Summary
Effector T-cells exhibiting features of either T helper 1 (Th1) or T follicular helper (Tfh) populations are essential to control experimental Plasmodium infection and are believed to be critical for resistance to clinical malaria. To determine whether Plasmodium-specific Th1- and Tfh-like effector cells generate memory populations that contribute to protection, we developed transgenic parasites that enable high-resolution study of anti-malarial memory CD4 T-cells in experimental models. We found that populations of both Th1- and Tfh-like Plasmodium-specific memory CD4 T-cells persist. Unexpectedly, Th1-like memory cells exhibit phenotypic and functional features of Tfh cells during recall and provide potent B-cell help and protection following transfer, characteristics that are enhanced following ligation of the T-cell co-stimulatory receptor OX40. Our findings delineate critical functional attributes of Plasmodium-specific memory CD4 T-cells and identify a host-specific factor that can be targeted to improve resolution of acute malaria and provide durable, long-term protection against Plasmodium parasite re-exposure.
eTOC Blurb
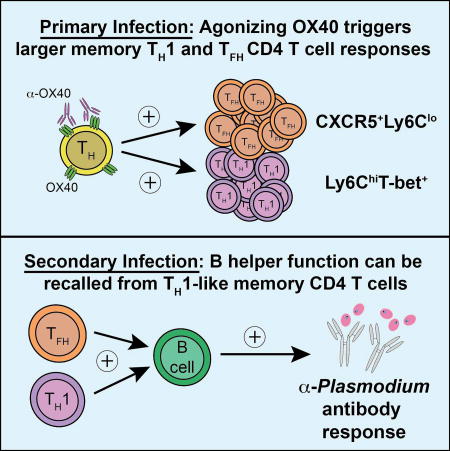
Th1 CD4 T cells are widely described as terminally differentiated with a relatively reduced capacity to form memory or support humoral immunity. Using experimental malaria models, Zander et al. show that potent proliferative and B cell helper activity unexpectedly resides within the Plasmodium-specific Th1-like memory CD4 T cell compartment.
Introduction
Plasmodium infections cause over 300 million cases of malaria and claim more than 400,000 lives annually (Organization, 2015). Although clinical immunity against the development of severe malaria can be established following repeated exposure, sterilizing immunity that protects individuals against re-infection rarely develops (Portugal et al., 2013, Crompton et al., 2014). Immune-mediated protection against repeated infection generally requires the activity of memory lymphocytes that develop following either primary infection or after vaccination (Jaigirdar and MacLeod, 2015, McKinstry et al., 2010). Elevated numbers of memory CD4 T cells are a hallmark of adaptive immune memory (Pepper and Jenkins, 2011). In addition to larger precursor frequencies, the capacity of memory CD4 T cells to rapidly control infection is associated with their enhanced trafficking patterns (Mueller et al., 2013), cytokine expression (Chandok et al., 2007), and ability to support humoral immunity (He et al., 2013). While adaptive immune memory is important for resistance to re-infection, the factors that govern memory CD4 T cell development, differentiation, and function remain less well understood. Moreover, one hypothesis to explain persistent and recurrent susceptibility to Plasmodium infection is that parasite-specific memory CD4 T cell populations are either numerically or functionally deficient. Addressing this question has been difficult, however, given the paucity of reagents necessary to study anti-Plasmodium CD4 T cell memory. Thus, the quantitative and qualitative features and mechanisms regulating the development of Plasmodium-specific memory CD4 T cells remain major knowledge gaps.
Resistance to clinical malarial disease is believed to require CD4 T cells exhibiting features of either T helper 1 (Th1) or T follicular helper (Tfh) cells (Jagannathan et al., 2014, Jagannathan et al., 2015, Obeng-Adjei et al., 2015). Plasmodium infection-induced Th1 cells secrete IFN-γ and stimulate macrophage phagocytic function respiratory burst, which aids in elimination of parasites and parasite-infected red blood cells (von der Weid and Langhorne, 1993). Tfh cells induce long-lived memory B cell and antibody-secreting plasma cell responses (Crotty, 2014). Experimental models have established the essential role of Th1 and Tfh cells in mediating host resistance to malaria (Carpio et al., 2015, Freitas do Rosario and Langhorne, 2012, Gwyer Findlay et al., 2014, Opata et al., 2015, Perez-Mazliah et al., 2015, Stephens et al., 2005, Stephens and Langhorne, 2010, Villegas-Mendez et al., 2013). In addition to secretion of IFN-γ, Th1 cells are characterized by expression of the transcriptional regulator T-bet (Szabo et al., 2000), lymphocyte antigen 6C (Ly6C) (Marshall et al., 2011, Yamanouchi et al., 1998), and the chemokine receptor CXCR3 (Sallusto and Lanzavecchia, 2000). Tfh cells are identified by expression of CXCR5 (Kim et al., 2001) and PD-1 (Haynes et al., 2007), secretion of IL-21 (Chtanova et al., 2004), and are regulated by the transcriptional repressor Bcl-6 (Crotty, 2011, Choi et al., 2013). An emerging literature supports that Plasmodium infection-induced CD4 T cell populations can exhibit mixed profiles that reflect both Tfh- and Th1-like phenotype and function. For example, Plasmodium infection-induced effector CD4 T cells can co-express effector cytokines IFN-γ and IL-21 (Carpio et al., 2015, Freitas do Rosario et al., 2012, Perez-Mazliah et al., 2015, Ryg-Cornejo et al., 2016) and effector CXCR5+CXCR3+ Tfh cells are preferentially expanded in P. falciparum-infected children during acute malaria (Obeng-Adjei et al., 2015).
The study of memory CD4 T cell responses against Plasmodium has been hampered by the lack of defined parasite-derived T cell epitopes that are necessary for their identification and interrogation of function. Thus, whether distinct Plasmodium-specific Th1- and Tfh-like memory CD4 T cell subsets either persist, exhibit plasticity during recall responses, or differentially support protective immunity are not well known. To overcome these obstacles and address these questions we generated transgenic P. yoelii parasites engineered to encode the hepatocyte-erythrocyte protein of 17kDa (Hep17) tagged with a dominant CD4 T cell epitope from lymphocytic choriomeningitis virus (LCMV). Hep17 is found in the parasitophorous vacuole, host cytoplasm, and on the surface of merozoites (Charoenvit et al., 1995), and immunization with recombinant Hep17 stimulates protective immunity (Charoenvit et al., 1999). With these reagents, we identified that both Th1- and Tfh-like Plasmodium-specific memory CD4 T cells form, persist, and can be distinguished by differential expression of Ly6C and CXCR5. Plasmodium-specific memory CD4 T cells within the CXCR3+Ly6C+ Th1-like subset exhibit unanticipated potent secondary expansion, protective capacity, and superior B cell helper function, features that were each enhanced by therapeutic ligation of the co-stimulatory molecule OX40 during acute malarial disease.
Results
Therapeutic ligation of OX40 during acute P. yoelii infection increases the number of parasite-specific memory CD4 T cells
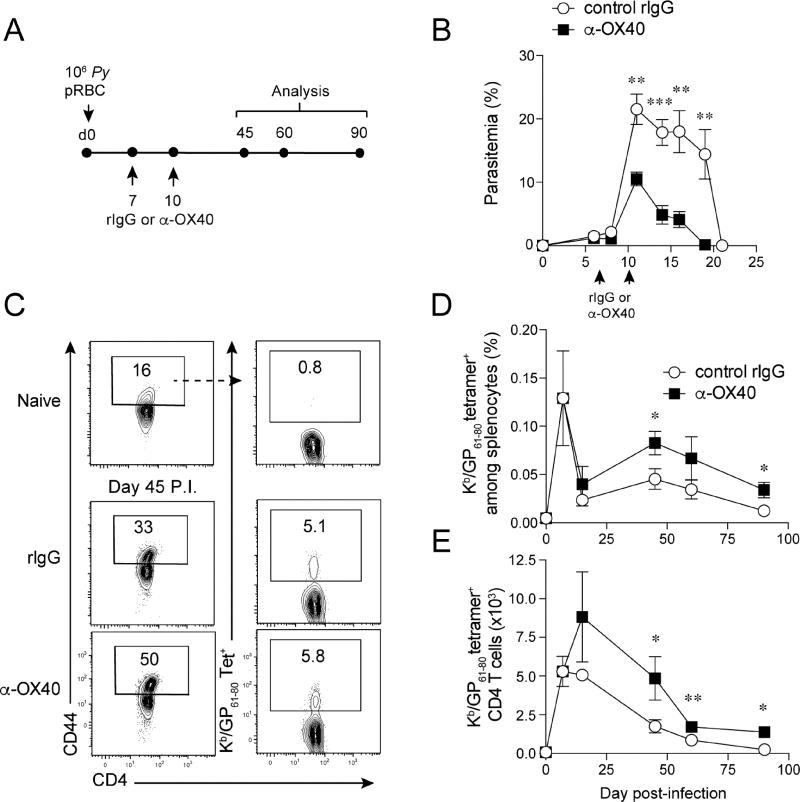
To determine the magnitude and kinetics of the parasite-specific CD4 T cell response during experimental malaria we infected groups of wild type C57BL/6 (WT B6) mice with recombinant P. yoelii parasites (Py-GP) engineered to express the immunodominant CD4 T cell epitope from LCMV (amino acids 61–80 of the glycoprotein, GP61–80; Fig. S1A,B). Two independent isolates of Py-GP each exhibited growth kinetics that closely match the parental strain (Fig. S1C). Given the dramatic increase in the expansion of effector CD4 T cells upon therapeutic ligation of OX40 during primary P. yoelii infection (Zander et al., 2015), we further tested whether agonizing the OX40 co-stimulatory molecule during acute experimental malaria (Fig. 1A) would promote the accumulation and long-term maintenance of Plasmodium-specific memory CD4 T cells. Endogenous GP61–80-specific and total/bulk Py-GP infection-induced splenic CD4 T cell responses (CD4+CD44hi) were quantified at effector (days 7 and 14 p.i.) and memory (days 45, 60, and 90 p.i.) time points. As we reported (Zander et al., 2015), stimulating OX40 during primary P. yoelii infection decreased peak parasitemia and accelerated Plasmodium parasite clearance (Fig. 1B). Anti-OX40 treatment also increased the proportion and total number of GP61–80-specific memory CD4 T cells by 2 to 3-fold (Fig. 1C–E). Of note, α-OX40 treatment shifted the peak of the effector CD4 T cell proliferative expansion from day 7 to day 14 p.i. (Fig. 1E). Consistent with the GP61–80-specific memory CD4 T cell responses, the frequency (Fig. S1D) and total number (Fig. S1E) of total Py-GP infection-induced memory CD4+ T cell populations were increased ~2-fold following α-OX40 treatment. As inflammatory cytokines can erode the quantity and function of memory T cell populations (Harty and Badovinac, 2008), we investigated whether the observed elevation in memory CD4 T cells following OX40 ligation was associated with reduced inflammation as a consequence of the truncated infection. However, OX40 ligation triggered elevated inflammatory reactions that were sustained through day 12 p.i. (Fig. S1F–I), despite the 2-fold reduction in parasite burden at this time point (Fig. 1B). These data demonstrate that accelerated clearance of blood-stage Plasmodium parasites induced by agonizing the OX40 co-stimulatory receptor is associated with an enhanced accumulation of parasite-specific memory CD4 T cells.
Figure 1. Therapeutic ligation of OX40 during acute P. yoelii infection increases the magnitude of the P. yoelii GP61–80-specific CD4 T cell response.
(A) Experimental design. Mice were infected with 1×106 transgenic Py-GP and administered either control rIgG or agonistic α-OX40 antibodies on days 7 and 10 p.i.
(B) Parasite growth and clearance kinetics.
(C) Proportions of tetramer+ GP61–80-specific splenic CD4+ T cells on day 45 p.i. in Py-GP-infected rIgG- and α-OX40-treated mice.
(D,E) Kinetics of the proportion (D) and total number (E) of GP61–80-specific CD4+ T cells.
Data (Mean +/− SEM) in (B,D,E) are pooled from 2–3 independent experiments per time point with 3–5 mice/group per experiment and were analyzed using multiple Student’s t tests while correcting for multiple comparisons using the Holm-Sidak method. *p<0.05 **p<0.01 ***p<0.0001. See also Figure S1
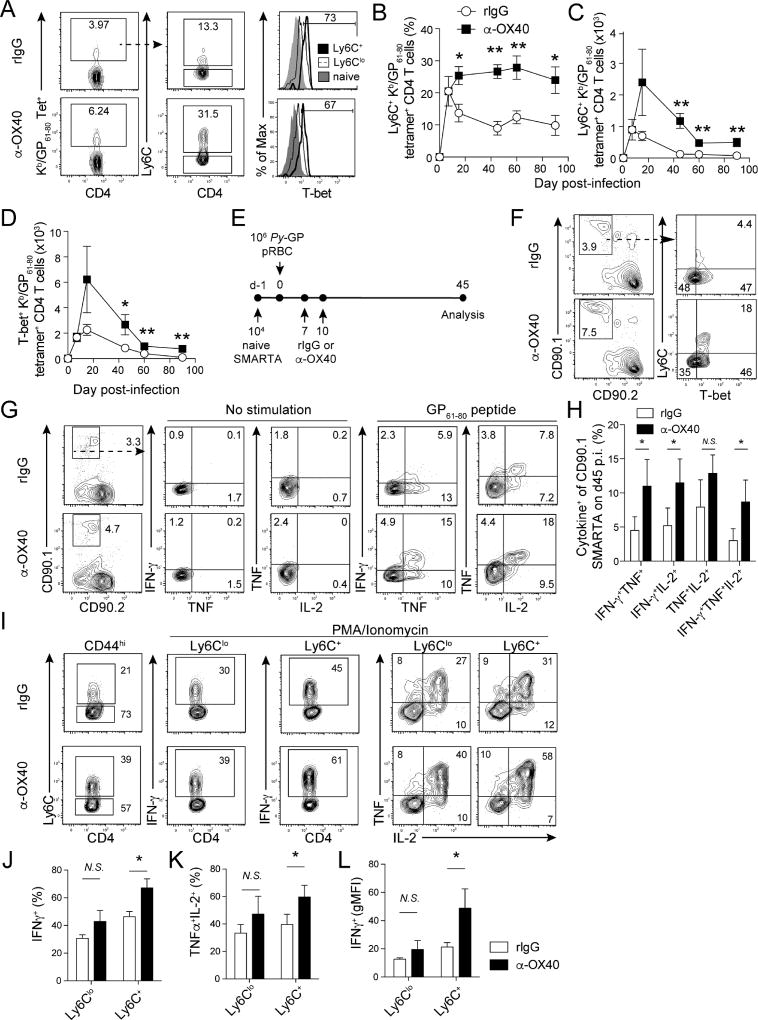
OX40 ligation during acute P. yoelii infection boosts the number of Ly6C+T-bet+ polyfunctional, Th1-like memory CD4 T cells
Both Th1 and Tfh cells contribute to Plasmodium parasite clearance (Langhorne et al., 1990, Perez-Mazliah and Langhorne, 2014, Perez-Mazliah et al., 2015, Spence and Langhorne, 2012, Stephens and Langhorne, 2010, Opata et al., 2015, Stephens et al., 2005, Stephens and Langhorne, 2006). Leishmania-specific Th1-like (Ly6C+T-bet+) effector CD4 T cells mediate enhanced parasite control (Peters et al., 2014) and we previously showed that therapeutic ligation of OX40 during primary P. yoelii infection expands effector Th1 cells (Zander et al., 2015). Thus, we next addressed whether agonistic α-OX40 treatment during an established Plasmodium infection affects the magnitude of Ly6C+ T-bet+ parasite-specific effector and memory CD4 T cell responses. By day 14 p.i., both proportions and total numbers of Ly6C+ Th1-like GP61–80-specific CD4 T cells were elevated 2 to 3-fold in α-OX40-treated mice, compared to rIgG-treated mice (Fig. 2A–C). Whereas Ly6C+ GP61–80-specific CD4 T cells in rIgG-treated mice gradually diminished over time, ligation of OX40 sustained the Ly6C+ GP61–80-specific CD4 T cell response through day 90 p.i. (Fig. 2B), which equated to a 7-fold increase in the total number of Ly6C+ GP61–80-specific memory CD4 T cells (Fig. 2C). The proportion of T-bet+ cells (Fig. 2A, right panels) and per cell expression of T-bet (gMFI, Fig. S2A) were highest among the Ly6C+ GP61–80-specific CD4 T cell subset. Consistent with elevated Ly6C+ Th1-like CD4 T cell numbers, we further identified increases in both the proportion (Fig. S2B) and total number (Fig. 2D) of T-bet+ GP61–80-specific CD4 T cells in α-OX40-treated mice, compared to rIgG-treated mice. Similar to NKT and anti-viral CD4 T cells (Marshall et al., 2011, Matsuda et al., 2006), T-bet is necessary (Fig. S2C,D), but not sufficient (e.g. Fig. 2F), for Ly6C expression on Plasmodium infection-induced CD4 T cells. Together, these data support that ligation of OX40 during primary P. yoelii infection promotes a 7-fold greater accumulation of T-bet-dependent Ly6C+ Th1-like memory CD4 T cells that persist for at least 90 days p.i.
Figure 2. Therapeutic ligation of OX40 during primary P. yoelii infection induces T-bet expression and expands polyfunctional Ly6C+ Th1-like memory CD4 T cell populations.
(A–D). Mice were infected with 1×106 transgenic Py-GP and administered either control rIgG or agonistic α-OX40 antibodies on days 7 and 10 p.i.
(A) Proportions of Ly6C+ GP61–80-specific CD4 T cells and their expression of T-bet relative to Ly6Clo GP61–80-specific CD4 T cells on d14 p.i.
(B–D) Kinetics of the proportion (B) and total number (C) of Ly6C+ GP61–80-specific CD4 T cells and total number of T-bet+ (D) GP61–80 -specific CD4 T cells in rIgG- and α-OX40-treated mice.
(E) Experimental design. 10,000 naïve CD90.1+ SMARTA CD4 T cells were transferred to naïve CD90.2+ mice. Recipients were infected with Py-GP and treated with either agonistic α-OX40 or control IgG antibodies on d7 and 10 p.i.
(F) Proportions of Ly6C+T-bet+ memory SMARTA cells on day 45 p.i.
(G) Proportions of memory SMARTA cells expressing IFN-γ, TNF, and IL-2 on day 45 p.i.
(H) Summary of cytokine profiles of memory SMARTA cells from rIgG- and α-OX40-treated mice on day 45 p.i.
(I–L) Expression of Ly6C (I), IFN-γ (I,L), and IL-2/TNF (K) by total polyclonal (CD44hi) memory CD4 T cells on day 45 p.i.
Data (Mean +/− SEM) in (B–D) are pooled from 2–3 independent experiments per time point with 3–5 mice/group per experiment and were analyzed using multiple Student’s t tests while correcting for multiple comparisons using the Holm-Sidak method. Data (Mean +/− SD) in (H–L) derive from 4–5 mice/group and are representative of two independent experiments. *p<0.05 **p<0.01 N.S. = not significant. See also Figure S2
Expression of Ly6C is associated with enhanced effector cytokine production among effector and memory CD4 T cells (Hu et al., 2015, Marshall et al., 2011). Thus, we next tested whether ligation of OX40 during the primary P. yoelii infection also impacted the capacity of parasite-specific memory CD4 T cells to express pro-inflammatory cytokines. To do this, we used CD4+ T cell receptor transgenic cells specific for the LCMV GP61–80 epitope (SMARTA cells (Oxenius et al., 1998)) (Fig. 2E). Forty-five days p.i., we isolated and stimulated donor-derived, memory SMARTA cells ex vivo and performed intracellular cytokine staining for IFN-γ, TNF and IL-2. Agonistic α-OX40-treatment doubled the number of T-bet+Ly6C+ SMARTA cells (Fig. 2F) and the proportion of SMARTA cells competent to express IFN-γ, TNF and IL-2 (Fig. 2G,H). We further verified that total polyclonal (CD11ahiCD44hi) Py-GP infection-induced Ly6C+ memory CD4 T cells recovered from both control rIgG- and α-OX40-treated mice also exhibited a greater capacity to produce IFN-γ or TNF and IL-2, compared to Ly6Clo cells. Elevated cytokine expression (Fig. 2I–K) and the per cell expression of IFN-γ (Fig. 2L) were 30–40% greater among CD4 T cells recovered from α-OX40-treated mice, compared to cells recovered from rIgG-treated mice, which was sustained for at least 2 months after parasite clearance (Fig. S2E). Thus, compared to Ly6Clo CD4 T cells, parasite-specific Ly6C+ memory CD4 T cells exhibit enhanced polyfunctional cytokine expression and therapeutic ligation of OX40 during acute P. yoelii infection promotes the accumulation and maintenance of polyfunctional Th1-like Ly6C+T-bet+ memory CD4 T cells.
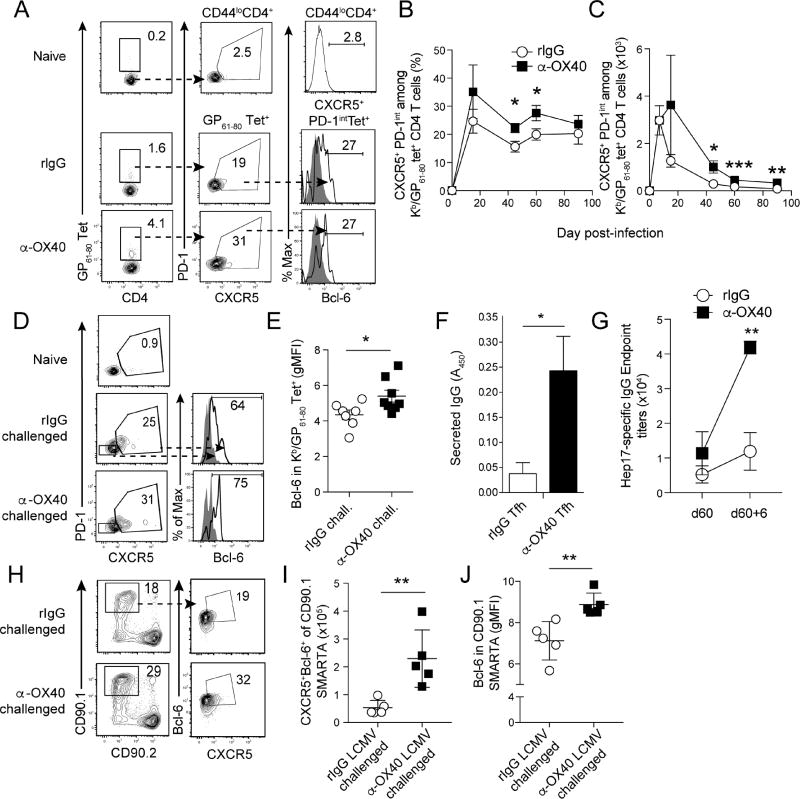
Ligation of OX40 during acute P. yoelii infection increases the number of parasite-specific CXCR5+ Tfh-like memory CD4 T cells that express Bcl-6 during recall responses
CXCR5+PD-1+Bcl-6+ Tfh cells are expanded during acute malaria and are critical for the secretion of isotype switched IgG and enhanced parasite control (Perez-Mazliah et al., 2015, Butler et al., 2012). Thus, we tested whether Plasmodium-specific Tfh cells have the capacity to form memory and persist. CXCR5 is the most reliable cell surface marker to track memory Tfh cells (Hale and Ahmed, 2015, Hale et al., 2013, Choi et al., 2013), as both PD-1 and Bcl-6 are down-regulated shortly after pathogen clearance (Pepper et al., 2011, Hale et al., 2013). Similarly, PD-1 and Bcl-6 also declined over the course of 60 days after Plasmodium infection (Fig. S3A–C). By contrast, the proportion of Tfh-like CXCR5+ GP61–80-specific CD4 T cells remained relatively stable and comprised ~20% of both effector (day 14 p.i.) and memory (>day 45 p.i.) populations (Fig. 3A,B). Ligation of OX40 during acute Plasmodium infection resulted in a 2–3-fold increase in the absolute number of CXCR5+ GP61–80-specific memory CD4 T cells that persisted through day 90 p.i. (Fig. 3C). At both effector and memory time points, the majority (85–95%) of Tfh-like CXCR5+ CD4 T cells are Ly6CloT-bet− Bcl-6+ (Fig. S3D–G) and Th1-like Ly6C+ cells are uniformly T-bet+CXCR5−Bcl-6− (Fig. S3E–G). Notably, substantial proportions of both effector and memory Ly6C+ Th1-like and CXCR5+Ly6Clo Tfh-like CD4 T cells express CXCR3 (Fig. S3H). These data support that the CXCR5−Ly6C+ and CXCR5+Ly6Clo phenotypes can be used to distinguish Th1- and Tfh-like Plasmodium-specific memory CD4 T cells, respectively. Moreover, CXCR3 expression may be a common signature of both Plasmodium-specific memory Th1 and Tfh cells, which is consistent with the identification of CXCR3+ effector Tfh cells in P. falciparum infected children (Obeng-Adjei et al., 2015).
Figure 3. Therapeutic ligation of OX40 during acute Plasmodium infection expands GP61–80-specific CXCR5+memory CD4 T cells that express Bcl-6 during recall.
(A–C) Mice were infected with 1×106 transgenic Py-GP and administered either control rIgG or agonistic α-OX40 antibodies on days 7 and 10 p.i. Proportions of CXCR5+PD-1intBcl-6+ memory CD4 T cell on day 60 p.i. (A). Kinetics of the proportion (B) and total number (C) of CXCR5+PD-1int memory CD4 T cells.
(D,E) Py-GP-immune mice were challenged with 5×106 Py-GP parasites on day 60 p.i. (D) Representative flow plots showing the proportion of CXCR5+PD-1int GP61–80 -specific CD4 Tfh cells and their expression of Bcl-6. (E) Summary data showing the relative amount (gMFI) of Bcl-6 in GP61–80-specific secondary effector CD4 T cells 6 days after challenge of Py-GP-immune mice.
(F) CD44hiCXCR5+PD-1int memory CD4 T cells from rIgG- and α-OX40-treated mice were co-cultured with naïve B cells in the presence of α-CD3ε and α-IgM. Supernatants were assayed for secreted IgG on day 7.
(G) Py-GP-immune mice were challenged as in D and Hep17-specific serum IgG was measured before and 6 days later.
(H–J) Py-GP-immune mice harboring memory CD90.1+ SMARTA cells were challenged with 2×106 p.f.u. of LCMV. Frequency (H), total number (I) and expression of Bcl-6 (J) were evaluated in secondary effector SMARTA cells. Data (Mean +/− SEM) in (B,C,E) are pooled from 2–3 independent experiments per time point with 3–5 mice/group per experiment and were analyzed using Student’s t tests while correcting for multiple comparisons using the Holm-Sidak method. Data (Mean +/− SD) in (F) derive from 4 replicates, represent 2 independent experiments, and were analyzed by Student’s t-test. *p<0.05 **p<0.01 ***p<0.0001. See also Figure S3
Ligation of OX40 during acute Plasmodium infection did not differentially modulate Bcl-6 expression in CXCR5+ GP61–80-specific memory CD4 T cells through day 60 p.i. (Fig. S3A,B). However, compared to rIgG-treated mice, high-dose Plasmodium re-challenge of α-OX40-treated immune mice (60 days after primary infection) markedly increased the proportion of CXCR5+PD-1intBcl-6+ parasite-specific secondary effector CD4 T cells (Fig. 3D,E). Consistent with this enhanced Tfh-like phenotype after recall, naive (IgD+IgM+) B cells were stimulated to isotype switch and secrete 5 to 6-fold greater amounts of IgG when co-cultured with CXCR5+PD-1int memory Tfh cells sort purified from α-OX40-treated mice, compared to memory Tfh cells purified from rIgG-treated donor mice (Fig. 3F). Regarding the epitope-tagged Hep17 protein, by day 60 p.i. we observed a 2-fold increase in Hep17-specific IgG titers in α-OX40-treated mice, compared to rIgG-treated mice (Fig. 3G). Furthermore, homologous high-dose challenge with Py-GP lead to a 4-fold increase in titers in the α-OX40-treated mice, compared to a 2-fold increase in rIgG-treated mice (Fig. 3G).
We further hypothesized that Plasmodium infection-induced GP61–80-specific memory CD4 T cells expanded by α-OX40-treatment would also exhibit enhanced Tfh responses after heterologous infection. To test this, LCMV clone 13 was used to challenge rIgG- and α-OX40-treated, Py-GP-immune mice harboring GP61–80-specific SMARTA TCR Tg memory CD4 T cells. Strikingly, numbers of CXCR5+Bcl-6+ secondary effector Tfh cells were elevated ~2-fold (Fig. 3H,I) and relative expression of Bcl-6 increased by >20% (Fig. 3J) in cells recovered from mice treated with α-OX40 during acute Plasmodium infection. The expansion of Tfh cells was further associated with 2-fold larger GC B cell responses in LCMV-challenged mice (Fig. S3I,J). These data show that parasite-specific Ly6CloCXCR5+ Tfh-like memory CD4 T cells persist at least 90 days following experimental malaria and that therapeutic ligation of OX40 during acute Plasmodium infection enhances the recall and function of parasite-specific memory Tfh-like CD4 T cells after either homologous or heterologous boosting.
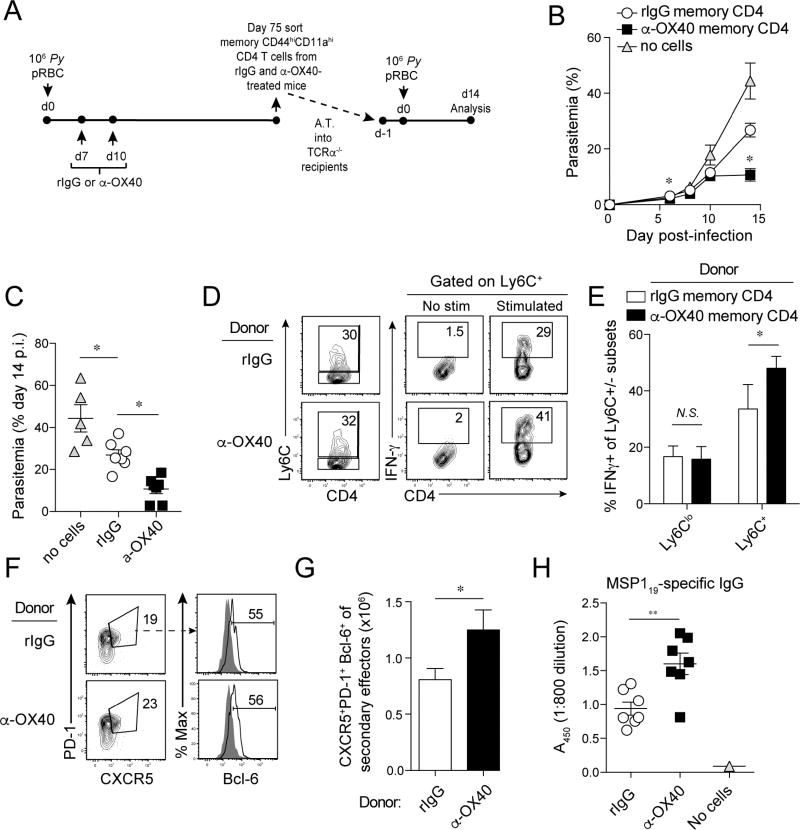
Ligation of OX40 during acute P. yoelii infection enhances the protective capacity of Plasmodium-specific memory CD4 T cells
To test the protective capacity of α-OX40-induced memory CD4 T cells, we sort-purified bulk memory CD4 T populations from rIgG- or α-OX40-treated mice and transferred 2×105 cells to T cell-deficient (Tcrα−/−) mice (Fig. 4A). Tcrα−/− recipients of memory CD4 T cells derived from either rIgG- or α-OX40 donors exhibited enhanced control of parasite replication, compared to mock-transfer Tcrα−/− mice (Fig. 4B). However, adoptive transfer of α-OX40 donor-derived memory CD4 T cells suppressed parasite growth by >2-fold compared to transfer of memory CD4 T cells derived from rIgG-treated donors (Fig. 4B,C). Suppression of parasite growth was associated with elevated IFN-γ expression by Ly6C+ secondary effectors (Fig. 4D,E). However, we also observed a 40–50% increase in the expansion of CXCR5+PD-1+Bcl-6+ secondary effector Tfh-like cells derived from α-OX40-treated memory CD4 T cells (Fig. 4F,G). Accordingly, merozoite surface protein (MSP)119-specific total IgG titers were elevated by >70% in Tcrα−/− mice seeded with memory CD4 T cells from α-OX40-treated donors (Fig. 4H). We repeated bulk transfer of memory CD4 T cells using recipient mice that lack both B and T cells (Rag1−/−). Donor-derived memory CD4 T cells engrafted and exhibited a CD62LloCD127loCD69hi effector phenotype in infected Rag1−/− recipients (Fig. S4A,B), yet we observed no differences in parasite control whether recipients were seeded with memory cells isolated from either rIgG- or α-OX40-treated donor mice (Fig. S4C). Furthermore, Rag1−/− mice from both experimental groups succumbed to P. yoelii infection between days 10 and 11 p.i. (Fig. S4D), supporting that B cells are essential for memory CD4 T cell-mediated protection following transfer. These results are consistent with previous studies showing that B cells are necessary for rescue of Plasmodium-infected Rag2−/− mice following CD4 T cell transfer (Stephens et al., 2005, Stephens and Langhorne, 2010). These data show that stimulating OX40 during a Plasmodium-specific primary effector CD4 T cell response boosts the protective capacity of memory populations, which is associated with amplified Tfh-like recall and enhanced B cell helper functions.
Figure 4. Therapeutic ligation of OX40 during acute Plasmodium infection enhanced both Th1- and Tfh-like recall responses and per cell protective capacity of memory CD4 T cells.
(A) Experimental design. Mice were infected with 1×106 Py and administered either control rIgG or α-OX40 antibodies on day 7 and 10 p.i. On 75 day p.i., memory CD4 T cells were sorted and transferred (2×105) into groups of Tcrα−/− mice. Recipients were challenged with P. yoelii and parasite growth kinetics were measured. Cells were analyzed on day 14 p.i. Statistical analyses (*p<0.05) reflect comparisons between recipients of memory cells from rIgG- and α-OX40-treated donor mice.
(B,C) Kinetics of parasite growth (B) and summary data (C) showing parasitemia on day 14 p.i.
(D–F) Proportions of donor-derived CD4 T cells expressing either Ly6C (D), IFN-γ (D,E), or CXCR5+PD1+ Tfh cells expressing Bcl-6 (F).
(G) Total number of CXCR5+PD1+Bcl-6+ Tfh cells recovered from recipients.
(H) MSP119-specific serum antibody titers in mice seeded with memory CD4 T cells derived from rIgG- and α-OX40-treated donors.
Data (Mean +/− SEM) in (B,C,E,G,H) are pooled from 2 independent experiments with 3–4 mice/group per experiment and were analyzed using either one-way ANOVA (B,C) or Student’s t tests (E,G,H). *p<0.05 **p<0.01. N.S. = not significant. See also Figure S4.
Plasmodium-specific, Th1-like Ly6C+ memory CD4 T cells exhibit Tfh function during recall responses
We observed both Th1- and Tfh-like activity following the transfer and recall of bulk memory CD4 T cells, suggesting that either substantial heterogeneity or functional plasticity may exist within the Plasmodium-specific memory CD4 T cell compartment. To evaluate the phenotypic stability and activity of Th1- and Tfh-like memory CD4 T cells during recall and determine if B cell helper function is restricted to the Tfh-like populations, performed adoptive transfer studies (Fig. 5A). To simplify sort-purification, we enriched Th1- and Tfh-like memory CD4 T cell populations based on differential expression of Ly6C, given that the development of Th1-like Ly6C+ memory CD4 T cells is T-bet-dependent (Fig. S2C,D and (Marshall et al., 2011, Matsuda et al., 2006)) and Tfh-like CXCR5+ Bcl-6+ memory CD4 T cells are only found within the Ly6Clo CD4 T cell population (Fig. S3D,E and S5A,B). Following their adoptive transfer and recall, Plasmodium-specific Ly6C+ memory CD4 T cells underwent secondary expansions that were numerically equivalent to Ly6Clo memory CD4 T cells (Fig. 5B,C and Fig. S5C), which was unexpected given reported proliferative deficits among Ly6C+ CD4 T cells (Hu et al., 2015, Marshall et al., 2011). Moreover, memory CD4 T cell populations derived from α-OX40-treated mice expanded ~4-fold more than memory CD4 T cells derived from control rIgG-treated donor mice, independent of Ly6C expression (Fig. 5C and Fig. S5C). Approximately 50% of secondary effectors expressed Ly6C following transfer and recall of Ly6Clo memory CD4 T cells (Fig. 5D). By contrast, ~25–35% of donor Ly6C+ memory CD4 T cells down-regulated Ly6C following transfer and recall (Fig. 5D and Fig. S5D), supporting that Plasmodium-specific Ly6C+ memory CD4 T cells retain the capacity to proliferate and exhibit a high degree of phenotypic inter-conversion between Ly6C+ and Ly6Clo memory CD4 T cells during recall in response to homologous infection. As expected, Ly6C+ secondary effector progeny of either Th1- or Tfh-like memory cells expressed more T-bet relative to their Ly6Clo counterparts (Fig. S5E). However, despite this apparent Th1-like bias, more than twice as many Ly6C+ secondary effectors co-expressed CXCR5 and Bcl-6, compared to Ly6Clo effector CD4 T cells (Fig. 5D,E and Fig. S5F). Thus, Ly6C+ secondary effectors derived from either Ly6C+ or Ly6Clo memory CD4 T cells appeared to more efficiently recall or acquire a Tfh-like phenotype during recall responses, suggesting that Tfh activity may reside within the Ly6C+ pool of secondary effectors.
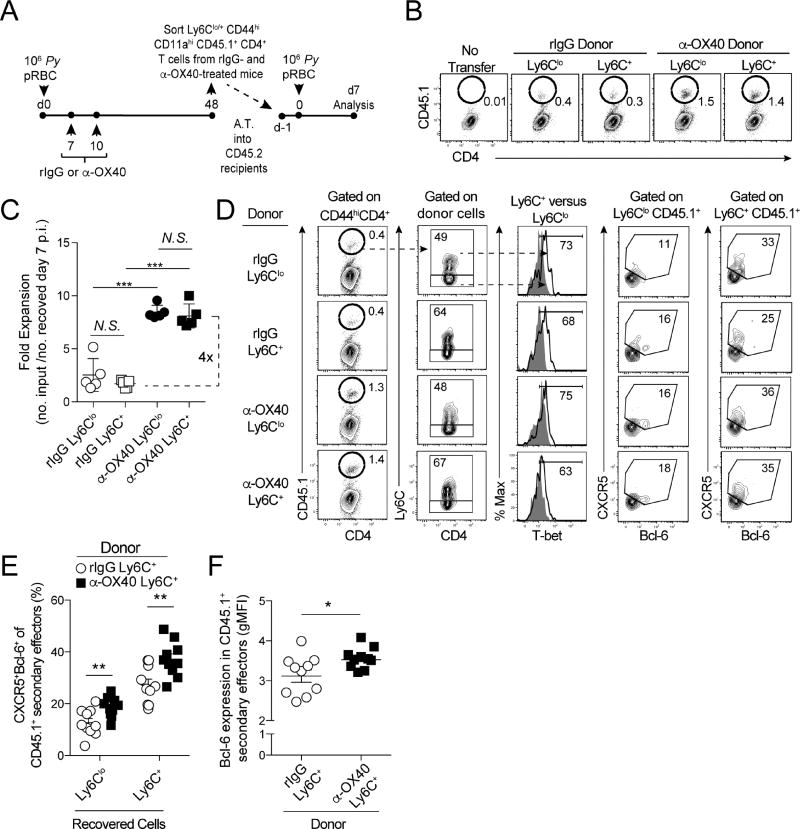
Figure 5. Both Ly6Clo and Ly6C+ Plasmodium-specific memory CD4 T cells can give rise to Tfh-like secondary effectors.
(A) Experimental design. CD45.1+ mice were infected with 1×106 Py and administered either control rIgG or α-OX40 on days 7 and 10 p.i. On day 48 p.i., 2×105 total (CD11ahiCD44hi) infection-induced Ly6C+ memory CD4+ T cells were sorted and transferred into congenic CD45.2+ mice. Recipient mice were infected with Py one day later. CD4 T cell responses were assessed on d7 p.i.
(B,C) Representative flow plots (B) and summary graph (C) showing expansion of CD45.1+ cells on day 7 p.i.
(D) Phenotype and CXCR5 and Bcl-6 expression among recovered CD45.1+ Ly6Clo and Ly6C+ secondary effector CD4 T cells.
(E) Summary data showing the proportion of Ly6Clo and Ly6C+ CD45.1+ secondary effector cells expressing CXCR5 and Bcl-6.
(F) Summary data of Bcl-6 expression (gMFI) in rIgG- and α-OX40-treated donor-derived CD45.1+ secondary effector CD4 T cells.
Data (Mean +/− SD) in (C) were analyzed using one-way ANOVA. Data (Mean +/− SEM) in (E–F) are pooled from 2 independent experiments with 5 mice/group and were analyzed using Student’s t tests. *p<0.05 **p<0.01 ***p<0.0001. See also Figure S5
Regarding the impact of α-OX40 treatment, both the proportion of CXCR5+Bcl-6+ Tfh cells (Fig. 5E and Fig. S5F) and the amount of Bcl-6 (Fig. 5F) were elevated ~25% in Ly6C+ secondary effectors derived from α-OX40-treated mice, compared to those derived from IgG-treated mice. Identical patterns were observed following recall of Py-GP GP61–80-specific memory CD4 T cells (Fig. S5G). Thus, both Ly6Clo and Ly6C+ Plasmodium-specific memory CD4 T cells are endowed with the capacity to give rise to Tfh-like secondary effectors. Collectively, our data support that phenotypically distinct Plasmodium-specific Th1- and Tfh-like memory CD4 T cell subsets display functional overlap during secondary recall responses. Moreover, our data show that Tfh-like qualities can be recalled from Ly6C+ Th1-like memory CD4 T cells, features that are enhanced by therapeutic ligation of OX40.
Parasite-specific, Th1-like Ly6C+ memory CD4 T cells exhibit potent B cell helper function
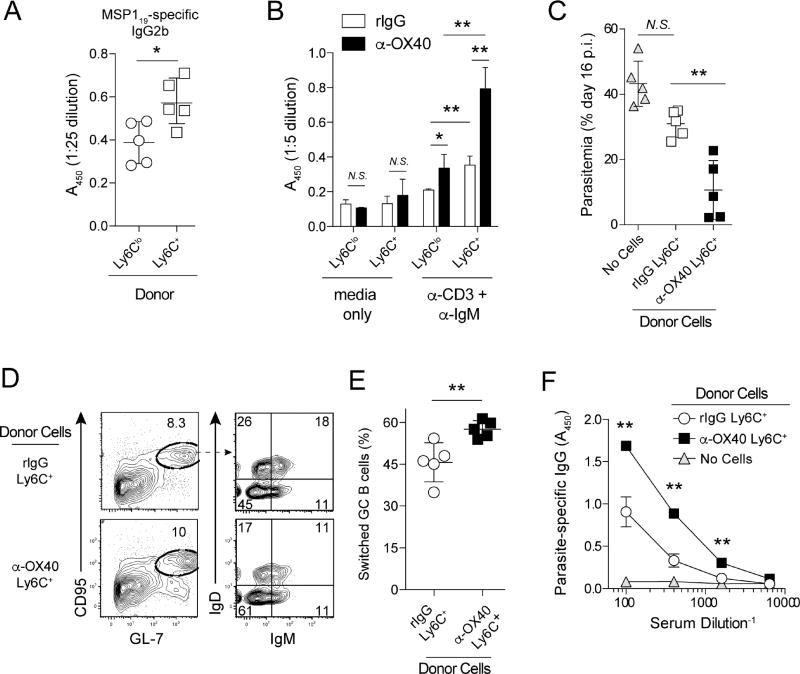
To directly compare the capacity of Ly6C+ Th1- and Ly6Clo Tfh-like memory CD4 T cells to support B cell helper function, and to determine whether OX40 stimulation imprinted on this function, we performed additional in vivo and in vitro B cell differentiation and activation studies (Sage and Sharpe, 2015). Strikingly, transfer of Th1-like Ly6C+ memory CD4 T cells stimulated elevated titers of parasite-specific IgG2b in recipient mice, compared to transfer of Tfh-like Ly6Clo memory CD4 T cells (Fig. 6A). Consistent with these in vivo data, Th1-like Ly6C+ memory CD4 T cells sort-purified from either rIgG- or α-OX40-treated mice exhibited superior B cell helper function, compared to their Ly6Clo counterparts (Fig. 6B). Of note, B cells cultured with Ly6C+ memory CD4 T cells sorted from α-OX40-treated mice secreted more IgG, compared to B cells cultured with Ly6C+ memory CD4 T cells sort-purified from rIgG-treated mice (Fig. 6B). In line with these in vitro studies, transfer of Ly6C+ memory CD4 T cells isolated from α-OX40-treated mice more effectively limited parasite replication in T cell-deficient recipients following P. yoelii challenge (Fig. 6C), which was associated with >50% increases in T cell-dependent germinal center B cell class-switching (Fig. 6D,E) and circulating titers of MSP119-specific IgG (Fig. 6F). Thus, Plasmodium-specific Ly6C+ Th1-like memory CD4 T cells exhibit significant functional heterogeneity and are capable of providing robust B cell help during recall responses. Collectively, our data provide both in vivo and in vitro evidence that Plasmodium-specific Th1-like Ly6C+T-bet+ memory CD4 T cells more effectively support secondary humoral immune reactions compared to Ly6Clo populations in which CXCR5+Bcl-6+ Tfh-like cells reside. Moreover, our data demonstrate that the host-specific co-stimulatory molecule OX40 can be therapeutically targeted during malaria to enhance the B cell helper function and protective capacity of polyfunctional Ly6C+ Th1-like memory CD4 T cells.
Figure 6. Ligation of OX40 during an acute P. yoelii infection bolsters the anti-parasitic and B cell helper capacity of Plasmodium-specific, Th1-like Ly6C+ memory CD4 T cells.
(A–F) Mice were infected with 1×106 Py and administered either control rIgG or agonistic α-OX40 antibodies on days 7 and 10 p.i.
(A) MSP119-specific serum IgG2b titers in Plasmodium-infected mice seeded with either Ly6Clo or Ly6Chi memory CD4 T cells.
(B) On day 50 p.i., Ly6C+ and Ly6Clo memory CD4 T cells were sort-purified from rIgG- and α-OX40-treated mice and co-cultured with naïve (CD19+IgDhi) B cells in the presence of α-CD3ε and α-IgM. Secreted IgG was measured on day 5.
(C) Ly6C+ and Ly6Clo memory CD4 T cells from rIgG- and α-OX40 treated-mice were sort-purified and transferred (2×105) into Tcrα−/− mice. Recipients were infected the following day. Parasite burdens on day 16 p.i. are shown.
(D,E) Proportion (D) and summary (E) of class-switched (IgDloIgMlo) germinal center B cells (GL-7+CD95+) in Tcrα−/− mice seeded with Th1-like Ly6C+ memory CD4 T cells derived from either rIgG- or α-OX40-treated donors.
(F) MSP119-specific serum IgG titers in memory CD4 T cell recipient Tcrα−/− mice on day 16 p.i.
Data (Mean +/− SD) in (A–F) are from 5 mice/group, represent 2–3 independent experiments, and were analyzed by either Student’s t tests (A,B, D–F) or one-way ANOVA (C). *p<0.05 **p<0.01. N.S. = not significant.
Discussion
Multiple studies show that OX40 signaling is important for promoting effector CD4 T cell proliferation and survival (Murata et al., 2000, Kopf et al., 1999, Soroosh et al., 2007, Gramaglia et al., 2000, Song et al., 2008, Song et al., 2007, Song et al., 2005). Our data show that OX40 ligation during established malaria can also enhance the function of memory CD4 T cells. OX40 signaling is known to activate the PI3K/Akt (Song et al., 2004), NfKB (Song et al., 2008), NFAT (So et al., 2006), and mTOR (Song et al., 2004) pathways, as well as regulate the activity of c-Myc (Haque et al., 2016). Each of these circuits modulates memory T cell formation (Angelosanto and Wherry, 2010, Hale et al., 2013, Youngblood et al., 2013a, Youngblood et al., 2013b). Understanding the mechanisms by which OX40, alone or in combination with other co-stimulatory (Wikenheiser et al., 2016) or co-inhibitory (Wykes and Lewin, 2017) pathways, impacts memory CD4 T cell function may be key for developing strategies to enhance protection against chronic infections or cancer.
The origins, phenotype, and function of memory CD4 T cells and their capacity for protection during recall remain enigmatic (Opata and Stephens, 2013, Seder et al., 2008). Following acute bacterial infection, CXCR5+PD-1int CD4 T cells exhibiting characteristics of either T central memory or Tfh cells have been identified (Pepper et al., 2011). Compared to other T cell subsets, including Th1-like Ly6C+T-bet+ CD4 T cells, Tfh-like CXCR5+Ly6CloT-betlo memory cells induced by acute viral infection exhibit enhanced longevity, elevated per cell proliferative capacity, and enhanced B cell helper function following their adoptive transfer and recall (Marshall et al., 2011, Hale et al., 2013). Following Leishmania infection, CXCR3+Ly6C+T-bethi effector CD4 T cells are defined as short-lived Th1 cells that mediate enhanced parasite control, yet exhibit limited capacity to form memory or undergo secondary expansion, compared to their less-differentiated CXCR3loLy6CloT-betlo counterparts (Peters et al., 2014). Here evidence is presented supporting that Plasmodium-specific Th1-like Ly6C+T-bet+ memory CD4 T cells exhibit equivalent or enhanced proliferative potential, CXCR5 and Bcl-6 expression, and B cell helper function during recall, compared to pools of Ly6Clo populations that harbor CXCR5+ memory Tfh cells. The proliferative potential of Plasmodium-specific Ly6C+ Th1-like memory CD4 T cells contrasts sharply with the reported inability of virus- and Leishmania-specific Ly6C+ Th1-like effector and memory CD4 T cells to proliferate (Hu et al., 2015, Marshall et al., 2011). The basis for the significant functional plasticity among Plasmodium-specific memory CD4 T cells may be linked to the distinct inflammatory environments and antigen presenting cell types expanded during malaria. Direct comparative studies will be required to delineate the molecular and transcriptional features of Plasmodium-specific Th1- and Tfh-like memory CD4 T cells that distinctly govern their development, plasticity, and protective function.
An emerging literature supports that anti-microbial effector CD4 T cells can exhibit mixed Th1- and Tfh-like phenotype and function (Carpio et al., 2015, Li et al., 2016, Nakayamada et al., 2011, Obeng-Adjei et al., 2015, Velu et al., 2016, Wikenheiser et al., 2016). Viral infections are linked to expansion of effector Tfh cells that express CXCR3 and co-produce IFN-γ and IL-21 following experimental SIV infection (Velu et al., 2016). IFN-γ+IL-21+ICOS+Bcl-6+ Mycobacterium tuberculosis-specific effector CD4 T cell populations have been identified (Li et al., 2016) and Toxoplasma gondii-specific effector CD4 T cells are reported to adopt a transitional stage characterized by co-expression of IL-21, Bcl-6, and T-bet (Nakayamada et al., 2011). Recent studies also identify Tfh cells exhibiting characteristics of Th17, Th1, and Th2 effector CD4 T cells in vivo (Morita et al., 2011, Obeng-Adjei et al., 2015) and in vitro experiments show that Th17-like effector Tfh cells exert potent B cell helper function, relative to Th1- and Th2-like effector Tfh cells (Lu et al., 2011, Mitsdoerffer et al., 2010). During chronic experimental malaria, CD4 T cells can co-express CXCR5, IL-21, IL-10 and IFN-γ (Carpio et al., 2015, Perez-Mazliah et al., 2015). Despite the consistent reports of expanded populations of effector Th1/Tfh-like CD4 T cells following microbial infection, whether these cells represent either distinct CD4 T cell subsets or a continuum of phenotype and function, and whether they retain capacity to form stable memory populations that distinctly mediate protective immunity have not been thoroughly addressed. Our data support that Plasmodium-specific Th1-like effector and memory cells are transcriptionally distinct from Tfh-like cells, which is consistent with recent data from others (Lonnberg et al., 2017, Hale et al., 2013). However, Plasmodium-specific Th1- and Tfh-like effector CD4 T cells exhibit phenotypic similarities, including expression of CXCR3. Our data show that this phenotype is stable through memory formation. Our data also show that Th1- and Tfh-like memory CD4 T cell subsets display functional overlap during recall responses, with both Th1- and Tfh-like memory subsets giving rise to secondary effectors that robustly proliferate and exhibit phenotypic and functional features of Tfh cells. Thus, substantial proliferative capacity and potent B cell helper activity unexpectedly resides within the Plasmodium-specific Ly6C+ Th1-like memory CD4 T cell pool.
Impaired humoral immunity during malaria is linked to expansions of circulating Th1-like effector Tfh cells (reviewed in (Hansen et al., 2016)). The acquisition of Th1-associated CXCR3, T-bet, and IFN-γ expression by pre-Tfh cells is reported to constrain their migration and development into competent B helper cells in follicles (Ioannidis et al., 2016, Ryg-Cornejo et al., 2016). We and others have shown that excessive IFN-γ limits humoral immunity (Zander et al., 2015, Ryg-Cornejo et al., 2016). In line with a potential deleterious effect of Tfh-derived IFN-γ expression, we recently identified that B cell-intrinsic IFN-γ signaling limits Plasmodium-specific GC B cell reactions (Guthmiller et al., 2016). Despite these analyses of effector Tfh responses, our current experiments show that potent B cell helper activity can be recalled from Th1-like, CXCR3+Ly6C+T-bet+ memory CD4 T cells, which can express IFN-γ ex vivo. However, whether Th1-like Tfh memory cells either express IFN-γ in vivo or whether IL-21 (Perez-Mazliah et al., 2015) or IL-10 (Guthmiller et al., 2016) counteract the activity of IFN-γ during recall responses remains unknown. Although IL-21 expression was undetectable in Tfh- and Th1-like memory CD4 T cells ex vivo (not shown), current work in our laboratory is focused on dissecting these functional parameters as well as the transcriptional networks regulating Plasmodium-specific effector and memory Tfh cell development in vivo. Such information will be critical for developing new strategies to enhance the formation and function of long-lived, Plasmodium-specific memory B cells and plasma cells and durable protection against malaria.
Experimental Procedures
Mice, parasites, viruses, and biologics
Male and female C57BL/6 wild type, Tbx21−/−, Rag1−/− and Tcrα−/− mice (6–8 weeks, 16–21g) were purchased from Jackson Laboratories. SMARTA mice were generously provided by Dorian McGavern (Scripps) via Allan Zajac (UAB). The University of Oklahoma Health Sciences Center and University of Iowa Institutional Animal Care and Use Committees approved all experiments. Plasmodium yoelii (clone 17XNL, BEI Resources/MR4/ATCC) was inoculated into mice intravenously. To generate the T cell epitope tagged transgenic parasites (see Fig. S1 and Table S1), a dispensable genetic locus (“p230p”) was stably targeted with an expression cassette of Hep17 protein tagged with the GP61–80 CD4 T cell epitope (amino acids 61–80 from LCMV glycoprotein). Standard methods (Jongco et al., 2006, Lindner et al., 2013) were used to create and integrate a linearized pDEF construct, select transgenic parasites, and obtain two pure, clonal populations. Parasitemia was measured using flow cytometry as previously described (Malleret et al., 2011). LCMV clone 13 (2×106 plaque forming units) was administered i.p. 50 µg of rIgG or α-OX40 antibody (clone OX86, BioXCell) were administered i.p. on the days indicated in figure legends.
Flow cytometry
T cells were stained with H2-Kb/GP61–80 tetramers (NIH) and antibodies against mouse CD4 (clone GK1.5, Biolegend), CD11a (clone M17/4, eBioscience), CD25 (clone PC61, Biolegend), CD44 (clone IM7, Tonbo), Ly6C (clone HK1.4, Biolegend), CXCR3 (clone CXCR3-173, Biolegend), and PD-1 (clone RMP1-30, Biolegend). B cells were stained with CD19 (clone 6D5, Tonbo), T and B cell activation antigen (clone GL-7, BD Biosciences), CD95 (clone Jo2, BD Biosciences), IgD (clone 11-26c.2a, BD Biosciences), and IgM (clone RMM-1, eBioscience). Cells were stimulated with either GP61–80 peptide antigen or PMA and ionomycin in the presence of monensin, permeabilized with cytofix/cytoperm (BD Bioscience), and stained with anti-mouse IFN-γ (clone XMG1.2, eBioscience), anti-IL-2 (clone JES6-5H4, eBioscience), and anti-TNF (clone MP6-XT22, eBioscience). For Tfh analyses, cells were stained with anti-CXCR5 (clone 2G8, BD Biosciences) followed by staining with fluorochrome-conjugated anti-CD4, anti-CD44, anti-Ly6C, anti-CXCR3, and anti-PD-1. Anti-T-bet (clone 4B-10, Biolegend) and anti-Bcl-6 (clone K112-91, BD Biosciences) were applied after fixation and permeabilization of cells using FoxP3/Transcription Factor Staining Buffer Kits (Tonbo). Data were acquired using either Stratedigm S1200Ex or BD LSR II flow cytometers and analyzed using FlowJo software (Tree Star, Inc.).
Cell sorting and adoptive transfer
CD4 T cells were enriched (AutoMACS Pro-Separator, Miltenyi), stained for various markers (CD90, CD4, Ly6C, CXCR5, and/or PD-1), and sort-purified using either FACSJazz or Aria II instruments (BD Biosciences). Memory CD4 T cell subsets were adoptively transferred (2×105) via tail vein.
CD4 T cell-B cell co-cultures
Memory cells were sorted and co-cultured with naïve (CD19+IgDhiCD44lo) B cells (1:1) for 5–7 days in 96 well round-bottom plates containing 5 µg/ml of anti-CD3ε (clone 145-2C11) and 5 µg/ml anti-mouse IgM (Jackson ImmunoResearch) as described (Sage and Sharpe, 2015).
ELISA
Plates (Nunc) were coated with recombinant P. yoelii MSP119 (BEI Resources/MR4/ATCC) and blocked with 2.5% BSA + 5% normal goat serum. MSP119-specific total IgG or IgG2b was detected using HRP-conjugated goat anti-mouse-IgG (Jackson ImmunoResearch). Plates were developed with SureBlue Reserve TMB Kit (KPL) and analyzed with a Spectra Max 340 (Molecular Devices).
Statistical Analyses
Tests of statistical significance were conducted using Prism 6 software (GrapPad) and either non-parametric unpaired Student’s t-tests or one-way ANOVA as indicated in figure legends.
Supplementary Material
Highlights.
Reagents generated to study Plasmodium-specific memory CD4 T cell development
Th1- and Tfh-like memory CD4 T cells exhibit functional overlap during recall
Potent B cell helper function can be recalled from Th1-like memory cells
Functions of parasite-specific memory CD4 T cells are enhanced by ligation of OX40
Acknowledgments
We thank Drs. Martin Richer (McGill University) and Lauren Zenewicz (OUHSC) for critical feedback. This work was supported by grants from the National Institutes of Health/National Institute of Allergy and Infectious Diseases (T32AI007633 to R.A.Z.; K22AI101039 and R01AI123341 to S.E.L.; R01AI125446 and R01AI127481 to N.S.B.), the American Heart Association (16PRE27660002 to J.J.G.), and the National Institutes of Health/National Institute of General Medical Sciences under grant number 8P20GM103447.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
RAZ designed, performed, analyzed, and interpreted experiments and wrote the paper. RV, ADP, JJG, and ACG performed, analyzed, and interpreted experiments. SEL and AMV assisted with the generation of the transgenic parasites. SHIK provided resources to generate the transgenic parasites. NSB conceived and generated transgenic parasites, designed, performed, analyzed, and interpreted experiments, and wrote the paper.
References
- Angelosanto JM, Wherry EJ. Transcription factor regulation of CD8+ T-cell memory and exhaustion. Immunol Rev. 2010;236:167–75. doi: 10.1111/j.1600-065X.2010.00927.x. [DOI] [PubMed] [Google Scholar]
- Butler NS, Moebius J, Pewe LL, Traore B, Doumbo OK, Tygrett LT, Waldschmidt TJ, Crompton PD, Harty JT. Therapeutic blockade of PD-L1 and LAG-3 rapidly clears established blood-stage Plasmodium infection. Nat Immunol. 2012;13:188–95. doi: 10.1038/ni.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpio VH, Opata MM, Montanez ME, Banerjee PP, Dent AL, Stephens R. IFN-gamma and IL-21 Double Producing T Cells Are Bcl6-Independent and Survive into the Memory Phase in Plasmodium chabaudi Infection. PLoS One. 2015;10:e0144654. doi: 10.1371/journal.pone.0144654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandok MR, Okoye FI, Ndejembi MP, Farber DL. A biochemical signature for rapid recall of memory CD4 T cells. J Immunol. 2007;179:3689–98. doi: 10.4049/jimmunol.179.6.3689. [DOI] [PubMed] [Google Scholar]
- Charoenvit Y, Majam VF, Corradin G, Sacci JB, Jr, Wang R, Doolan DL, Jones TR, Abot E, Patarroyo ME, Guzman F, Hoffman SL. CD4(+) T-cell- and gamma interferon-dependent protection against murine malaria by immunization with linear synthetic peptides from a Plasmodium yoelii 17-kilodalton hepatocyte erythrocyte protein. Infect Immun. 1999;67:5604–14. doi: 10.1128/iai.67.11.5604-5614.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charoenvit Y, Mellouk S, Sedegah M, Toyoshima T, Leef MF, De La Vega P, Beaudoin RL, Aikawa M, Fallarme V, Hoffman SL. Plasmodium yoelii: 17-kDa hepatic and erythrocytic stage protein is the target of an inhibitory monoclonal antibody. Exp Parasitol. 1995;80:419–29. doi: 10.1006/expr.1995.1054. [DOI] [PubMed] [Google Scholar]
- Choi YS, Yang JA, Yusuf I, Johnston RJ, Greenbaum J, Peters B, Crotty S. Bcl6 expressing follicular helper CD4 T cells are fate committed early and have the capacity to form memory. J Immunol. 2013;190:4014–26. doi: 10.4049/jimmunol.1202963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chtanova T, Tangye SG, Newton R, Frank N, Hodge MR, Rolph MS, Mackay CR. T follicular helper cells express a distinctive transcriptional profile, reflecting their role as non-Th1/Th2 effector cells that provide help for B cells. J Immunol. 2004;173:68–78. doi: 10.4049/jimmunol.173.1.68. [DOI] [PubMed] [Google Scholar]
- Crompton PD, Moebius J, Portugal S, Waisberg M, Hart G, Garver LS, Miller LH, Barillas-Mury C, Pierce SK. Malaria immunity in man and mosquito: insights into unsolved mysteries of a deadly infectious disease. Annu Rev Immunol. 2014;32:157–87. doi: 10.1146/annurev-immunol-032713-120220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29:621–63. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- Crotty S. T follicular helper cell differentiation, function, and roles in disease. Immunity. 2014;41:529–42. doi: 10.1016/j.immuni.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas Do Rosario AP, Lamb T, Spence P, Stephens R, Lang A, Roers A, Muller W, O'Garra A, Langhorne J. IL-27 promotes IL-10 production by effector Th1 CD4+ T cells: a critical mechanism for protection from severe immunopathology during malaria infection. J Immunol. 2012;188:1178–90. doi: 10.4049/jimmunol.1102755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas Do Rosario AP, Langhorne J. T cell-derived IL-10 and its impact on the regulation of host responses during malaria. Int J Parasitol. 2012;42:549–55. doi: 10.1016/j.ijpara.2012.03.010. [DOI] [PubMed] [Google Scholar]
- Gramaglia I, Jember A, Pippig SD, Weinberg AD, Killeen N, Croft M. The OX40 costimulatory receptor determines the development of CD4 memory by regulating primary clonal expansion. J Immunol. 2000;165:3043–50. doi: 10.4049/jimmunol.165.6.3043. [DOI] [PubMed] [Google Scholar]
- Guthmiller JJ, Graham AC, Zander RA, Pope RL, Butler NS. Cutting Edge: IL-10 Is Essential for the Generation of Germinal Center B Cell Responses and Anti-Plasmodium Humoral Immunity. J Immunol. 2016 doi: 10.4049/jimmunol.1601762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwyer Findlay E, Villegas-Mendez A, O'Regan N, De Souza JB, Grady LM, Saris CJ, Riley EM, Couper KN. IL-27 receptor signaling regulates memory CD4+ T cell populations and suppresses rapid inflammatory responses during secondary malaria infection. Infect Immun. 2014;82:10–20. doi: 10.1128/IAI.01091-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale JS, Ahmed R. Memory T follicular helper CD4 T cells. Front Immunol. 2015;6:16. doi: 10.3389/fimmu.2015.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale JS, Youngblood B, Latner DR, Mohammed AU, Ye L, Akondy RS, Wu T, Iyer SS, Ahmed R. Distinct memory CD4+ T cells with commitment to T follicular helper- and T helper 1-cell lineages are generated after acute viral infection. Immunity. 2013;38:805–17. doi: 10.1016/j.immuni.2013.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen DS, Obeng-Adjei N, Ly A, Ioannidis LJ, Crompton PD. Emerging concepts in T follicular helper cell responses to malaria. Int J Parasitol. 2016 doi: 10.1016/j.ijpara.2016.09.004. [DOI] [PubMed] [Google Scholar]
- Haque M, Song J, Fino K, Wang Y, Sandhu P, Song X, Norbury C, Ni B, Fang D, Salek-Ardakani S, Song J. C-Myc regulation by costimulatory signals modulates the generation of CD8+ memory T cells during viral infection. Open Biol. 2016;6:150208. doi: 10.1098/rsob.150208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harty JT, Badovinac VP. Shaping and reshaping CD8+ T-cell memory. Nat Rev Immunol. 2008;8:107–19. doi: 10.1038/nri2251. [DOI] [PubMed] [Google Scholar]
- Haynes NM, Allen CD, Lesley R, Ansel KM, Killeen N, Cyster JG. Role of CXCR5 and CCR7 in follicular Th cell positioning and appearance of a programmed cell death gene-1high germinal center-associated subpopulation. J Immunol. 2007;179:5099–108. doi: 10.4049/jimmunol.179.8.5099. [DOI] [PubMed] [Google Scholar]
- He J, Tsai LM, Leong YA, Hu X, Ma CS, Chevalier N, Sun X, Vandenberg K, Rockman S, Ding Y, Zhu L, Wei W, Wang C, Karnowski A, Belz GT, Ghali JR, Cook MC, Riminton DS, Veillette A, Schwartzberg PL, Mackay F, Brink R, Tangye SG, Vinuesa CG, Mackay CR, Li Z, Yu D. Circulating precursor CCR7(lo)PD-1(hi) CXCR5(+) CD4(+) T cells indicate Tfh cell activity and promote antibody responses upon antigen reexposure. Immunity. 2013;39:770–81. doi: 10.1016/j.immuni.2013.09.007. [DOI] [PubMed] [Google Scholar]
- Hu Z, Blackman MA, Kaye KM, Usherwood EJ. Functional heterogeneity in the CD4+ T cell response to murine gamma-herpesvirus 68. J Immunol. 2015;194:2746–56. doi: 10.4049/jimmunol.1401928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannidis LJ, Nie CQ, Ly A, Ryg-Cornejo V, Chiu CY, Hansen DS. Monocyte- and Neutrophil-Derived CXCL10 Impairs Efficient Control of Blood-Stage Malaria Infection and Promotes Severe Disease. J Immunol. 2016;196:1227–38. doi: 10.4049/jimmunol.1501562. [DOI] [PubMed] [Google Scholar]
- Jagannathan P, Eccles-James I, Bowen K, Nankya F, Auma A, Wamala S, Ebusu C, Muhindo MK, Arinaitwe E, Briggs J, Greenhouse B, Tappero JW, Kamya MR, Dorsey G, Feeney ME. IFNgamma/IL-10 co-producing cells dominate the CD4 response to malaria in highly exposed children. PLoS Pathog. 2014;10:e1003864. doi: 10.1371/journal.ppat.1003864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagannathan P, Nankya F, Stoyanov C, Eccles-James I, Sikyomu E, Naluwu K, Wamala S, Nalubega M, Briggs J, Bowen K, Bigira V, Kapisi J, Kamya MR, Dorsey G, Feeney ME. IFNgamma Responses to Pre-erythrocytic and Blood-stage Malaria Antigens Exhibit Differential Associations With Past Exposure and Subsequent Protection. J Infect Dis. 2015;211:1987–96. doi: 10.1093/infdis/jiu814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaigirdar SA, Macleod MK. Development and Function of Protective and Pathologic Memory CD4 T Cells. Front Immunol. 2015;6:456. doi: 10.3389/fimmu.2015.00456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongco AM, Ting LM, Thathy V, Mota MM, Kim K. Improved transfection and new selectable markers for the rodent malaria parasite Plasmodium yoelii. Mol Biochem Parasitol. 2006;146:242–50. doi: 10.1016/j.molbiopara.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Kim CH, Rott LS, Clark-Lewis I, Campbell DJ, Wu L, Butcher EC. Subspecialization of CXCR5+ T cells: B helper activity is focused in a germinal center-localized subset of CXCR5+ T cells. J Exp Med. 2001;193:1373–81. doi: 10.1084/jem.193.12.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopf M, Ruedl C, Schmitz N, Gallimore A, Lefrang K, Ecabert B, Odermatt B, Bachmann MF. OX40-deficient mice are defective in Th cell proliferation but are competent in generating B cell and CTL Responses after virus infection. Immunity. 1999;11:699–708. doi: 10.1016/s1074-7613(00)80144-2. [DOI] [PubMed] [Google Scholar]
- Langhorne J, Simon-Haarhaus B, Meding SJ. The role of CD4+ T cells in the protective immune response to Plasmodium chabaudi in vivo. Immunol Lett. 1990;25:101–7. doi: 10.1016/0165-2478(90)90099-c. [DOI] [PubMed] [Google Scholar]
- Li L, Jiang Y, Lao S, Yang B, Yu S, Zhang Y, Wu C. Mycobacterium tuberculosis-Specific IL-21+IFN-gamma+CD4+ T Cells Are Regulated by IL-12. PLoS One. 2016;11:e0147356. doi: 10.1371/journal.pone.0147356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindner SE, Mikolajczak SA, Vaughan AM, Moon W, Joyce BR, Sullivan WJ, Jr, Kappe SH. Perturbations of Plasmodium Puf2 expression and RNA-seq of Puf2-deficient sporozoites reveal a critical role in maintaining RNA homeostasis and parasite transmissibility. Cell Microbiol. 2013;15:1266–83. doi: 10.1111/cmi.12116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonnberg T, Svensson V, James KR, Fernandez-Ruiz D, Sebina I, Montandon R, Soon MS, Fogg LG, Nair AS, Liligeto U, Stubbington MJ, Ly LH, Bagger FO, Zwiessele M, Lawrence ND, Souza-Fonseca-Guimaraes F, Bunn PT, Engwerda CR, Heath WR, Billker O, Stegle O, Haque A, Teichmann SA. Single-cell RNA-seq and computational analysis using temporal mixture modelling resolves Th1/Tfh fate bifurcation in malaria. Sci Immunol. 2017;2 doi: 10.1126/sciimmunol.aal2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu KT, Kanno Y, Cannons JL, Handon R, Bible P, Elkahloun AG, Anderson SM, Wei L, Sun H, O'Shea JJ, Schwartzberg PL. Functional and epigenetic studies reveal multistep differentiation and plasticity of in vitro-generated and in vivo-derived follicular T helper cells. Immunity. 2011;35:622–32. doi: 10.1016/j.immuni.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malleret B, Claser C, Ong AS, Suwanarusk R, Sriprawat K, Howland SW, Russell B, Nosten F, Renia L. A rapid and robust tri-color flow cytometry assay for monitoring malaria parasite development. Sci Rep. 2011;1:118. doi: 10.1038/srep00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall HD, Chandele A, Jung YW, Meng H, Poholek AC, Parish IA, Rutishauser R, Cui W, Kleinstein SH, Craft J, Kaech SM. Differential expression of Ly6C and T-bet distinguish effector and memory Th1 CD4(+) cell properties during viral infection. Immunity. 2011;35:633–46. doi: 10.1016/j.immuni.2011.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda JL, Zhang Q, Ndonye R, Richardson SK, Howell AR, Gapin L. T-bet concomitantly controls migration, survival, and effector functions during the development of Valpha14i NKT cells. Blood. 2006;107:2797–805. doi: 10.1182/blood-2005-08-3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mckinstry KK, Strutt TM, Swain SL. The potential of CD4 T-cell memory. Immunology. 2010;130:1–9. doi: 10.1111/j.1365-2567.2010.03259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsdoerffer M, Lee Y, Jager A, Kim HJ, Korn T, Kolls JK, Cantor H, Bettelli E, Kuchroo VK. Proinflammatory T helper type 17 cells are effective B-cell helpers. Proc Natl Acad Sci U S A. 2010;107:14292–7. doi: 10.1073/pnas.1009234107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita R, Schmitt N, Bentebibel SE, Ranganathan R, Bourdery L, Zurawski G, Foucat E, Dullaers M, Oh S, Sabzghabaei N, Lavecchio EM, Punaro M, Pascual V, Banchereau J, Ueno H. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity. 2011;34:108–21. doi: 10.1016/j.immuni.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SN, Gebhardt T, Carbone FR, Heath WR. Memory T cell subsets, migration patterns, and tissue residence. Annu Rev Immunol. 2013;31:137–61. doi: 10.1146/annurev-immunol-032712-095954. [DOI] [PubMed] [Google Scholar]
- Murata K, Ishii N, Takano H, Miura S, Ndhlovu LC, Nose M, Noda T, Sugamura K. Impairment of antigen-presenting cell function in mice lacking expression of OX40 ligand. J Exp Med. 2000;191:365–74. doi: 10.1084/jem.191.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayamada S, Kanno Y, Takahashi H, Jankovic D, Lu KT, Johnson TA, Sun HW, Vahedi G, Hakim O, Handon R, Schwartzberg PL, Hager GL, O'Shea JJ. Early Th1 cell differentiation is marked by a Tfh cell-like transition. Immunity. 2011;35:919–31. doi: 10.1016/j.immuni.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeng-Adjei N, Portugal S, Tran TM, Yazew TB, Skinner J, Li S, Jain A, Felgner PL, Doumbo OK, Kayentao K, Ongoiba A, Traore B, Crompton PD. Circulating Th1-Cell-type Tfh Cells that Exhibit Impaired B Cell Help Are Preferentially Activated during Acute Malaria in Children. Cell Rep. 2015;13:425–39. doi: 10.1016/j.celrep.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opata MM, Carpio VH, Ibitokou SA, Dillon BE, Obiero JM, Stephens R. Early effector cells survive the contraction phase in malaria infection and generate both central and effector memory T cells. J Immunol. 2015;194:5346–54. doi: 10.4049/jimmunol.1403216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opata MM, Stephens R. Early Decision: Effector and Effector Memory T Cell Differentiation in Chronic Infection. Curr Immunol Rev. 2013;9:190–206. doi: 10.2174/1573395509666131126231209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Organization WH. World Malaria Report 2015. Geneva: World Health Organization; 2015. [Google Scholar]
- Oxenius A, Bachmann MF, Zinkernagel RM, Hengartner H. Virus-specific MHC-class II-restricted TCR-transgenic mice: effects on humoral and cellular immune responses after viral infection. Eur J Immunol. 1998;28:390–400. doi: 10.1002/(SICI)1521-4141(199801)28:01<390::AID-IMMU390>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Pepper M, Jenkins MK. Origins of CD4(+) effector and central memory T cells. Nat Immunol. 2011;12:467–71. doi: 10.1038/ni.2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper M, Pagan AJ, Igyarto BZ, Taylor JJ, Jenkins MK. Opposing signals from the Bcl6 transcription factor and the interleukin-2 receptor generate T helper 1 central and effector memory cells. Immunity. 2011;35:583–95. doi: 10.1016/j.immuni.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Mazliah D, Langhorne J. CD4 T-cell subsets in malaria: TH1/TH2 revisited. Front Immunol. 2014;5:671. doi: 10.3389/fimmu.2014.00671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Mazliah D, Ng DH, Freitas Do Rosario AP, Mclaughlin S, Mastelic-Gavillet B, Sodenkamp J, Kushinga G, Langhorne J. Disruption of IL-21 signaling affects T cell-B cell interactions and abrogates protective humoral immunity to malaria. PLoS Pathog. 2015;11:e1004715. doi: 10.1371/journal.ppat.1004715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters NC, Pagan AJ, Lawyer PG, Hand TW, Henrique Roma E, Stamper LW, Romano A, Sacks DL. Chronic parasitic infection maintains high frequencies of short-lived Ly6C+CD4+ effector T cells that are required for protection against re-infection. PLoS Pathog. 2014;10:e1004538. doi: 10.1371/journal.ppat.1004538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portugal S, Pierce SK, Crompton PD. Young lives lost as B cells falter: what we are learning about antibody responses in malaria. J Immunol. 2013;190:3039–46. doi: 10.4049/jimmunol.1203067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryg-Cornejo V, Ioannidis LJ, Ly A, Chiu CY, Tellier J, Hill DL, Preston SP, Pellegrini M, Yu D, Nutt SL, Kallies A, Hansen DS. Severe Malaria Infections Impair Germinal Center Responses by Inhibiting T Follicular Helper Cell Differentiation. Cell Rep. 2016;14:68–81. doi: 10.1016/j.celrep.2015.12.006. [DOI] [PubMed] [Google Scholar]
- Sage PT, Sharpe AH. In vitro assay to sensitively measure T(FR) suppressive capacity and T(FH) stimulation of B cell responses. Methods Mol Biol. 2015;1291:151–60. doi: 10.1007/978-1-4939-2498-1_13. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Lanzavecchia A. Understanding dendritic cell and T-lymphocyte traffic through the analysis of chemokine receptor expression. Immunol Rev. 2000;177:134–40. doi: 10.1034/j.1600-065x.2000.17717.x. [DOI] [PubMed] [Google Scholar]
- Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol. 2008;8:247–58. doi: 10.1038/nri2274. [DOI] [PubMed] [Google Scholar]
- So T, Song J, Sugie K, Altman A, Croft M. Signals from OX40 regulate nuclear factor of activated T cells c1 and T cell helper 2 lineage commitment. Proc Natl Acad Sci U S A. 2006;103:3740–5. doi: 10.1073/pnas.0600205103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Salek-Ardakani S, Rogers PR, Cheng M, Van Parijs L, Croft M. The costimulation-regulated duration of PKB activation controls T cell longevity. Nat Immunol. 2004;5:150–8. doi: 10.1038/ni1030. [DOI] [PubMed] [Google Scholar]
- Song J, Salek-Ardakani S, So T, Croft M. The kinases aurora B and mTOR regulate the G1-S cell cycle progression of T lymphocytes. Nat Immunol. 2007;8:64–73. doi: 10.1038/ni1413. [DOI] [PubMed] [Google Scholar]
- Song J, So T, Cheng M, Tang X, Croft M. Sustained survivin expression from OX40 costimulatory signals drives T cell clonal expansion. Immunity. 2005;22:621–31. doi: 10.1016/j.immuni.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Song J, So T, Croft M. Activation of NF-kappaB1 by OX40 contributes to antigen-driven T cell expansion and survival. J Immunol. 2008;180:7240–8. doi: 10.4049/jimmunol.180.11.7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soroosh P, Ine S, Sugamura K, Ishii N. Differential requirements for OX40 signals on generation of effector and central memory CD4+ T cells. J Immunol. 2007;179:5014–23. doi: 10.4049/jimmunol.179.8.5014. [DOI] [PubMed] [Google Scholar]
- Spence PJ, Langhorne J. T cell control of malaria pathogenesis. Curr Opin Immunol. 2012;24:444–8. doi: 10.1016/j.coi.2012.05.003. [DOI] [PubMed] [Google Scholar]
- Stephens R, Albano FR, Quin S, Pascal BJ, Harrison V, Stockinger B, Kioussis D, Weltzien HU, Langhorne J. Malaria-specific transgenic CD4(+) T cells protect immunodeficient mice from lethal infection and demonstrate requirement for a protective threshold of antibody production for parasite clearance. Blood. 2005;106:1676–84. doi: 10.1182/blood-2004-10-4047. [DOI] [PubMed] [Google Scholar]
- Stephens R, Langhorne J. Priming of CD4+ T cells and development of CD4+ T cell memory; lessons for malaria. Parasite Immunol. 2006;28:25–30. doi: 10.1111/j.1365-3024.2006.00767.x. [DOI] [PubMed] [Google Scholar]
- Stephens R, Langhorne J. Effector memory Th1 CD4 T cells are maintained in a mouse model of chronic malaria. PLoS Pathog. 2010;6:e1001208. doi: 10.1371/journal.ppat.1001208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–69. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- Velu V, Mylvaganam GH, Gangadhara S, Hong JJ, Iyer SS, Gumber S, Ibegbu CC, Villinger F, Amara RR. Induction of Th1-Biased T Follicular Helper (Tfh) Cells in Lymphoid Tissues during Chronic Simian Immunodeficiency Virus Infection Defines Functionally Distinct Germinal Center Tfh Cells. J Immunol. 2016;197:1832–42. doi: 10.4049/jimmunol.1600143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villegas-Mendez A, De souza JB, Lavelle SW, Gwyer Findlay E, Shaw TN, Van Rooijen N, Saris CJ, Hunter CA, Riley EM, Couper KN. IL-27 receptor signalling restricts the formation of pathogenic, terminally differentiated Th1 cells during malaria infection by repressing IL-12 dependent signals. PLoS Pathog. 2013;9:e1003293. doi: 10.1371/journal.ppat.1003293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Der Weid T, Langhorne J. The roles of cytokines produced in the immune response to the erythrocytic stages of mouse malarias. Immunobiology. 1993;189:397–418. doi: 10.1016/s0171-2985(11)80367-0. [DOI] [PubMed] [Google Scholar]
- Wikenheiser DJ, Ghosh D, Kennedy B, Stumhofer JS. The Costimulatory Molecule ICOS Regulates Host Th1 and Follicular Th Cell Differentiation in Response to Plasmodium chabaudi chabaudi AS Infection. J Immunol. 2016;196:778–91. doi: 10.4049/jimmunol.1403206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wykes MN, Lewin SR. Immune checkpoint blockade in infectious diseases. Nat Rev Immunol. 2017 doi: 10.1038/nri.2017.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanouchi S, Kuwahara K, Sakata A, Ezaki T, Matsuoka S, Miyazaki J, Hirose S, Tamura T, Nariuchi H, Sakaguchi N. A T cell activation antigen, Ly6C, induced on CD4+ Th1 cells mediates an inhibitory signal for secretion of IL-2 and proliferation in peripheral immune responses. Eur J Immunol. 1998;28:696–707. doi: 10.1002/(SICI)1521-4141(199802)28:02<696::AID-IMMU696>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Youngblood B, Hale JS, Ahmed R. T-cell memory differentiation: insights from transcriptional signatures and epigenetics. Immunology. 2013a;139:277–84. doi: 10.1111/imm.12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngblood B, Hale JS, Akondy R. Using epigenetics to define vaccine-induced memory T cells. Curr Opin Virol. 2013b;3:371–6. doi: 10.1016/j.coviro.2013.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zander RA, Obeng-Adjei N, Guthmiller JJ, Kulu DI, Li J, Ongoiba A, Traore B, Crompton PD, Butler NS. PD-1 Co-inhibitory and OX40 Co-stimulatory Crosstalk Regulates Helper T Cell Differentiation and Anti-Plasmodium Humoral Immunity. Cell Host Microbe. 2015;17:628–41. doi: 10.1016/j.chom.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.