Abstract
Objective
Impaired endothelial cell autophagy compromises shear-stress induced nitric oxide (NO) generation. We determined the responsible mechanism.
Approach and Results
Upon autophagy compromise in bovine aortic endothelial cells (ECs) exposed to shear-stress, a decrease in glucose uptake and EC glycolysis attenuated ATP production. We hypothesized that decreased glycolysis-dependent purinergic signaling via P2Y-1 receptors, secondary to impaired autophagy in ECs, prevents shear-induced p-eNOSS1177 and NO generation. Maneuvers that restore glucose transport and glycolysis (e.g., overexpression of GLUT1) or purinergic signaling (e.g., addition of exogenous ADP) rescue shear-induced p-eNOSS1177 and NO production in ECs with impaired autophagy. Conversely, inhibiting glucose-transport via GLUT1 siRNA, blocking purinergic signaling via ectonucleotidase-mediated ATP/ADP degradation (e.g., apyrase), or inhibiting P2Y1 receptors using pharmacological (e.g., MRS2179) or genetic (e.g., P2Y1-R siRNA) procedures, inhibits shear-induced p-eNOSS1177 and NO generation in ECs with intact autophagy. Supporting a central role for PKCδT505 in relaying the autophagy-dependent purinergic-mediated signal to eNOS, we find that: (i) shear-stress induced activating phosphorylation of PKCδT505 is negated by inhibiting autophagy; (ii) shear-induced p-eNOSS1177 and NO generation are restored in autophagy-impaired ECs via pharmacological (e.g., bryostatin) or genetic (e.g., CA-PKCδ) activation of PKCδT505 and (iii) pharmacological (e.g., rottlerin) and genetic (e.g., PKCδ siRNA) PKCδ inhibition prevents shear-induced p-eNOSS1177 and NO generation in ECs with intact autophagy. Key nodes of dysregulation in this pathway upon autophagy compromise were revealed in human arterial endothelial cells.
Conclusion
Targeted reactivation of purinergic signaling and/or PKCδ has strategic potential to restore compromised NO generation in pathologies associated with suppressed EC autophagy.
Introduction
Common among many vascular pathologies is an altered endothelial cell (EC) phenotype i.e., endothelial dysfunction.1, 2 A crucial aspect of EC dysfunction is compromised nitric oxide (NO) bioavailability, which results from decreased NO synthesis and/or increased NO degradation. Molecular mechanisms responsible for endothelial dysfunction are unclear, and new targets for therapeutic intervention are required.
Autophagy is a highly conserved trafficking process through which intracellular components, including soluble proteins and protein aggregates, carbohydrates, lipids, membranes, cytoskeletal components, and organelles, are delivered to lysosomes. Cargo delivered to lysosomes by autophagy is degraded by lysosomal acid hydrolases, producing metabolites that can be recycled for use in new biosynthetic reactions, or diverted to metabolic pathways that generate ATP. Autophagy therefore plays a critical role in maintaining cellular homeostasis.3, 4
Intriguing but limited evidence from human subjects suggests that compromised EC autophagy is associated with NO-mediated arterial dysfunction in the context of aging5 and type 2 diabetes mellitus (T2DM).6 However, concurrent risk factors associated with aging and T2DM have potential to impair eNOS activity independent from suppressed EC autophagy, and cannot be overlooked as contributing to the dysregulated EC phenotype.
Earlier we determined whether autophagy suppression per se compromises shear-stress evoked activation of eNOS and subsequent NO generation.7 Shear-stress increased autophagosome formation and NO generation in ECs. Genetic inhibition of autophagy by siRNA-mediated knockdown of Atg3 prevented shear-induced phosphorylation of eNOS at its positive regulatory site S1117 (p-eNOSS1177), negated NO generation, amplified reactive oxygen species (ROS) production, and unleashed pro-inflammatory and adhesive responses, indicating autophagy is critical for normal EC function. While recent studies have confirmed our first report that shear-stress increases indices of autophagy in ECs8, 9 and arteries,8 none have concurrently measured NO generation in the absence and presence of autophagy inhibition, and the mechanism(s) whereby compromised autophagy in ECs jeopardizes shear-induced NO generation is unknown. The purpose of the present study was to determine the mechanism whereby suppressed EC autophagy compromises shear-induced EC NO production.
Materials and Methods
This section is available in the online-only Data Supplement.
Results
Shear-stress increases autophagy and mitochondrial turnover in endothelial cells
Bovine aortic ECs were exposed to no shear (− shear) or ~ 20 dyn/cm2 for 3 h (+ shear). Shear-stress increased Atg3 (Fig 1A) and Atg5 (Supp IA) protein expression, LC3-II accumulation (Fig 1B) and p62 degradation (Supp IB), and LC3:GFP puncta formation (Fig 1C, E).
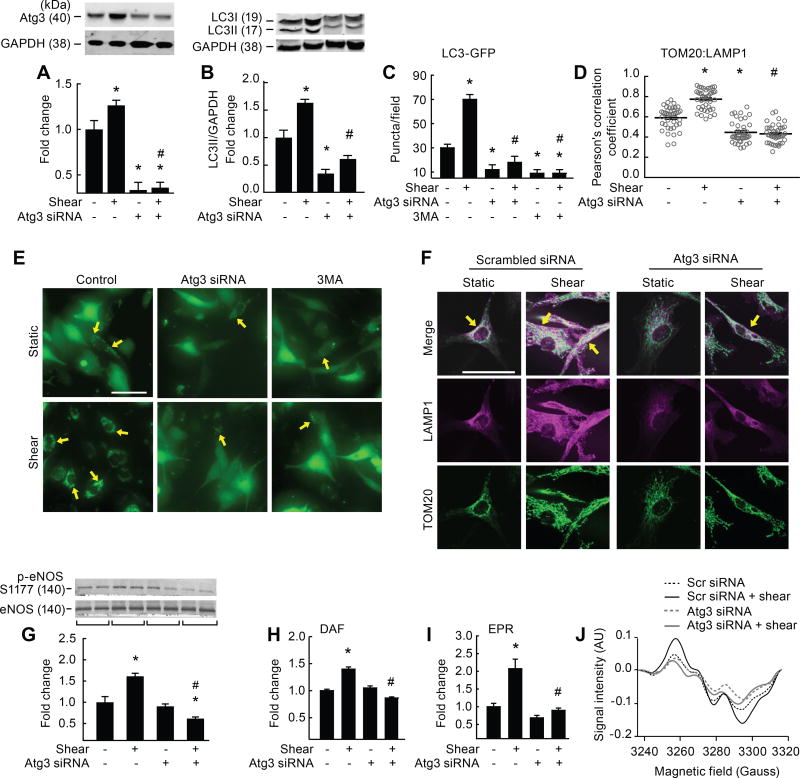
Figure 1. Genetic disruption of Atg3 impairs shear-stress induced autophagy, mitophagy, and NO generation.
Relative to static conditions, shear-stress increased Atg3 protein expression (A), LC3 II accumulation (B), LC3-GFP puncta formation (C, E), colocalization of TOM20 with LAMP1 (D, F), p-eNOSS1177 (G), and NO generation (H–J), in ECs transfected with scrambled siRNA (bar 1 vs. 2) but not in ECs transfected with Atg3 siRNA (bar 3 vs. 4), or in ECs after treatment with 3MA (C, bar 5 vs. 6). Images shown in E, F, and J represent mean data shown in C, D, and I, respectively. Fluorescence images in E, F were individually adjusted to maximize clarity. Calibration bar = 50 µm. For A, B (n=30), C, D (n=10), E, F (n=6), G, H (n=30), I, J (n=10). For A, B, G each n=1 × 10 cm petri dish. For C, D each n=1 well of a 24-well plate. For H, each n = 1 well of a 6-well plate. For C, D, E, F each n=10 cells per field × 10 fields per slide. For I, J each n = 3 wells of a 6-well plate. *p<0.05 vs. (−shear)(−Atg3 siRNA); # p<0.05 vs. (+shear)(−Atg3 siRNA).
Autophagy is an important regulator of mitochondrial turnover i.e., mitophagy.10 We observed shear-induced degradation of mitochondrial (m) -aconitase and TOM20 (Supp I C, D), together with colocalization of TOM20 with LAMP1 (Fig 1D, F), and TOM20 with LC3 (Supp I E, F). These latter findings demonstrate that mitochondrial turnover is elevated in response to shear stress.
Shear-induced LC3-II accumulation was greater, whereas p62 degradation was prevented, in the presence vs. the absence of Baf-A1 (Supp I G, H), confirming that shear stress increases autophagosome formation rather than decreases autophagosomal degradation by the lysosome.7 All indices of shear-induced EC autophagy and mitophagy we measured were prevented by repressing Atg3 (Fig 1 A–F; Supp I B–F) or Atg5 (Supp I A, I, J) protein expression. Using an alternative approach, shear-induced LC3:GFP puncta formation was negated by pharmacological inhibition of autophagy using the class III phosphatidylinositol 3-kinase (PI3K) inhibitor 3-methyladenine (3-MA; Fig 1C, E). These data indicate shear-stress increases EC autophagy and mitophagy., and demonstrate that genetic and pharmacological approaches we employed to limit autophagy do not alter cell viability (Supp II A–C).
Autophagy suppression limits shear-stress induced NO generation in endothelial cells
Shear-stress increased p-eNOSS1177 and NO generation in ECs transfected with scrambled but not Atg3 (Fig 1 G–J) or Atg5 siRNA (Supp III A, B). Impairment of autophagy exacerbated shear-induced ROS production (Supp IV A) and unleashed markers of inflammation and adhesion (Supp IV B–F). These findings confirm that vascular autophagy plays a critical role in maintaining NO bioavailability and regulating oxidant/antioxidant balance and inflammatory/anti-inflammatory balance in ECs.
Shear-induced phosphatase, kinase, and ROS signaling after autophagy suppression
Protein phosphatases and kinases respectively remove and add phosphate groups from target proteins11, 12 including eNOS.13–17 Vascular protein phosphatase 2A (PP2A) activity is increased in pathologies associated with suppressed p-eNOSS1177 and compromised endothelial function.16, 18–20 We observed no evidence that shear stress altered PP2A activation in the absence or presence of Atg3 siRNA.
To determine whether disrupted kinase signaling to eNOS might compromise NO synthesis secondary to repressed autophagy, we assessed p-AktS473, p-AktT308, p-ERK1/2, p-p38MAPK, and p-AMPKT172 ± Atg3 siRNA ± 180-min of shear stress (Supp IV A–F). Activating phosphorylation was not different in response to shear-stress ± autophagy repression, and we concluded that altered signaling from these kinases to eNOS is not responsible for compromised EC NO generation. It was surprising to us that impairment of autophagy per se elevated p-AMPKT172 (Supp V E) and downstream targets (not shown), and this was pursued (see below).
Exaggerated ROS production after Atg3 siRNA (Supp IV A) has potential to decrease the synthesis of NO and/or increase the destruction of NO and this was examined. Regarding NO synthesis, MAP kinases such as p38-MAPK and ERK are phosphorylated/stimulated by NADPH generated ROS in vessels from mice with type 2 diabetes which concurrently exhibit reduced p-eNOSS1177 and impaired vasorelaxation.21 However, shear-induced p-ERK1/2 and p-p38MAPK were not altered by autophagy compromise (Supp V C, D). Further, pharmacological inhibition of MEK using PD98059 (Supp V C, F) and ERK1/2 using FR180204 (not shown) did not restore blunted shear-induced p-eNOSS1177 (Supp V F) after autophagy suppression.
Amplified shear-induced ROS production after Atg3 siRNA has potential to precipitate NO destruction and this was investigated. After its formation, O2•− reacts rapidly with NO to form peroxynitrite (ONOO−).22 ONOO− causes tyrosine nitration. Nitrotyrosine (i.e., 3-NT) is an established estimate for ONOO− formation.22 3-NT formation assessed via immunoblot (Supp VI A, B) or ELISA (not shown) was not greater in ECs exposed to shear stress after autophagy compromise. To strengthen these findings, specific sources of ROS were inhibited in an attempt to restore shear-induced p-eNOSS1177 and NO generation in ECs after autophagy suppression. Mitochondria were targeted first because mitophagy was repressed after Atg3 siRNA (Fig 1D, F; Supp 1C, D), and it is not unreasonable to hypothesize that accumulation of these organelles might provide a potent source for ROS production. Even though mito-tempo was efficacious with regard to attenuating shear-induced ROS accumulation in ECs with compromised autophagy (Supp VI C), a restoration of p-eNOSS1177 and NO generation was not observed (Supp VI D, E). Likewise, while the intracellular ROS scavenger N-acetyl cysteine (NAC) prevented ROS accumulation in the context of autophagy repression (Supp VI F), neither p-eNOSS1177 nor NO generation were preserved in ECs exposed to shear stress (Supp VI G, H). Neither mito-tempo (Supp VII A) nor NAC (Supp VII B) altered shear-induced autophagy ± Atg3 siRNA. A similar pattern of results concerning ROS and p-eNOSS1177 was recapitulated with the intracellular O2•− scavenger tiron, the complex IV inhibitor potassium cyanide, and the NADPH oxidase inhibitor N-vanillynonanamide23, 24 (not shown). The ability of DCFDA fluorescence to detect ROS, and results concerning cell viability ± the various antioxidant treatments, are shown in Supp VII C and D, respectively.
Autophagy suppression compromises glycolysis in endothelial cells
Elevated p-AMPKT172 in ECs after autophagy compromise (Supp V E) suggests an energy stress. ECs derive ATP primarily from glycolysis.25, 26 We examined whether autophagy suppression evokes an energetic deficit in ECs. When ECs transfected with scrambled siRNA were exposed to shear-stress they displayed the expected increase in GLUT1 expression – the main glucose transporter in ECs, 3H-deoxyglucose uptake, and cellular ATP production. Relative to control ECs, each response was prevented in ECs transfected with Atg3 (Fig 2 A–C) or Atg5 siRNA (not shown). Because these data suggested that autophagy compromise impairs EC glycolysis, we measured the extracellular acidification rate (ECAR; a surrogate measure of lactic acid production) using the XF24 Seahorse Bioanalyzer.27 Compared to ECs exposed to no shear, shear-stress increased ECAR in ECs transfected with scrambled but not Atg3 siRNA, and results were similar under basal and maximal (i.e., + FCCP) conditions (Supp VIII). These findings motivated us to complete a “glycolysis stress test” i.e., ECAR was assessed in ECs challenged with shear stress ± autophagy suppression under the following conditions: 0 mM glucose, 5 mM glucose (to stimulate glycolysis, lactate production, and increase ECAR), 1 µM oligomycin (to inhibit mitochondrial ATP production), and 50 mM 2 deoxyglucose (to inhibit glycolysis) (Fig 2D). Basal, glucose-stimulated, and oligomycin-stimulated ECAR was suppressed after Atg3 siRNA. As expected, 2-deoxyglucose inhibited ECAR in ECs transfected with scrambled but not Atg3 siRNA. These findings collectively indicate that EC autophagy suppression impairs EC glycolysis.
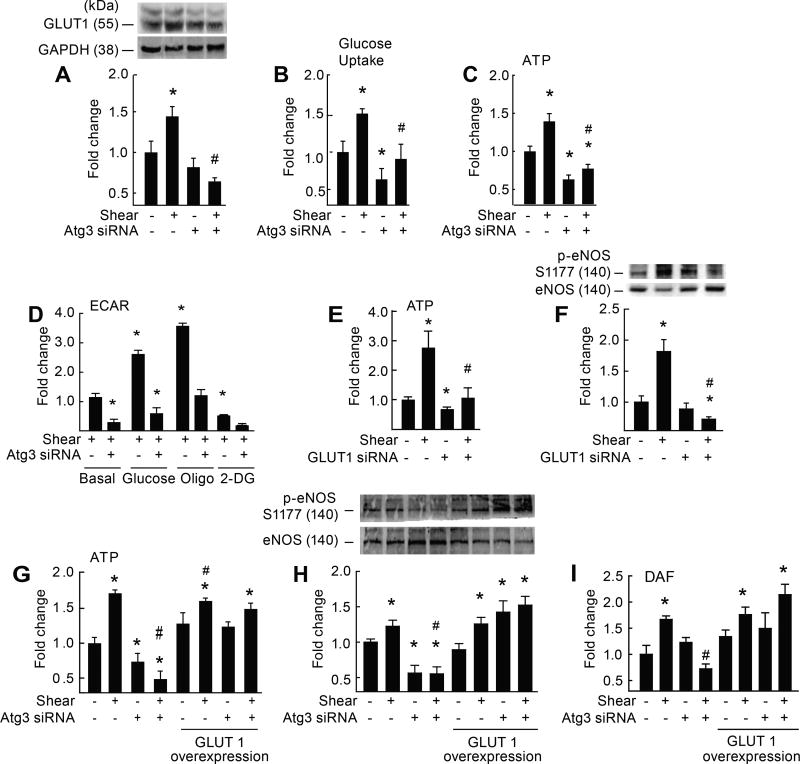
Figure 2. Genetic disruption of Atg3 impairs EC glycolysis and NO generation.
Relative to static conditions, shear-stress increased GLUT1 protein expression (A), glucose uptake (B), ATP production (C, G), p-eNOSS1177 (H) and NO generation (I) in ECs transfected with scrambled siRNA (bar 1 vs. 2) but not Atg3 siRNA (bar 3 vs. 4). For A–C (n=8–12). For A, each n = 1 × 10 cm petri dish). For B, C, each n = 1 well of a 6-well plate. For A–C *p<0.05 vs. (−shear)(−Atg3 siRNA); #p<0.05 vs. (+shear)(−Atg3 siRNA). Extracellular acidification rate (ECAR) was assessed in ECs exposed to shear stress ± Atg3 siRNA (D). Relative to basal conditions, ECs transfected with scrambled siRNA displayed increased ECAR in response to 5 mM glucose (bar 1 vs. 3) and 1 µM oligomycin (oligo; bar 1 vs. 5), but these responses were not observed in ECs after Atg3 siRNA (bar 2 vs. 4; bar 2 vs. 6, respectively). Relative to basal conditions, ECAR decreased upon treatment with 50 mM 2-DG in ECs transfected with scrambled (bar 1 vs. 7) but not Atg3 siRNA (bar 2 vs. 8). For D, n=3, each n= 1 seahorse plate. For D, *p<0.05 vs. basal condition (+shear)(−Atg3 siRNA i.e., bar 1); # p<0.05 vs. same condition (+shear)(−Atg3 siRNA). Relative to static conditions, shear-stress increased ATP (E) and p-eNOSS1177 (F) in ECs transfected with scrambled siRNA (bar 1 vs. 2) but not GLUT1 siRNA (bar 3 vs. 4). For E, n=12, each n = 1 well of a 6-well plate. For F, n=6, each n = 1 × 10 cm petri dish. For E, F *p<0.05 vs. (−shear)(−Atg3 siRNA); #p<0.05 vs. (+shear)(−Atg3 siRNA). After co-transfection with a plasmid vector to increase GLUT1 expression in ECs (G–I), the suppression of shear-induced ATP (G), p-eNOSS1177 (H), and NO generation (I) after autophagy repression (bar 2 vs. 4) was not observed (bar 6 vs. 8). For G–I, n=4, each n = 1 × 10 cm petri dish). For G–I *p<0.05 vs. (−shear)(−Atg3 siRNA); #p<0.05 vs. (+shear)(−Atg3 siRNA).
To determine whether impaired EC glycolysis after autophagy compromise might negate shear-induced NO generation, we first employed a loss of function approach. ECs with ~65% knockdown of GLUT1 via siRNA (Supp IX A) exhibited depressed shear-induced ATP production (Fig 2E) and were refractory to shear-induced p-eNOSS1177 (Fig 2F). Second, we used a gain of function approach. When ECs with suppressed autophagy were transfected with a plasmid vector that increased GLUT1 expression by ~50% (Supp IX B), shear-induced ATP production, p-eNOSS1177, and NO generation (Fig 2 G–I) were restored. Cell viability and indices of autophagy, respectively, were not altered by GLUT1 siRNA (Supp IX C, D) or GLUT1 overexpression (Supp IX E, F).
Autophagy suppression impairs purinergic mediated activation of eNOS
It is established that ECs produce ATP in response to shear stress, and that extracellular ATP/ADP signals via purinergic receptors to activate p-eNOSS1177 and NO generation.28, 29 As would be predicted from cellular ATP results shown in Fig 2C, 2E, and 2G, shear-induced extracellular ATP accumulation was : robust in ECs transfected with scrambled but not Atg3 siRNA (Fig 3A); prevented in control ECs transfected with GLUT1 siRNA (Fig 3B); and restored by GLUT1 overexpression in ECs with compromised autophagy (Fig 3C).
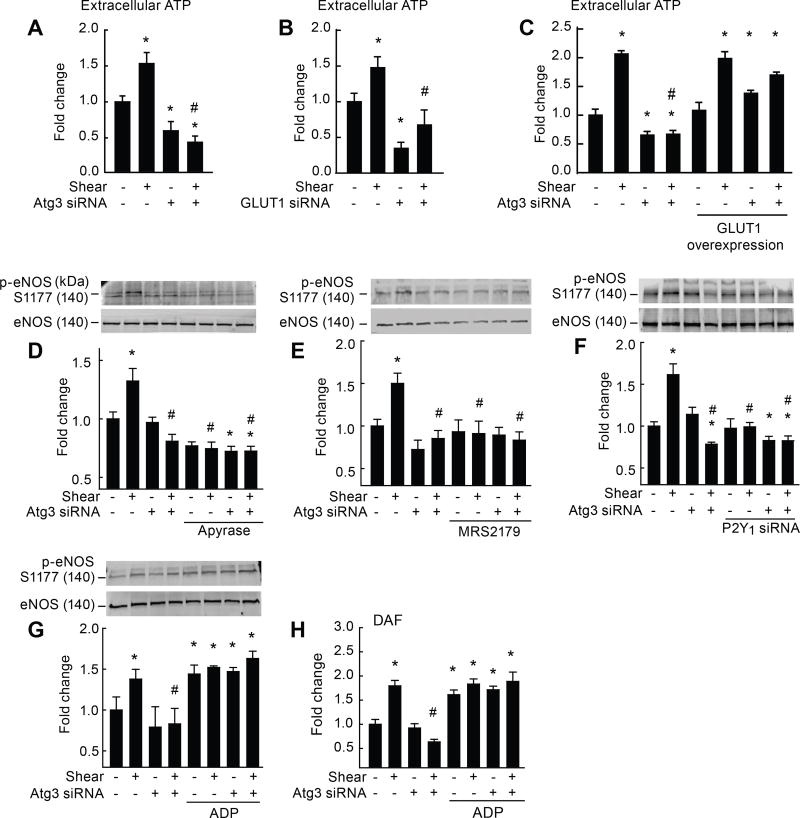
Figure 3. Genetic disruption of Atg3 limits purinergic-mediated activation of eNOS.
Relative to static conditions, shear-stress increased extracellular ATP accumulation (A–C), p-eNOSS1177 (D–G), and NO generation (H) in ECs transfected with scrambled siRNA (bar 1 vs. 2) but not Atg3 siRNA (A,C,D,E; bar 3 vs. 4) or GLUT1 siRNA (B; bar 3 vs. 4). After co-transfection with a plasmid vector to increase GLUT1 expression in ECs (C), the suppression of shear-induced ATP after autophagy compromise (bar 2 vs. 4) was normalized (bar 6 vs. 8). The ectonucleotidase apyrase (D), the pharmacological P2Y1-R blocker MRS2179 (E), and genetic disruption of P2Y1-R via siRNA (F) prevented shear-induced p-eNOSS1177 in ECs with intact Atg3 protein (bar 5 vs. 6). Conversely exogenous 2-methylthioadenosine diphosphate (ADP) restored shear-induced p-eNOSS1177 (G) and NO generation (H) in ECs with suppressed autophagy (bar 7 vs. 8). For A–C, H, n=6, each n = 1 well of a 6-well plate. For D–G, n=5, each n = 1 × 10 cm petri dish. * p<0.05 vs. (−shear)(−Atg3 siRNA); # p<0.05 vs. (+shear)(−Atg3 siRNA).
If limited extracellular ATP is responsible for suppressed shear-induced NO generation in ECs with compromised autophagy, then promoting extracellular ATP breakdown in ECs with intact autophagy, or blocking the dominant purinergic receptor in ECs with intact autophagy,30 should independently recapitulate the phenotype displayed by ECs with impaired autophagy. Supporting this notion, shear stress did not increase p-eNOSS1177 when ECs transfected with scrambled siRNA were treated concurrently with apyrase, an ectonucleotidase that hydrolyzes ATP to AMP and inorganic phosphate (Fig 3D), or MRS2179, a P2Y1-receptor blocker (Fig 3E).31 A genetic approach was used to strengthen these findings. When the P2Y1-receptor was silenced by ~45% via siRNA in ECs with intact autophagy (Supp X A), shear stress was incapable of activating eNOS when compared to results from ECs transfected with scrambled siRNA (Fig 3F). Importantly, P2Y1-receptor expression was not different ± shear stress ± Atg3 siRNA (Supp X A), and neither apyrase nor P2Y1-receptor siRNA altered LC3-II:GAPDH or cell viability (Supp X B–D).
To validate that limited extracellular ATP contributes importantly to preventing shear-induced NO generation in ECs with compromised autophagy, we provided exogenous ADP to the cellular milieu in an attempt to restore NO production. ADP rescued shear-induced p-eNOSS1177 (Fig 3G) and NO generation (Fig 3H) in ECs with suppressed autophagy, and neither LC3-II:GAPDH (Supp X E) nor cell viability (Supp X D) were altered by this treatment.
Autophagy suppression impairs purinergic mediated activation of eNOS via PKCδT505
To this point our data indicate that when autophagy is suppressed in ECs, a glycolytic defect limits shear-induced ATP production to an extent that purinergic-mediated activation of eNOS is compromised. We sought to identify the link between P2Y1 activation and eNOS phosphorylation. An exploration of the literature revealed that extracellular nucleotide-mediated activation of p-eNOSS1177 in human umbilical vein endothelial cells exposed to shear stress occurs via p-PKCδT505 30. Because we did not assess this kinase originally, historical cellular homogenates treated ± shear ± Atg3 siRNA were retrieved, re-examined, and results indicated that shear-induced p-PKCδT505 was prevented in ECs with suppressed autophagy (Fig 4A). Confirming the importance of this kinase in the context of the current experimental conditions, eNOS activation was refractory to shear stress in ECs transfected with PKCδ vs. scrambled siRNA (Fig 4B; producing ~40% reduction in PKCδ gene expression, Supp XI A), and in ECs with intact autophagy treated with the PKCδ inhibitor rottlerin (not shown).
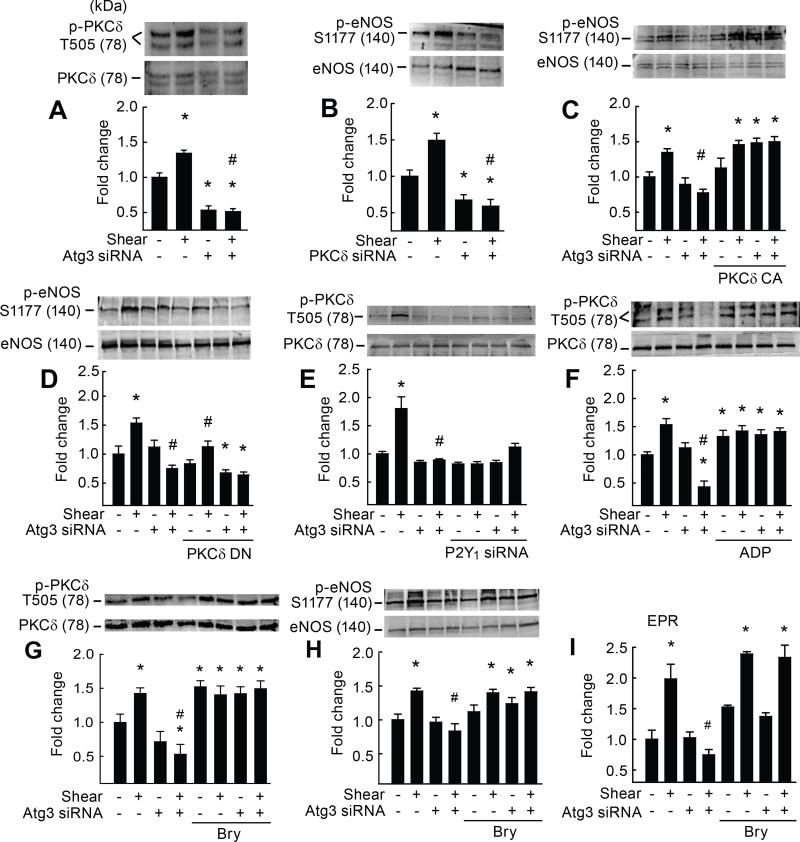
Figure 4. Defective purinergic-mediated p-PKCδT505 activation of eNOS after autophagy compromise is normalized by genetic and pharmacological approaches.
Relative to static conditions, shear-stress increased p-PKCδT505 (A,E,F,G) and p-eNOSS1177 (C,D,H) in ECs transfected with scrambled siRNA (bar 1 vs. 2) but not Atg3 siRNA (bar 3 vs. 4). Relative to static conditions, shear-stress increased p-eNOSS1177 in ECs transfected with scrambled but not PKCδ siRNA (B) (bar 2 vs. 4). Suppressed shear-induced p-eNOSS1177 after Atg3 siRNA was restored in ECs co-transfected with constitutively active (CA) PKCδ (bar 7 vs. 8; C) but not dominant-negative (DN) PKCδ (D). Suppressed shear-induced p-PKCδT505 after Atg3 siRNA (A,E,F,G) could be recapitulated in ECs with intact Atg3 protein that were transfected with P2Y1-R siRNA (bar 5 vs. 6; E) or restored in ECs with autophagy compromise by ADP (bar 7 vs. 8; F). Suppressed shear-induced p-PKCδT505, p-eNOSS1177, and NO generation in ECs transfected with Atg3 siRNA was restored by the PKCδ agonist bryostatin-1 (bry; bar 4 vs. 8, G, H, I). For A–H, n=5, each n = 1 × 10 cm petri dish. For I, n=6, each n = 3 wells of a 6-well plate. *p<0.05 vs. (−shear)(−Atg3 siRNA); # p<0.05 vs. (+shear)(−Atg3 siRNA).
Using a gain-of-function approach, when ECs with suppressed autophagy were transfected with constitutively-active (CA) PKCδ, and subsequently challenged with shear-stress, p-eNOSS1177 (Fig 4C) and NO generation (Supp XI B, C) were restored, and indices of autophagy (Supp XI D) were not altered. Consistent with these findings, when ECs with suppressed autophagy were transfected with dominant negative (DN) PKCδ, and subsequently challenged with shear-stress, p-eNOSS1177 was not restored (Fig 4D), and indices of autophagy (Supp XI E) were unaltered. Protein expression was ~ 65% greater or ~ 50% greater in ECs transfected with CA or DN PKCδ, respectively (Supp XI F, G). Representative EPR traces and cell death for all treatments are shown in Supp XII A–C.
Next we confirmed that p-PKCδT505 is a downstream target of purinergic mediated signaling in our system. Specifically, phosphorylation of this kinase was refractory to shear stress in ECs with intact autophagy after transfection with P2Y1-receptor vs. scrambled siRNA (Fig 4E). Substantiating these findings, shear-stress evoked robust p-PKCδT505 (and p-eNOSS1177 and NO generation; Fig 3 G,H) in ECs with repressed autophagy that were treated concurrently with exogenous ADP (Fig 4F).
Bryostatin-1 is a small molecule that binds with and directly activates PKCδ.32–34 To determine whether pharmacological activation might preserve NO production in the context of suppressed autophagy, ECs ± Atg3 siRNA were treated ± shear stress ± bryostatin-1. Defective shear-induced PKCδT505, p-eNOSS1177, and NO generation after autophagy compromise were restored by concurrent treatment with bryostatin-1 (Fig 4 G–I; Supp Fig XIII A, B). Taken together, our findings indicate that impaired EC autophagy represses glycolysis to an extent that extracellular ATP accumulation is limited, and purinergic signaling via the P2Y1-R and PKCδ to eNOS is blunted.
Translation to human endothelial cells and intact mice
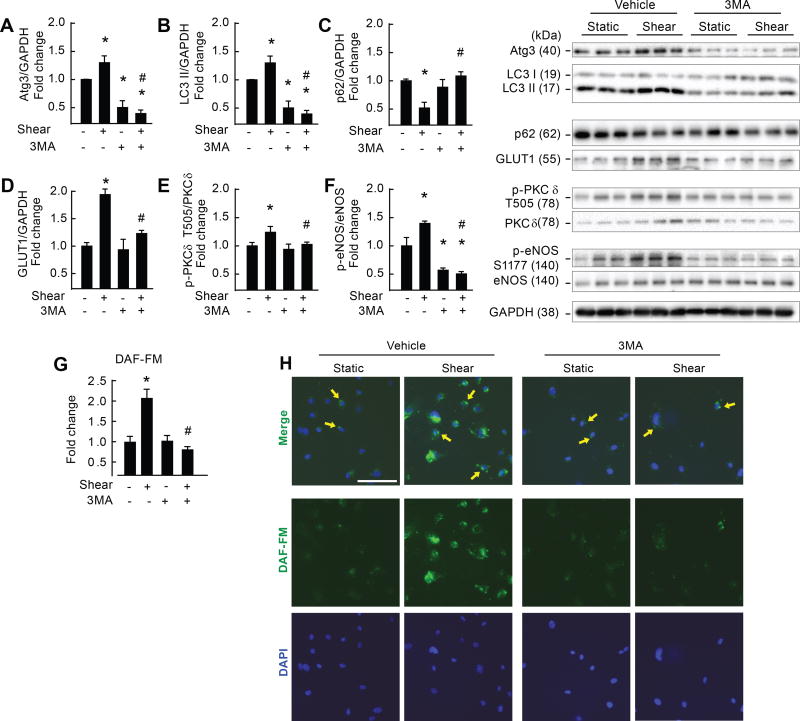
We determined if major findings observed in BAECs might be observed in human cells. Human arterial endothelial cells (HAECs) were treated ± 20 dyn/cm2 for 3 h ± 5 mM 3-MA. Shear-stress increased Atg3:GAPDH, LC3-II:GAPDH, p62 degradation, GLUT1:GAPDH, p-PKCδT505 : PKC, p-eNOSS1177 : eNOS, and NO generation in the absence but not the presence of autophagy inhibition (Fig 5 A–H). 3-MA did not alter cell viability (Supp Fig XIV A–C). These data are congruent with results from BAECs and demonstrate strong proof of concept for translational relevance.
Figure 5. Pharmacological inhibition of autophagy impairs shear-stress induced NO generation in human arterial endothelial cells.
Relative to static conditions, shear-stress increased Atg3 (A), LC3 II (B), GLUT1 (D), p-PKCδT505 (E) and p-eNOSS1177 (F) protein expression, NO generation (G), and p62 degradation (C) (bar 1 vs. 2). All responses were prevented by concurrent treatment with 3MA (bar 3 vs. 4). Images shown in the “merge” portion of H represent mean data shown in G. Calibration bar = 100 µm. For A–F, n=3, each n= 2 wells of a 6-well plate. For G and H, n=2, each n = 1 well of a 4 well chamber slide. *p<0.05 vs. (−shear)(− 3MA); # p<0.05 vs. (+shear)(− 3MA).
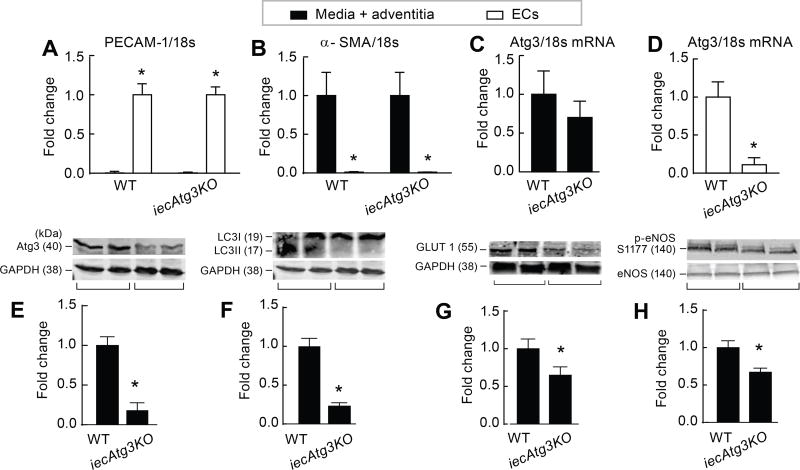
Next we examined whether major findings from ECs studied in vitro could be recapitulated in mice with compromised EC autophagy (Fig 6 A–D). Arterial ECs obtained from mice with tamoxifen-inducible deletion of Atg3 specifically in endothelial cells (iecAtg3KO mice) exhibit the anticipated reduction in Atg3 protein expression and LC3II:GAPDH, concurrent with suppressed GLUT1:GAPDH and p-eNOSS1177:eNOS, vs. results obtained from wild type (WT) controls (Fig 6 E–H). Thus, several key nodes of dysregulation observed in BAECs and HAECs studied in vitro after genetic and/or pharmacological repression of autophagy are recapitulated in vivo in mice with inducible disruption of EC autophagy. Studies are ongoing to determine the physiological and pathophysiological relevance of these findings.
Figure 6. iecAtg3KO mice exhibit impaired endothelial cell autophagy, GLUT1 protein expression, and p-eNOS protein expression vs. WT mice.
To assess mRNA, endothelial cells (ECs) and media + adventitia were isolated from iliac arteries of 5-month old iecAtg3KO and WT mice treated 30-days earlier with tamoxifen. Purity of the EC and media + adventitia fraction was confirmed by PECAM (A) and α-SMA (B) staining, respectively. Atg3/18S mRNA was similar in the media + adventitia component of both groups (C), but was lower in the EC fraction of iecAtg3KO vs. WT mice (D). For A–D, n=4 mice per group. *p<0.05 vs. media + adventitia (A); vs. EC (B); vs. WT (D). EC protein was assessed in a different cohort of 5-month old mice treated 30 days earlier with tamoxifen. Atg3:GAPDH (E), LC3 II:GAPDH (F), GLUT1 : GAPDH (G), and p-eNOS : eNOS (H) was lower in ECs from iecAtg3KO vs. WT mice. For E–H, n=3, each n = ECs obtained from 4 entire aortae. *p<0.05 vs. WT.
Discussion
An understanding of the role that autophagy plays in maintaining cytoplasmic homeostasis in the context of health and disease is evolving. Evidence exists that autophagy can exert protective or adverse effects, and the respective response appears to be dynamic, context specific, and cell autonomous. Compared to other tissues, relatively little is known concerning vascular autophagy in general, and EC autophagy in particular,35–37 but insight concerning the clinical relevance of EC autophagy is emerging. For example, the absence of endothelial autophagy markedly increased vascular lipid accumulation to an extent that elevated atherosclerotic burden in ApoE−/− mice.38
Our goal was to elucidate the molecular mechanisms whereby repressed EC autophagy compromises EC NO generation. Rationale for investigating this issue is strong. For example, primary arterial ECs from 61–71 y old (i.e., “old”) humans and aortae from 27–28 month old mice display markers of impaired autophagy and indices of lower NO bioavailability vs. the appropriate controls.5 Likewise, peripheral venous ECs obtained from diabetic patients exhibit depressed autophagy and blunted insulin-stimulated p-eNOSS1177.6 While these interesting studies provide proof of concept that impaired EC autophagy might precipitate compromised EC NO generation, the contribution from concurrent risk factors associated with aging and T2DM, with potential to impair eNOS activity independent from suppressed EC autophagy in primary ECs, cannot be overlooked.
Earlier we reported that after transfection with Atg3 siRNA, ECs were refractory to shear-induced p-eNOSS1177 and NO generation, ROS accumulation was amplified, and inflammatory cytokine production was unmasked.7 These findings were substantiated in the present study, extended to include autophagy suppression via Atg5 siRNA and class III PI3K inhibition using 3-MA (Fig 1, Supp I, III), and solid evidence is provided that key findings can be translated to human arterial endothelial cells (Fig 5) and intact mice (Fig 6). In this study we provide evidence for a unique mechanism whereby compromised autophagy impairs shear-induced NO generation.
Our first novel finding is that autophagy suppression impairs EC glycolysis. An initial exploration of candidate pathways that might be responsible for impaired NO synthesis and/or augmented NO destruction in ECs exposed to shear-stress after Atg3 siRNA revealed that neither altered kinase signaling to eNOS, ROS-mediated eNOS enzyme disruption, nor ROS-mediated conversion of NO to peroxynitrite were accountable. ECs derive ATP primarily from glycolysis and when the resulting metabolic end-product lactate is exported from the cell, the pH of the extracellular milieu is lowered25, 26. By quantifying the extracellular acidification rate in ECs under basal conditions, and in response to glucose stimulation, ATP synthase inhibition, and glycolysis inhibition, our suspicion of impaired EC glycolysis after autophagy suppression was supported (Fig 2). Consistent with this, shear-induced expression of GLUT1, together with increased glucose uptake, cellular ATP production, and extracellular ATP accumulation, was prevented in ECs after Atg3 siRNA. Importantly, shear-induced NO generation in ECs after autophagy suppression could be rescued by GLUT1 overexpression, and congruent with these observations, shear-induced NO production was prevented in control ECs after GLUT1 siRNA. Taken together, we believe these data are the first to indicate that autophagy suppression impairs EC glycolysis.
Our second original finding is that autophagy repression in ECs precludes purinergic mediated eNOS phosphorylation and NO generation. Extracellular nucleotides have important biological roles as signaling molecules that regulate cellular functions under physiological and pathophysiological conditionsreviewed in 29. eNOS is regulated at multiple levels39–42, including via nucleotide activation of purinergic receptors.29, 30, 43, 44 Indeed, shear-stress stimulates the release of ATP in ECs30, 43, 45, 46 and its hydrolysis product ADP is an important ligand of purinergic receptor –mediated eNOS phosphorylation and NO generation.30 Of the P2Y (G-protein coupled receptors) and P2X (calcium channels) receptors located on ECs, the importance of P2Y receptor subtype 1 (P2Y1-receptor) mediated eNOS activation and NO generation has been demonstrated in human umbilical vein ECs30, bovine aortic ECs43, 44, and cerebro microvascular ECs47. Because shear-stress failed to elevate cellular and extracellular ATP levels in ECs with suppressed autophagy, we thought it reasonable to test whether purinergic signaling to eNOS was compromised under these conditions. Pharmacological and genetic interference of the P2Y1 receptor in ECs with intact autophagy phenocopied results from ECs with compromised autophagy concerning shear-induced eNOS activation and NO generation, demonstrating the importance of purinergic signaling in general, and this receptor subtype in particular. Consistent with these results, exogenous ADP restored shear-induced p-eNOSS1177 and NO generation in ECs with suppressed autophagy (Fig 3). These findings are the first to demonstrate that shear-induced purinergic mediated activation of eNOS and resultant NO generation is impaired in ECs with suppressed autophagy.
The third important finding presented here is that PKCδ links purinergic-mediated signaling via the P2Y1 receptor to eNOS activation and NO generation. The PKC family of serine-threonine kinases includes conventional, novel, and atypical isoforms.48 As the obtained data directed our focus toward purinergic signaling, we became aware of evidence from human umbilical vein ECs that shear stress increases extracellular nucleotide-mediated activation of p-eNOSS1177 via phosphorylation of the novel PKC isoform δ at threonine 505 (i.e., p-PKCδT505)30. We observed that shear-stress increases p-PKCδT505 in ECs transfected with scrambled but not Atg3 siRNA, and confirmed that p-PKCδT505 is a downstream target of P2Y1-R mediated signaling. Using a loss-of-function approach, eNOS activation was refractory to shear stress in ECs transfected with PKCδ vs. scrambled siRNA, and in ECs with intact autophagy treated with the PKCδ inhibitor rottlerin. Gain-of-function procedures substantiated these findings. Specifically, when ECs with suppressed autophagy were transfected with CA PKCδ, or treated with a small molecule activator of PKC (i.e., bryostatin-1), p-eNOSS1177 and NO generation were restored in response to shear-stress (Fig 4). The bryostatins are a family of 20 macrolide natural products isolated from the marine bryozoan Bugula neritina.34 The biologically active constituent bryostatin-1 binds to the regulatory C1 domain of the PKC isoforms,49 and clinical trials have investigated the therapeutic utility of bryostatin-1 in the context of neurodegenerative diseases.33, 50 Here we provide evidence that genetic and pharmacological approaches to activate PKC can restore shear-stress mediated eNOS phosphorylation and NO generation in the context of impaired EC autophagy.
EC metabolism is an emerging but understudied therapeutic target. Metabolic abnormalities in ECs have potential to dysregulate vascular function in the context of numerous vascular pathologies. Initiating events that disrupt EC metabolism to an extent that precipitates EC dysfunction in the context of aging51 and diabetes52 are unclear. Here we present solid evidence that impaired autophagy suppresses EC glycolysis, resulting in deficient ATP synthesis and release, and defective purinergic signaling to eNOS via PKCδ. Many nodes of dysregulation in this pathway were revealed in human arterial endothelial cells exposed to shear stress after pharmacological impairment of autophagy (Fig 5), and several are recapitulated in adult mice with inducible disruption of autophagy specifically in ECs (Fig 6). Current studies are investigating the functional role of EC autophagy in preclinical models of aging and diabetes.
Supplementary Material
Highlights.
Inhibiting autophagosome formation in bovine aortic endothelial cells (ECs) diminishes shear stress activation of eNOS and subsequent NO generation, while amplifying inflammation and exaggerating oxidant stress.
Compromised EC autophagy impairs EC glycolysis, ATP production, and P2Y1-R mediated signaling to eNOS via PKCδ, to ultimately attenuate shear-induced NO generation.
This phenotype can be recapitulated in ECs with intact autophagy, and rescued in ECs with genetic repression of autophagy, by manipulating nodes in this signaling cascade.
Key nodes of dysregulation in this pathway upon autophagy compromise were revealed in human arterial endothelial cells after pharmacological suppression of autophagy, and in adult mice with temporal deletion of EC autophagy.
Targeted reactivation of purinergic signaling and/or PKCδ has strategic potential to restore compromised NO generation in pathologies associated with suppressed EC autophagy.
Acknowledgments
Drs. QJ Zhang, E Dale Abel, and BK Kishore are thanked for their scientific input throughout this study. Ms. Diana Lim is thanked for preparing the figures.
Sources of funding: JDS was supported by the American Heart Association (AHA:16GRNT31050004), National Institutes of Health (NIH:RO3AGO52848; 2R15HL091493), American Diabetes Association (ADA: 1-12-BS-208, ADA 7-08-RA-164), and Seed Grants from the UU Office of the Vice-President for Research, the UU College of Health, the UU Center on Aging, the UU Diabetes and Metabolism Center, and the Diabetes Research Center at Washington University at Saint Louis, Grant No. 5 P30 DK020579. SB was supported by NIH 1R01DK098646-01A1 and an AHA Scientist Development Grant. KP was supported by a Postdoctoral Fellowship from the AHA, Western States Affiliate. RR was supported by NIH P01 HL-091830, VA RR&D Merit Grants E6910-R and E1697-R, and VA RR&D SPiRE Grant R1433-P. Student support was provided by the American Physiological Society (APS) Undergraduate Summer Research Program, the APS Undergraduate Research Excellence Fellowship Program, the AHA, Western States Affiliate, Undergraduate Student Summer Research Program, and the UU Undergraduate Research Opportunities Program.
Abbreviations
- 3-MA
3-methyladenine
- ADP
Adenosine diphosphate
- Akt
Protein kinase B
- AMPK
5’ AMP activated protein kinase
- Atg3
Autophagocytosis associated protein 3
- ATP
Adenosine triphosphate
- BAF-A1
Bafilomycin A-1
- Bry
Bryostatin-1
- DAF-FM
4-amino-5-methylamino-2′,7′-difluorofluorescein diacetate
- DCFDA
2’,7’–dichlorofluorescin diacetate
- DEA NONOate
2-(N,N-Diethylamino)-diazenolate-2-oxide diethylammonium salt
- DETC
Sodium diethyldithiocarbamate trihydrate
- EC
Endothelial cell
- ECAR
Extracellular acidification rate
- eNOS
Endothelial nitric oxide synthase
- ERK
Extracellular signal regulated kinase
- FCCP
Carbonyl cyanide-4-(trifluoromethoxy) phenylhydrazone
- GAPDH
Glyceraldehyde 3-phosphate dehydrogenase
- Hsp90
Heat shock protein 90
- ICAM-1
Intracellular adhesion molecule -1
- IL-8
Interleukin-8
- KCN
Potassium cyanide
- LAMP1
Lysosome associated membrane protein1
- LC3
Microtubule-associated protein light chain 3
- L-NMMA
NG-monomethyl L-arginine citrate
- MCP-1
Monocyte chemoattractant protein-1
- MEK
Mitogen activated protein kinase
- 2-Me-ADP
2-(Methylthio) adenosine 5'-diphosphate
- MRS2179
2'-Deoxy-N6-methyladenosine 3',5'-bisphosphate tetrasodium salt
- NAC
N-acetyl cysteine
- NADPH
Nicotinamide adenine dinucleotide phosphate
- NO
Nitric oxide
- NVN
N-vanillynonanamide
- OCR
Oxygen consumption rate
- P2Y1
P2Y purinoceptor 1
- P38MAPK
p38 Mitogen activated protein kinase
- PI3K
Phosphatidylinositol 3-kinase
- PKCδ
Protein Kinase C delta
- PP2A
Protein phosphatase 2A
- ROS
Reactive oxygen species
- T2DM
Type 2 Diabetes mellitus
- TOM20
Translocase of outer membrane 20
- VCAM-1
Vascular adhesion molecule-1
Footnotes
Abstracts of data contained in this manuscript were presented at The Cardiovascular Forum for Promoting Excellence and Young Investigators, 2013; Experimental Biology 2014, 2015, 2016, 2017; Keystone Symposia on Cellular and Molecular Biology 2014, 2015, 2017; Scientific Sessions of the American Diabetes Association 2014.
Disclosures: None
References
- 1.Zhang Y, Janssens SP, Wingler K, Schmidt HH, Moens AL. Modulating endothelial nitric oxide synthase: a new cardiovascular therapeutic strategy. Am J Physiol Heart Circ. Physiol. 2011;301:H634–H646. doi: 10.1152/ajpheart.01315.2010. [DOI] [PubMed] [Google Scholar]
- 2.Triggle CR, Hollenberg M, Anderson TJ, Ding H, Jiang Y, Ceroni L, Wiehler WB, Ng ESM, Ellis A, Andrews K, McGuire JJ, Pannirselvam M. The endothelium in health and disease- a target for therapeutic intervention. J Sm Musc. 2003;39:249–267. doi: 10.1540/jsmr.39.249. [DOI] [PubMed] [Google Scholar]
- 3.Kuma A, Mizushima N. Physiological role of autophagy as an intracellular recycling system: with an emphasis on nutrient metabolism. Semin Cell Dev Biol. 2010;21:683–690. doi: 10.1016/j.semcdb.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Mizushima N. Physiological functions of autophagy. Curr Top Microbiol Immunol. 2009;335:71–84. doi: 10.1007/978-3-642-00302-8_3. [DOI] [PubMed] [Google Scholar]
- 5.Larocca TJ, Henson GD, Thorburn A, Sindler AL, Pierce GL, Seals DR. Translational evidence that impaired autophagy contributes to arterial ageing. J Physiol. 2012;590:3305–3316. doi: 10.1113/jphysiol.2012.229690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fetterman JL, Holbrook M, Flint N, Feng B, Bretomicronn-Romero R, Linder EA, Berk BD, Duess MA, Farb MG, Gokce N, Shirihai OS, Hamburg NM, Vita JA. Restoration of autophagy in endothelial cells from patients with diabetes mellitus improves nitric oxide signaling. Atherosclerosis. 2016;247:207–217. doi: 10.1016/j.atherosclerosis.2016.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bharath LP, Mueller R, Li Y, Ruan T, Kunz D, Goodrich R, Mills T, Deeter L, Sargsyan A, Anandh Babu PV, Graham TE, Symons JD. Impairment of autophagy in endothelial cells prevents shear-stress-induced increases in nitric oxide bioavailability. Can J Physiol Pharmacol. 2014;92:605–612. doi: 10.1139/cjpp-2014-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo F, Li X, Peng J, Tang Y, Yang Q, Liu L, Wang Z, Jiang Z, Xiao M, Ni C, Chen R, Wei D, Wang GX. Autophagy regulates vascular endothelial cell eNOS and ET-1 expression induced by laminar shear stress in an ex vivo perfused system. Ann Biomed Eng. 2014;42:1978–1988. doi: 10.1007/s10439-014-1033-5. [DOI] [PubMed] [Google Scholar]
- 9.Liu J, Bi X, Chen T, Zhang Q, Wang SX, Chiu JJ, Liu GS, Zhang Y, Bu P, Jiang F. Shear stress regulates endothelial cell autophagy via redox regulation and Sirt1 expression. Cell Death Dis. 2015;6:e1827–1837. doi: 10.1038/cddis.2015.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kubli DA, Gustafsson AB. Mitochondria and mitophagy: the yin and yang of cell death control. Circ Res. 2012;111:1208–1221. doi: 10.1161/CIRCRESAHA.112.265819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei Q, Xia Y. Proteasome inhibition down-regulates endothelial nitric-oxide synthase phosphorylation and function. J Biol Chem. 2006;281:21652–21659. doi: 10.1074/jbc.M602105200. [DOI] [PubMed] [Google Scholar]
- 12.Shi Y. Serine/threonine phosphatases: mechanism through structure. Cell. 2009;139:468–484. doi: 10.1016/j.cell.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 13.Park SY, Ives SJ, Gifford JR, Andtbacka RH, Hyngstrom JR, Reese V, Layec G, Bharath LP, Symons JD, Richardson RS. Impact of age on the vasodilatory function of human skeletal muscle feed arteries. Am J Physiol Heart Circ Physiol. 2016;310:H217–H225. doi: 10.1152/ajpheart.00716.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang QJ, McMillin SL, Tanner JM, Palionyte M, Abel ED, Symons JD. Endothelial nitric oxide synthase phosphorylation in treadmill-running mice: role of vascular signalling kinases. J Physiol. 2009;587:3911–3920. doi: 10.1113/jphysiol.2009.172916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Symons JD, McMillin SL, Riehle C, et al. Contribution of insulin and Akt1 signaling to endothelial nitric oxide synthase in the regulation of endothelial function and blood pressure. Circ Res. 2009;104:1085–1094. doi: 10.1161/CIRCRESAHA.108.189316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang QJ, Holland WL, Wilson L, et al. Ceramide mediates vascular dysfunction in diet-induced obesity by PP2A-mediated dephosphorylation of the eNOS-Akt complex. Diabetes. 2012;61:1848–1859. doi: 10.2337/db11-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bharath LP, Ruan T, Li Y, et al. Ceramide-initiated protein phosphatase 2A activation contributes to arterial dysfunction in vivo. Diabetes. 2015;64:3914–3926. doi: 10.2337/db15-0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu Y, Song P, Xu J, Zhang M, Zou MH. Activation of protein phosphatase 2A by palmitate inhibits AMP-activated protein kinase. J Biol Chem. 2007;282:9777–9788. doi: 10.1074/jbc.M608310200. [DOI] [PubMed] [Google Scholar]
- 19.Smith AR, Visioli F, Frei B, Hagen TM. Age-related changes in endothelial nitric oxide synthase phosphorylation and nitric oxide dependent vasodilation: evidence for a novel mechanism involving sphingomyelinase and ceramide-activated phosphatase 2A. Aging Cell. 2006;5:391–400. doi: 10.1111/j.1474-9726.2006.00232.x. [DOI] [PubMed] [Google Scholar]
- 20.Du Y, Kowluru A, Kern TS. PP2A contributes to endothelial death in high glucose: inhibition by benfotiamine. Am J Physiol Regul Integr Comp Physiol. 2010;299:R1610–R1617. doi: 10.1152/ajpregu.00676.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kassan M, Choi SK, Galan M, Lee YH, Trebak M, Matrougui K. Enhanced p22phox expression impairs vascular function through p38 and ERK1/2 MAP kinase-dependent mechanisms in type 2 diabetic mice. Am J Physiol Heart Circ Physiol. 2014;306:H972–H980. doi: 10.1152/ajpheart.00872.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brar SS, Kennedy TP, Whorton AR, Sturrock AB, Huecksteadt TP, Ghio AJ, Hoidal JR. Reactive oxygen species from NAD(P)H:quinone oxidoreductase constitutively activate NF-kappaB in malignant melanoma cells. Am J Physiol Cell Physiol. 2001;280:C659–C676. doi: 10.1152/ajpcell.2001.280.3.C659. [DOI] [PubMed] [Google Scholar]
- 24.Berridge MV, Tan AS. Cell-surface NAD(P)H-oxidase: relationship to trans-plasma membrane NADH-oxidoreductase and a potential source of circulating NADH-oxidase. Antioxid Redox Signal. 2000;2:277–288. doi: 10.1089/ars.2000.2.2-277. [DOI] [PubMed] [Google Scholar]
- 25.Harjes U, Bensaad K, Harris AL. Endothelial cell metabolism and implications for cancer therapy. Br J Cancer. 2012;107:1207–1212. doi: 10.1038/bjc.2012.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dodson M, Darley-Usmar V, Zhang J. Cellular metabolic and autophagic pathways: traffic control by redox signaling. Free Radic Biol Med. 2013;63:207–221. doi: 10.1016/j.freeradbiomed.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bricker DK, Taylor EB, Schell JC, et al. A mitochondrial pyruvate carrier required for pyruvate uptake in yeast, Drosophila, and humans. Science. 2012;337:96–100. doi: 10.1126/science.1218099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burnstock G. Release of vasoactive substances from endothelial cells by shear stress and purinergic mechanosensory transduction. J Anat. 1999;194:335–342. doi: 10.1046/j.1469-7580.1999.19430335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peti-Peterdi J, Kishore BK, Pluznick JL. Regulation of Vascular and Renal Function by Metabolite Receptors. Annu Rev Physiol. 2016;78:391–414. doi: 10.1146/annurev-physiol-021115-105403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.da Silva CG, Specht A, Wegiel B, Ferran C, Kaczmarek E. Mechanism of purinergic activation of endothelial nitric oxide synthase in endothelial cells. Circulation. 2009;119:871–879. doi: 10.1161/CIRCULATIONAHA.108.764571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bultmann R, Tuluc F, Starke K. On the suitability of adenosine 3'-phosphate 5'-phosphosulphate as a selective P2Y receptor antagonist in intact tissues. Eur J Pharmacol. 1998;359:95–101. doi: 10.1016/s0014-2999(98)00600-1. [DOI] [PubMed] [Google Scholar]
- 32.Sud N, Kumar S, Wedgwood S, Black SM. Modulation of PKCdelta signaling alters the shear stress-mediated increases in endothelial nitric oxide synthase transcription: role of STAT3. Am J Physiol Lung Cell Mol Physiol. 2009;296:L519–L526. doi: 10.1152/ajplung.90534.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun MK, Alkon DL. Bryostatin-1: pharmacology and therapeutic potential as a CNS drug. CNS Drug Rev. 2006;12:1–8. doi: 10.1111/j.1527-3458.2006.00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wender PA, Nakagawa Y, Near KE, Staveness D. Computer-guided design, synthesis, and protein kinase C affinity of a new salicylate-based class of bryostatin analogs. Org Lett. 2014;16:5136–5139. doi: 10.1021/ol502491f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lavandero S, Troncoso R, Rothermel BA, Martinet W, Sadoshima J, Hill JA. Cardiovascular autophagy: concepts, controversies, and perspectives. Autophagy. 2013;9:1455–1466. doi: 10.4161/auto.25969. [DOI] [PubMed] [Google Scholar]
- 36.Lavandero S, Chiong M, Rothermel BA, Hill JA. Autophagy in cardiovascular biology. J Clin Invest. 2015;125:55–64. doi: 10.1172/JCI73943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Meyer GR, Grootaert MO, Michiels CF, Kurdi A, Schrijvers DM, Martinet W. Autophagy in vascular disease. Circ Res. 2015;116:468–479. doi: 10.1161/CIRCRESAHA.116.303804. [DOI] [PubMed] [Google Scholar]
- 38.Torisu K, Singh KK, Torisu T, Lovren F, Liu J, Pan Y, Quan A, Ramadan A, Al-Omran M, Pankova N, Boyd SR, Verma S, Finkel T. Intact endothelial autophagy is required to maintain vascular lipid homeostasis. Aging Cell. 2016;15:187–191. doi: 10.1111/acel.12423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sessa WC. eNOS at a glance. J Cell Sci. 2004;117:2427–2429. doi: 10.1242/jcs.01165. [DOI] [PubMed] [Google Scholar]
- 40.Shaul PW. Regulation of endothelial nitric oxide: location, location, location. Annu Rev Physiol. 2002;64:749–774. doi: 10.1146/annurev.physiol.64.081501.155952. [DOI] [PubMed] [Google Scholar]
- 41.Fulton D, Gratton JP, Sessa WC. Post-translational control of endothelial nitric oxide synthase: why isn't calcium/calmodulin enough? J Pharmacol Exp Ther. 2001;299:818–824. [PubMed] [Google Scholar]
- 42.Dudzinski DM, Michel T. Life history of eNOS: partners and pathways. Cardiovasc Res. 2007;75:247–260. doi: 10.1016/j.cardiores.2007.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Andrews AM, Jaron D, Buerk DG, Barbee KA. Shear stress-induced NO production is dependent on ATP autocrine signaling and capacitative calcium entry. Cell Mol Bioeng. 2014;7:510–520. doi: 10.1007/s12195-014-0351-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kirby PL, Buerk DG, Parikh J, Barbee KA, Jaron D. Mathematical model for shear stress dependent NO and adenine nucleotide production from endothelial cells. Nitric Oxide. 2016;52:1–15. doi: 10.1016/j.niox.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bodin P, Burnstock G. Evidence that release of adenosine triphosphate from endothelial cells during increased shear stress is vesicular. J Cardiovasc Pharmacol. 2001;38:900–918. doi: 10.1097/00005344-200112000-00012. [DOI] [PubMed] [Google Scholar]
- 46.Yegutkin G, Bodin P, Burnstock G. Effect of shear stress on the release of soluble ecto-enzymes ATPase and 5'-nucleotidase along with endogenous ATP from vascular endothelial cells. Br J Pharmacol. 2000;129:921–926. doi: 10.1038/sj.bjp.0703136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Webb TE, Feolde E, Vigne P, Neary JT, Runberg A, Frelin C, Barnard EA. The P2Y purinoceptor in rat brain microvascular endothelial cells couple to inhibition of adenylate cyclase. Br J Pharmacol. 1996;119:1385–1392. doi: 10.1111/j.1476-5381.1996.tb16050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parker PJ, Murray-Rust J. PKC at a glance. J Cell Sci. 2004;117:131–132. doi: 10.1242/jcs.00982. [DOI] [PubMed] [Google Scholar]
- 49.Kazanietz MG. Novel "nonkinase" phorbol ester receptors: the C1 domain connection. Mol Pharmacol. 2002;61:759–767. doi: 10.1124/mol.61.4.759. [DOI] [PubMed] [Google Scholar]
- 50.Sun MK, Hongpaisan J, Lim CS, Alkon DL. Bryostatin-1 restores hippocampal synapses and spatial learning and memory in adult fragile × mice. J Pharmacol Exp Ther. 2014;349:393–401. doi: 10.1124/jpet.114.214098. [DOI] [PubMed] [Google Scholar]
- 51.Donato AJ, Morgan RG, Walker AE, Lesniewski LA. Cellular and molecular biology of aging endothelial cells. J Mol Cell Cardiol. 2015;89:122–135. doi: 10.1016/j.yjmcc.2015.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.de Zeeuw P, Wong BW, Carmeliet P. Metabolic adaptations in diabetic endothelial cells. Circ J. 2015;79:934–941. doi: 10.1253/circj.CJ-15-0230. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.