Abstract
Alteration of microbiota has been associated with intestinal, inflammatory, and neurological diseases. Abundance of “good bacteria” such as Bifidobacterium, or their products have been generally believed to be beneficial for any diseases, while “bad bacteria” such as pathogenic Helicobacter pylori are assumed to be always detrimental for hosts. However, this is not the case when we compare and contrast the association of the gut microbiota with two neurological diseases, multiple sclerosis (MS) and Alzheimer’s disease (AD). Following H. pylori infection, pro-inflammatory T helper (Th)1 and Th17 immune response are initially induced to eradicate bacteria. However, H. pylori evades the host immune response by inducing Th2 cells and regulatory T cells (Tregs) that produce anti-inflammatory interleukin (IL)-10. Suppression of anti-bacterial Th1/Th17 cells by Tregs may enhance gastric H. pylori propagation, followed by a cascade reaction involving vitamin B12 and folic acid malabsorption, plasma homocysteine elevation, and reactive oxygen species induction. This can damage the blood-brain barrier (BBB), leading to accumulation of amyloid-β in the brain, a hallmark of AD. On the other hand, this suppression of pro-inflammatory Th1/Th17 responses to H. pylori has protective effects on the hosts, since it prevents uncontrolled gastritis as well as suppresses the induction of encephalitogenic Th1/Th17 cells, which can mediate neuroinflammation in MS. The above scenario may explain why chronic H. pylori infection is positively associated with AD, while it is negatively associated with MS. Lastly, we list “10 pitfalls of microbiota studies”, which will be useful for evaluating and designing clinical and experimental microbiota studies.
Keywords: 16S rRNA sequencing, CNS demyelinating diseases, Experimental autoimmune encephalomyelitis (EAE), Inflammatory bowel diseases (IBD), Theiler’s murine encephalomyelitis virus (TMEV)-induced demyelinating disease (TMEV-IDD)
Graphical abstract
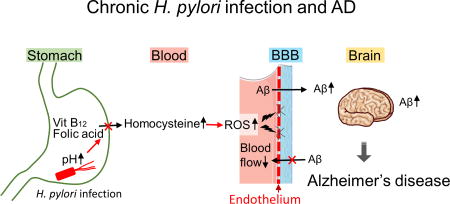
Helicobacter pylori infection increases gastric pH, followed by a cascade reaction involving vitamin B12 and folic acid malabsorption, plasma homocysteine elevation, and reactive oxygen species (ROS) induction. This can damage the blood-brain barrier (BBB), leading to accumulation of amyloid-β in the brain, a hallmark of Alzheimer’s disease (AD).
“The wolf shall live with the lamb,
the leopard shall lie down with the kid,
the calf and the lion and the fatling together,
and a little child shall lead them.” - Isaiah 11.61
Introduction
In the “Peaceable Kingdom” of the Bible, humans co-exist peacefully with carnivorous and herbivorous animals. Although it sounds unrealistic, in the human gut, archaea, bacteria, fungi, parasites, and viruses live peacefully as members of commensal microbes, beyond the “kingdom”, and even beyond the domain. In the classification of life, there are three domains: Bacteria, Archaea, and Eukarya (Eukaryotes),2 while viruses are not included into any domains. Each domain is subdivided into the following ranks: the phylum, class, order, family, genus, and species. In Bible’s Peaceable Kingdom, human belongs to the domain Eukarya, kingdom Animalia, phylum Chordata, class Mammalia, order Primates, while all the other animals also belong to the class Mammalia. Wolf, leopards, and lion belong to the order Carnivora (wolves, family Canidae; leopards and lions, family Felidae), and lamb (sheep) and calf (cattle) belong to the order Artiodactyla, family Bovidae (sheep, genus Ovis; cattle, genus Bos). In addition to mammals, the phylum Chordata includes vertebrata including fish and frog, and non-vertebrata, such as sea urchin and sea anemones.
In humans, the gut microbiota consists of approximately 1,000 species of bacteria, five genera of archaea, 66 genera of fungi, and as yet undetermined families of viruses including bacteriophages.3 Currently, most microbiota studies focused on the community of bacteria (bacteriome), but not the other taxa.4,5 Healthy gut bacteriome mainly consists of two major phyla, Bacteroidetes and Firmicutes, and three minor phyla, Actinobacteria, Proteobacteria, and Verrucomicrobia (Table 1).6,7 The phylum Actinobacteria is a group of mostly Gram-positive bacteria, generally, which consists of 222 genera, such as the genera Actinomyces, Collinsella, and Streptomyces.8 The phylum Bacteroidetes is a group of Gram-negative bacteria, which consists of 128 genera, such as the genera Alistipes, Bacteroides, and Prevotella.9 The phylum Firmicutes is a group of Gram-positive bacteria, which consists of 241 genera, including well-known pathogenic bacteria, such as the genera Bacillus, Clostridium, Staphylococcus, and Streptococcus.10 The phylum Proteobacteria is the largest group of Gram-negative bacteria, which consists of 452 genera that include a variety of pathogenic bacteria, such as the genera Brucella, Escherichia, Helicobacter, and Salmonella.11 The phylum Verrucomicrobia is a group of Gram-negative bacteria with wart-like prosthecae, which consists of 12 genera, including the genus Akkermansia.12
Table 1.
Classification of bacteria associated with MS and its animal models
| Phylum | Class | Order | Family | Genus | Species |
|---|---|---|---|---|---|
| Actinobacteria | Coriobacteriia | Coriobacteriales | Coriobacteriaceae | Adlercreutzia, Collinsella | |

|
|||||
| Bacteroidetes | Bacteroidia | Bacteroidales | Bacteroidaceae | Bacteroides | fragilis |

|
Porphyromonadaceae | Butyricimonas, Parabacteroides | |||
| Prevotellaceae | Alloprevotella | ||||
| Prevotella | copri | ||||
| Rikenellaceae | Alistipes | ||||
| S24-7 | |||||
| Firmicutes | Bacilli | Lactobacillales | Streptococcaceae | Streptococcus | |

|
Clostridia | Clostridiales | Segmented filamentous bacteria | ||
| Christensenellaceae | |||||
| Clostridiaceae | Clostridium | perfringens | |||
| Eubacteriaceae | Eubacterium | ||||
| Lachnospiraceae | Anaerostipes, Blautia | ||||
| Ruminococcaceae | Anaerotruncus, Faecalibacterium | ||||
| Proteobacteria | α-proteobacteria | Rhizobiales | Brucellaceae | Mycoplana | |

|
β-proteobacteria | Burkholderiales | Oxalobacteraceae | Undibacterium | oligocarboniphilum |
| δ-proteobacteria | Desulfovibrionales | Desulfovibrionaceae | Bilophila, Desulfovibrio | ||
| ε-proteobacteria | Campylobacterales | Helicobacteraceae | Helicobacter | pylori | |
| γ-proteobacteria | Enterobacteriales | Enterobacteriaceae | |||
| Pasteurellales | Pasteurellaceae | Haemophilus | |||
| Pseudomonadales | Pseudomonadaceae | Pseudomonas | |||
| Verrucomicrobia | Verrucomicrobiae | Verrucomicrobiales | Akkermansiaceae | Akkermansia | |

|
|||||
Phyla Actinobacteria and Firmicutes are Gram-positive, while phyla Bacteroidetes Proteobacteria, and Verrucomicrobia are Gram-negative.
Since the introduction of next generation sequencing, there is a growing number of studies on the association between microbiota and diseases.13 In this review article, we will first introduce how gut microbiota changes have been associated with a variety of diseases, and move on to discuss the role of gut microbiota in multiple sclerosis (MS), its related neuromyelitis optica (NMO) and animal models. Then, we will propose the contrasting roles of Helicobacter pylori infection between MS versus Alzheimer’s disease (AD). Lastly, we will list “10 pitfalls of microbiota studies” for microbiota study design and evaluation.
Microbiota changes and diseases
“Dysbiosis”, an altered state of the bacterial community, has been associated with health conditions and diseases. While antibiotics are used to treat bacterial infections in humans and animals,14 continuous use of broad-spectrum antibiotics can induce changes of the gut microbiota.15 Antibiotics treatment decreases native bacterial species and disrupts the bacterial interactions, which potentially leads to the growth of harmful species, such as Clostridium difficile,16–18 resulting in antibiotic-associated diarrhea (AAD).16 “Probiotics” containing Lactobacillus species, such as L. reuteri, which naturally inhabits the mammalian gut, has been clinically tried to prevent AAD.19
The gut microbiota has been shown to play a crucial role in induction of several immune components, such as T helper (Th)1720 and mucosal-associated invariant T (MAIT) cells.21,22 Thus, changes in the gut microbiota have been associated with inflammatory diseases, particularly in the gastrointestinal tract (Table 2).23 Inflammatory bowel diseases (IBD) have been considered to reflect interactions between microbes and the host;24 changes in the gut microbiota has been reported in both ulcerative colitis and Crohn’s disease.25,26 Early childhood exposure to antibiotics is associated with an increased risk for Crohn’s disease in which microbial diversity is diminished.27,28 Necrotizing enterocolitis (NEC) is another disease associated with the alteration of gut microbiota, although the precise pathogenesis is unclear. NEC is primarily seen in premature infants,29,30 whose clinical signs include feeding intolerance, increased gastric residuals, abdominal distension, and bloody stools.31 Microbiome studies showed increased relative abundance of the phylum Proteobacteria, and decreased phyla Firmicutes and Bacteroidetes.32 L. reuteri supplementation may reduce the risk of NEC.33
Table 2.
Diseases associated with gut microbiota
| Disease | Microbiota association | References |
|---|---|---|
| Antibiotic-associated diarrhea (AAD) | Clostridium difficile (pF)↑ Lactobacillus reuteri (pF) therapy | 16,23 |
| Inflammatory bowel disease (IBD): ulcerative colitis and Crohn’s disease | Patient-specific fecal microbiota changes Species: Escherichia coli (pP)↑, Proteus vulgaris (pP)↑, Enterobacter cowanii (pP)↑, Serratia marcescens (pP)↑, Candida tropicalis (kF)↑ | 25–27,138 |
| Necrotizing enterocolitis (NEC) | Lactobacillus reuteri (pF) therapy Phylum: Proteobacteria↑, Firmicutes↓, Bacteroidetes↓ | 29,30,33 |
| Extra-intestinal diseases | Liver diseases, atopic diseases, diabetes mellitus, rheumatoid arthritis, multiple sclerosis, and/or Alzheimer’s disease may be influenced by antibiotics treatment, microbiota changes, or Helicobacter pylori (pP) infection | 34–42 |
Abbreviations: kF, kingdom Fungi; pF, phylum Firmicutes; pP, phylum Proteobacteria
Changes of the gut microbiota have also been suggested to affect distant anatomical sites. Representative extra-intestinal diseases, which have been associated with the gut microbiota, are listed in Table 2, including liver diseases,34–37 atopic diseases,38 diabetes mellitus (DM),39–41 rheumatoid arthritis,42 MS, and AD.
Gut microbiota in MS and its animal models
Gut microbiota in MS
MS is an inflammatory demyelinating disease in the central nervous system (CNS).43 Although the precise pathomechanism is unclear, autoimmunity, genetic background, and environmental factors, such as infections and latitude, appear to contribute to disease onset and exacerbation.44 Among environmental factors, the gut microbiota has also been proposed to be associated with the pathogenesis of MS.13,45,46 In high-income countries, lifestyle westernization, including food, water, and sanitation, has decreased several infectious diseases, such as viral hepatitis, and helminth infestations, while chronic inflammatory and autoimmune diseases, including MS and IBD, have been increased.47 Particularly, “western diet”, rich in fat and salt, has been associated with the increased incidence of MS and IBD.48,49 Fatty acids as well as sodium chloride (NaCl) have been shown to increase Th 17 cells, decrease regulatory T cells (Tregs), and exacerbate an animal model for MS.
Changes of the gut microbiota have been investigated in MS, by sequencing 16S ribosomal (r) RNA that is encoded in bacteria and archaea, but in neither fungi nor viruses.45 Case-control studies demonstrated that the microbiome of MS patients differs from that of controls, although it is unknown whether the altered microbiota is a cause or result of development of MS (Table 3). In MS, reproducible changes of microbial taxa are limited, partly because each study often analyzed microbiome at different taxonomic ranks. For example, some studies indicated the changes at the phylum and genus levels, while most studies showed the data neither at the order nor class level; the data in each study sometimes are incomparable. At the phylum level, Miyake et al.50 reported decreased abundance of the phyla Firmicutes (e.g., genera Faecalibacterium and Anaerostipes) and Bacteroidetes (e.g., genus Prevotella) in the fecal microbiome of relapsing-remitting (RR)-MS patients, compared with healthy controls (HC). Inconsistent with Miyake’s findings, Chen et al. reported decreased phyla Bacteroidetes (e.g., genera Parabacteroides and Prevotella) and Actinobacteria (e.g., genera Adlercreutzia and Collinsella) as well as increased phyla Firmicutes (e.g., genus Blautia) and Proteobacteria (e.g., genera Pseudomonas, Mycoplana, and Haemophilus) in RR-MS patients, compared with HC.51 On the other hand, in pediatric RR-MS, Tremlett et al.52 reported an increase in the phylum Actinobacteria, but not in the other phyla.
Table 3.
Microbiota in MS and its animal models
| Demyelinating diseases | References | |
|---|---|---|
| Multiple sclerosis (MS) | ||
| • | Phylum level: Actinobacteria (pA)↑↓, Bacteroidetes (pB)↓, Firmicutes (pF)↑↓, Proteobacteria (pP)↑↓, Verrucomicrobia (pV)↑ | 50–54 |
| Family level: Coriobacteriaceae (pA)↓, Bacteroidaceae (pB)↓, S24-7 (pB)↓, Christensenellaceae (pF)↑, Lachnospiraceae (pF)↓, Ruminococcaceae (pF)↓, Desulfovibrionaceae (pP)↑, Enterobacteriaceae (pP)↑, Helicobacteraceae (pP)↓, Akkermansiaceae (pV)↑ | ||
| Genus level: Adlercreutzia(pA)↓, Collinsella (pA)↓, Butyricimonas (pB)↓, Parabacteroides (pB)↓, Prevotella (pB)↓, Blautia (pF)↑, Haemophilis (pP)↑, Helicobacter (pP)↓, Mycoplana (pP)↑, Pseudomonas (pP)↑, Akkermansia (pV)↑ | ||
| Species level: Clostridium perfringens (pF)↓, Helicobacter pylori (pP)↓ | ||
| Experimental autoimmune encephalomyelitis (EAE) | ||
| • | Members of the phyla Firmicutes (pF) and Proteobacteria (pP) were increased in rats, but not in mice | 67,68 |
| • | High fat diet reduced members of families Prevotellaceae (pB) and S24-7 (pB) | 68 |
| • | SPF mice developed more severe EAE than GF mice | 62,66 |
| • | Oral antibiotic treatment suppressed EAE | 64 |
| • | SPF MOG-TCR Tg mice developed spontaneous EAE, while GF MOG-TCR Tg mice did not develop EAE | 66 |
| • | Polysaccharide A derived from Bacteroides fragilis (pB) suppressed EAE | 63 |
| Theiler’s murine encephalomyelitis virus-induced demyelinating disease (TMEV-IDD) | ||
| • | Phylum level: Bacteroidetes (pB)↑↓, Firmicutes (pF)↑↓ | 82 |
| Family level: Rikenellaceae (pB)↑, Eubacteriaceae (pF)↑, Streptococcaceae (pF)↓ | ||
| Genus level: Alistipes (pB)↑, Eubacterium (pF)↑, Streptococcus (pF)↓ | ||
| • | Oral antibiotic treatment did not influence demyelination | 82 |
Abbreviations: MS, multiple sclerosis; pA, phylum Actinobacteria; pB, phylum Bacteroidetes; pF, phylum Firmicutes; pP, phylum Proteobacteria; pV, phylum Verrucomicrobia; SPF, specific pathogen-free; GF, germ-free; MOG-TCR Tg, myelin oligodendrocyte glycoprotein-specific T cell receptor transgenic
At the genus level, Jangi et al.53 reported a significant increase of the genus Akkermansia (phylum Verrucomicrobia) and a decrease of the genus Butyricimonas (phylum Bacteroidetes) in RR-MS patients. At the species level, Rumah et al.54 reported unexpectedly that Clostridium perfringens type A (phylum Firmicutes) was present in 23% of MS patients, compared with 53% of healthy controls, while C. perfringens type B (natural host, ruminant animals) was detected in one MS patient. Since C. perfringens type A can cause food poisoning and gas gangrene, the reduction of such a potential pathogenic bacterium in MS patients is intriguing.
Gut microbiota in NMO
Zamvil’s group has proposed that the gut microbiota is also associated with NMO.55,56 They found that T cells from NMO patients responded to aquaporin (AQP) 4 peptide, p63–76, greater than those from HC. Since p63–76 contains “10 residues with 90% homology” to a sequence p204–217 within C. perfringens adenosine triphosphate-binding cassette (ABC) transporter permease (TP), the authors suggested a potential pathogenic role of Clostridium species in NMO. Here, it should be noted that the “90% homology” is between the AQP4 p66–75 and ABC-TP p207–217, neither of which is T cell epitope but only a portion of the epitope. Since 1) the real homology between AQP4 p63–76 and ABC-TP p204–217 is only 64% (9 of 14) and 2) there is no evidence that C. perfringens infection induces T cell responses to ABC-TP, it is unlikely that the immune response to C. perfringens could lead to generation of cross-reactive responses to AQP4. In addition, while the authors reported “a robust proliferative T-cell response to p61–80 in all 15 NMO patients, Matsuya et al.57 found that only one in 12 NMO patients had the p61–80-specific T cell proliferative response. More recently, Zamvil’s group analyzed the gut microbiome in NMO, comparing with HC and MS samples, and detected 42 operational taxonomic units (OTUs) which were differentially detected only between NMO versus HC, not MS versus HC.58 Among 42 OTUs, Enterobacteriaceae of unknown species (4.08-fold) and Prevotella copri (0.11-fold) were the most and least abundant compared with HC, while C. perfringens was only 1.12-fold more abundant than HC (its P value was the second least, though).
Gut microbiota in EAE
Experimental autoimmune encephalomyelitis (EAE) is an autoimmune model for MS. EAE can be induced by sensitization with myelin components, such as myelin oligodendrocyte glycoprotein (MOG) and myelin proteolipid protein (PLP).59 The presence of the gut microbiota has been shown to affect EAE induction. In wild-type C57BL/6 mice sensitized with MOG, germ-free mice showed less severe EAE than specific pathogen-free (SPF) mice, while germ-free mice transplanted with Th17-cell-inducing segmented filamentous bacteria (SFB) (phylum Firmicutes, order Clostridiales, strong similarity with the genus Clostridium)60,61 were more susceptible to EAE than control germ-free mice.62 Ochoa-Rapáraz et al.63 demonstrated that polysaccharide A derived from Bacteroides fragilis suppressed EAE. Oral antibiotics administration in C57BL/6 mice prior to EAE induction reduced the clinical signs by enhancing interleukin (IL)-10 production from B cells.64 In transgenic SJL/J mice expressing MOG-specific T cell receptor on CD4+ T cells,65 gut commensal microbiota is required for induction of spontaneous EAE, although germ-free wild-type SJL/J mice sensitized with MOG showed only delayed onset compared with SPF wild-type mice.66
Stanisavljević et al.67 reported that some members of the phylum Firmicutes and Undibacterium oligocarboniphilum (phylum Proteobacteria) were increased in feces of rats with EAE. On the other hand, Haghikia et al.68 demonstrated that EAE itself did not alter the microbiome, while high fat diet exacerbated EAE with a reduction of the families Prevotellaceae and S24-7 (proposed family name is “Candidatus Homeothermaceae”69) of the phylum Bacteroidetes in feces of C57BL/6 mice. Although the precise pathophysiology of how fatty acids together with microbiota could contribute to CNS inflammation in EAE is unclear, these results are intriguing since 1) some fatty acids are generated in the gut as fermentation products of dietary fibers by commensal bacteria,70,71 and 2) MS-like CNS inflammation is induced in X-linked adrenoleukodystrophy, whose principal biochemical alteration is the accumulation of very long-chain fatty acids.72
Gut microbiota, viral infections, and a viral model for MS
In viral infections, the gut microbiota has been shown to promote viral replication. Kuss et al.73 showed that the intestinal microbiota can promote enteric replication of poliovirus (order Picornavirales, family Picornaviridae, genus Enterovirus), since orally antibiotic-treated mice had lower susceptibility to poliovirus-induced disease with decreased viral replication. Since poliovirus can bind certain bacterial lipopolysaccharide (LPS) and peptidoglycan, the interactions may enhance the infectivity of poliovirus, although the exact pathomechanism remains unclear. Kane et al.74 showed that LPS from the gut microbiota was a key factor for successful transmission of mouse mammary tumor virus (MMTV, family Retroviridae, genus Betaretrovirus) from mother to offspring mice, since antibiotics-treatment of the mother prevented the viral transmission. The LPS-bound MMTV activated dendritic cells (DCs) and macrophages via toll-like receptor (TLR) 4, which induced IL-10. The production of IL-10 may inhibit anti-viral immune responses, resulting in the successful viral transmission. Jones et al.75 demonstrated that histo-blood group antigens (HBGAs) derived from enteric bacteria were required for effective norovirus infection in B cells. In an in vitro infection model of human norovirus (family Caliciviridae, genus Norovirus), viral replication in B cells was higher in the presence of HBGAs than in the absence of HBGAs by enhancing the attachment to B cells. Furthermore, in an in vivo model of mouse norovirus, antibiotic-treated mice had significantly lower viral titers in the intestine compared with the controls.
Theiler’s murine encephalomyelitis virus (TMEV, family Picornaviridae, genus Cardiovirus) has been used to induce a viral model for MS.76–80 Since TMEV is a natural enteric pathogen in mice, the virus can infect the intestine.81 Carrillo-Salinas et al.82 monitored the changes in the gut microbiome in TMEV-infected SJL/J mice, where relative abundances of bacteria differed significantly compared with uninfected control mice at the phylum and genus levels. The oral administration of antibiotics of broad spectrum depleted the gut microbiota and enhanced viral replication in the CNS with 50% mortality (TMEV infection alone did not kill any mice), while no clinical or histological effects were observed during the chronic phase. Since the numbers of CD4+ and CD8+ T cells decreased in the cervical and mesenteric lymph nodes and the CNS, the depletion of the gut microbiota seemed to suppress anti-viral immunity, resulting in fatal acute viral infection, although anti-virus specific immune responses were not investigated in this study.
Helicobacter pylori infection in MS and AD
H. pylori infection in gastric and extra-gastric diseases
In the above section, it is intriguing that the presence of a potential pathogenic C. perfringens type A in feces was lower in MS than in HC. Similar negative association between a pathogen and MS has been reported in Helicobacter pylori infection. H. pylori is a spiral-shaped, flagellated, highly motile Gram-negative bacterium that selectively colonizes the human stomach (Fig. 1).83,84 H. pylori belongs to the phylum Proteobacteria, class Epsilonproteobacteria, order Campylobacterales, family Helicobacteraceae, genus Helicobacter. H. pylori infects approximately 50% of the world’s population, and its persistent infection in the gastric mucosa is etiologically associated with peptic ulcer, chronic gastritis, gastric adenocarcinoma, and gastric mucosa-associated lymphoid tissue (MALT) lymphoma.85–87 The standard therapy for eradication of H. pylori is treatment with a proton pump inhibitor and antibiotics, such as amoxicillin, clarithromycin and metronidazole.87,88 H. pylori eradication has been shown to reduce the incidence of gastric cancer.89,90 Mice and Mongolian gerbils are often used for H. pylori infection studies.91–93 Although H. pylori Sydney strain 1 (SS1) chronically infects mice and induces antibodies against H. pylori, infected mice showed only mild clinical signs and inflammation without development of gastric cancer. On the other hand, H. pylori-infected Mongolian gerbils had severe inflammation in the stomach and developed gastric cancer.92 Mouse and gerbil models reflect asymptomatic infection and symptomatic gastritis in humans, respectively.
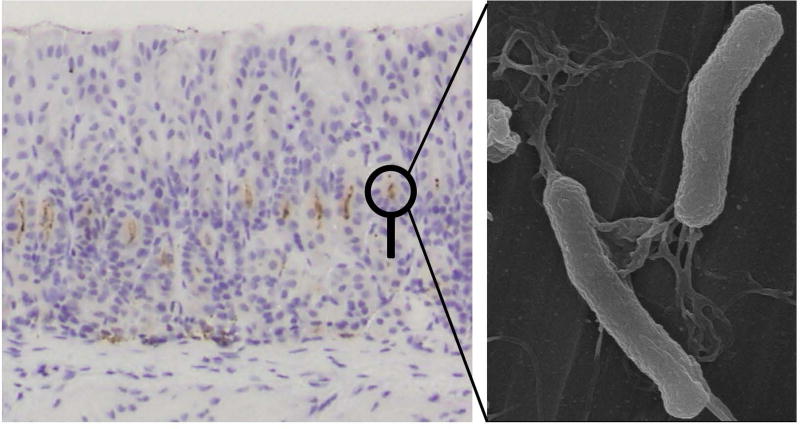
Figure 1.
Chronically Helicobacter pylori infected mouse stomach and electrion microscopic image of H. pylori. (Left) Light micrograph of H. pylori detected by immunohistochemistry (Thermo Fisher Scientific, Fremont, CA) (brown dots) in the stomach of CD1 mouse, 6 months after H. pylori inoculation. Note that inflammation and tissue damage were not detected despite the presence of H. pylori. (Right) Electron micrograph of H. pylori with the spiral shape and multiple flagella. Image was taken by an ultra-high resolution scanning electron microscope S-900 (Hitachi, Ibaraki, Japan) at Kindai University Faculty of Medicine (Osaka, Japan).
H. pylori infection has also been associated with extra-gastric diseases. Idiopathic thrombocytopenic purpura (ITP) is a well-known disease associated with H. pylori infection;94 H. pylori eradication results in a significant increase in the platelet counts in ITP patients. Although a link is not as strong as that of ITP, H. pylori infection has also been associated with cardiovascular diseases (CVD), immune-mediated diseases, and neurological diseases. Similar to ITP, H. pylori infection appears to increase the risk of CVD,95 DM,96 and AD.97–99 In contrast, H. pylori infection seems to decrease the risk of asthma,100–102 IBD,103 and MS.104–106 Thus, H. pylori infection may play contrasting roles in two neurological diseases: a detrimental role for AD and a protective role for MS.
H. pylori infection is a protective factor against MS
Since 2007, Kira’s group has demonstrated that H. pylori seropositivity rates are lower in MS than in controls in Japan.44,107,108 In the UK, the seroprevalence of H. pylori was half in MS patients compared with that of HC,106 while Pedrini et al.105 found that H. pylori seropositivity was lower in female patients, but not in male patients, compared with controls, using 550 Caucasian MS serum samples in Australia. Jaruvongvanich et al.104 conducted meta-analysis of six observational studies from Japan, China, Iran, Greece, India, and Australia, involving 1902 participants. They demonstrated a significant lower prevalence of H. pylori infection in MS patients. On the other hand, in NMO, H. pylori seropositivity rates were significantly higher than controls.109 Furthermore, H. pylori seropositivity was significantly higher in AQP4 antibody-positive patients than in AQP4 antibody-negative patients.110
Experimentally, Cook et al.106 tested whether infection of live H. pylori could affect EAE. C57BL/6 mice were infected orally with 1 × 109 colony-forming units (CFU) of the SS1 strain of H. pylori every third days and sensitized with MOG for EAE induction, 3 weeks after H. pylori infection. H. pylori-infected mice exhibited lower clinical signs with decreased levels of MOG-specific lymphoproliferation and reduced frequencies of Th1 and Th17 cells in the CNS and spleen, compared with the controls. Thus, H pylori infection could ameliorate EAE by regulating immune responses to MOG. In addition, flow cytometric analyses of spleen CD4+ cells showed decreased frequencies of Th1 and Th17 cells as well as interferon (IFN)-γ and IL-17 producing cells (following PMA/ionomycin incubation111) in infected mice, suggesting that general immune responses were also changed by H. pylori infection. Boziki et al.112 examined the effects of inactivated H. pylori in EAE. C57BL/6 mice developed EAE by the standard approach, sensitization with MOG emulsified in incomplete Freund’s adjuvant (IFA) containing inactivated Mycobacterium tuberculosis (known as complete Freund’s adjuvant, CFA). In contrast, sensitization with MOG emulsified in IFA containing inactivated H. pylori failed to induce EAE in C57BL/6 mice.91 Thus, inactivated H. pylori may not have adjuvant effects.
Although these results are consistent with clinical seroprevalence of H. pylori in MS, the experimental setting may not reproduce H. pylori infection in humans. Generally, human H. pylori infection is established in their childhood by 4-years old,113,114 while the environmental factors during early life have been proposed to affect MS susceptibility. In Cook’s study,106 mice were sensitized with MOG only 3 weeks after H. pylori infection. At this early phase of H. pylori infection, pro-inflammatory Th1 responses have been shown to function as a major effector cell.115 During the chronic phase, anti-inflammatory Treg/Th2 responses become predominant with production of IL-10. It will be intriguing to test how chronic H. pylori infection affects EAE, for example, by transferring encephalitogenic T cells into chronically infected mice (passive EAE), which may provide clinically relevant information about the association between H. pylori infection and MS.
H. pylori infection is associated with AD progression
AD is a progressive neurodegenerative disorder that is the most common form of dementia. The two histological features that define AD are neurofibrillary tangles and extracellular β-amyloid peptide (Aβ) deposits within senile plaques in the CNS. Unlike MS, H. pylori infection has been positively associated with AD. Significantly high prevalence of H. pylori infection in AD patients has been reported in Europe and East Asia except Japan.97–99,116
To evaluate the effect of H. pylori eradication on the progression of AD, Chang et al. analyzed the data of patients who diagnosed of AD and peptic ulcer with (n=675) or without (n=863) H. pylori eradication, in which AD patients received triple or quadruple therapy with proton pump inhibitor or H2 receptor blocker, antibiotics (clarithromycin, metronidazole, amoxicillin, or tetracycline) or with bismuth (83Bi). Compared with no H. pylori eradication, H. pylori eradication was associated with a decreased risk of AD progression. Interestingly, in this study, there were significantly lower comorbidities of CVD and DM in AD patients with H. pylori eradication than those with no H. pylori eradication. Although no animal research has been conducted to investigate the association between H. pylori infection and AD, mouse models of AD117 will be useful to clarify the role of H. pylori infection in AD.
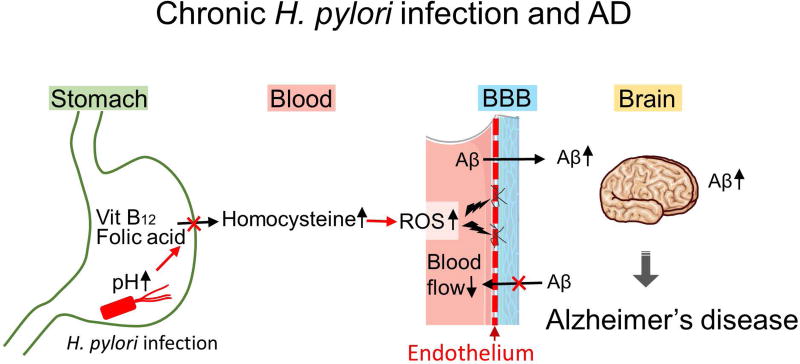
Blood-brain barrier (BBB) breakdown is one of characteristics of neuroimaging and neuropathology of MS, which can be visualized by gadolinium enhancement MRI or albumin and immunoglobulin (Ig) immunostaining of brain sections. Although such substantial BBB breakdown is not seen in AD, dysfunction of BBB has been demonstrated in AD and its animal models.118 Since BBB restricts the transport of peptides from the periphery to the brain, BBB dysfunction can lead to accumulation of peripheral Aβ in the brain and/or decreased clearance of brain Aβ. We hypothesize the mechanism by which H. pylori infection leads to dysfunction of BBB (Fig. 2). First, chronic H. pylori infection increases pH in the stomach due to the parietal cell loss caused by atrophic gastritis and intestinal metaplasia.119–121 The pH change decreases the absorption of vitamin B12 and folic acid, which increases homocysteine in the blood. Homocysteine is a metabolic intermediate of methionine, while vitamin B12 and folic acid metabolite (N5-methyltetrahydrofolate) function as coenzymes when homocysteine is recycled into methionine or converted into cysteine. Thus, the deficiencies of vitamin B12 and folic acid increase the blood homocysteine level. Auto-oxidation of homocysteine generates hydrogen peroxide, which damages vascular endothelial cells, a component of BBB;122 homocysteine-induced endothelial toxicity has been demonstrated in isolated aorta and endothelia cells.123 Then, subsequent BBB dysfunction and blood flow decrease caused by the high homocysteine level in the blood result in increased Aβ accumulation.124 The high serum homocysteine level has been proposed to be a risk factor of AD and vascular diseases.125,126 In addition, as described above, H. pylori infection is associated with increased comorbidities of CVD and DM, both of which can also cause BBB dysfunction.118
Figure 2.
H. pylori infection may contribute to progression of Alzheimer’s disease (AD). Chronic H. pylori infection has been known to increase gastric pH. This pH change decreases absorption of vitamin B12 and folic acid absorption, while it increases the blood homocysteine level. Auto-oxidation of homocysteine generates reactive oxygen species (ROS), which damages vascular endothelial cells, leading to blood-brain barrier (BBB) dysfunction and blood flow reduction. BBB dysfunction can not only increase the accumulation of amyloid-β (Aβ) from the periphery, but also decrease the clearance of Aβ from the brain, contributing to progression of AD.
Distinct roles of H. pylori between MS versus AD
Although the gut microbiome has not been investigated in AD, some infections with bacteria, including spirochetes (Borrelia burgdorferi and Treponema pallidum127,128) and Chlamydophila pneumoniae,129,130 have been associated with AD. Furthermore, Chlamydophila pneumoniae detection in brain tissues using a PCR method revealed that 74% of AD patients were positive while that of controls was 11%.130 Using an AD model, APPSWE/PS1ΔE9 mice, Minter et al.131 demonstrated reduced amyloid plaque deposition with significant changes in the gut microbiome by long-term treatment with a cocktail of eight antibiotics for the duration of 6-month lifespan. They suggested that gut microbiota diversity may impact Aβ deposition.
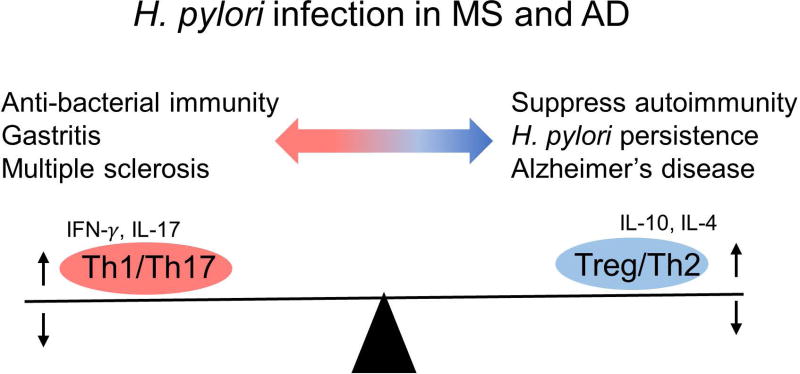
While the beneficial effect of antibiotics treatment on the murine AD model is similar to that on EAE models, why the effect of H. pylori infection on MS and AD is opposite (Table 4)? There are two possible factors contributing to the distinct roles of H. pylori infection in the two neurological diseases. First, in H pylori infection, higher gastric inflammation correlates with lower bacterial loads. H. pylori can control both innate and acquired immune responses in the hosts. H. pylori has been shown to activate, manipulate, and evade pathogen recognition receptors (PRRs), such as TLRs and C-type lectin receptors, on DCs. If H. pylori activates pro-inflammatory genes and cytokines via PRRs on DCs, the DCs induce anti-bacterial Th1/Th17 responses that contribute to eradication of H. pylori.132,133 However, uncontrolled Th1/Th17 responses could induce immune-mediated gastritis (immunopathology).86 In contrast, if H. pylori induces anti-inflammatory genes and cytokines by manipulating PRR pathways in DCs, the DCs induce anti-bacterial Treg responses with anti-inflammatory cytokine IL-10 production. IL-10 suppresses anti-bacterial Th1/Th17 responses, facilitating H. pylori persistence. However, this immunosuppression is protective for hosts since it prevents gastritis. Thus, Tregs act as a double-edged sword in H. pylori infection (Fig. 3).79
Table 4.
Microbial and immune responses of multiple sclerosis and Alzheimer’s disease
| Multiple sclerosis | Alzheimer's disease | |
|---|---|---|
| Microbiota dysbiosis | + | ? |
| H. pylori infection | protection | disease progression |
| T cell infiltration | +++ | − |
| Immune response | Th1/Th17 | innate |
| Vascular/BBB dysfunction | +++ | ++ |
Abbreviations: H. pylori, Helicobacter pylori; BBB, blood brain barrier; Th, T helper
Figure 3.
H. pylori infection in multiple sclerosis (MS) and Alzheimer’s disease (AD). Chronic H. pylori infection changes the T helper (Th) cell subset balance toward Treg/Th2 responses, suppressing MS and gastritis, both of which are mediated by pro-inflammatory Th1/Th17 responses. On the other hand, increased Treg/Th2 responses can suppress anti-bacterial Th1/Th17 immunity, which leads to persistent H. pylori infection, resulting in BBB dysfunction and AD progression (see Fig. 2).
Second, although both MS and AD have often been described as CNS diseases with “neuroinflammation”,134 substantial perivascular T cell infiltration is seen in MS, but not in AD. While microglia and astrocytes (resident innate cells) are activated in both MS and AD, pro-inflammatory peripheral cellular immune responses, particularly Th1 and Th17 cells, contribute to the pathogenesis only in MS. Thus, increased Treg/Th2 response in individuals with persistent H. pylori infection can suppress encephalitogenic Th1/Th17, protecting from MS. On the other hand, although Th2 cells help antibody production, anti-H. pylori antibody has no role in eliminating this bacterium; suppression of cellular Th1/Th17 immunity leads to propagation of H. pylori, which subsequently leads to BBB dysfunction and AD progression as discussed above. Similarly, H. pylori-induced exacerbation of NMO can be explained by enhancement of humoral immunity, i.e. enhanced production of anti-AQP4 antibody.
Are the effects of H. pylori infection in MS and AD accompanied with changes of the gut microbiota? It is controversial whether H. pylori infection affects the gut microbiota. Although a higher gastric pH of infected humans can increase the number and diversity of gastric microbiota,135 H. pylori infection has been reported to cause no or little effect on gastric microbiota in most human or animal studies.136 Recently, however, Kienesberger et al. reported that gastric H. pylori infection altered the gastric and intestinal microbiota in mice.137
“10 pitfalls of microbiota studies”
Gut microbiota studies have a number of potential pitfalls. In the last section of this review, we have listed the potential “10 pitfalls of microbiota studies”, which will be helpful in evaluating and planning the microbiota study (Table 5).
Table 5.
10 pitfalls in microbiota studies
| Pitfalls | References |
|---|---|
| 1. The term ”microbiota” does not include fungi or viruses | 138,139,142 |
| 2. Inappropriate usage of microbial taxonomy / classification | 143,144 |
| 3. The ratio of fecal bacterial taxa underrepresents the gut microbiota | 145–147 |
| 4. Microbiota changes can be the cause or outcome of disease | 3 |
| 5. Discrepancy between microbiota studies versus PID | 148–152 |
| 6. Microbiota influenced by age, gender, and country | 3,138,153,154 |
| 7. Probiotics/prebiotics are not always beneficial for hosts | 156,157 |
| 8. Effect of antibiotics treatment on systemic microbiota and immune system | 21,82,158,160–163,165 |
| 9. FMT methodology and safety | 167–170 |
| 10. Tailor-made gut microbiota therapy | 171–173 |
Abbreviations: FMT, fecal microbiome transplantation; PID, primary immunodeficiency diseases; and Treg, regulatory T cells
1. The term “microbiota” does not include fungi, viruses, and parasites
Although the term “microbiota” should include bacteria, archaea, fungi, viruses, protozoa, and helminths, the majority of the microbiota studies focused on the bacterial community (bacteriome), sequencing conserved 16S rRNAs, but not on the fungal (mycobiome) or viral (virome) community. Bacteriome and mycobiome studies demonstrated the significant intrakingdom and interkingdom microbial correlations.138 In addition, the association between MS and Candida species (kingdom Fungi) has been reported.139 The virome represents the viral component of the microbiome, which includes viruses infecting not only the hosts but also bacteria (bacteriophages). Currently, the virome analyses still need establishment of a standard pipeline for the sample preparation and sequence analyses, since 1) the factors involving the sample preparation,140 such as centrifugation, temperature, and filtration, for viruses are different from those for bacteria: e.g., the standard sample preparation protocols for the gut bacteriome is good to harvest bacteriophages localized in bacterial cell bodies, but bad for cell-free virions,141 2) viruses lack universally conserved genomic regions, and 3) single reference viral genome database containing all eukaryotic DNA/RNA viruses and bacteriophage is not available for identification of viruses in the virome.142
2. Inappropriate usage of microbial taxonomy / classification
In most gut microbiota studies, it is obvious that some researchers do not pay attention to the bacterial taxonomy or classification system. As we discussed in the introduction, there are taxonomic ranks classifying the bacteria in the following order; phylum, class, order, family, genus, and species. However, it is not unusual even in the top journal articles, discussing the phylum and genus levels indiscrimately; for example, in a sentence, “We found an increase in the phylum Firmicutes, which supports the theory of importance of bacteria belonging to the clostridial group.” (here, the authors do not care which taxonomic rank their “clostridial group” means), “clostridial group” can be the class Clostridia, the order Clostridiales, the family Clostridiaceae, or the genus Clostridium. While phylogenetic distances among the different kingdoms are not the same, the above sentence is as obscure as the following sentence: “We found an increase of the phylum Chordata, which supports the theory of importance of sea anemones and/or of herbivorous animals.” The inappropriate bacterial taxonomic description is partly due to the changing bacterial taxonomy system; the changes at the phylum levels are not uncommon, while no consensus information about bacterial classification is readly available.143,144 For example, Collins et al. classified the genus Clostridium into 14 clusters in 1994, and discussed “need of major revision”, since some clusters including IV and XIVa, consisted of phenotypically heterogeneous bacteria. Thus, the Clostridium cluster system does not provide precise information about the bacterial classification, yet it is still widely used. This is in contrast to the viral taxonomy, updated regularly by the International Committee on Taxonomy of Viruses (ICTV), whose reports are available online for free (https://talk.ictvonline.org/taxonomy/).
3. The ratio of fecal bacterial taxa underrepresents the gut microbiota
In most human studies, stool samples have been used to investigate the gut microbiome. The fecal microbiome, however, may not reflect the gut microbiome,145 since the bacteriome of the digestive tract differs in each portion. Steams et al.145 demonstrated that the bacterial communities of colon biopsy samples were distinct from those in stool samples. In addition, most microbiome studies showed only the ratio of bacterial taxa in the content of organs, such as saliva and stool, but did not quantify the total numbers of bacteria, which require information about many parameters, such as the total volume of the content, weight, water content, and intestinal transit time.146 Although microbiome associated with mucosa of the organs are more biological significance than those in the content of the organs,147 mucosal microbiome analysis requires biopsy of the mucosal samples, which is not feasible in many human studies.
4. Microbiota changes can be the cause or outcome of disease
The changes in microbiota can be the cause or outcome of disease.3 This is true even in digestive diseases whose connection with gut microbiota appears to be straightforward.3 For example, dysbiosis may only reflect constipation or diarrhea, which changes the gut and colonic transit, and fecal output.
5. Discrepancy between experimental microbiota studies versus clinical primary immunodeficiency diseases (PID)
In microbiota research, despite the key defense role of innate immune components including neutrophils and macrophages, acquired immune components, particularly IgA, Th17 cells, and Tregs, have been studied more extensively in experimental mice, which have been proposed to influence the gut microbiota and their related diseases.148 Clinically, however, reports of primary immunodeficiency diseases (PID)149 sometimes do not support the roles of such immune components. For example, gain-of-function (GOF) mutations in the signal transducer and activator of transcription (STAT) 1 result in imbalanced STAT signaling, reducing Th17 cells.150 The patients with the GOF STAT1 mutation is characterized by susceptibility to oral and esophageal Candida infections, while candidiasis rarely appeared in other parts of the gastrointestinal tract; neither bacterial infections nor diarrhea is characteristic among the patients.
IgA deficiency is the most common PID;151 e.g. the incidence in the Arabian peninsula and Spain is 1:143 and 1:163, respectively. Although some individuals with IgA deficiency are susceptible to infectious and immune-mediated diseases or have altered Escherichia coli strain phylogenetic group distribution,152 most people with IgA deficiency is asymptomatic and healthy.
6. Microbiota is influenced by many factors, including age, gender, and country
Gut microbiota has been shown to be influenced by many factors, such as the genetic background of the hosts, diet, age, gender, and country.3,138,153 Among the taxonomic ranks, from the phylum to the genus, there is no consensus on the bacterial components of “healthy gut microbiota” or its alteration (dysbiosis) that can be applicable for all individuals, while it may be possible to find the stable bacterial components/amounts at the genus or species level. Hoarau et al. compared the gut microbiome between 1) patients with Crohn’s disease, 2) their healthy family members, and 3) unrelated healthy individuals living in the same area.138 They demonstrated that the difference in micobiome between 1) and 2) was smaller the difference between 2) and 3). Although gender deference does not seem to affect microbiome in general, there are some reports that disease susceptibility may be associated with the differences in gut microbiota between male and female mice.154
7. Probiotics/prebiotics are not always beneficial for hosts
In public, some groups of bacteria, Bifidobacterium and Lactobacillus, are regarded as “good bacteria”, while other groups of bacteria, including Clostridium, are regarded as “bad bacteria”. “Good bacteria” called as “probiotics” as well as “prebiotics” that favors propagation of “good bacteria”, including high fiber diet and breast feeding, seem to be beneficial for any conditions from gastrointestinal diseases to neurological diseases, while bad bacteria and dysbiosis are always bad for any conditions. This is not necessary the case. As we reviewed the above, H. pylori and C. perfringens infections may protect from MS. Infant botulism is the acute, flaccid paralysis caused by Clostridium botulinum; notably, the infant is the only family member who is ill with a broad peak from 2 to 4 months of age155 despite the fact that the normal human infant microbiota contains mainly Bifidobacterium and Bacteroides species. In addition, identified risk factors for infant botulism include breast-feeding and the ingestion of honey.156
In prebiotics field, modern western diet has been linked to recent increases of the prevalence of many diseases. Aging has been shown to reduce diversity of the gut microbiota with increased bad bacteria (opportunistic species and pathobionts) and reduced good bacteria producing short-chain fatty acids. Although this change usually considered “dysbiosis”, one may suggest that this can be adaptations to the aged condition.157 Currently, we do not know whether “western diet” is good or bad for senescence; at least, the average life expectancy is higher in industrialized western countries than developing countries where people are eating more prebiotics in their eating habit.
8. Effect of antibiotics treatment on systemic microbiota and immune system
To investigate the role of microbiota, microbiota has been depleted by antibiotics, experimentally. In many mouse studies, antibiotics are provided through the most facile means available, for example, through the animal’s water supply.158 While oral administration of non-absorbable antibiotics, such as neomycin147,159–161 and vancomycin,21 can affect mainly the gut microbiota, some studies often use highly efficacious absorbable drugs, such as metronidazole73–75 and trimethoprim/sulfamethoxazole (TMP-SMX),154 for complete microbiota depletion (TMP-SMX can deplete even some fungi).82 Here, it should be taken into account the systemic effects of the absorbable antibiotics, such as changes in other microbiota (e.g., altered microbiota in the nasal cavity may change CNS viral infection through the olfactory route, while lung inflammation has been shown to suppress EAE162) and immunomodulatory effects (e.g., TMP-SMX can cause hematologic and allergic adverse effects, while minocycline can suppress microglia163). On the other hand, diet and dietary supplements, some of which are known as prebiotics, have also been shown to alter microbiota. For example, resveratrol, a natural polyphenol compound,164 is known to have anti-oxidant and anti-inflammatory effects; more recently, resveratrol has been shown to suppress IBD models with alteration of the gut microbiota.165 Thus, in some pathological conditions, the influence on microbiota needs to be considered, once such diet/dietary supplements are proved to have antibiotic/prebiotic functions.
9. Fecal microbiome transplantation (FMT) methodology and safety
Experimentally, to assess whether the microbiota is responsible for disease phenotypes, fecal microbiome transplantation (FMT) has been used. One standard protocol is that FMT from the donor to recipient is performed through oral gavage, while an alternate protocol is co-housing and/or litter swaps (also referred to as cross-fostering) of the two mouse strains, since mice are coprophagic; co-housing allows the microbiota of all the animals within the same cage to homogenize.166 These protocols have the disadvantage that some microbes, such as fastidious anaerobic bacteria or enveloped viruses, could not survive the fecal preparation or in the gastric acid.
Clinically, FMT has been reported to be effective in several diseases, particularly Clostridium difficile infection.167 Although the term “transplantation” sounds safe, FMT is, after all, to infect humans with large numbers and species of archaea, bacteria, fungi, and viruses whose components and pathogenicity are largely unknown. For example, recently, even archaea has been suggested to be a human pathogen,168 while archaea had been believed to be non-pathogenic. Giant viruses, such as mimivirus, are a part of the gut microbiota. The potential pathology of giant viruses is unknown, while they are frequently missed by virome studies that use 0.22 µm filters.169
Wang et al.170 conducted systematic review on a total of 1089 patients receiving FMT in 50 publications, and concluded that serious adverse events, including death and viral infections, are not rare. When live or inactivated pathogens are given to humans, for example, as vaccine for infectious disease or helminth therapy in MS,171 adverse effects have been extensively investigated, even though such treatment usually involves only one known microbial species. In addition, historically transmission of infectious diseases by medical procedures and human behavior, including blood transfusion, sexual intercourse, breast feeding, and kiss, have been extensively investigated. Thus, the safety of FMT should be thoroughly investigated for more widely future clinical application.
10. Tailor-made gut microbiota therapy
To avoid severe adverse effects of FMT and probiotics treatment, tailor-made treatment is required, when considering all the above points. For example, L. reuteri has been clinically tested in other gastrointestinal diseases in children, prophylactically172 or therapeutically.173,174 We are currently conducting a randomized controlled trial to see whether L. reuteri DSM 17938 can be effective for pediatric chronic constipation. Targeting one microbe and/or its product on one specific disease condition among defined age-group of recipients will be one safe approach to find the individualized therapeutic and prophylactic intervention of human health and diseases associated with the gut microbiota.
Acknowledgments
This work was supported by grants from the Science Research Promotion Fund from the Promotion and Mutual Aid Corporation for Private Schools of Japan (F. Sato), the Faculty Assistance and Development Research Grant from the Kindai University Research Enhancement Grant (F. Sato & S. Omura), BioGaia AB Clinical Research Fund 2016 (M. Fujita), the National Institute of General Medical Sciences COBRE Grant (P30-GM110703, I. Tsunoda), the Japan Society for the Promotion of Science [JSPS, Grant-in-Aid for Scientific Research (B)-MEXT KAKENHI Grant #15K08975 (A.-M. Park), Grant-in-Aid for Young Scientists (B) KAKENHI, JP17K15628 (F. Sato), and Grants-in-Aid for Scientific Research-KAKENHI, 16H07356 (I. Tsunoda)]. We thank Ms. Namie Sakiyama for excellent technical assistance.
Glossary
Abbreviations
- AAD
antibiotic-associated diarrhea
- ABC
adenosine triphosphate-binding cassette
- Aβ
amyloid-beta
- AD
Alzheimer’s disease
- AQP
aquaporin
- BBB
blood-brain barrier
- CFA
complete Freund’s adjuvant
- CFU
colony-forming unit
- CNS
central nervous system
- CVD
cardiovascular diseases
- DC
dendritic cell
- DM
diabetes mellitus
- EAE
experimental autoimmune encephalomyelitis
- FMT
fecal microbiome transplantation
- GOF
gain-of-function
- HBGAs
histo-blood group antigens
- HC
healthy controls
- IBD
inflammatory bowel diseases
- ICTV
International Committee on Taxonomy of Viruses
- IFA
incomplete Freund’s adjuvant
- IFN
interferon
- Ig
immunoglobulin
- IL
interleukin
- ITP
idiopathic thrombocytopenic purpura
- LPS
lipopolysaccharide
- MAIT
mucosal-associated invariant T
- MALT
mucosa-associated lymphoid tissue
- MMTV
mouse mammary tumor virus
- MOG
myelin oligodendrocyte glycoprotein
- MS
multiple sclerosis
- NEC
necrotizing enterocolitis
- NMO
neuromyelitis optica
- OTU
operational taxonomic unit
- PID
primary immunodeficiency diseases
- PLP
proteolipid protein
- PMA
phorbol 12-myristate 13-acetate
- RR
relapsing-remitting
- rRNA
ribosomal RNA
- SFB
segmented filamentous bacteria
- SPF
specific pathogen-free
- SS1
Sydney strain 1
- STAT
signal transducer and activator of transcription
- Th
T helper
- TLR
toll-like receptor
- TMEV
Theiler’s murine encephalomyelitis virus
- TMP-SMX
trimethoprim/sulfamethoxazole
- TP
transporter permease
- Tregs
regulatory T cells
Footnotes
An authorship declaration: All authors are in agreement with the content of the manuscript. Each author wrote the following sections: A-M.P, H. pylori; S.O, MS; M.F, introduction; F.S, EAE and TMEV; and I.T, pitfalls.
Conflict of interests
Authors declare no Conflict of Interests for this article.
References
- 1.Isaiah . The Book of Isaiah. The HarperCollins study Bible : New Revised Standard Version, with the Apocraphal/Deuterocanonical books. In: Meeks WA, editor. Fully rev. and updated. 1. New York, NY: HarperCollins Publishers, Inc; 1993. p. 1030. [Google Scholar]
- 2.Pace NR. Time for a change. Nature. 2006;441:289. doi: 10.1038/441289a. [DOI] [PubMed] [Google Scholar]
- 3.Kverka M, Tlaskalová-Hogenová H. Intestinal Microbiota: Facts and Fiction. Dig Dis. 2017;35:139–147. doi: 10.1159/000449095. [DOI] [PubMed] [Google Scholar]
- 4.Sam QH, Chang MW, Chai LYA. The Fungal Mycobiome and Its Interaction with Gut Bacteria in the Host. Int J Mol Sci. 2017;18:230. doi: 10.3390/ijms18020330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whiteside SA, Razvi H, Dave S, Reid G, Burton JP. The microbiome of the urinary tract--a role beyond infection. Nat Rev Urol. 2015;12:81–90. doi: 10.1038/nrurol.2014.361. [DOI] [PubMed] [Google Scholar]
- 6.Human Microbiome Project C. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lloyd-Price J, Abu-Ali G, Huttenhower C. The healthy human microbiome. Genome Med. 2016;8:51. doi: 10.1186/s13073-016-0307-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao B, Gupta RS. Conserved indels in protein sequences that are characteristic of the phylum Actinobacteria. Int J Syst Evol Microbiol. 2005;55:2401–2412. doi: 10.1099/ijs.0.63785-0. [DOI] [PubMed] [Google Scholar]
- 9.Johnson EL, Heaver SL, Walters WA, Ley RE. Microbiome and metabolic disease: revisiting the bacterial phylum Bacteroidetes. J Mol Med (Berl) 2017;95:1–8. doi: 10.1007/s00109-016-1492-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davey L, Halperin SA, Lee SF. Thiol-Disulfide Exchange in Gram-Positive Firmicutes. Trends Microbiol. 2016;24:902–915. doi: 10.1016/j.tim.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 11.Gupta RS. The phylogeny of proteobacteria: relationships to other eubacterial phyla and eukaryotes. FEMS Microbiol Rev. 2000;24:367–402. doi: 10.1111/j.1574-6976.2000.tb00547.x. [DOI] [PubMed] [Google Scholar]
- 12.Hedlund BP, Gosink JJ, Staley JT. Verrucomicrobia div. nov., a new division of the bacteria containing three new species of Prosthecobacter. Antonie Van Leeuwenhoek. 1997;72:29–38. doi: 10.1023/a:1000348616863. [DOI] [PubMed] [Google Scholar]
- 13.Forbes JD, Van Domselaar G, Bernstein CN. The Gut Microbiota in Immune-Mediated Inflammatory Diseases. Front Microbiol. 2016;7:1081. doi: 10.3389/fmicb.2016.01081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nathan C. Antibiotics at the crossroads. Nature. 2004;431:899–902. doi: 10.1038/431899a. [DOI] [PubMed] [Google Scholar]
- 15.Jernberg C, Lofmark S, Edlund C, Jansson JK. Long-term ecological impacts of antibiotic administration on the human intestinal microbiota. ISME J. 2007;1:56–66. doi: 10.1038/ismej.2007.3. [DOI] [PubMed] [Google Scholar]
- 16.Beaugerie L, Petit J-C. Antibiotic-associated diarrhoea. Best Pract Res Clin Gastroenterol. 2004;18:337–352. doi: 10.1016/j.bpg.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Carman RJ, Simon MA, Fernandez H, Miller MA, Bartholomew MJ. Ciprofloxacin at low levels disrupts colonization resistance of human fecal microflora growing in chemostats. Regul Toxicol Pharmacol. 2004;40:319–326. doi: 10.1016/j.yrtph.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 18.Guarner F, Malagelada J-R. Gut flora in health and disease. Lancet. 2003;361:512–519. doi: 10.1016/S0140-6736(03)12489-0. [DOI] [PubMed] [Google Scholar]
- 19.Schneiderhan J, Master-Hunter T, Locke A. Targeting gut flora to treat and prevent disease. J Fam Pract. 2016;65:34–38. [PubMed] [Google Scholar]
- 20.Ivanov II, Atarashi K, Manel N, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yokote H, Miyake S, Croxford JL, Oki S, Mizusawa H, Yamamura T. NKT cell-dependent amelioration of a mouse model of multiple sclerosis by altering gut flora. Am J Pathol. 2008;173:1714–1723. doi: 10.2353/ajpath.2008.080622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eckle SBG, Corbett AJ, Keller AN, et al. Recognition of Vitamin B Precursors and Byproducts by Mucosal Associated Invariant T Cells. J Biol Chem. 2015;290:30204–30211. doi: 10.1074/jbc.R115.685990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sekirov I, Tam NM, Jogova M, et al. Antibiotic-induced perturbations of the intestinal microbiota alter host susceptibility to enteric infection. Infect Immun. 2008;76:4726–4736. doi: 10.1128/IAI.00319-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hansen JJ. Immune Responses to Intestinal Microbes in Inflammatory Bowel Diseases. Curr Allergy Asthma Rep. 2015;15:61. doi: 10.1007/s11882-015-0562-9. [DOI] [PubMed] [Google Scholar]
- 25.Mondot S, Kang S, Furet JP, et al. Highlighting new phylogenetic specificities of Crohn's disease microbiota. Inflamm Bowel Dis. 2011;17:185–192. doi: 10.1002/ibd.21436. [DOI] [PubMed] [Google Scholar]
- 26.Wills ES, Jonkers DMAE, Savelkoul PH, Masclee AA, Pierik MJ, Penders J. Fecal microbial composition of ulcerative colitis and Crohn's disease patients in remission and subsequent exacerbation. PLoS One. 2014;9:e90981. doi: 10.1371/journal.pone.0090981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manichanh C, Rigottier-Gois L, Bonnaud E, et al. Reduced diversity of faecal microbiota in Crohn's disease revealed by a metagenomic approach. Gut. 2006;55:205–211. doi: 10.1136/gut.2005.073817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hviid A, Svanström H, Frisch M. Antibiotic use and inflammatory bowel diseases in childhood. Gut. 2011;60:49–54. doi: 10.1136/gut.2010.219683. [DOI] [PubMed] [Google Scholar]
- 29.Sodhi C, Richardson W, Gribar S, Hackam DJ. The development of animal models for the study of necrotizing enterocolitis. Dis Model Mech. 2008;1:94–98. doi: 10.1242/dmm.000315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin PW, Stoll BJ. Necrotising enterocolitis. Lancet. 2006;368:1271–1283. doi: 10.1016/S0140-6736(06)69525-1. [DOI] [PubMed] [Google Scholar]
- 31.Yee WH, Soraisham AS, Shah VS, et al. Incidence and timing of presentation of necrotizing enterocolitis in preterm infants. Pediatrics. 2012;129:e298–304. doi: 10.1542/peds.2011-2022. [DOI] [PubMed] [Google Scholar]
- 32.Pammi M, Cope J, Tarr PI, et al. Intestinal dysbiosis in preterm infants preceding necrotizing enterocolitis: a systematic review and meta-analysis. Microbiome. 2017;5:31. doi: 10.1186/s40168-017-0248-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Athalye-Jape G, Rao S, Patole S. Lactobacillus reuteri DSM 17938 as a Probiotic for Preterm Neonates: A Strain-Specific Systematic Review. JPEN J Parenter Enteral Nutr. 2016;40:783–794. doi: 10.1177/0148607115588113. [DOI] [PubMed] [Google Scholar]
- 34.Abu-Shanab A, Quigley EMM. The role of the gut microbiota in nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2010;7:691–701. doi: 10.1038/nrgastro.2010.172. [DOI] [PubMed] [Google Scholar]
- 35.Mutlu E, Keshavarzian A, Engen P, Forsyth CB, Sikaroodi M, Gillevet P. Intestinal dysbiosis: a possible mechanism of alcohol-induced endotoxemia and alcoholic steatohepatitis in rats. Alcohol Clin Exp Res. 2009;33:1836–1846. doi: 10.1111/j.1530-0277.2009.01022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yan AW, Fouts DE, Brandl J, et al. Enteric dysbiosis associated with a mouse model of alcoholic liver disease. Hepatology. 2011;53:96–105. doi: 10.1002/hep.24018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen Y, Yang F, Lu H, et al. Characterization of fecal microbial communities in patients with liver cirrhosis. Hepatology. 2011;54:562–572. doi: 10.1002/hep.24423. [DOI] [PubMed] [Google Scholar]
- 38.Wang M, Karlsson C, Olsson C, et al. Reduced diversity in the early fecal microbiota of infants with atopic eczema. J Allergy Clin Immunol. 2008;121:129–134. doi: 10.1016/j.jaci.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 39.Larsen N, Vogensen FK, van den Berg FWJ, et al. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS One. 2010;5:e9085. doi: 10.1371/journal.pone.0009085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qin J, Li Y, Cai Z, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490:55–60. doi: 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- 41.Kemppainen KM, Ardissone AN, Davis-Richardson AG, et al. Early childhood gut microbiomes show strong geographic differences among subjects at high risk for type 1 diabetes. Diabetes Care. 2015;38:329–332. doi: 10.2337/dc14-0850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang X, Zhang D, Jia H, et al. The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. Nat Med. 2015;21:895–905. doi: 10.1038/nm.3914. [DOI] [PubMed] [Google Scholar]
- 43.Sato F, Omura S, Martinez NE, Tsunoda I. In: Animal models for multiple sclerosis. Minagar A, editor. Burlington, MA: Neuroinflammation: Elsevier; 2011. pp. 55–79. [Google Scholar]
- 44.Kira J. Genetic and environmental backgrounds responsible for the changes in the phenotype of MS in Japanese subjects. Mult Scler Relat Disord. 2012;1:188–195. doi: 10.1016/j.msard.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 45.Budhram A, Parvathy S, Kremenchutzky M, Silverman M. Breaking down the gut microbiome composition in multiple sclerosis. Mult Scler. 2017;23:628–636. doi: 10.1177/1352458516682105. [DOI] [PubMed] [Google Scholar]
- 46.Adamczyk-Sowa M, Medrek A, Madej P, Michlicka W, Dobrakowski P. Does the Gut Microbiota Influence Immunity and Inflammation in Multiple Sclerosis Pathophysiology? J Immunol Res. 2017;2017:7904821. doi: 10.1155/2017/7904821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ehlers S, Kaufmann SHE. Participants of the 99th Dahlem Conference. Infection, inflammation, and chronic diseases: consequences of a modern lifestyle. Trends Immunol. 2010;31:184–190. doi: 10.1016/j.it.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 48.Jorg S, Grohme DA, Erzler M, et al. Environmental factors in autoimmune diseases and their role in multiple sclerosis. Cell Mol Life Sci. 2016;73:4611–4622. doi: 10.1007/s00018-016-2311-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Esposito S, Bonavita S, Sparaco M, Gallo A, Tedeschi G. The role of diet in multiple sclerosis: A review. Nutr Neurosci. 2017:1–14. doi: 10.1080/1028415X.2017.1303016. [DOI] [PubMed] [Google Scholar]
- 50.Miyake S, Kim S, Suda W, et al. Dysbiosis in the gut microbiota of patients with multiple sclerosis, with a striking depletion of species belonging to Clostridia XIVa and IV clusters. PLoS One. 2015;10:e0137429. doi: 10.1371/journal.pone.0137429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen J, Chia N, Kalari KR, et al. Multiple sclerosis patients have a distinct gut microbiota compared to healthy controls. Sci Rep. 2016;6:28484. doi: 10.1038/srep28484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tremlett H, Fadrosh DW, Faruqi AA, et al. Gut microbiota in early pediatric multiple sclerosis: a case-control study. Eur J Neurol. 2016;23:1308–1321. doi: 10.1111/ene.13026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jangi S, Gandhi R, Cox LM, et al. Alterations of the human gut microbiome in multiple sclerosis. Nat Commun. 2016;7:12015. doi: 10.1038/ncomms12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rumah KR, Linden J, Fischetti VA, Vartanian T. Isolation of Clostridium perfringens type B in an individual at first clinical presentation of multiple sclerosis provides clues for environmental triggers of the disease. PLoS One. 2013;8:e76359. doi: 10.1371/journal.pone.0076359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hauser SL, Chan JR, Oksenberg JR. Multiple sclerosis: Prospects and promise. Ann Neurol. 2013;74:317–327. doi: 10.1002/ana.24009. [DOI] [PubMed] [Google Scholar]
- 56.Varrin-Doyer M, Spencer CM, Schulze-Topphoff U, et al. Aquaporin 4-specific T cells in neuromyelitis optica exhibit a Th17 bias and recognize Clostridium ABC transporter. Ann Neurol. 2012;72:53–64. doi: 10.1002/ana.23651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Matsuya N, Komori M, Nomura K, et al. Increased T-cell immunity against aquaporin-4 and proteolipid protein in neuromyelitis optica. Int Immunol. 2011;23:565–573. doi: 10.1093/intimm/dxr056. [DOI] [PubMed] [Google Scholar]
- 58.Cree BAC, Spencer CM, Varrin-Doyer M, Baranzini SE, Zamvil SS. Gut microbiome analysis in neuromyelitis optica reveals overabundance of Clostridium perfringens. Ann Neurol. 2016;80:443–447. doi: 10.1002/ana.24718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Martinez NE, Sato F, Omura S, Minagar A, Alexander JS, Tsunoda I. Immunopathological patterns from EAE and Theiler's virus infection: Is multiple sclerosis a homogenous 1-stage or heterogenous 2-stage disease? Pathophysiology. 2013;20:71–84. doi: 10.1016/j.pathophys.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ericsson AC, Hagan CE, Davis DJ, Franklin CL. Segmented filamentous bacteria: commensal microbes with potential effects on research. Comp Med. 2014;64:90–98. [PMC free article] [PubMed] [Google Scholar]
- 61.Collins MD, Lawson PA, Willems A, et al. The phylogeny of the genus Clostridium: proposal of five new genera and eleven new species combinations. Int J Syst Bacteriol. 1994;44:812–826. doi: 10.1099/00207713-44-4-812. [DOI] [PubMed] [Google Scholar]
- 62.Lee YK, Menezes JS, Umesaki Y, Mazmanian SK. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4615–4622. doi: 10.1073/pnas.1000082107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ochoa-Repáraz J, Mielcarz DW, Ditrio LE, et al. Central nervous system demyelinating disease protection by the human commensal Bacteroides fragilis depends on polysaccharide A expression. J Immunol. 2010;185:4101–4108. doi: 10.4049/jimmunol.1001443. [DOI] [PubMed] [Google Scholar]
- 64.Ochoa-Repáraz J, Mielcarz DW, Haque-Begum S, Kasper LH. Induction of a regulatory B cell population in experimental allergic encephalomyelitis by alteration of the gut commensal microflora. Gut Microbes. 2010;1:103–108. doi: 10.4161/gmic.1.2.11515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pöllinger B, Krishnamoorthy G, Berer K, et al. Spontaneous relapsing-remitting EAE in the SJL/J mouse: MOG-reactive transgenic T cells recruit endogenous MOG-specific B cells. J Exp Med. 2009;206:1303–1316. doi: 10.1084/jem.20090299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Berer K, Mues M, Koutrolos M, et al. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature. 2011;479:538–541. doi: 10.1038/nature10554. [DOI] [PubMed] [Google Scholar]
- 67.Stanisavljević S, Lukić J, Soković S, et al. Correlation of Gut Microbiota Composition with Resistance to Experimental Autoimmune Encephalomyelitis in Rats. Front Microbiol. 2016;7:2005. doi: 10.3389/fmicb.2016.02005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Haghikia A, Jörg S, Duscha A, et al. Dietary Fatty Acids Directly Impact Central Nervous System Autoimmunity via the Small Intestine. Immunity. 2015;43:817–829. doi: 10.1016/j.immuni.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 69.Ormerod KL, Wood DL, Lachner N, et al. Genomic characterization of the uncultured Bacteroidales family S24-7 inhabiting the guts of homeothermic animals. Microbiome. 2016;4:36. doi: 10.1186/s40168-016-0181-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ganapathy V, Thangaraju M, Prasad PD, Martin PM, Singh N. Transporters and receptors for short-chain fatty acids as the molecular link between colonic bacteria and the host. Curr Opin Pharmacol. 2013;13:869–874. doi: 10.1016/j.coph.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 71.Kim CH, Park J, Kim M. Gut microbiota-derived short-chain Fatty acids, T cells, and inflammation. Immune Netw. 2014;14:277–288. doi: 10.4110/in.2014.14.6.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ferrer I, Aubourg P, Pujol A. General aspects and neuropathology of X-linked adrenoleukodystrophy. Brain Pathol. 2010;20:817–830. doi: 10.1111/j.1750-3639.2010.00390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kuss SK, Best GT, Etheredge CA, et al. Intestinal microbiota promote enteric virus replication and systemic pathogenesis. Science. 2011;334:249–252. doi: 10.1126/science.1211057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kane M, Case LK, Kopaskie K, et al. Successful transmission of a retrovirus depends on the commensal microbiota. Science. 2011;334:245–249. doi: 10.1126/science.1210718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jones MK, Watanabe M, Zhu S, et al. Enteric bacteria promote human and mouse norovirus infection of B cells. Science. 2014;346:755–759. doi: 10.1126/science.1257147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tsunoda I, Sato F, Omura S, Fujita M, Sakiyama N, Park A-M. Three immune-mediated disease models induced by Theiler's virus: multiple sclerosis, seizures, and myocarditis. Clin Exp Neuroimmunol. 2016;7:330–345. doi: 10.1111/cen3.12341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tsunoda I, Omura S, Sato F, Kusunoki S, Fujita M, Park A-M, Hasanovic F, Yanagihara R, Nagata S. Neuropathogenesis of Zika virus infection: Potential roles of antibody-mediated pathology. Acta Medica Kindai Univ. 2016;41:37–52. [PMC free article] [PubMed] [Google Scholar]
- 78.Omura S, Kawai E, Sato F, et al. Bioinformatics multivariate analysis determined a set of phase-specific biomarker candidates in a novel mouse model for viral myocarditis. Circ Cardiovasc Genet. 2014;7:444–454. doi: 10.1161/CIRCGENETICS.114.000505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Martinez NE, Karlsson F, Sato F, et al. Protective and detrimental roles for regulatory T cells in a viral model for multiple sclerosis. Brain Pathol. 2014;24:436–451. doi: 10.1111/bpa.12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Uhde A-K, Herder V, Akram Khan M, et al. Viral Infection of the Central Nervous System Exacerbates Interleukin-10 Receptor Deficiency-Mediated Colitis in SJL Mice. PLoS One. 2016;11:e0161883. doi: 10.1371/journal.pone.0161883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tsunoda I, Libbey JE, Fujinami RS. Theiler's murine encephalomyelitis virus attachment to the gastrointestinal tract is associated with sialic acid binding. J Neurovirol. 2009;15:81–89. doi: 10.1080/13550280802380563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Carrillo-Salinas FJ, Mestre L, Mecha M, et al. Gut dysbiosis and neuroimmune responses to brain infection with Theiler's murine encephalomyelitis virus. Sci Rep. 2017;7:44377. doi: 10.1038/srep44377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Park A-M, Li Q, Nagata K, et al. Oxygen tension regulates reactive oxygen generation and mutation of Helicobacter pylori. Free Radic Biol Med. 2004;36:1126–1133. doi: 10.1016/j.freeradbiomed.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 84.Park A-M, Nagata K, Sato EF, Tamura T, Shimono K, Inoue M. Mechanism of strong resistance of Helicobacter pylori respiration to nitric oxide. Arch Biochem Biophys. 2003;411:129–135. doi: 10.1016/s0003-9861(02)00691-4. [DOI] [PubMed] [Google Scholar]
- 85.Atherton JC. The pathogenesis of Helicobacter pylori-induced gastro-duodenal diseases. Annu Rev Pathol. 2006;1:63–96. doi: 10.1146/annurev.pathol.1.110304.100125. [DOI] [PubMed] [Google Scholar]
- 86.Salama NR, Hartung ML, Müller A. Life in the human stomach: persistence strategies of the bacterial pathogen Helicobacter pylori. Nat Rev Microbiol. 2013;11:385–399. doi: 10.1038/nrmicro3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pereira M-I, Medeiros JA. Role of Helicobacter pylori in gastric mucosa-associated lymphoid tissue lymphomas. World J Gastroenterol. 2014;20:684–698. doi: 10.3748/wjg.v20.i3.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tsujimae M, Yamashita H, Hashimura H, et al. A Comparative Study of a New Class of Gastric Acid Suppressant Agent Named Vonoparazan versus Esomeprazole for the Eradication of Helicobacter pylori. Digestion. 2016;94:240–246. doi: 10.1159/000454762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ford AC, Forman D, Hunt R, Yuan Y, Moayyedi P. Helicobacter pylori eradication for the prevention of gastric neoplasia. Cochrane Database Syst Rev. 2015;7:CD005583. doi: 10.1002/14651858.CD005583.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lee YC, Chiang TH, Chou CK, et al. Association Between Helicobacter pylori Eradication and Gastric Cancer Incidence: A Systematic Review and Meta-analysis. Gastroenterology. 2016;150:1113–1124. e1115. doi: 10.1053/j.gastro.2016.01.028. [DOI] [PubMed] [Google Scholar]
- 91.Lee A, O'Rourke J, De Ungria MC, Robertson B, Daskalopoulos G, Dixon MF. A standardized mouse model of Helicobacter pylori infection: introducing the Sydney strain. Gastroenterology. 1997;112:1386–1397. doi: 10.1016/s0016-5085(97)70155-0. [DOI] [PubMed] [Google Scholar]
- 92.Zhang S, Moss SF. Rodent models of Helicobacter infection, inflammation, and disease. Methods Mol Biol. 2012;921:89–98. doi: 10.1007/978-1-62703-005-2_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Park A-M, Hagiwara S, Hsu DK, Liu F-T, Yoshie O. Galectin-3 Plays an Important Role in Innate Immunity to Gastric Infection by Helicobacter pylori. Infect Immun. 2016;84:1184–1193. doi: 10.1128/IAI.01299-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Goni E, Franceschi F. Helicobacter pylori and extragastric diseases. Helicobacter. 2016;21(Suppl 1):45–48. doi: 10.1111/hel.12340. [DOI] [PubMed] [Google Scholar]
- 95.Lai C-Y, Yang T-Y, Lin C-L, Kao C-H. Helicobacter pylori infection and the risk of acute coronary syndrome: a nationwide retrospective cohort study. Eur J Clin Microbiol Infect Dis. 2015;34:69–74. doi: 10.1007/s10096-014-2207-7. [DOI] [PubMed] [Google Scholar]
- 96.Yang GH, Wu JS, Yang YC, Huang YH, Lu FH, Chang CJ. Gastric Helicobacter pylori infection associated with risk of diabetes mellitus, but not prediabetes. J Gastroenterol Hepatol. 2014;29:1794–1799. doi: 10.1111/jgh.12617. [DOI] [PubMed] [Google Scholar]
- 97.Malaguarnera M, Bella R, Alagona G, Ferri R, Carnemolla A, Pennisi G. Helicobacter pylori and Alzheimer's disease: a possible link. Eur J Intern Med. 2004;15:381–386. doi: 10.1016/j.ejim.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 98.Roubaud-Baudron C, Krolak-Salmon P, Quadrio I, Mégraud F, Salles N. Impact of chronic Helicobacter pylori infection on Alzheimer's disease: preliminary results. Neurobiol Aging. 2012;33:1009, e1011–1009. doi: 10.1016/j.neurobiolaging.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 99.Tsolaki F, Kountouras J, Topouzis F, Tsolaki M. Helicobacter pylori infection, dementia and primary open-angle glaucoma: are they connected? BMC Ophthalmol. 2015;15:24. doi: 10.1186/s12886-015-0006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chen Y, Blaser MJ. Inverse associations of Helicobacter pylori with asthma and allergy. Arch Intern Med. 2007;167:821–827. doi: 10.1001/archinte.167.8.821. [DOI] [PubMed] [Google Scholar]
- 101.Lim JH, Kim N, Lim SH, et al. Inverse Relationship Between Helicobacter Pylori Infection and Asthma Among Adults Younger than 40 Years: A Cross-Sectional Study. Medicine (Baltimore) 2016;95:e2609. doi: 10.1097/MD.0000000000002609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Arnold IC, Dehzad N, Reuter S, et al. Helicobacter pylori infection prevents allergic asthma in mouse models through the induction of regulatory T cells. J Clin Invest. 2011;121:3088–3093. doi: 10.1172/JCI45041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Papamichael K, Konstantopoulos P, Mantzaris GJ. Helicobacter pylori infection and inflammatory bowel disease: is there a link? World J Gastroenterol. 2014;20:6374–6385. doi: 10.3748/wjg.v20.i21.6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jaruvongvanich V, Sanguankeo A, Jaruvongvanich S, Upala S. Association between Helicobacter pylori infection and multiple sclerosis: A systematic review and meta-analysis. Mult Scler Relat Disord. 2016;7:92–97. doi: 10.1016/j.msard.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 105.Pedrini MJF, Seewann A, Bennett KA, et al. Helicobacter pylori infection as a protective factor against multiple sclerosis risk in females. J Neurol Neurosurg Psychiatry. 2015;86:603–607. doi: 10.1136/jnnp-2014-309495. [DOI] [PubMed] [Google Scholar]
- 106.Cook KW, Crooks J, Hussain K, et al. Helicobacter pylori infection reduces disease severity in an experimental model of multiple sclerosis. Front Microbiol. 2015;6:52. doi: 10.3389/fmicb.2015.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li W, Minohara M, Su JJ, et al. Helicobacter pylori infection is a potential protective factor against conventional multiple sclerosis in the Japanese population. J Neuroimmunol. 2007;184:227–231. doi: 10.1016/j.jneuroim.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 108.Kira J. Helicobacter pylori infection might prove the hygiene hypothesis in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2015;86:591–592. doi: 10.1136/jnnp-2014-309759. [DOI] [PubMed] [Google Scholar]
- 109.Li W, Minohara M, Piao H, et al. Association of anti-Helicobacter pylori neutrophil-activating protein antibody response with anti-aquaporin-4 autoimmunity in Japanese patients with multiple sclerosis and neuromyelitis optica. Mult Scler. 2009;15:1411–1421. doi: 10.1177/1352458509348961. [DOI] [PubMed] [Google Scholar]
- 110.Long Y, Gao C, Qiu W, et al. Helicobacter pylori infection in Neuromyelitis Optica and Multiple Sclerosis. Neuroimmunomodulation. 2013;20:107–112. doi: 10.1159/000345838. [DOI] [PubMed] [Google Scholar]
- 111.Martinez NE, Sato F, Omura S, et al. RORγt, but not T-bet, overexpression exacerbates an autoimmune model for multiple sclerosis. J Neuroimmunol. 2014;276:142–149. doi: 10.1016/j.jneuroim.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Boziki B, Grigoriadis N, Deretzi G, et al. Helicobacter pylori immunomodulative properties in a mouse model of multiple sclerosis. Immuno-gastroenterology. 2012;1:34–39. [Google Scholar]
- 113.Maheshwari P, Eslick GD. Bacterial infection and Alzheimer's disease: a meta-analysis. J Alzheimers Dis. 2015;43:957–966. doi: 10.3233/JAD-140621. [DOI] [PubMed] [Google Scholar]
- 114.Bhuiyan TR, Qadri F, Saha A, Svennerholm A-M. Infection by Helicobacter pylori in Bangladeshi children from birth to two years: relation to blood group, nutritional status, and seasonality. Pediatr Infect Dis J. 2009;28:79–85. doi: 10.1097/INF.0b013e31818a5d9d. [DOI] [PubMed] [Google Scholar]
- 115.Larussa T, Leone I, Suraci E, Imeneo M, Luzza F. Helicobacter pylori and T Helper Cells: Mechanisms of Immune Escape and Tolerance. J Immunol Res. 2015;2015:981328. doi: 10.1155/2015/981328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shiota S, Murakami K, Yoshiiwa A, et al. The relationship between Helicobacter pylori infection and Alzheimer's disease in Japan. J Neurol. 2011;258:1460–1463. doi: 10.1007/s00415-011-5957-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hall AM, Roberson ED. Mouse models of Alzheimer's disease. Brain Res Bull. 2012;88:3–12. doi: 10.1016/j.brainresbull.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders. Nat Rev Neurosci. 2011;12:723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ruiz B, Correa P, Fontham ETH, Ramakrishnan T. Antral atrophy, Helicobacter pylori colonization, and gastric pH. Am J Clin Pathol. 1996;105:96–101. doi: 10.1093/ajcp/105.1.96. [DOI] [PubMed] [Google Scholar]
- 120.Derakhshan MH, El-Omar E, Oien K, et al. Gastric histology, serological markers and age as predictors of gastric acid secretion in patients infected with Helicobacter pylori. J Clin Pathol. 2006;59:1293–1299. doi: 10.1136/jcp.2005.036111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mitchell DR, Derakhshan MH, Wirz AA, et al. The gastric acid pocket is attenuated in H. pylori infected subjects. Gut. 2016 doi: 10.1136/gutjnl-2016-312638. [DOI] [PubMed] [Google Scholar]
- 122.Loscalzo J. The oxidant stress of hyperhomocyst(e)inemia. J Clin Invest. 1996;98:5–7. doi: 10.1172/JCI118776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lang D, Kredan MB, Moat SJ, et al. Homocysteine-induced inhibition of endothelium-dependent relaxation in rabbit aorta: role for superoxide anions. Arterioscler Thromb Vasc Biol. 2000;20:422–427. doi: 10.1161/01.atv.20.2.422. [DOI] [PubMed] [Google Scholar]
- 124.Bell RD, Zlokovic BV. Neurovascular mechanisms and blood-brain barrier disorder in Alzheimer's disease. Acta Neuropathol. 2009;118:103–113. doi: 10.1007/s00401-009-0522-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Clarke R, Smulders Y, Fowler B, Stehouwer CD. Homocysteine, B-vitamins, and the risk of cardiovascular disease. Semin Vasc Med. 2005;5:75–76. doi: 10.1055/s-2005-872393. [DOI] [PubMed] [Google Scholar]
- 126.Seshadri S, Beiser A, Selhub J, et al. Plasma homocysteine as a risk factor for dementia and Alzheimer's disease. N Engl J Med. 2002;346:476–483. doi: 10.1056/NEJMoa011613. [DOI] [PubMed] [Google Scholar]
- 127.Miklossy J. Chronic inflammation and amyloidogenesis in Alzheimer's disease -- role of Spirochetes. J Alzheimers Dis. 2008;13:381–391. doi: 10.3233/jad-2008-13404. [DOI] [PubMed] [Google Scholar]
- 128.Miklossy J, Kis A, Radenovic A, et al. Beta-amyloid deposition and Alzheimer's type changes induced by Borrelia spirochetes. Neurobiol Aging. 2006;27:228–236. doi: 10.1016/j.neurobiolaging.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 129.Balin BJ, Appelt DM. Role of infection in Alzheimer's disease. J Am Osteopath Assoc. 2001;101:S1–6. [PubMed] [Google Scholar]
- 130.Gérard HC, Dreses-Werringloer U, Wildt KS, et al. Chlamydophila (Chlamydia) pneumoniae in the Alzheimer's brain. FEMS Immunol Med Microbiol. 2006;48:355–366. doi: 10.1111/j.1574-695X.2006.00154.x. [DOI] [PubMed] [Google Scholar]
- 131.Minter MR, Zhang C, Leone V, et al. Antibiotic-induced perturbations in gut microbial diversity influences neuro-inflammation and amyloidosis in a murine model of Alzheimer's disease. Sci Rep. 2016;6:30028. doi: 10.1038/srep30028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bhuiyan TR, Islam MM, Uddin T, et al. Th1 and Th17 responses to Helicobacter pylori in Bangladeshi infants, children and adults. PLoS One. 2014;9:e93943. doi: 10.1371/journal.pone.0093943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Shi Y, Liu X-F, Zhuang Y, et al. Helicobacter pylori-induced Th17 responses modulate Th1 cell responses, benefit bacterial growth, and contribute to pathology in mice. J Immunol. 2010;184:5121–5129. doi: 10.4049/jimmunol.0901115. [DOI] [PubMed] [Google Scholar]
- 134.Sato F, Omura S, Jaffe SL, Tsunoda I. Role of CD4+ T lymphocytes in pathophysiology of multiple sclerosis. In: Minagar A, editor. Multiple sclerosis: a mechanistic view. London, UK: Elsevier Inc; 2016. pp. 41–69. [Google Scholar]
- 135.Engstrand L, Lindberg M. Helicobacter pylori and the gastric microbiota. Best Pract Res Clin Gastroenterol. 2013;27:39–45. doi: 10.1016/j.bpg.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 136.Sheh A, Fox JG. The role of the gastrointestinal microbiome in Helicobacter pylori pathogenesis. Gut Microbes. 2013;4:505–531. doi: 10.4161/gmic.26205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kienesberger S, Cox LM, Livanos A, et al. Gastric Helicobacter pylori Infection Affects Local and Distant Microbial Populations and Host Responses. Cell Rep. 2016;14:1395–1407. doi: 10.1016/j.celrep.2016.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hoarau G, Mukherjee PK, Gower-Rousseau C, et al. Bacteriome and Mycobiome Interactions Underscore Microbial Dysbiosis in Familial Crohn's Disease. Mbio. 2016;7:e01250–16. doi: 10.1128/mBio.01250-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Benito-León J, Pisa D, Alonso R, Calleja P, Díaz-Sánchez M, Carrasco L. Association between multiple sclerosis and Candida species: evidence from a case-control study. Eur J Clin Microbiol Infect Dis. 2010;29:1139–1145. doi: 10.1007/s10096-010-0979-y. [DOI] [PubMed] [Google Scholar]
- 140.Conceicão-Neto N, Zeller M, Lefrére H, et al. Modular approach to customise sample preparation procedures for viral metagenomics: a reproducible protocol for virome analysis. Sci Rep. 2015;5:16532. doi: 10.1038/srep16532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tong M, Jacobs J, McHardy I, Braun J. Current Protocols in Immunology. John Wiley and Sons, Inc; 2014. Sampling of intestinal microbiota and targeted amplification of bacterial 16S rRNA genes for microbial ecologic analysis; pp. 7.41.41–47.41.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rampelli S, Soverini M, Turroni S, et al. ViromeScan: a new tool for metagenomic viral community profiling. BMC Genomics. 2016;17:165. doi: 10.1186/s12864-016-2446-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Whitman WB. Bergey's Manual of Systematics of Archaea and Bacteria. John Wiley & Sons, Inc; 2001. [Google Scholar]
- 144.Skerman VBD, McGowan V, Sneath PHA. Approved Lists of Bacterial Names and Amended edition of the Approved Lists of Bacterial Names (vol 30, pg 225, 1980) International Journal of Systematic Bacteriology. 1997;47:1271–1272. [Google Scholar]
- 145.Stearns JC, Lynch MDJ, Senadheera DB, et al. Bacterial biogeography of the human digestive tract. Sci Rep. 2011;1:170. doi: 10.1038/srep00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Grasa L, Abecia L, Forcen R, et al. Antibiotic-Induced Depletion of Murine Microbiota Induces Mild Inflammation and Changes in Toll-Like Receptor Patterns and Intestinal Motility. Microb Ecol. 2015;70:835–848. doi: 10.1007/s00248-015-0613-8. [DOI] [PubMed] [Google Scholar]
- 147.Puhl NJ, Uwiera RRE, Yanke LJ, Selinger LB, Inglis GD. Antibiotics conspicuously affect community profiles and richness, but not the density of bacterial cells associated with mucosa in the large and small intestines of mice. Anaerobe. 2012;18:67–75. doi: 10.1016/j.anaerobe.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 148.Sutherland DB, Suzuki K, Fagarasan S. Fostering of advanced mutualism with gut microbiota by Immunoglobulin A. Immunol Rev. 2016;270:20–31. doi: 10.1111/imr.12384. [DOI] [PubMed] [Google Scholar]
- 149.Picard C, Al-Herz W, Bousfiha A, et al. Primary Immunodeficiency Diseases: an Update on the Classification from the International Union of Immunological Societies Expert Committee for Primary Immunodeficiency 2015. J Clin Immunol. 2015;35:696–726. doi: 10.1007/s10875-015-0201-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Depner M, Fuchs S, Raabe J, et al. The Extended Clinical Phenotype of 26 Patients with Chronic Mucocutaneous Candidiasis due to Gain-of-Function Mutations in STAT1. J Clin Immunol. 2016;36:73–84. doi: 10.1007/s10875-015-0214-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yazdani R, Azizi G, Abolhassani H, Aghamohammadi A. Selective IgA Deficiency: Epidemiology, Pathogenesis, Clinical Phenotype, Diagnosis, Prognosis and Management. Scand J Immunol. 2017;85:3–12. doi: 10.1111/sji.12499. [DOI] [PubMed] [Google Scholar]
- 152.Nowrouzian FL, Friman V, Adlerberth I, Wold AE. Different phylogenetic profile and reduced mannose-sensitive adherence capacity characterize commensal Escherichia coli in IgA deficient individuals. Microb Pathog. 2013;61–62:62–65. doi: 10.1016/j.micpath.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 153.Nishijima S, Suda W, Oshima K, et al. The gut microbiome of healthy Japanese and its microbial and functional uniqueness. DNA Res. 2016;23:125–133. doi: 10.1093/dnares/dsw002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Miller PG, Bonn MB, Franklin CL, Ericsson AC, McKarns SC. TNFR2 Deficiency Acts in Concert with Gut Microbiota To Precipitate Spontaneous Sex-Biased Central Nervous System Demyelinating Autoimmune Disease. J Immunol. 2015;195:4668–4684. doi: 10.4049/jimmunol.1501664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Arnon SS. Botulism (Clostridium Botulinum) In: Kliegman RM, Jenson HB, Behrman RE, Stanton BF, editors. Nelson textbook of pediatrics. 18. Philadelphia: Saunders; 2007. pp. 1224–1227. [Google Scholar]
- 156.Midura TF. Update: infant botulism. Clin Microbiol Rev. 1996;9:119–125. doi: 10.1128/cmr.9.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Biagi E, Rampelli S, Turroni S, Quercia S, Candela M, Brigidi P. The gut microbiota of centenarians: Signatures of longevity in the gut microbiota profile. Mech Ageing Dev. 2016 doi: 10.1016/j.mad.2016.12.013. [DOI] [PubMed] [Google Scholar]
- 158.Gibson MK, Crofts TS, Dantas G. Antibiotics and the developing infant gut microbiota and resistome. Curr Opin Microbiol. 2015;27:51–56. doi: 10.1016/j.mib.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Koureleas S, Arvaniti A, Stavropoulos M, Scopa CD, Vagianos CE. The effect of non-absorbable antibiotics on intestinal bacterial translocation and endotoxemia in experimental obstructive jaundice. Annals of Gastroenterology. 2000;13:31–36. [Google Scholar]
- 160.Nevado R, Forcén R, Layunta E, Murillo MD, Grasa L. Neomycin and bacitracin reduce the intestinal permeability in mice and increase the expression of some tight-junction proteins. Rev Esp Enferm Dig. 2015;107:672–676. doi: 10.17235/reed.2015.3868/2015. [DOI] [PubMed] [Google Scholar]
- 161.Ichinohe T, Pang IK, Kumamoto Y, et al. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc Natl Acad Sci U S A. 2011;108:5354–5359. doi: 10.1073/pnas.1019378108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kanayama M, Danzaki K, He Y-W, Shinohara ML. Lung inflammation stalls Th17-cell migration en route to the central nervous system during the development of experimental autoimmune encephalomyelitis. Int Immunol. 2016;28:463–469. doi: 10.1093/intimm/dxw013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Libbey JE, Kennett NJ, Wilcox KS, White HS, Fujinami RS. Interleukin-6, produced by resident cells of the central nervous system and infiltrating cells, contributes to the development of seizures following viral infection. J Virol. 2011;85:6913–6922. doi: 10.1128/JVI.00458-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sato F, Martinez NE, Shahid M, Rose JW, Carlson NG, Tsunoda I. Resveratrol exacerbates both autoimmune and viral models of multiple sclerosis. Am J Pathol. 2013;183:1390–1396. doi: 10.1016/j.ajpath.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Laurell A, Sjöberg K. Prebiotics and synbiotics in ulcerative colitis. Scand J Gastroenterol. 2017;52:477–485. doi: 10.1080/00365521.2016.1263680. [DOI] [PubMed] [Google Scholar]
- 166.McCoy K, Geuking M, Ronchi F. Gut microbiome standardization in control and experimental mice. Current Protocols in Immunology. Vol. 23. John Wiley and Sons, Inc; 2017. pp. 23.21.21–23.21.13. [DOI] [PubMed] [Google Scholar]
- 167.Brandt LJ, Borody TJ, Campbell J. Endoscopic fecal microbiota transplantation: “first-line” treatment for severe clostridium difficile infection? J Clin Gastroenterol. 2011;45:655–657. doi: 10.1097/MCG.0b013e3182257d4f. [DOI] [PubMed] [Google Scholar]
- 168.Sakiyama Y, Kanda N, Higuchi Y, et al. New type of encephalomyelitis responsive to trimethoprim/sulfamethoxazole treatment in Japan. Neurol Neuroimmunol Neuroinflamm. 2015;2:e143. doi: 10.1212/NXI.0000000000000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lagier J-C, Million M, Hugon P, Armougom F, Raoult D. Human gut microbiota: repertoire and variations. Front Cell Infect Microbiol. 2012;2:136. doi: 10.3389/fcimb.2012.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wang S, Xu M, Wang W, et al. Systematic Review: Adverse Events of Fecal Microbiota Transplantation. PLoS One. 2016;11:e0161174. doi: 10.1371/journal.pone.0161174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Voldsgaard A, Bager P, Garde E, et al. Trichuris suis ova therapy in relapsing multiple sclerosis is safe but without signals of beneficial effect. Mult Scler. 2015;21:1723–1729. doi: 10.1177/1352458514568173. [DOI] [PubMed] [Google Scholar]
- 172.Urbańska M, Szajewska H. The efficacy of Lactobacillus reuteri DSM 17938 in infants and children: a review of the current evidence. Eur J Pediatr. 2014;173:1327–1337. doi: 10.1007/s00431-014-2328-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Urbańska M, Gieruszczak-Bialek D, Szajewska H. Systematic review with meta-analysis: Lactobacillus reuteri DSM 17938 for diarrhoeal diseases in children. Aliment Pharmacol Ther. 2016;43:1025–1034. doi: 10.1111/apt.13590. [DOI] [PubMed] [Google Scholar]
- 174.Szajewska H, Urbanska M, Chmielewska A, Weizman Z, Shamir R. Meta-analysis: Lactobacillus reuteri strain DSM 17938 (and the original strain ATCC 55730) for treating acute gastroenteritis in children. Benef Microbes. 2014;5:285–293. doi: 10.3920/BM2013.0056. [DOI] [PubMed] [Google Scholar]