to 43.6 Gy to point A by intra-cavitary implant with radium or its equivalent if treated with LDR. If HDR was used, the dose to point A was 27 to 31.5 Gy over 5 fractions [14].
It is worth emphasizing the changes in radiation techniques both for external beam fields and inclusion of HDR brachytherapy required education and quality assurance and occurred between the execution of GOG protocols 113/120 and GOG protocols 165/219.
Anatomic fields for pelvic radiation are defined by the GOG and include an upper margin of the L-5 vertebrae and a lower margin of the mid portion of the obturator foramen or the lowest extent of disease with a 3 cm margin for anterior and posterior radiation fields. Laterally the anterior and posterior radiation fields extended 1.5 to 2 cm. beyond the pelvic brim. For right and left lateral radiation fields, the anterior border was the pubic symphysis and the posterior border was the space between the S2 and S3 vertebrae. The posterior border was placed behind the sacrum starting with GOG 165. The superior and inferior margins for lateral radiation fields were the same as the anterior and posterior radiation fields.
Interstitial BT was not allowed on GOG protocols.
3.3. Statistical analysis
The data collected included patient demographics, clinicopathologic information, chemotherapy administration, radiation therapy completion, adverse events, and survival outcomes. Completion of brachytherapy was determined by the ratio of the mean dose to point A. The association of age with measures of chemoradiation tolerance, toxicity, and survival was evaluated. Categorical variables were compared among the various age groups by the Pearson chi-square test [15], and continuous variables by the Kruskal–Wallis test [16]. Survival was estimated using the Kaplan–Meier [17] method. The Cox proportional hazards model [18] was used to evaluate age and other independent prognostic factors and to estimate their covariate-adjusted effects on progression-free survival (PFS) and overall survival (OS). The association of age with toxicity counts was evaluated with the covariate-adjusted Poisson regression model. The association of age with measures of chemoradiation tolerance was evaluated with the covariate-adjusted logistic regression model. The nonlinearity of the effect of continuous variables was assessed using restricted cubic splines. All statistical tests were two-tailed with the significance level set at α = 0.05. Statistical analyses were performed using the R programming language and environment [19].
4. Results
4.1. Demographics
1319 patients were included in this analysis. The demographic and pathology characteristics of this large cohort of women treated on clinical trials with concomitant chemotherapy and radiation is displayed in Table 1 divided by age. In the overall cohort, the majority were Caucasian (61%), while 21% were Black and 12% Hispanic. Median age was 47.7 years. The vast majority of trial participants had squamous cell carcinoma (87.4%) and just over half were enrolled as stage IIB (55%). The second most common stage enrolled was IIIB at 34%. Medicaid or uninsured comprised 41% of the cohort.
Table 1.
Patient demographics and tumor characteristics by age.
| N | <40.0 N = 336 |
40.0–50.0 N = 403 |
50.0–60.0 N = 322 |
60.0–70.0 N = 195 |
≥70.0 N = 63 |
Test statistic | |
|---|---|---|---|---|---|---|---|
| Payer | 1319 | p < 0.001a | |||||
| Private | 26.8% (90) | 38.2% (154) | 40.7% (131) | 21.5% (42) | 7.9% (5) | ||
| Medicare | 1.2% (4) | 0.7% (3) | 1.6% (5) | 20.0% (39) | 54.0% (34) | ||
| Medicare + private | 0.6% (2) | 0.5% (2) | 0.6% (2) | 6.2% (12) | 11.1% (7) | ||
| Medicaid | 28.3% (95) | 19.9% (80) | 17.4% (56) | 10.3% (20) | 9.5% (6) | ||
| Medicaid + Medicare | 0.9% (3) | 0.0% (0) | 1.2% (4) | 2.1% (4) | 1.6% (1) | ||
| Military or veterans | 3.3% (11) | 0.7% (3) | 3.1% (10) | 2.1% (4) | 0.0% (0) | ||
| Other government | 5.4% (18) | 5.0% (20) | 5.3% (17) | 5.1% (10) | 3.2% (2) | ||
| Self/uninsured | 23.2% (78) | 25.8% (104) | 18.6% (60) | 23.1% (45) | 3.2% (2) | ||
| Unknown | 10.4% (35) | 9.2% (37) | 11.5% (37) | 9.7% (19) | 9.5% (6) | ||
| Race/ethnicity | 1319 | p = 0.014a | |||||
| White | 56.5% (190) | 62.0% (250) | 63.0% (203) | 62.6% (122) | 55.6% (35) | ||
| Black | 24.7% (83) | 20.3% (82) | 19.6% (63) | 21.5% (42) | 19.0% (12) | ||
| Hispanic | 16.1% (54) | 11.9% (48) | 9.3% (30) | 10.8% (21) | 17.5% (11) | ||
| Asian | 1.2% (4) | 3.2% (13) | 6.2% (20) | 4.6% (9) | 7.9% (5) | ||
| Other | 1.5% (5) | 2.5% (10) | 1.9% (6) | 0.5% (1) | 0.0% (0) | ||
| Performance status | 1319 | p = 0.006a | |||||
| Normal, asymptomatic | 65.5% (220) | 71.0% (286) | 64.6% (208) | 65.1% (127) | 47.6% (30) | ||
| Symptomatic, ambulatory | 31.5% (106) | 25.1% (101) | 32.3% (104) | 30.3% (59) | 41.3% (26) | ||
| Symptomatic, in bed | 3.0% (10) | 4.0% (16) | 3.1% (10) | 4.6% (9) | 11.1% (7) | ||
| Histology | 1319 | p = 0.91a | |||||
| Squamous | 85.1% (286) | 88.1% (355) | 88.5% (285) | 88.7% (173) | 85.7% (54) | ||
| Adenosquamous | 6.5% (22) | 5.2% (21) | 5.0% (16) | 5.6% (11) | 4.8% (3) | ||
| Adenocarc., other | 8.3% (28) | 6.7% (27) | 6.5% (21) | 5.6% (11) | 9.5% (6) | ||
| Tumor size cm | 1308 | 5.0 6.0 7.5 | 5.0 6.0 7.0 | 5.0 6.0 7.0 | 4.7 6.0 7.0 | 4.0 5.2 6.0 | p = 0.003b |
| FIGO stage | 1319 | p = 0.144a | |||||
| IB | 6.5% (22) | 5.2% (21) | 4.7% (15) | 3.1% (6) | 1.6% (1) | ||
| IIA | 1.2% (4) | 1.2% (5) | 3.1% (10) | 1.0% (2) | 3.2% (2) | ||
| IIB | 61.9% (208) | 52.9% (213) | 52.8% (170) | 51.3% (100) | 50.8% (32) | ||
| IIIA | 1.2% (4) | 0.7% (3) | 1.2% (4) | 2.1% (4) | 1.6% (1) | ||
| IIIB | 27.1% (91) | 36.5% (147) | 34.2% (110) | 37.9% (74) | 39.7% (25) | ||
| IVA | 2.1% (7) | 3.5% (14) | 4.0% (13) | 4.6% (9) | 3.2% (2) | ||
| Tumor grade (differentiation) | 1319 | p = 0.769a | |||||
| Good | 8.0% (27) | 6.9% (28) | 6.2% (20) | 5.6% (11) | 7.9% (5) | ||
| Moderate | 58.3% (196) | 62.8% (253) | 59.6% (192) | 62.6% (122) | 65.1% (41) | ||
| Poor | 31.0% (104) | 28.8% (116) | 33.2% (107) | 30.3% (59) | 27.0% (17) | ||
| Not graded | 2.7% (9) | 1.5% (6) | 0.9% (3) | 1.5% (3) | 0.0% (0) |
Numbers after percents are frequencies.
Test used: Pearson test
Test used: Kruskal-Wallis test.
When evaluated by age, there was no difference in tumor grade, stage or histology. Not surprisingly, statistically significantly more women in the older age categories had Medicare as a payer source (p < 0.001) and more women in the older age categories were enrolled with poorer performance status (p = 0.006). There were statistically significant differences in race/ethnicity with more women ≥70 years of age being Hispanic or Asian race than as compared to younger groups.
4.2. Treatment received
The chemotherapy received varied by study protocol. Details of the treatments received are presented in Table 2 categorized by age. In the cohort as a whole, only 16% of patients required a chemotherapy dose modification and only 5% were delayed. The median length of radiation duration including brachytherapy was 57 days. The lower and upper quartiles were 51 and 66 days, respectively. Nineteen percent of patients required a radiation dose modification and 37% of the enrolled patients had either minor (21%) or major (16%) radiation protocol deviations. BT was not completed in 15%.
Table 2.
Patient treatment details by age.
| N | <40.0 N = 336 |
40.0–50.0 N = 403 |
50.0–60.0 N = 322 |
60.0–70.0 N = 195 |
≥70.0 N = 63 |
Test statistic | |
|---|---|---|---|---|---|---|---|
| Treatment | 1319 | p = 0.179a | |||||
| RT | 3.0% (10) | 1.2% (5) | 1.2% (4) | 2.1% (4) | 1.6% (1) | ||
| RT + 5FU | 12.2% (41) | 11.4% (46) | 12.1% (39) | 7.7% (15) | 25.4% (16) | ||
| RT + HU | 12.8% (43) | 12.4% (50) | 15.2% (49) | 14.4% (28) | 9.5% (6) | ||
| RT + CDDP | 37.8% (127) | 42.2% (170) | 37.9% (122) | 45.1% (88) | 34.9% (22) | ||
| RT + CDDP + 5FU + HU | 21.4% (72) | 17.4% (70) | 18.3% (59) | 17.9% (35) | 19.0% (12) | ||
| RT + CDDP + TPZ | 12.8% (43) | 15.4% (62) | 15.2% (49) | 12.8% (25) | 9.5% (6) | ||
| Cycles of chemotherapy | 1319 | 5.0 5.0 6.0 | 5.0 5.0 6.0 | 5.0 5.0 6.0 | 5.0 5.0 6.0 | 4.5 6.0 6.0 | p = 0.382b |
| Chemo. dose modification | 1226 | p = 0.276a | |||||
| No | 80.4% (251) | 86.1% (322) | 84.8% (257) | 86.2% (156) | 82.1% (46) | ||
| Yes | 19.6% (61) | 13.9% (52) | 15.2% (46) | 13.8% (25) | 17.9% (10) | ||
| Chemo. dose delay | 1228 | p = 0.422a | |||||
| No | 93.5% (290) | 96.5% (362) | 94.1% (286) | 95.6% (174) | 94.7% (54) | ||
| Yes | 6.5% (20) | 3.5% (13) | 5.9% (18) | 4.4% (8) | 5.3% (3) | ||
| Chemo. completed | 1319 | p = 0.105a | |||||
| No | 47.0% (158) | 39.0% (157) | 39.8% (128) | 47.2% (92) | 41.3% (26) | ||
| Yes | 53.0% (178) | 61.0% (246) | 60.2% (194) | 52.8% (103) | 58.7% (37) | ||
| Mean dose to pt. A | 1208 | 3000 3931 4005 | 3000 3533 4001 | 3000 3875 4002 | 2998 3750 4000 | 2998 3435 4002 | p = 0.054b |
| Mean dose to pt. B | 1207 | 842 1024 1174 | 794 927 1121 | 803 948 1118 | 786 918 1082 | 768 959 1121 | p < 0.001b |
| Length of RT treatment weeks | 1305 | 7.14 8.14 9.29 | 7.28 8.14 9.29 | 7.29 8.14 9.43 | 7.29 8.29 9.57 | 7.15 8.14 9.29 | p = 0.24b |
| RT dose modification | 1319 | p = 0.424a | |||||
| No | 79.2% (266) | 83.9% (338) | 81.4% (262) | 79.0% (154) | 77.8% (49) | ||
| Yes | 20.8% (70) | 16.1% (65) | 18.6% (60) | 21.0% (41) | 22.2% (14) | ||
| RT protocol deviation | 1308 | p = 0.022a | |||||
| None | 66.4% (221) | 63.8% (256) | 58.4% (187) | 64.4% (125) | 61.7% (37) | ||
| Minor | 17.1% (57) | 23.7% (95) | 22.8% (73) | 21.1% (41) | 11.7% (7) | ||
| Major | 16.5% (55) | 12.5% (50) | 18.8% (60) | 14.4% (28) | 26.7% (16) | ||
| Brachytherapy completed | 1319 | p < 0.001a | |||||
| No | 13.1% (44) | 11.9% (48) | 15.2% (49) | 20.0% (39) | 34.9% (22) | ||
| Yes | 86.9% (292) | 88.1% (355) | 84.8% (273) | 80.0% (156) | 65.1% (41) |
Numbers after percents are frequencies.
Test used: Pearson test.
Test used: Kruskal–Wallis test.
When evaluated by age, there were no statistically significant differences in number of cycles of chemotherapy received, need for chemotherapy dose modification, need for chemotherapy dose delay or completion of assigned chemotherapy. There was a trend towards the oldest age group (>70) receiving less mean dose to point A (p = 0.054). Mean dose delivered to point B varied significantly between the age groups but did not correlate with age (p < 0.001). Interestingly, there were significantly more major radiation protocol deviations among the oldest age group (27% in those ≥70 as compared to 17% in those <40; p = 0.022). There was also significantly less completion of BT among the oldest population (35% in those ≥70 as compared to 13% in those <40; p < 0.001). BT completion was determined by evaluating ratio of the reported dose given to point A over the planned point A dose. If this ratio was ≥0.85, then the dose is considered “fully acceptable” according to the GOG radiotherapy manual. If the ratio was <0.85, then the brachytherapy dose was considered incomplete.
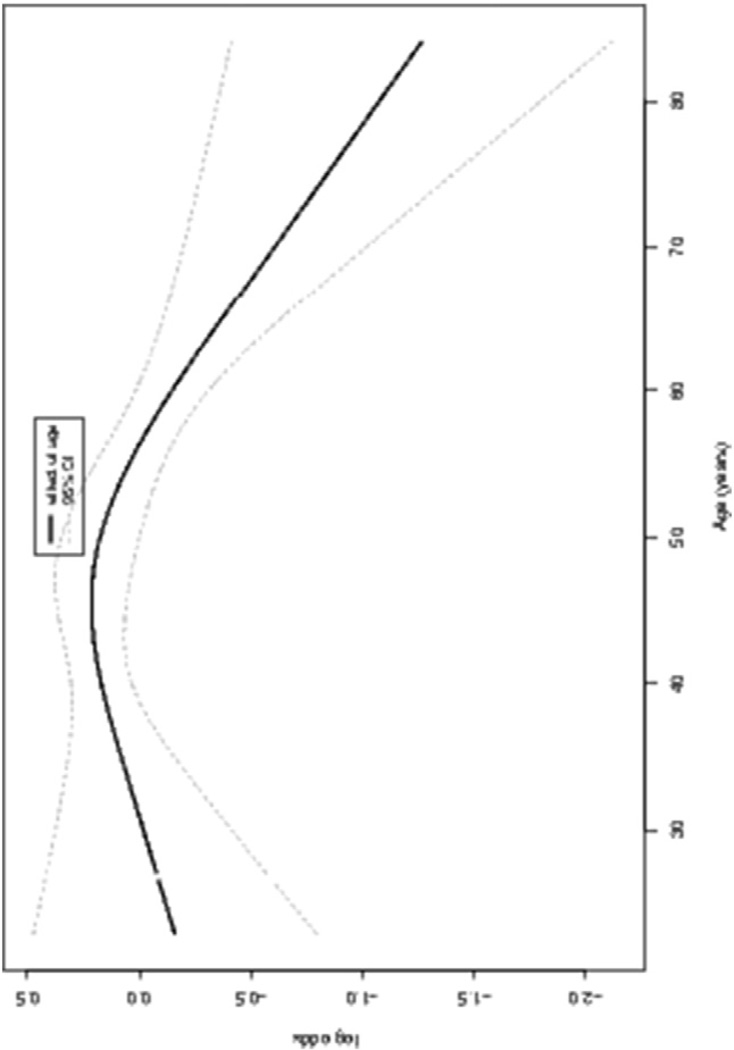
Supplemental Table 1 summarizes the logistic regression model of the association of brachytherapy completion with age along with other important clinical factors including, insurance type, race, performance status, stage and specific GOG protocol participation. Age was a significant factor associated with completion of BT (p = 0.118) along with BMI (p = 0.0112), performance status (p = 0.0016) and stage (0.0007) but not specific protocol participation (p = 0.1211). Fig. 1 shows the partial effects plot of age on the log odds of BT completion. The increase in log odds of BT before age ≈ 50 years is not significant given the wide confidence intervals, but after 50 the log odds of BT appears to decrease sharply. More specifically, the odds of completion of BT decreased approximately 5% for each increasing year of age after about 50 years (OR = 0.95, 95% CI: 0.95–0.99). Age was only significant in the model for BT completion, not in other models evaluating measures of chemoradiation tolerance.
Fig. 1.
Plot of the partial effect of age on the log odds of brachytherapy completion.
4.3. Toxicity
Toxicity data was collected and grouped by type and age category. Toxicities recorded included white blood count, peripheral and central neurotoxicity, other hematologic, skin, genitourinary, lymphatic, gastrointestinal/hepatic, pulmonary and cardiovascular toxicities as well as symptoms of stomatitis/pharyngitis, fever, and allergy. For toxicities of any grade ≥ 1, there were no toxicities that varied significantly by age with the exception of lymphatic toxicity which occurred more frequently among the oldest age group (10% in those ≥70 as compared to 3% < 40; p = 0.006)
For the same toxicities grade 3 or greater, the only category that varied with age was cardiovascular toxicity which increased with advancing age (6% for those ≥70 as compared to 1% <40; p = 0.019; Table 3). A Poisson model of the association of severe toxicity counts with age was developed (Supplemental Table 2)and age was not significantly associated with severe toxicity counts nor overall toxicities. Variables that were found to be associated with overall toxicities included body mass index (p = 0.0007), stage (p = 0.0137) and specific protocol (p < 0.0001). Of the 4 included studies, GOG protocol 219 was significantly associated with more severe toxicities as compared to the referent protocol which was GOG 113 (HR 0.395 (0.715, 0.614)). Neither GOG 165 nor GOG 120 reported toxicities significantly different than those reported in GOG 113.
Table 3.
Grade 3 or greater toxicities by age category.
| N | <40.0 N = 336 |
40.0–50.0 N = 403 |
50.0–60.0 N = 322 |
60.0–70.0 N = 195 |
≥70.0 N = 63 |
Test statistic | |
|---|---|---|---|---|---|---|---|
| Severe toxicity | 1319 | ||||||
| WBC | 24.1% (81) | 22.3% (90) | 27.0% (87) | 34.4% (67) | 25.4% (16) | p = 0.030 | |
| Peripheral neuropathy | 0.3% (1) | 0.2% (1) | 0.3% (1) | 0.0% (0) | 0.0% (0) | p = 0.943 | |
| Neurologic, auditory, visual | 5.4% (18) | 6.0% (24) | 5.0% (16) | 5.1% (10) | 4.8% (3) | p = 0.979 | |
| Other hematologic | 21.7% (73) | 21.6% (87) | 22.0% (71) | 29.7% (58) | 27.0% (17) | p = 0.169 | |
| Skin | 3.3% (11) | 4.5% (18) | 5.0% (16) | 3.6% (7) | 3.2% (2) | p = 0.807 | |
| Genitourinary/renal | 5.1% (17) | 4.0% (16) | 2.2% (7) | 2.6% (5) | 6.3% (4) | p = 0.210 | |
| Lymphatic | 0.0% (0) | 0.0% (0) | 0.0% (0) | 0.5% (1) | 0.0% (0) | p = 0.217 | |
| Gastrointestinal, hepatic | 13.4% (45) | 17.4% (70) | 15.5% (50) | 19.5% (38) | 23.8% (15) | p = 0.165 | |
| Stomatitis/pharyngitis | 0.0% (0) | 0.2% (1) | 0.0% (0) | 0.5% (1) | 0.0% (0) | p = 0.554 | |
| Constitutional, fever | 3.9% (13) | 2.5% (10) | 5.3% (17) | 4.1% (8) | 7.9% (5) | p = 0.176 | |
| Pulmonary | 0.6% (2) | 1.5% (6) | 0.6% (2) | 1.5% (3) | 3.2% (2) | p = 0.321 | |
| Allergy, immunological | 0.0% (0) | 0.5% (2) | 0.3% (1) | 0.0% (0) | 0.0% (0) | p = 0.603 | |
| Cardiovascular | 0.9% (3) | 1.5% (6) | 0.9% (3) | 2.1% (4) | 6.3% (4) | p = 0.019 | |
| Other | 10.7% (36) | 8.7% (35) | 10.2% (33) | 13.8% (27) | 23.8% (15) | p = 0.006 |
N is the number of non–missing values. Test used: Pearson test.
4.4. Outcomes
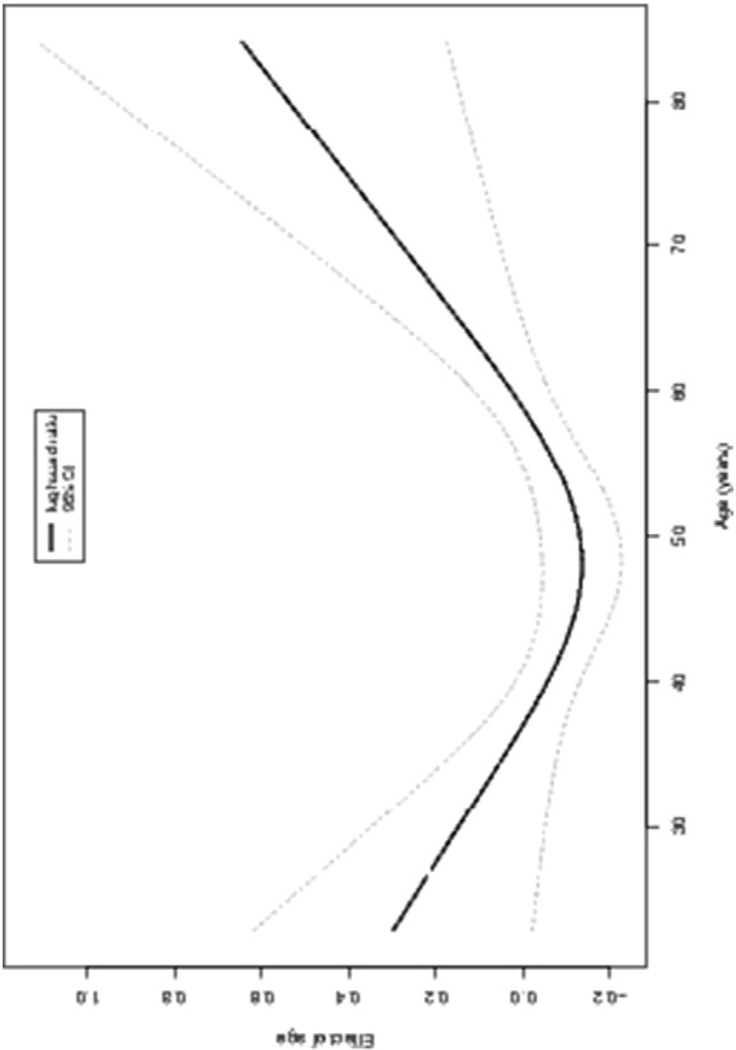
Age is significantly associated with OS (all-cause) and significantly nonlinear. Fig. 2 shows the partial effects plot of age on the log hazard ratio. The decrease in risk before age 50 years is not significant given the wide confidence intervals, but after 50 the risk appears to increase sharply. The increase in risk is 2% (HR 1.02; 95% CI, 1.01–1.04) for every 1-year increase in age, with the other variables held constant. That is, patients in the age range >50 years with a 1-year increase from a lower value have a 2% increase in risk of death from any cause.
Fig. 2.
Plot of the partial effect of age on the log hazard ratio of overall survival (all cause) model.
However, multivariate analysis of age and disease-specific PFS and OS models found age was not significant in these models, indicating that the increase in risk of death was due to etiologies other than cervical cancer. Multivariate analysis did find that race (p = 0.0390), poor performance status (p = 0.0178), nonsquamous histology (p = 0.0009), size of tumor (p = 0.003), higher stage (p < 0.0001) and pelvic lymph nodes (p=0.0057) were all associated with disease specific progression free survival. Disease specific overall survival was only associated with race (p = 0.0185), lower performance status (p = 0.0032), non-squamous histology (p = 0.0121), stage (p < 0.0001), grade (p = 0.0299) and pelvic lymph nodes (p = 0.0010). Insurance status, or lack thereof, was evaluated and not found to be significantly associated with either all cause or disease specific survival. Race was only significant in that non-white, non-black patients had improved disease specific overall survival as compared to white patients while outcomes for black patients were indistinguishable from those of white.
5. Discussion
There is conflicting data on treatment and outcomes among older women with local–regionally advanced cervical cancer. This disparity stems from the different populations studied. Here, we present an ancillary data analysis of 4, Phase I–III trials including over 1300 women and demonstrate that there were not great differences in disease specific survival or toxicity when patients greater and <65 were treated in a standard fashion. Population based studies would suggest the opposite, that outcomes are worse and toxicities more severe with increasing age [6–8].
Our study did concur with the findings of several of these studies that treatments did differ with age – which is a surprising finding given that these patients were all enrolled on a clinical trial with standard treatment prescribed. The most concerning finding here was the absence of or incomplete intra-cavitary BT seen with increasing age that will be addressed below.
The demographics of cervical cancer among older women were nicely summarized by Sharma et al. in their SEER based analysis of treatment and outcomes for cervix cancer. In a cohort of over 28,000 women, 27% were diagnosed at age 60 or greater. These older women were more frequently non-White (p < 0.05) and more frequently had advanced disease (p < 0.0001) [6]. Our study also demonstrated racial differences with age (p = 0.014) with more Hispanic and Asian women in the oldest age category but no differences in stage distribution (p = 0.14). Both of these later two points are likely entirely due to selection for the trials and don’t represent population based data.
In this analysis of over 1300 patients treated with CCRT, we demonstrated no difference by age in chemotherapy completion or chemotherapy delays. This reinforces the results Kunos et al. [4] reported including only patients on GOG-120 and 165 where he reported that after controlling for other clinical factors, patients age 55 and older were no more likely to stop protocol therapy early than those <55 years of age (OR 1.16; 95% CI 0.7–1.189; p = 0.552). Interestingly, his analysis did find that Black race was associated with failure to complete therapy as compared to Caucasian patients with an OR of 2.13; 95% CI 1.22–3.74; p = 0.029) [4]. In looking at this population further, there were no toxicities or other factors that could explain this finding. In this analysis including both GOG-113 and 219, we find no association with treatment completion and race.
Radiation experience did appear to change with advancing age. We reported significantly more major radiation deviations in the oldest group; 27% in those ≥70 years of age as compared to 17% in those <40; p = 0.022. We don’t have details for these deviations but many of them may be related to the increasing rate of intracavitary BT (ICBT) omission with age. In our analysis, over 30% of patients >70 did not complete ICBT as compared to 13% in those <40; p <0.001. Although the importance of ICBT has been recognized for decades, the omission of ICBT in older patients has not been well reported until recently. Sharma et al. reporting from the SEER database found that use of ICBT declined with age from 66.7% in those <50 years old to 58.9% in those 70–79 and 46.3% in women >70 [6]. This translates into ICBT omission in 41% for those women 70 and older, which is not dissimilar to what our current study reports for women >65. Yanazume et al. evaluated 85 patients with local-regional cervix cancer age 70–89. They categorized ICBT as complete, incomplete or impractical. Incomplete ICBT referred to discontinuation of otherwise technically feasible radiation delivery and impractical refers to technically challenging or infeasible delivery. In this cohort, 25% of patients could not receive or did not complete ICBT. Multivariate analysis found that non squamous cell cancer, incomplete ICBT and positive lymph nodes were all prognostic for PFS [20]. When they evaluated the 25% who did not receive or complete ICBT (n = 17), 15 of these were due to technical difficulty while only 2 were due to not proceeding with technically feasible delivery. In their analysis they found nulliparity (p = 0.014) and tumor size (p = 0.007) to both be independent predictors for failure to complete or receive ICBT [20].
Other authors have reported on ICBT completion with fairly consistent results (Table 4), the exception being Chakraborty reporting on a cohort of patients treated in Northern Kerala, India. This report was evaluating a new modality of external beam therapy so there may be selection bias here [21]. The more disturbing of the reports is the population based analysis by Sharma et al. where over 40% of patients >70 don’t receive ICBT for their cervix cancer [6].
Table 4.
Reported prevalence of incomplete intracavitary brachytherapy (ICBT) among older patients with cervical cancer.
| Study | Age | n | Incomplete ICBT (%) |
Explanation |
|---|---|---|---|---|
| Moore et al. (2016) |
>70 | 63 | 34.9% | Not known |
| Yanazume et al. (2014) |
70–89 | 85 | 25% | 88% due to technical issues |
| Chakraborty et al. (2014) |
≥65 | 23 | 13% | 2 pts. refused and 1 pt. had poor response to tx |
| Sharma et al. (2011) (SEER) |
70–79 ≥80 |
1099 611 |
41.1% 53.7% |
Other than age, predictors for ICBT were race, year of diagnosis, area of residence and stage |
In our current re-analysis of patients previously treated on GOG trials, we cannot know whether the deviations were due to technical feasibility or other reasons. One can postulate that, among a population of patients enrolled on a clinical trial, the reasons for incomplete intracavitary BT may well have been technical difficulties with using standard tandem and ovoids in older patients with vaginal stenosis and age related anatomic changes. Quality assurance for BT administered during participation in all 4 protocols included submission of orthogonal simulation films taken following intracavitary insertion. In addition, participating sites had to demonstrate the ability to achieve an accuracy of ±3% in measuring the output of their therapy units and ±5% in delivering prescribed dose. While the standards set for radiation administration were high, the analysis of the orthogonal simulation films is not available for this analysis and would be highly informative as to the question of whether BT was attempted or even feasible in some participants. It suggests the need to consider alternative strategies for BT administration as well as alternative coding should these strategies prove difficult.
Toxicities reported were not significantly different by age in this analysis with the exception of any grade lymphatic disorders that were more common with increasing age. Unlike prior analyses, there was no increase in the hematologic toxicities with increasing age. None of these studies allowed or included granulocyte stimulating factors as a part of their protocols so the reason for this is likely good patient selection for clinical trials in the older age ranges and more hematologic tolerance of therapies. Interestingly, toxicities did differ between the 4 included studies with GOG 219 having 1.5 times as many reported severe toxicities as the referent study GOG 113. It is unclear whether this result is real, or rather a result of improved toxicity reporting in this most recent Phase III trial.
As stated in the results, there was as association between increasing age >50 and all-cause mortality with an increased risk of 2% (HR 1.02; 95% CI, 10.1–10.4) for every 1-year increase in age with other variables held constant. When considering disease specific survival, however, age was not significant. This is similar to the findings of Kunos et al. which included some of this current population [4] but very different than population based analyses which find that women 70–79 years old (HR 1.12; 95% CI 1.01–1.24) and ≥80 years old (HR 1.47; 95% CI 1.29– 1.68) with stage IIB–IVA disease were more likely to die of their cancers than younger women [6]. Other than disease specific characteristics such as tumor stage and presence of pelvic lymph nodes, the only other factors that appeared to impact disease specific survival were performance status and race. The impact of race, specifically black race on survival with cervical cancer has been well documented. In an analysis of linked US Census and SEER database information, black women with cervical cancer had a higher likelihood of dying from their disease as compared to white women even after adjustment for socioeconomic status (HR 1.09 (CI: 1.03–1.15)). Etiologies for this disparate survival are under investigation but include later stage at diagnosis and access to care among others [22]. Our study did not reproduce these findings as black women had survival outcomes that were indistinguishable from white patients. This is an encouraging finding and may be due to selection/enrollment bias of women, regardless of race, who have access to care centers with dedicated clinical trials programs in cervical cancer. Our analysis did look at insurance status as a surrogate for socioeconomic status and found no association with any of the endpoints under study, however, payer status may be a poor surrogate for this measure.
Our study demonstrates that in a selected population of older women treated on study, the toxicities were not meaningfully different with increasing age, completion of external beam did not differ and disease specific survival was similar. Somewhat disturbingly, we found that approximately 30% of patients enrolled on treatment specified trials failed to complete BT, mirroring what is seen in population-based data. With the increasing proportion of our population living into their 7th 8th and 9th decades and the bimodal distribution of cervical cancer incidence, we will see more of these older women with cervical cancer and we need to discern best practices. This group has been poorly studied in clinical trials and under treated based on population bases data. This has been attributed to multiple factors including physician bias, presence of multiple medical co-morbidities and a belief that elderly patients are more vulnerable and prone to toxicity [4, 7, 23]. The GOG launched a registration trial known as GOG-247, which was a prospective, multi-institutional observational trial evaluating baseline characteristics of patients who presented with treatment naïve cervical or uterine cancer and how these factors impact enrollment on a clinical trial. What was striking here is that patients over the age of 71 were less likely to have a trial available when compared with patients under the age of 41 (OR 0.4; 95% CI 0.2–0.8; p = 0.0097). This doesn’t mean these patients were ineligible, they were just being seen at a place where a trial wasn’t available. GOG-247 did note that multiple co-morbid illnesses were negatively associated with eligibility for a clinical trial (p = 00019) but this was not reported for age [24].
This study is important and reassuring in showing that increasing age does not appear to result in increasing adverse effects among patients participating in GOG/NRG clinical trials. The problem is one of extrapolation to the general population. All patients participating in these studies met strict entry criteria (performance status; renal function; bone marrow function, et cetera) beyond just stage or absence of aortic lymph node involvement. It is anticipated that elderly women who have accumulated co-existing medical problems may have greater toxicity and less adherence to planned therapy. Future population-based studies will be important to determine whether age is an independent risk factor for adverse effects and/or poor outcome.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ygyno.2016.08.317.
Box 1. Highlights.
Among older patients, toxicities and completion of chemo-RT for cervix cancer are not well defined.
This ancillary NRG study demonstrates few differences in reported toxicities during chemo-RT by age.
Brachytherapy was not completed in 35% of patients ≥70 as compared to 13% of pts < 40.
Disease specific survival did not differ among patients < 70 years old and those ≥ 70 years old.
Footnotes
This study was supported by National Cancer Institute grants to the Gynecologic Oncology Group (GOG) Administrative Office (CA 27469), the Gynecologic Oncology Group Statistical Office (CA 37517), NRG Oncology (1U10 CA180822) and NRG Operations (U10CA180868). The following Gynecologic Oncology Group member institutions participated in the primary treatment studies: Wayne State University/Karmanos Cancer Institute, University of Oklahoma Health Sciences Center, Penn State Milton S. Hershey Medical Center, University of Southern California, University of Miami School of Medicine, Indiana University Hospital/Melvin and Bren Simon Cancer Center, Abington Memorial Hospital, University of Rochester Medical Center, Georgetown University Hospital, Tufts-New England Medical Center, Stanford University Medical Center, Fox Chase Cancer Center, Wake Forest University Health Sciences, Woman’s Hospital, Long Beach Memorial Medical Center-Todd Cancer Institute, St. Francis Hospital, Advocate Lutheran General Hospital, State University of New York Downstate Medical Center, Eastern Virginia Medical Center, Washington University School of Medicine, Cooper Hospital University Medical Center, University of Mississippi Medical Center, Case Western Reserve University, University of North Carolina at Chapel Hill, Tacoma General Hospital, University of Colorado Cancer Center - Anschutz Cancer Pavilion, University of Texas Southwestern Medical Center, Ohio State University Comprehensive Cancer Center, University of California at Los Angeles Health System, Medical University of South Carolina, Walter Reed National Military Medical Center, University of Alabama at Birmingham, Oregon Health Sciences University, University of California Medical Center at Irvine - Orange Campus, University of Cincinnati, Eastern Pennsylvania GYN/ONC Center, P.C, University of Massachusetts Memorial Health Care, University of Arizona, Mayo Clinic, Emory University Clinic, Abramson Cancer Center of the University of Pennsylvania, University of Chicago, The New York Hospital, Cornell Medical Center, Ellis Fischel Cancer Center, University of Iowa Hospitals and Clinics, Moffitt Cancer Center and Research Institute, University of Virginia, North Shore University Hospital, Gynecologic Oncology Network/Brody School of Medicine, Women and Infants Hospital, Women’s Cancer Center of Nevada, University of New Mexico, Yale University, University of Hawaii, Virginia Commonwealth University, Meharry Medical College Minority Based CCOP, Rush University Medical Center, Cleveland Clinic Foundation, MD Anderson Cancer Center, Fox Chase Cancer Center at Virtua Memorial Hospital of Burlington County, Aurora Women’s Pavilion of Aurora West Allis Medical Center, Roswell Park Cancer Institute, University of Pittsburgh Cancer Institute (UPCI), Memorial Sloan Kettering, New York University Medical Center, Carle Cancer Center, Delaware/Christiana Care CCOP, and St. Vincent Hospital.
Conflicts of interest
Dr. Kathleen Moore has consultancy positions with Astra Zeneca, Clovis, Genentech/Roche, Immunogen, and Merrimack. She also receives payment for development of educational presentations from Genentech.
Dr. Junzo Chino has a Board Membership at NanoScint, Inc. He also has Grants/grant pending, as well as payment for lectures including service on speakers’ bureaus with Varian Medical Systems. He also has patents (planned, pending or issued) and stock/stock options with NanoScint, Inc.
Dr. David Miller receives grant monies from the Gynecologic Oncology Group.
Dr. David Moore receives payment for lectures including service on speakers’ bureaus from AstraZeneca.
Dr. Frederick Stehman receives grant monies from NCI/NIH and Gynecologic Oncology Group.
All other co-authors have no conflicts to declare.
References
- 1.U.S. Census Bureau, C.R.N. 2012 from http://www.aoa.gov/AoARoot/Aging_Statis-tics/Census_Population/census2010/Index.aspx.
- 2.A.C. Socity, American Cancer Society. Atlanta: Cancer Facts & Figures; 2016. [Google Scholar]
- 3.Apostolides AD, Apostolides IK. The US Cancer Program and specific types of cancer, 1975–2007. A Failure. Townsend Lett. 2011 [Google Scholar]
- 4.Kunos C, et al. Retrospective analysis of concomitant Cisplatin during radiation in patients aged 55 years or older for treatment of advanced cervical cancer: a gynecologic oncology group study. Int. J. Gynecol. Cancer. 2009;19(7):1258–1263. doi: 10.1111/IGC.0b013e3181b33ace. [DOI] [PubMed] [Google Scholar]
- 5.Landoni F, et al. Randomised study of radical surgery versus radiotherapy for stage Ib-IIa cervical cancer. Lancet. 1997;350(9077):535–540. doi: 10.1016/S0140-6736(97)02250-2. [DOI] [PubMed] [Google Scholar]
- 6.Sharma C, et al. Patterns of care and treatment outcomes for elderly women with cervical cancer. Cancer. 2012;118(14):3618–3626. doi: 10.1002/cncr.26589. [DOI] [PubMed] [Google Scholar]
- 7.Wright JD, et al. Endometrial cancer in the oldest old: tumor characteristics, patterns of care, and outcome. Gynecol. Oncol. 2011;122(1):69–74. doi: 10.1016/j.ygyno.2011.02.040. [DOI] [PubMed] [Google Scholar]
- 8.Goodheart M, et al. Chemoradiation for invasive cervical cancer in elderly patients: outcomes and morbidity. Int. J. Gynecol. Cancer. 2008;18(1):95–103. doi: 10.1111/j.1525-1438.2007.00967.x. [DOI] [PubMed] [Google Scholar]
- 9.Alektiar KM, et al. Is endometrial carcinoma intrinsically more aggressive in elderly patients? Cancer. 2003;98(11):2368–2377. doi: 10.1002/cncr.11830. [DOI] [PubMed] [Google Scholar]
- 10.Pignon T, et al. Age is not a limiting factor for radical radiotherapy in pelvic malignancies. Radiother. Oncol. 1997;42(2):107–120. doi: 10.1016/s0167-8140(96)01861-0. [DOI] [PubMed] [Google Scholar]
- 11.Stehman FB, et al. Hydroxyurea, 5-fluorouracil infusion, and cisplatin adjunct to radiation therapy in cervical carcinoma: a phase I-II trial of the Gynecologic Oncology Group. Gynecol. Oncol. 1997;66(2):262–267. doi: 10.1006/gyno.1997.4761. [DOI] [PubMed] [Google Scholar]
- 12.Rose PG, et al. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N. Engl. J. Med. 1999;340(15):1144–1153. doi: 10.1056/NEJM199904153401502. [DOI] [PubMed] [Google Scholar]
- 13.Lanciano R, et al. Randomized comparison of weekly cisplatin or protracted venous infusion of fluorouracil in combination with pelvic radiation in advanced cervix cancer: a gynecologic oncology group study. J. Clin. Oncol. 2005;23(33):8289–8295. doi: 10.1200/JCO.2004.00.0497. [DOI] [PubMed] [Google Scholar]
- 14.DiSilvestro PA, et al. Phase III randomized trial of weekly cisplatin and irradiation versus cisplatin and tirapazamine and irradiation in stages IB2, IIA, IIB, IIIB, and IVA cervical carcinoma limited to the pelvis: a Gynecologic Oncology Group study. J. Clin. Oncol. 2014;32(5):458–464. doi: 10.1200/JCO.2013.51.4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.K. P., On the criterion that a given system of deviations from the probable in the case of a correlated system of variables is such that it can be reasonably supposed to have arisen from random sampling. Philos. Mag. Ser. 1900;50(5):157–175. [Google Scholar]
- 16.Association, K.W.a.W.W.J.o.t.A.S. Use of Ranks in One-criterion Variance Analysis. 1952;47:583–621. [Google Scholar]
- 17.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J. Am. Stat. Assoc. 1958;53:457–481. [Google Scholar]
- 18.DR C. Regression models and life-tables. J. R. Stat. Soc. Ser. B Methodol. 1972;34:187–220. [Google Scholar]
- 19.R.C.T. R, A language and environment for statistical computing. R Foundation for Statistical Computing. 2013 [Google Scholar]
- 20.Yanazume Y, et al. Major causes of impractical brachytherapy in elderly patients with uterine cervical cancer. J. Obstet. Gynaecol. Res. 2014;40(6):1725–1732. doi: 10.1111/jog.12387. [DOI] [PubMed] [Google Scholar]
- 21.Santam Chakraborty GM, Dessai S, Patil VM. How well do elderly patients with cervical cancer tolerate definitive radiochemotherapy using RapidArc? Results from an institutional audit comparing elderly versus younger patients. ecancermedicalscience. 2014;8(484):1–13. doi: 10.3332/ecancer.2014.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheppard CS, et al. Assessment of mediators of racial disparities in cervical cancer survival in the United States. Int. J. Cancer. 2016;138(11):2622–2630. doi: 10.1002/ijc.29996. [DOI] [PubMed] [Google Scholar]
- 23.Caires IQ, et al. Definitive chemoradiotherapy for advanced cervical cancer: should it be different in the elderly? Eur. J. Obstet. Gynecol. Reprod. Biol. 2015;192:86–89. doi: 10.1016/j.ejogrb.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 24.Brooks SE, et al. Patient and physician factors associated with participation in cervical and uterine cancer trials: an NRG/GOG247 study. Gynecol. Oncol. 2015;138(1):101–108. doi: 10.1016/j.ygyno.2015.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]