Abstract
Background
Allergy vaccines should be easily applicable, safe, and efficacious. For Bet v 1–mediated birch pollen and associated food allergies, a single wild-type allergen does not provide a complete solution.
Objective
We aimed to combine immunologically relevant epitopes of Bet v 1 and the 2 clinically most important related food allergens from apple and hazelnut to a single hybrid protein, termed MBC4.
Methods
After identification of T cell epitope–containing parts on each of the 3 parental allergens, the hybrid molecule was designed to cover relevant epitopes and evaluated in silico. Thereby a mutation was introduced into the hybrid sequence, which should alter the secondary structure without compromising the immunogenic properties of the molecule.
Results
MBC4 and the parental allergens were purified to homogeneity. Analyses of secondary structure elements revealed substantial changes rendering the hybrid de facto nonreactive with patients’ serum IgE. Nevertheless, the protein was monomeric in solution. MBC4 was able to activate T-cell lines from donors with birch pollen allergy and from mice immunized with the parental allergens. Moreover, on immunization of mice and rabbits, MBC4 induced cross-reactive IgG antibodies, which were able to block the binding of human serum IgE.
Conclusion
Directed epitope rearrangements combined with a knowledge-based structural modification resulted in a protein unable to bind IgE from allergic patients. Still, properties to activate specific T cells or induce blocking antibodies were conserved. This suggests that MBC4 is a suitable vaccine candidate for the simultaneous treatment of Bet v 1 and associated food allergies.
Keywords: Birch pollen allergy, Bet v 1, birch pollen–associated food allergy, allergy vaccine candidate, molecular allergology
Birch pollen allergy is dominated by a single disease-eliciting allergen, namely Bet v 1, with reactivity rates of greater than 90% among patients with birch pollen allergy. Moreover, it has been reported that more than 70% of patients with birch pollen allergy react to at least 1 Bet v 1–associated allergenic food source, including pomaceous and stone fruits, vegetables, nuts, and legumes. With 80% reactivity among patients with food allergy, apple was most frequently recognized, followed by hazelnut, which triggered allergic symptoms in 59% of patients with pollen-food syndrome (PFS).1
For the sustainable treatment of birch pollen allergy, it has been shown that extract-based therapeutics can be successfully substituted by purified rBet v 1 applied either by means of subcutaneous or sublingual immunotherapy.2,3 However, successful birch pollen allergen-specific immunotherapy (AIT) does not necessarily correlate with the amelioration of concomitant food allergies. In a study by Kinaciyan et al,4 SLIT performed with birch pollen extract induced protective Bet v 1–specific IgG4 antibodies in test subjects, which were not cross-reactive with Mal d 1 from apple. Moreover, the treatment reduced exclusively the responsiveness of Bet v 1–specific T cells, and the T-cell response to Mal d 1 remained practically unaltered.4
In a follow-up the presence of specific blocking IgG4 antibodies, as well as the IgG4/IgE ratio, was linked to food tolerance. Such blocking antibodies can be either innately present in food-tolerant patients or can be induced through AIT.1,5 The majority of AIT-induced IgG4 is thought to recognize IgE epitopes on the allergens; however, also allergen-specific unique IgG4 specificities, which do not overlap with the IgE epitope, will eventually develop during therapy.5
In light of the above, it is necessary to tackle Bet v 1–associated food allergies at both the B- and T-cell levels, and AIT based on wild-type (WT) Bet v 1 seems insufficient for this task. Therefore the structure of Bet v 1, which is immunologically distinct from the structures of the related food allergens from apple and hazelnut, might be the cause of this observation.6
Thus we aimed to design a hybrid molecule by combining immunologically relevant epitopes of Mal d 1 from apple and Cor a 1.04 from hazelnut with the major birch pollen allergen. Recently, we successfully developed a strategy to produce a vaccine candidate for patients who are multisensitized to Bet v 1–like Fagales pollen allergens by generating a hybrid molecule. Sequence regions of the 5 most important allergens from birch, hazel, alder, oak, and hornbeam were combined into a single protein that showed reduced binding of patients’ IgE but preserved immunogenicity.7 In analogy to this study, we identified T-cell epitopes on Mal d 1, Bet v 1, and Cor a 1.04 and combined T cell–reactive stretches to a hybrid protein (MBC) showing the same overall length as the parental allergens. To reduce IgE binding of the protein, we generated a fold variant of our hybrid (MBC4) by introducing a mutation previously identified as important for Bet v 1 to adopt its native fold.8,9 This particular mutation was shown to conserve sufficient potential IgG-reactive secondary structure elements on the surface of Bet v 1 to efficiently induce blocking antibodies while preexisting IgE epitopes would be eliminated. In addition, the structural modification should not affect the immunogenic properties of the fold variant.
Methods
Patients and sera
Patients with birch pollen allergy and concomitant PFS were selected based on case history, positive skin prick test responses, and/or in vitro IgE detection (CAP System; Thermo Fisher Scientific, Phadia AB, Uppsala, Sweden; see Table E1 in this article’s Online Repository at www.jacionline.org). Inclusion criteria were a CAP class of greater than 3 to birch and greater than 1 to apple and hazelnut. Experiments with patients’ sera were approved by the Ethics Committee of the University of Vienna (EK028/2006) and Salzburg (415-E/1398/4-2011). Written informed consent was obtained from all subjects included in the study.
Design, in silico evaluation, and cloning of MBC and MBC4
Hybrid proteins were designed and evaluated in silico, as described in the Methods section in this article’s Online Repository at www.jacionline.org. Thereafter, the hybrid allergen MBC was cloned by means of PCR recombination of 3 overlapping fragments from the genes Mal d 1.0108 (AF126402), Bet v 1.0102 (X77266), and Cor a 1.0401 (AF136945), respectively. Subsequently, the gene was cloned into a pET28b vector (Novagen, Merck Millipore, Billerica, Mass). By using MBC as a template, a mutation was introduced in the Bet v 1.0102 part of the molecule to generate the MBC4. Moreover, in MBC4 the C-terminal cysteine of Cor a 1.0401 was mutated to a serine to abrogate dimerization.6 A detailed description is provided in the Methods section in this article’s Online Repository.
Expression and purification of recombinant proteins
The parental allergens Bet v 1.0101, Mal d 1.0108, and Cor a 1.0401 were produced as nonfusion proteins, as described, and are referred to as Bet v 1, Mal d 1, and Cor a 1.04, respectively, in the following.6,8 Recombinant MBC was expressed in Escherichia coli and produced from the soluble fraction, whereas MBC4 was produced from inclusion E coli bodies and refolded after purification. Both methods are described in the Methods section in this article’s Online Repository. rBet v 1.0201 used as reference material was produced, as previously described.10 Endotoxin levels of recombinant proteins were determined by using HEK-Blue mTLR4 (InvivoGen, San Diego, Calif) cell assays, according to the manufacturer’s instructions.
Physicochemical analyses of recombinant proteins
Recombinant proteins were analyzed, as previously described, in terms of quantity, primary and secondary structure, aggregation behavior, and ligand binding by means of amino acid analysis, mass spectrometry, circular dichroism (CD) and Fourier transform infrared spectroscopy, dynamic light scattering, and 1-anilino-8-naphthalene sulfonate (ANS)–binding assays, respectively.6,8
In vitro assessment of endosomal/lysosomal proteolysis
The proteolytic stability of all proteins was determined by using degradome assays, as previously described.11 Briefly, the microsomal fraction of the JAWS II cells (ATCC no. CRL-11904) was obtained by means of ultracentrifugation. Incubation of 5 μg of protein and 7.5 μg of microsomes in 100 mmol/L citrate buffer (pH 4.8) and 2 mmol/L dithiothreitol was performed for 0, 0.5, 1, 3, 6, 12, 24, and 48 hours at 37°C. The reaction was stopped at 95°C for 5 minutes. Samples were analyzed by using SDS-PAGE and tandem mass spectrometry.
Human T-cell studies
Bet v 1–specific T-cell lines (TCLs) were expanded from PBMCs of patients with birch pollen allergy, as previously described.12 TCLs were stimulated with recombinant proteins or a panel of synthetic 12-mer peptides covering the sequence of Bet v 1.0101 in the presence of irradiated (60 Gr) autologous PBMCs as antigen-presenting cells (APCs). Stimulation indices were calculated as the ratio between counts per minute obtained in cultures with T cells plus APCs and antigenic stimulus and counts per minute obtained in T-cell cultures containing only APCs.
Antibody-binding analyses
Binding of allergic patients’ serum IgE to parental allergens, as well as MBC4, was compared by using ELISAs and mediator release assays with rat basophil leukemia (RBL) 2H3 cells transfected with the α chain of the FcεRI receptor and passively sensitized with patients’ IgE, as described in the Methods section in this article’s Online Repository. Antigen-specific murine IgG1 and IgG2a levels were determined by means of ELISAs, and murine IgE levels were determined by means of mediator release assays, respectively. Both methods are described in the Methods section in this article’s Online Repository.
Mouse immunization model
Eight-week-old female BALB/c mice purchased from Janvier (Saint Berthevin, France) were housed under specific pathogen-free conditions. Mice were immunized with 5 μg of protein adsorbed to Alu-Gel S (Serva, Heidelberg, Germany) in 2 injections of 50 μL administered subcutaneously into the backs of the animals and boosted on days 7, 14, and 21. Sera were collected on days −3 and 28, respectively. Five animals per group were tested. At day 28, animals were killed, and lymphocytes were harvested from the spleen, as previously described.13 Briefly, spleens were homogenized, and after erythrocyte lysis, cells were counted and used for cytokine analysis. ELISpot assays were performed according to the manufacturer’s instructions (Merck Millipore). Splenocytes (2 × 105 cells/well) were restimulated with either 40 μg/mL parental allergen or MBC4 protein, respectively, and secreted cytokines were analyzed by using matched pair mAbs for IL-4, IL-5, and IFN-γ detection (eBioscience, San Diego, Calif). Splenocyte supernatants were analyzed by using the ProcartaPlex multiplex system, according to the manufacturer’s instructions (eBioscience), and measurements were performed with the MAGPIX System (Merck Millipore). All mouse experiments were conducted according to the national guidelines approved by the Austrian Federal Ministry of Science, Research and Economy (BMWF-66.012/0010-II/3b/2013).
Rabbit immunization model
Two New Zealand white rabbits were immunized with 200 μg of MBC4 adsorbed to Alu-Gel S (Serva) in a volume of 500 μL; booster immunizations were given on days 14, 28, and 42; and final bleeding was performed on day 69 (Charles River, Chatillon sur Chalaronne, France). Rabbit total IgG was purified from immune sera through Protein G Sepharose (GE Healthcare, Fairfield, Conn). Inhibition mediator release assays were performed with sera of patients with Bet v 1–mediated birch pollen allergy and concomitant oral allergy syndrome to apple and hazelnut. Bet v 1 (0.1 ng) or Cor a 1.04 and Mal d 1 (1 ng), respectively, were preincubated with 15 μg of total rabbit IgG for 1 hour in Tyrode buffer at room temperature and thereafter used to trigger mediator release of IgE-loaded RBL cells. Serum from a patient with ragweed pollen allergy was used as a control; here, mediator release was trigger with 1 ng of Amb a 1 after preincubation with rabbit IgG.
Results
In silico design and cloning of hybrid molecules
The hybrid molecule MBC was designed by combining T cell epitope–containing stretches of Mal d 1, Bet v 1, and Cor a 1.04, respectively (see Fig E1 in this article’s Online Repository at www.jacionline.org). In general, high levels of T-cell cross-reactivity between Bet v 1 and homologous food allergens have been reported.14–17 To assemble the hybrid allergen, we used the isoform Bet v 1.0102, formerly Bet v 1d, which shows limited IgE reactivity but high immunogenicity.16 Within Bet v 1, T-cell epitopes are scattered throughout the entire sequence,12 whereas the central region of Bet v 1 was shown to be important for the structure of the allergen.8 T-cell epitope mapping of Mal d 1 (isoform 0108) revealed a clustering of epitopes at the N-terminus and within the central region of the allergen.14 Moreover, hazelnut Cor a 1.04 harbors a unique immunodominant T-cell epitope (residues 142-153) that shows limited cross-reactivity with pollen allergens but cross-reacts with the carrot homologue of Bet v 1, Dau c 1.15
Taking this into consideration, the arrangement of the protein stretches were Mal d 1, Bet v 1, and Cor a 1.04. For the crossover events, structurally conserved regions were selected. Therefore primers were designed spanning those regions and coding at the 5′ end for the N-terminal and at the 3′ end for the C-terminal fusion partner. By using these primer pairs, regions of the 3 parental allergens with overlapping ends were amplified and recombined to full-length genes by using PCR.
We applied statistical scoring functions, which are versatile tools in structural bioinformatics, to derive a qualitative ranking of the fit of the different allergen sequences to the canonical Bet v 1 fold. This allowed a knowledge-based evaluation and prediction of the structural behavior of hybrid molecules. One hundred models were generated per allergen sequence. Randomly sampled pairs of models were superimposed, resulting in a root mean square deviation of 0.3 Å (SD, 0.2 Å 10,000 samples), indicating that the models closely resemble the Bet v 1 fold. Statistical scoring functions were applied to determine how well different sequences would fit to a given structure. The higher the score, the less the given fold represents the native folding behavior of Bet v 1–like proteins. We found that the sequence of the hybrid molecule MBC was still compatible with that of the canonical Bet v 1 fold. Thus a variant of MBC, MBC4, was designed by incorporating amino acids previously identified as detrimental for the Bet v 1 structure into the backbone of MBC.8 This resulted in a protein that had clearly the lowest compatibility with the canonical Bet v 1 fold (see Fig E2, A, in this article’s Online Repository at www.jacionline.org). To reduce the effect of the local structure on the score, we removed the pair interactions between residues close in sequence (sequence distance, <10 residues). The differences in the scores between MBC4 and the other allergens remained, indicating the occurrence of larger structural rearrangements (see Fig E2, B).
Recombinant production of the hybrid proteins
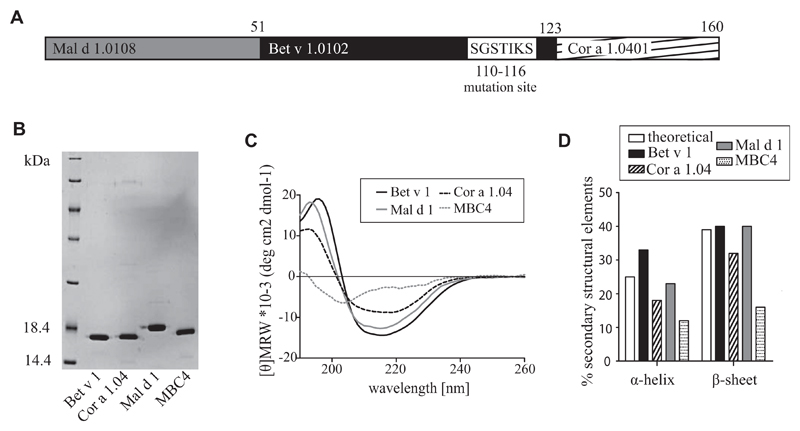
First, the hybrid protein MBC was produced in E coli (see Fig E3, A, in this article’s Online Repository at www.jacionline.org), purified to homogeneity, and subjected to screening by using CD spectroscopy (see Fig E3, B). Both CD spectra from Bet v 1 and MBC showed identical curve shape, whereas differences in amplitudes accounted for different ratios of secondary structure elements. As predicted, CD data confirmed that MBC still contained a high percentage of native secondary structure elements.8 Comparison of patients’ IgE binding of MBC with the low IgE-binding isoform Bet v 1.0102 by using ELISA revealed an almost identical IgE response (see Fig E3, C). The residual IgE binding was considered a risk factor for a potential therapeutic application. Our in silico data indicated that by introducing a mutation into the backbone of MBC (see Fig E1), the resulting molecule MBC4 (Fig 1) should not be able to adopt the typical Bet v 1 structure.8 Previously, Bet v 1 has been altered by using the same strategy. The resulting protein, termed BM4, did not react with patients’ serum IgE levels; however, in a mouse model the molecule was able to induce IgG antibodies cross-reactive with Bet v 1.8 The hybrid MBC4, as well as parental WT allergens, were produced in E coli and purified to homogeneity (Fig 1, B). Protein identity was confirmed by means of mass spectrometry (see Fig E4, A, in this article’s Online Repository at www.jacionline.org). Endotoxin levels were found to be less than 3 EU/mg recombinant protein (see Fig E4, B, in this article’s Online Repository at www.jacionline.org).
Fig 1.
A, Schematic representation of MBC4 sequence. B, Coomassie-stained 15% SDS-PAGE of purified proteins. One microgram per lane was loaded. C, CD spectra of parental allergens compared with MBC4 were recorded at 20°C and are presented as mean residue molar ellipticity after baseline correction. D, Fourier transform infrared spectra recorded at 20°C were used to calculate secondary structure elements.
MBC4 is monomeric in solution showing unique secondary structure elements
In CD measurements MBC4 showed the typical curve shape of proteins with high amounts of unordered structural elements, whereas the curves of the parental WT allergens were indicative for the typical Bet v 1–like fold (Fig 1, C). This was confirmed by means of Fourier transform infrared measurements, which indicated an α-helix content of 12% for MBC4 compared with 33% for Bet v 1, 23% for Mal d 1, and 18% for Cor a 1.04 (Fig 1, D). The β-sheet structures of MBC4 accounted for 16% compared with 40% in Bet v 1, 40% in Mal d 1, and 32% in Cor a 1.04. The substrates deoxycholate and ANS were previously reported to bind to the hydrophobic cavity, which is typical for members of the Bet v 1 allergen family.18 Thus ANS displacement assays were performed to analyze whether this structural property was conserved in MBC4. In our experimental setup we could not measure ligand binding to MBC4 indicative of an inaccessible cavity, which is very likely a result of the structural changes induced into the molecule (see Fig E5 in this article’s Online Repository at www.jacionline.org). The aggregation state of the 4 proteins was determined by using dynamic light scattering measurements (see Fig E4, C). Bet v 1 was found to be monomeric, with a hydrodynamic radius of approximately 1.8 nm, as well as Mal d 1 and Cor a 1.04, represented as a mixture of monomers and cysteine-induced multimers,6 whereas 99% of MBC4 was found in a monomeric state in solution, showing a hydrodynamic radius of 3.2 nm and indicating the partially unfolded nature of the fold variant.
Binding of patients’ IgE to MBC4 is drastically reduced
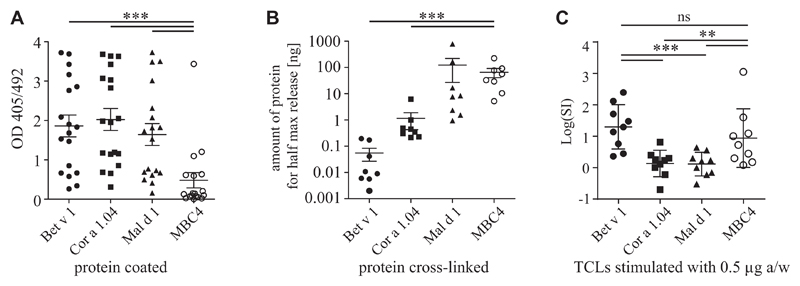
IgE binding to MBC4 was compared with parental allergens in ELISA by using sera of patients with Bet v 1 allergy with PFS to apple and hazelnut (Fig 2, A). The mean IgE binding to MBC4 (0.48 OD units) was significantly lower than the IgE binding to Bet v 1 (1.86), Mal d 1 (1.64), or Cor a 1.04 (2.02). Mediator release assays with RBL cells confirmed the data, whereas in mean 0.055 ng/mL Bet v 1 was necessary to trigger half-maximal mediator release of cells passively sensitized with patients’ serum IgE. There was a requirement for 1.16 ng/mL Cor a 1.04, 123.4 ng/mL Mal d 1, and 65.82 ng/mL MBC4 to obtain the same levels of basophil activation, indicating a 1196-fold reduction in allergenicity of MBC4 compared with the highly reactive allergen Bet v 1 (Fig 2, B).
Fig 2.
A and B, IgE ELISA (Fig 2, A) and mediator release assays (Fig 2, B) using sera of donors with birch pollen allergy with PFS to apple and hazelnut. C, Log(SI) values of TCLs from donors with birch pollen allergy stimulated with 0.5 μg of antigen per well. P values of less than .05 calculated with ANOVA were considered significant. Data of mediator release assays were transformed before analysis (y = log[y]). **P < .01 and ***P < .001. ns, Not significant; SI, stimulation index.
MBC4 effectively activates TCLs from allergic donors
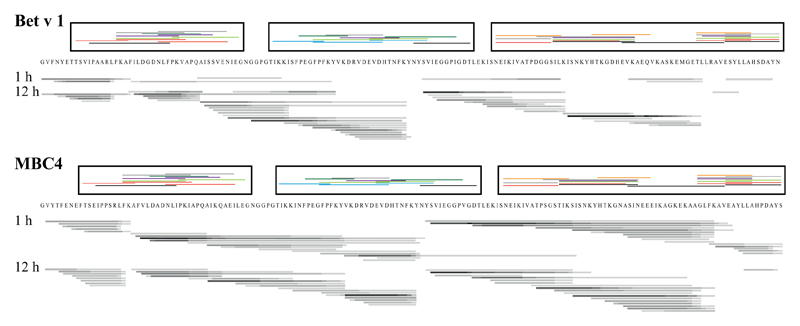
Bet v 1–specific TCLs were stimulated with either Bet v 1, Mal d 1, Cor a 1.04, or MBC4 (Fig 2, C, and see Table E2 in this article’s Online Repository at www.jacionline.org). As expected, Bet v 1 was a significantly stronger T-cell stimulus than Mal d 1 and Cor a 1.04. Although MBC4 was less potent in activating T cells than Bet v 1, the T cell–stimulating capacity was approximately 3-fold increased compared with that of the 2 food allergens. Because the TCLs were generated on stimulation of PBMCs from donors with birch pollen allergy with Bet v 1, this might induce a T-cell bias, which could explain the higher T cell–activating property of MBC4 compared with that of both food allergens. However, epitope mapping of Bet v 1–specific TCLs with overlapping 12-mer peptides revealed T-cell epitopes spreading the entire sequence, including the parts replaced by Mal d 1 and Cor a 1.04, respectively (Fig 3). To compare antigen processing of MBC4 with Bet v 1, either protein was digested with a cocktail of proteases from dendritic cells, and the degradation patterns were compared at different time points. Two representative time points are shown in Fig 3, and a high-resolution image showing 5 different time points for MBC4 is provided in Fig E6 in this article’s Online Repository at www.jacionline.org. Detailed analyses of the degradation pattern of Bet v 1, Mal d 1, and Cor a 1.04 have recently been published.6,11 Degradations of Bet v 1–like molecules with endosomal/lysosomal proteases usually result in formation of peptide clusters over a period of more than 24 hours. This was also observed here. Initially, MBC4 degraded faster than Bet v 1, resulting in more peptides available for MHC class II loading after 1 hour; however, after 12 hours, we could not find any difference in the peptide pattern of both proteins. Moreover, we found essentially the same degradation pattern for both molecules, suggesting that the endosomal/lysosomal degradation process is comparable. The peptide clusters found for both proteins overlapped very well with peptides that were identified in epitope mapping experiments with TCLs of donors with birch pollen allergy. The data indicate that MBC4 will be processed similarly to its parental allergens, resulting in the effective activation of allergen-specific T cells.
Fig 3.
Bars underneath protein sequences show peptide clusters derived from in vitro endosomal/lysosomal proteolysis: black, frequently occurring peptides; gray, peptides of medium frequency; light gray, rare peptides. Peptide clusters above each sequence (boxed) represent TCL-reactive peptides from donors with birch pollen allergy (each color represents 1 donor).
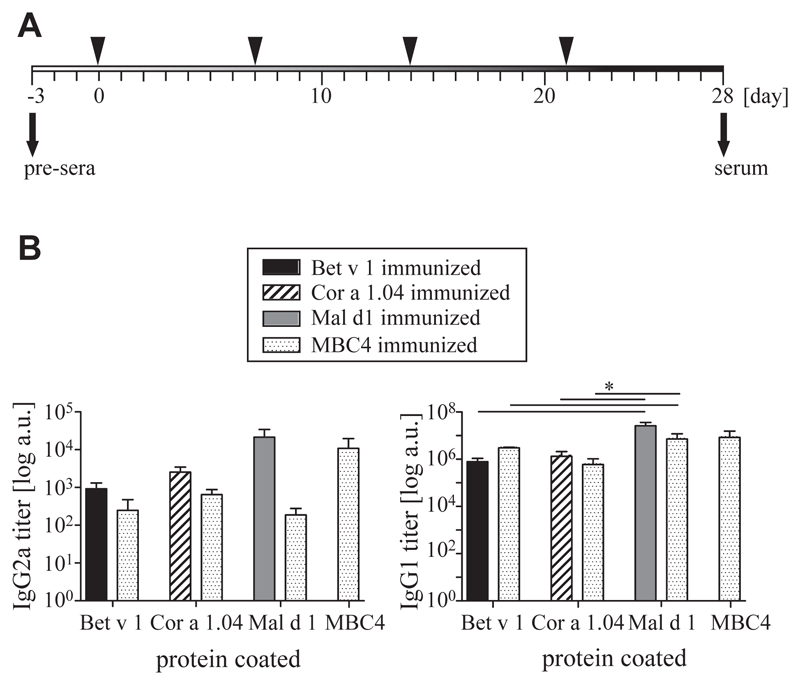
MBC4 is able to induce cross-reactive IgG antibodies in a mouse model
BALB/c mice were immunized either with parental allergens or with MBC4 (Fig 4, A). After 3 booster injections, both IgG1 and IgG2a titers were determined by means of ELISA (Fig 4, B). Therefore parental WT allergens were coated, and sera of mice immunized with the parental allergen were compared with sera of MBC4-immunized animals. Moreover, anti-MBC4 antibody titers were determined. For IgG1, we found that MBC4 immunization induced the highest titers against itself and against Mal d 1, followed by Bet v 1 and Cor a 1.04. There was no significant difference between titers induced by immunizations with WT allergens or by MBC4 immunization when tested with the respective immobilized WT proteins. For IgG2a, the picture was essentially similar; however, titers were generally lower, and MBC4 immunization led to higher antibody titers against Bet v 1 and Cor a 1.04 than Mal d 1.
Fig 4.
A, Schematic representation of immunization schedule. B, Murine IgG1 and IgG2a antibody titers were determined as means ± SEMs by using ELISA. Statistical analysis was performed by using ANOVA, and *P values of less than .05 were considered significant.
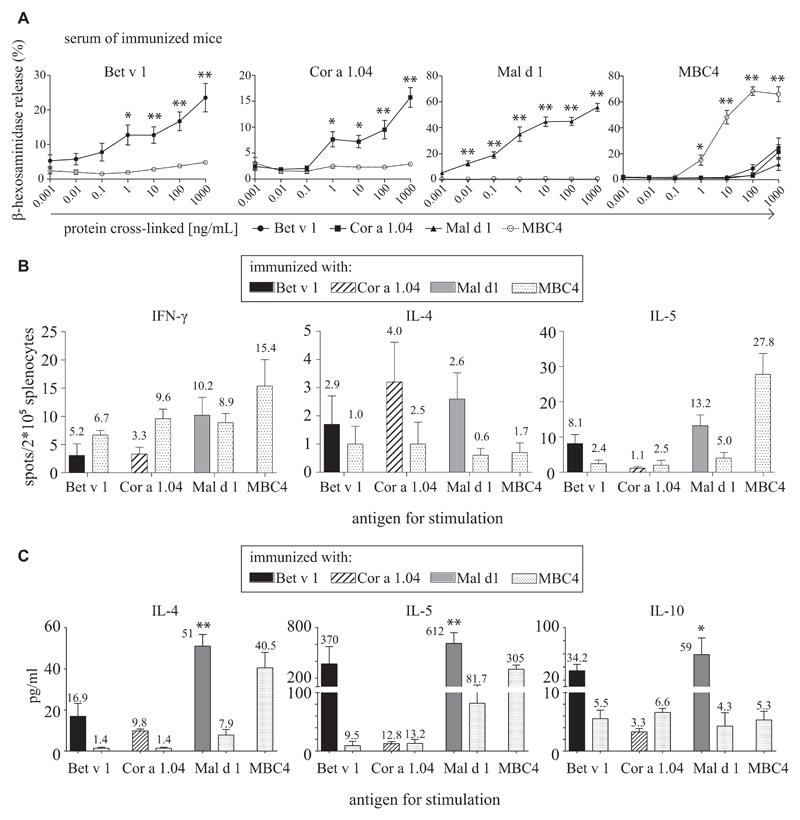
Murine immunization-induced IgE antibodies show significantly lower cross-reactivity
Despite the highly cross-reactive IgG response, murine immunization-induced IgE antibodies showed a statistically significant lower cross-reactivity (Fig 5, A). In mediator release assays we found that stimulation of cells passively sensitized with murine Bet v 1 immune sera with Bet v 1 itself led to a mean maximal activation of 23.5%, whereas stimulation with MBC4 activated 4.8% of the cells. Similar results were found for Mal d 1 and Cor a 1 (58.9% vs 0.9% and 15.8% vs 2.9% mediator release), respectively. Although MBC4 induced high levels of IgE against itself, the cross-reactivity with Bet v 1, Cor a 1, and Mal d 1 was significantly reduced (68.3% vs 8.6%, 3.2%, and 3.5% activation).
Fig 5.
A, RBL cells were passively sensitized with sera of immunized mice, and mediator release was triggered by addition of antigen. B, Splenocytes of immunized animals cultured in the presence of antigen- and cytokine-producing cells were identified in ELISpot assays. C, Splenocyte supernatants were analyzed with a multiplex approach. P values of less than .05 were considered significant. *P < .05 and **P < .01.
Immunization with MBC4 recruits both cross-reactive TH1 and TH2 cells in mice
Splenocytes of immunized animals were stimulated by the addition of protein antigens, and cytokine-producing cells were detected in ELISpot assays (Fig 5, B). Therefore splenocytes of mice immunized with parental WT allergens were stimulated with the respective allergen or with MBC4, and numbers of INF-γ–, IL-4–, and IL-5–producing cells were determined. No significant differences between parental allergens and MBC4 were found. Furthermore, we analyzed the supernatant of restimulated splenocytes and found essentially the same picture. Splenocytes of WT allergen-immunized mice could be stimulated with MBC4 in most cases, although to a lower level than with the immunogen. Restimulation with MBC4 could also induce IL-10 in splenocytes, which, with the exception of Mal d 1–immunized animals, did not significantly differ between MBC4 and the respective immunogen (Fig 5, C).
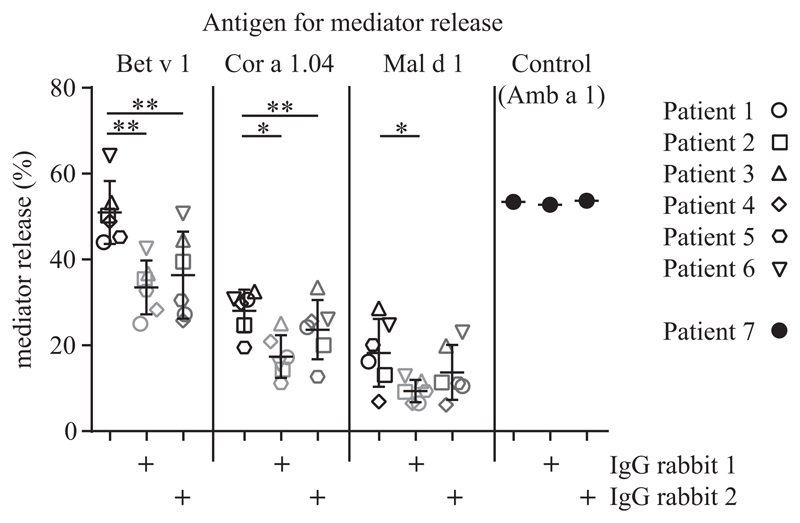
MBC4 immunization is able to induce cross-reactive blocking IgG in rabbits
Two New Zealand white rabbits were immunized with MBC4 to test whether the antigen could induce blocking IgG against parental allergens. Total rabbit IgG was purified by means of affinity chromatography and used to block mediator release in RBL assays. Therefore cells were passively sensitized with sera of patients with Bet v 1 allergy with concomitant food allergies to apple and hazelnut. The addition of rabbit IgG to either parental allergen could significantly reduce mediator release (Fig 6). MBC4-induced blocking antibodies were specific because they did not inhibit mediator release of a patient with ragweed allergy serum triggered by the addition of the major ragweed pollen allergen Amb a 1.
Fig 6.
RBL cells were passively sensitized with sera of patients with Bet v 1–mediated birch pollen allergy and concomitant oral allergy syndrome (open symbols) or the serum of a patient with ragweed allergy (solid symbols). Mediator release was triggered by addition of antigen in the presence or absence of IgG isolated from MBC4-immunized rabbits. P values of less than .05 calculated with ANOVA were considered significant. *P < .05 and **P < .01.
Discussion
Ideally, allergy treatment should be specific, efficacious, safe, and easily applicable.19 Especially with widely cross-reactive allergens, simplicity becomes a major challenge. In the case of birch pollen allergy, the simultaneous treatment of concomitant pollen and food allergies seems essential; however, extract-based therapy, even though successful for pollen allergy, does not necessarily ameliorate food-related symptoms of Bet v 1–associated allergic reactions.4,20,21 Therefore it is unlikely that a purified single allergen, such as Bet v 1, will show better performance. Moreover, pollen-related food allergens seem to have unique immunologic properties distinct from Bet v 1.6 Because AIT with a cocktail of different allergens might have disadvantages over a single molecule in terms of reproducibility and production costs, we aimed to produce a hybrid protein by recombining parts of Mal d 1, Bet v 1, and Cor a 1.04 that contained the most relevant T-cell epitopes.12,14,15 Introducing point mutations further allowed us to modify the 3-dimensonal structure of the hybrid, abrogating the IgE-binding properties of the WT allergens but preserving their T-cell reactivity: a crucial step to reduce IgE binding and retain efficacy of a potential therapeutic agent.
The MBC4 hybrid protein displayed a significant change of secondary structure elements compared with the parental allergens. Nevertheless, MBC4 was monomeric in solution. Because of the fold variation, the hydrophobic cavity, a characteristic feature of Bet v 1–like proteins, was blocked. The structural rearrangements translated into a strongly reduced IgE-binding capacity of MBC4 so that in mediator release assays using RBL cells loaded with patients’ serum IgE, the protein showed an 1196-fold reduced allergenic activity compared with Bet v 1.
Bet v 1–specific TCLs containing T cells specific for a variety of epitopes spreading the entire sequence of the major birch pollen allergen were stimulated to investigate its T cell–activating properties. MBC4 was slightly less active than Bet v 1; however, it still represented a more potent stimulus than either of the parental WT food allergens. In addition, endosomal/lysosomal proteases were used to degrade MBC4 in vitro. When comparing the acquired peptide spectra with Bet v 1, essentially the same pattern was obtained. Moreover, in our murine model splenocytes of animals immunized with the WT allergens were isolated and stimulated with either the immunogen or MBC4. In ELISpot assays we did not find any differences in the stimulatory capacity of MBC4 compared with the parental allergens. When analyzing the splenocyte supernatants, we saw essentially the same pattern. Splenocytes of WT allergen–immunized mice could be stimulated with MBC4, and we could also detect some IL-10 in the supernatants. All of this indicates that the epitope recombination preserved (not to say expanded) the T-cell epitope repertoire of MBC4. Therefore it should allow the induction of specific T-cell tolerance to the individual allergens in a therapeutic setup.
It is generally accepted that the induction of blocking antibodies is a hallmark of AIT.22 Therefore proteins have to be immunogenic and, in case a modified allergen derivative is used for vaccination, able to induce antibodies cross-reactive with the WT allergen. In this case MBC4-induced antibodies should be reactive to Bet v 1 and associated food allergens. We established a mouse model, immunized the animals with MBC4, and tested the antibody cross-reactivity to WT allergens. MBC4 was very immunogenic and induced high IgG1 and IgG2a antibody titers reactive to each of the parental WT allergens. Interestingly, the IgE antibodies induced by MBC4 immunization were not cross-reactive. Therefore we speculate that the structural rearrangements within MBC4 prohibit the binding and induction of cross-reactive IgE; however, within MBC4, sufficient secondary structure elements were preserved to stimulate a potent cross-reactive IgG response. This was also verified in a rabbit model in which MBC4 immunization induced blocking antibodies against all 3 WT allergens, which could significantly inhibit allergen-induced degranulation of passively sensitized basophils.
Clinical studies with recombinant allergens, especially modified proteins, are still rare.23 Moreover, the treatment of food allergies remains especially problematic. Here we present a modified allergen derivative with drastically reduced IgE-binding properties but retained immunogenicity for the simultaneous treatment of birch pollen and associated food allergies. The concept can serve as an archetype for modern AIT vaccine design and will prove itself especially valuable to approach problems of cross-reactivity frequently encountered in allergen families.
Supplementary Material
Clinical implications.
Targeted epitope recombination in combination with a knowledge-based structural modification allowed the production of a single molecule as an allergy vaccine candidate to tackle birch pollen and associated food allergies.
Acknowledgments
Supported by the Austrian Science Fund (FWF) grant L688, the Austrian National Bank (ÖNB) grant 12533, and the priority program “Allergy-Cancer-BioNano Research Centre” of the University of Salzburg.
Abbreviations used
- AIT
Allergen-specific immunotherapy
- ANS
1-Anilino-8-naphthalene sulfonate
- AP
Alkaline phosphatase
- APC
Antigen-presenting cell
- CD
Circular dichroism
- PDB
Protein Data Bank
- PFS
Pollen-food syndrome
- RBL
Rat basophil leukemia
- TCL
T-cell line
- WT
Wild-type
Footnotes
Disclosure of potential conflict of interest: H. Hofer received travel support from the European Academy of Allergy and Clinical Immunology (EAACI). M. Himly is an employee of the University of Salzburg, receives grant support from the Austrian Science Fund, and is the inventor of and patent holder for hypoallergenic molecules. T. Hawranek serves on the board for ALK-Abelló, Leti, and Novartis and receives payments for lectures from ALK-Abelló. B. Bohle receives grant support from the Austrian Science Funds and Christian Doppler Laboratory for Immunomodulation. F. Ferreira serves on the board form AllergenOnline Database, SIAF, and HAL Allergy. M. Wallner receives grant support from the Austrian National Bank. The rest of the authors declare that they have no relevant conflicts of interest.
The CrossMark symbol notifies online readers when updates have been made to the article such as errata or minor corrections
References
- 1.Geroldinger-Simic M, Zelniker T, Aberer W, Ebner C, Egger C, Greiderer A, et al. Birch pollen-related food allergy: clinical aspects and the role of allergen-specific IgE and IgG4 antibodies. J Allergy Clin Immunol. 2011;127:616–22.e1. doi: 10.1016/j.jaci.2010.10.027. [DOI] [PubMed] [Google Scholar]
- 2.Pauli G, Larsen TH, Rak S, Horak F, Pastorello E, Valenta R, et al. Efficacy of recombinant birch pollen vaccine for the treatment of birch-allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2008;122:951–60. doi: 10.1016/j.jaci.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 3.Nony E, Bouley J, Le Mignon M, Lemoine P, Jain K, Horiot S, et al. Development and evaluation of a sublingual tablet based on recombinant Bet v 1 in birch pollenallergic patients. Allergy. 2015;70:795–804. doi: 10.1111/all.12622. [DOI] [PubMed] [Google Scholar]
- 4.Kinaciyan T, Jahn-Schmid B, Radakovics A, Zwolfer B, Schreiber C, Francis JN, et al. Successful sublingual immunotherapy with birch pollen has limited effects on concomitant food allergy to apple and the immune response to the Bet v 1 homolog Mal d 1. J Allergy Clin Immunol. 2007;119:937–43. doi: 10.1016/j.jaci.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 5.Subbarayal B, Schiller D, Mobs C, de Jong NW, Ebner C, Reider N, et al. Kinetics, cross-reactivity, and specificity of Bet v 1-specific IgG4 antibodies induced by immunotherapy with birch pollen. Allergy. 2013;68:1377–86. doi: 10.1111/all.12236. [DOI] [PubMed] [Google Scholar]
- 6.Roulias A, Pichler U, Hauser M, Himly M, Hofer H, Lackner P, et al. Differences in the intrinsic immunogenicity and allergenicity of Bet v 1 and related food allergens revealed by site-directed mutagenesis. Allergy. 2014;69:208–15. doi: 10.1111/all.12306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pichler U, Hauser M, Hofer H, Himly M, Hoflehner E, Steiner M, et al. Allergen hybrids—next generation vaccines for Fagales pollen immunotherapy. Clin Exp Allergy. 2014;44:438–49. doi: 10.1111/cea.12250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wallner M, Hauser M, Himly M, Zaborsky N, Mutschlechner S, Harrer A, et al. Reshaping the Bet v 1 fold modulates T(H) polarization. J Allergy Clin Immunol. 2011;127:1571–8.e9. doi: 10.1016/j.jaci.2011.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pichler U, Asam C, Weiss R, Isakovic A, Hauser M, Briza P, et al. The fold variant BM4 is beneficial in a therapeutic Bet v. 1 mouse model. Biomed Res Int. 2013;2013:832404. doi: 10.1155/2013/832404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wallner M, Himly M, Neubauer A, Erler A, Hauser M, Asam C, et al. The influence of recombinant production on the immunologic behavior of birch pollen isoallergens. PLoS One. 2009;4:e8457. doi: 10.1371/journal.pone.0008457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Egger M, Jurets A, Wallner M, Briza P, Ruzek S, Hainzl S, et al. Assessing protein immunogenicity with a dendritic cell line-derived endolysosomal degradome. PLoS One. 2011;6:e17278. doi: 10.1371/journal.pone.0017278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jahn-Schmid B, Radakovics A, Luttkopf D, Scheurer S, Vieths S, Ebner C, et al. Bet v 1142-156 is the dominant T-cell epitope of the major birch pollen allergen and important for cross-reactivity with Bet v 1-related food allergens. J Allergy Clin Immunol. 2005;116:213–9. doi: 10.1016/j.jaci.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 13.Bauer R, Scheiblhofer S, Kern K, Gruber C, Stepanoska T, Thalhamer T, et al. Generation of hypoallergenic DNA vaccines by forced ubiquitination: preventive and therapeutic effects in a mouse model of allergy. J Allergy Clin Immunol. 2006;118:269–76. doi: 10.1016/j.jaci.2006.03.033. [DOI] [PubMed] [Google Scholar]
- 14.Kitzmuller C, Zulehner N, Roulias A, Briza P, Ferreira F, Fae I, et al. Correlation of sensitizing capacity and T-cell recognition within the Bet v 1 family. J Allergy Clin Immunol. 2015;136:151–8. doi: 10.1016/j.jaci.2014.12.1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bohle B, Radakovics A, Luttkopf D, Jahn-Schmid B, Vieths S, Ebner C. Characterization of the T cell response to the major hazelnut allergen, Cor a 1.04: evidence for a relevant T cell epitope not cross-reactive with homologous pollen allergens. Clin Exp Allergy. 2005;35:1392–9. doi: 10.1111/j.1365-2222.2005.02332.x. [DOI] [PubMed] [Google Scholar]
- 16.Ferreira F, Hirtenlehner K, Jilek A, Godnik-Cvar J, Breiteneder H, Grimm R, et al. Dissection of immunoglobulin E and T lymphocyte reactivity of isoforms of the major birch pollen allergen Bet v 1: potential use of hypoallergenic isoforms for immunotherapy. J Exp Med. 1996;183:599–609. doi: 10.1084/jem.183.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferreira F, Ebner C, Kramer B, Casari G, Briza P, Kungl AJ, et al. Modulation of IgE reactivity of allergens by site-directed mutagenesis: potential use of hypoallergenic variants for immunotherapy. FASEB J. 1998;12:231–42. doi: 10.1096/fasebj.12.2.231. [DOI] [PubMed] [Google Scholar]
- 18.Kofler S, Asam C, Eckhard U, Wallner M, Ferreira F, Brandstetter H. Crystallographically mapped ligand binding differs in high and low IgE binding isoforms of birch pollen allergen bet v 1. J Mol Biol. 2012;422:109–23. doi: 10.1016/j.jmb.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jutel M, Agache I, Bonini S, Burks AW, Calderon M, Canonica W, et al. International consensus on allergy immunotherapy. J Allergy Clin Immunol. 2015;136:556–68. doi: 10.1016/j.jaci.2015.04.047. [DOI] [PubMed] [Google Scholar]
- 20.Mauro M, Russello M, Incorvaia C, Gazzola G, Frati F, Moingeon P, et al. Birch-apple syndrome treated with birch pollen immunotherapy. Int Arch Allergy Immunol. 2011;156:416–22. doi: 10.1159/000323909. [DOI] [PubMed] [Google Scholar]
- 21.Bolhaar ST, Tiemessen MM, Zuidmeer L, van Leeuwen A, Hoffmann-Sommergruber K, Bruijnzeel-Koomen CA, et al. Efficacy of birch-pollen immunotherapy on cross-reactive food allergy confirmed by skin tests and double-blind food challenges. Clin Exp Allergy. 2004;34:761–9. doi: 10.1111/j.1365-2222.2004.1939.x. [DOI] [PubMed] [Google Scholar]
- 22.Akdis M, Akdis CA. Mechanisms of allergen-specific immunotherapy: multiple suppressor factors at work in immune tolerance to allergens. J Allergy Clin Immunol. 2014;133:621–31. doi: 10.1016/j.jaci.2013.12.1088. [DOI] [PubMed] [Google Scholar]
- 23.Ferreira F, Wolf M, Wallner M. Molecular approach to allergy diagnosis and therapy. Yonsei Med J. 2014;55:839–52. doi: 10.3349/ymj.2014.55.4.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.