Abstract
Selectins mediate rolling of leukocytes by rapid formation and dissociation of selectin–ligand bonds, which are assumed to require high mechanical strength to prevent premature dissociation by the forces applied in shear flow. This assumption is based largely on the observation that increasing wall shear stress increases only modestly the dissociation of transient leukocyte tethers on very low selectin densities. P-selectin binds to the N-terminal region of P-selectin glycoprotein ligand-1 (PSGL-1), a mucin on leukocytes. Both PSGL-1 and P-selectin are extended homodimers. We perfused transfected cells expressing wild-type dimeric PSGL-1 or a chimeric monomeric form of PSGL-1 on immobilized dimeric or monomeric forms of P-selectin. Cells expressing dimeric or monomeric PSGL-1 tethered to P-selectin at equivalent rates. However, cells expressing dimeric PSGL-1 established more stable rolling adhesions, which were more shear resistant and exhibited less fluctuation in rolling velocities. On low densities of dimeric P-selectin, increasing wall shear stress more rapidly increased transient tether dissociation of cells expressing monomeric PSGL-1 than dimeric PSGL-1. Tether dissociation on low densities of monomeric P-selectin was even more shear sensitive. We conclude that dimerization of both PSGL-1 and P-selectin stabilizes tethering and rolling, probably by increasing rebinding within a bond cluster. Because transient tethers may have more than one bond, the mechanical strength of selectin–ligand bonds is likely to be lower than initially estimated. Tether strength may rely more on bond clusters to distribute applied force.
During inflammation, leukocytes first tether to and roll on the blood vessel wall (1, 2). This adhesive event is mediated primarily by binding of the selectins to cell-surface glycoconjugates. L-selectin, expressed on most leukocytes, binds to ligands on endothelial cells and other leukocytes. P- and E-selectin, expressed on activated platelets and/or endothelial cells, bind to ligands on leukocytes, platelets, and some endothelial cells.
Leukocyte rolling in shear flow requires rapid formation and breakage of adhesive bonds that are subjected to applied force (3). Surface plasmon resonance measurements reveal that soluble, monomeric P-, and L-selectin bind to physiological ligands with very rapid kon and koff values (4, 5). These rapid intrinsic binding kinetics are important, but mechanical properties of selectin–ligand interactions also affect rolling, because adherent cells must resist premature detachment under force (6, 7). The lifetimes of transient tethers of leukocytes on selectins at densities too low to support rolling have been measured as a function of wall shear stress or force applied to the tether (6, 8–12). The lifetimes appear to obey first-order dissociation kinetics and are independent of selectin or ligand density; this suggests, but does not prove, that the tethers represent single selectin–ligand bonds. There are two caveats to this assumption. First, tethers supported by multibond clusters can have lifetime distributions that are similar to those of single-bond tethers; that is, they may appear to obey first-order dissociation kinetics (13). Thus, the inherent errors in measuring tether lifetimes make it difficult to discriminate tethers supported by single or multiple bonds. Second, independence of selectin or ligand density is expected even for multibond lifetimes if the selectin and/or ligand are clustered (13). Bond clusters may be particularly important for stabilizing cell rolling, especially at higher wall shear stresses. The number of bonds formed during tethering and rolling may be a function of the local distribution as well as the overall density of receptors and ligands. Some adhesion molecules are concentrated in microvillus tips or clathrin-coated pits (14–16). Dimerization of adhesion receptors and ligands is another potential mechanism favoring bond clusters.
The physiological ligand for P-selectin on leukocytes is P-selectin glycoprotein ligand-1 (PSGL-1) (2, 17). The preferential binding of P-selectin to PSGL-1 makes this receptor–ligand pair an excellent model system to address the biochemical and biophysical features that contribute to cell tethering and rolling in shear flow. Both P-selectin and PSGL-1 are extended membrane glycoproteins that form homodimers. P-selectin isolated from human platelets consists of dimers and oligomers in nonionic detergents (18). Dimerization is apparently mediated through interactions of the transmembrane domains, because recombinant soluble forms of P-selectin that lack the transmembrane domain are monomeric. Crosslinking studies confirm that P-selectin forms dimers in the cell membrane (19). Under static conditions, more leukocytes adhere to low matched densities of dimeric P-selectin than of monomeric P-selectin (18). PSGL-1 is a mucin that forms homodimers covalently linked by a disulfide bond between a single extracellular cysteine in each subunit (20), which is located at the junction with the transmembrane domain (21, 22). Mutation of this cysteine was reported to eliminate dimerization of PSGL-1 on intact cells and to prevent binding of fluid-phase P-selectin to PSGL-1 on the cells (21). This finding was surprising in that soluble monomeric P-selectin binds with appreciable affinity to a soluble N-terminal proteolytic fragment of PSGL-1 and to soluble glycosulfopeptides modeled after the N terminus of PSGL-1 (22, 23). Furthermore, mutation of the cysteine in PSGL-1 still allows it to form noncovalent dimers that can be crosslinked on the cell surface (22). Noncovalent dimerization is mediated through interactions of the transmembrane domains. Creation of a chimeric form of PSGL-1 by substituting its transmembrane domain with that of CD43, which also removes the cysteine residue, yields an apparently monomeric form of PSGL-1 that cannot be crosslinked but still binds well to P-selectin (22). These combined results indicate that dimerization of PSGL-1 is not a prerequisite for binding to P-selectin. However, it has not been established whether dimerization of PSGL-1 or P-selectin modulates cell adhesion in flow.
In this paper, we directly compare the tethering and rolling of cells expressing dimeric or monomeric PSGL-1 on substrates of dimeric or monomeric P-selectin. Our results indicate that dimerization stabilizes cell rolling in shear flow. The dissociation kinetics of transient tethers formed through interactions of monomeric P-selectin and PSGL-1 are much more sensitive to applied force than those formed through interactions of dimeric P-selectin and PSGL-1. Thus, transient tethers that appear to obey first-order dissociation kinetics need not represent one bond. Our data support the importance of bond clusters in supporting tethering and rolling and suggest that the mechanical strength of individual selectin–ligand bonds may be lower than the initial estimates.
Materials and Methods
Cells.
Human neutrophils were isolated as described (15). Transfected Chinese hamster ovary (CHO) cells expressing α1, 3-fucosyltransferase VII and core-2 β1, 6-N-acetylglucosaminyltransferase-l were transfected with cDNA for wild-type PSGL-1 or for CD43TMD PSGL-1, a chimeric molecule in which the transmembrane domain of PSGL-1 was replaced with the transmembrane domain of CD43 (11, 22). Transfected cells resistant to G418, hygromycin, and Zeocin (Invitrogen) were selected for matched expression of PSGL-1 by using anti-PSGL-1 mAbs PL1 and PL2 (15). Human K562 cells transfected with cDNAs for wild-type or CD43TMD PSGL-1 were also selected for matched expression of PSGL-1. To create sialyl Lewis x (sLex) determinants on the cell surface, 5–10 × 106 K562 cells were treated with 1 mM GDP-fucose/20 milliunits/ml of α1, 3-fucosyltransferase VI (Calbiochem)/10 mM MnCl2 in 0.5 ml Hanks' balanced salt solution (HBSS) containing Ca2+ and Mg2+, plus 0.1% human serum albumin (HBSS/HSA) for 45 min at 37°C. Matched levels of cell-surface sLex epitopes were confirmed by flow cytometric analysis with the anti-sLex mAb HECA-452 (24).
Cell Accumulation, Shear Resistance, and Tethering in Shear Flow.
CHO cells or K562 cells expressing wild-type or CD43TMD PSGL-1 (106/ml in HBSS/HSA) were perfused over adsorbed membrane-derived P-selectin (mP-selectin) (18, 25) in a parallel-plate flow chamber. Site densities of P-selectin were determined by binding of radiolabeled anti-P-selectin mAb G1 (15). The accumulated number of rolling cells was measured with a videomicroscopy system coupled to a digitized image analysis system (Inovision, Durham, NC) (11). To measure resistance to detachment, transfected cells were allowed to accumulate at 0.5 dyn/cm2 (1 dyn = 10 μN). Wall shear stress was increased every 30 s, and the percentage of remaining adherent cells was determined. The rate that cells tethered to mP-selectin was measured during the first 60 s of perfusion (11). Cells that detached in less than 30 frames were defined as transient tethers. Cells that remained attached for at least 30 frames were defined as rolling tethers, because greater than 90% of these cells rolled after the 30-frame observation period.
Velocity Measurements.
Rolling velocities were measured by tracking an individual cell frame by frame, an interval of 0.033 s, in the direction of flow. For each P-selectin-PSGL-1 interaction, at least 60 cells were tracked, each for up to 5 s, to yield a total observation time of 300 s (11). The frame-by-frame velocity data were used to calculate the mean velocity and the variance of velocity for each cell over the 5-s period. The pooled data from all cells were used to calculate the mean velocity and variance of velocity for the cell population.
Determination of Dissociation Rate Constants and Mechanical Strength of Transient Tethers.
Transient tether durations were measured on very low site densities of mP-selectin or recombinant soluble P-selectin (sP-selectin) (11, 18). The natural log of the number of cells that remained bound as a function of time after initiation of tethering was plotted. For first-order dissociation kinetics, the resultant plot is a straight line, and the slope = −koff. Multibond tethers may also dissociate in a pseudofirst-order fashion, although the slope in such a nearly linear plot would not represent the −koff of the individual bonds in the cluster. Nevertheless, the measured apparent koff allowed us to compare the dissociation kinetics of tethers mediated through monomeric and dimeric P-selectin-PSGL-1 interactions. Five independent measurements of koff were made for the interaction of neutrophils or of CHO cells transfected with wild-type or CD43TMD PSGL-1 with either mP-selectin or sP-selectin. The dependence of koff on applied force was assumed to follow the Bell equation (26): koff = k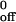 exp (aFb/kT), where k
exp (aFb/kT), where k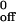 is the dissociation rate in the absence of applied force, a is the reactive compliance, Fb is the force on the bond, k is Boltzman's constant, and T is the absolute temperature. The term Ft was substituted for Fb, because the tether may represent more than one bond. For CHO cells, the force on the tether was calculated (27) by using a cell diameter of 20 μm and assigning a tether angle of 50°. For neutrophils, the force on the tether was calculated on the basis of a tether angle of 62°, which was derived after directly measuring the lever arm of the tether (9). The Bell equation was fit to the koff vs. Ft data by using the exponential curve fitting function in Excel (Microsoft), which returns the best-fit parameter values for k
is the dissociation rate in the absence of applied force, a is the reactive compliance, Fb is the force on the bond, k is Boltzman's constant, and T is the absolute temperature. The term Ft was substituted for Fb, because the tether may represent more than one bond. For CHO cells, the force on the tether was calculated (27) by using a cell diameter of 20 μm and assigning a tether angle of 50°. For neutrophils, the force on the tether was calculated on the basis of a tether angle of 62°, which was derived after directly measuring the lever arm of the tether (9). The Bell equation was fit to the koff vs. Ft data by using the exponential curve fitting function in Excel (Microsoft), which returns the best-fit parameter values for k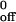 and a. The values from each of the five experiments were then averaged. Statistical significance, assessed by two independent methods (11), was assumed for P < 0.05.
and a. The values from each of the five experiments were then averaged. Statistical significance, assessed by two independent methods (11), was assumed for P < 0.05.
Results
Tethering and Rolling of Transfected Cells Expressing Wild-Type or CD43TMD PSGL-1 on mP-selectin.
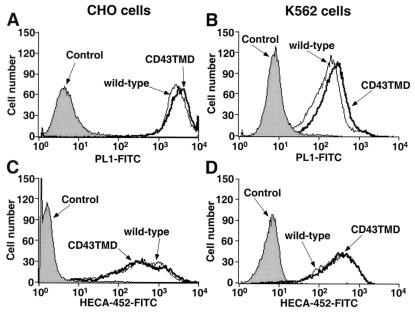
To examine whether dimerization of PSGL-1 affects tethering and rolling of cells on P-selectin in shear flow, we compared cells expressing wild-type dimeric PSGL-1 with cells expressing CD43TMD PSGL-1. CD43TMD PSGL-1 appears to be monomeric on the cell surface, because unlike wild-type PSGL-1, it cannot be chemically crosslinked (22). Transfected CHO cells express a form of PSGL-1 that binds to P-selectin if the cells coexpress 3-fucosyltransferase VII and 6-N-acetylglucosaminyltransferase-l, which permits construction of a required core-2 O-glycan capped with sLex on the N-terminal region of PSGL-1 (23, 28). CHO cell clones expressing matched densities of PSGL-1 were identified by flow cytometry with PL1, a mAb to PSGL-1 (Fig. 1A). Equivalent expression of sLex was confirmed by flow cytometry with HECA-452, a mAb to sLex (Fig. 1C). We also prepared transfected K562 cells expressing matched levels of wild-type or CD43TMD PSGL-1 (Fig. 1B). K562 cells express a form of PSGL-1 that can bind to P-selectin if they are cotransfected with a cDNA for α1,3-fucosyltransferase VII (29). As an alternative method of generating sLex, we incubated transfected K562 cells expressing PSGL-1 with exogenous GDP-fucose and human α1, 3-fucosyltransferase VI to force fucosylation of surface glycoconjugates. Flow cytometry with a mAb to sLex confirmed equivalent fucosylation of K562 cells expressing wild-type or CD43TMD PSGL-1 (Fig. 1D).
Figure 1.
Expression of PSGL-1 and sLex on transfected CHO and K562 cells. Cells were incubated with the anti-PSGL-1 mAb PL1, the anti-sLex mAb HECA-452, or an isotype-matched control mAb. Bound antibody was detected with FITC-conjugated goat-antimouse IgG/IgM.
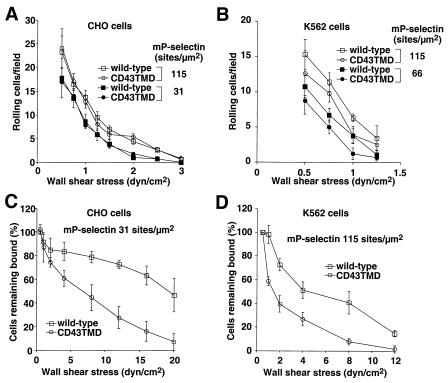
We analyzed rolling of the transfected cells on adsorbed mP-selectin, the membrane form of P-selectin isolated from human platelets. Rolling required a specific interaction of the N-terminal region of PSGL-1 with P-selectin, because it was blocked by the anti-PSGL-1 mAb PL1 or the anti-P-selectin mAb G1 (data not shown). Comparable numbers of CHO cells expressing wild-type or CD43TMD PSGL-1 rolled on mP-selectin at all wall shear stresses and P-selectin densities examined (Fig. 2A and data not shown). Compared with K562 cells expressing wild-type PSGL-1, fewer K562 cells expressing CD43TMD PSGL-1 rolled on mP-selectin, although the differences were not statistically significant when assessed by Student's t test at individual data points (Fig. 2B). To examine the stability of rolling, we allowed cells to accumulate on mP-selectin at 0.5 dyne/cm2 and then subjected the cells to stepwise increases in wall shear stress. Compared with cells expressing wild-type PSGL-1, cells expressing CD43TMD PSGL-1 detached more rapidly in response to increasing wall shear stress (Fig. 2 C and D). For CHO cells, this difference was particularly evident at a relatively low mP-selectin density (31 sites/μm2) (Fig. 2C). The difference was less marked at an mP-selectin density of 66 sites/μm2, and it disappeared at 115 sites/μm2 (data not shown).
Figure 2.
Rolling of cells expressing wild-type or CD43TMD PSGL-1 on mP-selectin. (A and B) CHO cells or K562 cells expressing wild-type or CD43TMD PSGL-1 were perfused at the indicated wall shear stress over mP-selectin at the indicated density. After 4 min, the number of rolling cells was quantified. (C and D) CHO cells or K562 cells expressing wild-type or CD43TMD PSGL-1 were allowed to accumulate on mP-selectin at 0.5 dyne/cm2. Wall shear stress was increased every 30 s, and the percentage of remaining adherent cells was determined. The data represent the mean ± SD of three to six experiments.
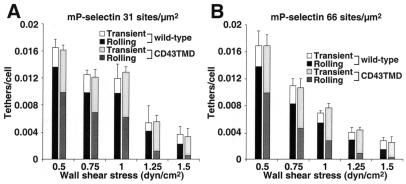
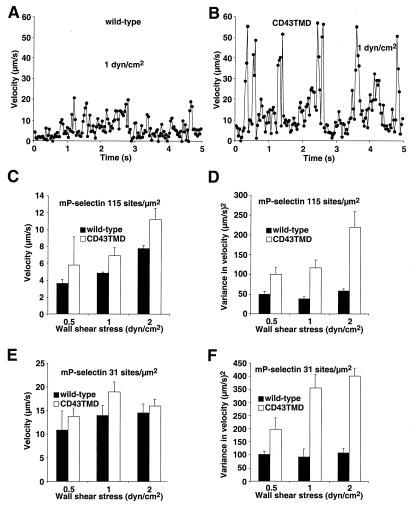
We then examined the initial tethering of cells to mP-selectin (Fig. 3). CHO cells expressing wild-type or CD43TMD PSGL-1 tethered to mP-selectin at equivalent rates, suggesting that both molecules were comparably distributed on the cell surface, probably on microvillous tips (15). However, CHO cells expressing wild-type PSGL-1 converted a higher percentage of the initial tethers into rolling adhesions. As an additional measure of rolling stability, we tracked the displacements of CHO cells expressing wild-type or CD43TMD PSGL-1 on mP-selectin between successive video frames. Each displacement was divided by the time interval of 0.033 s to derive the velocity. Fig. 4 A and B show the velocity at each frame for a representative CHO cell expressing wild-type or CD43TMD PSGL-1 rolling on mP-selectin at 1 dyne/cm2. The cell expressing wild-type PSGL-1 rolled with much smaller fluctuations in velocity than the cell expressing CD43TMD PSGL-1. To quantify the rolling behavior, the frame-by-frame velocity data were used to calculate the mean velocity and the variance in velocity for each cell over a 5-s period. The pooled data from at least 60 cells were used to calculate the mean velocity and variance of velocity for a cell population at various wall shear stresses and mP-selectin densities. Under the conditions examined, the mean rolling velocities of cells expressing wild-type PSGL-1 appeared to be lower than those of cells expressing CD43TMD PSGL-1, although the differences were not statistically significant when assessed by Student's t test at individual data points (Fig. 4 C and E). However, the variance of velocity was significantly lower for cells expressing wild-type PSGL-1 than for cells expressing CD43TMD PSGL-1 (Fig. 4 D and F). This difference was also observed when we compared cells expressing wild-type PSGL-1 at half the density of the cells expressing CD43TMD PSGL-1 (data not shown). The more irregular velocities of cells expressing CD43TMD PSGL-1 suggest that they are less able to balance the rates of forming new bonds with the rates of breaking old bonds with P-selectin. The functional consequences of this defect become more pronounced at lower P-selectin densities or at higher wall shear stresses.
Figure 3.
Rate of tethering of cells expressing wild-type or CD43TMD PSGL-1 on mP-selectin. CHO cells expressing wild-type or CD43TMD PSGL-1 were perfused over mP-selectin at 66 sites/μm2 (A) or 31 sites/μm2 (B). The number of cells that tethered to mP-selectin during the first 60 s was quantified and normalized by dividing by the number of cells delivered across the field of view in the focal plane of the substrate. The percentage of tethers that were transient or that were converted to rolling adhesion is also indicated. The data represent the mean ± SD of three experiments. At each wall shear stress, the difference in percentage of cells that converted to rolling adhesion between wild-type and CD43TMD PSGL-1 was significant at P < 0.05.
Figure 4.
Rolling velocities of cells expressing wild-type or CD43TMD PSGL-1 on mP-selectin. (A and B) Frame-by-frame velocities of representative cells rolling at 1 dyn/cm2. (C–F) Mean velocities and variances of velocities for cell populations rolling on mP-selectin at the indicated densities. The data were derived from three to four experiments. At each shear stress, the difference in variance of velocity between wild-type and CD43TMD PSGL-1 was significant at P < 0.05.
Kinetics of Dissociation and Mechanical Strength of Transient Tethers of Cells Expressing Wild-type or CD43TMD PSGL-1 on mP-selectin or sP-selectin.
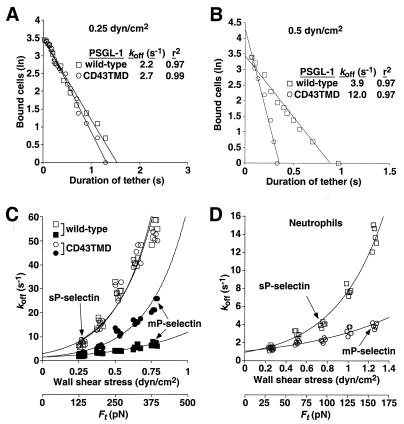
Under flow conditions, a cell expressing PSGL-1 can theoretically tether to immobilized P-selectin through a single selectin–ligand bond. However, PSGL-1 and P-selectin are both dimers, which could promote formation of dimeric bonds. To examine this possibility, we measured the lifetimes of transient tethers of CHO cells expressing wild-type or CD43TMD PSGL-1 on very low densities of mP-selectin, which consists of dimers or oligomers, or on very low densities of recombinant sP-selectin, which is monomeric. We also measured the transient tether lifetimes of human neutrophils, which express native dimeric PSGL-1, on mP-selectin or sP-selectin. All transient tethers were eliminated by addition of anti-PSGL-1 mAb PL1 or anti-P-selectin mAb G1 (data not shown). Fig. 5 A and B show representative data at two different wall shear stresses for cells tethered through wild-type or CD43TMD PSGL-1 to mP-selectin. The tether lifetimes appeared to obey first-order dissociation kinetics. In the examples shown, the apparent dissociation rate, or koff, was similar for tethers formed by wild-type or CD43TMD PSGL-1 at 0.25 dyne/cm2. However, the apparent koff was 3-fold lower for wild-type than for CD43TMD PSGL-1 at 0.5 dyn/cm2.
Figure 5.
Kinetics of dissociation and mechanical strength of transient tethers of cells expressing wild-type or CD43TMD PSGL-1 on very low densities of mP-selectin or sP-selectin. (A and B) Representative pseudofirst-order dissociation kinetics for transient tethers of CHO cells expressing wild-type or CD43TMD PSGL-1 on mP-selectin at the indicated wall shear stress. (C and D) Effect of increasing wall shear stress on tether dissociation rates for transfected CHO cells or neutrophils on mP-selectin or sP-selectin. Each group of points represents a single wall shear stress from each of five independent experiments. The points were displaced horizontally so that all points could be seen. The data were fit to the Bell equation (26).
The durations of transient tethers were measured in five independent experiments for each P-selectin-PSGL-1 interaction over a range of wall shear stresses. The measured tether dissociation rates were plotted against wall shear stress, and the data were fit to the Bell equation, a theoretical relationship between koff and the force on the bond, Fb (26). Because the tether may represent more than one bond, we substituted the term Ft for Fb (Fig. 5 C and D). The fit yielded values for k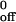 , i.e., the koff in the absence of force and for a, the reactive compliance, which is inversely proportional to the mechanical stability of the tether (Table 1). For CHO cells, the value for k
, i.e., the koff in the absence of force and for a, the reactive compliance, which is inversely proportional to the mechanical stability of the tether (Table 1). For CHO cells, the value for k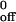 for the interaction of wild-type PSGL-1 with mP-selectin was slightly lower than for the other interactions. For neutrophils, the k
for the interaction of wild-type PSGL-1 with mP-selectin was slightly lower than for the other interactions. For neutrophils, the k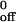 was similar for the interaction with mP-selectin or sP-selectin. In response to increasing wall shear stress, the dissociation rates of tethers on mP-selectin increased significantly more rapidly for CHO cells expressing CD43TMD PSGL-1 than for CHO cells expressing wild-type PSGL-1 (Fig. 5C). This indicates that tethers formed by binding of CD43TMD PSGL-1 to mP-selectin had less mechanical strength than those formed by binding of wild-type PSGL-1 to mP-selectin. The kinetics and mechanical properties of single bonds between mP-selectin and wild-type or CD43TMD PSGL-1 should not differ, because the substitution of the transmembrane domain should not alter the posttranslational modifications in the N-terminal domain of PSGL-1 that are required for binding to P-selectin. Furthermore, fluid-phase sP-selectin binds equivalently to wild-type and CD43TMD PSGL-1 (22). This suggests that transient tethers formed by binding of CD43TMD PSGL-1 to mP-selectin contained fewer bonds than tethers formed by binding of wild-type PSGL-1 to mP-selectin. Remarkably, the dissociation rates of tethers formed by binding of wild-type or CD43TMD PSGL-1 to monomeric sP-selectin increased even faster in response to increasing wall shear stress (Fig. 5C). Neutrophil tether dissociation rates also increased much more rapidly on sP-selectin than on mP-selectin as wall shear stress was increased (Fig. 5D). These differences were reflected in an increase in a, the reactive compliance, for tethers of CHO cells or neutrophils on sP-selectin compared with those on mP-selectin (Table 1). The statistical significance of the observed differences was confirmed by nonlinear regression analysis and by the method of maximum likelihood. The value for Ft is an estimate that requires knowledge of the lever arm of the tether and the angle of the tether with the substrate. Errors in Ft may explain differences in a between CHO cells and neutrophils but will not affect the differences observed for distinct molecular interactions in the same cell type. These data demonstrate that dimerization of PSGL-1 and P-selectin enhances the mechanical strength of transient tethers, most likely by increasing the number of bonds.
was similar for the interaction with mP-selectin or sP-selectin. In response to increasing wall shear stress, the dissociation rates of tethers on mP-selectin increased significantly more rapidly for CHO cells expressing CD43TMD PSGL-1 than for CHO cells expressing wild-type PSGL-1 (Fig. 5C). This indicates that tethers formed by binding of CD43TMD PSGL-1 to mP-selectin had less mechanical strength than those formed by binding of wild-type PSGL-1 to mP-selectin. The kinetics and mechanical properties of single bonds between mP-selectin and wild-type or CD43TMD PSGL-1 should not differ, because the substitution of the transmembrane domain should not alter the posttranslational modifications in the N-terminal domain of PSGL-1 that are required for binding to P-selectin. Furthermore, fluid-phase sP-selectin binds equivalently to wild-type and CD43TMD PSGL-1 (22). This suggests that transient tethers formed by binding of CD43TMD PSGL-1 to mP-selectin contained fewer bonds than tethers formed by binding of wild-type PSGL-1 to mP-selectin. Remarkably, the dissociation rates of tethers formed by binding of wild-type or CD43TMD PSGL-1 to monomeric sP-selectin increased even faster in response to increasing wall shear stress (Fig. 5C). Neutrophil tether dissociation rates also increased much more rapidly on sP-selectin than on mP-selectin as wall shear stress was increased (Fig. 5D). These differences were reflected in an increase in a, the reactive compliance, for tethers of CHO cells or neutrophils on sP-selectin compared with those on mP-selectin (Table 1). The statistical significance of the observed differences was confirmed by nonlinear regression analysis and by the method of maximum likelihood. The value for Ft is an estimate that requires knowledge of the lever arm of the tether and the angle of the tether with the substrate. Errors in Ft may explain differences in a between CHO cells and neutrophils but will not affect the differences observed for distinct molecular interactions in the same cell type. These data demonstrate that dimerization of PSGL-1 and P-selectin enhances the mechanical strength of transient tethers, most likely by increasing the number of bonds.
Table 1.
Dissociation rates and reactive compliance values for P-selectin–PSGL-1 tethers
| PSGL-1 |
k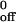 , s−1 , s−1
|
a, Å | |
|---|---|---|---|
| mP-selectin | Wild-type (CHO cells) | 1.2 ± 0.2 | 0.20 ± 0.03 |
| CD43TMD (CHO cells) | 2.0 ± 0.8 | 0.34 ± 0.01 | |
| Wild-type (neutrophils) | 1.0 ± 0.1 | 0.42 ± 0.03 | |
| sP-selectin | Wild-type (CHO cells) | 2.8 ± 0.3 | 0.35 ± 0.01 |
| CD43TMD (CHO cells) | 2.6 ± 0.3 | 0.33 ± 0.01 | |
| Wild-type (neutrophils) | 1.1 ± 0.1 | 0.70 ± 0.05 |
Values for k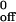 and a were derived from Fig. 5 C and D. The data represent the mean ± SD of five experiments.
and a were derived from Fig. 5 C and D. The data represent the mean ± SD of five experiments.
Discussion
To mediate leukocyte rolling in flow, selectin–ligand bonds are thought to associate and dissociate rapidly and to have high mechanical strength that resists premature dissociation by force. Here we show that dimerization of both PSGL-1 and P-selectin stabilizes rolling by increasing the strength of adhesive tethers. Dimerization probably strengthens tethers by favoring formation of dimeric bonds, even at P-selectin densities that are too low to support rolling. Thus, transient tethers most likely represent more than one bond, even if their lifetimes appear to follow first-order dissociation kinetics and to be independent of selectin or ligand density. This suggests that the more conservative term “tether” should be substituted for the widely used term “tether bond” (6, 8–12). Our data also suggest that the strengths of individual selectin–ligand bonds may be less than those originally estimated by transient tether lifetime analysis.
Under the conditions studied, comparable numbers of CHO cells or K562 cells expressing dimeric wild-type PSGL-1 or monomeric CD43TMD PSGL-1 rolled on P-selectin. However, cells expressing CD43TMD PSGL-1 detached more readily in response to increasing wall shear stress. CHO cells expressing wild-type or CD43TMD PSGL-1 tethered at similar rates, but cells expressing CD43TMD PSGL-1 were less efficient at converting tethers to rolling adhesions, and rolling was much more irregular, as assessed by the higher variance in rolling velocities. The tethering rate is proportional to the rate of forming the initial bond, provided that the bond is sufficiently strong and long-lived to be observed. By comparison, the efficiency of converting tethers to rolling and the stability of rolling depend on whether front tethers form before rear tethers dissociate. Dimerization of PSGL-1 and P-selectin may be most important for stabilizing rolling, particularly at lower P-selectin or PSGL-1 densities or at higher wall shear stresses. During flow, forming the initial bond between a dimeric P-selectin and a dimeric PSGL-1 is likely to have the same low probability as forming the initial bond between two monomeric P-selectin and two monomeric PSGL-1 molecules. This may explain why CHO cells expressing wild-type or CD43TMD PSGL-1 tethered at similar rates. For dimeric molecules, however, formation of the second bond should be greatly favored because of the close proximity of the binding partners. This will distribute the force over both bonds and thus increase the average bond lifetime. If one bond dissociates, there is an opportunity for it to rebind, because the cell remains tethered by the other bond. The net effect is to prolong the lifetime of the initial tether, which allows time for the cell to form new bonds that may themselves be dimeric. This should result in more regular and more shear-resistant rolling adhesion, as we observed.
Analysis of transient tether lifetimes strongly suggests that dimerization of PSGL-1 and P-selectin increases the number of bonds in cell tethers. Transient tethers supported by interactions of wild-type PSGL-1 with mP-selectin showed the least sensitivity to applied force, whereas transient tethers supported by interactions of wild-type or CD43TMD PSGL-1 with sP-selectin showed the greatest sensitivity. Intermediate sensitivity to force was observed for tethers supported by interactions of CD43TMD PSGL-1 with mP-selectin. The precise number of bonds supported by each class of tether is not known. The simplest interpretation is that dimeric, or double, bonds support tethers between wild-type PSGL-1 and mP-selectin, whereas monomeric, or single, bonds support tethers between wild-type or CD43TMD PSGL-1 with sP-selectin. The sparse number of monomeric sP-selectin molecules on the substrate may support only single bonds, even if PSGL-1 on the tethered cell is dimeric. The tethers supported by interactions of CD43TMD PSGL-1 with mP-selectin may represent a mixture of single and double bonds. Although the mP-selectin molecules are sparsely distributed, an individually adsorbed dimer or oligomer might interact with two closely spaced monomeric PSGL-1 molecules, especially at the relatively high levels of PSGL-1 expressed on the CHO cells. Microvillous extension or extrusion of membrane tethers may lower the force applied to the tether (30, 31) but will not affect the conclusion that dimeric molecules form more bonds in cell tethers than monomeric molecules.
It is noteworthy that previous measurements of transient tethers mediated by interactions of P-selectin with PSGL-1 used adsorbed mP-selectin isolated from human platelets, which is dimeric or oligomeric, or P-selectin expressed on transfected cells, which is probably dimeric (6, 11, 12). Thus the tethers analyzed in these studies probably represented at least two bonds, and the reported strength of these “tether bonds” likely overestimated the strength of an individual bond. Furthermore, our results suggest that the transient tethers previously measured for interactions of E-selectin and L-selectin with their respective ligands may not represent single bonds (8–11). That multibond tethers appeared to follow first-order dissociation kinetics, which seemed to be independent of the selectin or ligand density, highlights that these criteria are not sufficient to discriminate adhesion through single or multiple bonds. Without knowing the number of bonds in a tether, kinetic and mechanical properties derived from tether lifetime analysis should not be referred to as intrinsic properties of a single bond, but rather as apparent properties of a single tether, even if the tether behaves as a quantal binding unit. The Bell equation has been proposed to govern the force dependence of dissociation rate of selectin–ligand bonds, on the basis of analyzing lifetimes of neutrophil transient tethers on mP-selectin at different wall shear stresses (12). These tethers, however, probably contained more than one bond, and here we show that the Bell equation can fit tether lifetime vs. wall shear stress data for both mP-selectin and sP-selectin, despite differences in bond number in the tethers. Without knowing the number of bonds in the tethers, an apparent agreement between the data and the Bell equation should not be taken as sufficient proof of the model. Furthermore, the zero-force extrapolation of tether dissociation rates, i.e., the k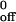 values, should be interpreted with caution, because it cannot be assumed that single or multiple bonds respond uniformly to all applied forces.
values, should be interpreted with caution, because it cannot be assumed that single or multiple bonds respond uniformly to all applied forces.
Do single selectin–ligand bonds have significant mechanical strength? For tethers of wild-type PSGL-1 on sP-selectin, the reactive compliance, which is inversely proportional to the tether strength, is nearly twice that of the tethers on mP-selectin. If the tether on sP-selectin is a single bond, then the strength of the bond is about half that originally proposed. If the tether on sP-selectin represents more than one bond, then the strength of the individual bond will be even lower. Selectin–ligand bonds have been considered to require high mechanical strength to prevent premature dissociation in shear flow (6, 9). Here we show that stable rolling requires high mechanical strength of the tethers; this requirement can be met by dimerization of P-selectin and PSGL-1 in lieu of high mechanical strength of the individual molecular bonds. Artificial crosslinking of L-selectin also increases rolling stability (32), and it is possible that L- and E-selectin or other selectin ligands form dimers or oligomers in the cell membrane. Molecular self-association is likely to function in cooperation with other methods for clustering selectins or selectin ligands. PSGL-1 and L-selectin cluster in microvillous tips (14, 15), probably through linkages with cytoskeletal elements (33), and P-selectin clusters in clathrin-coated pits of endothelial cells (16). The local cell-surface concentrations of selectins and selectin ligands, even in cells where the global densities are not high, may increase bond numbers in tethers and thus increase the lifetimes of the tethers. This may be particularly important for L-selectin, which has a much higher intrinsic rate of dissociation from its ligands (4). The modest mechanical strength of the L-selectin–PSGL-1 interaction measured by dynamic force spectroscopy implies that L-selectin tethers require multibond clusters to prevent premature cell detachment under flow (34).
Acknowledgments
We thank Cindy Carter, Michael McDaniel, and Kelsey Kennedy for technical assistance. This work was supported by National Institutes of Health Grants HL 34363, AI 44902, and AI 48075. V.R. is the recipient of a postdoctoral fellowship from the Heartland Affiliate of the American Heart Association.
Abbreviations
- CHO
Chinese hamster ovary
- PSGL-1
P-selectin glycoprotein ligand-1
- mP-selectin
membrane-derived P-selectin
- sP-selectin
soluble P-selectin
- sLex
sialyl Lewis x
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
See commentary on page 10023.
References
- 1.Vestweber D, Blanks J E. Physiol Rev. 1999;79:181–213. doi: 10.1152/physrev.1999.79.1.181. [DOI] [PubMed] [Google Scholar]
- 2.McEver, R. P. (2001) Thromb. Haemostasis, in press.
- 3.Lawrence M B, Springer T A. Cell. 1991;65:859–873. doi: 10.1016/0092-8674(91)90393-d. [DOI] [PubMed] [Google Scholar]
- 4.Nicholson M W, Barclay A N, Singer M S, Rosen S D, Van der Merwe P A. J Biol Chem. 1998;273:763–770. doi: 10.1074/jbc.273.2.763. [DOI] [PubMed] [Google Scholar]
- 5.Mehta P, Cummings R D, McEver R P. J Biol Chem. 1998;273:32506–32513. doi: 10.1074/jbc.273.49.32506. [DOI] [PubMed] [Google Scholar]
- 6.Alon R, Hammer D A, Springer T A. Nature (London) 1995;374:539–542. doi: 10.1038/374539a0. [DOI] [PubMed] [Google Scholar]
- 7.Chen S Q, Springer T A. J Cell Biol. 1999;144:185–200. doi: 10.1083/jcb.144.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Puri K D, Chen S, Springer T A. Nature (London) 1998;392:930–933. doi: 10.1038/31954. [DOI] [PubMed] [Google Scholar]
- 9.Alon R, Chen S Q, Puri K D, Finger E B, Springer T A. J Cell Biol. 1997;138:1169–1180. doi: 10.1083/jcb.138.5.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alon R, Chen S, Fuhlbrigge R, Puri K D, Springer T A. Proc Natl Acad Sci USA. 1998;95:11631–11636. doi: 10.1073/pnas.95.20.11631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramachandran V, Nollert M U, Qiu H, Liu W, Cummings R D, Zhu C, McEver R P. Proc Natl Acad Sci USA. 1999;96:13771–13776. doi: 10.1073/pnas.96.24.13771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen S Q, Springer T A. Proc Natl Acad Sci USA. 2001;98:950–955. doi: 10.1073/pnas.98.3.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu, C., Long, M. & Bongrand, P. (2001) Ann. Biomed. Eng., in press. [DOI] [PubMed]
- 14.Picker L J, Warnock R A, Burns A R, Doerschuk C M, Berg E L, Butcher E C. Cell. 1991;66:921–933. doi: 10.1016/0092-8674(91)90438-5. [DOI] [PubMed] [Google Scholar]
- 15.Moore K L, Patel K D, Bruehl R E, Fugang L, Johnson D A, Lichenstein H S, Cummings R D, Bainton D F, McEver R P. J Cell Biol. 1995;128:661–671. doi: 10.1083/jcb.128.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Setiadi H, Sedgewick G, Erlandsen S L, McEver R P. J Cell Biol. 1998;142:859–871. doi: 10.1083/jcb.142.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McEver R P, Cummings R D. J Clin Invest. 1997;100:485–492. doi: 10.1172/JCI119556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ushiyama S, Laue T M, Moore K L, Erickson H P, McEver R P. J Biol Chem. 1993;268:15229–15237. [PubMed] [Google Scholar]
- 19.Barkalow F J, Barkalow K L, Mayadas T N. Blood. 2000;96:3070–3077. [PubMed] [Google Scholar]
- 20.Moore K L, Stults N L, Diaz S, Smith D L, Cummings R D, Varki A, McEver R P. J Cell Biol. 1992;118:445–456. doi: 10.1083/jcb.118.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Snapp K R, Craig R, Herron M, Nelson R D, Stoolman L M, Kansas G S. J Cell Biol. 1998;142:263–270. doi: 10.1083/jcb.142.1.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Epperson T K, Patel K D, McEver R P, Cummings R D. J Biol Chem. 2000;275:7839–7853. doi: 10.1074/jbc.275.11.7839. [DOI] [PubMed] [Google Scholar]
- 23.Leppanen A, Mehta P, Ouyang Y-B, Ju T, Helin J, Moore K L, van Die I, Canfield W M, McEver R P, et al. J Biol Chem. 1999;274:24838–24848. doi: 10.1074/jbc.274.35.24838. [DOI] [PubMed] [Google Scholar]
- 24.Picker L J, Kishimoto T K, Smith C W, Warnock R A, Butcher E C. Nature (London) 1991;349:796–799. doi: 10.1038/349796a0. [DOI] [PubMed] [Google Scholar]
- 25.Moore K L, Varki A, McEver R P. J Cell Biol. 1991;112:491–499. doi: 10.1083/jcb.112.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bell G I. Science. 1978;200:618–627. doi: 10.1126/science.347575. [DOI] [PubMed] [Google Scholar]
- 27.Goldman A J, Cox R G, Brenner H. Chem Eng Sci. 1967;22:653–660. [Google Scholar]
- 28.Li F, Wilkins P P, Crawley S, Weinstein J, Cummings R D, McEver R P. J Biol Chem. 1996;271:3255–3264. [PubMed] [Google Scholar]
- 29.Snapp K R, Wagers A J, Craig R, Stoolman L M, Kansas G S. Blood. 1997;89:896–901. [PubMed] [Google Scholar]
- 30.Shao J Y, Ting-Beall H P, Hochmuth R M. Proc Natl Acad Sci USA. 1998;95:6797–6802. doi: 10.1073/pnas.95.12.6797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmidtke D W, Diamond S L. J Cell Biol. 2000;149:719–729. doi: 10.1083/jcb.149.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li X, Steeber D A, Tang M L K, Farrar M A, Perlmutter R M, Tedder T F. J Exp Med. 1998;188:1385–1390. doi: 10.1084/jem.188.7.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kansas G S, Ley K, Munro J M, Tedder T F. J Exp Med. 1993;177:833–838. doi: 10.1084/jem.177.3.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Evans E, Leung A, Hammer D, Simon S. Proc Natl Acad Sci USA. 2001;98:3784–3789. doi: 10.1073/pnas.061324998. . (First Published March 13, 2001; 10.1073/pnas.061324998) [DOI] [PMC free article] [PubMed] [Google Scholar]