SUMMARY
The basement membrane (BM), a sheet of extracellular matrix lining the basal side of epithelia is essential for epithelial cell function and integrity. Yet, the mechanisms that control the basal restriction of BM proteins are poorly understood. In epithelial cells, a specialized pathway is dedicated to restrict the deposition of BM proteins basally. Here we report the identification of a factor in this pathway, a homolog of the mammalian guanine nucleotide exchange factor (GEF) Mss4, that we named Stratum. The loss of Stratum leads to the missecretion of BM proteins at the apical side of the cells, forming aberrant layers in close contact with the plasma membrane. Interestingly, we found that Rab8GTPase acts downstream of Stratum in this process. Together, our results uncover the importance of this GEF/Rab complex in specifically coordinating the basal restriction of BM proteins, a critical process for the establishment and maintenance of epithelial cell polarity.
Keywords: Basement membrane, Cell Polarity, GEF, Mss4, RabIF, Rab8, Drosophila, Oogenesis
eTOC Blurb
The proper placement of the basement membrane at the basal side of epithelial cells is essential for cellular function. Devergne et al. show that Stratum, a homolog of the mammalian GEF Mss4/RabIF, and its downstream effector Rab8GTPase are essential for the basal sorting of BM proteins in polarized epithelial cells.
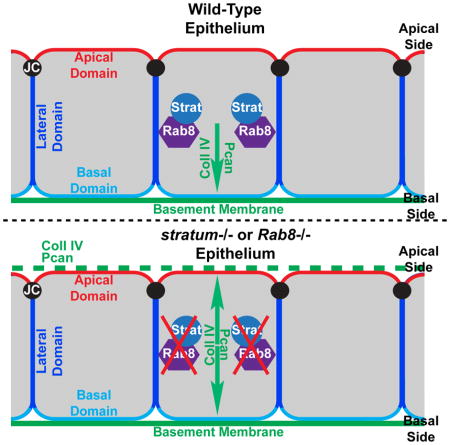
INTRODUCTION
One of the common characteristics of epithelial tissues is the presence of a specialized sheet of extracellular matrix (ECM) at their basal side, called the basement membrane (BM). BMs are cell-adherent extracellular scaffolds composed of proteins such as type IV Collagens (Coll IV), Laminins, and heparan sulfate proteoglycans such as Perlecan (Pcan) (Yurchenco, 2011). BMs interact with the basal side of epithelial cells via cellular receptors such as Integrin and Dystroglycan (Yurchenco, 2011). In addition to providing tissue support, BMs are also essential for embryonic and organ morphogenesis and adult functions (Li et al., 2003; Miner and Yurchenco, 2004; Yurchenco, 2011). Notably, the BM has been shown to act as a signaling platform for the regulation of epithelial polarity. The BM can direct the orientation of the apico-basal axis of epithelial cells, resulting in the formation of a basal domain on the side contacting the BM and an apical domain on the opposite side (O’Brien et al., 2001; Schneider et al., 2006; Yu et al., 2005; Zuk and Matlin, 1996). Importantly, the loss of integrity and misregulation of the BM has been associated with tumor metastasis (Valastyan and Weinberg, 2011). Despite the significance of the BM in both normal and abnormal epithelial cells, the molecular mechanisms ensuring the accurate basal secretion of BM proteins remain largely elusive.
Epithelial cells exhibit a pronounced apico-basal polarity. Polarized intracellular trafficking is a critical process required to establish and maintain epithelial cell polarity by delivering newly synthesized and recycled proteins to their correct destinations (Apodaca et al., 2012; Rodriguez-Boulan et al., 2005). In polarized epithelial cells, a pathway is specifically dedicated to the basal restriction of BM components. It is composed of the GEF Crag (Calmodulin binding protein related to a Rab3 GDP/GTP exchange protein) and its GTPase Rab10, as well as the phosphoinositide PI(4,5)P2 and the protease-like protein Scarface (Denef et al., 2008; Devergne et al., 2014; Lerner et al., 2013; Sorrosal et al., 2010).
To study the mechanisms leading to the basal restriction of BM proteins in polarized epithelial cells, we use the highly polarized follicular epithelium (FE) of the Drosophila melanogaster ovary as a model system. The FE consists of a monolayer epithelium composed of highly polarized cells, called follicle cells (FCs), which surrounds the germline cells (Figure 1A). As is typical of epithelial cells, FCs contain different membrane domains: an apical domain facing the germline, a basolateral domain and junctional domains (Horne-Badovinac and Bilder, 2005). Components of the BM, such as Pcan and Coll IV, are actively secreted basally by FCs during egg chamber maturation (Figure 1A,B), thus establishing the FE as an excellent model for the basal restriction of BM proteins in epithelial cells.
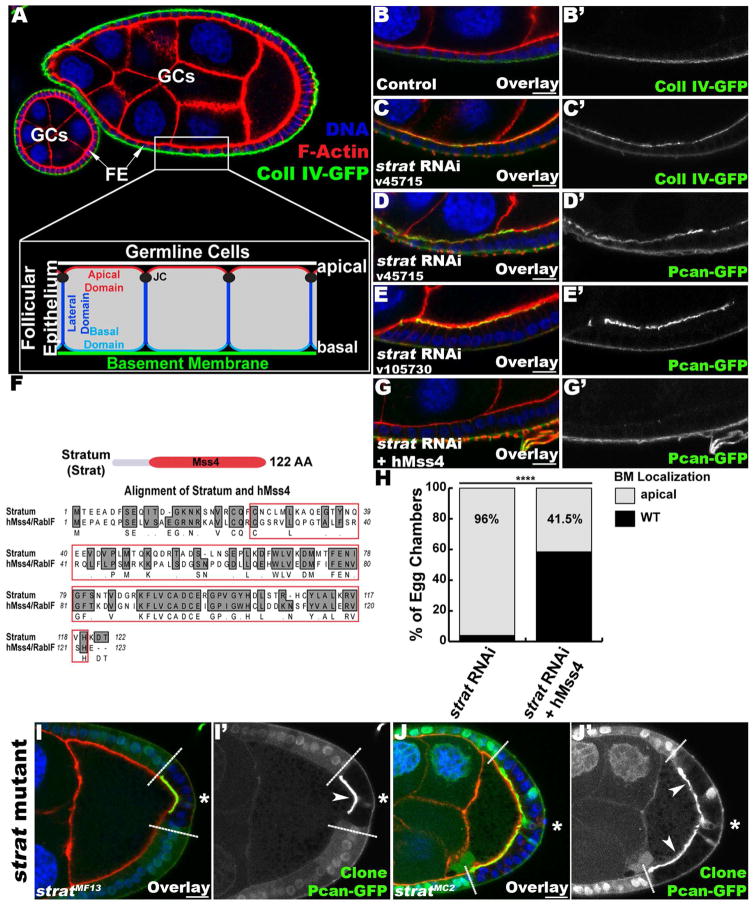
Figure 1. Stratum controls the basal secretion of BM proteins in polarized epithelial cells.
(A) Overview of an egg chamber and schematic representation of the follicular epithelium (FE) polarity domains and germline cells (GCs). Longitudinal (Lg) section through a wildtype egg chamber expressing Coll IV-GFP (green), stained for F-Actin (red) and DNA (blue). (B–E) Lg-sections through follicle cell (FC) layers expressing Coll IV-GFP (green, B, C) or Pcan-GFP (green, D, E), stained for F-Actin (red) and DNA (blue). (B) Control FE. (C–E) FE expressing two different RNAi constructs against strat (CG7787) with traffic jam-Gal4 (tj-Gal4). In strat knockdown FCs, Coll IV-GFP (C′) and Pcan-GFP (D′, E′) accumulate apically. (F) Strat protein schematic and amino acid sequence alignment between Drosophila Strat and human Mss4 (hMss4/RabIF). Strat protein contains an Mss4 domain (red). (G–H) Stratum is the Drosophila homolog of human Mss4. (G) Lg-sections through FC layers expressing Pcan-GFP IV, stained for F-Actin (red) and DNA (blue). FE expressing an RNAi construct against strat (CG7787) and hMss4 with traffic jam-Gal4 (tj-Gal4). (H) The expression of hMss4 in strat-knocked down FCs leads to a decrease in the number of egg chambers exhibiting apical mislocalization of BM proteins, indicating a partial rescue of the phenotype observed in strat RNAi FCs. n≥100; ****, p<0.0001. (I–J) Lg-section through egg chambers containing strat mutant (I, stratMF13; J, stratMC2) FC clones expressing Pcan-GFP (green) and stained for F-Actin (red) and DNA (blue). strat mutant FCs are marked by the absence of intracellular GFP (green, dashed lines indicate clonal boundaries; asterisks (*) specify homozygous mutant FCs). In strat mutant cells, the polarized distribution of BM is disrupted, as revealed by the strong accumulation of Pcan-GFP at the apical side of FCs (arrowheads, I′ and J′). Bars, 10 μm.
Using this model system, we identified a GEF/RabGTPase complex, composed of the GEF Stratum (Strat) and the Rab8GTPase, which controls the basal restriction of BM proteins in polarized epithelial cells. The loss of one of these partners leads to the apical mislocalization of BM components. While Rab8GTPase has a diffuse cytoplasmic localization In the FE, Strat is basally enriched, suggesting that Strat restricts Rab8GTPase activation basally, leading to basal secretion of BM proteins. In addition, we show that other factors involved in polarized BM deposition, including PI(4,5)P2 and Crag, control intracellular levels of Strat.
RESULTS AND DISCUSSION
The putative GEF Stratum, a homolog of mammalian Mss4/RabIF, is critical for the polarized deposition of basement membrane in epithelial cells
The GEF Crag and its RabGTPase partner Rab10 play critical roles in directing the basal secretion of BM proteins in polarized epithelial cells (Denef et al., 2008; Lerner et al., 2013). In order to identify new factors that control the polarized intracellular trafficking and secretion of BM proteins, we utilized a Drosophila protein interaction map (DPiM) to find Rab10 interacting partners (Guruharsha et al., 2011). One strong interactor was a putative GEF encoded by the gene CG7787. Since GEFs are critical for the control of intracellular trafficking, we set out to study its role in BM secretion.
In order to test the involvement of CG7787 in BM polarity, we knocked down the expression of CG7787 in FCs by RNAi (Figure 1C–E). We monitored the distribution of BM proteins using the GFP-protein trap lines Pcan-GFP (Morin et al., 2001) and Coll IV-GFP (Buszczak et al., 2007) that reflect the endogenous localization of these proteins (Denef et al., 2008; Devergne et al., 2014). CG7787-depleted epithelial cells present an accumulation of Pcan and Coll IV on both their basal and apical surfaces, indicating that CG7787 is required for polarized BM deposition (Figure 1C, D and E compare to B). Since BM proteins accumulate in an apical sheet in CG7787-depleted cells, we named the gene stratum (strat).
strat encodes a GEF, based on predicted conserved protein domains, that belongs to the MSS4 family of proteins (Figure 1F). In particular, mammalian Mss4 (mammalian suppressor of yeast Sec4), also called RABIF (Rab Interacting Factor), has been shown to interact with Rabs belonging to the same subfamily, including Rab1, Rab3, Rab8 and Rab10, all of which are involved in secretion (Burton et al., 1994). In order to assess whether Strat is the Drosophila homolog of mammalian Mss4/RabIF, we expressed human Mss4/RabIF (hMss4) in strat-knocked down FCs. hMss4 expression partially rescues the defects associated with the loss of Strat (Figure 1G, H), indicating that Strat is the functional homolog of human Mss4/RabIF.
To confirm the phenotype observed in strat RNAi expressing FCs, we generated four strat mutant lines by ethylmethane sulfonate (EMS) mutagenesis (Figure S1, Experimental Procedures). We then generated homozygous mutant FC clones using the Flp/FRT system (Experimental Procedure). In strat mutant FCs, Pcan accumulates apically (Figure 1I, J). Expression of a full-length strat transgene rescued this mislocalization phenotype, indicating that we indeed generated strat mutant alleles (Figure S1C, D). Overall, this data confirms Stratum as an essential factor for the basal restriction of BM proteins in epithelial cells. Moreover, quantification of the BM mislocalization phenotype observed in strat mutant clones indicates that the apical accumulation of BM components progressively increases during egg chamber maturation (Figure S1B).
However, we found that Strat does not globally control the apico-basal polarity of FCs. The polarized distribution of other classes of proteins that undergo polarized intracellular trafficking localize normally (Figure S2), indicating that Strat is a member of a pathway specifically dedicated to the polarized sorting of BM proteins in epithelial cells.
In FCs depleted of Stratum, BM proteins are secreted apically and interact closely with the epithelial plasma membrane
A better understanding of this biological pathway requires a careful analysis of its different members. To do so, we decided to better characterize the apical mislocalization of BM components observed in strat mutant FCs. We used super resolution three-dimensional structured illumination microscopy (3D-SIM) and expressed the plasma membrane marker mCD8-RFP exclusively in the FE to observe the distribution of Coll IV-GFP in relation to mCD8-RFP (Figure 2). 3D-SIM has a resolution of 120 nm in xy axes, allowing us to precisely determine the distribution of BM proteins with respect to the FE plasma membrane. We quantified the spatial distribution and levels of these components by measuring fluorescence intensity with an optical section through FCs. The basal and apical membranes can be visualized by the two most extreme red mCD8-RFP peaks (Figure 2 marked by *, red). As expected in WT FCs, only one peak of Coll IV (CollIV-GFP, green) was observed on the basal side and it co-localizes tightly with the basal plasma membrane of the cells; additionally, no apical Coll IV peak was observed (Figure 2A).
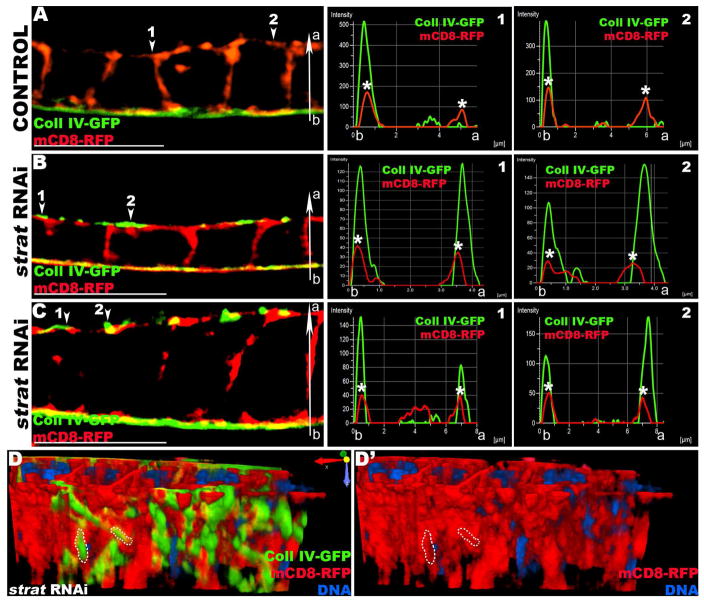
Figure 2. Stratum depleted FCs secrete BM proteins apically.
(A–C) 3D-SIM reconstructions and micrographs of Lg-sections through a control (A) or strat RNAi (B, C) FE expressing Coll IV-GFP (green) and the plasma membrane marker mCD8-RFP (red) exclusively in the FCs. The distributions of green (Coll IV-GFP) and red (mCD8-RFP) pixels along the basal-apical axis (b-a) at different positions of the epithelium (arrowheads) are plotted in histograms to the right of the corresponding micrograph. The x-axis represents the distance along the b-a axis (μm); the y-axis represents arbitrary pixel intensity. The basal and apical plasma membranes are marked with asterisks (*). (A) In control FE, Coll IV-GFP is only secreted at the basal side of FCs and its tightly associated with the basal plasma membrane. Histograms show the distribution of pixels along the b-a axis (red and green pixel peaks overlap). (B–C) In strat RNAi cuboidal (B) or columnar (C) FCs, Coll IV accumulates on both apical and basal sides. At the apical side, Coll IV and the plasma membrane are closely associated, although distinct layers (arrowheads). Histograms, showing the distribution of pixels along the b-a axis, reveal that Coll IV is tightly associated with both basal and apical plasma membranes (red and green peaks overlap on both sides) and accumulates apically outside of cells (green peaks detected apically to red peaks). (D) 3D reconstruction of a strat RNAi FE (same as C). View facing the apical side of the FE. Orientation reference is shown. Coll IV covers the apical FE plasma membrane, indicating that Coll IV is secreted apically outside of the cell (see dotted lines). Bars, 10 μm.
In contrast, in strat-knocked down FCs (Figure 2B, C), an additional Coll IV peak was observed at the apical membrane that is also tightly associated with the plasma membrane. Moreover, the Coll IV peak is apical to the apical plasma membrane peak, indicating that Coll IV is also found outside of the apical plasma membrane. Thus, the loss of Strat leads to the apical secretion of BM proteins. These data, confirmed by 3D reconstruction (Figure 2D), suggest that the putative GEF Strat is not required for secretion per se, but for the directionality of secretion. The same observation was made in Crag-knocked down FCs, suggesting that Crag and Strat are both involved in directionality of secretion (Figure S3).
3D-SIM imaging also revealed that the apical deposition of Coll IV is different from its basal deposition. The pixel distribution shows that the “sheet” of Coll IV is thicker apically (between 300 and 600 nM) than basally (less than 100 nM, below the resolution of SIM). The apical FC membrane contains microvilli and is therefore topologically thicker than the basal membrane. Moreover, it is unknown whether the mechanisms needed to establish a properly assembled BM are present apically. Altogether, these data suggest that the loss of Strat leads to the apical secretion of Coll IV, which associates with the apical plasma membrane with an aberrant organization.
Stratum is a putative GEF for Rab8 during polarized BM deposition
Next, we set out to identify the RabGTPase(s) that function(s) with Stratum in polarized BM deposition. Since Rab10 is involved in polarized BM secretion in epithelial cells, we first tested Rab10 as a potential Strat interactor.
To access whether Rab10 functions downstream of Stratum, we used a constitutively active form of Rab10 (Rab10CA) (Zhang et al., 2007). Constitutively active forms of RabGTPases remain bound to GTP, and thus, do not require a GEF for activation. If Strat functions as a GEF for Rab10 in polarized BM deposition, the expression of Rab10CA (YFP-Rab10CA) may rescue the apical mislocalization of BM proteins observed in strat-deficient cells. Surprisingly, the expression of Rab10CA in strat-knocked FCs did not rescue the BM mislocalization phenotype (Figure 3B, compare to 3A, and 3F). Although a negative result is difficult to interpret, this data might suggest one of the following: (1) Strat is not a GEF for Rab10 during polarized BM deposition; (2) Strat functions as a GEF for (an)other Rab(s), which also control(s) this process; or (3) the expression of Rab10CA is not strong enough to suppress the strat phenotype. Since we are able to detect the expression of YFP-tagged Rab10CA in the FE, the latter hypothesis seems unlikely.
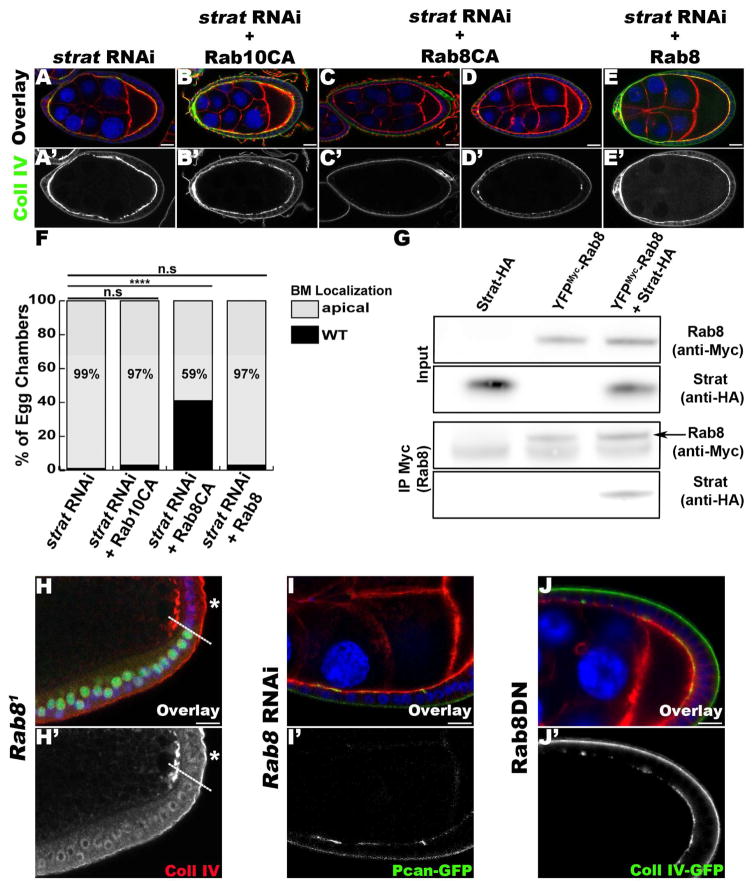
Figure 3. Rab8 acts downstream of Stratum in polarized BM deposition.
(A–E) Lg-sections through egg chambers expressing Coll IV-GFP (green), stained for F-Actin (red) and DNA (blue). The FEs of egg chambers express strat RNAi (A), strat RNAi and Rab10CA (B), strat RNAi and Rab8CA (C–D) or strat RNAi and Rab8 (E) using tj-Gal4. (F) Quantification of BM mislocalization observed in A–D (n≥76; ****, p<0.0001; n.s., non-significant). (G) YFPMYC-Rab8 interacts with Strat-HA in ovary extracts. (H) Lg-section through egg chambers containing strat mutant FC clones (dashed line indicates clonal boundary; asterisks (*) specify homozygous mutant FCs) and stained for α1-Collagen IV (red) and DNA (blue). (I–J) Lg-sections through FE expressing Pcan-GFP (I, green) or Coll IV-GFP (J, green), stained for F-Actin (red) and DNA (blue) and expressing Rab8 RNAi (I) or Rab8DN (J). Bars, 10 μm.
To determine if Strat interacts with other RabGTPases during BM polarity, we turned to Rab8. In Drosophila, Rab8 and Rab10 are paralogs, sharing an amino acid sequence identity of 67%. In addition, mammalian Mss4/RabIF can act as a GEF for Rab8a (Itzen et al., 2006; Wixler et al., 2011). First, we examined the effects of Rab8 on BM proteins. In FCs mutant for Rab8 (Figure 3H), knocked down for Rab8 (Figure 3I), or expressing a dominant negative form of Rab8 (Rab8DN, Figure 3J), we observed mislocalization of Pcan or Coll IV, indicating that Rab8 is also involved in polarized BM secretion in epithelial cells. To assess if Rab8 acts downstream of Strat, we expressed a constitutively active (CA) form of Rab8 (YFP-Rab8CA) in strat knockdown FCs (Zhang et al., 2007). This resulted in a partial rescue of the phenotype associated with the loss of strat, suggesting that Rab8 acts downstream of Strat in the process of polarized BM deposition (Figure 3C, D and F). In addition, the expression of wildtype full length Rab8 did not rescue the phenotype associated with the loss of Strat, suggesting that Strat activates the GTPase activity of Rab8 (Figure 3E, F).
Finally, to determine whether Strat and Rab8 interact physically, we performed co-IP of tagged Rab8 (YFPMYC-Rab8) (Dunst et al., 2015) and Stratum (Strat-HA) and found that they interact in ovary extracts (Figure 3G). Altogether, these results suggest that Strat acts as a GEF for Rab8 during the basal restriction of BM deposition. This conclusion is supported by the data that Rab8a interacts with Mss4 in mammalian cells and has weak GEF activity for Rab8a in vitro (Itzen et al., 2006; Wixler et al., 2011). We also showed that Strat phenotype is rescued by hMss4, suggesting that Strat and hMss4 share similar activities. Thus, we identified another GEF/Rab complex, Strat/Rab8, in addition to Crag/Rab10, involved in BM deposition in epithelial cells.
The epithelial distribution of Stratum suggests it is required to restrict Rab8 activity basally
Three different non-exclusive mechanisms have been proposed to explain the basal secretion of BM proteins: (1) BM-containing vesicles are directly targeted to the basal side of polarized cells; (2) BM-containing vesicles are blocked apically; and (3) BM proteins are secreted on both sides of epithelial cells, but are degraded or endocytosed apically. The intracellular localization of components involved in this process, such as Stratum and Rab8, may provide insight into how these factors restrict BM proteins basally. First, we assessed the subcellular localization of Rab8 using endogenously tagged YFP-Myc-tagged-Rab8 (YFPMyc-Rab8) (Figure 4A). In the FE, YFPMyc-Rab8 is detected diffusely throughout the cytoplasm and is non-polarized during early and mid-stages of oogenesis (Figure 4A″). YFPMyc-Rab8 accumulates in intracellular puncta, which may represent endosomes and/or vesicles (Figure 4A″). More specifically, Rab8 partially co-localizes with early and recycling endosome and Golgi markers (Figure S4). This subcellular localization is consistent with the known role of Rab8 in regulating vesicular transport from the Golgi to the plasma membrane (Wandinger-Ness and Zerial, 2014). YFPMyc-Rab8 becomes slightly enriched at the basal side of the FCs starting in stages 9 to 10. In contrast to Rab8, Strat (Strat-HA) has a diffuse intracellular localization earlier in oogenesis but quickly assumes a pronounced polarized distribution, accumulating basally in FCs (Figure 4A″, A‴). This observation suggests that Strat restricts the activity of Rab8 basally to allow proper basal deposition of BM proteins. Alternatively, since mammalian Mss4 protein has been shown to have only weak GTPase activity compared to other GEFs, Mss4 may act as a chaperone, allowing interacting Rab proteins to be properly activated where and when they are needed in the cell (Itzen et al., 2006; Wixler et al., 2011). Therefore, Strat may play one or both of these roles to restrict Rab8 activity basally and thus direct BM protein-containing vesicles towards the basal side of the cell.
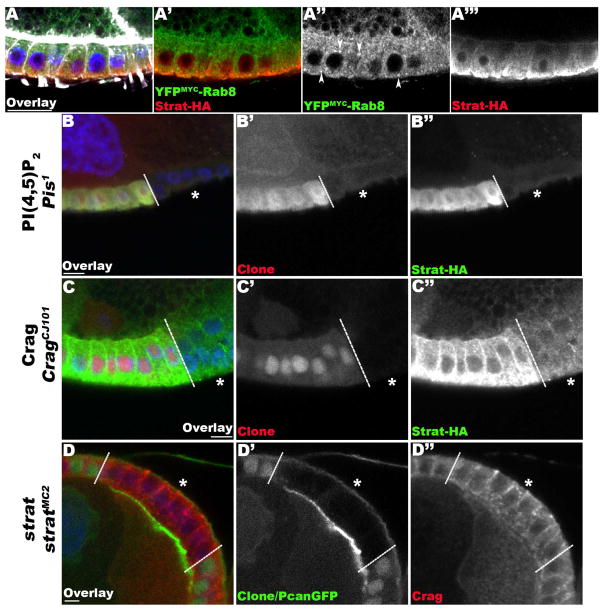
Figure 4. Stratum acts in a pathway dedicated to the basal restriction of BM proteins.
(A) Lg-section through egg chambers expressing YFPMYC-Rab8 (green) and Strat-HA (red), stained for F-Actin (white) and DNA (blue). Confocal Enhanced Resolution (ER) micrographs reveal that Rab8 has a diffuse cytoplasmic localization and accumulates in intracellular structures (arrowheads; A′, A″). Conversely, Strat-HA is basally distributed (A′, A‴). (B–C) Lg-section through the FE of an egg chamber containing Pis1 (B) and CragCJ101 (C) mutant clones, marked by the absence of intracellular RFP, expressing Strat-HA (green), and stained for DNA (blue). (D) Lg-section through the FE of an egg chamber containing a stratMC2 mutant clone, marked by the absence of intracellular GFP, expressing Pcan-GFP (green) and stained for DNA (blue). Dashed lines indicate clonal boundaries; asterisks (*) specify homozygous mutant FCs. In Pis and Crag mutant FCs, Strat-HA levels are overall reduced (B″, C″). However, the distribution of Crag is not significantly affected in strat mutant FCs (D″). Bars, 10 μm.
The polarized localization of Strat differs from Crag, which accumulates at apical and lateral membranes, suggesting that Crag blocks the apical secretion of BM proteins. Both Crag and Stratum GEFs have critical roles to restrict BM proteins basally; however, they are structurally, and perhaps functionally, very different. Crag is a 187 kDa multidomain protein composed of 3 DENN domains with GEF activity, a Calmodulin binding domain, and a conserved C-terminal domain. In contrast, Stratum is 14 kDa and composed of a single Mss4 domain with weak GEF activity. In view of these structural differences, it is unlikely that Crag and Stratum have the same interactors, regulators and effectors. In addition, the strikingly different localization of these factors also suggests independent roles in this process, as Crag is localized to lateral and apical membranes and Stratum to the basal side of cells. Yet together, these proteins allow the specific basal restriction of BM components in epithelial cells.
PI(4,5)P2 and Crag control Strat levels in FCs
Recently, we showed that the proper intracellular distribution of Crag is dependent on the phosphoinositide PI(4,5)P2. A decrease in PI(4,5)P2 levels leads to a loss of Crag apico-basal distribution and the mislocalization of BM proteins (Devergne et al., 2014). To assess the role of other members of the pathway, such as PI(4,5)P2 and Crag on Strat localization, we determined the distribution of Strat-HA in Pis and Crag mutant FCs (Figure 4B, C). As we previously observed for Crag, a decrease in PI(4,5)P2 levels in Pis mutant FCs, leads to reduced levels of Strat (Figure 4B″). This phenotype is observed in 49% of mutant clones (n=35). The same decrease of Strat can also be observed in Crag mutant FCs (in 47% of mutant clones, n=71, Figure 4C″). These data suggest that both PIP2 levels and Crag control the levels and distribution of Strat. Since we previously showed that PIP2 controls Crag localization, the decrease of Strat observed in Pis mutant FCs might be due to the loss of Crag. However, the distribution and levels of Crag are not significantly affected in strat mutant FCs (Figure 4D″). Overall, the loss of Strat observed in the mutant backgrounds highlights the existence of a regulatory mechanism between the two GEF/Rab complexes dedicated to the polarized secretion of BM proteins and should be further investigated in the future.
In conclusion, we identified Strat, the homolog of mammalian GEF Mss4/RabIF, and Rab8GTPase as essential regulators in the basal sorting of BM proteins in polarized epithelial cells. This GEF/Rab complex partners to correctly deliver BM protein-containing vesicles basally, an essential process for epithelial cell function. Our previous work identified an apical complex involved in this process containing Crag/Rab10 and depending on PIP2. Here we found a more basally localized complex, consisting of Strat and Rab8 also required for the exclusive basal localization of BM proteins. Interestingly, we found that these complexes do not function redundantly, but both complexes are required independently. Recently, a third complex involving Rab10 and Ehbp1 has been described to deliver BM proteins to the baso-lateral side of the follicle cells in a late differentiation process involved in egg chamber elongation (Isabella and Horne-Badovinac, 2016), These findings reveal that the proper positioning of BM proteins is handled by the cell in more complex regulatory pathways than was previously realized.
EXPERIMENTAL PROCEDURES
Fly Stocks and Genetics
Details on the generation of strat mutant alleles and transgenic lines (UASp-Strat, UASp-Strat-HA and UASp-hMss4) can be found in Supplemental Experimental Procedures. The following Drosophila stocks were also used: CragCJ101 (Denef et al., 2008); Pis1 (Wang and Montell, 2006); Rab81 (Giagtzoglou et al., 2012); UASp-YFP-Rab8, UASp-YFP-Rab8T22N (Rab8DN), UASp-YFP-Rab8Q67L (Rab8CA), and UASp-YFP-Rab10Q68L (Rab10CA) (Zhang et al., 2007); YFPMYC-Rab8 (Dunst et al., 2015);. UAS-RNAi-Crag (TRIP line HMS00241); UAS-dicer2 and UAS-mCD8-RFP (Bloomington Stock Center); tj-Gal4 (a gift from D. Bilder); GR1-Gal4 (Goentoro et al., 2006). The RNAi lines for strat (v45715, v105730) and Rab8 (v28092) were from the Vienna Drosophila RNAi Center. The protein trap lines Vkg-GFP (CC00791) (Buszczak et al., 2007) and Pcan-GFP (ZCL1700) (Morin et al., 2001) were obtained from Flytrap. Follicle cell clones for Strat, Rab8, Crag and Pis were induced using the UAS-Flipase/FRT method (Harrison and Perrimon, 1993).
Immunofluorescence Stainings
Egg chambers were immunostained as described previously (Ghiglione et al., 2002) and mounted in Aqua-Poly/Mount (Polysciences). The following primary antibodies were used: rat anti-HA (3F10, 1:100, Roche), rabbit anti-GFP (AB3080P, 1:500, Millipore), mouse anti-cMyc (9E10, 1:10, DHSB), rat anti-Crag (1:200, see Supplemental Experimental Procedures), guinea pig anti-Coll IV α1-chain (Cgc25c, 1:500, (Shahab et al., 2015)). Secondary antibodies used were Alexa Fluor 488-, 568-, or 647-conjugated (1:400, Molecular Probes). Alexa Fluor 546 Phalloidin (1:500) and Alexa Fluor 647 Phalloidin (1:100, Molecular Probes) were used to stain F-Actin. DNA was stained using Hoechst (10μg/mL, Molecular Probes).
Microscopy Imaging
Confocal microscopy was performed using a Nikon A1 laser scanning confocal microscope. In Figures 4A and S4, to enhance spatial resolution, we used the Nikon enhanced resolution (ER) module (Nikon A1 ER using a 60X 1.4NA oil objective). Raw data for ER were processed using the Richardson-Lucy deconvolution algorithm of Nikon Elements software. Super resolution microscopy (3D-SIM) was performed using a Nikon N-SIM. All data for 3D-SIM were captured used a Nikon CFI Apo TIRF 100X 1.49NA oil objective. 3D-SIM image stacks were sectioned using a 200 nm Z-step size. Raw data for 3D-SIM were reconstructed and analyzed using Nikon Elements software.
Statistical Tests
Statistical significance of differences between mutant and WT localization of BM proteins (Figures 1H and 3F) were determined by the Chi square test.
Co-Immunoprecipitation
Co-IP was performed using ovary lysates expressing endogenously-tagged YFPMYC-Rab8 and/or Strat-HA using the UAS/Gal4 system. Additional details can be found in Supplemental Experimental Procedures.
Supplementary Material
Highlights.
Stratum (Strat) controls the basal restriction of basement membrane (BM) proteins
Loss of Strat leads to the apical missecretion of BM components
Rab8 acts downstream of Strat to deliver BM proteins to the basal side of the cell
Strat/Rab8 act in a pathway dedicated to the basal restriction of BM proteins
Acknowledgments
We are grateful to the Developmental Studies Hybridoma Bank, Vienna Drosophila RNAi Center, Flytrap, M. J. Ringuette and the Bloomington Stock Center for providing flies and antibodies; Gary Laevsky for excellent support with confocal and SIM microscopy; Megan Gladwin and members of the Schüpbach and Wieschaus labs for feedback and advice. We also thank Julie Merkle and Shelby Blythe for helpful comments on manuscript. This work was supported by the Howard Hughes Medical Institute and by NIH Grant R01 GM077620.
Footnotes
AUTHORS CONTRIBUTIONS
OD and TS conceived and designed the experiments and analyzed the data. OD, TS and GHS performed the experiments. OD and TS wrote the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Apodaca G, Gallo LI, Bryant DM. Role of membrane traffic in the generation of epithelial cell asymmetry. Nat Cell Biol. 2012;14:1235–1243. doi: 10.1038/ncb2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton JL, Burns ME, Gatti E, Augustine GJ, De Camilli P. Specific interactions of Mss4 with members of the Rab GTPase subfamily. EMBO J. 1994;13:5547–5558. doi: 10.1002/j.1460-2075.1994.tb06892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buszczak M, Paterno S, Lighthouse D, Bachman J, Planck J, Owen S, Skora AD, Nystul TG, Ohlstein B, Allen A, et al. The carnegie protein trap library: a versatile tool for Drosophila developmental studies. Genetics. 2007;175:1505–1531. doi: 10.1534/genetics.106.065961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denef N, Chen Y, Weeks SD, Barcelo G, Schüpbach T. Crag regulates epithelial architecture and polarized deposition of basement membrane proteins in Drosophila. Dev Cell. 2008;14:354–364. doi: 10.1016/j.devcel.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devergne O, Tsung K, Barcelo G, Schüpbach T. Polarized deposition of basement membrane proteins depends on Phosphatidylinositol synthase and the levels of Phosphatidylinositol 4,5-bisphosphate. Proc Natl Acad Sci U S A. 2014;111:7689–7694. doi: 10.1073/pnas.1407351111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunst S, Kazimiers T, von Zadow F, Jambor H, Sagner A, Brankatschk B, Mahmoud A, Spannl S, Tomancak P, Eaton S, et al. Endogenously Tagged Rab Proteins: A Resource to Study Membrane Trafficking in Drosophila. Dev Cell. 2015;33:351–365. doi: 10.1016/j.devcel.2015.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiglione C, Devergne O, Georgenthum E, Carballes F, Medioni C, Cerezo D, Noselli S, Carballès F, Médioni C, Cerezo D, et al. The Drosophila cytokine receptor Domeless controls border cell migration and epithelial polarization during oogenesis. Development. 2002;129:5437–5447. doi: 10.1242/dev.00116. [DOI] [PubMed] [Google Scholar]
- Giagtzoglou N, Yamamoto S, Zitserman D, Graves HK, Schulze KL, Wang H, Klein H, Roegiers F, Bellen HJ. dEHBP1 controls exocytosis and recycling of Delta during asymmetric divisions. J Cell Biol. 2012;196:65–83. doi: 10.1083/jcb.201106088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goentoro LA, Reeves GT, Kowal CP, Martinelli L, Schupbach T, Shvartsman SY. Quantifying the Gurken morphogen gradient in Drosophila oogenesis. Dev Cell. 2006;11:263–272. doi: 10.1016/j.devcel.2006.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guruharsha KG, Rual JF, Zhai B, Mintseris J, Vaidya P, Vaidya N, Beekman C, Wong C, Rhee DY, Cenaj O, et al. A Protein Complex Network of Drosophila melanogaster. Cell. 2011;147:690–703. doi: 10.1016/j.cell.2011.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison DA, Perrimon N. Simple and efficient generation of marked clones in Drosophila. Curr Biol. 1993;3:424–433. doi: 10.1016/0960-9822(93)90349-s. [DOI] [PubMed] [Google Scholar]
- Horne-Badovinac S, Bilder D. Mass transit: epithelial morphogenesis in the Drosophila egg chamber. Dev Dyn. 2005;232:559–574. doi: 10.1002/dvdy.20286. [DOI] [PubMed] [Google Scholar]
- Isabella AJ, Horne-Badovinac S. Rab10-Mediated Secretion Synergizes with Tissue Movement to Build a Polarized Basement Membrane Architecture for Organ Morphogenesis. Dev Cell. 2016;38:47–60. doi: 10.1016/j.devcel.2016.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itzen A, Pylypenko O, Goody RS, Alexandrov K, Rak A. Nucleotide exchange via local protein unfolding--structure of Rab8 in complex with MSS4. EMBO J. 2006;25:1445–1455. doi: 10.1038/sj.emboj.7601044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner DW, McCoy D, Isabella AJ, Mahowald AP, Gerlach GF, Chaudhry Ta, Horne-Badovinac S. A Rab10-Dependent Mechanism for Polarized Basement Membrane Secretion during Organ Morphogenesis. Dev Cell. 2013;24:159–168. doi: 10.1016/j.devcel.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Edgar D, Fassler R, Wadsworth W, Yurchenco PD. The role of laminin in embryonic cell polarization and tissue organization. Dev Cell. 2003;4:613–624. doi: 10.1016/s1534-5807(03)00128-x. [DOI] [PubMed] [Google Scholar]
- Miner JH, Yurchenco PD. Laminin functions in tissue morphogenesis. Annu Rev Cell Dev Biol. 2004;20:255–284. doi: 10.1146/annurev.cellbio.20.010403.094555. [DOI] [PubMed] [Google Scholar]
- Morin X, Daneman R, Zavortink M, Chia W. A protein trap strategy to detect GFP-tagged proteins expressed from their endogenous loci in Drosophila. Proc Natl Acad Sci U S A. 2001;98:15050–15055. doi: 10.1073/pnas.261408198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien LE, Jou TS, Pollack aL, Zhang Q, Hansen SH, Yurchenco P, Mostov KE. Rac1 orientates epithelial apical polarity through effects on basolateral laminin assembly. Nat Cell Biol. 2001;3:831–838. doi: 10.1038/ncb0901-831. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Boulan E, Kreitzer G, Musch A. Organization of vesicular trafficking in epithelia. Nat Rev Mol Cell Biol. 2005;6:233–247. doi: 10.1038/nrm1593. [DOI] [PubMed] [Google Scholar]
- Schneider M, Khalil AA, Poulton J, Castillejo-Lopez C, Egger-Adam D, Wodarz A, Deng WM, Baumgartner S. Perlecan and Dystroglycan act at the basal side of the Drosophila follicular epithelium to maintain epithelial organization. Development. 2006;133:3805–3815. doi: 10.1242/dev.02549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahab J, Baratta C, Scuric B, Godt D, Venken KJT, Ringuette MJ. Loss of SPARC dysregulates basal lamina assembly to disrupt larval fat body homeostasis in Drosophila melanogaster. Dev Dyn. 2015;244:540–552. doi: 10.1002/dvdy.24243. [DOI] [PubMed] [Google Scholar]
- Sorrosal G, Pérez L, Herranz H, Milán M. Scarface, a secreted serine protease-like protein, regulates polarized localization of laminin A at the basement membrane of the Drosophila embryo. EMBO Rep. 2010;11:373–379. doi: 10.1038/embor.2010.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valastyan S, Weinberg Ra. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147:275–292. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandinger-Ness A, Zerial M. Rab Proteins and the Compartmentalization of the Endosomal system. Cold Spring Harb Perspect Biol. 2014;6:1–25. doi: 10.1101/cshperspect.a022616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Montell C. A phosphoinositide synthase required for a sustained light response. J Neurosci. 2006;26:12816–12825. doi: 10.1523/JNEUROSCI.3673-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wixler V, Wixler L, Altenfeld A, Ludwig S, Goody RS, Itzen A. Identification and characterisation of novel Mss4-binding Rab GTPases. Biol Chem. 2011;392:239–248. doi: 10.1515/BC.2011.022. [DOI] [PubMed] [Google Scholar]
- Yu W, Datta A, Leroy P, O’Brien LE, Mak G, Jou TS, Matlin KS, Mostov KE, Zegers MM. Beta1-integrin orients epithelial polarity via Rac1 and laminin. Mol Biol Cell. 2005;16:433–445. doi: 10.1091/mbc.E04-05-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurchenco PD. Basement membranes: cell scaffoldings and signaling platforms. Cold Spring Harb Perspect Biol. 2011:3. doi: 10.1101/cshperspect.a004911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Schulze KL, Hiesinger PR, Suyama K, Wang S, Fish M, Acar M, Hoskins RA, Bellen HJ, Scott MP. Thirty-one flavors of Drosophila rab proteins. Genetics. 2007;176:1307–1322. doi: 10.1534/genetics.106.066761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuk A, Matlin KS. Apical beta 1 integrin in polarized MDCK cells mediates tubulocyst formation in response to type I collagen overlay. J Cell Sci. 1996;109(Pt 7):1875–1889. doi: 10.1242/jcs.109.7.1875. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.