Abstract
Background
Enterovirus D68 (EV-D68) has been infrequently reported historically, and is typically associated with isolated cases or small clusters of respiratory illness. Beginning in August, 2014, increases in severe respiratory illness associated with EV-D68 were reported across the USA. We aimed to describe the clinical, epidemiological, and laboratory features of this outbreak, and to better understand the role of EV-D68 in severe respiratory illness.
Methods
We collected regional syndromic surveillance data for epidemiological weeks 23 to 44, 2014, (June 1 to Nov 1, 2014) and hospital admissions data for epidemiological weeks 27 to 44, 2014, (June 29 to Nov 1, 2014) from three states: Missouri, Illinois and Colorado. Data were also collected for the same time period of 2013 and 2012. Respiratory specimens from severely ill patients nationwide, who were rhinovirus-positive or enterovirus-positive in hospital testing, were submitted between Aug 1, and Oct 31, 2014, and typed by molecular sequencing. We collected basic clinical and epidemiological characteristics of EV-D68 cases with a standard data collection form submitted with each specimen. We compared patients requiring intensive care with those who did not, and patients requiring ventilator support with those who did not. Mantel-Haenszel χ2 tests were used to test for statistical significance.
Findings
Regional and hospital-level data from Missouri, Illinois, and Colorado showed increases in respiratory illness between August and September, 2014, compared with in 2013 and 2012. Nationwide, 699 (46%) of 1529 patients tested were confirmed as EV-D68. Among the 614 EV-D68-positive patients admitted to hospital, age ranged from 3 days to 92 years (median 5 years). Common symptoms included dyspnoea (n=513 [84%]), cough (n=500 [81%]), and wheezing (n=427 [70%]); 294 (48%) patients had fever. 338 [59%] of 574 were admitted to intensive care units, and 145 (28%) of 511 received ventilator support; 322 (52%) of 614 had a history of asthma or reactive airway disease; 200 (66%) of 304 patients with a history of asthma or reactive airway disease required intensive care compared with 138 (51%) of 270 with no history of asthma or reactive airway disease (p=0·0004). Similarly, 89 (32%) of 276 patients with a history of asthma or reactive airway disease required ventilator support compared with 56 (24%) of 235 patients with no history of asthma or reactive airway disease (p=0·039).
Interpretation
In 2014, EV-D68 caused widespread severe respiratory illness across the USA, disproportionately affecting those with asthma. This unexpected event underscores the need for robust surveillance of enterovirus types, enabling improved understanding of virus circulation and disease burden.
Funding
None.
Introduction
Enteroviruses (family Picornaviridae) are associated with a wide range of clinical manifestations including respiratory illness, febrile illness, or neurological illness. Enterovirus D68 (EV-D68) differs from other enteroviruses in that it is primarily detected in association with respiratory illness and shows biological similarity to some rhinoviruses;1 most diagnostic PCR assays cannot distinguish rhinovirus from enterovirus. The full spectrum of EV-D68 disease remains unclear. No vaccines or specific treatments are available for EV-D68 infection, and clinical care is supportive.
EV-D68 was initially isolated in 1962,2 and was first reported to the National Enterovirus Surveillance System (NESS) in the USA in 1987.3 NESS passively collects voluntary laboratory reports of enterovirus and parechovirus types. During 1987–2005, 26 sporadic cases of EV-D68 were reported to NESS.3 Reporting subsequently increased, with 94 reports during 2006–13; a peak of 50 reports in 2009 coincided with small clusters of EV-D68 associated with acute respiratory illness.4 Many small, localised clusters of EV-D68 were reported before 2014 in the USA4, 5, 6 and worldwide.4, 7, 8, 9, 10, 11, 12, 13, 14 Most clusters have included fewer than 30 cases and, as such, epidemiological and clinical descriptions of the virus have been limited; one of the largest reported outbreaks was in Japan in 2010, where more than 120 cases were detected.4 Circulating strains of EV-D68 are genetically diverse and seem to be distributed globally.13
Research in context.
Evidence before this study
Since first isolated in 1962, EV-D68 has been reported and investigated only rarely and sporadically and, as such, past publications on the virus are limited. We searched MEDLINE using search terms such as “enterovirus”, “enterovirus 68”, “HEV-68”, “EV-68”, and “EV-D68” for all EV-D68 reports up to the start of the outbreak (September, 2014). Some laboratory studies were identified, as were several case studies and case series that described basic clinical features of the infection. In the past decade, reports from the USA and globally have increased and typically describe small and localised clusters of EV-D68 infections. Although basic epidemiological and clinical features are described, these clusters typically included less than 30 individuals with little information available.
Added value of this study
This is the largest outbreak of EV-D68 recognised to date, and our study shows that EV-D68 can cause widespread severe respiratory illness. Furthermore, this investigation characterises epidemiological and clinical features of severe EV-D68 infection in a large group of patients, and suggests that a history of asthma or reactive airway disease is associated with illness severity.
Implications of all the available evidence
Clinicians and public health professionals should be aware that EV-D68 can cause severe respiratory illness. Enhanced surveillance for EV-D68 and investigation of identified cases is recommended to improve our understanding of EV-D68 circulation and its contribution to respiratory disease.
On Aug 19, 2014, the US Centers for Disease Control and Prevention (CDC) was notified by investigators at Children's Mercy Hospital in Kansas City, MO, USA, (CMH-Kansas City-MO) of an increase in patients with severe respiratory illness requiring intensive care. An increase was also noted in detections of rhinovirus or enterovirus by a multiplex PCR assay in nasopharyngeal specimens obtained during Aug 5–19. On Aug 23 and 29, CDC was notified of similar increases in children's hospitals in Chicago, IL, and Aurora, CO, respectively. Sequencing of specimens from selected cases of severe respiratory illness from Kansas City and Chicago identified EV-D68 as the predominant pathogen.15 In response to these findings, we launched a broader nationwide investigation to further describe the clinical, epidemiological, and laboratory features of this outbreak, and to better understand the role of EV-D68 in severe respiratory illness.
Methods
Regional and hospital data collection
To describe trends in respiratory illness, we collected two types of data: regional syndromic surveillance data and hospital admissions data. These data were available from three states that had reported large clusters early in the investigation: Missouri, Illinois, and Colorado. To describe the clinical and epidemiological characteristics of EV-D68 cases, we collected nationwide patient-level data with a patient summary form submitted with clinical specimens for virus typing.
The Electronic Surveillance System for the Early Notification of Community-based Epidemics (ESSENCE) collates and transmits chief complaint data (the primary symptom that a patient provides as the reason for seeking medical care) from emergency departments to public health authorities in many US states for syndromic surveillance.16 Data for emergency department visits and admissions for respiratory illness were collected from paediatric and adult hospitals in northern Illinois, via ESSENCE. Data for emergency department visits in northwest Missouri were also collected via ESSENCE. All chief complaints falling under the respiratory syndrome in ESSENCE were included; “AcuteBronchitis”, “CongestionChest”, “Cough”, “DifficultyBreathing”, “Hemoptysis”, “Laryngitis”, “LowerRespiratoryInfection”, “CongestionNasal”, “OtitisMedia”, “Pneumonia”, “ShortnessOfBreath”, “SoreThroat”, “UpperRespiratoryInfection”, or “Wheezing”. Regional outpatient data from the Aurora-Boulder Metro area of Colorado were collected from routine influenza-like illness surveillance systems, with the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) code for acute upper respiratory infections of unspecified site (465.9). These three states provided data for epidemiological weeks 23 to 44 of 2012, 2013, and 2014, including the months of June to October.
Hospital admissions data for respiratory illness were available from the University of Chicago Comer Children's Hospital (CCH-Chicago-IL) using respiratory ICD-9-CM codes with Medicare severity diagnosis-related groups17 and from Children's Hospital Colorado in Aurora (CHC-Aurora-CO) using chief complaints (the data from CHC-Aurora-CO were subsequently described elsewhere18). Data for paediatric intensive care unit admissions for respiratory illness were collected from CCH-Chicago-IL with respiratory ICD-9-CM codes with Medicare severity diagnosis-related groups and from CMH-Kansas City-MO with a primary diagnosis of asthma. Hospitals provided data for epidemiological weeks 27 to 44 of 2012, 2013, and 2014, including the months of July to October.
Investigation of patients with severe respiratory illness
Following early reports of severe respiratory illness associated with EV-D68, case finding was widened through a national health advisory, a report in The Epidemic Information Exchange (EpiX), and national calls to clinicians and state epidemiologists. Because EV-D68 typing assays were not widely available, the CDC used molecular sequencing to type available specimens submitted by local and state health departments; state public health laboratories able to type EV-D68 also submitted results. Initially, samples submitted to the CDC were collected from patients with severe respiratory illness who were positive by hospital testing for either rhinovirus or enterovirus. Subsequently, to better understand the outbreak scope, sampling expanded to include specimens from states without previously confirmed cases, and samples from new populations and new locations within states.
We developed a standard one-page data collection form for each specimen submitted to the CDC (appendix pp 1–2). We also received data from the states that performed molecular sequencing on site. Variables included demographics, underlying illness, signs and symptoms, and measures of clinical severity. Patients with either a specimen collection date or a symptom onset date between Aug 1, and Oct 31, 2014, were included. To better understand severity of illness, only patients who were reported to be admitted to hospital were included. A patient was considered to have had fever if stated on the data collection form or if a temperature was reported as greater than 38·0°C (100·4°F). We excluded patients reported as having an emergency department visit only. When date of admission was known, we excluded patients whose specimen date was more than 7 days before or after admission to hospital.
We did RNA extraction from clinical specimens, enterovirus VP1-specific RT-PCR, sequencing, and virus identification as described previously.19 Based on initial phylogenetic analyses of VP1 (336 bp), representatives of the EV-D68 lineages from Aug 1, and Oct 31, 2014, were selected for genome sequencing.20 Complete VP1 gene sequences (927 bp) were abstracted from the EV-D68 genomes (GenBank KM851225-KM851231).
The complete VP1 gene sequences from cases in 2014 were compared with EV-D68 VP1 gene sequences from GenBank. Phylogenetic relationships were inferred by the neighbour-joining and maximum likelihood methods implemented in MEGA (version 6.0).21 Support for specific tree topologies was estimated by bootstrap analysis with 500 pseudoreplicate datasets.
Statistical analysis
To understand factors associated with more severe illness, we compared patients using two markers of severity: intensive care unit admission and ventilator support (non-invasive bilevel or continuous positive pressure ventilation [NIPPV], intubation, or extracorporeal membrane oxygenation [ECMO]). The percentage of severe cases was compared by epidemiological variables. Mantel-Haenszel χ2 tests were used to test for statistical significance. Descriptive and analytical data analyses were done with SAS 9.3 (SAS Institute, Cary, NC, USA).
Role of the funding source
This investigation formed part of an emergency public health response by the US Centers for Disease Control and Prevention (CDC). CDC authors were involved in data collection, data analysis, data interpretation, and writing of the report. CMM and ATC had access to the full data set. CMM had full access to all of the data and the final responsibility to submit for publication.
Results
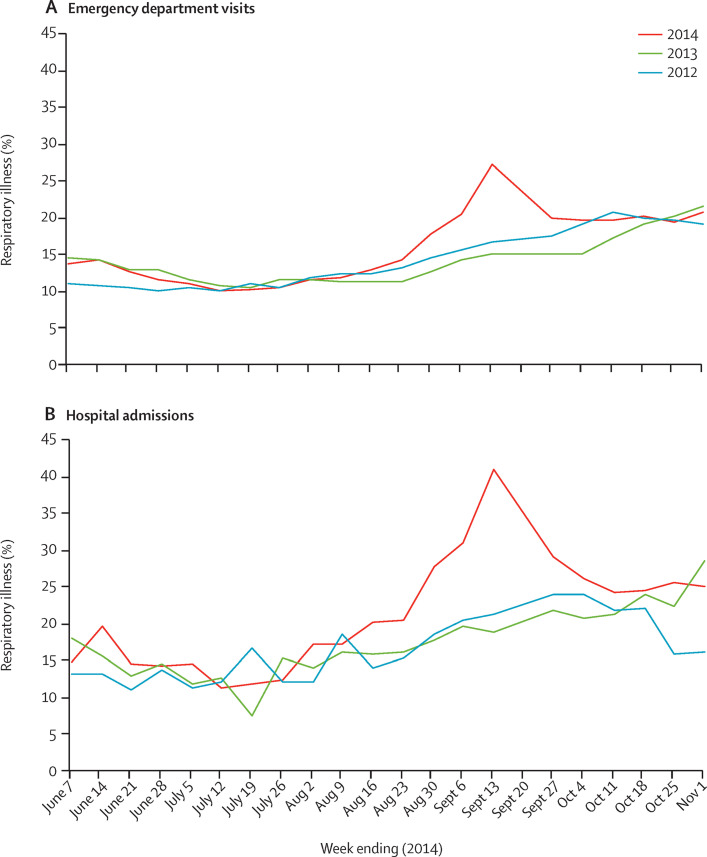
In Illinois, Missouri, and Colorado, respiratory illness reports for August and September were higher in 2014 compared with the same period in 2013 and 2012 (figure 1 ; appendix p 3). In northern Illinois, chief complaint data indicated increases in both paediatric emergency department visits and hospital admissions for respiratory illness during this period (figure 1) compared with the same months in 2012 and 2013. Overall, the duration of the apparent increase varied between these three locations, lasting roughly 3–6 weeks (figure 1; appendix p 3).
Figure 1.
Syndromic surveillance of respiratory illness among patients younger than 18 years of age in northern Illinois, by year
(A) Visits to the emergency department for respiratory illness and (B) hospital admissions for respiratory illness are depicted as a percentage of total visits or admissions, respectively. Data for respiratory illness from hospitals in northern Illinois were extracted from ESSENCE Surveillance System. Data for the same time period in 2012, 2013, and 2014 were extracted for comparison.
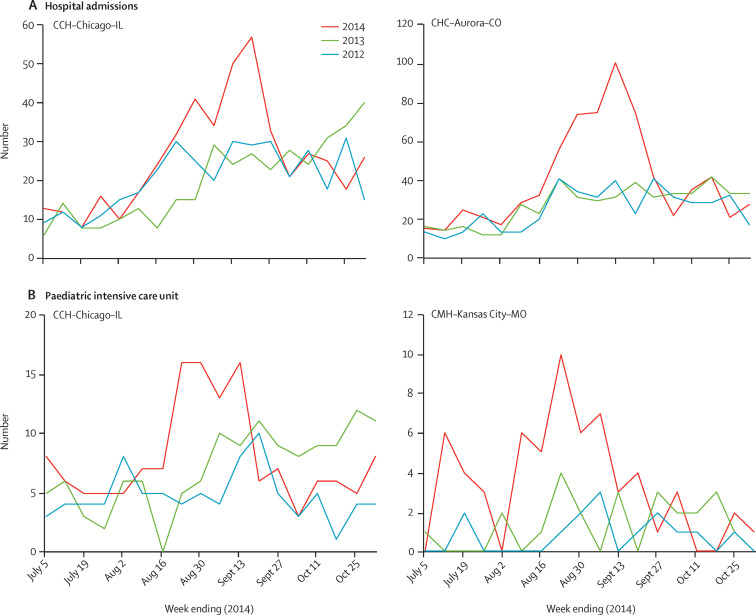
Compared with 2012 and 2013, increases were noted between August and September, 2014, in both hospital admissions for respiratory illness (figure 2A ) and paediatric intensive care unit admissions for respiratory illness (figure 2B). The number of respiratory hospital admissions between weeks 32 and 39 (Aug 3, and Sept 27, 2014) increased by 61% in CCH-Chicago-IL and 95% in CHC-Aurora-CO, compared with the average of the same period in 2012 and 2013. Respiratory admissions to paediatric intensive care units, for this period (Aug 3–Sept 27) increased by 73% in CCH-Chicago-IL and by 282% in CMH-Kansas City-MO.
Figure 2.
Respiratory illness detected in three hospitals in the USA, by year
Data for hospital admissions (A) and paediatric intensive care unit admissions (B) for respiratory illness were collected from three hospitals: University of Chicago Comer Children's Hospital, IL, USA (CCH-Chicago-IL), Children's Mercy Hospital in Kansas City, MO, USA (CMH-Kansas City-MO), and Children's Hospital Colorado (CHC-Aurora-CO). Data were collected for the same time periods in 2014, 2013, and 2012, for comparison. Available data are shown; comparative hospital admission data were not available from Children's Mercy Hospital, and comparative paediatric intensive care unit admission data were not available from Children's Hospital Colorado.
Throughout the USA, respiratory specimens were collected from 1529 patients between Aug 1, and Oct 31, 2014, and submitted to CDC for typing by molecular sequencing. Of the 1573 specimens received from 1529 patients, 1365 [87%] specimens submitted were nasopharyngeal swabs, although other specimen types included nasopharyngeal washes, oropharyngeal swabs, nasal swabs, nasal washes, bronchoalveolar lavages, and bronchial washes. At CDC, 699 (46%) of 1529 patients were confirmed as EV-D68-positive. No other single enterovirus or rhinovirus type was identified in more than 4% of patients tested at CDC. The remaining patients were positive for other enterovirus species (56 [4%] of 1529) or rhinoviruses (500 [33%] of 1529). Three patients (<1%) were positive for enterovirus or rhinovirus, with no specific virus identified, and 271 (18%) were negative for rhinoviruses or enteroviruses by sequencing.
The 699 EV-D68-positive patients were reported from 41 states and the District of Columbia. Additional patients were identified by the New York State Public Health Laboratory (n=149), the California Department of Public Health (n=57), the Indiana State Department of Health (n=19), and the Minnesota Department of Health (n=3). Overall, 927 patients tested positive for EV-D68 by molecular sequencing between August, and October, 2014, and were reported to CDC.
Demographic and clinical data were available for 764 patients, whose specimen date or symptom onset date was between Aug 1, and Oct 31, 2014. Those with no reported respiratory symptoms (n=52), those who were not known to be admitted to hospital (n=89) and, if known, those whose specimen date was more than 7 days before or after hospital admission (n=9) were excluded; data were analysed for 614 patients (table 1 ). In patients with EV-D68, other pathogens were detected infrequently by hospital testing. Reported ages ranged from 3 days to 92 years (median 5 years). Symptoms included dyspnoea (n=513 [84%]), cough (n=500 [81%]), and wheezing (n=427 [70%]); 294 (48%) patients had fever. More than half of patients (322 [52%] of 614) had a history of asthma or reactive airway disease, although wheezing was also noted in patients (169 [58%] of 292) with no previous history of asthma or reactive airway disease. 39 (6%) of 614 cases were reported as being born prematurely (gestational age of 36 weeks or younger). 12 (31%) of these 39 patients were younger than 1 year of age at symptom onset; seven (58%) of these 12 required intensive care.
Table 1.
Epidemiological and clinical characteristics of EV-D68-positive patients that were admitted to hospital (N=614)
| Number of patients/total reported* | |
|---|---|
| Sex | |
| Female | 250/611 (41%) |
| Male | 361/611 (59%) |
| Race | |
| American Indian or Alaskan Native | 5/363 (1%) |
| Asian | 14/363 (4%) |
| Black or African American | 110/363 (30%) |
| Native Hawaiian or Pacific Islander | 2/363 (<1%) |
| White | 232/363 (64%) |
| Ethnicity | |
| Hispanic | 72/304 (24%) |
| Non-Hispanic | 232/304 (76%) |
| Age group (years) | |
| <1 | 51/609 (8%) |
| 1–4 | 209/609 (34%) |
| 5–11 | 251/609 (41%) |
| 12–17 | 59/609 (10%) |
| ≥18 | 39/609 (6%) |
| Comorbidities | |
| Asthma or reactive airway disease | 322/614 (52%) |
| Prematurity | 39/614 (6%) |
| Cardiac disease | 15/614 (2%) |
| Immunocompromised | 10/614 (2%) |
| Bronchopulmonary dysplasia | 7/614 (1%) |
| Symptoms | |
| Dyspnoea | 513/614 (84%) |
| Cough | 500/614 (81%) |
| Wheezing | 427/614 (70%) |
| Tachypnoea | 296/614 (48%) |
| Fever | 294/614 (48%) |
| Retractions | 261/614 (43%) |
| Rhinorrhoea | 232/614 (38%) |
| Vomiting | 137/614 (22%) |
| Sore throat | 78/614 (13%) |
| Lethargy | 57/614 (9%) |
| Diarrhoea | 23/614 (4%) |
| Cyanosis | 19/614 (3%) |
| Chills | 18/614 (3%) |
| Seizure | 9/614 (1%) |
| Rash | 9/614 (1%) |
| Co-detections† | |
| Streptococcus pneumoniae | 5/31 (16%) |
| Legionella pneumophila | 2/46 (4%) |
| Adenovirus | 7/407 (2%) |
| Parainfluenza virus | 4/394 (1%) |
| Respiratory syncytial virus | 4/430 (1%) |
| Human metapneumovirus | 1/385 (<1%) |
| Imaging | |
| Abnormal chest radiograph‡ | 281/465 (60%) |
| Abnormal chest CT scan | 17/114 (15%) |
| Treatment | |
| Bronchodilators | 498/542 (92%) |
| Antibiotics | 235/516 (46%) |
| Clinical course | |
| Hypoxic in room air | 383/533 (72%) |
| Supplemental oxygen given | 436/544 (80%) |
| Admitted to intensive care unit | 338/574 (59%) |
| Required ventilation§ | 145/511 (28%) |
| Non-invasive ventilation (eg, BiPAP, CPAP) | 118/520 (23%) |
| Intubated | 40/502 (8%) |
| ECMO | 8/495 (2%) |
| Outcome | |
| Died | 5/499 (1%) |
Data are n/N (%).
Denominators represent the number of patients for whom a response was provided at the time of case investigation.
With data from the patient summary form, there were no reported co-detections of Chlamydophila pneumoniae (n=255 tested), human coronavirus (n=320 tested), influenza A virus (n=418 tested), influenza B virus (n=415 tested), or Mycoplasma pneumoniae (n=262 tested); denominators represent patients reported as tested for the specific pathogen.
Typical findings included peribronchial thickening, atelectasis, or interstitial markings consistent with reactive airway disease.
Required non-invasive ventilation, intubation, or ECMO; these designations are not mutually exclusive. BiPAP=bilevel positive airway pressure. CPAP=continuous positive airway pressure. ECMO=extracorporeal membrane oxygenation.
Nearly two-thirds of patients (338 [59%] of 574) were admitted to intensive care units, and 145 (28%) of 511 received ventilator support (table 2 ). Patients with a history of asthma or reactive airway disease were more likely to be admitted to the intensive care unit (p=0·0004) and were more likely to require ventilator support (p=0·039) than those without. These findings remained significant when stratifying individually for sex or prematurity. When stratified by age group, patients with a history of asthma or reactive airway disease were more likely to require intensive care than those without (p=0·0019), but there was less evidence for an association with ventilator support (p=0·059). Females were more likely to require ventilator support than males (p<0·0001), although there was no difference in intensive care unit admissions between sexes. This finding remained significant when stratifying individually for a history of asthma or reactive airway disease, age group, or prematurity.
Table 2.
Patients with severe disease by medical history, sex, and age group
|
Admission to the intensive care unit |
Ventilator support |
||||
|---|---|---|---|---|---|
| n/N (%) | p value | n/N (%) | p value | ||
| History of asthma or reactive airway disease | |||||
| Yes | 200/304 (66%) | .. | 89/276 (32%) | .. | |
| No | 138/270 (51%) | 0·0004 | 56/235 (24%) | 0·039 | |
| Prematurity | |||||
| Yes | 24/39 (62%) | .. | 11/35 (31%) | .. | |
| No | 314/535 (59%) | 0·73 | 134/476 (28%) | 0·68 | |
| Sex | |||||
| Female | 143/233 (61%) | .. | 80/209 (38%) | .. | |
| Male | 194/338 (57%) | 0·34 | 65/299 (22%) | <0·0001 | |
| Age group (years) | |||||
| <1 | 28/49 (57%) | .. | 15/45 (33%) | .. | |
| 1–4 | 102/192 (53%) | 0·62 | 36/170 (21%) | 0·089 | |
| 5–11 | 144/234 (62%) | 0·57 | 56/206 (27%) | 0·41 | |
| 12–17 | 43/58 (74%) | 0·065 | 24/51 (47%) | 0·17 | |
| ≥18 | 18/36 (50%) | 0·52 | 12/34 (35%) | 0·86 | |
| Total admitted to hospital | 338/574 (59%) | .. | 145/511 (28%) | .. | |
Severity was defined in two ways: 1) being admitted to an intensive care unit or 2) requiring ventilator support (NIPPV [non-invasive bilevel or continuous positive pressure ventilation], intubation, or ECMO [extracorporeal membrane oxygenation]). Mantel-Haenszel χ2 p values were calculated as indicated. The p value for age group analysis represents a comparison of each age group with those younger than 1 year of age.
Among cases for whom survival outcome was reported, five (1%) of 499 were reported as fatal. The contribution of EV-D68 to the deaths of these individuals is unknown. Ages of fatal cases ranged from 5 months to 21 years. One individual had a history of asthma or reactive airway disease, one had Duchenne muscular dystrophy, and one had sickle cell trait.
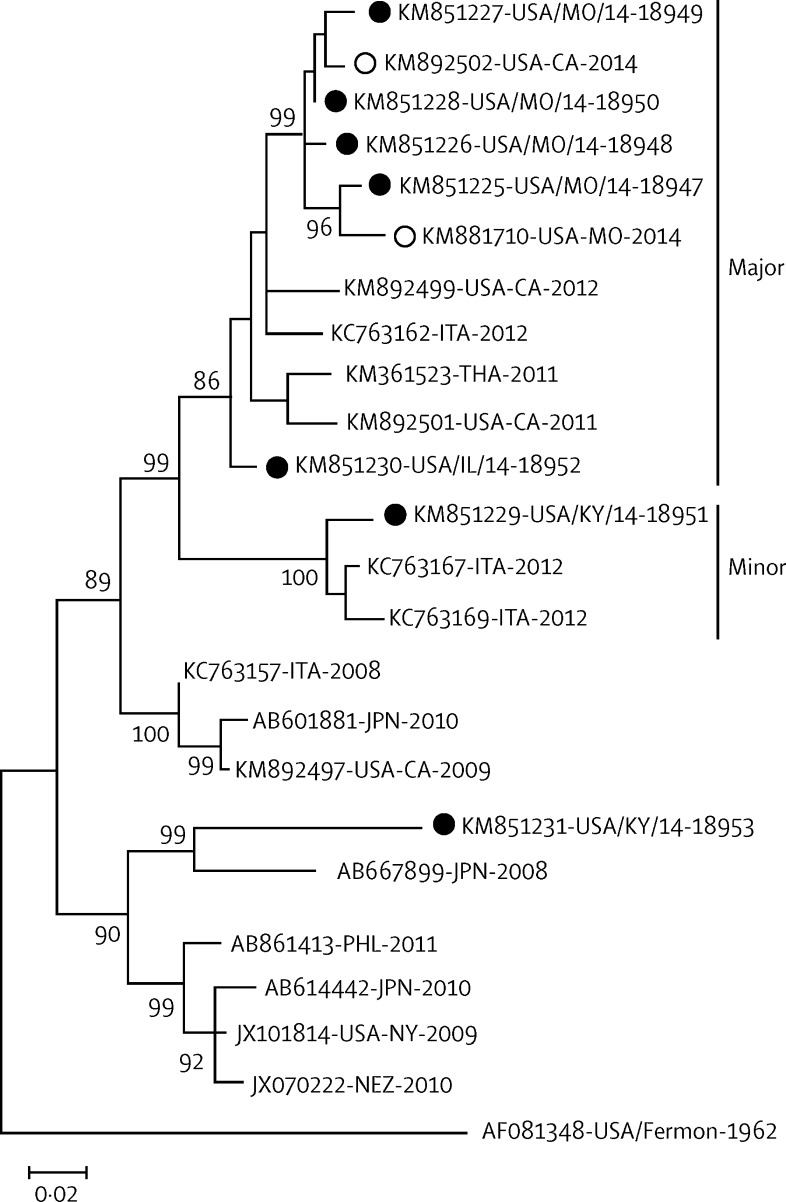
Sequence analyses of the EV-D68 strains collected in the USA in 2014 showed major and minor lineages cocirculating at the time of the outbreak; a representative summary of these data is provided in figure 3 . Most patients (560 [92%] of 606) had strains in the major lineage. The major lineage viruses were closely related to strains identified in the USA, Asia, and southern Europe in 2011, 2012, and 2013. Minor lineage strains accounted for 7% of EV-D68 cases. The minor lineage was closely related to strains identified in southern Europe in 2012. Both the major and minor lineages were detected across many US states, and were not localised to a given region. Patients with severe illness, including the need for intensive care, were part of both lineages. One patient was infected with an EV-D68 strain that was distantly related to some strains from the Philippines, Japan, the USA, and New Zealand isolated during 2008–10. All cocirculating 2014 EV-D68 outbreak strains identified at CDC are represented in the seven EV-D68 genomes previously described.20
Figure 3.
Maximum-likelihood phylogeny of EV-D68 strains associated with respiratory disease clusters or outbreaks from 2008 to 2014
Complete viral protein 1 gene sequences from the US 2014 outbreak (solid circles are EV-D68 KM85851225-KM851231 sequenced at CDC;20 hollow circles are other US 2014 EV-D68 VP1 genes from GenBank) were compared with EV-D68 strains from Europe and Asia. The major (>91% of cases) and minor (>7% of cases) cocirculating outbreak strains are indicated. Bootstrap values >80% are shown. The scale bar represents genetic change in base substitutions per site. A single EV-D68, distantly related to an older lineage, was identified in Kentucky. ITA=Italy. THA=Thailand. JPN=Japan. PHL=Philippines. NEZ=New Zealand.
Discussion
Beginning in August, 2014, reports of severe respiratory illness predominantly affecting children increased compared with previous years, resulting in a substantial burden of hospital admissions. Between August and October, almost half of the respiratory specimens sequenced from patients already known to be enterovirus-positive or rhinovirus-positive were identified as EV-D68. This outbreak is the largest and most widespread outbreak of EV-D68 reported to date. Among a select number of patients with available clinical data, illness was indicative of a non-specific respiratory disease, often in the absence of fever, indistinguishable from asthma exacerbation related to other causes. Most patients required intensive care. Half of the patients had a history of asthma or reactive airway disease, and these individuals were more likely to require intensive care or ventilator support.
Between Aug 1, and Oct 31, 2014, 927 EV-D68 cases were detected across the USA. These cases are a substantial underestimate of the true count, because only a selection of patients with severe illness was tested. Phylogenetic analysis showed that the EV-D68 viruses circulating at that time were from two lineages, both of which were detected across the USA, suggesting that this outbreak was widespread and not likely to be a series of smaller localised clusters. The reason for the widespread circulation of EV-D68 in 2014 is unknown. Generally, enteroviruses are considered ubiquitous in the community, but with unpredictable annual variations in circulation and outbreak patterns.3 EV-D68 strains identified during this outbreak are very similar to those detected sporadically over the past few years in the USA and elsewhere, indicating that the 2014 EV-D68 outbreak is unlikely to be the result of a novel virus strain. More detailed phylogenetic analyses of EV-D68 strains are ongoing. Testing practices probably played a part in increased detection and case ascertainment. Historically, EV-D68 typing by either neutralisation or molecular sequencing has been done by only a few specialised laboratories, restricting the number of cases identified. Recent introduction of multipathogen PCR panels in many large hospitals, however, has greatly expanded the detection of enteroviruses generically, especially in respiratory illness in which enteroviruses were not previously routinely considered. This has indirectly contributed to the recent increase in reporting. The substantial rise in respiratory illness detected by syndromic surveillance, however, suggests that increased testing is unlikely to be the only contributor. Studies from across the world that have retrospectively typed stored specimens suggest that EV-D68 circulation has been present at low levels in recent years.8, 10, 12, 22, 23, 24, 25, 26
The spectrum of respiratory illness caused by EV-D68 is not well defined. Because of the sudden increase in reports of severe respiratory illness, our investigation focused primarily on further describing this entity. However, existing syndromic surveillance detected increases in both inpatient and outpatient respiratory illness during the outbreak period in the three states investigated. This highlights the importance of such systems in characterizing outbreaks. For the detection of EV-D68 going forward, afebrile presentations should be considered in the design of such systems, because many aim to detect influenza-like illness and include fever as a detection criterion. The full scope of EV-D68 circulation, both geographical and temporal, remains unknown. Enterovirus season in temperate climates is typically summer and autumn, but some cases can occur into the winter.3 Because of few data, EV-D68 seasonality is not well characterised. Most EV-D68 cases reported have fallen within this typical enterovirus season,4, 6, 10, 12 although cases have been detected at other times of year.3, 7, 9
Although most EV-D68 reports so far have been in association with respiratory illness, the full spectrum of disease is unknown. As examples, EV-D68 was detected in the cerebrospinal fluid of a patient with acute flaccid paralysis in the USA in 2005,3 and in one fatal case involving complications of the nervous system in the USA in 2008.27 Additionally, in a similar timeframe with the increase in respiratory illness described in our investigation, there was an increase of acute flaccid myelitis in children across the USA.18, 28, 29 EV-D68 was detected in respiratory specimens of some of these individuals but no virus, including EV-D68, was detected in the cerebrospinal fluid. As such, the contribution of EV-D68 to this neurological illness is not clear and the cause of acute flaccid myelitis is still being elucidated.
Our description of hospital cases with respiratory illness showed dyspnoea, cough, and wheezing as predominant symptoms. Typical patient presentations were indistinguishable from asthma exacerbation, which has been previously associated with EV-D68 infection.30 Furthermore, almost half of the patients admitted to hospital had a previous history of asthma or reactive airway disease. In view that the prevalence of asthma in the USA was 8·4% in 2010,31 our data suggest that this is a possible risk factor for admission to hospital with EV-D68. Once admitted, these individuals were more likely to require intensive care or ventilator support. In the future, a comparison of EV-D68 characteristics to those of the other viruses detected, including various rhinovirus types, would also be of interest.
This report is subject to several limitations. We describe syndromic and hospital data from three states only, and the trends noted might not be representative of all states. Additionally, the increases noted in these data in 2014 compared with 2013 and 2012 might have been influenced by increased media and public awareness during a nationwide outbreak. Although emergency department visits might be affected by this attention, we would not expect a substantial effect on patients admitted to hospital. Due to the regional syndromic data being reported in aggregate, we were also not able to directly compare these findings to the patient-level data received. During this investigation, specimen collection and testing focused on severely ill patients and therefore we were unable to describe the full severity spectrum of respiratory illness. The criteria for specimen submission was not standardised and might not be representative of all those admitted to hospital and, furthermore, collection priorities changed among the states through the course of the investigation. Additionally, data completeness was not uniform and some variables, such as race, ethnicity, and detections of other pathogens, were less well reported. For the latter, testing algorithms were not standardised and probably varied among hospitals and providers across the country. An age-specific selection bias might have also been introduced in that most reporting facilities were children's hospitals. Samples from adults were sought and tested, but awareness might have been increased in the paediatric settings. Previous reports would suggest that a higher number of adult cases might have been expected,3, 4, 12 although these studies were not focused on severe disease. Paediatric intensive care unit admission criteria might have varied across the country, possibly introducing a misclassification of severity in our comparative analysis; however, our results are strengthened by the consistent observation of the effect of asthma or reactive airway disease noted by use of two severity methods.
We show that EV-D68 was a cause of widespread, severe respiratory illness during 2014, disproportionately affecting those with a history of asthma or reactive airway disease. As a result of this outbreak, a real-time EV-D68-specific RT-PCR assay has been developed by US CDC that might substantially improve EV-D68 detection. Implementation in diagnostic laboratories nationwide, combined with increased reporting to enterovirus surveillance systems, could lead to a better understanding of EV-D68 circulation and burden.
Acknowledgments
Acknowledgments
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention.
Contributors
CMM analysed the data. CMM and JTW wrote the report. CMM, JTW, WAN, MSO, and SIG interpreted the data. CMM, WAN, SLR, BAB, MSO, EL, ES, FH, MAJ, JES, RS, GT, DJ, EO, AP, CC, SH, SG, MP, SRD, A-CN, LM, and JBN collected the data. ATC managed the data. DRF, BR, MAP, and JFS contributed to the strategic direction of the investigation. All authors were involved in critical revision of the final manuscript.
Declaration of interests
MSO and WAN report a patent pending (Nix WA, Oberste MS. Detection and identification of enteroviruses by semi-nested amplification of the enterovirus VP1 protein. US patent number 7,247,457, issued July 24, 2007. Australian patent number 2005201742, issued July 28, 2011). LM reports grants from Centers for Disease Control and Prevention, during the conduct of the study. All other authors declare no competing interests.
Supplementary Material
References
- 1.Oberste MS, Maher K, Schnurr D. Enterovirus 68 is associated with respiratory illness and shares biological features with both the enteroviruses and the rhinoviruses. J Gen Virol. 2004;85:2577–2584. doi: 10.1099/vir.0.79925-0. [DOI] [PubMed] [Google Scholar]
- 2.Schieble JH, Fox VL, Lennette EH. A probable new human picornavirus associated with respiratory diseases. Am J Epidemiol. 1967;85:297–310. doi: 10.1093/oxfordjournals.aje.a120693. [DOI] [PubMed] [Google Scholar]
- 3.Khetsuriani N, Lamonte-Fowlkes A, Oberst S, Pallansch MA, Centers for Disease Control and Prevention Enterovirus surveillance—United States, 1970–2005. Morb MMWR Surveill Summ. 2006;55:1–20. [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention Clusters of acute respiratory illness associated with human enterovirus 68–Asia, Europe, and United States, 2008–2010. MMWR Morb Mortal Wkly Rep. 2011;60:1301–1304. [PubMed] [Google Scholar]
- 5.Jacobson LM, Redd JT, Schneider E. Outbreak of lower respiratory tract illness associated with human enterovirus 68 among American Indian children. Pediatr Infect Dis J. 2012;31:309–312. doi: 10.1097/INF.0b013e3182443eaf. [DOI] [PubMed] [Google Scholar]
- 6.Tokarz R, Kapoor V, Wu W. Longitudinal molecular microbial analysis of influenza-like illness in New York City, May 2009 through May 2010. Virol J. 2011;8:288. doi: 10.1186/1743-422X-8-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Todd AK, Hall RJ, Wang J. Detection and whole genome sequence analysis of an enterovirus 68 cluster. Virol J. 2013;10:103. doi: 10.1186/1743-422X-10-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Imamura T, Suzuki A, Lupisan S. Molecular evolution of Enterovirus 68 detected in the Philippines. PLoS one. 2013;8:e74221. doi: 10.1371/journal.pone.0074221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lauinger IL, Bible JM, Halligan EP, Aarons EJ, MacMahon E, Tong CYW. Lineages, sub-lineages and variants of enterovirus 68 in recent outbreaks. PLoS One. 2012;7:e36005. doi: 10.1371/journal.pone.0036005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ikeda T, Mizuta K, Abiko C. Acute respiratory infections due to enterovirus 68 in Yamagata, Japan between 2005 and 2010. Microbiol Immunol. 2012;56:139–143. doi: 10.1111/j.1348-0421.2012.00411.x. [DOI] [PubMed] [Google Scholar]
- 11.Imamura T, Fuji N, Suzuki A. Enterovirus 68 among children with severe acute respiratory infection, the Philippines. Emerg Infect Dis. 2011;17:1430–1435. doi: 10.3201/eid1708.101328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meijer A, Van Der Sanden S, Snijders BEP. Emergence and epidemic occurrence of enterovirus 68 respiratory infections in The Netherlands in 2010. Virology. 2012;423:49–57. doi: 10.1016/j.virol.2011.11.021. [DOI] [PubMed] [Google Scholar]
- 13.Tokarz R, Firth C, Madhi SA. Worldwide emergence of multiple clades of enterovirus 68. J Gen Virol. 2012;93:1952–1958. doi: 10.1099/vir.0.043935-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piralla A, Girello A, Grignani M. Phylogenetic characterization of enterovirus 68 strains in patients with respiratory syndromes in Italy. J Med Virol. 2014;86:1590–1593. doi: 10.1002/jmv.23821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Midgley CM, Jackson MA, Selvarangan R. Severe respiratory illness associated with enterovirus d68 - missouri and illinois, 2014. MMWR Morb Mortal Wkly Rep. 2014;63:798–799. [PMC free article] [PubMed] [Google Scholar]
- 16.Lewis MD, Pavlin JA, Mansfield JL. Disease outbreak detection system using syndromic data in the greater Washington DC area. Am J Prev Med. 2002;23:180–186. doi: 10.1016/s0749-3797(02)00490-7. [DOI] [PubMed] [Google Scholar]
- 17.Medicare Medicare.gov Hospital Compare, 2015. https://www.medicare.gov/hospitalcompare/Resources/Glossary.html (accessed July 29, 2015).
- 18.Messacar K, Schreiner TL, Maloney JA. A cluster of acute flaccid paralysis and cranial nerve dysfunction temporally associated with an outbreak of enterovirus D68 in children in Colorado, USA. Lancet. 2015;385:1662–1671. doi: 10.1016/S0140-6736(14)62457-0. [DOI] [PubMed] [Google Scholar]
- 19.Nix WA, Oberste MS, Pallansch MA. Sensitive, seminested PCR amplification of VP1 sequences for direct identification of all enterovirus serotypes from original clinical specimens. J Clin Microbiol. 2006;44:2698–2704. doi: 10.1128/JCM.00542-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown BA, Nix WA, Sheth M, Frace M, Oberste MS. Seven strains of Enterovirus D68 detected in the United States during the 2014 severe respiratory disease outbreak. Genome Announc. 2014:e01201–e01214. doi: 10.1128/genomeA.01201-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar S, Tamura K, Jakobsen IB, Nei M. MEGA2: molecular evolutionary genetics analysis software. Bioinformatics. 2001;17:1244–1245. doi: 10.1093/bioinformatics/17.12.1244. [DOI] [PubMed] [Google Scholar]
- 22.Meijer A, Benschop K, Donker G, van der Avoort H. Continued seasonal circulation of enterovirus D68 in the Netherlands, 2011–2014. Euro Surveill. 2014;19:20935. doi: 10.2807/1560-7917.es2014.19.42.20935. [DOI] [PubMed] [Google Scholar]
- 23.Lu QB, Wo Y, Wang HY. Detection of enterovirus 68 as one of the commonest types of enterovirus found in patients with acute respiratory tract infection in China. J Med Microbiol. 2014;63:408–414. doi: 10.1099/jmm.0.068247-0. [DOI] [PubMed] [Google Scholar]
- 24.Linsuwanon P, Puenpa J, Suwannakarn K. Molecular epidemiology and evolution of human enterovirus serotype 68 in Thailand, 2006–2011. PLoS One. 2012;7:e35190. doi: 10.1371/journal.pone.0035190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reiche J, Bottcher S, Diedrich S. Low-level circulation of enterovirus D68-associated acute respiratory infections, Germany, 2014. Emerg Infect Dis. 2015;21:837–841. doi: 10.3201/eid2105.141900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poelman R, Scholvinck EH, Borger R, Niesters HG, van Leer-Buter C. The emergence of enterovirus D68 in a Dutch University Medical Center and the necessity for routinely screening for respiratory viruses. J Clin Virol. 2015;62:1–5. doi: 10.1016/j.jcv.2014.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kreuter JD, Barnes A, McCarthy JE. A fatal central nervous system enterovirus 68 infection. Arch Pathol Lab Med. 2011;135:793–796. doi: 10.5858/2010-0174-CR.1. [DOI] [PubMed] [Google Scholar]
- 28.Division of Viral Diseases. National Centers for Immunization and Respiratory Diseases, CDC. Division of Vector-Borne Diseases. Division of High-Consequence Pathogens and Pathology. National Center for Emerging and Zoonotic Infectious Diseases CDC. Children's Hospital Colorado; Council of State and Territorial Epidemiologists Notes from the field: acute flaccid myelitis among persons aged ≤21 years—United States, August 1–November 13, 2014. MMWR Morb Mortal Wkly Rep. 2015;63:1243–1244. [PMC free article] [PubMed] [Google Scholar]
- 29.Greninger AL, Naccache SN, Messacar K. A novel outbreak enterovirus D68 strain associated with acute flaccid myelitis cases in the USA (2012–14): a retrospective cohort study. Lancet Infect Dis. 2015;15:671–682. doi: 10.1016/S1473-3099(15)70093-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hasegawa S, Hirano R, Okamoto-Nakagawa R, Ichiyama T, Shirabe K. Enterovirus 68 infection in children with asthma attacks: virus-induced asthma in Japanese children. Allergy. 2011;66:1618–1620. doi: 10.1111/j.1398-9995.2011.02725.x. [DOI] [PubMed] [Google Scholar]
- 31.Moorman JE, Akinbami LJ, Bailey CM. National surveillance of asthma: United States, 2001–2010. Vital Health Stat 3. 2012;35:1–67. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.