Abstract
Objective
To characterize the endocrine phenotype of patients with Shwachman-Diamond syndrome (SDS).
Study design
Clinically indicated endocrine screening data from 43 patients with SDS or SDS-like presentation were analyzed according to sex, age, and genetic testing. In addition to 25 patients with biallelic Shwachman-Bodian-Diamond syndrome (SBDS) gene mutations, we evaluated 18 patients with cytopenias who were receiving pancreatic enzyme replacement but were without SBDS mutation. We performed a retrospective review of growth records and clinically indicated endocrine evaluations.
Results
Of patients with SBDS mutations, 2 had low stimulated growth hormone levels, 2 had mildly elevated thyrotropin levels, 5 had abnormal glucose levels, and 1 had an elevated follicle-stimulating hormone level (post transplantation). In contrast, 1 patient without SBDS mutations had postprandial hyperglycemia and 3 had mildly low free thyroxine levels without short stature. Endocrine abnormalities were identified in 19% of short patients and 26% of the whole group. Of patients with SBDS mutations, 56% had a height expressed in SD units from the mean for age and sex of <−1.8, in contrast to only 12% of patients without SBDS mutations (38% of the whole group). Body mass index z score was significantly greater in the group with SBDS mutations (P < .001).
Conclusion
Although short stature was more common in patients with SBDS mutations, no consistent endocrine phenotype was observed in patients with SDS regardless of genetic testing.
Many patients with congenital bone marrow failure syndromes have short stature and other endocrine abnormalities. For instance, Diamond-Blackfan anemia is frequently associated with poor growth in response to chronic steroid therapy, but even patients without a history of significant steroid therapy are often constitutionally short. Fanconi anemia is frequently associated not only with short stature but also with growth hormone (GH) deficiency (GHD), mild hypothyroidism, premature gonadal failure, and deficiency of first-phase insulin release, resulting in glucose intolerance and overt diabetes during illness.1–3 In contrast, the endocrine profile of Shwachman-Diamond syndrome (SDS) has not been well characterized. Other features of SDS are well described, including frequent infections, pancreatic insufficiency, and metaphyseal chondrodysplasia.4 In addition, most patients with SDS have intermittent or persistent neutropenia, 40% develop anemia, and 35% develop thrombocytopenia.5 Tri-lineage bone marrow failure occurs rarely.6 Risk for cancer is elevated, with an estimated 15–30% risk for developing myelodysplasia or leukemia.7,8 Up to 90% of affected patients have biallelic genetic mutations identified in the Shwachman-Bodian-Diamond syndrome (SBDS) gene. Some patients have clinical features consistent with SDS (“SDS-like”) but have no identifiable genetic mutation.
Along with the mentioned characteristics, short stature is recognized as a common feature of SDS.5 In addition, single case reports describe a few patients with SDS who have been identified with GHD, as well as several with type 1 diabetes.9–15 As specific endocrine phenotypes have been identified in other inherited bone marrow failure syndromes, we sought to characterize endocrine function in a series of patients who met clinical criteria for SDS.
Methods
The study was a retrospective review of clinically obtained endocrinologic assessments of 43 patients with clinical features of SDS attending the Bone Marrow Failure Clinic of Cincinnati Children’s Hospital Medical Center. Patients were assessed by an endocrinologist for clinical evaluation (including pubertal staging) and laboratory endocrine evaluation. Clinically indicated endocrine screening data from 43 patients with SDS or SDS-like presentation were entered in an institutional review board–approved database. Data were analyzed according to sex, age, and genetic testing.
Four of the patients with gene mutations had undergone hematopoietic cell transplantation (HCT) at the time of endocrine screening. Details of HCT treatment were available in all 4 evaluated after HCT. Chemoimmunotherapy included alemtuzumab,16 fludarabine, and melphalan.
Mutations in SBDS were confirmed by clinically available gene testing in 25 patients, and testing was normal in the other patients. The 18 patients without SBDS mutations were patients referred to our center for evaluation of possible SDS. The diagnosis of SDS-like presentation was made on the basis of evidence of marrow dysfunction as well as pancreatic dysfunction. Marrow dysfunction was defined as presence of hypoproductive cytopenias including neutropenia (absolute neutrophil count <1500/μL), anemia, or idiopathic macrocytosis or thrombocytopenia (<150 000/μL). Pancreatic dysfunction was diagnosed on the basis of either laboratory documentation of pancreatic insufficiency (abnormal pancreatic isoamylase, trypsinogen, or on measurement of pancreatic enzymes after stimulation with cholecystokinin) or improvement of symptomatology with pancreatic enzyme therapy. It is expected that only about 10% of clinically diagnosed patients with SDS will be gene negative. However, because we work at a referral center for suspected SDS, we see a large number of gene-negative patients, representative of our interest in this subgroup of patients. Thus, our series cannot be presumed to be typical of what is found in the general population.
Endocrinologic Assessment
Endocrinologic assessment included full medical history, weight, height by stadiometer, physical examination with pubertal staging, and blood for clinically indicated laboratory studies. Not all tests were performed on every patient. Endocrine laboratory testing included fasting and postprandial glucose, oral glucose tolerance tests (OGTTs) with glucose and insulin measurement at each time point, glycosylated hemoglobin (HbA1c), thyroid function tests (thyroxine, free thyroxine [FT4], thyrotropin [TSH]), GH screening (insulin-like growth factor 1 [IGF-I], insulin-like growth factor binding protein 3), and pubertal hormone levels when appropriate (luteinizing hormone, follicle-stimulating hormone [FSH], estradiol, testosterone). Morning cortisol and stimulated GH levels were assayed when clinically indicated, based on slow growth velocity. Radiographs of the left hand and wrist were obtained for assessment of bone maturation. Assays were performed at the clinical laboratory of Cincinnati Children’s Hospital Medical Center. Results were interpreted with respect to age-appropriate reference ranges established in the laboratory.
We defined endocrine outcome data as follows: Height was expressed in SD units from the mean for age and sex. Body mass index (BMI; weight divided by height squared) was also expressed in SD units from the mean for age and sex. Glucose regulation was considered abnormal if fasting glucose was >100 mg/dL, peak glucose (postprandial or on OGTT) was >140 mg/dL, or HbA1c (high-pressure liquid chromatography) was >6.3% (upper limit of normal for our assay). Postprandial insulin (chemiluminescent immunoassay) was considered elevated if >80 mg/dL on OGTT. Thyroid function was abnormal if TSH (microparticle enzyme immunoassay) was >4 mU/L (upper limit of normal for our assay) or FT4 (equilibrium dialysis) was <1 ng/dL (upper limit of normal for our assay). GH secretion was suspicious for deficiency if stimulated peak GH on 2 standard stimulation tests was <10 ng/mL or if IGF-I (radioimmunoassay) or insulin-like growth factor binding protein 3 (chemiluminescent immunoassay) was below the normal range for age. Puberty was considered to be abnormal if there was no clinical evidence of puberty by age 13 years in female patients or 14 years in male patients, or if FSH (chemiluminescent immunoassay) was >18 mIU/mL (upper limit of normal for our assay).
Data were managed and analyzed using SAS version 9.3 (SAS Institute, Cary, North Carolina). Median values with the associated IQRs and overall ranges are reported due to the sample sizes for the interval variables. Number and percentage are reported for the categorical variables. Group differences were initially tested using Wilcoxon rank sum and Fisher exact tests for continuous and categorical variables as appropriate. Because there were siblings from the same families, analyses were repeated using a mixed-model approach, incorporating a logit link and generalized estimating equations for the categorical variables.
Results
The studied population included 43 (19 female patients and 24 male patients, age range 1–19.7 years) (Table I). All patients underwent genetic testing for SBDS, and 25 had identified SBDS gene mutations. Of these, most (77%) were on treatment with pancreatic enzymes at the time of evaluation. Four patients, all with gene mutations, had undergone HCT.
Table I.
Clinical characteristics of patients with SDS by sex, according to genetic tests
| Sex | SBDS biallelic mutation | n | Chronologic age, y | Mid-parental height, cm | Height z score | BMI z score |
|---|---|---|---|---|---|---|
| Male | Yes | 14 | 8.5 | 184 | −1.8 | +0.0 |
| (4.3, 9.9) | (171, 187) | (−2.9, −1.5) | (−0.7, 0.7) | |||
| [2 to 14.6] | [171 to 187] | [−3.8 to −1.3] | [−1.6 to +4.8] | |||
| n = 3 | ||||||
| No | 10 | 9.8 | 177 | −1.0 | −0.3 | |
| (5.4, 10.8) | (172, 177) | (−1.5, −0.7) | (−1.6, 0.1) | |||
| [2.5 to 15.7] | [172 to 182] | [−1.9 to +0.0] | [−1.8 to +2.2] | |||
| n = 5 | ||||||
| Total | 24 | 8.7 | 177 | −1.6 | −0.3 | |
| (4.9, 10.6) | (172, 183) | (−1.9, −1.3) | (−1.1, 0.7) | |||
| [2 to 15.7] | [171 to 187] | [−3.8 to +0] | [−1.8 to +4.8] | |||
| n = 8 | ||||||
| Female | Yes | 11 | 4.4 | 160 | −1.9 | 0.2 |
| (3.6, 15.3) | n = 1 | (−2.5, 0) | (−1.2, 0.8) | |||
| [1 to 19.7] | [−5.0 to 0.6 | (−1.8 to +1.8) | ||||
| No | 8 | 5.0 | 159 | −0.3 | −1.5 | |
| (2.8, 8.0) | n = 1 | (−1.3, 0.7) | (−1.9, 0.4) | |||
| [1.2 to 13.5] | [−1.5 to +1.3] | [−2.0 to +3.2] | ||||
| Total | 19 | 4.8 | 159 | −1.0 | −0.0 | |
| (3.2, 12.3) | (159, 160) | (−2.2, 0) | (−1.6, 0.4) | |||
| [1 to 19.7] | [159 to 160] | [−5 to +1.3] | [−2.0 to +3.2] | |||
| n = 2 |
Values shown are as median (IQR: 25th, 75th) [range] and n if number included was less than the whole subgroup.
Of the 18 patients without SBDS mutations, 17 patients had evidence of marrow dysfunction, most commonly intermittent neutropenia, requiring filgrastim therapy in 9 patients. Sixteen were receiving pancreatic enzyme replacement with clinical improvement, of whom 7 had laboratory testing documenting pancreatic insufficiency. Two patients who partially met criteria were included, as they had similar symptomatology as their siblings who met full criteria and almost certainly have the same disorder with a slightly different phenotype. These included 1 patient with normal blood counts and abnormal isoamylase levels, who had a sibling with intermittent neutropenia requiring filgrastim treatment, failure to thrive with low isoamylase requiring enzyme replacement, and hypogammaglobulinemia. The other patient had intermittent neutropenia requiring filgrastim therapy, without documented pancreatic insufficiency; however, her sibling also had intermittent neutropenia requiring filgrastim therapy, pancreatic insufficiency, and failure to thrive.
There were 7 sets of siblings. Within the sibling groups, SBDS status was consistent in all sets; 2 sets had SBDS mutations and 5 sets did not. Three sets of siblings were male, 2 sets were female, and 2 were of mixed sex.
Fasting glucose, insulin, and HbA1c were normal, with the exception of 1 patient who was on an insulin pump for type 1 diabetes mellitus. This patient had positive islet cell antibodies and a family history of type 1 diabetes but no family history of SDS. When postprandial glucose level >140 mg/dL was used to define abnormal glucose, this was present in 21% of male patients and 5% of female patients assessed (Table II).
Table II.
Percentage of patients with SDS with abnormal endocrine status according to sex and genetic testing
| SBDS biallelic mutation | Sex | GH (ng/dL) screen (low IGF-I or stimulated GH <10), % | Thyroid (TSH >4 mU/L or FT4 <1 ng/dL), % | Pubertal (FSH >18 mIU/L), % | Abnormal glucose (PP glucose >140 mg/dL or FPG >100 mg/dL or HbA1c >6.3), % | Height z score (<1.8), % |
|---|---|---|---|---|---|---|
| Yes | Female | 12 (1 of 8) | 0 (0 of 11) | 0 (0 of 3) | 11 (1 of 9) | 64 (7 of 11) |
| Male | 8 (1 of 12) | 14 (2 of 14) | 25 (1 of 4) | 36 (4 of 11) | 50 (7 of 14) | |
| Total | 10 (2 of 20) | 8 (2 of 25) | 14 (1 of 7) | 25 (5 of 20) | 56 (14 of 25) | |
| No | Female | 0 (0 of 4) | 50 (3 of 6) | 0 (0 tested) | 0 (0 of 7) | 0 (0 of 7) |
| Male | 0 (0 of 6) | 0 (0 of 9) | 0 (0 of 2) | 11 (1 of 9) | 20 (2 of 10) | |
| Total | 0 (0 of 10) | 20 (3 of 15) | 0 (0 of 2) | 6 (1 of 16) | 12 (2 of 17) | |
| SBDS yes versus SBDS no (P value) | .54 | .34 | NA | .03 | .03 | |
| SBDS versus population expected (5%) (P value) | .68 | .02 | NA | .001 | <.0001 |
FPG, fasting plasma glucose; PP glucose, postprandial glucose 2 h after food or oral glucose challenge. Values shown are percentage abnormal (number abnormal of number tested).
Thyroid function was mildly abnormal in 2 male patients and 3 female patients, with TSH >4 mU/L in 1 male patient and 2 female patients who had normal FT4, and low FT4 in 1 male patient and 1 female patient who had normal TSH. In male patients, median TSH was 2.2 mU/L (range 0.8–5.9 mU/L); in female patients, median TSH was 2.0 mU/L (range 0.2–4.4 mU/L) (Table II).
Only 10 of the patients were of pubertal age, and they were each appropriately in puberty. Of them, only 1 male patient (who underwent prior HCT) had gonadotropin elevation (FSH 33.6 mIU/L), suggestive of partial gonadal impairment after HCT (Table II).
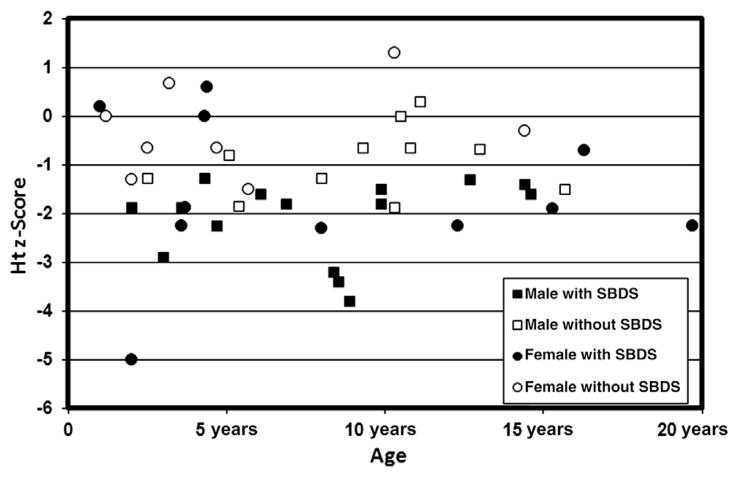
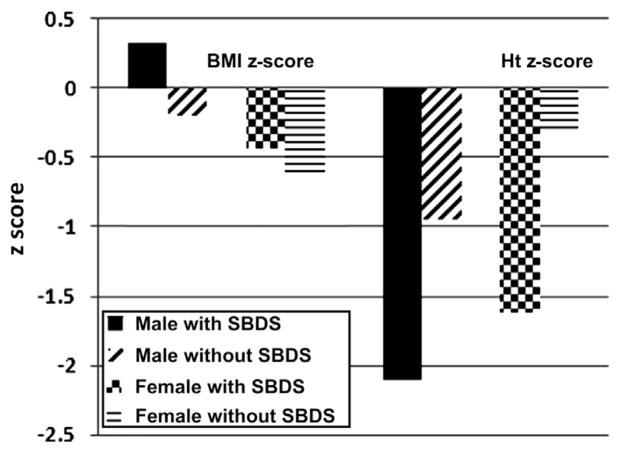
Of the 15 with birth information, 80% of the patients were born full term with a weight and length appropriate for gestational age. Mean mid-parental height was similar to the mean for the general population (Table I). Median Ht z score was shorter than expected in both male (−1.6) and female (−1.0) patients, with a broad range (−3.8 to +0.0 in male patients, −5.0 to +1.3 in female patients) (Table I, Figure 1). Of the male patients, 9 of 24 (38%) had an Ht z score <−1.8. Of the female patients, 7 of 18 (39%) had an Ht z score <−1.8. The remaining 63% of the patients had heights in the normal range. Height was shorter for age in patients with SBDS mutations than in patients without SBDS mutations (P < .001). Overall, 56% (64% of female patients, 50% of male patients) of patients with SBDS mutations and 12% (0% of female patients, 20% of male patients) of patients without SBDS mutations had an Ht z score <−1.8 (Figure 2; available at www.jpeds.com).
Figure 1.
Ht z score according to genetic testing, chronologic age, and sex, in 43 patients with SDS.
Figure 2.
BMI, BMI z score, and Ht z score according to genetic testing and sex, in 43 patients with SDS.
Information regarding skeletal dysplasia was available in 30 patients, and dysplasia was present in 8 (27%). Eight of 17 (47%) patients with SBDS mutations demonstrated dysplasia, but it was not seen in the patients without SBDS mutations. In the population of patients with short stature, 7 of 14 evaluable patients (50%) demonstrated skeletal dysplasia.
Stimulated peak GH level was low in 1 male patient and 1 female patient (peak GH level 4.3 and 2.3 ng/mL, respectively). Their Ht z score was −1.6 and −2.3, respectively. The male patient also had neonatal hypoglycemia. The female patient incidentally also had Chiari 1 malformation and had been treated with GH since infancy. Small stature could be explained on a hormonal basis in only 2 of the 16 (12%) short patients, or 5% of the whole group. This included 1 patient with GHD and 1 patient with mild hypothyroidism (Table II).
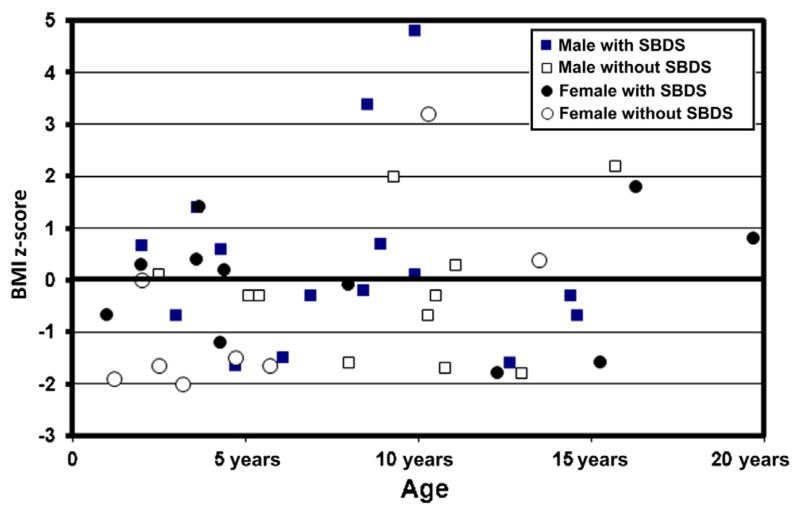
BMI showed a wide variation when expressed as BMI z score. Median BMI z score was close to normal, at −0.3 for male patients (range −1.8 to 4.8) and 0.0 for female patients (range −2 to 3.2) (Table I, Figure 3). However, 5 male patients (21%) and 5 female patients (28%) (overall 23%) were relatively thin (BMI z score <−1.5), and 3 male patients and 1 female patient (overall 10%) were relatively overweight (BMI z score >2.0) (Figure 3). BMI z score <−1.5 was observed in 16% of patients with SBDS mutations and 35% of patients without. Overall, patients with SDS were not thin.
Figure 3.
BMI z score according to genetic testing, chronological age, and sex, in 43 patients with SDS.
Only 25% of the SDS patients showed at least 1 endocrine abnormality excluding short stature. This included 6 (25%) of the male patients and 5 (28%) of the female patients, 7 (28%) of the gene-positive and 4 (24%) of patients without SBDS mutations.
The mixed-model analysis, taking into consideration the potential family effect of siblings within family, revealed the same results as the analyses by sex and SBDS biallelic mutation status. There were no statistically significant sex × SBDS interactions, and the only statistically significant main effects were SBDS for Ht z score (P = .02), short stature defined as Ht z score <−1.8 (P = .03), and sex for Ht z score (Table II). Comparison with an anticipated general population rate of endocrine abnormalities of 5% demonstrates a significant increase in endocrine abnormalities in our cohort compared with the general population in thyroid, glucose, and height abnormalities (P = .02 and .001 and <.0001, respectively). The presence of any endocrine abnormality in our cohort compared with the general population was also highly significant (P < .0001).
Discussion
We report endocrine function in a large group of patients with SDS. We found that 38% of the whole group of patients with SDS had short stature and that height was shorter for age in those with genetically confirmed SDS than in those with no genetic marker. The patients with SDS did not, as a group, tend to be thin. Twenty-five percent of the male patients and only 6% of the female patients showed mild glucose elevation after eating. The patients with SDS did not have a consistent profile of endocrine abnormality, with 26% showing any endocrine abnormality excluding short stature. Two individual patients presented exceptions—1 with type 1 diabetes and 1 with Chiari and GHD. Although this occurrence exceeded that expected in an unaffected normal population, there was not a consistent pattern of type of endocrine abnormality.
Previous studies of endocrine function in children and adults with SDS consist mostly of reports of small numbers of patients with SDS, with limited endocrinologic evaluations. A report by Ginzberg et al evaluated growth in patients with clinically diagnosed SDS, before the advent of molecular diagnosis.5 Frequency of short stature was similar to our findings, with 56% of patients having heights below the 3rd percentile for age.5 In addition, 47% of those patients were also below the 3rd percentile for weight.5 In contrast to their findings, we found normal BMI in the majority of our patients. This difference could reflect use of the BMI z score instead of weight percentile, or it could reflect improved nutritional status in patients currently receiving therapy. Importantly, short stature in our series did not appear to be due to failure to thrive or being underweight, as might be expected with malabsorption related to pancreatic insufficiency.
Metaphyseal chondrodysplasia, a known feature of SDS, may contribute to the smaller stature in about one-third of patients with SDS. However, small stature in SDS should not be dismissed as simply a part of the syndrome, as we identified a treatable etiology in 2 (12%) of the short patients and in 7 (16%) of the whole SDS group. Additionally, GHD has been previously reported in 2 individuals with SDS.15,17 One GH-deficient patient who had associated hypogamma-globulinemia responded to GH treatment,17 and another did not.15 Children and adolescents with SDS who have short stature or slow growth velocity should be referred for endocrine evaluation. In instances where an endocrine etiology for short stature can be identified, thyroid hormone or GH therapy should be initiated.
Children and adolescents with SDS, however, do have a predisposition toward malignancy, specifically acute myeloid leukemia. Therefore, GH therapy should be used only when there is convincing evidence of GHD. We recommend that patients with SDS who are being considered for GH therapy have a bone marrow examination with cytogenetics and focused fluorescence in situ hybridization (monosomy 7, del 7q, del 20q, trisomy 8) before the start of GH therapy and yearly thereafter to document absence of a cytogenetic clone, dysplasia, or leukemia. In conditions like SDS, with a known malignancy risk, GH doses should be adjusted to keep IGF-I concentrations near the middle of the normal range.
In a prior report, hypogonadotrophic hypogonadism in a 12-year-old boy with SDS was treated with hormone replacement; however, no further details were provided.18 Delayed puberty was described in the 6 oldest patients (3 adults and 3 adolescents) of a 21-patient cohort with SDS.18 In contrast, in our cohort, puberty was present in all 10 adolescent patients, although 1 had FSH elevation consistent with partial primary hypogonadism after transplantation.
Case reports of individuals with SDS and type 1 diabetes have been described.9–14 Gana et al reported an occurrence rate of diabetes of 3.23% in 62 gene-positive individuals with SBDS from the Italian registry, a 30-fold increase over that of the general population.9 Our cohort included 1 of 25 (4%) individuals with SBDS mutations with a concomitant diagnosis of type 1 diabetes. Additionally, there are 4 other patients described in the literature who had clinically diagnosed SDS and concomitant diabetes of varying types.5,19–21 However, we did not have any such patients in our cohort, as none of our 18 patients without SBDS mutations had diabetes. Our 6 patients with mild glucose abnormalities could be at risk for developing diabetes as adults.
Based on our results, there is not a consistent endocrine phenotype in patients with SDS, although there is a tendency toward short stature. Short stature appears to be more associated with genotype than with any specific endocrine abnormality or low BMI. Certainly, endocrine findings in SDS cannot be generalized to other inherited bone marrow failure syndromes, which seem to have a more clearly defined endocrine phenotype. In contrast, two-thirds of patients with Fanconi anemia have mild hypothyroidism, most have deficiency of first-phase insulin release with associated glucose intolerance, and many have GHD or gonadal failure.1–3,22
This study remains limited by its retrospective nature, as a review of existing clinical data in which complete evaluations were not performed in every patient. In some instances, the number of results available was small. Further prospective studies may be warranted to validate and confirm these findings. In the meantime, in the clinical setting, monitoring growth and optimizing nutrition remain important, and additional endocrine testing should be performed if clinically indicated.
Glossary
- BMI
Body mass index
- FSH
Follicle-stimulating hormone
- FT4
Free thyroxine
- GH
Growth hormone
- GHD
Growth hormone deficiency
- HbA1c
Glycosylated hemoglobin
- HCT
Hematopoietic cell transplantation
- IGF-I
Insulin-like growth factor-1
- OGTT
Oral glucose tolerance test
- SBDS
Shwachman-Bodian-Diamond syndrome
- SDS
Shwachman-Diamond syndrome
- TSH
Thyrotropin
Footnotes
The authors declare no conflicts of interest.
References
- 1.Elder DA, D’Alessio DA, Eyal O, Mueller R, Smith FO, Kansra AR, et al. Abnormalities in glucose tolerance are common in children with Fanconi anemia and associated with impaired insulin secretion. Pediatr Blood Cancer. 2008;51:256–60. doi: 10.1002/pbc.21589. [DOI] [PubMed] [Google Scholar]
- 2.Eyal O, Blum S, Mueller R, Smith FO, Rose SR. Improved growth velocity during thyroid hormone therapy in children with Fanconi anemia and borderline thyroid function. Pediatr Blood Cancer. 2008;51:652–6. doi: 10.1002/pbc.21673. [DOI] [PubMed] [Google Scholar]
- 3.Rose SR, Myers KC, Rutter MM, Mueller R, Khoury JC, Mehta PA, et al. Endocrine phenotype of children and adults with Fanconi anemia. Pediatr Blood Cancer. 2012;59:690–6. doi: 10.1002/pbc.24095. [DOI] [PubMed] [Google Scholar]
- 4.Burroughs L, Woolfrey A, Shimamura A. Shwachman-Diamond syndrome: a review of the clinical presentation, molecular pathogenesis, diagnosis, and treatment. Hematol Oncol Clin North Am. 2009;23:233–48. doi: 10.1016/j.hoc.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ginzberg H, Shin J, Ellis L, Morrison J, Ip W, Dror Y, et al. Shwachman syndrome: phenotypic manifestations of sibling sets and isolated cases in a large patient cohort are similar. J Pediatr. 1999;135:81–8. doi: 10.1016/s0022-3476(99)70332-x. [DOI] [PubMed] [Google Scholar]
- 6.Alter BP. Aplastic anemia, pediatric aspects. Oncologist. 1996;1:361–6. [PubMed] [Google Scholar]
- 7.Alter BP, Giri N, Savage SA, Peters JA, Loud JT, Leathwood L, et al. Malignancies and survival patterns in the National Cancer Institute inherited bone marrow failure syndromes cohort study. Br J Haematol. 2010;150:179–88. doi: 10.1111/j.1365-2141.2010.08212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenberg PS, Zeidler C, Bolyard AA, Alter BP, Bonilla MA, Boxer LA, et al. Stable long-term risk of leukaemia in patients with severe congenital neutropenia maintained on G-CSF therapy. Br J Haematol. 2010;150:196–9. doi: 10.1111/j.1365-2141.2010.08216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gana S, Sainati L, Frau MR, Monciotti C, Poli F, Cannioto Z, et al. Shwachman-Diamond syndrome and type 1 diabetes mellitus: more than a chance association? Exp Clin Endocrinol Diabetes. 2011;119:610–2. doi: 10.1055/s-0031-1275699. [DOI] [PubMed] [Google Scholar]
- 10.Rosendahl J, Teich N, Mossner J, Edelmann J, Koch CA. Compound heterozygous mutations of the SBDS gene in a patient with Shwachman-Diamond syndrome, type 1 diabetes mellitus and osteoporosis. Pancreatology. 2006;6:549–54. doi: 10.1159/000096978. [DOI] [PubMed] [Google Scholar]
- 11.Kamoda T, Saito T, Kinugasa H, Iwasaki N, Sumazaki R, Mouri Y, et al. A case of Shwachman-Diamond syndrome presenting with diabetes from early infancy. Diabetes Care. 2005;28:1508–9. doi: 10.2337/diacare.28.6.1508. [DOI] [PubMed] [Google Scholar]
- 12.Kawashima H, Ushio M, Aritaki K, Kashiwagi Y, Watanabe C, Nishimata S, et al. Discordant endocrinopathy in a sibling with Shwachman-Diamond syndrome. J Trop Pediatr. 2006;52:445–7. doi: 10.1093/tropej/fml050. [DOI] [PubMed] [Google Scholar]
- 13.Mack DR, Forstner GG, Wilschanski M, Freedman MH, Durie PR. Shwachman syndrome: Exocrine pancreatic dysfunction and variable phenotypic expression. Gastroenterology. 1996;111:1593–602. doi: 10.1016/s0016-5085(96)70022-7. [DOI] [PubMed] [Google Scholar]
- 14.Fatih Akdogan M, Altay M, Denizli N, Gucun M, Tanrikulu S, Duranay M. A rare case: Shwachman-Diamond syndrome presenting with diabetic ketoacidosis. Endocrine. 2011;40:146–7. doi: 10.1007/s12020-011-9460-7. [DOI] [PubMed] [Google Scholar]
- 15.Goeteyn M, Oranje A, Vuzevski V, de Groot R, van Suijlekom-Smit L. Ichthyosis, exocrine pancreatic insufficiency, impaired neutrophil chemotaxis, growth retardation, and metaphyseal dysplasia (Shwachman syndrome). Report of a case with extensive skin lesions (clinical, histological, and ultrastructural findings) Arch Dermatol. 1991;127:225–30. [PubMed] [Google Scholar]
- 16.Bhatla D, Davies SM, Shenoy S, Harris RE, Crockett M, Shoultz L, et al. Reduced-intensity conditioning is effective and safe for transplantation of patients with Shwachman-Diamond syndrome. Bone Marrow Transplant. 2008;42:159–65. doi: 10.1038/bmt.2008.151. [DOI] [PubMed] [Google Scholar]
- 17.Kornfeld SJ, Kratz J, Diamond F, Day NK, Good RA. Shwachman-Diamond syndrome associated with hypogammaglobulinemia and growth hormone deficiency. J Allergy Clin Immunol. 1995;96:247–50. doi: 10.1016/s0091-6749(95)70014-5. [DOI] [PubMed] [Google Scholar]
- 18.Raj AB, Bertolone SJ, Barch MJ, Hersh JH. Chromosome 20q deletion and progression to monosomy 7 in a patient with Shwachman-Diamond syndrome without MDS/AML. J Pediatr Hematol Oncol. 2003;25:508–9. doi: 10.1097/00043426-200306000-00018. [DOI] [PubMed] [Google Scholar]
- 19.Filippi L, Tronchin M, Pezzati M, Chiti G, Dani C, Vichi G, et al. Shwachman syndrome in a preterm newborn associated with transient diabetes mellitus. J Pediatr Gastroenterol Nutr. 2002;34:219–23. doi: 10.1097/00005176-200202000-00022. [DOI] [PubMed] [Google Scholar]
- 20.Shmerling D, Prader A, Hitzig W, Giedion A, Hadorn B, Kühni M. The syndrome of exocrine pancreatic insufficiency, neutropenia, metaphyseal dysostosis and dwarfism. Helv Paediatr Acta. 1969;24:547–75. [PubMed] [Google Scholar]
- 21.Aggett P, Cavanagh N, Matthew D, Pincott J, Sutcliffe J, Harries J. Shwachman’s syndrome. A review of 21 cases. Arch Dis Child. 1980;55:331–47. doi: 10.1136/adc.55.5.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sherafat-Kazemzadeh R, Mehta SN, Care MM, Kim MO, Williams DA, Rose SR. Small pituitary size in children with Fanconi anemia. Pediatr Blood Cancer. 2007;49:166–70. doi: 10.1002/pbc.21148. [DOI] [PubMed] [Google Scholar]