Abstract
Increased levels of tumor necrosis factor (TNF) α have been linked to a number of pulmonary inflammatory diseases including asthma, chronic obstructive pulmonary disease (COPD), acute lung injury (ALI)/acute respiratory distress syndrome (ARDS), sarcoidosis, and interstitial pulmonary fibrosis (IPF). TNFα plays multiple roles in disease pathology by inducing an accumulation of inflammatory cells, stimulating the generation of inflammatory mediators, and causing oxidative and nitrosative stress, airway hyperresponsiveness and tissue remodeling. TNF-targeting biologics, therefore, present a potentially highly efficacious treatment option. This review summarizes current knowledge on the role of TNFα in pulmonary disease pathologies, with a focus on the therapeutic potential of TNFα-targeting agents in treating inflammatory lung diseases.
Keywords: TNF, pulmonary disease, lung injury, biologics, inflammation
1. Introduction
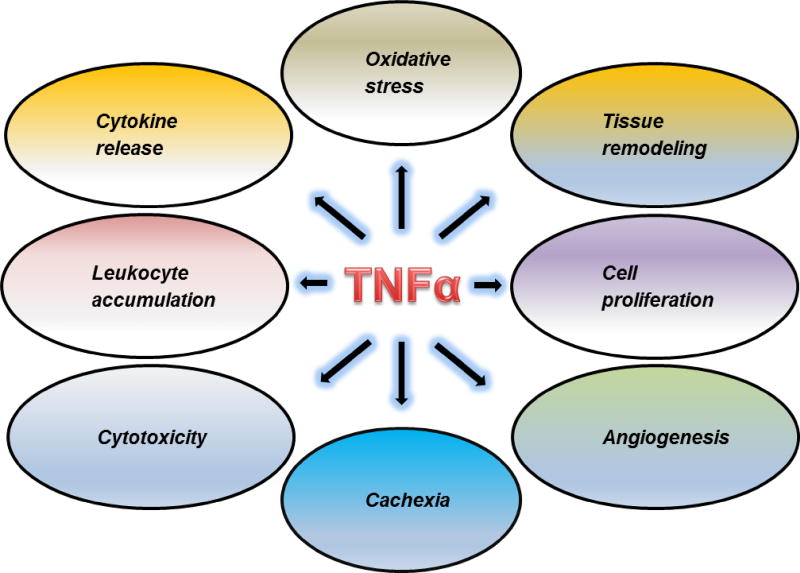
Proinflammatory cytokines including tumor necrosis factor (TNF) α, interleukin (IL)-1, IL-6 and are key modulators of inflammation that initiate and drive many pulmonary pathologies and diseases. TNFα is especially important as its actions are numerous and quite diverse. These include stimulating leukocyte accumulation, proliferation and differentiation at the sites of injury and infection as well as oxidative stress, necrosis, apoptosis, angiogenesis, and tissue remodeling (Figure1) (Aggarwal, 2003; Mukhopadhyay et al., 2006). TNFα is primarily produced by macrophages and monocytes (Aggarwal et al., 2012; Suzuki et al., 2013). It is synthesized as a cell surface bound precursor, known as transmembrane TNFα (tmTNFα), a homotrimer of 26 KDa subunits. tmTNFα is cleaved by TNFα-converting enzyme (TACE) to a biologically active soluble form (sTNFα), a homotrimer of 17 KDa subunits (Horiuchi et al., 2010). TNFα is a hormone-like peptide that can act locally in an autocrine or paracrine manner or at distant sites by entering the bloodstream. Many inflammatory mediators stimulate the production of TNFα including bacterially-derived lipopolysaccharide (LPS), interleukin (IL)-1, IL-2, interferon-γ, granulocyte macrophage colony stimulating factor and platelet derived growth factor, as well as TNFα itself (Barbara et al., 1996; Semenzato, 1990; Suzuki et al., 2013).
Fig. 1.
Multiple roles of TNFα in the pathophysiology of inflammatory diseases.
The multiple activities of TNFα are mediated via binding to cell surface receptors. Two types of structurally distinct TNF receptors have been identified: Type 1 or TNFRI (a 55-kDa protein) and Type 2 or TNFRII (a 75 kDa protein) (Aggarwal, 2003). TNFRI contains a death domain and is expressed on most cell types. In contrast, TNFRII expression is limited mainly to immune cells, endothelial cells, and nerve cells (Aggarwal et al., 2012). Although the presence of a TNF receptor is a prerequisite for biological responses, there doesn’t appear any relationship between the number of receptors and the magnitude of responses to TNFα (Semenzato, 1990). Both TNFRI and TNFRII bind TNFα, as well as lymphotoxin, a cytotoxic protein secreted by lymphoid cells, with approximately equal affinity. TNFα binding to its receptors initiates a signaling cascade involving mitogen-activated protein kinase and c-Jun Nterminal kinase, culminating in activation of the transcription factors, nuclear factor-kappa B (NF-κB) and activated protein 1 (AP-1) (Garg and Aggarwal, 2002). Activation of these intracellular signals is important in TNFα-mediated apoptosis, differentiation and proliferation, and production of proinflammatory proteins including IL-6, IL-8, IL-18, chemokines, inducible nitric oxide synthase, cyclooxygenase and lipoxygenase enzymes, as well as TNF, itself (Aggarwal et al., 2012). Downregulation of inflammation is associated with shedding of the extracellular domain of TNFRs and decreases in TNFα activity.
2. Biological activities of TNFα
TNFα is identical to cachectin, a peptide recognized for its ability to induce fever and wasting (Aggarwal et al., 2012; Clark, 2007). As a promoter of cachexia, TNFα inhibits the synthesis of lipoprotein lipase, an enzyme that cleaves fatty acids from triglycerides (Clark, 2007). TNFα is also a master regulator of inflammation which is an important component of its pathogenic actions. TNFα is a neutrophil and eosinophil chemoattractant, and it stimulates the production of macrophage chemokines, such as CCL2. It also promotes inflammation by upregulating adhesion molecules important in leukocyte trafficking to inflammatory sites, including intracellular leukocyte adhesion molecule, endothelial leukocyte adhesion molecule-1, and vascular cell adhesion molecule-1(Kelly et al., 2007). In addition, TNFα stimulates the release of eicosanoids and platelet activating factor (Camussi et al., 1991; Kelly et al., 2007), which contribute to inflammation by promoting vasodilatation, and leukocyte adhesion and migration to sites of injury (Camussi et al., 1991; Michel et al., 2014; Semenzato, 1990). Oxidative and nitrosative stress are hallmarks of inflammatory diseases (Laskin et al., 2010; Thomas, 2001). TNFα is thought to be a major inducer of oxidative and nitrosative stress in inflammatory cells (Blaser et al., 2016; Rahman, 2000). This leads to the activation of redox sensitive transcription factors including NF-κB and AP-1 which upregulate inflammatory gene expression. TNFα also depletes intracellular glutathione which contributes to its prooxidant effects (Obrador et al., 1998; Rahman, 2000).
Increases in TNFα are associated with cytotoxicity, a response mediated by its binding to the p55 receptor (TNFRI). This results in recruitment of TNFR-associated death domain and Fas associated death domain and caspases, key pro-apoptotic enzymes (Aggarwal et al., 2012). TNFα can also activate caspases and promote apoptosis by stimulating mitochondria to release reactive oxygen species, cytochrome c and Bax, and by activating sphingomyelinases (Aggarwal et al., 2012; Garcia-Ruiz et al., 2003). Both soluble and transmembrane TNFα are equally effective in inducing apoptosis (Klimp et al., 2002).
TNFα is also a potent mitogen stimulating proliferation of epithelial cells (Lu et al., 1997). This is thought to be due in part to activation of the transcription factor AP-1 and upregulation of cyclin-D1 (Mukhopadhyay et al., 2009; Rahman, 2000). TNFα-mediated proliferation is thought to contribute to epithelial thickening and pulmonary fibrosis (Allen and Spiteri, 2002; Sasaki et al., 2000). TNFα also promotes fibrosis by inducing focal accumulation of fibroblasts and collagen deposition. It upregulates expression of matrix metalloproteinases and transforming growth factor (TGF) β, which are involved in tissue remodeling and fibrogenesis (Oikonomou et al., 2006; Piguet, 1990; Sasaki et al., 2000; Sullivan et al., 2005).
3. TNFα inhibitors
The recognition that TNFα plays a key role in inflammatory diseases and pathologies has led to the development of a number of drugs and biologics that target TNFα. Both chimeric mouse/humanized monoclonal anti-TNFα antibody (infliximab) and fully human monoclonal anti-TNFα antibodies (golimumab and adalimumab) have been developed; these bind to and inactivate membrane bound and soluble TNFα. Etanercept, a soluble fusion protein consisting of two p75 TNF receptors attached to an Fc fragment of human IgG1, is also available which mainly binds and inactivates sTNFα. Etanercept has better avidity and affinity for sTNFα than tmTNFα, and does not induce complement activation. In contrast, monoclonal anti-TNFα antibodies (i.e., infliximab, golimumab and adalimumab) bind to both monomeric and trimeric soluble and transmembrane forms of TNFα, which can activate complement cascade resulting in cytotoxicity (Liang et al., 2013). Despite differences in their mode of administration, efficacy and safety profile, these TNFα targeting agents have effectively been used to treat patients with TNFα-associated diseases such as Crohn’s disease, psoriatic arthritis, rheumatoid arthritis and ankylosing spondylitis with minimal toxicity (Hasegawa et al., 2001; Raychaudhuri and Raychaudhuri, 2009).
4. Role of TNFα in pulmonary diseases
Macrophages are the major source of TNFα in the lung; however, epithelial cells, eosinophils, and mast cells also have the capacity to release TNFα upon activation (Finotto et al., 1994; Gosset et al., 1991; Herfs et al., 2012; Khair et al., 1994). Increased levels of TNFα have been linked to a number of pulmonary inflammatory diseases including asthma, chronic obstructive pulmonary disease (COPD), acute lung injury (ALI)/acute respiratory distress syndrome (ARDS), sarcoidosis, and interstitial pulmonary fibrosis (IPF). Each of these pathologies is characterized by airway injury, inflammation, and bronchial and parenchymal remodeling. TNFα contributes to these inflammatory diseases by recruiting inflammatory cells, stimulating the generation of inflammatory mediators, increasing oxidative and nitrosative stress, and inducing airway hyperresponsiveness (Anticevich et al., 1995; Choi et al., 2005; Herfs et al., 2012; Hughes et al., 1995; Shah et al., 1995). In this review, we describe the role of TNFα in pulmonary disease pathologies, with a focus on the therapeutic potential of TNFα-targeting agents in treating pulmonary diseases (Table 1).
Table 1.
Summary of Studies of TNFα Targeting Agents in Inflammatory Lung Diseases
| Disease/ pathology |
Treatment | Rodent/ human |
References | Outcome |
|---|---|---|---|---|
|
| ||||
| Asthma | Etanercept | human | Berry et al., 2006; Holgate et al., 2011; Howarth et al., 2005; Morjaria et al., 2008 | Improvement in asthma symptoms, lung function and quality of life |
| mouse | Dejager et al., 2015 | Reversal of GC insensitivity | ||
| Anti-TNF antibody | mouse | Busse et al., 2009; Kim et al., 2006 | Reduced lung inflammation and mucus cell metaplasia | |
| Infliximab | rat | Cai et al., 2011 | Reduced airway inflammation and hyperreactivity | |
| mouse | Deveci et al., 2008 | Reduced inflammatory cell accumulation and decreased cytokine and chemokine release | ||
| human | Taille et al., 2013 | Improved asthma control and reduced frequency of exacerbations | ||
| Adalimumab | mouse | Catal et al., 2014 | Attenuation of lung damage | |
| Golimumab | human | Wenzel et al., 2009 | No significant effect on forced expiratory volume in 1 sec. or asthma exacerbations | |
| TNFα antisense | mouse | Luo et al., 2012 | Reduced inflammatory cell infiltration and mucus secretion | |
|
| ||||
| COPD | Etanercept and infliximab | human | Suissa et al., 2008 | Reduced COPD exacerbations following etanercept treatment |
| Etanercept | human | Aaron et al., 2013 | Reduced acute COPD exacerbations; equally effective as prednisone | |
| Infliximab | human | Dentener et al., 2008; Rennard et al., 2007; van der Vaart et al., 2005 | No significant effect on quality of life, inflammation or disease exacerbations | |
|
| ||||
| ALI/ARDS | Anti-TNFα monoclonal antibody | human | Abraham et al., 1998; Abraham et al., 1995 | No significant effect on mortality in septic shock patients |
| mouse | Bertok et al., 2012 | p55 receptor-specific domain antibody but not anti-TNFα antibody inhibited lung injury, edema and inflammation | ||
| pig | Mullen et al., 1993 | Combined ibuprofen and anti-TNFα antibody protect against ALI | ||
| p55 TNFR fusion protein | human | Abraham et al., 1997 | Reduction in mortality in patients with severe sepsis | |
| Etanercept | rat | Guthmann et al., 2009 | Hyperoxia-induced lung injury and BAL cell content inhibited | |
|
| ||||
| Pulmonary sarcoidosis | Infliximab | human | Baughman et al., 2006; Chebib et al., 2014; Rossman et al., 2006; Russell et al., 2013; Sweiss et al., 2005 | Improvement in FVC and pulmonary disease |
| Adalimumab | human | Callejas-Rubio et al., 2006; Milman et al., 2012; Sweiss et al., 2014 | Decrease in dyspnea, cough and disease pathology. Improvement in FVC and Borg dyspnea score | |
| Golimumab | human | Judson et al., 2014 | No significant effect | |
| Etanercept | human | Utz et al., 2003 | Terminated early due to treatment failure | |
|
| ||||
| IPF | Etanercept | human | Raghu et al., 2008 | Reduction in disease progression |
| Pirfenidone | human | Azuma et al., 2011; King et al., 2014; Maher, 2010; Takeda et al., 2014; Taniguchi et al., 2011 | Reduction in vital capacity decline, disease progression and increase in progression-free survival, suppression of cough and dyspnea | |
|
| ||||
| Experimental models of lung injury | Anti-TNFα antibody | mouse | Bhalla et al., 2002; Piguet et al., 1990; Shvedova et al., 1996 | Inhibition of ozone, silica or cotton dust induced lung injury, decreased inflammatory cytokine release, inflammation and fibrosis |
| rat | Malaviya et al., 2015 | Marked inhibition in vesicant-induced lung injury, oxidative stress, numbers of cytotoxic, proinflammatory macrophages and fibrosis | ||
| Infliximab | rat | Zhang et al., 2011 | Reduced airway inflammation and improvement in cigarette smoke-induced histopathology; protection from emphysema | |
| Pentoxifylline | rat | Sunil et al., 2014 | Inhibition of vesicant-induced lung injury, inflammation and oxidative stress | |
Asthma
Asthma is a chronic lung inflammatory disease characterized by persistent eosinophilic inflammation, airway hyperreactivity, mucus secretion and reversible airway obstruction. Increased levels of TNFα have been described in the airways of patients with severe asthma (Bradding et al., 1994; Howarth et al., 2005; Noguchi et al., 2002). Alveolar macrophages and peripheral blood monocytes isolated from patients with asthma produce increased amounts of TNFα and TACE, and express higher levels of TNFα receptors (Berry et al., 2006). In patients with allergic asthma, high sputum TNFα levels are observed within 24 h of allergen challenge (Keatings et al., 1997); TNFα has also been reported to increase during asthma exacerbations or after allergen challenge in patients suffering from asthma (Thomas, 2001). In healthy individuals, inhalation of recombinant TNFα increases airway hyperresponsiveness and sputum neutrophils (Thomas, 2001; Thomas and Heywood, 2002). This is thought to be due to a direct effect of TNFα on airway smooth muscle cells and release of leukotrienes (Anticevich et al., 1995; Choi et al., 2005). Blood monocytes and alveolar macrophages from asthmatic subjects have been reported to produce increased amounts of TNFα, as well as IL-8 and granulocyte macrophage colony stimulating factor, following LPS stimulation, when compared to normal subjects, suggesting selective augmentation of cytokine production (Hallsworth et al., 1994).
Animal studies have confirmed that TNFα plays a role in the pathophysiology of asthma and bronchial hyperresponsiveness. Thus, exposure of rats to endotoxin upregulates TNFα production by bronchial epithelial cells and alveolar macrophages, a response associated with bronchial hyperresponsiveness (Ermert et al., 2003; Kips et al., 1992). Ovalbumin-induced asthma in rats is also associated with increases in TNFα in serum and lung (Cai et al., 2011). Additionally, mice lacking TNFα or TNFR are protected from lung inflammation, mucus secretion and late airway hyperresponsiveness in an ovalbumin-induced model of asthma, a response mimicked by administration of anti-TNFα antibody to wild type animals (Busse et al., 2009; Choi et al., 2005).
The efficacy of antagonizing TNFα in asthma has been evaluated both in animal models and in humans. In animal models of asthma, the results of anti-TNFα antibody treatment have been encouraging. In ovalbumin challenged rodents infliximab inhibited airway smooth muscle hyperreactivity and inflammatory damage (Cai et al., 2011; Deveci et al., 2008). Monoclonal anti-TNFα antibody treatment of mice also mitigated house dust induced allergic inflammation, including increases in eosinophils, lymphocytes, macrophages, and neutrophils in bronchoalveolar lavage (BAL), and histopathological changes in the lung (Kim et al., 2006). Methacholine-induced airway hyperreactivity was also inhibited by infliximab. Similarly, in a murine model of acute asthma, adalimumab therapy reduced lung inflammation and inflammatory cell infiltration (Catal et al., 2014). Local administration of TNFα antisense nucleotide has also been reported to suppress allergic inflammation in mice by reducing release of TNFα and other Th2 cytokines, mucus secretion and inflammatory cell influx (Luo et al., 2012). Airway mucus cell metaplasia and hyperresponsiveness were also inhibited by monoclonal anti-TNF antibody in mice (Busse et al., 2009). In another study, etanercept was found to restore the therapeutic efficacy of glucocorticoids (GC) in an ovalbumin induced GC-insensitive mouse models of airway hyperinflammation (Dejager et al., 2015).
In humans with asthma the efficacy of antagonizing TNFα is less clear. Howarth et al. (2005) reported that treatment of patients with severe asthma with etanercept was associated with improvement in asthma symptoms, lung function and bronchial hyperresponsiveness. Additionally, in patients with corticosteroid refractory asthma, both etanercept and infliximab were reported to improve asthma, lung inflammation, lung function and quality of life and to reduce the frequency of asthma exacerbations and hospitalizations (Berry et al., 2006; Morjaria et al., 2008; Taille et al., 2013). Infliximab was also observed to reduce asthma exacerbations in patients with moderate asthma (Erin et al., 2006). Conversely, etanercept had no effect in patients with moderate-to-severe persistent asthma (Holgate et al., 2011). Similarly, in a large multicenter trial of patients with severe asthma, golimumab treatment for 12 months failed to demonstrate a favorable risk-benefit profile (Wenzel et al., 2009). Together these findings suggest that TNFα targeting may particularly be an effective strategy to treat severe or refractory asthma.
COPD
COPD is an inflammatory disease characterized by chronic progressive airway obstruction due to prominent localization of inflammatory cells including neutrophils, macrophages, T cells and mast cells in the airways and thickening of the airway walls (Aoshiba and Nagai, 2004). In transgenic mice overexpressing TNFα, progressive histopathologic changes are observed including chronic inflammation, thickened interstitium, alveolar air space enlargement, septal wall destruction and bronchiolitis, consistent with emphysema, and increases in collagen; these mice also develop fibrosis (Fujita et al., 2001; Lundblad et al., 2005; Miyazaki et al., 1995). Alveolar macrophages from cigarette smokers and patients with COPD release increased quantities of TNFα (Chung, 2005; Lim et al., 2000). In addition, higher levels of TNFα and sTNFRII are observed in BAL from chronic smokers and sputum from patients with COPD. Moreover, levels of TNFα and sTNFRII increase further during COPD exacerbations (Chung, 2005; Keatings et al., 1996; Woodruff et al., 2014). Sputum levels of sTNFRII have been reported to be inversely related to forced expiratory volume 1 in patients with COPD, and serve as a prognostic biomarker for the risk of exacerbation (Takabatake et al., 2000; Vernooy et al., 2002; Woodruff et al., 2014). TNFα is thought to contribute to COPD by upregulating expression of adhesion molecules resulting in inflammatory cell influx, and increased production of matrix metalloproteinases and tenascin, which promote tissue damage and remodeling (Chung, 2001). TNFα may also contribute to cachexia in COPD patients via activation of the transcription factor, NF-κB (Wagner, 2008).
Despite evidence suggesting a role of TNFα in COPD disease pathology, TNFα antagonists have shown only limited clinical efficacy. In patients with RA and COPD, etanercept, but not infliximab, reduced COPD hospitalizations (Suissa et al., 2008). Conversely, in a randomized double blind controlled trial, etanercept had no beneficial effect for treatment of acute exacerbations of COPD (Aaron et al., 2013). Similarly, in controlled studies, infliximab had no beneficial effect in patients with mild, moderate or severe COPD (Rennard et al., 2007; van der Vaart et al., 2005). Infliximab has been reported to have minor effects on systemic inflammation in cachectic patients with COPD, but local inflammation was unaffected (Dentener et al., 2008). The reason for the lack of efficacy of TNF antagonists in COPD is unclear. COPD is a heterogeneous inflammatory disease mediated by multiple cytokines; it is possible that blocking just one cytokine is not sufficient to control the disease pathology (Barnes, 2007; Matera et al., 2010).
ALI/ARDS
ALI and ARDS are life-threatening manifestations of an inflammatory response of the lung to various insults, and are characterized by severe hypoxemia, diffuse infiltration in the chest X-ray, and a substantial reduction in pulmonary compliance (Matuschak and Lechner, 2010). The early inflammatory phase of ALI, characterized by alveolar epithelial and endothelial barrier dysfunction, hemorrhage and protein rich pulmonary edema is followed by a proliferative phase involving alveolar epithelial cell proliferation, interstitial fibrosis and air space obliteration (Tomashefski, 2000; Vasudevan et al., 2004). Later in the pathology, fibrosis and emphysema are observed, along with loss of normal lung structure. ARDS represents a more severe and late phase of ALI.
Increased levels of TNFα have been detected in BAL, serum and epithelial lining fluid from patients with ALI and ARDS (Antonelli et al., 1994; Bauer et al., 2000; Hamacher et al., 2002; Li et al., 2010; Reper and Heijmans, 2015; Roten et al., 1991; Singh et al., 2015; Suter et al., 1992; Vaillant et al., 1996). Higher serum levels of TNFα during early stages of ALI are associated with increased mortality at 2–3 months (Makabe et al., 2012). Higher levels of TNFα and sTNFRII in BAL are observed in patients in early stage ALI, when compared to patients with late phase ARDS (Hamacher et al., 2002). Pulmonary microvascular endothelial cells isolated from ARDS patients also express increased levels of TNFRII relative to microvascular endothelial cells from control patients (Grau et al., 1996). A multicenter study (Parsons et al., 2005) demonstrated that increased baseline plasma sTNFRI and sTNFRII levels are strongly associated with mortality and morbidity in ALI patients. Moreover, genetic variations in TNF receptor-associated factor 6 gene are linked to susceptibility to ALI in patients with sepsis (Song et al., 2012). In vitro, BAL from early stage ALI patients induced endothelial cell cytotoxicity, suggesting a role of TNFα in endothelial-interstitial barrier dysfunction (Hamacher et al., 2002).
In animal models of ALI or ARDS induced by endotoxin, mechanical ventilation, or extracorporeal circulation, TNFα has been implicated in disease pathogenesis (Kao et al., 2006; Li et al., 2013; Matuschak and Lechner, 2010). As observed in patients with ALI, TNFα accumulates rapidly in large quantities in the lung after injury in animals, and is considered an initiating cytokine in early disease pathology (Li et al., 2013). Pretreatment of rats with etanercept protected against hyperoxia-induced ALI, which is characterized by increases in TNFα and TNFRI, and disruption of alveolar-epithelial barrier functions (Guthmann et al., 2009). Monoclonal anti-TNFα antibody alone, or in combination with ibuprofen has also been reported to attenuate ALI in pigs (Mullen et al., 1993). Mice lacking TNFRI were protected from ventilation or acid-induced ALI, whereas TNFRII knockout mice developed pulmonary edema (Maniatis et al., 2012; Wilson et al., 2007). Similarly, inhibition of TNFR p55 using a specific antibody mitigated ventilation, or endotoxin plus ventilation-induced ALI, while anti-TNFα antibody was ineffective (Bertok et al., 2012). Consistent with these findings, clinical trials of monoclonal anti-TNFα antibody in patients with sepsis-induced ALI did not improve survival (Abraham et al., 1998; Abraham et al., 1995), whereas p55 TNFR fusion protein treatment of patients with severe septic shock reduced mortality (Abraham et al., 1997). These studies suggest that divergent effects of TNFα binding to p55 and p75 receptors. Thus, while ligand activation of the p55 receptor promotes lung inflammation, activation of the p75 receptor is protective; this difference may underlie the lack of efficacy of non-selective anti-TNFα treatment in ALI.
Pulmonary sarcoidosis
Pulmonary sarcoidosis is a disease of unknown etiology characterized by the presence of granulomatous inflammation which can progress to fibrosis (Amin et al., 2014; Crommelin et al., 2014). Increased numbers of CD4+ T cells and inflammatory macrophages are present in the lungs of sarcoidosis patients which may contribute to disease progression (Allen et al., 1998; Oswald-Richter et al., 2013). Lung fibrosis in sarcoidosis is an irreversible process which, in combination with ongoing inflammation, leads to bronchial distortion, cystic changes and loss of normal lung architecture and function (Mornex et al., 1994; Rozy et al., 2006). TNFα, released by alveolar macrophages, is known to play a role in promoting inflammation and Th1 driven granuloma formation and propagation (Bachwich et al., 1986; Baughman et al., 1990; Nunes et al., 2005). Increased levels of TNFα are observed in exhaled breath condensate and BAL from patients with pulmonary sarcoidosis; moreover, macrophages isolated from patients with sarcoidosis express relatively greater levels of TACE, and release increased amounts of TNFα (Baughman et al., 1990; Rozy et al., 2006). Plasma levels of TNFα are also increased in patients with sarcoidosis (Baydur et al., 2011).
Pentoxifylline, a methylxanthine phosphodiesterase inhibitor known to block TNFα production (Fernandes et al., 2008), has been shown to downregulate spontaneous or LPS-induced TNFα release from alveolar macrophages isolated from sarcoidosis patients (Tong et al., 2003). Inhibition of TNFα using infliximab or adalimumab significantly improved symptoms in patients with chronic sarcoidosis (Baughman et al., 2006; Callejas-Rubio et al., 2008; Chebib et al., 2014; Crommelin et al., 2014; Rossman et al., 2006; Russell et al., 2013; Sweiss et al., 2005); specifically, infliximab improved forced vital capacity (FVC) after 24 weeks of therapy (Baughman et al., 2006). Results with humanized anti-TNF antibody in sarcoidosis have been mixed. In an open label, single center study in patients with refractory sarcoidosis, treatment with adalimumab stabilized or improved FVC, 6-minute walk test distance of 50 m or greater, Borg dyspnea score and Physician’s and Patient’s Global Assessment (Sweiss et al., 2014). Improvement in overall health status compared to baseline was noted after 24 weeks, a response which persisted for at least 52 weeks. A reduction in disease severity following adalimumab treatment has also been described in patients with recalcitrant sarcoidosis or prednisone- and methotrexate-resistant sarcoidosis (Callejas-Rubio et al., 2006; Milman et al., 2012). In contrast, in a randomized, double-blind, placebo-controlled trial of pulmonary sarcoidosis, golimumab failed to improve FVC at week 16 or 28 compared with placebo (Judson et al., 2014). Treatment with etanercept was also ineffective in treating pulmonary sarcoidosis (Utz et al., 2003).
Idiopathic pulmonary fibrosis
Idiopathic pulmonary fibrosis is a chronic, progressive, life threatening lung disease of unknown etiology (Gross and Hunninghake, 2001). It is clinically characterized by a progressive decline in pulmonary function (Raghu et al., 2008). Increased levels of TNFα have been observed experimentally in the lungs of animals with pulmonary fibrosis and patients with IPF (Altintas et al., 2016; Lopez-de la Mora et al., 2015; Losa Garcia et al., 1999; Lozo Vukovac et al., 2014; Pan et al., 1996; Riha et al., 2004; Schupp et al., 2015; Vaillant et al., 1996; Zhang et al., 1993; Ziegenhagen et al., 1998). Lung tissue and alveolar macrophages isolated from patients with IPF release increased amounts of TNFα and sTNF receptors compared to healthy subjects (Cu et al., 2009; Piguet et al., 1993; Zhang et al., 1993). Additionally, in rodents, targeting TNFα attenuates pulmonary fibrosis suggesting role of TNFα in fibrogenesis (Malaviya et al., 2015; Piguet et al., 1993; Piguet and Vesin, 1994; Sunil et al., 2014; Thrall et al., 1997; Zhang et al., 1993).
Raghu et al. (2008) investigated the efficacy of etanercept in a randomized, prospective, double-blind, placebo-controlled, multicenter exploratory trial in patients with clinically progressive IPF. Although etanercept had no significant effect on FVC and lung diffusing capacity, it did decrease the rate of disease progression. Similarly, in clinical trials, pirfenidone, a non-peptide synthetic molecule with TNFα inhibitory activity, slowed disease progression, improved survival, and attenuated decreases in FVC in IPF patients (Azuma et al., 2011; King et al., 2014; Maher, 2010; Takeda et al., 2014; Taniguchi et al., 2011). Partial pressure of arterial oxygen level at rest and exercise capacity, as evaluated by the 6-min walk test were also improved following pirfenidone treatment (Hagmeyer et al., 2016; Miyamoto et al., 2016). In addition to inhibiting TNFα, pirfenidone reduced lipid peroxidation and oxidative stress, and suppressed TGFβ production (Salazar-Montes et al., 2008). The improved efficacy of pirfenidone in IPF patients, when compared to etanercept, may be attributed to these additional actions.
Experimental models of acute lung injury and fibrosis
Exposure of humans and experimental animals to pulmonary irritants including ozone, particulate matter, cigarette smoke, silica, bleomycin, chlorine or mustard vesicants induces an acute inflammatory response in the lung characterized by multifocal inflammatory lesions, macrophage accumulation, perivascular and peribronchial edema, interstitial thickening, cytotoxicity, bronchiectasis and bronchiolization of alveolar walls which in long term may lead to alterations in lung function, tissue remodeling and pulmonary fibrosis (Churg et al., 2009; Fakhrzadeh et al., 2004; Padilla-Carlin et al., 2011; Pendino et al., 1995; Razavi et al., 2013; Weinberger et al., 2011; Wollin et al., 2014; Yadav et al., 2010). Evidence suggests that macrophage-derived TNFα plays role in these pathogenic responses (Fakhrzadeh et al., 2008; Gossart et al., 1996; Lim et al., 2000; Malaviya et al., 2010; Michael et al., 2013; Weinberger et al., 2011). Both macrophages and epithelial cells release TNFα after exposure to ozone, mustards, silica or particulate matter (Barrett et al., 1999; Karacsonyi et al., 2009; Laskin et al., 2003; Michael et al., 2013; Osterlund et al., 2005; Ovrevik et al., 2009; Rusznak et al., 1996). Silica-induced TNFα expression has been shown to persist for more than 70 days in the lungs of mice (Piguet et al., 1990). In vitro TNF induces binding of particulates to rat tracheal explants (Xie et al., 2000). In rodents, this is associated with an increase in TNFRI signaling in the lung; TNFRII expression is also upregulated (Cho et al., 2007; Ortiz et al., 1999). Consistent with a role of TNFα in lung injury and disease pathology are findings that TNFRI−/−, TNFRII−/−, or TNFRI/II−/− mice are protected from ozone, silica or vesicant-induced lung injury and fibrosis (Cho et al., 2001; Laskin et al., 1998; Ortiz et al., 2001; Pryhuber et al., 2003; Sunil et al., 2011). Similarly, treatment of rodents with pentoxifylline or anti-TNFα antibody attenuates histopathological alterations in the lung, inflammatory cytokine release, alveolar cell apoptosis and the development of emphysema following exposure to various pulmonary toxicants (Bhalla et al., 2002; Malaviya et al., 2015; Shvedova et al., 1996; Sunil et al., 2014; Zhang et al., 2011). Damage to the alveolar-epithelial barrier, measured by increases in BAL protein and cell content following mustard exposure, along with expression of the oxidative stress markers, heme oxygenase-1 and lipocalin-2, is also reduced by pharmacologic inhibition of TNFα, (Malaviya et al., 2015; Sunil et al., 2014). Treatment of rats with anti-TNFα antibody also reduces mustard-induced increases in expression of the profibrotic mediator, TGFβ. This is associated with a marked inhibition of mustard-induced collagen deposition in the lung and fibrosis (Malaviya et al., 2015). Similar findings have been described in a silica-induced lung injury model (Piguet et al., 1990). In murine lung epithelial cells, anti-TNFα antibody inhibits silica-induced chemokine release and oxidative stress (Barrett et al., 1999). Taken together, these findings provide strong support for a role of TNFα in pulmonary injury and fibrosis induced by environmental toxicants and chemical threat agents.
5. Summary and conclusions
TNFα is a key mediator of local damage and inflammation in the lung. Several specific TNFα antagonists, including etanercept, infliximab, adalimumab and golimumab are currently used clinically for treatment of immune-inflammatory diseases. Although these agents are potent neutralizers of TNFα bioactivity, their efficacy varies in different pulmonary diseases. Whereas etanercept is more effective in reducing severe asthma or COPD, infliximab and adalimumab are efficacious in treating sarcoidosis. Fundamental differences in the molecular structure, dosing method and schedule, binding characteristics, and mode of action of TNF targeting agents are potentially responsible for the observed variability in clinical responses of these agents. Impairment of mechanical barriers of the lung may also play role. In injured lung, disease pathology is associated with increases in epithelial permeability, squamous metaplasia, increases in goblet cells, inflammatory cells and protein rich pulmonary edema. Together, these changes may hinder overall drug availability. Differences in expression of other inflammatory molecules or heterogeneity of the disease may also play a role. Nonetheless, significant therapeutic responses of TNFα neutralizing agents in lung injury and inflammation are intriguing. Further research on the role of TNFα in acute and chronic pulmonary diseases may help to develop successful treatment strategies using TNF targeting agents.
Acknowledgments
This work was supported by National Institutes of Health Grants U54AR055073, R01ES004738, and P30ES005022.
Abbreviations
- TNFα
tumor necrosis factor
- tmTNFα
transmembrane TNFα
- sTNFα
soluble TNFα
- TACE
TNFα-converting enzyme
- LPS
lipopolysaccharide
- IL
interleukin
- NF-κB
nuclear factor-kappa B
- AP-1
activated protein 1
- TGFβ
transforming growth factor β
- COPD
chronic obstructive pulmonary disease
- ALI
acute lung injury
- ARDS
acute respiratory distress syndrome
- IPF
interstitial pulmonary fibrosis
- BAL
bronchoalveolar lavage
- GC
glucocorticoids
- FVC
forced vital capacity.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6. Conflict of interest
The authors declare no conflict of interest.
References
- Aaron SD, Vandemheen KL, Maltais F, Field SK, Sin DD, Bourbeau J, et al. TNFα antagonists for acute exacerbations of COPD: a randomised double-blind controlled trial. Thorax. 2013;68:142–148. doi: 10.1136/thoraxjnl-2012-202432. [DOI] [PubMed] [Google Scholar]
- Abraham E, Anzueto A, Gutierrez G, Tessler S, San Pedro G, Wunderink R, et al. Double-blind randomised controlled trial of monoclonal antibody to human tumour necrosis factor in treatment of septic shock. NORASEPT II Study Group. Lancet. 1998;351:929–933. [PubMed] [Google Scholar]
- Abraham E, Glauser MP, Butler T, Garbino J, Gelmont D, Laterre PF, et al. p55 Tumor necrosis factor receptor fusion protein in the treatment of patients with severe sepsis and septic shock. A randomized controlled multicenter trial. Ro 45-2081 Study Group. JAMA. 1997;277:1531–1538. [PubMed] [Google Scholar]
- Abraham E, Wunderink R, Silverman H, Perl TM, Nasraway S, Levy H, et al. Efficacy and safety of monoclonal antibody to human tumor necrosis factor α in patients with sepsis syndrome. A randomized, controlled, double-blind, multicenter clinical trial. TNF-α MAb Sepsis Study Group. JAMA. 1995;273:934–941. [PubMed] [Google Scholar]
- Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat. Rev. Immunol. 2003;3:745–756. doi: 10.1038/nri1184. [DOI] [PubMed] [Google Scholar]
- Aggarwal BB, Gupta SC, Kim JH. Historical perspectives on tumor necrosis factor and its superfamily: 25 years later, a golden journey. Blood. 2012;119:651–665. doi: 10.1182/blood-2011-04-325225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JT, Bloor CA, Knight RA, Spiteri MA. Expression of insulin-like growth factor binding proteins in bronchoalveolar lavage fluid of patients with pulmonary sarcoidosis. Am. J. Respir. Cell Mol. Biol. 1998;19:250–258. doi: 10.1165/ajrcmb.19.2.3080. [DOI] [PubMed] [Google Scholar]
- Allen JT, Spiteri MA. Growth factors in idiopathic pulmonary fibrosis: relative roles. Respir. Res. 2002;3:13. doi: 10.1186/rr162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altintas N, Erboga M, Aktas C, Bilir B, Aydin M, Sengul A, et al. Protective effect of infliximab, a tumor necrosis factor-α inhibitor, on bleomycin-induced lung fibrosis in rats. Inflammation. 2016;39:65–78. doi: 10.1007/s10753-015-0224-z. [DOI] [PubMed] [Google Scholar]
- Amin EN, Closser DR, Crouser ED. Current best practice in the management of pulmonary and systemic sarcoidosis. Ther. Adv. Respir. Dis. 2014;8:111–132. doi: 10.1177/1753465814537367. [DOI] [PubMed] [Google Scholar]
- Anticevich SZ, Hughes JM, Black JL, Armour CL. Induction of human airway hyperresponsiveness by tumour necrosis factor-α. Eur. J. Pharmacol. 1995;284:221–225. doi: 10.1016/0014-2999(95)00463-u. [DOI] [PubMed] [Google Scholar]
- Antonelli M, Raponi G, Lenti L, Severi L, Capelli O, Riccioni L, et al. Leukotrienes and alpha tumor necrosis factor levels in the bronchoalveolar lavage fluid of patient at risk for the adult respiratory distress syndrome. Minerva Anestesiol. 1994;60:419–426. [PubMed] [Google Scholar]
- Aoshiba K, Nagai A. Differences in airway remodeling between asthma and chronic obstructive pulmonary disease. Clin. Rev. Allergy Immunol. 2004;27:35–43. doi: 10.1385/CRIAI:27:1:035. [DOI] [PubMed] [Google Scholar]
- Azuma A, Taguchi Y, Ogura T, Ebina M, Taniguchi H, Kondoh Y, et al. Exploratory analysis of a phase III trial of pirfenidone identifies a subpopulation of patients with idiopathic pulmonary fibrosis as benefiting from treatment. Respir. Res. 2011;12:143. doi: 10.1186/1465-9921-12-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachwich PR, Lynch JP, 3rd, Larrick J, Spengler M, Kunkel SL. Tumor necrosis factor production by human sarcoid alveolar macrophages. Am. J. Pathol. 1986;125:421–425. [PMC free article] [PubMed] [Google Scholar]
- Barbara JA, Van ostade X, Lopez A. Tumour necrosis factor-alpha (TNF-α): the good, the bad and potentially very effective. Immunol. Cell Biol. 1996;74:434–443. doi: 10.1038/icb.1996.73. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. Unexpected failure of anti-tumor necrosis factor therapy in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2007;175:866–867. doi: 10.1164/rccm.200702-253ED. [DOI] [PubMed] [Google Scholar]
- Barrett EG, Johnston C, Oberdorster G, Finkelstein JN. Silica-induced chemokine expression in alveolar type II cells is mediated by TNF-α-induced oxidant stress. Am. J. Physiol. 1999;276:L979–988. doi: 10.1152/ajplung.1999.276.6.L979. [DOI] [PubMed] [Google Scholar]
- Bauer TT, Monton C, Torres A, Cabello H, Fillela X, Maldonado A, et al. Comparison of systemic cytokine levels in patients with acute respiratory distress syndrome, severe pneumonia, and controls. Thorax. 2000;55:46–52. doi: 10.1136/thorax.55.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baughman RP, Drent M, Kavuru M, Judson MA, Costabel U, du Bois R, et al. Infliximab therapy in patients with chronic sarcoidosis and pulmonary involvement. Am. J. Respir. Crit. Care Med. 2006;174:795–802. doi: 10.1164/rccm.200603-402OC. [DOI] [PubMed] [Google Scholar]
- Baughman RP, Strohofer SA, Buchsbaum J, Lower EE. Release of tumor necrosis factor by alveolar macrophages of patients with sarcoidosis. J. Lab. Clin. Med. 1990;115:36–42. [PubMed] [Google Scholar]
- Baydur A, Alavy B, Nawathe A, Liu S, Louie S, Sharma OP. Fatigue and plasma cytokine concentrations at rest and during exercise in patients with sarcoidosis. Clin. Respir. J. 2011;5:156–164. doi: 10.1111/j.1752-699X.2010.00214.x. [DOI] [PubMed] [Google Scholar]
- Berry MA, Hargadon B, Shelley M, Parker D, Shaw DE, Green RH, et al. Evidence of a role of tumor necrosis factor α in refractory asthma. N. Engl. J. Med. 2006;354:697–708. doi: 10.1056/NEJMoa050580. [DOI] [PubMed] [Google Scholar]
- Bertok S, Wilson MR, Morley PJ, de Wildt R, Bayliffe A, Takata M. Selective inhibition of intra-alveolar p55 TNF receptor attenuates ventilator-induced lung injury. Thorax. 2012;67:244–251. doi: 10.1136/thoraxjnl-2011-200590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhalla DK, Reinhart PG, Bai C, Gupta SK. Amelioration of ozone-induced lung injury by anti-tumor necrosis factor-α. Toxicol. Sci. 2002;69:400–408. doi: 10.1093/toxsci/69.2.400. [DOI] [PubMed] [Google Scholar]
- Blaser H, Dostert C, Mak TW, Brenner D. TNF and ROS crosstalk in inflammation. Trends Cell Biol. 2016;26:249–261. doi: 10.1016/j.tcb.2015.12.002. [DOI] [PubMed] [Google Scholar]
- Bradding P, Roberts JA, Britten KM, Montefort S, Djukanovic R, Mueller R, et al. Interleukin-4, -5, and-6 and tumor necrosis factor-α in normal and asthmatic airways: evidence for the human mast cell as a source of these cytokines. Am. J. Respir. Cell Mol. Biol. 1994;10:471–480. doi: 10.1165/ajrcmb.10.5.8179909. [DOI] [PubMed] [Google Scholar]
- Busse PJ, Zhang TF, Schofield B, Kilaru S, Patil S, Li XM. Decrease in airway mucous gene expression caused by treatment with anti-tumor necrosis factor α in a murine model of allergic asthma. Ann. Allergy Asthma Immunol. 2009;103:295–303. doi: 10.1016/S1081-1206(10)60528-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y, Cao YX, Lu SM, Xu CB, Cardell LO. Infliximab alleviates inflammation and ex vivo airway hyperreactivity in asthmatic E3 rats. Int. Immunol. 2011;23:443–451. doi: 10.1093/intimm/dxr032. [DOI] [PubMed] [Google Scholar]
- Callejas-Rubio JL, Lopez-Perez L, Ortego-Centeno N. Tumor necrosis factor-alpha inhibitor treatment for sarcoidosis. Ther. Clin. Risk Manag. 2008;4:1305–1313. doi: 10.2147/tcrm.s967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callejas-Rubio JL, Ortego-Centeno N, Lopez-Perez L, Benticuaga MN. Treatment of therapy-resistant sarcoidosis with adalimumab. Clin. Rheumatol. 2006;25:596–597. doi: 10.1007/s10067-005-0037-9. [DOI] [PubMed] [Google Scholar]
- Camussi G, Albano E, Tetta C, Bussolino F. The molecular action of tumor necrosis factor-α. Eur. J. Biochem. 1991;202:3–14. doi: 10.1111/j.1432-1033.1991.tb16337.x. [DOI] [PubMed] [Google Scholar]
- Catal F, Mete E, Tayman C, Topal E, Albayrak A, Sert H. A human monoclonal anti-TNF alpha antibody (adalimumab) reduces airway inflammation and ameliorates lung histology in a murine model of acute asthma. Allergol. Immunopathol. (Madr) 2014;43:14–18. doi: 10.1016/j.aller.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Chebib N, Piegay F, Traclet J, Mion F, Mornex JF. Improvement with infliximab of a disseminated sarcoidosis in a patient with Crohn's disease. Case Rep. Pulmonol. 2014;2014:1–4. doi: 10.1155/2014/368780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HY, Morgan DL, Bauer AK, Kleeberger SR. Signal transduction pathways of tumor necrosis factor--mediated lung injury induced by ozone in mice. Am. J. Respir. Crit. Care Med. 2007;175:829–839. doi: 10.1164/rccm.200509-1527OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HY, Zhang LY, Kleeberger SR. Ozone-induced lung inflammation and hyperreactivity are mediated via tumor necrosis factor-α receptors. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001;280:L537–546. doi: 10.1152/ajplung.2001.280.3.L537. [DOI] [PubMed] [Google Scholar]
- Choi IW, Sun K, Kim YS, Ko HM, Im SY, Kim JH, et al. TNF-α induces the late-phase airway hyperresponsiveness and airway inflammation through cytosolic phospholipase A2 activation. J. Allergy Clin. Immunol. 2005;116:537–543. doi: 10.1016/j.jaci.2005.05.034. [DOI] [PubMed] [Google Scholar]
- Chung KF. Cytokines in chronic obstructive pulmonary disease. Eur. Respir. J. Suppl. 2001;34:50s–59s. [PubMed] [Google Scholar]
- Chung KF. Inflammatory mediators in chronic obstructive pulmonary disease. Curr. Drug Targets Inflamm. Allergy. 2005;4:619–625. doi: 10.2174/156801005774912806. [DOI] [PubMed] [Google Scholar]
- Churg A, Zhou S, Preobrazhenska O, Tai H, Wang R, Wright JL. Expression of profibrotic mediators in small airways versus parenchyma after cigarette smoke exposure. Am. J. Respir. Cell Mol. Biol. 2009;40:268–276. doi: 10.1165/rcmb.2007-0367OC. [DOI] [PubMed] [Google Scholar]
- Clark IA. How TNF was recognized as a key mechanism of disease. Cytokine Growth Factor Rev. 2007;18:335–343. doi: 10.1016/j.cytogfr.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Crommelin HA, Vorselaars AD, van Moorsel CH, Korenromp IH, Deneer VH, Grutters JC. Anti-TNF therapeutics for the treatment of sarcoidosis. Immunotherapy. 2014;6:1127–1143. doi: 10.2217/imt.14.65. [DOI] [PubMed] [Google Scholar]
- Cu A, Ye Q, Sarria R, Nakamura S, Guzman J, Costabel U. N-acetylcysteine inhibits TNF-α, sTNFR, and TGF-β1 release by alveolar macrophages in idiopathic pulmonary fibrosis in vitro. Sarcoidosis Vasc. Diffuse Lung Dis. 2009;26:147–154. [PubMed] [Google Scholar]
- Dejager L, Dendoncker K, Eggermont M, Souffriau J, Van Hauwermeiren F, Willart M, et al. Neutralizing TNFα restores glucocorticoid sensitivity in a mouse model of neutrophilic airway inflammation. Mucosal. Immunol. 2015;8:1212–1225. doi: 10.1038/mi.2015.12. [DOI] [PubMed] [Google Scholar]
- Dentener MA, Creutzberg EC, Pennings HJ, Rijkers GT, Mercken E, Wouters EF. Effect of infliximab on local and systemic inflammation in chronic obstructive pulmonary disease: a pilot study. Respiration. 2008;76:275–282. doi: 10.1159/000117386. [DOI] [PubMed] [Google Scholar]
- Deveci F, Muz MH, Ilhan N, Kirkil G, Turgut T, Akpolat N. Evaluation of the anti-inflammatory effect of infliximab in a mouse model of acute asthma. Respirology. 2008;13:488–497. doi: 10.1111/j.1440-1843.2008.01278.x. [DOI] [PubMed] [Google Scholar]
- Erin EM, Leaker BR, Nicholson GC, Tan AJ, Green LM, Neighbour H, et al. The effects of a monoclonal antibody directed against tumor necrosis factor-α in asthma. Am. J. Respir. Crit. Care Med. 2006;174:753–762. doi: 10.1164/rccm.200601-072OC. [DOI] [PubMed] [Google Scholar]
- Ermert M, Pantazis C, Duncker HR, Grimminger F, Seeger W, Ermert L. In situ localization of TNFα/β, TACE and TNF receptors TNF-R1 and TNF-R2 in control and LPS-treated lung tissue. Cytokine. 2003;22:89–100. doi: 10.1016/s1043-4666(03)00117-0. [DOI] [PubMed] [Google Scholar]
- Fakhrzadeh L, Laskin JD, Laskin DL. Ozone-induced production of nitric oxide and TNF-α and tissue injury are dependent on NF-κB p50. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004;287:L279–285. doi: 10.1152/ajplung.00348.2003. [DOI] [PubMed] [Google Scholar]
- Fakhrzadeh L, Laskin JD, Laskin DL. Regulation of caveolin-1 expression, nitric oxide production and tissue injury by tumor necrosis factor-α following ozone inhalation. Toxicol. Appl. Pharmacol. 2008;227:380–389. doi: 10.1016/j.taap.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes JL, de Oliveira RT, Mamoni RL, Coelho OR, Nicolau JC, Blotta MH, et al. Pentoxifylline reduces pro-inflammatory and increases anti-inflammatory activity in patients with coronary artery disease--a randomized placebo-controlled study. Atherosclerosis. 2008;196:434–442. doi: 10.1016/j.atherosclerosis.2006.11.032. [DOI] [PubMed] [Google Scholar]
- Finotto S, Ohno I, Marshall JS, Gauldie J, Denburg JA, Dolovich J, et al. TNF-α production by eosinophils in upper airways inflammation (nasal polyposis) J. Immunol. 1994;153:2278–2289. [PubMed] [Google Scholar]
- Fujita M, Shannon JM, Irvin CG, Fagan KA, Cool C, Augustin A, et al. Overexpression of tumor necrosis factor-α produces an increase in lung volumes and pulmonary hypertension. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001;280:L39–49. doi: 10.1152/ajplung.2001.280.1.L39. [DOI] [PubMed] [Google Scholar]
- Garcia-Ruiz C, Colell A, Mari M, Morales A, Calvo M, Enrich C, et al. Defective TNF-α-mediated hepatocellular apoptosis and liver damage in acidic sphingomyelinase knockout mice. J. Clin. Invest. 2003;111:197–208. doi: 10.1172/JCI16010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg AK, Aggarwal BB. Reactive oxygen intermediates in TNF signaling. Mol. Immunol. 2002;39:509–517. doi: 10.1016/s0161-5890(02)00207-9. [DOI] [PubMed] [Google Scholar]
- Gossart S, Cambon C, Orfila C, Seguelas MH, Lepert JC, Rami J, et al. Reactive oxygen intermediates as regulators of TNF-α production in rat lung inflammation induced by silica. J. Immunol. 1996;156:1540–1548. [PubMed] [Google Scholar]
- Gosset P, Tsicopoulos A, Wallaert B, Vannimenus C, Joseph M, Tonnel AB, et al. Increased secretion of tumor necrosis factor α and interleukin-6 by alveolar macrophages consecutive to the development of the late asthmatic reaction. J. Allergy Clin. Immunol. 1991;88:561–571. doi: 10.1016/0091-6749(91)90149-i. [DOI] [PubMed] [Google Scholar]
- Grau GE, Mili N, Lou JN, Morel DR, Ricou B, Lucas R, et al. Phenotypic and functional analysis of pulmonary microvascular endothelial cells from patients with acute respiratory distress syndrome. Lab. Invest. 1996;74:761–770. [PubMed] [Google Scholar]
- Gross TJ, Hunninghake GW. Idiopathic pulmonary fibrosis. N. Engl. J. Med. 2001;345:517–525. doi: 10.1056/NEJMra003200. [DOI] [PubMed] [Google Scholar]
- Guthmann F, Wissel H, Rustow B. Early subcutaneous administration of etanercept (Enbrel) prevents from hyperoxia-induced lung injury. Exp. Lung Res. 2009;35:770–780. doi: 10.3109/01902140902887430. [DOI] [PubMed] [Google Scholar]
- Hagmeyer L, Treml M, Priegnitz C, Randerath WJ. Successful concomitant therapy with pirfenidone and nintedanib in idiopathic pulmonary fibrosis: a case report. Respiration. 2016;91:327–332. doi: 10.1159/000444690. [DOI] [PubMed] [Google Scholar]
- Hallsworth MP, Soh CP, Lane SJ, Arm JP, Lee TH. Selective enhancement of GM-CSF, TNF-α, IL-1β and IL-8 production by monocytes and macrophages of asthmatic subjects. Eur. Respir. J. 1994;7:1096–1102. [PubMed] [Google Scholar]
- Hamacher J, Lucas R, Lijnen HR, Buschke S, Dunant Y, Wendel A, et al. Tumor necrosis factor-α and angiostatin are mediators of endothelial cytotoxicity in bronchoalveolar lavages of patients with acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2002;166:651–656. doi: 10.1164/rccm.2109004. [DOI] [PubMed] [Google Scholar]
- Hasegawa A, Takasaki W, Greene MI, Murali R. Modifying TNFα for therapeutic use: a perspective on the TNF receptor system. Mini Rev. Med. Chem. 2001;1:5–16. doi: 10.2174/1389557013407214. [DOI] [PubMed] [Google Scholar]
- Herfs M, Hubert P, Poirrier AL, Vandevenne P, Renoux V, Habraken Y, et al. Proinflammatory cytokines induce bronchial hyperplasia and squamous metaplasia in smokers: implications for chronic obstructive pulmonary disease therapy. Am. J. Respir. Cell Mol. Biol. 2012;47:67–79. doi: 10.1165/rcmb.2011-0353OC. [DOI] [PubMed] [Google Scholar]
- Holgate ST, Noonan M, Chanez P, Busse W, Dupont L, Pavord I, et al. Efficacy and safety of etanercept in moderate-to-severe asthma: a randomised, controlled trial. Eur. Respir. J. 2011;37:1352–1359. doi: 10.1183/09031936.00063510. [DOI] [PubMed] [Google Scholar]
- Horiuchi T, Mitoma H, Harashima S, Tsukamoto H, Shimoda T. Transmembrane TNF-α: structure, function and interaction with anti-TNF agents. Rheumatology (Oxford) 2010;49:1215–1228. doi: 10.1093/rheumatology/keq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howarth PH, Babu KS, Arshad HS, Lau L, Buckley M, McConnell W, et al. Tumour necrosis factor (TNFα) as a novel therapeutic target in symptomatic corticosteroid dependent asthma. Thorax. 2005;60:1012–1018. doi: 10.1136/thx.2005.045260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JM, Stringer RS, Black JL, Armour CL. The effects of tumour necrosis factor α on mediator release from human lung. Pulm. Pharmacol. 1995;8:31–36. doi: 10.1006/pulp.1995.1004. [DOI] [PubMed] [Google Scholar]
- Judson MA, Baughman RP, Costabel U, Drent M, Gibson KF, Raghu G, et al. Safety and efficacy of ustekinumab or golimumab in patients with chronic sarcoidosis. Eur. Respir. J. 2014;44:1296–1307. doi: 10.1183/09031936.00000914. [DOI] [PubMed] [Google Scholar]
- Kao SJ, Wang D, Lin HI, Chen HI. N-acetylcysteine abrogates acute lung injury induced by endotoxin. Clin. Exp. Pharmacol. Physiol. 2006;33:33–40. doi: 10.1111/j.1440-1681.2006.04320.x. [DOI] [PubMed] [Google Scholar]
- Karacsonyi C, Shanmugam N, Kagan E. A clinically relevant in vitro model for evaluating the effects of aerosolized vesicants. Toxicol. Lett. 2009;185:38–44. doi: 10.1016/j.toxlet.2008.11.015. [DOI] [PubMed] [Google Scholar]
- Keatings VM, Collins PD, Scott DM, Barnes PJ. Differences in interleukin-8 and tumor necrosis factor-α in induced sputum from patients with chronic obstructive pulmonary disease or asthma. Am. J. Respir. Crit. Care Med. 1996;153:530–534. doi: 10.1164/ajrccm.153.2.8564092. [DOI] [PubMed] [Google Scholar]
- Keatings VM, O'Connor BJ, Wright LG, Huston DP, Corrigan CJ, Barnes PJ. Late response to allergen is associated with increased concentrations of tumor necrosis factor-α and IL-5 in induced sputum. J. Allergy Clin. Immunol. 1997;99:693–698. doi: 10.1016/s0091-6749(97)70032-0. [DOI] [PubMed] [Google Scholar]
- Kelly M, Hwang JM, Kubes P. Modulating leukocyte recruitment in inflammation. J. Allergy Clin. Immunol. 2007;120:3–10. doi: 10.1016/j.jaci.2007.05.017. [DOI] [PubMed] [Google Scholar]
- Khair OA, Devalia JL, Abdelaziz MM, Sapsford RJ, Tarraf H, Davies RJ. Effect of Haemophilus influenzae endotoxin on the synthesis of IL-6, IL-8, TNF-α and expression of ICAM-1 in cultured human bronchial epithelial cells. Eur. Respir. J. 1994;7:2109–2116. doi: 10.1183/09031936.94.07122109. [DOI] [PubMed] [Google Scholar]
- Kim J, McKinley L, Natarajan S, Bolgos GL, Siddiqui J, Copeland S, et al. Anti-tumor necrosis factor-α antibody treatment reduces pulmonary inflammation and methacholine hyper-responsiveness in a murine asthma model induced by house dust. Clin. Exp. Allergy. 2006;36:122–132. doi: 10.1111/j.1365-2222.2005.02407.x. [DOI] [PubMed] [Google Scholar]
- King TE, Jr, Bradford WZ, Castro-Bernardini S, Fagan EA, Glaspole I, Glassberg MK, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N. Engl. J. Med. 2014;370:2083–2092. doi: 10.1056/NEJMoa1402582. [DOI] [PubMed] [Google Scholar]
- Kips JC, Tavernier J, Pauwels RA. Tumor necrosis factor causes bronchial hyperresponsiveness in rats. Am. Rev. Respir. Dis. 1992;145:332–336. doi: 10.1164/ajrccm/145.2_Pt_1.332. [DOI] [PubMed] [Google Scholar]
- Klimp AH, de Vries EG, Scherphof GL, Daemen T. A potential role of macrophage activation in the treatment of cancer. Crit. Rev. Oncol. Hematol. 2002;44:143–161. doi: 10.1016/s1040-8428(01)00203-7. [DOI] [PubMed] [Google Scholar]
- Laskin DL, Heck DE, Laskin JD. Role of inflammatory cytokines and nitric oxide in hepatic and pulmonary toxicity. Toxicol. Lett. 1998;102–103:289–293. doi: 10.1016/s0378-4274(98)00316-6. [DOI] [PubMed] [Google Scholar]
- Laskin DL, Morio L, Hooper K, Li TH, Buckley B, Turpin B. Peroxides and macrophages in the toxicity of fine particulate matter in rats. Res. Rep. Health Eff. Inst. 2003:1–51. discussion 53–63. [PubMed] [Google Scholar]
- Laskin DL, Sunil VR, Fakhrzadeh L, Groves A, Gow AJ, Laskin JD. Macrophages, reactive nitrogen species, and lung injury. Ann. N. Y. Acad. Sci. 2010;1203:60–65. doi: 10.1111/j.1749-6632.2010.05607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li BQ, Sun HC, Nie SN, Shao DB, Liu HM, Qian XM. Effect of penehyclidine hydrochloride on patients with acute lung injury and its mechanisms. Chin. J. Traumatol. 2010;13:329–335. [PubMed] [Google Scholar]
- Li T, Luo N, Du L, Zhou J, Zhang J, Gong L, et al. Tumor necrosis factor-α plays an initiating role in extracorporeal circulation-induced acute lung injury. Lung. 2013;191:207–214. doi: 10.1007/s00408-012-9449-x. [DOI] [PubMed] [Google Scholar]
- Liang S, Dai J, Hou S, Su L, Zhang D, Guo H, et al. Structural basis for treating tumor necrosis factor α (TNFα)-associated diseases with the therapeutic antibody infliximab. J. Biol. Chem. 2013;288:13799–13807. doi: 10.1074/jbc.M112.433961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S, Roche N, Oliver BG, Mattos W, Barnes PJ, Chung KF. Balance of matrix metalloprotease-9 and tissue inhibitor of metalloprotease-1 from alveolar macrophages in cigarette smokers. Regulation by interleukin-10. Am. J. Respir. Crit. Care Med. 2000;162:1355–1360. doi: 10.1164/ajrccm.162.4.9910097. [DOI] [PubMed] [Google Scholar]
- Lopez-de la Mora DA, Sanchez-Roque C, Montoya-Buelna M, Sanchez-Enriquez S, Lucano-Landeros S, Macias-Barragan J, et al. Role and new insights of pirfenidone in fibrotic diseases. Int. J. Med. Sci. 2015;12:840–847. doi: 10.7150/ijms.11579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losa Garcia JE, Rodriguez FM, Martin de Cabo MR, Garcia Salgado MJ, Losada JP, Villaron LG, et al. Evaluation of inflammatory cytokine secretion by human alveolar macrophages. Mediators Inflamm. 1999;8:43–51. doi: 10.1080/09629359990711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozo Vukovac E, Lozo M, Mise K, Gudelj I, Puljiz Z, Jurcev-Savicevic A, et al. Bronchoalveolar pH and inflammatory biomarkers in newly diagnosed IPF and GERD patients: a case-control study. Med. Sci. Monit. 2014;20:255–261. doi: 10.12659/MSM.889800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu G, Beuerman RW, Zhao S, Sun G, Nguyen DH, Ma S, et al. Tumor necrosis factor-α and interleukin-1 induce activation of MAP kinase and SAP kinase in human neuroma fibroblasts. Neurochem. Int. 1997;30:401–410. doi: 10.1016/s0197-0186(96)00075-7. [DOI] [PubMed] [Google Scholar]
- Lundblad LK, Thompson-Figueroa J, Leclair T, Sullivan MJ, Poynter ME, Irvin CG, et al. Tumor necrosis factor-α overexpression in lung disease: a single cause behind a complex phenotype. Am. J. Respir. Crit. Care Med. 2005;171:1363–1370. doi: 10.1164/rccm.200410-1349OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Pang Z, Zhu Q, Cai X, Yin Y, Wang M, et al. Locally instilled tumor necrosis factor-α antisense oligonucleotide inhibits allergic inflammation via the induction of Tregs. J. Gene Med. 2012;14:374–383. doi: 10.1002/jgm.2631. [DOI] [PubMed] [Google Scholar]
- Maher TM. Pirfenidone in idiopathic pulmonary fibrosis. Drugs Today (Barc) 2010;46:473–482. doi: 10.1358/dot.2010.46.7.1488336. [DOI] [PubMed] [Google Scholar]
- Makabe H, Kojika M, Takahashi G, Matsumoto N, Shibata S, Suzuki Y, et al. Interleukin-18 levels reflect the long-term prognosis of acute lung injury and acute respiratory distress syndrome. J. Anesth. 2012;26:658–663. doi: 10.1007/s00540-012-1409-3. [DOI] [PubMed] [Google Scholar]
- Malaviya R, Sunil VR, Cervelli J, Anderson DR, Holmes WW, Conti ML, et al. Inflammatory effects of inhaled sulfur mustard in rat lung. Toxicol. Appl. Pharmacol. 2010;248:89–99. doi: 10.1016/j.taap.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaviya R, Sunil VR, Venosa A, Verissimo VL, Cervelli JA, Vayas KN, et al. Attenuation of nitrogen mustard-induced pulmonary injury and fibrosis by anti-tumor necrosis factor-α antibody. Toxicol. Sci. 2015;148:71–88. doi: 10.1093/toxsci/kfv161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis NA, Sfika A, Nikitopoulou I, Vassiliou AG, Magkou C, Armaganidis A, et al. Acid-induced acute lung injury in mice is associated with P44/42 and c-Jun N-terminal kinase activation and requires the function of tumor necrosis factor α receptor I. Shock. 2012;38:381–386. doi: 10.1097/SHK.0b013e3182690ea2. [DOI] [PubMed] [Google Scholar]
- Matera MG, Calzetta L, Cazzola M. TNF-α inhibitors in asthma and COPD: we must not throw the baby out with the bath water. Pulm. Pharmacol. Ther. 2010;23:121–128. doi: 10.1016/j.pupt.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Matuschak GM, Lechner AJ. Acute lung injury and the acute respiratory distress syndrome: pathophysiology and treatment. MO. Med. 2010;107:252–258. [PMC free article] [PubMed] [Google Scholar]
- Michael S, Montag M, Dott W. Pro-inflammatory effects and oxidative stress in lung macrophages and epithelial cells induced by ambient particulate matter. Environ. Pollut. 2013;183:19–29. doi: 10.1016/j.envpol.2013.01.026. [DOI] [PubMed] [Google Scholar]
- Michel O, Dinh PH, Doyen V, Corazza F. Anti-TNF inhibits the airways neutrophilic inflammation induced by inhaled endotoxin in human. BMC Pharmacol. Toxicol. 2014;15:60. doi: 10.1186/2050-6511-15-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milman N, Graudal N, Loft A, Mortensen J, Larsen J, Baslund B. Effect of the TNF-α inhibitor adalimumab in patients with recalcitrant sarcoidosis: a prospective observational study using FDG-PET. Clin. Respir. J. 2012;6:238–247. doi: 10.1111/j.1752-699X.2011.00276.x. [DOI] [PubMed] [Google Scholar]
- Miyamoto A, Morokawa N, Takahashi Y, Ogawa K, Takeyasu M, Murase K, et al. Marked improvement with pirfenidone in a patient with idiopathic pulmonary fibrosis. Intern. Med. 2016;55:657–661. doi: 10.2169/internalmedicine.55.5259. [DOI] [PubMed] [Google Scholar]
- Miyazaki Y, Araki K, Vesin C, Garcia I, Kapanci Y, Whitsett JA, et al. Expression of a tumor necrosis factor-α transgene in murine lung causes lymphocytic and fibrosing alveolitis. A mouse model of progressive pulmonary fibrosis. J. Clin. Invest. 1995;96:250–259. doi: 10.1172/JCI118029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morjaria JB, Chauhan AJ, Babu KS, Polosa R, Davies DE, Holgate ST. The role of a soluble TNFalpha receptor fusion protein (etanercept) in corticosteroid refractory asthma: a double blind, randomised, placebo controlled trial. Thorax. 2008;63:584–591. doi: 10.1136/thx.2007.086314. [DOI] [PubMed] [Google Scholar]
- Mornex JF, Leroux C, Greenland T, Ecochard D. From granuloma to fibrosis in interstitial lung diseases: molecular and cellular interactions. Eur. Respir. J. 1994;7:779–785. doi: 10.1183/09031936.94.07040779. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay S, Hoidal JR, Mukherjee TK. Role of TNFα in pulmonary pathophysiology. Respir. Res. 2006;7:125. doi: 10.1186/1465-9921-7-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay S, Mukherjee S, Stone WL, Smith M, Das SK. Role of MAPK/AP-1 signaling pathway in the protection of CEES-induced lung injury by antioxidant liposome. Toxicology. 2009;261:143–151. doi: 10.1016/j.tox.2009.05.010. [DOI] [PubMed] [Google Scholar]
- Mullen PG, Windsor AC, Walsh CJ, Blocher CR, Fisher BJ, Leeper-Woodford SK, et al. Combined ibuprofen and monoclonal antibody to tumor necrosis factor-α attenuate hemodynamic dysfunction and sepsis-induced acute lung injury. J. Trauma. 1993;34:612–620. doi: 10.1097/00005373-199305000-00002. [DOI] [PubMed] [Google Scholar]
- Noguchi E, Yokouchi Y, Shibasaki M, Inudou M, Nakahara S, Nogami T, et al. Association between TNFA polymorphism and the development of asthma in the Japanese population. Am. J. Respir. Crit. Care Med. 2002;166:43–46. doi: 10.1164/rccm.2110052. [DOI] [PubMed] [Google Scholar]
- Nunes H, Soler P, Valeyre D. Pulmonary sarcoidosis. Allergy. 2005;60:565–582. doi: 10.1111/j.1398-9995.2005.00778.x. [DOI] [PubMed] [Google Scholar]
- Obrador E, Navarro J, Mompo J, Asensi M, Pellicer JA, Estrela JM. Regulation of tumour cell sensitivity to TNF-induced oxidative stress and cytotoxicity: role of glutathione. Biofactors. 1998;8:23–26. doi: 10.1002/biof.5520080105. [DOI] [PubMed] [Google Scholar]
- Oikonomou N, Harokopos V, Zalevsky J, Valavanis C, Kotanidou A, Szymkowski DE, et al. Soluble TNF mediates the transition from pulmonary inflammation to fibrosis. PLoS One. 2006;1:e108. doi: 10.1371/journal.pone.0000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz LA, Lasky J, Gozal E, Ruiz V, Lungarella G, Cavarra E, et al. Tumor necrosis factor receptor deficiency alters matrix metalloproteinase 13/tissue inhibitor of metalloproteinase 1 expression in murine silicosis. Am. J. Respir. Crit. Care Med. 2001;163:244–252. doi: 10.1164/ajrccm.163.1.2002123. [DOI] [PubMed] [Google Scholar]
- Ortiz LA, Lasky J, Lungarella G, Cavarra E, Martorana P, Banks WA, et al. Upregulation of the p75 but not the p55 TNF-α receptor mRNA after silica and bleomycin exposure and protection from lung injury in double receptor knockout mice. Am. J. Respir. Cell Mol. Biol. 1999;20:825–833. doi: 10.1165/ajrcmb.20.4.3193. [DOI] [PubMed] [Google Scholar]
- Osterlund C, Lilliehook B, Ekstrand-Hammarstrom B, Sandstrom T, Bucht A. The nitrogen mustard melphalan activates mitogen-activated phosphorylated kinases (MAPK), nuclear factor-κB and inflammatory response in lung epithelial cells. J. Appl. Toxicol. 2005;25:328–337. doi: 10.1002/jat.1070. [DOI] [PubMed] [Google Scholar]
- Oswald-Richter KA, Richmond BW, Braun NA, Isom J, Abraham S, Taylor TR, et al. Reversal of global CD4+ subset dysfunction is associated with spontaneous clinical resolution of pulmonary sarcoidosis. J. Immunol. 2013;190:5446–5453. doi: 10.4049/jimmunol.1202891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovrevik J, Lag M, Holme JA, Schwarze PE, Refsnes M. Cytokine and chemokine expression patterns in lung epithelial cells exposed to components characteristic of particulate air pollution. Toxicology. 2009;259:46–53. doi: 10.1016/j.tox.2009.01.028. [DOI] [PubMed] [Google Scholar]
- Padilla-Carlin DJ, Schladweiler MC, Shannahan JH, Kodavanti UP, Nyska A, Burgoon LD, et al. Pulmonary inflammatory and fibrotic responses in Fischer 344 rats after intratracheal instillation exposure to Libby amphibole. J. Toxicol. Environ. Health A. 2011;74:1111–1132. doi: 10.1080/15287394.2011.586940. [DOI] [PubMed] [Google Scholar]
- Pan LH, Ohtani H, Yamauchi K, Nagura H. Co-expression of TNFα and IL-1β in human acute pulmonary fibrotic diseases: an immunohistochemical analysis. Pathol. Int. 1996;46:91–99. doi: 10.1111/j.1440-1827.1996.tb03584.x. [DOI] [PubMed] [Google Scholar]
- Parsons PE, Matthay MA, Ware LB, Eisner MD. Elevated plasma levels of soluble TNF receptors are associated with morbidity and mortality in patients with acute lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005;288:L426–431. doi: 10.1152/ajplung.00302.2004. [DOI] [PubMed] [Google Scholar]
- Pendino KJ, Meidhof TM, Heck DE, Laskin JD, Laskin DL. Inhibition of macrophages with gadolinium chloride abrogates ozone-induced pulmonary injury and inflammatory mediator production. Am. J. Respir. Cell Mol. Biol. 1995;13:125–132. doi: 10.1165/ajrcmb.13.2.7542894. [DOI] [PubMed] [Google Scholar]
- Piguet PF. Is “tumor necrosis factor” the major effector of pulmonary fibrosis? Eur. Cytokine Netw. 1990;1:257–258. [PubMed] [Google Scholar]
- Piguet PF, Collart MA, Grau GE, Sappino AP, Vassalli P. Requirement of tumour necrosis factor for development of silica-induced pulmonary fibrosis. Nature. 1990;344:245–247. doi: 10.1038/344245a0. [DOI] [PubMed] [Google Scholar]
- Piguet PF, Ribaux C, Karpuz V, Grau GE, Kapanci Y. Expression and localization of tumor necrosis factor-α and its mRNA in idiopathic pulmonary fibrosis. Am. J. Pathol. 1993;143:651–655. [PMC free article] [PubMed] [Google Scholar]
- Piguet PF, Vesin C. Treatment by human recombinant soluble TNF receptor of pulmonary fibrosis induced by bleomycin or silica in mice. Eur. Respir. J. 1994;7:515–518. doi: 10.1183/09031936.94.07030515. [DOI] [PubMed] [Google Scholar]
- Pryhuber GS, Huyck HL, Baggs R, Oberdorster G, Finkelstein JN. Induction of chemokines by low-dose intratracheal silica is reduced in TNFR I (p55) null mice. Toxicol. Sci. 2003;72:150–157. doi: 10.1093/toxsci/kfg018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghu G, Brown KK, Costabel U, Cottin V, du Bois RM, Lasky JA, et al. Treatment of idiopathic pulmonary fibrosis with etanercept: an exploratory, placebo-controlled trial. Am. J. Respir. Crit. Care Med. 2008;178:948–955. doi: 10.1164/rccm.200709-1446OC. [DOI] [PubMed] [Google Scholar]
- Rahman I. Regulation of nuclear factor-κB, activator protein-1, and glutathione levels by tumor necrosis factor-α and dexamethasone in alveolar epithelial cells. Biochem. Pharmacol. 2000;60:1041–1049. doi: 10.1016/s0006-2952(00)00392-0. [DOI] [PubMed] [Google Scholar]
- Raychaudhuri SP, Raychaudhuri SK. Biologics: target-specific treatment of systemic and cutaneous autoimmune diseases. Indian. J. Dermatol. 2009;54:100–109. doi: 10.4103/0019-5154.53175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razavi SM, Ghanei M, Salamati P, Safiabadi M. Long-term effects of mustard gas on respiratory system of Iranian veterans after Iraq-Iran war: a review. Chin. J. Traumatol. 2013;16:163–168. [PubMed] [Google Scholar]
- Rennard SI, Fogarty C, Kelsen S, Long W, Ramsdell J, Allison J, et al. The safety and efficacy of infliximab in moderate to severe chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2007;175:926–934. doi: 10.1164/rccm.200607-995OC. [DOI] [PubMed] [Google Scholar]
- Reper P, Heijmans W. High-frequency percussive ventilation and initial biomarker levels of lung injury in patients with minor burns after smoke inhalation injury. Burns. 2015;41:65–70. doi: 10.1016/j.burns.2014.05.007. [DOI] [PubMed] [Google Scholar]
- Riha RL, Yang IA, Rabnott GC, Tunnicliffe AM, Fong KM, Zimmerman PV. Cytokine gene polymorphisms in idiopathic pulmonary fibrosis. Intern. Med. J. 2004;34:126–129. doi: 10.1111/j.1444-0903.2004.00503.x. [DOI] [PubMed] [Google Scholar]
- Rossman MD, Newman LS, Baughman RP, Teirstein A, Weinberger SE, Miller W, Jr, et al. A double-blinded, randomized, placebo-controlled trial of infliximab in subjects with active pulmonary sarcoidosis. Sarcoidosis Vasc. Diffuse Lung Dis. 2006;23:201–208. [PubMed] [Google Scholar]
- Roten R, Markert M, Feihl F, Schaller MD, Tagan MC, Perret C. Plasma levels of tumor necrosis factor in the adult respiratory distress syndrome. Am. Rev. Respir. Dis. 1991;143:590–592. doi: 10.1164/ajrccm/143.3.590. [DOI] [PubMed] [Google Scholar]
- Rozy A, Czerniawska J, Stepniewska A, Wozbinska B, Goljan A, Puscinska E, et al. Inflammatory markers in the exhaled breath condensate of patients with pulmonary sarcoidosis. J. Physiol. Pharmacol. 2006;57(Suppl. 4):335–340. [PubMed] [Google Scholar]
- Russell E, Luk F, Manocha S, Ho T, O'Connor C, Hussain H. Long term follow-up of infliximab efficacy in pulmonary and extra-pulmonary sarcoidosis refractory to conventional therapy. Semin. Arthritis Rheum. 2013;43:119–124. doi: 10.1016/j.semarthrit.2012.10.008. [DOI] [PubMed] [Google Scholar]
- Rusznak C, Devalia JL, Sapsford RJ, Davies RJ. Ozone-induced mediator release from human bronchial epithelial cells in vitro and the influence of nedocromil sodium. Eur. Respir. J. 1996;9:2298–2305. doi: 10.1183/09031936.96.09112298. [DOI] [PubMed] [Google Scholar]
- Salazar-Montes A, Ruiz-Corro L, Lopez-Reyes A, Castrejon-Gomez E, Armendariz-Borunda J. Potent antioxidant role of pirfenidone in experimental cirrhosis. Eur. J. Pharmacol. 2008;595:69–77. doi: 10.1016/j.ejphar.2008.06.110. [DOI] [PubMed] [Google Scholar]
- Sasaki M, Kashima M, Ito T, Watanabe A, Izumiyama N, Sano M, et al. Differential regulation of metalloproteinase production, proliferation and chemotaxis of human lung fibroblasts by PDGF, interleukin-1β and TNF-α. Mediators Inflamm. 2000;9:155–160. doi: 10.1080/09629350020002895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schupp JC, Binder H, Jäger B, Cillis G, Zissel G, Müller-Quernheim J, et al. Macrophage activation in acute exacerbation of idiopathic pulmonary fibrosis. PLoS One. 2015;10:e0116775. doi: 10.1371/journal.pone.0116775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenzato G. Tumour necrosis factor: a cytokine with multiple biological activities. Br. J. Cancer. 1990;61:354–361. doi: 10.1038/bjc.1990.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah A, Church MK, Holgate ST. Tumour necrosis factor alpha: a potential mediator of asthma. Clin. Exp. Allergy. 1995;25:1038–1044. doi: 10.1111/j.1365-2222.1995.tb03249.x. [DOI] [PubMed] [Google Scholar]
- Shvedova AA, Satoh T, Tollerud D, Guevarra L, Karol MH. Elevated levels of IL-6, INF-γ, and TNF-α in mice in response to cotton dust are modulated by anti-TNF-α antiserum. Exp. Lung Res. 1996;22:149–161. doi: 10.3109/01902149609050844. [DOI] [PubMed] [Google Scholar]
- Singh S, Grover V, Christie L, Charles P, Kelleher P, Shah PL. A comparative study of bronchoscopic microsample probe versus bronchoalveolar lavage in patients with burns-related inhalational injury, acute lung injury and chronic stable lung disease. Respiration. 2015;89:19–26. doi: 10.1159/000368367. [DOI] [PubMed] [Google Scholar]
- Song Z, Yao C, Yin J, Tong C, Zhu D, Sun Z, et al. Genetic variation in the TNF receptor-associated factor 6 gene is associated with susceptibility to sepsis-induced acute lung injury. J. Transl. Med. 2012;10:166. doi: 10.1186/1479-5876-10-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suissa S, Ernst P, Hudson M. TNF-α antagonists and the prevention of hospitalisation for chronic obstructive pulmonary disease. Pulm. Pharmacol. Ther. 2008;21:234–238. doi: 10.1016/j.pupt.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Sullivan DE, Ferris M, Pociask D, Brody AR. Tumor necrosis factor-α induces transforming growth factor-β1 expression in lung fibroblasts through the extracellular signal-regulated kinase pathway. Am. J. Respir. Cell Mol. Biol. 2005;32:342–349. doi: 10.1165/rcmb.2004-0288OC. [DOI] [PubMed] [Google Scholar]
- Sunil VR, Patel-Vayas K, Shen J, Gow AJ, Laskin JD, Laskin DL. Role of TNFR1 in lung injury and altered lung function induced by the model sulfur mustard vesicant, 2-chloroethyl ethyl sulfide. Toxicol. Appl. Pharmacol. 2011;250:245–255. doi: 10.1016/j.taap.2010.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunil VR, Vayas KN, Cervelli JA, Malaviya R, Hall L, Massa CB, et al. Pentoxifylline attenuates nitrogen mustard-induced acute lung injury, oxidative stress and inflammation. Exp. Mol. Pathol. 2014;97:89–98. doi: 10.1016/j.yexmp.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter PM, Suter S, Girardin E, Roux-Lombard P, Grau GE, Dayer JM. High bronchoalveolar levels of tumor necrosis factor and its inhibitors, interleukin-1, interferon, and elastase, in patients with adult respiratory distress syndrome after trauma, shock, or sepsis. Am. Rev. Respir. Dis. 1992;145:1016–1022. doi: 10.1164/ajrccm/145.5.1016. [DOI] [PubMed] [Google Scholar]
- Suzuki J, Hamada E, Shodai T, Kamoshida G, Kudo S, Itoh S, et al. Cytokine secretion from human monocytes potentiated by P-selectin-mediated cell adhesion. Int. Arch. Allergy Immunol. 2013;160:152–160. doi: 10.1159/000339857. [DOI] [PubMed] [Google Scholar]
- Sweiss NJ, Noth I, Mirsaeidi M, Zhang W, Naureckas ET, Hogarth DK, et al. Efficacy results of a 52-week trial of adalimumab in the treatment of refractory sarcoidosis. Sarcoidosis Vasc. Diffuse Lung Dis. 2014;31:46–54. [PMC free article] [PubMed] [Google Scholar]
- Sweiss NJ, Welsch MJ, Curran JJ, Ellman MH. Tumor necrosis factor inhibition as a novel treatment for refractory sarcoidosis. Arthritis Rheum. 2005;53:788–791. doi: 10.1002/art.21468. [DOI] [PubMed] [Google Scholar]
- Taille C, Poulet C, Marchand-Adam S, Borie R, Dombret MC, Crestani B, et al. Monoclonal anti-TNF-α antibodies for severe steroid-dependent asthma: a case series. Open Respir. Med. J. 2013;7:21–25. doi: 10.2174/1874306401307010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takabatake N, Nakamura H, Inoue S, Terashita K, Yuki H, Kato S, et al. Circulating levels of soluble Fas ligand and soluble Fas in patients with chronic obstructive pulmonary disease. Respir. Med. 2000;94:1215–1220. doi: 10.1053/rmed.2000.0941. [DOI] [PubMed] [Google Scholar]
- Takeda Y, Tsujino K, Kijima T, Kumanogoh A. Efficacy and safety of pirfenidone for idiopathic pulmonary fibrosis. Patient Prefer. Adherence. 2014;8:361–370. doi: 10.2147/PPA.S37233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi H, Kondoh Y, Ebina M, Azuma A, Ogura T, Taguchi Y, et al. The clinical significance of 5% change in vital capacity in patients with idiopathic pulmonary fibrosis: extended analysis of the pirfenidone trial. Respir. Res. 2011;12:93. doi: 10.1186/1465-9921-12-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas PS. Tumour necrosis factor-α: the role of this multifunctional cytokine in asthma. Immunol. Cell Biol. 2001;79:132–140. doi: 10.1046/j.1440-1711.2001.00980.x. [DOI] [PubMed] [Google Scholar]
- Thomas PS, Heywood G. Effects of inhaled tumour necrosis factor alpha in subjects with mild asthma. Thorax. 2002;57:774–778. doi: 10.1136/thorax.57.9.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrall RS, Vogel SN, Evans R, Shultz LD. Role of tumor necrosis factor-α in the spontaneous development of pulmonary fibrosis in viable motheaten mutant mice. Am. J. Pathol. 1997;151:1303–1310. [PMC free article] [PubMed] [Google Scholar]
- Tomashefski JF., Jr Pulmonary pathology of acute respiratory distress syndrome. Clin. Chest Med. 2000;21:435–466. doi: 10.1016/s0272-5231(05)70158-1. [DOI] [PubMed] [Google Scholar]
- Tong Z, Dai H, Chen B, Abdoh Z, Guzman J, Costabel U. Inhibition of cytokine release from alveolar macrophages in pulmonary sarcoidosis by pentoxifylline: comparison with dexamethasone. Chest. 2003;124:1526–1532. doi: 10.1378/chest.124.4.1526. [DOI] [PubMed] [Google Scholar]
- Utz JP, Limper AH, Kalra S, Specks U, Scott JP, Vuk-Pavlovic Z, et al. Etanercept for the treatment of stage II and III progressive pulmonary sarcoidosis. Chest. 2003;124:177–185. doi: 10.1378/chest.124.1.177. [DOI] [PubMed] [Google Scholar]
- Vaillant P, Menard O, Vignaud JM, Martinet N, Martinet Y. The role of cytokines in human lung fibrosis. Monaldi Arch. Chest Dis. 1996;51:145–152. [PubMed] [Google Scholar]
- van der Vaart H, Koeter GH, Postma DS, Kauffman HF, ten Hacken NH. First study of infliximab treatment in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2005;172:465–469. doi: 10.1164/rccm.200501-147OC. [DOI] [PubMed] [Google Scholar]
- Vasudevan A, Lodha R, Kabra SK. Acute lung injury and acute respiratory distress syndrome. Indian. J. Pediatr. 2004;71:743–750. doi: 10.1007/BF02730667. [DOI] [PubMed] [Google Scholar]
- Vernooy JH, Kucukaycan M, Jacobs JA, Chavannes NH, Buurman WA, Dentener MA, et al. Local and systemic inflammation in patients with chronic obstructive pulmonary disease: soluble tumor necrosis factor receptors are increased in sputum. Am. J. Respir. Crit. Care Med. 2002;166:1218–1224. doi: 10.1164/rccm.2202023. [DOI] [PubMed] [Google Scholar]
- Wagner PD. Possible mechanisms underlying the development of cachexia in COPD. Eur. Respir. J. 2008;31:492–501. doi: 10.1183/09031936.00074807. [DOI] [PubMed] [Google Scholar]
- Weinberger B, Laskin JD, Sunil VR, Sinko PJ, Heck DE, Laskin DL. Sulfur mustard-induced pulmonary injury: therapeutic approaches to mitigating toxicity. Pulm. Pharmacol. Ther. 2011;24:92–99. doi: 10.1016/j.pupt.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel SE, Barnes PJ, Bleecker ER, Bousquet J, Busse W, Dahlen SE, et al. A randomized, double-blind, placebo-controlled study of tumor necrosis factor-α blockade in severe persistent asthma. Am. J. Respir. Crit. Care Med. 2009;179:549–558. doi: 10.1164/rccm.200809-1512OC. [DOI] [PubMed] [Google Scholar]
- Wilson MR, Goddard ME, O'Dea KP, Choudhury S, Takata M. Differential roles of p55 and p75 tumor necrosis factor receptors on stretch-induced pulmonary edema in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007;293:L60–68. doi: 10.1152/ajplung.00284.2006. [DOI] [PubMed] [Google Scholar]
- Wollin L, Maillet I, Quesniaux V, Holweg A, Ryffel B. Antifibrotic and anti-inflammatory activity of the tyrosine kinase inhibitor nintedanib in experimental models of lung fibrosis. J. Pharmacol. Exp. Ther. 2014;349:209–220. doi: 10.1124/jpet.113.208223. [DOI] [PubMed] [Google Scholar]
- Woodruff PG, Chatila W, Connett JE, Criner GJ, Curtis JL, Dransfield MT, et al. Tumour necrosis factor receptor-75 and risk of COPD exacerbation in the azithromycin trial. Eur. Respir. J. 2014;43:295–298. doi: 10.1183/09031936.00140613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie C, Reusse A, Dai J, Zay K, Harnett J, Churg A. TNF-α increases tracheal epithelial asbestos and fiberglass binding via a NF-κB-dependent mechanism. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000;279:L608–614. doi: 10.1152/ajplung.2000.279.3.L608. [DOI] [PubMed] [Google Scholar]
- Yadav AK, Bracher A, Doran SF, Leustik M, Squadrito GL, Postlethwait EM, et al. Mechanisms and modification of chlorine-induced lung injury in animals. Proc. Am. Thorac. Soc. 2010;7:278–283. doi: 10.1513/pats.201001-009SM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XY, Zhang C, Sun QY, Li D, Luo RR, Wan ZF, et al. Infliximab protects against pulmonary emphysema in smoking rats. Chin. Med. J. (Engl) 2011;124:2502–2506. [PubMed] [Google Scholar]
- Zhang Y, Lee TC, Guillemin B, Yu MC, Rom WN. Enhanced IL-1β and tumor necrosis factor-α release and messenger RNA expression in macrophages from idiopathic pulmonary fibrosis or after asbestos exposure. J. Immunol. 1993;150:4188–4196. [PubMed] [Google Scholar]
- Ziegenhagen MW, Schrum S, Zissel G, Zipfel PF, Schlaak M, Muller-Quernheim J. Increased expression of proinflammatory chemokines in bronchoalveolar lavage cells of patients with progressing idiopathic pulmonary fibrosis and sarcoidosis. J. Investig. Med. 1998;46:223–231. [PubMed] [Google Scholar]