Abstract
Pannexins form channels at the plasma membrane surface that establish a pathway for communication between the cytosol of individual cells and their extracellular environment. By doing so, pannexin signaling dictates several physiological functions, but equally underlies a number of pathological processes. Indeed, pannexin channels drive inflammation by assisting in the activation of inflammasomes, the release of pro-inflammatory cytokines, and the activation and migration of leukocytes. Furthermore, these cellular pores facilitate cell death, including apoptosis, pyroptosis and autophagy. The present paper reviews the roles of pannexin channels in inflammation and cell death. In a first part, a state-of-the-art overview of pannexin channel structure, regulation and function is provided. In a second part, the mechanisms behind their involvement in inflammation and cell death are discussed.
Keywords: pannexin, inflammation, apoptosis, pyroptosis, autophagy
1. Introduction
Inflammation and cell death result from tissue responses against infections, chemical insults and physical injuries. In multicellular organisms, a wide range of signaling pathways act in concert in order to control these pathological processes [1–3]. Indeed, communication circuits govern the spread of signals over the tissue and modulate inflammatory and cell death responses in cells surrounding the site of injury. A key event in both processes is the release of adenosine-5’-triphosphate (ATP) in the extracellular milieu. Apoptotic cells release ATP and uridine-5’-triphosphate (UTP) at the earliest stages of death, which act as “find-me” signals to attract monocytes, macrophages and microglia to the area of insult [4–7]. Moreover, binding of ATP to purinergic P2 receptors at the plasma membrane surface was shown to underlie the activation of the nucleotide-binding oligomerization domain receptors (NLR) pyrin domain-containing 3 (NLRP3) inflammasome [8–10], the amplification of the extravasation and emigration responses of neutrophils [11] and cytolysis-pore formation during pyroptosis [12]. On the other hand, extracellular ATP liberation promotes neuronal cell death during ischemic conditions [13, 14] and, when induced by chemotherapeutic drugs, tumor cell death [15–17]. In the last decade, pannexin (Panx) channels have been identified as key mediators of extracellular ATP release. The Panx family consists of 3 members, namely Panx1, Panx2 and Panx3 [18]. Among those, Panx1 has yet been most extensively studied. In fact, the activation of Panx1 channels during pathological conditions is triggered by several signals, including increases in extracellular K+ and intracellular Ca2+ concentration [Ca2+]i, caspase-mediated cleavage, c-Jun N-terminal kinases [19] and the Src family of tyrosine kinases [20, 21]. Moreover, in metastasic breast cancer cells, the appearance of the Panx1 truncated form, namely Panx11-89, leads to an open stage of the Panx1 wild channels [22]. This is probably due to the lack of the carboxyterminal (CT) tail of Panx11-89 [22]. Opening of Panx1 channels has been observed in several other disease processes, such as in Crohn’s disease [23], brain ischemia [14], melanoma [24], endotoxic shock [12], epilepsy [25] and upon infection with human immunodeficient virus (HIV) [26]. The present paper will review the roles of Panx channels in the processes of inflammation and cell death related to most of these pathological conditions.
2. Pannexin channels: basic features
2.1. Structural properties
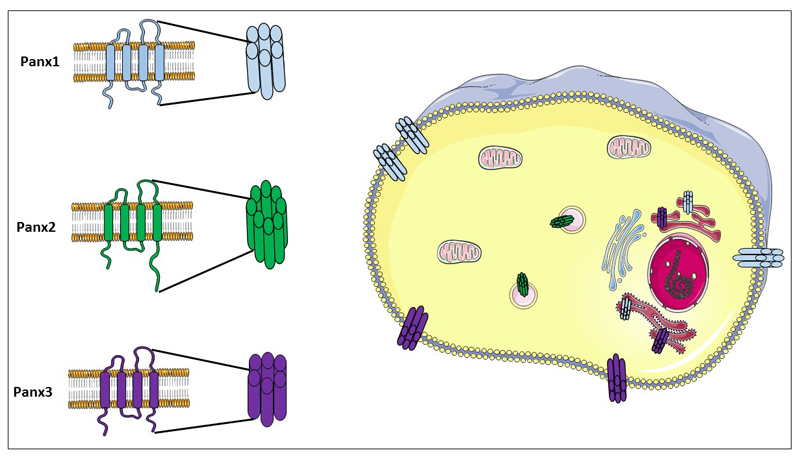
Panx proteins have been discovered in 2000 as homologs of the innexin family of invertebrate gap junction proteins [27]. Of the 3 Panx members characterized in human and mouse, Panx1 is most widespread and has been detected in brain, spleen, skin, cartilage, bladder, kidney, liver, lung, colon, erythrocytes, platelets and immune cells [28–37]. In contrast, Panx2 expression seems to be confined to the nervous system [18, 30], yet a recent study suggests that this protein is also highly abundant in non-neuronal mouse tissues, albeit being preferentially located in the intracellular compartment [38]. This has been linked to the presence of Panx2 in the membrane of endosomal vesicles [39] and points to its possible role in intracellular signaling rather than extracellular communication. Moreover, it has been reported that heteromeric channels consisting of Panx1 and Panx2 can be formed at the plasma membrane of Xenopus oocytes [40] with an attenuated functional activity as observed by a decreased dye uptake and currents from whole cell recording patch clamp [30, 41]. This suggests that Panx2 may also have a function in the modulation of Panx1 activity. Panx3 is present in cartilage, skin and bone [28, 42]. Throughout the years, Panx proteins have been frequently studied together with connexin (Cx) proteins. Panx and Cx proteins indeed share the same topology consisting of 4 transmembrane domains, 2 extracellular loops, 1 intracellular loop, 1 cytosolic aminoterminal tail and 1 cytosolic CT tail (Figure 1). However, no sequence homology exists between both types of proteins [43]. Within the Panx family, the aminoterminal tail is highly conserved, whereas the CT tail shows considerable sequence variability [43]. Unlike Panx2, which has a longer CT tail, Panx1 and Panx3 appear to be closely related to each other [43]. Although it has been generally accepted that Panx proteins gather in hexameric channels, reminiscent of Cx hemichannels, it has been demonstrated that Panx2 may assemble in octameric cellular pores [44, 45] (Figure 1). While Cx hemichannels can interact with unapposed counterparts occurring on neighboring cells to form intercellular gap junctions [46, 47], it is currently highly debated whether Panx channels retain this characteristic [48, 49]. In this respect, Bruzzone and group first demonstrated the ability of Panx1 to form gap junctions in paired Xenopus oocytes [30]. Similarly, Lai and team showed dye coupling in glioma cells transfected with Panx1-green fluorescent protein [50]. In addition, intercellular Ca2+ movement through Panx1-based gap junctions was reported in human prostate cancer epithelial transfected with Panx1-green fluorescent protein cells [51]. Sahu and co-workers described dye transfer and electrical coupling in HeLa cells transfected with Panx1-enhanced green fluorescent protein and Panx3-IRES2-enhanced green flurescent protein [52]. More recent evidence, however, favors gap junction-independent functions of these proteins in different cell types and tissues. Thus, immunocytochemistry analysis has revealed a more diffuse staining pattern of Panx1 and Panx3 in a number of tissues, such as mouse spleen and human skin, respectively [28]. Moreover, electron microscopy studies of hippocampus showed the lack of Panx1 interaction with neighboring cells [53]. Furthermore, Panx1 channels have been found to be functional in the cell membrane of single cells, including erythrocytes, macrophages, neutrophils, T-cells and Kupffer cells [32, 34–37]. Operational Panx1 channels have also been detected in the apical pole of the airway epithelial cells [54] or in the postsynaptic site of neurons [53], thereby not participating in cell-to-cell contact. The main reason suggested to underlie the absence of gap junctional coupling via Panxs lies in the presence of glycosylation patterns at the extracellular loop regions, which may impede docking of Panx channels of adjacent cells [45]. Nevertheless, a recent study performed on various Panx1-transfected cell lines demonstrates that Panx1 can be differentially glycosylated depending on the cell type, leading to cell-specific formation of Panx1-based gap junctions [52].
Figure 1. Pannexin architecture, channel configuration and subcellular localization.
The Panx family encompasses of Panx1, Panx2 and Panx3. Panx proteins all share a common structure consisting of 4 transmembrane domains, 2 extracellular loops, 1 intracellular loop, 1 cytosolic aminoterminal tail and 1 cytosolic CT. While it has been suggested that Panx2 gathers in octameric channels, Panx channels are usually composed of 6 Panx proteins. Panx1 and Panx3 reside both in the plasma membrane surface and the endoplasmic reticulum. Panx2 has been identified in the membrane of endosomal vesicles.
2.2. Regulatory properties
2.2.1. Transcriptional regulation
Panx1 and Panx3 genes are located on human chromosome 11 and mouse chromosome 9, whereas Panx2 is found on human chromosome 22 and mouse chromosome 15 [18]. The human Panx1 gene bears 4 introns and 5 exons, which drive the generation of 2 isoforms from alternative spliced exon 5, called Panx1a and Panx1b, containing 2 intracellular C-terminus lengths due to the insertion of a 4 amino acid sequence [18]. Another human variant displays a single deletion of a single valine at position 377, yielding Panx1bv, which, like the other Panx1 variants, remains at the plasma membrane [55]. Furthermore, 2 novel Panx1 isoforms generated from alternative splicing at exons 2 and 4 have been identified in rat pituitary cells [56]. Both splice variants are located in intracellular compartments and inhibit the ATP-releasing capacity of full length Panx1 when co-expressed [56]. The presence of Panx1 splice variants may be due to differential regulation of the expression of the Panx1 gene. Thus, this gene contains a promoter located in a highly conserved CpG island, which possesses multiple transcriptional start sites, but that lacks core promoter elements. This supports the notion that transcription factors can initiate the processing of Panx1 gene. In fact, the transcription factors E26 transformation-specific translocation variant 4 and 3’,5’-cyclic adenosine monophosphate response element-binding protein are known to bind to the Panx1 gene promoter [57]. Likewise, the Panx3 gene promoter is regulated by the transcription factor runt-related transcription factor 2 in bone [42]. Only 1 Panx2 splice variant has been described in humans, namely Panx2alt2 [18]. In addition to classical cis/trans regulation, gene expression is also controlled by epigenetic mechanisms, including DNA methylation and reversible histone modifications. In this context, Panx1 gene expression has been found to depend, at least in part, on DNA methylation, as Panx1 mRNA levels can be upregulated by the demethylating agent 5-azacytidine [57]. A recent study showed that Panx1 gene transcription is regulated by histone modifications rather than DNA methylation upon nerve injury [58]. In particular, histone markers associated with transcriptional activation, like H3K4me3 and H3K9ac, became more prominent in this condition at the expense of hallmarks of gene silencing, such as H3K9me2 and H3K27me3 [58].
2.2.2. Posttranslational regulation
Posttranslational modifications, such as phosphorylation, N-glycosylation and S-nitrosylation, have been reported to affect Panx trafficking and channel gating [59]. Whereas phosphorylation is a major regulator of the Cx life cycle and activity, and although potential phosphorylation sites have been identified in Panx1 and Panx3 [28], this posttranslational modification is considered to play only a minor role in Panx metabolism and functionality [28, 45]. Nevertheless, the Src family of tyrosine kinases were firstly identified to promote Panx1 opening by phosphorylation due to the activation of N-methyl-D-aspartate receptors in anoxic conditions in the neurons [20]. These results were recently confirmed by Weilinger and group in a study in which the phosphorylation site at a highly conserved tyrosine residue was identified, namely tyrosine residue Y308, which constitutes a putative target site for the Src family of tyrosine kinases and that favors channel opening [60]. This phosphorylation site differs in venous endothelial cells in the presence of tumor necrosis factor alpha (TNFα), in which the site Y198 was identified [21]. This difference may reside in the activation mechanisms and cell types used in both studies. Moreover, N-glysosylation seems a critical determinant in Panx physiology [59]. Several potential glycosylation sites are present in Panx1 and Panx3 [28], yet only 1 has been confirmed for both proteins, namely asparagine residues N254 in Panx1 and N71 in Panx3 occurring at the extracellular side [28]. Panx2 is also a glycoprotein with a predicted N-glycosylation site at asparagine residue N86 in the first extracellular loop [41]. Panx1 and Panx3 exist in 3 glysosylated variants, including a non-glycosylated core protein (Gly0), a high-mannose glysosylated protein (Gly1) and an extensively glycosylated species (Gly2) [28, 41, 45]. Glycosylation of Panx1 and Panx3 has been suggested to control cellular trafficking to the plasma membrane surface [45], where they preferentially reside in their Gly2 form. This glycosylation status avoids the docking between 2 adjacent channels, thus impeding gap junction formation [45]. S-nitrosylation is another posttranslational modification described to take place on Panx1 proteins. In this light, the S-nitrosylation by S-nitrosoglutathione of the cysteine groups 40 and 346 in Panx1 results in the inhibition of extracellular ATP release [61]. In addition, nitric oxide suppresses Panx1 channel activity by activating the 3’,5’-cyclic guanosine monophosphate-protein kinase G pathway [62]. The latter is the result of phosphorylation at S206 site carried by protein kinase G, which may be allosterically modulated by the phosphorylation at S394 [62]. Recently, Panx3, but not Panx2, has also been identified as a substrate for S-nitrosylation. S-nitrosylation of Panx1 and Panx3 can be reversed by the reducing agent dithiothreitol [63].
2.3. Functional properties
Panx channels can be activated in both physiological and pathological conditions. Although Panx1 channels open at positive potential, a number of physiological stimuli have been identified to promote their opening at membrane resting potential. These include the elevation of [Ca2+]i [64], ATP binding to purinergic P2 receptors, such as P2Y receptors [64], as well as ionotropic receptors, like P2X7 receptors [34], oxygen deprivation [20, 32, 65, 66] and mechanical stress [32, 67]. A more recently identified mediator of Panx1 channel opening is insulin [68]. The opening of Panx channels allows the permeation of ions and small molecules in the size range of approximately 1.5 kDa [67, 69], of which ATP is the best studied molecule. ATP signaling through Panx1 channels underlies various physiological functions, such as mucociliary lung clearance [54, 70], differentiation of olfactory sensory neurons [71], glucose uptake in insulin-stimulated adipocytes [68], and potentiation of muscle contraction [72] and control of blood flow [32, 73] in skeletal muscle fibers. Moreover, due to its presence in postsynaptic neurons, Panx1 has been hypothesized to play a role in the synaptic processes in brain [13]. Similar to Panx1 channels, Panx3 can mediate ATP release and has been implicated in the differentiation of chondrocytes by the modulation of intracellular ATP/cyclic adenosine monophosphate levels [74]. Strikingly, Panx1 channels have been reported to lack ATP permeability although acting as anion selective-channels [75]. This discrepancy might be explained by the conductance stage of the channel, which depends on the conformation of the channel. First evidence with Xenopus oocytes showed the presence of a large unitary conductance of approximately 500 pS that allows the release of ATP as well as multiple subconductance stages [67]. These results were confirmed by others studies identifying similar unitary large conductance associated with ATP release and dye flux [32, 66]. In contrast, 2 recent papers reported a low conductance state of ~70 pS that is accompanied by anion-selectivity [75, 76], albeit with a permeability rank order for different anions. It is important to note that anion-selective channels can be permeable to ATP [76]. More recently, Wang and group confirmed the presence of 2 different channel conformations with different permeabilities in Xenopus oocytes expressing Panx1 [77]. The low conductance, which is ATP impermeable, is approximately 50 pS, while the high conductance is 500 pS and ATP-permeable. They appear to be the result of different experimental conditions applied to activate these channels, namely positive voltage and high extracellular K+ levels, respectively [77]. Due to the high voltage that is needed to activate the low conductance channel, it is suggested that the high conductance channel is likely to represent the most predominant configuration in both physiological and pathological conditions [77]. Modification of Panx1 channel confirmation by different stimuli is reflected by differential accessibility of the Panx1 CT cysteine residue C426 to thiol reagents, such as maleimidobutyrylbiocitins. This reagent irreversibly modifies cysteines and can obstruct channel flux when the reactive cysteine is close to the pore. This Panx1 CT cysteine reacts with thiol reagents in the low conductance (voltage-gated) conformation, but does not modify channel conductance in the high conductance (K+-gated) conformation [77]. However, the possible link between channel conductance and ATP permeability is disputed, as it has been demonstrated that dye uptake and ATP release by apoptotic cells expressing CT-truncated Panx1, which forms low-conductance (~75 pS) channels [4, 78]. In addition, the observation that Panx1 channel is an anion-selective channel does not exclude the possibility of cation flux. In this light, by using inside-out patch recordings with asymmetrical concentration of K+ and ATP across the membrane, Bao and co-workers noted that the reversal potential of Panx1 current is less positive than expected for K+ and ATP. This suggests cationic as well as ATP permeation [67]. Hence, both studies support the lack of ionic selectivity of Panx1 channels. Furthermore, Panx1 channels are reported to transport anionic (e.g. fluorescein and Lucifer Yellow) as well as cationic dyes (e.g. To-Pro and Yo-Pro) [4, 32], and dye uptake was inhibited by Panx1 channels blockers or Panx1 gene knock-out, while being enhanced by Panx1 overexpression [4, 32, 79]. In addition, both Romanov and group and Ma and team based their conclusions on anion selectivity by the measurement of the reversal potential of Panx1 currents during the substitution of cations and anions in the extracellular environment [75, 76]. The reversal potential was unchanged when Na+ was substituted by the larger cation, N-methyl-D-glucamine+, but was noticeably shifted when Cl- was replaced by other anions [75, 76]. At first sight, this indicates anion, but not cation permeation through Panx1 channels. However, an alternative interpretation could be an equal selectivity for both Na+ and N-methyl-D-glucamine+, thereby favoring both cationic and anionic transport. A final piece of evidence for the cationic permeation was the identification of a Ca2+ leak from the endoplasmic reticulum mediated by Panx1 and Panx3 channels [51, 80], which, in case of Panx3 channels contributes to osteoblast differentiation [80, 81]. Despite their relevant physiological roles, Panx1 and Panx3 gene deletions as well as double Panx1-Panx2 ablation in mice do not result in overt abnormalities in the anatomy or viability of the animals [82–84]. Nevertheless, Panx3+/- x Panx3+/- crosses generates offspring with a Panx3-/- genetic background and reduced litter size [82]. The mechanism behind this observation remains obscure. Possibly, the deficiency of a particular Panx species could be compensated by other Panx family members. In this context, Panx3 levels are upregulated in the dorsal skin of Panx1 whole body knock-out mice [85].
3. Roles of pannexin channels in inflammation
3.1. Inflammasome activation and release of pro-inflammatory cytokines
A key event in the initial phase of inflammation includes the extracellular release of ATP from the injured tissue, ultimately promoting the maturation and subsequent secretion of the pro-inflammatory cytokines, including interleukin 1 alpha and 1 beta (IL1α/β) as well as IL18 [8, 34]. It is generally accepted that this process requires 2 signals corresponding with canonical inflammasome activation (Figure 2A). The first signal relies on the activation of nuclear factor kappa beta (NF-κB), which is triggered by the binding of damage-associated molecular patterns (DAMPs) or pathogen-associated molecular patterns (PAMPs) to Toll-like receptors (TLRs) [86]. NF-κB can also be activated by cytokines of the TNF family, CD40 ligand, B-cell activating factor and receptor activator of NF-κB ligand [86]. Activation of NF-κB induces the expression of TNFα and the premature forms of IL1β and IL18 [86]. The second signal involves the formation of the inflammasome and concomitant activation of caspase 1. The latter then cleaves the premature forms of IL1β and IL18, yielding the fully active cytokines, which are subsequently released into the extracellular environment to perform their inflammatory actions [8]. A number of inflammasome variants have been identified based on NLR identity, namely NLRP3, NLRP1 and NLR caspase activation and recruitment domain (CARD) containing 4 (NLRC4) [87], of which the NLRP3 inflammasome has been most extensively studied [88–90]. NLRP3 forms a complex with apoptosis-associated speck-like protein containing a C-terminal caspase recruitment domain (ASC) and pro-caspase 1 [88, 91]. The expression of NLRP3 components is tightly controlled by NF-κB [92] and it is activated by a decrease in intracellular K+ concentration [93, 94]. In fact, the binding of extracellular ATP to P2X7 receptors in macrophages results in the cellular influx of Ca2+ ions and efflux of K+ ions [34]. The latter not only turns on the NLRP3 inflammasome, but, together with the alterations in the intracellular Ca2+ levels, also opens Panx1 channels, which then release ATP in the extracellular space [34]. This perpetuates P2X7 receptor stimulation, yet simultaneously ATP may inhibit Panx1 channels in a negative feedback loop [95]. In these conditions, Panx1 was identified to constitute the large pore induced by the activation of P2X7 receptors, and both channels were found to co-immunoprecipitate in HEK293 cells [34]. This supports a direct interaction at the plasma membrane between both channels. In contrast, microglia, the immunogenic cells of the brain, release ATP through Panx1 channels upon lipopolysaccharide (LPS) stimulation, which in turn activates P2Y1 receptors, resulting in Ca2+ release from the intracellular stores [96]. Alternatively, Panx1 channel opening may be the consequence of particle internalization involving uric acid, silica and Alum crystals, though the mechanism is not clear [97] (Figure 2A). Internalized particles fuse with lysosomes to form phagolysosomes, leading to destabilization of the phagolysosome membrane and subsequent release of cysteine cathepsins and cathepsin B [97]. This process could precede the ATP release mechanism by Panx channels and the activation of P2X7 receptors, and hence the assembly of the NLRP3 inflammasome [97]. In contrast to this finding is the observation that leakage of cathepsin B from the phagolysosome can be directly involved in the activation of the NLRP3 inflammasome to activate caspase 1 [98]. Another DAMP that regulates NLRP3 inflammasomes is asbestos, which promotes the secretion of IL1β by the activation of nicotinamide adenine dinucleotide phosphate oxidase upon phagocytosis [99] (Figure 2A). This generates reactive oxygen species that assist in the activation of the NLRP3 inflammasome [99]. On the other hand, open Panx1 channels may serve as a pathway for cellular uptake of muramyl dipeptide, a constituent of gram-negative and gram-positive bacteria [100] (Figure 2A). Muramyl dipeptide participates in both signals of inflammasome activation. Thus, muramyl dipeptide binds to nucleotide-binding oligomerization domain 2, which enhances the activation of NF-κB [100]. This also potentiates the induction of the NLRP1 inflammasome, and therefore the cleavage of caspase 1 [100, 101]. The latter mechanism equally applies to the Anthrax lethal toxin [101] (Figure 2A). Interestingly, Panx1 channel functional opening is accompanied by high levels of Panx1 expression due to histone modifications in neurons, which potentiates IL1β release [58]. The exact role of Panx1 in the activation of the inflammasome and thus the release of IL1β and IL18 is not clear. Inhibition of Panx1 channel opening by the prototypical Panx1 channel inhibitor 10Panx1 impedes the release of IL1β in mouse J774 macrophages, human THP-1 macrophages and human alveolar macrophages in the presence of LPS and ATP [34]. These results have been reproduced in mouse peritoneal macrophages and are comparable to caspase 1 inhibition [8]. Moreover, Panx1 gene silencing in a mouse model of hepatic ischemia-reperfusion results in a decrease of IL1β serum levels [37]. These findings collectively suggest an important role for Panx1 channels in inflammasome activation. In contrast, upon activation of the inflammatory cascade in mouse bone marrow-derived macrophages lacking Panx1, no defect in inflammasome activation or secretion of IL1β or IL18 could be recorded [102]. Nevertheless, various stimuli to activate the different inflammasomes in cells have been used, such as silica and Alum crystals to activate NLRP3, Salmonella thyphimurium and flagelin for NLRC4 and plasmid DNA to activate absent in melanoma 2 (AIM2). These results show that Panx1 is unlikely to be strictly necessary to activate NLRP3 inflammasome in the presence of silica or Alum crystals. Moreover, similar findings have been obtained with peritoneal macrophages deficient in Panx1/Panx2 exposed to LPS and ATP [84]. This discrepancy may be due to Panx2. Although Panx2 has not yet been identified in macrophages, it was recently found to be ubiquitously expressed in different tissues [38]. The absence of Panx1 and Panx2 did not interfere with the secretion of IL1β, but did in Panx1 inhibition, which could point to a role of Panx2 in the modulation of the inflammatory response. Important to note is that the concentrations used in these studies varied by 1000-fold, being more concentrated in macrophages deficient in Panx1 and Panx2. In addition, LPS induces the generation of reactive oxygen species [103]. Hence, it can be hypothesized that the secretion of IL1β is due to the generation of reactive oxygen species by the high concentration of LPS. Alternatively, in other immune cells, such as neutrophils, the activation of the inflammasome upon LPS and ATP administration has been linked directly to the activation of P2X7 receptors without considering the possible involvement of Panx1 [104]. This highlights not only the novelty of the role of Panx1 in the canonical inflammasome activation, but also the controversy within the different studies.
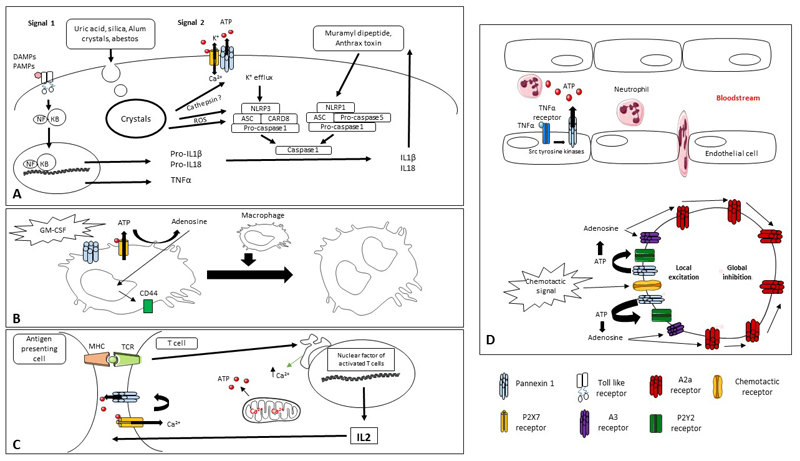
Figure 2. Role of Panx1 channels in inflammation.
The inflammatory response is activated upon injury. (A) Induction of the inflammasome occurs in 2 steps. The first step relies on the activation of TLRs by DAMPs and PAMPs to switch on gene transcription of TNFα and the premature forms of IL1β and IL18. The second step depends on the interaction between P2X7 receptors and Panx1 channels to promote oligomerization and hence activation of the inflammasome, followed by caspase 1-mediated cleavage and extracellular release of IL1β and IL18. (B) Multinucleated macrophage formation takes place following ATP release through P2X7 receptors and subsequent conversion into adenosine in order to enhance the expression of the fusion factor CD44, while Panx1 allows the permeabilization of the plasma membrane, thereby enabling fusion. (C) The release of Ca2+ by the endoplasmic reticulum contributes to the synthesis of ATP by mitochondria and the opening of Panx1 channels. ATP released through Panx1 channels binds to P2X7 receptors, which in turn activates Panx1 channels. T-cells secrete IL2 and become proliferative. (D) Endothelial cells secrete ATP via Panx1 channels, which stimulates the production of vascular cell adhesion molecule 1 and thus the extravasation of the immune cells. In the presence of chemotactic stimuli, ATP liberated by Panx1 channels induces local excitation in the leading face of migrating neutrophils due to the activation of P2Y2 and A3 receptors. By contrast, the activation of A2a receptors facilitates global inhibition in the rest of the cell (ATP, adenosine-5´-triphosphate; GM-CSF, granulocyte macrophage colony-stimulating factor; IL, interleukin; MHC, major histocompatibility complex; NLRP3, Nod-like receptor pyrin domain-containing 3; Panx1, pannexin 1; P2, purinergic receptor; ER, endoplasmic reticulum; TCR, T-cell receptor; TLR, Toll-like receptor).
3.2. Activation and migration of leukocytes
Besides inducing the inflammasome, ATP also influences the activation and chemoattraction of leukocytes to the site of injury in order to amplify the immune response. Panx1 channels are involved in the generation of multinucleated macrophages [105], the activation of T-cells [36] and the emigration of leukocytes during inflammation [21, 35]. Multinucleated giant cells arise from the fusion of macrophages in homeostatic and disease conditions. Although controversial, some evidence supports their action in normal tissue development or as host defense in a variety of pathological circumstances [106, 107]. In this respect, fusion of macrophages depends, at least in part, on activation of P2X7 receptors [108]. In fact, both P2X7 receptors and Panx1 channels promote the generation of multinucleated macrophages [105] (Figure 2B). Unlike inflammasome activation, P2X7 receptors and Panx1 channels might act independently of each other in the presence of granulocyte macrophage colony-stimulating factor to enhance multinucleated macrophage formation, as ATP release is unchanged in Panx1-deficient macrophages compared to wild-type counterparts [105]. In contrast, decreased ATP release has been observed in peritoneal macrophages with a deletion in P2X7 receptors [105]. Upon release, ATP is transformed into adenosine, which in turn may upregulate the expression of CD44, a membrane protein relevant for the fusion of macrophages [105]. Panx1 channels are involved in membrane permeabilization, thereby facilitating the fusion of the cells [105]. These data demonstrate that Panx1 channels and P2X7 receptors can act individually to activate different signaling pathways, which trigger the generation of multinucleated macrophages.
The activation of T-cells is mediated by the interaction between α and β T-cell receptors (TCR) and an immunogenic peptide bound to the major histocompatibility complex in antigen-presenting cells. This antigen recognition favors the activation of T-cells in an ATP-dependent manner. It has been speculated that in a first step, the interaction between both receptors, TCRs and antigen bound to major histocompatibility complex, stimulates the uptake of Ca2+ by mitochondria to synthesize ATP [36] (Figure 2C). In addition, Panx1 channel opening due to elevated [Ca2+]i promotes ATP release [36]. This mechanism guarantees an increase in extracellular ATP levels that in turn serves as an autocrine stimulus to activate P2X receptors [36]. This constitutes a positive feedback loop, since the opening of P2X receptors allows the cellular influx of Ca2+, thereby maintaining Panx1 channel opening in mouse and human CD4+ T-cells as well as in Jurkat cells [36, 109]. Moreover, Panx1 channels themselves may contribute to Ca2+ entry to further stimulate their opening. Consequently, T-cells produce IL2, activate the transcription factor nuclear factor of activated T-cells and become proliferative [36, 110] (Figure 2C). The influx of Ca2+ and ATP production are highly dependent on the strength of the activation mediated by TCRs. In this respect, inhibition of P2 receptors prevents autoreactive expansion of activated T-cells, while overproduction of ATP due to calreticulin deficiency, a major Ca2+-buffering chaperone at the lumen of the endoplasmic reticulum, results in a lowered threshold for T-cell activation and immunopathology [36, 111]. This points to the beneficial effects elicited by the inhibition of this pathway to prevent T-cell activation in immunological diseases. In diabetes mellitus type I and inflammatory bowel disease, the blockage of this signaling cascade by the administration of oxidized ATP limits the secretion of pro-inflammatory cytokines, avoids the response of T-cells to TCR stimulation and ameliorates the outcome of T-cell-mediated inflammation in vivo [36].
Recruitment of inflammatory cells to the site of injury is driven by a plethora of signals that influence cell-cell interactions between vascular endothelial cells and circulating leukocytes. In acute inflammation induced by TNFα, Panx1 channels have been found to assist in adhesion and extravasation of leukocytes through the vascular wall [21] (Figure 2D). This is substantiated by the observation that deletion of Panx1 expression impedes elevation of vascular cell adhesion molecule 1 production, which can be overcome in the presence of exogenous ATP. These results suggest that P2 receptors are also involved in the cascade of events induced upon acute inflammation [21]. An important immune barrier between the blood and the cerebrospinal fluid in brain is constituted by the choroid plexus, which contains native immune cells, called epiplexus cells. In case of injury, ATP release through Panx1 channels in epithelial cells attracts epiplexus cells, though the exact mechanism of their activation by ATP is unknown [112]. Furthermore, Panx1 channels have been reported to play a role in the chemotaxis response of neutrophils. Indeed, the activation of the chemoattractant receptors on the surface of neutrophils controls Panx1-induced ATP release, which subsequently stimulates P2Y2 receptors [35]. Moreover, ATP is transformed into adenosine by membrane enzymes, like ectonucleoside triphosphate diphosphohydrolase 1, and triggers A3 receptors. Both P2Y2 and A3 receptors turn on signaling pathways that contribute to chemotactic responses at the front of the migrating cells [11] (Figure 2D). By contrast, adenosine also stimulates A2A receptors to promote a global inhibitory signal in the neutrophils [35] (Figure 2D). This suggests that Panx1 represents a link between chemoattractant receptors and the amplification of the initial chemotactic response in neutrophils in order to contribute to cell migration. In compliance with this view, Panx1 channels mediate the migration of inflammatory cells to the site of injury to boost the immune response [11, 35].
4. Roles of pannexin channels in cell death
4.1. Apoptosis
Apoptosis is the best characterized form of programmed cell death, which drives removal of defunct cells in both physiological and pathological conditions [113]. Apoptosis is typified by a number of morphological changes, including cell shrinkage, chromatin condensation, formation of apoptotic bodies and engulfment by macrophages. Most of these modifications in cytoarchitecture result from the actions of caspases, which either initiate or execute the apoptotic response by proteolytically cleaving a variety of cellular proteins [113]. The 2 major apoptotic pathways that can lead to caspase activation are the intrinsic cascade and the extrinsic pathway [114]. The former is mainly mediated by mitochondria along with the B-cell lymphoma-2 family of pro-apoptotic and anti-apoptotic proteins [115]. In contrast, the extrinsic signaling pathway is triggered by the binding of extracellular death ligands, such as Fas ligand, TNFα or TNF-related apoptosis-inducing ligand, to their corresponding receptors at the plasma membrane [114]. Panx1 channels are implicated in different steps of apoptosis. In the early phases, activation of Panx1 channels contributes to the increase in plasma membrane permeability, as judged on selective dye uptake in nascent apoptotic cells [4, 116]. In particular, Panx1 channel activation has been detected in cells beginning to bleb [4]. A recent study showed that Panx1 channels maintain cellular integrity during this programmed cell death process, as evidenced by the occurrence of cellular disassembly in apoptotic bodies upon inactivation of Panx1 channels [117]. However, it has been observed that Panx1 knock-down has no effect on the progression of apoptosis [4]. This complies with extracellular ATP and UTP release, taking place at early, but not in late phases of apoptosis, in order to recruit monocytes and macrophages [4, 5] (Figure 3). ATP release from apoptotic cells is also important to initiate bone resorption [118]. On the other hand, Panx1 channels can occur at the endoplasmic reticulum, where they form Ca2+-permeable pores that contribute to Ca2+ leakage [51]. This may favor mitochondrial Ca2+ uptake and thus, the formation of the permeability transition pore, which conveys cytochrome c to the cytosol to initiate apoptosis [119, 120] (Figure 3). Unlike inflammation, Panx1 channel opening related to apoptosis is not dependent on P2 receptors or on the presence of ATP in the extracellular environment [117]. Rather, cleavage of the cytosolic CT tail of Panx1 by caspases 3 and 7 results in the irreversible opening of these channels, as shown by drastically increased dye uptake and ATP release in Jurkat cells with a truncated Panx1 CT tail [4, 5, 102]. The latter seems to inhibit channel functioning by the interaction with a specific site inside the channel pore [121]. Consequently, Panx1 channel activation is mediated by 2 events, namely caspase cleavage and the dissociation of a region immediately downstream of the caspase site in the CT region [121]. Recently, lethal effects have been described in Xenopus laevis oocytes and Neuro2A cells carrying Panx1 with truncation lengths ranging from 370 to 393 amino acids [122]. This includes the 375-378 amino acid caspase cleavage area, which further triggers the opening of these channels during cell death [122].
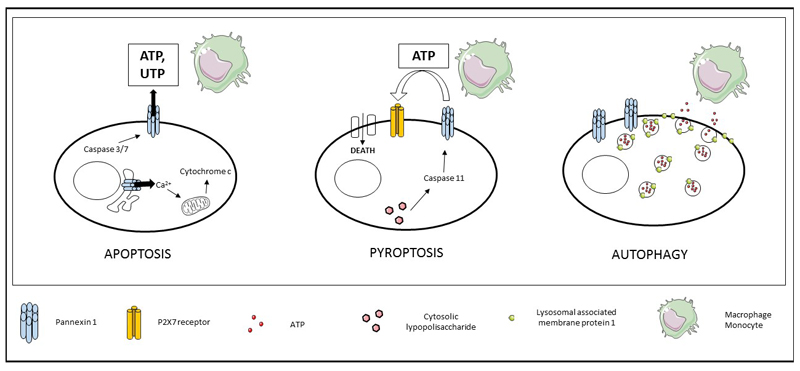
Figure 3. Role of Panx1 channels in cell death.
The opening of Panx1 channels due to the action of caspases 3 and 7 in early apoptosis facilitates the release of ATP and UTP to attract macrophages and monocytes, thereby maintaining cellular integrity and facilitating Ca2+ release from the endoplasmic reticulum to induce translocation of cytochrome c into the cytosol. In pyroptosis, ATP release through Panx1 channels activates P2X7 receptors, which underlies the formation of a membrane pore that triggers cytolysis. In autophagy, the presence of Panx1 channels and lysosomal associated membrane protein 1 favor lysosomal binding to the plasma membrane to release ATP and attract macrophages (ATP, adenosine-5´-triphosphate; Panx, pannexin; P2, purinergic receptor; UTP, uridine-5’-triphosphate).
4.2. Pyroptosis
Pyroptosis is an inflammatory cell death mode characterized by the activation of inflammatory caspases, cell swelling and rapid destabilization of the plasma membrane [123]. In case of bacterial infection, disruption of infected cells contributes to the release of pathogens and subsequent phagocytosis by neutrophils [123]. At present, macrophages and dendritic cells have been identified to undergo pyroptosis [124, 125]. A crucial event in this process has been initially attributed to the activation of caspase 1 [126, 127]. Nevertheless, new data show that mice lacking caspase 1, generated through stem cell technology, are also deficient in caspase 11 [128]. Therefore, caspase 11 may be responsible for some phenotypes previously believed to be due to caspase 1, such as shock following endotoxic challenge [129]. This is in accordance with earlier studies highlighting the importance of caspase 11 activation in the induction of pyroptosis [128–130]. The activation of mouse caspase 11 and related human caspases 4 and 5 is due to their direct binding to intracellular LPS [128, 131] (Figure 3). As holds for caspases 3 and 7, caspase 11 is able to cleave the CT moiety of Panx1, leading to channel opening and extracellular ATP release, which in turn activates P2X7 receptors to cause cytotoxicity [12] (Figure 3). The sensitivity of P2X7 receptor activation is increased by the presence of cytosolic LPS and extracellular ATP, resulting in the formation of a pore in the cell membrane that triggers cytolysis [12] (Figure 3). Furthermore, the secretion of ATP into the extracellular environment attracts macrophages that phagocyte pyroptotic cells [6]. Pyroptosis has also been linked to the activation of NLRP3 inflammasomes, though this is independent of P2X7 receptor activation [12]. The opening of Panx1 channels by caspase 11-mediated cleavage during pyroptosis induces K+ efflux and subsequent activation of NLRP3 inflammasomes to process and release IL1β [12]. This suggests that Panx1 channels could fulfill a dual function in pyroptosis, namely in the activation of P2X7 receptors to mediate cell death and in the induction of potassium efflux without P2X7 receptor involvement to turn on NLRP3 inflammasomes. Genetic ablation of Panx1 has been found to reduce the mortality rate in animal models of endotoxic shock [12]. Of note, neutrophils do not undergo pyroptosis under Salmonella enterica serovar typhimurium infection, but rather sustain IL1β production [125]. This mechanism is caspase 11 independent and mainly implies the contribution of the NLRC4 inflammasome [125]. Overall, these results show that absence of Panx1 channel activation could prevent pyroptosis in neutrophils.
4.3. Autophagy
Autophagy is considered as a self-degradative process in which dysfunctional or redundant cellular components are removed by lysosomes [132]. Basal levels of autophagy contribute to the maintenance of homeostasis [133]. However, in stress conditions, this process ensures cell survival by allowing energy production and the recycling of cytoplasmic components that provides the cells with building stones required for newly synthetized molecules. Although seemingly a cytoprotective mechanism, autophagy prolongs cell survival for a short time, yet upon persistent stimulation, it may promote cell death. This mode of cell death is referred to as cell death with autophagy, instead of cell death by autophagy, due to the fact that cells do not normally die by autophagy [134]. In fact, there are different stimuli that can trigger autophagy, including some chemotherapeutics [135]. Autophagic cells release ATP, which serves as a “find-me” signal for dendritic cells and neutrophils [15, 136]. Cells exposed to chemotherapeutics undergo autophagy, store ATP in autolysosome vesicles and release it through exocytosis by a mechanism that requires the translocation of lysosomal associated membrane protein 1 into the plasma membrane (Figure 3). This translocation is facilitated by the action of caspases and Panx1 channels, though this process is not well understood [135]. Extracellular ATP not only attracts immune cells, but also contributes to the activation of inflammasomes in macrophages, while engulfing dying cells in a Panx1-dependent mechanism [136].
5. Conclusions and perspectives
This paper has reviewed the involvement of Panx channels in inflammation and cell death. Although this research is still in its infancy, several critical roles have already been attributed to Panx channels in these pathological processes. Despite many knowledge gaps and the involvement of a myriad of underlying mechanisms, it appears that opening of Panx1 channels correlates with activation and/or perpetuation of inflammation and cell death. Consequently, interfering with Panx1 channel opening might be considered as a novel therapeutic approach. Indeed, inflammation and cell death accompany a wide spectrum of acute and chronic diseases, thereby rendering Panx1 channels plausible drug targets. In this respect, a number of chemotherapeutic drugs induce Panx1 channel opening to favor cell death by apoptosis, pyroptosis and autophagy [15–17]. This triggers ATP release and thus the initiation of the immunogenic cell death process in order to control residual tumors [137, 138]. By contrast, epigenetic silencing of Panx1 production in cultured melanoma cells reduces the expression of malignant melanoma markers [24]. In a mouse model of ischemia, suppression of Panx1 channels as well as genetic ablation of Panx1 expression result in diminished motor symptoms and brain infarcts [14]. This is in contrast to the observation that double Panx1-Panx2 ablation in mice produces smaller brain infarcts with improved motor symptoms upon ischemic stroke compared to wild-type or single Panx1 deleted counterparts [84, 139]. More recently, deletion of Panx3 was reported to protect against surgically induced osteoarthritis in mice [82]. It is tempting to speculate that this will necessitate different therapeutic strategies to either promote or inhibit Panx channel opening (Table 1). In parallel with clinical exploration, further fundamental and translational research in the Panx field will also require improved experimental tools and models. Unfortunately, the pharmacological agents currently used for this purpose lack Panx channel specificity and, at least in some cases, can also affect Cx hemichannels and gap junctions [55]. In this context, due to the increasing success of mimetic peptides targeting Cx hemichannels, the mimetic peptide 10Panx1 was developed. This peptide reproduces a specific amino acid sequence in the extracellular loop of Panx1 [140]. Although this peptide inhibits Panx1 currents, dye uptake and ATP release, it also interacts with Cx46 [69, 140]. Alternative approaches include the use of small interference RNA duplexes and knock-out mice. The absence of major abnormalities in mice genetically deficient in Panx1, Panx2 or Panx3 does not necessarily mean that these channels do not have physiological relevance. In this respect, mice deficient in Panx1 display distinct behavioral changes, including enhanced anxiety, impaired object recognition and spatial learning [141]. Moreover, mice with conditional Panx1 knock-out in the cochlea show hearing loss [142]. This is reminiscent of a recent report describing sensorineural hearing loss, intellectual disability and skeletal defects, such as kyphoscoliosis and primary ovarian failure, in a 17-year-old female with a homozygous mutation in the Panx1 gene, whereby arginine at position 217 is replaced by histidine. This results in a 50% reduction of Panx1 channel opening without any effect on cellular trafficking nor on Panx1 expression [143]. Such studies clearly illustrate the clinical potential of Panx signaling and concomitantly underscore the importance of Panx research in the upcoming years.
Table 1. Potential therapeutic effect of pannexin channels.
(ATP, adenosine-5´-triphosphate; HIV, human immunodeficient virus; IFNγ, interferon gamma; IL, interleukin; Panx, pannexin; TCR, T-cell receptor; TNFα, tumor necrosis factor alpha).
| Disease | Model | Pannexin expression | Modes of inhibition or activation of pannexin channel opening | Outcome from the activation or inhibition of pannexin channel opening | References |
|---|---|---|---|---|---|
| Leukemia | Human Jurkat T cells | High levels of Panx1 protein | Panx1 channel activation by the chemotherapeutic drugs staurosporine, doxorubicin and etoposide | Tumor cell death by apoptosis | [16, 17] |
| Breast cancer | Human MDA-MB-231 cells and mouse 4.T1.2 cells | Not determined | P2X4/P2X7/Panx1 channel activation by Ivermectin | Tumor cell death by autophagy and pyroptosis | [16, 17] |
| Human metastatic breast cancer CN-LM1A and MDA-LM2 cell lines | High levels of Panx1 together with its truncated form, Panx11-89, proteins on the plasma membrane | Panx1 channel inhibition by 10Panx1 and pharmacologically by carbenoxolone | Reduced viable cells and reduced lung-metastatic colonization in mice | [22] | |
| Glioma | Rat C6 glioma cells | Lack to express any Panx transcripts | Panx1 channel activation induced by plasmid transfection | Flattened cell morphology, reduced cell motility, increased dye coupling between cells and reduced size and number of colony formation in soft agar | [50] |
| Panx2 cytoplasmic expression induced by transfection with Murine Stem Cell Virus vector | Flattened cell morphology and reduced proliferation. Reduced size and number of colony formation in soft agar. Reduced tumor size when injected in nude mice | [144] | |||
| Melanoma | Mouse BL6 melanoma cell line | High levels of Panx1 protein | Panx1 channel inhibition by silencing Panx1 gene expression | Reduced cell motility, proliferation, vimentin β-catenin (localized intracellularly) and dye uptake. Reduced tumor size in the Avian embryo metastasis assay | [24] |
| Brain ischemia | Mouse C57 primary neurons and mouse C57 brain slices, Panx1 knockout mice, double ablated Panx1/Panx2 mice | High levels of Panx1 protein | The single inhibitory effect exerted by pharmacologically blocking and genetic deletion of Panx1 was described to have an effect on brain ischemia, yet the double ablation Panx1/Panx2 mouse was reported to counteract stroke | Reduced neuronal death, motor symptoms and brain infarcts | [14, 84, 139, 140] |
| Crohns's disease | C57Bl/6 mice with chemically induced intestinal inflammation and human tissue from patients with Crohns's disease | Human myenteric and submucosal neurons highly express Panx1, its levels are reduced in the myenteric plexus from Crohn's disease patients | Pharmacologically blocking of Panx1 | Functional control of the colonic musculature | [23] |
| Epilepsy | Primary astrocytes derived from C57Bl/6 mice, mice brain slices and wild-type and Panx1 knockout C57Bl/6 mice | Panx1 is expressed by astrocytes and neurons from the hippocampus | Genetic deletion of Panx1 | Diminished seizures outcomes | [25] |
| Endotoxic shock | Mouse bone marrow derived macrophages transfected with ultrapure LPS from Salmonella Minnesota RE595, C57Bl/6 mice primed with Escherichia coli O111:B4 LPS | High levels of Panx1 protein in mouse bone marrow derived macrophages | Genetic deletion of Panx1 | Reduced mouse mortality rate | [12] |
| HIV | HIV isolates R5 and X4 infect human CD4+ T-lymphocytes | T-lymphocytes express Panx1 proteins | Silencing of the Panx1 gene and 10Panx1 | Reduction in HIV replication in human CD4+ T-lymphocytes | [26] |
| Diabetes mellitus type I | Mice RAG-2-/- with the adoptive transfer of influenza hemagglutinin-specific transgenic TCR 6.5 CD4+ T-cells | T-lymphocytes express Panx1 proteins | Administration of oxidized ATP | Normal blood glucose levels, reduced influenza hemagglutinin-specific transgenic TCR 6.5 CD4+ T cells in the spleen and pancreas, none-detectable CD69+ TCR 6.5+ cells in the pancreas. Reduced levels of TNFα, IFNγ and IL6 | [36] |
| Inflammatory bowel disease | Mice cd3ε-/- injected with naïve CD4+ T-cells | T-cells express Panx1 proteins | Administration of oxidized ATP | Similar bowel wall to healthy mice, reduced inflammatory score, diminished CD4+ effector/memory subset and CD69+ cells in mesenteric lymph nodes and spleens, reduced number of cells that secret IL2, IL17, IFNγ and TNFα | [36] |
| Osteo-arthritis | Mice lacking Panx3 specifically in the cartilage and global Panx3 knockout mice with a destabilization of the median meniscus | High levels of Panx3 in the cartilage | Genetic deletion of Panx3 | Mice lacking Panx3 are resistant to develop osteo-arthritis | [82] |
Acknowledgements
This work was funded by grants of the European Research Council (Starting Grant 335476), the Fund for Scientific Research-Flanders (FWO grants G009514N and G010214N), the University Hospital of the Vrije Universiteit Brussel-Belgium (“Willy Gepts Fonds” UZ-VUB), the University of São Paulo-Brazil and the São Paulo Research Foundation-Brazil (FAPESP SPEC grant 2013/50420-6).
Abbreviations
- ASC
apoptosis-associated speck-like protein containing a C-terminal caspase recruitment domain
- ATP
adenosine-5’-triphosphate
- [Ca2+]i
intracellular calcium concentration
- CARD
caspase activation and recruitment domain
- CT
carboxyterminal
- Cx
connexin
- DAMP(s)
damage-associated molecular pattern(s)
- HIV
human immunodeficient virus
- IL1α/1β/2/17/18
interleukin1 alpha, 1 beta, 2 and 18
- LPS
lipopolysaccharide
- NF-κB
nuclear factor kappa beta
- NLR
nucleotide-binding oligomerization domain receptor
- NLRC4
NLR caspase activation and recruitment domain (CARD) containing 4
- NLRP1/3
NLR pyrin domain-containing 1 and 3
- PAMP(s)
pathogen-associated molecular pattern(s)
- Panx
pannexin
- TCR(s)
T-cell receptor(s)
- TLR(s)
Toll-like receptor(s)
- TNFα
tumor necrosis factor alpha
- UTP
uridine-5’-triphosphate.
References
- 1.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008 Jul;454(7203):428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 2.Tait SW, Ichim G, Green DR. Die another way--non-apoptotic mechanisms of cell death. J Cell Sci. 2014 May;127:2135–2144. doi: 10.1242/jcs.093575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weerasinghe P, Buja LM. Oncosis: an important non-apoptotic mode of cell death. Exp Mol Pathol. 2012 Dec;93(3):302–308. doi: 10.1016/j.yexmp.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 4.Chekeni FB, Elliot MR, Sandilos JK, Walk SF, Kinchen JM, Lazarowski ER, Armstrong AJ, Penuela S, Laird DW, Salvesen GS, Isakson BE, et al. Pannexin 1 channels mediate 'find-me' signal release and membrane permeability during apoptosis. Nature. 2010 Oct;467(7317):863–867. doi: 10.1038/nature09413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elliott MR, Chekeni FB, Trampont PC, Lazarowski ER, Kadl A, Walk SF, Park D, Woodsonm RI, Ostankovich M, Sharma P, Lysiak JJ, et al. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature. 2009 Sep;461(7261):282–286. doi: 10.1038/nature08296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Q, Imamura R, Motani K, Kushiyama H, Nagata S, Suda T. Pyroptotic cells externalize eat-me and release find-me signals and are efficiently engulfed by macrophages. Int Immunol. 2013 Jun;25(6):363–372. doi: 10.1093/intimm/dxs161. [DOI] [PubMed] [Google Scholar]
- 7.Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005 Jun;8(6):752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- 8.Pelegrin P, Barroso-Gutierrez C, Surprenant A. P2X7 receptor differentially couples to distinct release pathways for IL-1beta in mouse macrophage. J Immunol. 2008 Jun;180(11):7147–7157. doi: 10.4049/jimmunol.180.11.7147. [DOI] [PubMed] [Google Scholar]
- 9.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010 Mar;140(6):805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 10.Kanneganti TD, Lamkanfi M, Kim YG, Chen G, Park JH, Franchi L, Vandenabeele P, Núñez G. Pannexin-1-mediated recognition of bacterial molecules activates the cryopyrin inflammasome independent of Toll-like receptor signaling. Immunity. 2007 Apr;26(4):433–443. doi: 10.1016/j.immuni.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 11.Chen Y, Corriden R, Inoue Y, Yip L, Hashiguchi N, Zinkernagel A, Nizet V, Insel PA, Junger WG. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science. 2006 Dec;314(5806):1792–1795. doi: 10.1126/science.1132559. [DOI] [PubMed] [Google Scholar]
- 12.Yang D, He Y, Muñoz-Planillo R, Liu Q, Núñez G. Caspase-11 Requires the Pannexin-1 Channel and the Purinergic P2X7 Pore to Mediate Pyroptosis and Endotoxic Shock. Immunity. 2015 Nov;43(5):923–932. doi: 10.1016/j.immuni.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cisneros-Mejorado A, Pérez-Samartín A, Gottlieb M, Matute C. ATP signaling in brain: release, excitotoxicity and potential therapeutic targets. Cell Mol Neurobiol. 2015 Jan;35(1):1–6. doi: 10.1007/s10571-014-0092-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cisneros-Mejorado A, Gottlieb M, Cavaliere F, Magnus T, Knoch-Nolte F, Scemes E, Pérez-Samartín A, Matute C. Blockade of P2X7 receptors or pannexin-1 channels similarly attenuates postischemic damage. J Cereb Blood Flow Metab. 2015 May;35(5):843–850. doi: 10.1038/jcbfm.2014.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Michaud M, Martins I, Sukkurwala AQ, Adjemian S, Ma Y, Pellegatti P, Shen S, Kepp O, Scoazec M, Mignot G, Rello-Varona S, et al. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science. 2011 Dec;334(6062):1573–1577. doi: 10.1126/science.1208347. [DOI] [PubMed] [Google Scholar]
- 16.Boyd-Tressler A, Penuela S, Laird DW, Dubyak GR. Chemotherapeutic drugs induce ATP release via caspase-gated pannexin-1 channels and a caspase/pannexin-1-independent mechanism. J Biol Chem. 2014 Sep;289(39):27246–27263. doi: 10.1074/jbc.M114.590240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Draganov D, Gopalakrishna-Pillai S, Chen YR, Zuckerman N, Moeller S, Wang C, Ann D, Lee PP. Modulation of P2X4/P2X7/Pannexin-1 sensitivity to extracellular ATP via Ivermectin induces a non-apoptotic and inflammatory form of cancer cell death. Sci Rep. 2015 Nov;5:16222. doi: 10.1038/srep16222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baranova A, Ivanov D, Petrash N, Pestova A, Skoblov M, Kelmanson I, Shagin D, Nazarenko S, Geraymovyvh E, Litvin O, Tiunova A, et al. The mammalian pannexin family is homologous to the invertebrate innexin gap junction proteins. Genomics. 2004 Apr;83(4):706–716. doi: 10.1016/j.ygeno.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 19.Xiao F, Waldrop SL, Bronk SF, Gores GJ, Davis LS, Kilic G. Lipoapoptosis induced by saturated free fatty acids stimulates monocyte migration: a novel role for Pannexin1 in liver cells. Purinergic Signal. 2015 Sep;11(3):347–359. doi: 10.1007/s11302-015-9456-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weilinger NL, Tang PL, Thompson RJ. Anoxia-induced NMDA receptor activation opens pannexin channels via Src family kinases. J Neurosci. 2012 Sep;32(36):12579–12588. doi: 10.1523/JNEUROSCI.1267-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lohman AW, Leskov IL, Butcher JT, Johnstone SR, Stokes TA, Begandt D, DeLalio LJ, Best AK, Penuela S, Leitinger N, Ravichandran KS, et al. Pannexin 1 channels regulate leukocyte emigration through the venous endothelium during acute inflammation. Nat Commun. 2015 Aug;6:7965. doi: 10.1038/ncomms8965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Furlow PW, Zhang S, Soong TD, Halberg N, Goodarzi H, Mangrum C, Wu YG, Elemento O, Tavazoie SF. Mechanosensitive pannexin-1 channels mediate microvascular metastatic cell survival. Nat Cell Biol. 2015 Jul;17(7):943–952. doi: 10.1038/ncb3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gulbransen BD, Basashati M, Hirota SA, Gui X, Roberts JA, MacDonald JA, Muruve DA, McKay DM, Beck PL, Mawe GM, Thompson RJ, et al. Activation of neuronal P2X7 receptor-pannexin-1 mediates death of enteric neurons during colitis. Nat Med. 2012 Mar;18(4):600–604. doi: 10.1038/nm.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Penuela S, Gyenis L, Ablack A, Churko JM, Berger AC, Litchfield DW, Lewis JD, Laird DW. Loss of pannexin 1 attenuates melanoma progression by reversion to a melanocytic phenotype. J Biol Chem. 2012 Aug;287(34):29184–29193. doi: 10.1074/jbc.M112.377176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Santiago MF, Veliskova J, Patel NK, Lutz SE, Caille D, Charollais A, Meda P, Scemes E. Targeting pannexin1 improves seizure outcome. PLoS One. 2011 Sep;6(9):e25178. doi: 10.1371/journal.pone.0025178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Orellana JA, Velasquez S, Williams DW, Sáez JC, Berman JW, Eugenin EA. Pannexin1 hemichannels are critical for HIV infection of human primary CD4+ T lymphocytes. J Leukoc Biol. 2013 Sep;94(3):399–407. doi: 10.1189/jlb.0512249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Panchin Y, Kelmanson I, Matz M, Lukyanov K, Usman N, Lukyanov S. A ubiquitous family of putative gap junction molecules. Curr Biol. 2000 Jun;10(13):R473–R474. doi: 10.1016/s0960-9822(00)00576-5. [DOI] [PubMed] [Google Scholar]
- 28.Penuela S, Bhalla R, Gong XQ, Cowan KN, Celetti SJ, Cowan BJ, Bai D, Shao Q, Laird DW. Pannexin 1 and pannexin 3 are glycoproteins that exhibit many distinct characteristics from the connexin family of gap junction proteins. J Cell Sci. 2007 Nov;120:3772–3783. doi: 10.1242/jcs.009514. [DOI] [PubMed] [Google Scholar]
- 29.Hanstein R, Negoro H, Patel NK, Charollais A, Meda P, Spray DC, Suadicani SO, Scemes E. Promises and pitfalls of a Pannexin1 transgenic mouse line. Front Pharmacol. 2013 May;4:61. doi: 10.3389/fphar.2013.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bruzzone R, Hormuzdi SG, Barbe MT, Herb A, Monyer H. Pannexins, a family of gap junction proteins expressed in brain. Proc Natl Acad Sci U S A. 2003 Nov;100(23):13644–13649. doi: 10.1073/pnas.2233464100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Diezmos EF, Sandow SL, Markus I, Shevy Perera D, Lubowski DZ, King DW, Bertrand PP, Liu L. Expression and localization of pannexin-1 hemichannels in human colon in health and disease. Neurogastroenterol Motil. 2013 Jun;25(6):e395–405. doi: 10.1111/nmo.12130. [DOI] [PubMed] [Google Scholar]
- 32.Locovei S, Bao L, Dahl G. Pannexin 1 in erythrocytes: function without a gap. Proc Natl Acad Sci U S A. 2006 May;103(20):7655–7659. doi: 10.1073/pnas.0601037103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taylor KA, Wright JR, Vial C, Evans RJ, Mahaut-Smith MP. Amplification of human platelet activation by surface pannexin-1 channels. J Thromb Haemost. 2014 Jun;12(6):987–998. doi: 10.1111/jth.12566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pelegrin P, Surprenant A. Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. EMBO J. 2006 Nov;25(21):5071–5082. doi: 10.1038/sj.emboj.7601378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bao Y, Chen Y, Ledderose C, Li L, Junger WG. Pannexin 1 channels link chemoattractant receptor signaling to local excitation and global inhibition responses at the front and back of polarized neutrophils. J Biol Chem. 2013 Aug;288(31):22650–22657. doi: 10.1074/jbc.M113.476283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schenk U, Westendorf AM, Radaelli E, Casati A, Ferro M, Fumagalli M, Verderio C, Scanziani E, Grassi F. Purinergic control of T cell activation by ATP released through pannexin-1 hemichannels. Sci Signal. 2008 Sep;1(39):ra6. doi: 10.1126/scisignal.1160583. [DOI] [PubMed] [Google Scholar]
- 37.Kim HY, Kim SJ, Lee SM. Activation of NLRP3 and AIM2 inflammasomes in Kupffer cells in hepatic ischemia/reperfusion. FEBS J. 2015 Jan;282(2):259–270. doi: 10.1111/febs.13123. [DOI] [PubMed] [Google Scholar]
- 38.Le Vasseur M, Lelowski J, Bechberger JF, Sin WC, Naus CC. Pannexin 2 protein expression is not restricted to the CNS. Front Cell Neurosci. 2014 Nov;8:392. doi: 10.3389/fncel.2014.00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boassa D, Nguyen P, Hu J, Ellisman MH, Sosinsky GE. Pannexin2 oligomers localize in the membranes of endosomal vesicles in mammalian cells while Pannexin1 channels traffic to the plasma membrane. Front Cell Neurosci. 2015 Feb;8:468. doi: 10.3389/fncel.2014.00468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bruzzone R, Barbe MT, Jakob NJ, Monyer H. Pharmacological properties of homomeric and heteromeric pannexin hemichannels expressed in Xenopus oocytes. J Neurochem. 2005 Mar;92(5):1033–1043. doi: 10.1111/j.1471-4159.2004.02947.x. [DOI] [PubMed] [Google Scholar]
- 41.Penuela S, Bhalla R, Naq K, Laird DW. Glycosylation regulates pannexin intermixing and cellular localization. Mol Biol Cell. 2009 Oct;20(20):4313–4323. doi: 10.1091/mbc.E09-01-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bond SR, Lau A, Penuela S, Sampaio AV, Underhill TM, Laird DW, Naus CC. Pannexin 3 is a novel target for Runx2, expressed by osteoblasts and mature growth plate chondrocytes. J Bone Miner Res. 2011 Dec;26(12):2911–2922. doi: 10.1002/jbmr.509. [DOI] [PubMed] [Google Scholar]
- 43.Yen MR, Saier MH. Gap junctional proteins of animals: the innexin/pannexin superfamily. Prog Biophys Mol Biol. 2007 May;94(1–2):5–14. doi: 10.1016/j.pbiomolbio.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ambrosi C, Gassmann O, Pranskevich JN, Boassa D, Smock A, Wang J, Dahl G, Steinem C, Sosinsky GE. Pannexin1 and Pannexin2 channels show quaternary similarities to connexons and different oligomerization numbers from each other. J Biol Chem. 2010 Aug;285(32):24420–24431. doi: 10.1074/jbc.M110.115444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boassa D, Ambrosi C, Qiu F, Dahl G, Gaietta G, Sosinsky G. Pannexin1 channels contain a glycosylation site that targets the hexamer to the plasma membrane. J Biol Chem. 2007 Oct;282(43):31733–31743. doi: 10.1074/jbc.M702422200. [DOI] [PubMed] [Google Scholar]
- 46.Vinken M, Henkens T, De Rop E, Fraczek J, Vanhaecke T, Rogiers V. Biology and pathobiology of gap junctional channels in hepatocytes. Hepatology. 2008 Mar;47(3):1077–1088. doi: 10.1002/hep.22049. [DOI] [PubMed] [Google Scholar]
- 47.Lohman AW, Isakson BE. Differentiating connexin hemichannels and pannexin channels in cellular ATP release. FEBS Lett. 2014 Apr;588(8):1379–1388. doi: 10.1016/j.febslet.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sosinsky GE, Boassa D, Dermietzel R, Dufy HS, Laird DW, MacVicar B, Naus CC, Penuela S, Scemes E, Spray DC, Thompson RJ, et al. Pannexin channels are not gap junction hemichannels. Channels (Austin) 2011 May-Jun;5(3):193–197. doi: 10.4161/chan.5.3.15765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dahl G, Locovei S. Pannexin: to gap or not to gap, is that a question? IUBMB Life. 2006 Jul;58(7):409–419. doi: 10.1080/15216540600794526. [DOI] [PubMed] [Google Scholar]
- 50.Lai CP, Bechberger JF, Thompson RJ, MacVicar BA, Bruzzone R, Naus CC. Tumor-suppressive effects of pannexin 1 in C6 glioma cells. Cancer Res. 2007 Feb;67(4):1545–1554. doi: 10.1158/0008-5472.CAN-06-1396. [DOI] [PubMed] [Google Scholar]
- 51.Vanden Abeele F, Bidaux G, Gordienko D, Beck B, Panchin YV, Baranova AV, Ivanov DV, Skryma R, Prevarskaya N. Functional implications of calcium permeability of the channel formed by pannexin 1. J Cell Biol. 2006 Aug;174(4):535–546. doi: 10.1083/jcb.200601115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sahu G, Sukumaran S, Bera AK. Pannexins form gap junctions with electrophysiological and pharmacological properties distinct from connexins. Sci Rep. 2014 Apr;4:4955. doi: 10.1038/srep04955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zoidl G, Petrasch-Parwez E, Ray A, Meier C, Bunse S, Habbes HW, Dahl G, Dermietzel R. Localization of the pannexin1 protein at postsynaptic sites in the cerebral cortex and hippocampus. Neuroscience. 2007 Apr;146(1):9–16. doi: 10.1016/j.neuroscience.2007.01.061. [DOI] [PubMed] [Google Scholar]
- 54.Ransford GA, Fregien N, Qiu F, Dahl G, Conner GE, Salathe M. Pannexin 1 contributes to ATP release in airway epithelia. Am J Respir Cell Mol Biol. 2009 Nov;41(5):525–534. doi: 10.1165/rcmb.2008-0367OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ma W, Hui H, Pelegrin P, Surprenant A. Pharmacological characterization of pannexin-1 currents expressed in mammalian cells. J Pharmacol Exp Ther. 2009 Feb;328(2):409–418. doi: 10.1124/jpet.108.146365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li S, Tomić M, Stojilkovic SS. Characterization of novel Pannexin 1 isoforms from rat pituitary cells and their association with ATP-gated P2X channels. Gen Comp Endocrinol. 2011 Nov;174(2):202–210. doi: 10.1016/j.ygcen.2011.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dufresne J, Cyr DG. Regulation of the pannexin-1 promoter in the rat epididymis. Biol Reprod. 2014 Dec;91(6):143. doi: 10.1095/biolreprod.114.122168. [DOI] [PubMed] [Google Scholar]
- 58.Zhang Y, Laumet G, Chen SR, Hittelman WN, Pan HL. Pannexin-1 Up-regulation in the Dorsal Root Ganglion Contributes to Neuropathic Pain Development. J Biol Chem. 2015 Jun;290(23):14647–14655. doi: 10.1074/jbc.M115.650218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Johnstone SR, Billaud M, Lohman AW, Taddeo EP, Isakson BE. Posttranslational modifications in connexins and pannexins. J Membr Biol. 2012 Jun;245(5–6):319–332. doi: 10.1007/s00232-012-9453-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weilinger NL, Lohman AW, Rakai BD, Ma EM, Bialecki J, Maslieieva V, Rilea T, Bandet MV, Ikuta NT, Scott L, Colicos MA, et al. Metabotropic NMDA receptor signaling couples Src family kinases to pannexin-1 during excitotoxicity. Nat Neurosci. 2016 Mar;19(3):432–442. doi: 10.1038/nn.4236. [DOI] [PubMed] [Google Scholar]
- 61.Lohman AW, Weaver JL, Billaud M, Sandilos JK, Griffiths R, Straub AC, Penuela S, Leitinger N, Laird DW, Bayliss DA, Isakson BE. S-nitrosylation inhibits pannexin 1 channel function. J Biol Chem. 2012 Nov;287(47):39602–39612. doi: 10.1074/jbc.M112.397976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Poornima V, Vallabhaneni S, Mukhopadhyay M, Bera AK. Nitric oxide inhibits the pannexin 1 channel through a cGMP-PKG dependent pathway. Nitric Oxide. 2015 May;47:77–84. doi: 10.1016/j.niox.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 63.Penuela S, Lohman AW, Lai W, Gyenis L, Litchfield DW, Isakson BE, Laird DW. Diverse post-translational modifications of the pannexin family of channel-forming proteins. Channels (Austin) 2014;8(2):124–130. doi: 10.4161/chan.27422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Locovei S, Wang J, Dahl G. Activation of pannexin 1 channels by ATP through P2Y receptors and by cytoplasmic calcium. FEBS Lett. 2006 Jan;580(1):239–244. doi: 10.1016/j.febslet.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 65.Sridharan M, Adderley SP, Bowles EA, Egan TM, Stephenson AH, Ellsworth M, Sprague RS. Pannexin 1 is the conduit for low oxygen tension-induced ATP release from human erythrocytes. Am J Physiol Heart Circ Physiol. 2010 Oct;299(4):H1146–H1152. doi: 10.1152/ajpheart.00301.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thompson RJ, Zhou N, MacVicar BA. Ischemia opens neuronal gap junction hemichannels. Science. 2006 May;312(5775):924–927. doi: 10.1126/science.1126241. [DOI] [PubMed] [Google Scholar]
- 67.Bao L, Locovei S, Dahl G. Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS Lett. 2004 Aug;572(1–3):65–68. doi: 10.1016/j.febslet.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 68.Adamson SE, Meher AK, Chiu Y, Sandilos JK, Oberholtzer NP, Walker NN, Hargett SR, Seaman SA, Peirce-Cottler SM, Isakson BE, McNamara CA, et al. Pannexin 1 is required for full activation of insulin-stimulated glucose uptake in adipocytes. Molecular Metabolism. 2015 Sep;4(9):610–618. doi: 10.1016/j.molmet.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang J, Ma M, Locovei S, Keane RW, Dahl G. Modulation of membrane channel currents by gap junction protein mimetic peptides: size matters. Am J Physiol Cell Physiol. 2007 Sep;293(3):C1112–C1119. doi: 10.1152/ajpcell.00097.2007. [DOI] [PubMed] [Google Scholar]
- 70.Krick S, Wang J, St-Pierre M, Gonzalez C, Dahl G, Salathe M. Dual Oxidase 2 (Duox2) Regulates Pannexin 1-mediated ATP Release in Primary Human Airway Epithelial Cells via Changes in Intracellular pH and Not H2O2 Production. J Biol Chem. 2016 Mar;291(12):6423–6432. doi: 10.1074/jbc.M115.664854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kurtenbach S, Whyte-Fagundes P, Gelis L, Kurthenbach S, Brazil E, Zoidl C, Hatt H, Shestopalov VI, Zoidl G. Investigation of olfactory function in a Panx1 knock out mouse model. Front Cell Neurosci. 2014 Sep;8:266. doi: 10.3389/fncel.2014.00266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Riquelme MA, Cea LA, Vega JL, Boric MP, Monyer H, Bennett MV, Frank M, Willecke K, Sáez JC. The ATP required for potentiation of skeletal muscle contraction is released via pannexin hemichannels. Neuropharmacology. 2013 Dec;75:594–603. doi: 10.1016/j.neuropharm.2013.03.022. [DOI] [PubMed] [Google Scholar]
- 73.Billaud M, Lohman AW, Straub AC, Looft-Wilson R, Johnstone SR, Araj CA, Best AK, Chekeni FB, Ravichandran KS, Penuela S, Laird DW, et al. Pannexin1 regulates α1-adrenergic receptor-mediated vasoconstriction. Circ Res. 2011 Jun;109(1):80–85. doi: 10.1161/CIRCRESAHA.110.237594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Iwamoto T, Nakamura T, Doyle A, Ishikawa M, de Vega S, Fukumoto S, Yamada Y. Pannexin 3 regulates intracellular ATP/cAMP levels and promotes chondrocyte differentiation. J Biol Chem. 2010 Jun;285(24):18948–18958. doi: 10.1074/jbc.M110.127027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Romanov RA, Bystrova MF, Rogachevskaya OA, Sadovnikov VB, Shestopalov VI, Kolesnikov SS. The ATP permeability of pannexin 1 channels in a heterologous system and in mammalian taste cells is dispensable. J Cell Sci. 2012 Nov;125(22):5514–5523. doi: 10.1242/jcs.111062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ma W, Compan V, Zheng W, Martin E, North RA, Verkhrasky A, Surprenant A. Pannexin 1 forms an anion-selective channel. Pflugers Arch. 2012 Apr;463(4):585–592. doi: 10.1007/s00424-012-1077-z. [DOI] [PubMed] [Google Scholar]
- 77.Wang J, Ambrosi C, Qiu F, Jackson DG, Sosinsky G, Dahl G. The membrane protein Pannexin1 forms two open-channel conformations depending on the mode of activation. Sci Signal. 2014 Jul;7(335):ra69. doi: 10.1126/scisignal.2005431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chiu YH, Ravichandran KS, Bayliss DA. Intrinsic properties and regulation of Pannexin 1 channel. Channels (Austin) 2014 Jan;8(2):103–109. doi: 10.4161/chan.27545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dolmatova E, Spagnol G, Boassa D, Baum JR, Keith K, Ambrosi C, Kontaridis MI, Sorgen PL, Sosinsky GE, Dufy HS. Cardiomyocyte ATP release through pannexin 1 aids in early fibroblast activation. Am J Physiol Heart Circ Physiol. 2012 Nov;303(10):H1208–H1218. doi: 10.1152/ajpheart.00251.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ishikawa M, Iwamoto T, Nakamura T, Doyle A, Fukumoto S, Yamada Y. Pannexin 3 functions as an ER Ca(2+) channel, hemichannel, and gap junction to promote osteoblast differentiation. J Cell Biol. 2011 Jun;193(7):1257–1274. doi: 10.1083/jcb.201101050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ishikawa M, Williams GL, Ikeuchi T, Sakai K, Fukumoto S, Yamada Y. Pannexin 3 and connexin 43 modulate skeletal development through their distinct functions and expression patterns. J Cell Sci. 2016 Mar;129(5):1018–1030. doi: 10.1242/jcs.176883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Moon PM, Penuela S, Barr K, Khan S, Pin CL, Welch I, Attur M, Abramson SB, Laird DW, Beier F. Deletion of Panx3 Prevents the Development of Surgically Induced Osteoarthritis. J Mol Med (Berl) 2015 Aug;93(8):845–856. doi: 10.1007/s00109-015-1311-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Anselmi F, Hernandez VH, Crispino G, Seydel A, Ortolano S, Roper SD, Kessaris N, Richarson W, Rickheit G, Filippov MA, Monyer H, et al. ATP release through connexin hemichannels and gap junction transfer of second messengers propagate Ca2+ signals across the inner ear. Proc Natl Acad Sci U S A. 2008 Dec;105(48):18770–18775. doi: 10.1073/pnas.0800793105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bargiotas P, Krenz A, Hormuzdi SG, Ridder DA, Herb A, Barakat W, Penuela S, von Engelhardt J, Monyer H, Schwaninger M. Pannexins in ischemia-induced neurodegeneration. Proc Natl Acad Sci U S A. 2011 Dec;108(51):20772–20777. doi: 10.1073/pnas.1018262108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Penuela S, Kelly JJ, Churko JM, Barr KJ, Berger AC, Laird DW. Panx1 regulates cellular properties of keratinocytes and dermal fibroblasts in skin development and wound healing. J Invest Dermatol. 2014 Jul;134(7):2026–2035. doi: 10.1038/jid.2014.86. [DOI] [PubMed] [Google Scholar]
- 86.Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009 Dec;1(6):a001651. doi: 10.1101/cshperspect.a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ting JP, Willingham SB, Bergstralh DT. NLRs at the intersection of cell death and immunity. Nat Rev Immunol. 2008 May;8(5):372–379. doi: 10.1038/nri2296. [DOI] [PubMed] [Google Scholar]
- 88.Franchi L, Eigenbrod T, Muñoz-Planillo R, Nuñez G. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat Immunol. 2009 Mar;10(3):241–247. doi: 10.1038/ni.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhou Y, Lu M, Du RH, Qiao C, Jiang CY, Zhang KZ, Ding JH, Hu G. MicroRNA-7 targets Nod-like receptor protein 3 inflammasome to modulate neuroinflammation in the pathogenesis of Parkinson's disease. Mol Neurodegener. 2016 Apr;11:28. doi: 10.1186/s13024-016-0094-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jo EK, Kim JM, Shin DM, Sasakawa C. Molecular mechanisms regulating NLRP3 inflammasome activation. Cell Mol Immunol. 2016 Mar;13(2):148–159. doi: 10.1038/cmi.2015.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002 Aug;10(2):417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 92.Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, Fernandez-Alnemri T, Wu J, Monks BG, Fitzgerald KA, Hornung V, et al. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009 Jul;183(2):787–791. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Muñoz-Planillo R, Kuffa P, Martínez-Colón G, Smith BL, Rajendiran TM, Núñez G. K+ efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity. 2013 Jun;38(6):1142–1153. doi: 10.1016/j.immuni.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pétrilli V, Papin S, Dostert C, Mayor A, Martinon F, Tschopp J. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 2007 Sep;14(9):1583–1589. doi: 10.1038/sj.cdd.4402195. [DOI] [PubMed] [Google Scholar]
- 95.Qiu F, Dahl G. A permeant regulating its permeation pore: inhibition of pannexin 1 channels by ATP. Am J Physiol Cell Physiol. 2009 Feb;296(2):C250–255. doi: 10.1152/ajpcell.00433.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Orellana JA, Montero TD, von Bernhardi R. Astrocytes inhibit nitric oxide-dependent Ca(2+) dynamics in activated microglia: involvement of ATP released via pannexin 1 channels. Glia. 2013 Dec;61(12):2023–2037. doi: 10.1002/glia.22573. [DOI] [PubMed] [Google Scholar]
- 97.Riteau N, Baron L, Villeret B, Guillou N, Savigny F, Ryffel B, Rassendren F, Le Bert M, Gombault A, Couillin I. ATP release and purinergic signaling: a common pathway for particle-mediated inflammasome activation. Cell Death Dis. 2012 Oct;3:e403. doi: 10.1038/cddis.2012.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, Fitzgerald KA, Latz E. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008 Aug;9(8):847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dostert C, Pétrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008 May;320(5876):674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Marina-García N, Franchi L, Kim YG, Miller D, McDonald C, Boons GJ, Núñez G. Pannexin-1-mediated intracellular delivery of muramyl dipeptide induces caspase-1 activation via cryopyrin/NLRP3 independently of Nod2. J Immunol. 2008 Mar;180(6):4050–4057. doi: 10.4049/jimmunol.180.6.4050. [DOI] [PubMed] [Google Scholar]
- 101.Hsu LC, Ali SR, McGillivray S, Tsenq PH, Mariathasan S, Humke EW, Eckmann L, Powell JJ, Nizet V, Dixit VM, Karin M. A NOD2-NALP1 complex mediates caspase-1-dependent IL-1beta secretion in response to Bacillus anthracis infection and muramyl dipeptide. Proc Natl Acad Sci U S A. 2008 Jun;105(22):7803–7808. doi: 10.1073/pnas.0802726105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Qu Y, Misaghi S, Newton K, Gilmour LL, Louie S, Cupp JE, Dubyak GR, Hackos D, Dixit VM. Pannexin-1 is required for ATP release during apoptosis but not for inflammasome activation. J Immunol. 2011 Jun;186(11):6553–6561. doi: 10.4049/jimmunol.1100478. [DOI] [PubMed] [Google Scholar]
- 103.de Souza LF, Barreto F, da Silva EG, Andrades ME, Guimarães EL, Behr GA, Moreira JC, Bernard EA. Regulation of LPS stimulated ROS production in peritoneal macrophages from alloxan-induced diabetic rats: involvement of high glucose and PPARgamma. Life Sci. 2007 Jun;81(2):153–159. doi: 10.1016/j.lfs.2007.04.035. [DOI] [PubMed] [Google Scholar]
- 104.Karmakar M, Katsnelson MA, Dubyak GR, Pearlman E. Neutrophil P2X7 receptors mediate NLRP3 inflammasome-dependent IL-1β secretion in response to ATP. Nat Commun. 2016 Feb;7:10555. doi: 10.1038/ncomms10555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lemaire I, Falzoni S, Zhang B, Pellegatti P, Di Virgilio F. The P2X7 receptor and Pannexin-1 are both required for the promotion of multinucleated macrophages by the inflammatory cytokine GM-CSF. J Immunol. 2011 Oct;187(7):3878–3887. doi: 10.4049/jimmunol.1002780. [DOI] [PubMed] [Google Scholar]
- 106.McNally AK, Anderson JM. Multinucleated giant cell formation exhibits features of phagocytosis with participation of the endoplasmic reticulum. Exp Mol Pathol. 2005 Oct;79(2):126–135. doi: 10.1016/j.yexmp.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 107.McNally AK, Anderson JM. Macrophage fusion and multinucleated giant cells of inflammation. Adv Exp Med Biol. 2011 Jan;713:97–111. doi: 10.1007/978-94-007-0763-4_7. [DOI] [PubMed] [Google Scholar]
- 108.Lemaire I, Falzoni S, Leduc N, Zhang B, Pellegatti P, Adinolfi E, Chiozzi P, Di Virgilio F. Involvement of the purinergic P2X7 receptor in the formation of multinucleated giant cells. J Immunol. 2006 Nov;177(10):7257–7265. doi: 10.4049/jimmunol.177.10.7257. [DOI] [PubMed] [Google Scholar]
- 109.Woehrle T, Yip L, Elkhal A, Sumi Y, Chen Y, Yao Y, Insel PA, Junger WG. Pannexin-1 hemichannel-mediated ATP release together with P2X1 and P2X4 receptors regulate T-cell activation at the immune synapse. Blood. 2010 Nov;116(18):3475–3484. doi: 10.1182/blood-2010-04-277707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yip L, Woehrle T, Corriden R, Hirsh M, Chen Y, Inoue Y, Ferrari V, Insel PA, Junger WG. Autocrine regulation of T-cell activation by ATP release and P2X7 receptors. FASEB J. 2009 Jun;23(6):1685–1693. doi: 10.1096/fj.08-126458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Porcellini S, Traggiai E, Schenk U, Ferrera D, Matteoli M, Lanzavecchia A, Michalak M, Grassi F. Regulation of peripheral T cell activation by calreticulin. J Exp Med. 2006 Feb;203(2):461–471. doi: 10.1084/jem.20051519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Maslieieva V, Thompson RJ. A critical role for pannexin-1 in activation of innate immune cells of the choroid plexus. Channels (Austin) 2014 Mar;8(2):131–141. doi: 10.4161/chan.27653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007 Jun;35(4):495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ashe PC, Berry MD. Apoptotic signaling cascades. Prog Neuropsychopharmacol Biol Psychiatry. 2003 Apr;27(2):199–214. doi: 10.1016/S0278-5846(03)00016-2. [DOI] [PubMed] [Google Scholar]
- 115.Czabotar PE, Lessene G, Strasser A, Adams JM. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat Rev Mol Cell Biol. 2014 Jan;15(1):49–63. doi: 10.1038/nrm3722. [DOI] [PubMed] [Google Scholar]
- 116.Zhang L, Deng T, Sun Y, Liu K, Yang Y, Zheng X. Role for nitric oxide in permeability of hippocampal neuronal hemichannels during oxygen glucose deprivation. J Neurosci Res. 2008 Aug;86(10):2281–2291. doi: 10.1002/jnr.21675. [DOI] [PubMed] [Google Scholar]
- 117.Poon IK, Chiu YH, Armstrong AJ, Kinchen JM, Juncadella IJ, Bayliss DA, Ravichandran KS. Unexpected link between an antibiotic, pannexin channels and apoptosis. Nature. 2014 Mar;507(7492):329–334. doi: 10.1038/nature13147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cheung WY, Fritton JC, Morgan SA, Seref-Ferlengez Z, Basta-Pljakic J, Thi MM, Suadicani SO, Spray DC, Majeska RJ, Schaffler MB. Pannexin-1 and P2X7-Receptor Are Required for Apoptotic Osteocytes in Fatigued Bone to Trigger RANKL Production in Neighboring Bystander Osteocytes. J Bone Miner Res. 2016 Apr;31(4):890–899. doi: 10.1002/jbmr.2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: the calcium-apoptosis link. Nat Rev Mol Cell Biol. 2003 Jul;4(7):552–565. doi: 10.1038/nrm1150. [DOI] [PubMed] [Google Scholar]
- 120.Szalai G, Krishnamurthy R, Hajnóczky G. Apoptosis driven by IP(3)-linked mitochondrial calcium signals. EMBO J. 1999 Nov;18(22):6349–6361. doi: 10.1093/emboj/18.22.6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sandilos JK, Chiu YH, Chekeni FB, Armstrong AJ, Walk SF, Ravichandran KS, Bayliss DA. Pannexin 1, an ATP release channel, is activated by caspase cleavage of its pore-associated C-terminal autoinhibitory region. J Biol Chem. 2012 Mar;287(14):11303–11311. doi: 10.1074/jbc.M111.323378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Engelhardt K, Schmidt M, Tenbusch M, Dermietzel R. Effects on channel properties and induction of cell death induced by c-terminal truncations of pannexin1 depend on domain length. J Membr Biol. 2015 Apr;248(2):285–294. doi: 10.1007/s00232-014-9767-4. [DOI] [PubMed] [Google Scholar]
- 123.de Gassart A, Martinon F. Pyroptosis: Caspase-11 Unlocks the Gates of Death. Immunity. 2015 Nov;43(5):835–837. doi: 10.1016/j.immuni.2015.10.024. [DOI] [PubMed] [Google Scholar]
- 124.Edgeworth JD, Spencer J, Phalipon A, Griffin GE, Sansonetti PJ. Cytotoxicity and interleukin-1beta processing following Shigella flexneri infection of human monocyte-derived dendritic cells. Eur J Immunol. 2002 May;32(5):1464–1471. doi: 10.1002/1521-4141(200205)32:5<1464::AID-IMMU1464>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 125.Chen KW, Groß CJ, Sotomayor FV, Stacey KJ, Tschopp J, Sweet MJ, Schroder K. The neutrophil NLRC4 inflammasome selectively promotes IL-1β maturation without pyroptosis during acute Salmonella challenge. Cell Rep. 2014 Jul;8(2):570–582. doi: 10.1016/j.celrep.2014.06.028. [DOI] [PubMed] [Google Scholar]
- 126.Miao EA, Rajan JV, Aderem A. Caspase-1-induced pyroptotic cell death. Immunol Rev. 2011 Sep;243(1):206–214. doi: 10.1111/j.1600-065X.2011.01044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol. 2009 Feb;7(2):99–109. doi: 10.1038/nrmicro2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hagar JA, Powell DA, Aachoui Y, Ernst RK, Miao EA. Cytoplasmic LPS activates caspase-11: implications in TLR4-independent endotoxic shock. Science. 2013 Sep;341(6151):1250–1253. doi: 10.1126/science.1240988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kayagaki N, Warming S, Lamkanfi M, Vande Walle L, Louie S, Dong J, Newborn K, Qu Y, Liu J, Heldens S, Zhang J, et al. Non-canonical inflammasome activation targets caspase-11. Nature. 2011 Oct;479(7371):117–121. doi: 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- 130.Kayagaki N, Wong MT, Stowe IB, Ramani SR, Gonzalez LC, Akashi-Takamura S, Miyake K, Zhang J, Lee WP, Muszynski A, Forrberg LS, et al. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science. 2013 Sep;341(6151):1246–1249. doi: 10.1126/science.1240248. [DOI] [PubMed] [Google Scholar]
- 131.Shi J, Zhao Y, Wang Y, Gao W, Ding J, Li P, Hu L, Shao F. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature. 2014 Oct;514(7521):187–192. doi: 10.1038/nature13683. [DOI] [PubMed] [Google Scholar]
- 132.Glick D, Barth S, Macleod KF. Autophagy: cellular and molecular mechanisms. J Pathol. 2010 May;221(1):3–12. doi: 10.1002/path.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mariño G, Madeo F, Kroemer G. Autophagy for tissue homeostasis and neuroprotection. Curr Opin Cell Biol. 2011 Apr;23(2):198–206. doi: 10.1016/j.ceb.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 134.Kroemer G, Levine B. Autophagic cell death: the story of a misnomer. Nat Rev Mol Cell Biol. 2008 Dec;9(12):1004–1010. doi: 10.1038/nrm2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Martins I, Wang Y, Michaud M, Ma Y, Sukkurwala AQ, Shen S, Kepp O, Métivier D, Galluzi L, Perfettini JL, Zitvogel L, et al. Molecular mechanisms of ATP secretion during immunogenic cell death. Cell Death Differ. 2014 Jan;21(1):79–91. doi: 10.1038/cdd.2013.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ayna G, Krysko DV, Kaczmakek A, Petrovski G, Vandenabeele P, Fésüs L. ATP release from dying autophagic cells and their phagocytosis are crucial for inflammasome activation in macrophages. PLoS One. 2012 Jun;7(6):e40069. doi: 10.1371/journal.pone.0040069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ghiringhelli F, Apetoh L, Tesniere A, Aymeric L, Ma Y, Ortiz C, Vermaelen K, Panaretakis T, Mignot G, Ullrich E, Perfettini JL, et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med. 2009 Oct;15(10):1170–1178. doi: 10.1038/nm.2028. [DOI] [PubMed] [Google Scholar]
- 138.Apetoh L, Ghiringhelli F, Tesniere A, Criollo A, Ortiz C, Lidereau R, Mariette C, Chaput N, Mira JP, Delaloge S, André F, et al. The interaction between HMGB1 and TLR4 dictates the outcome of anticancer chemotherapy and radiotherapy. Immunol Rev. 2007 Dec;220:47–59. doi: 10.1111/j.1600-065X.2007.00573.x. [DOI] [PubMed] [Google Scholar]
- 139.Bargiotas P, Krenz A, Monyer H, Schwaninger M. Functional outcome of pannexin-deficient mice after cerebral ischemia. Channels (Austin) 2012 Nov;6(6):453–456. doi: 10.4161/chan.22315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Orellana JA, Froger N, Jiang JX, Bennett MV, Naus CC, Giaume C, Sáez JC. ATP and glutamate released via astroglial connexin 43 hemichannels mediate neuronal death through activation of pannexin 1 hemichannels. J Neurochem. 2011 Sep;118(5):826–840. doi: 10.1111/j.1471-4159.2011.07210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Prochnow N, Abdulazim A, Kurtenbach S, Wildförster V, Dvoriantchikova G, Hanske J, Petrasch-Parwez E, Shestopalov VI, Dermietzel R, Manahan-Vaughan D, Zoidl G. Pannexin1 stabilizes synaptic plasticity and is needed for learning. PLoS One. 2012 Dec;7(12):e51767. doi: 10.1371/journal.pone.0051767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhao HB, Zhu Y, Liang C, Chen J. Pannexin 1 deficiency can induce hearing loss. Biochem Biophys Res Commun. 2015 Jul;463(1–2):143–147. doi: 10.1016/j.bbrc.2015.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Shao Q, Lindstrom K, Shi R, Kelly J, Schroeder A, Juusola J, Levine KL, Esseltine JL, Penuela S, Jackson MF, Laird DW. A germline variant in PANX1 has reduced channel function and is associated with multisystem dysfunction. J Biol Chem. 2016 Jun;291(24):12432–12443. doi: 10.1074/jbc.M116.717934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lai CP, Bechberger JF, Naus CC. Pannexin2 as a novel growth regulator in C6 glioma cells. Oncogene. 2009 Dec;28(49):4402–4408. doi: 10.1038/onc.2009.283. [DOI] [PubMed] [Google Scholar]