Abstract
Introduction
Obesity is a potential risk factor for erectile dysfunction (ED). MicroRNAs (miRNAs) regulate the expression of genes involved in various pathophysiologic processes.
Aim
To identify the miRNA profile in the corpus cavernosum (CC) of obese rats with ED and elucidate the potential function of miRNA in the pathogenesis of ED.
Methods
Obesity was induced in rats by a high-fat diet. After the erectile function test, experimental animals were divided into two groups: obese rats with ED and obese rats with normal erectile function. The CCs from these rats were collected for miRNA microarray analysis. The results were verified by real-time polymerase chain reaction analysis. Subsequently, the targets of differentially expressed miRNAs were predicted. Bioinformatics analysis was applied to predict the functions of differentially expressed miRNAs in ED. Apomorphine-induced penile erection and intracavernous pressure measurements were used to evaluate the effects of miRNA on the erectile function of rats.
Main Outcome Measures
MiRNA expression in the CC of obese rats with ED and those with normal erectile function was detected by miRNA microarray analysis. Candidate miRNAs were validated by real-time polymerase chain reaction. Bioinformatics analysis was used to predict the functions of miRNAs. Apomorphine-induced penile erection and intracavernous pressure measurements were used to reflect the erectile function of rats.
Results
Sixty-eight miRNAs were differentially expressed in the CC of obese rats with ED (≥1.5-fold change). The real-time polymerase chain reaction results were consistent with the miRNA microarray analysis results. Specifically, miR-328a was significantly upregulated in rats with ED compared with control rats and was chosen for functional evaluation in the pathogenesis of ED. Overexpression of miR-328a noticeably decreased the erectile response to apomorphine and the expression of heme oxygenase-1.
Conclusion
MiRNAs are involved in the pathogenesis of obesity-related ED. MiR-328a might facilitate the induction of ED.
Bai Y, Zhang L, Jiang Y, et al. Identification and Functional Verification of MicroRNAs in the Obese Rat With Erectile Dysfunction. Sex Med 2017;5:e261–e271.
Key Words: Obesity, Erectile Dysfunction, MicroRNA Microarray Analysis, MiR-328a
Introduction
The World Health Organization has defined obesity as an abnormal or excessive accumulation of body fat that presents a risk to health. The incidence of obesity has increased rapidly in recent years. Among the complications induced by obesity, erectile dysfunction (ED) is the most common disorder that occurs in approximately 40% of men and lowers the quality of their lives.1, 2, 3, 4
Obesity-related ED is a neurovascular process that involves vasoconstriction, endothelial dysfunction, smooth muscle decrease, and androgen deficiency.5 Drug therapy, especially cyclic nucleotide phosphodiesterase (PDE) inhibitors, is common for the clinical management of ED. PDE inhibitors regulate intracellular cyclic adenosine monophosphate and cyclic guanosine monophosphate (cGMP) levels through the control of cyclic nucleotides. Although the clinical application of PDE inhibitors has achieved an important milestone in ED management, there are cases in which this strategy fails to work and the incidence of ED continues to increase. In a Swedish cohort of men, the use of PDE type 5 inhibitors statistically significantly increased the risk of malignant melanoma.6 Therefore, it is very important to elucidate the pathogenesis of ED and develop novel therapeutic strategies.
MicroRNAs (miRNAs) are short non-coding transcripts of approximately 22 nucleotides that have considerable potential as diagnostic and therapeutic tools against many diseases by regulating gene expression at the post-transcriptional level.7, 8, 9 Altered expression of miRNAs was observed in the corpus cavernosum (CC) of aging rats and diabetic mice with ED.10, 11 Upregulation of miR-200a attenuated endothelial function through the inhibition of sirtuin type 1 (SIRT1).11 Therefore, miRNAs could be involved in the pathogenesis of ED. However, little is known about the alteration of miRNAs in obese rats with ED. In this study, we identified the miRNA alteration in the CC of obese rats with ED compared with obese rats with normal erectile function and assessed the potential function of miRNAs in the ED process.
Aims
The present study was designed to identify the miRNA profile in the CC of obese rats with ED and elucidate the potential function of miRNA in the pathogenesis of ED.
Methods
Ethical Concerns
All experiments were performed in accordance with Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No 85-23, revised 1996).
Establishment of High-Fat Diet–Induced Obesity Rat Model
The obesity rat model was established based on the method described by Levin and Dunnmeynell12 and Wang et al.13 Sixty male Sprague-Dawley rats (180 ± 10 g) were housed on a 12-hour light-dark cycle and fed a standard chow diet (69% carbohydrate, 20% protein, and 5% fat) for 1 week to adapt to the diet. Then, 45 rats were randomly switched to a high-fat diet (HFD; 59% carbohydrate, 20% protein, and 15% fat, which is from the recipe of the American Institute of Nutrition and the Association of Official Analytical Chemists, with slight modification). According to the method described by Levin and Dunnmeynell, after 12 weeks, 15 obese rats with the greatest body weight gain were obtained. Apomorphine (APO) was used to estimate penile erectile function in control and obese rats. Epididymal, peritoneal, and mesenteric fat weights of six rats from each group were tested randomly for additional obesity estimates. Triglyceride, total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, fasting blood glucose, hemoglobin A1c, serum insulin, and testosterone levels from six rats from each group were measured to determine metabolic profiles.
APO-Induced Penile Erection in Rat
APO (Sigma, St Louis, MO, USA) was dissolved in saline with ascorbic acid 0.2 mg/mL at a concentration of 10 μg/mL to induce erection. After a 10-minute habituation period, rats were injected with APO 80 μg/kg subcutaneously in the loose skin at the back of the neck. Observation was done for 30 minutes. Emergence of the engorged glans penis and distal shaft was noted as an erection. The rate of erection and yawn of rats in each group were recorded.14
Intracavernous Pressure Measurement
Intracavernous pressure (ICP) was measured to assess erectile function before and during cavernosal nerve electrical stimulation in obese and control rats. Rats were anesthetized with an intraperitoneal injection of chloral hydrate (3 mL/kg). Polyethylene-50 tubing connected to a pressure transducer (Aerospace Medical Engineering Institute, Beijing, China) was cannulated into the carotid artery for the measurement of mean systemic arterial pressure (MAP). To perform the ICP measurement, cavernous nerves were exposed through a midline laparotomy and were electrically stimulated at a frequency of 10 Hz with a pulse width of 5 ms and at 5 V for 60 seconds with resting periods of 5 minutes between subsequent stimulations.15 The ratio of peak ICP to MAP (ICP/MAP) was used to evaluate erectile function.
Hematoxylin and Eosin and Immunofluorescence Histochemistry Staining
Hematoxylin and eosin staining was used to test the CC structure of normal rats and obese rats with ED. Frozen sections of the mid-penis were fixed in 4% paraformaldehyde for 30 minutes. After rinsing with phosphate buffered saline (PBS; pH = 7.5), sections were stained with hematoxylin for 5 minutes, differentiated by 1% hydrochloric acid and ethanol for 10 seconds, and then eosin staining solution was added for 2 minutes. Stained sections were examined under a microscope (BX53, Olympus, Tokyo, Japan).
Immunofluorescence histochemistry was performed to test α-smooth muscle actin expression in the CC of normal rats and obese rats with ED. After fixing in 4% paraformaldehyde for 30 minutes, frozen sections of the mid-penis were blocked with bovine serum albumin for 30 minutes at 37°C. Then, they were incubated with α-smooth muscle actin (1:100; Elabscience, Houston, TX, USA) antibody overnight at 4°C. After extensive washing with PBS, sections were incubated with Alexa-594–conjugated secondary antibodies (1:400; Bioss, Woburn, MA, USA) with fluorescence and 4,6-diamidino-2-phenylindole nuclear staining at room temperature away from the light. Fluorescence microscopic images were acquired using a microscope (BX53). Image analysis was performed by computerized densitometry using Image-Pro Plus 6.0 (Media Cybernetics, Silver Spring, MD, USA). To quantify smooth muscle, the percentage of α-smooth muscle actin indicating a positive area within the CC was analyzed.
MiRNA Microarray Analysis
Total RNA of the CC from obese rats with or without ED was harvested and extracted using Trizol reagent (Invitrogen, San Diego, CA, USA) according to the manufacturer’s instructions. The concentration and purity of RNA were determined by measuring absorbance in a spectrophotometer at 260 and 280 nm. Quantified RNA samples were labeled using the miRCURYTM array labeling kit (Exiqon, Vedbaek, Denmark) and hybridized on the miRCURYTM array microarray kit (Exiqon). After washing, the microarrays were scanned with a GenePix4000B microarray scanner (Axon Instruments, Sunnyvale, CA, USA) and analyzed with Pro 6.0 software (Axon Instruments). Expression data were normalized using median normalization. After normalization, average values of the replicate spots of each miRNA were used for statistical analysis. Differentially expressed miRNAs were identified as a fold change greater than 1.5.
Real-Time Polymerase Chain Reaction
Total RNA was extracted from the CC of normal rats, obese rats with normal erectile function, and obese rats with ED using Trizol reagent (Invitrogen). First-strand cDNA was synthesized using a reverse transcription kit (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s instructions. Real-time polymerase chain reaction (RT-PCR) analysis was performed on an ABI 7500 fast RT-PCR system (Applied Biosystems), with U6 as an internal control. The RT-PCR conditions were as follows: 95°C for 5 minutes followed by 40 cycles at 95°C for 10 seconds, 60°C for 20 seconds, and 72°C for 20 seconds. Relative RNA expression data were analyzed using the 2−ΔΔCt method.
Functional Assignment to Potential Targets of Differentially Expressed miRNAs
The predicted targets of differentially expressed miRNAs were obtained from the TargetScan website (http://www.targetscan.org/mamm_31/). Gene ontology (GO) classification and Kyoto Encyclopedia Genes and Genomes (KEGG) enrichment of identified target genes were used to evaluate the functions of differentially expressed miRNA. For all functional analyses, the Fisher exact test corrected by the false discovery rate method was used to determine the probability that each biological function assigned to that dataset was due to chance alone.
MiRNA Transfection In Vivo
MiR-328a, miR-NC, and miR-328a inhibitor (an antisense oligodeoxyribonucleotide of miR-328a, AMO-328a, and ACGGAAGGGCAGAGAGGGCCAG) were diluted in RNase-free sterile water at a concentration of 600 μg/mL. MiRNAs (30 μg) were transfected to the CC of normal rats with the base diet (n = 10 for each group) using a transfection reagent (Invitrogen) two times at an interval of 48 hours. The control group was administered with the transfection reagent.
Western Blot
After transfection for 48 hours, total protein extracted from the CC was obtained. Protein samples were separated by electrophoresis in 10% sodium dodecylsulfate polyacrylamide gels and the protein was transferred to polyvinylidene difluoride membranes (Millipore, Bedford, MA, USA). Membranes were blocked with 5% non-fat dry milk in PBS for 2 hours. Samples were incubated overnight at 4°C with primary antibodies for heme oxygenase-1 (HO-1; 1:500; Abcam, Cambridge, MA, USA). The next day, membranes were washed three times, each time for 10 minutes with PBS containing 0.5% Tween 20, and then incubated with secondary antibodies (1:10,000; ZSGB, Beijing, China) for 1 hour at room temperature. Membranes were rinsed with PBS before scanning. Images were captured on the Gel Doc XR System (Bio-Rad, Richmond, CA, USA). Western blotting bands were quantified using Quantity One software (Bio-Rad). Glyceraldehyde 3-phosphate dehydrogenase (1:1,000; ZSGB) served as an internal control.
Data Analysis
Data are expressed as mean ± standard error of the mean and were analyzed with SPSS 13.0 (SPSS, Inc, Chicago, IL, USA). Statistical comparison between the two groups was performed using the Student t-test. The relation between two dichotomous variables was calculated by the Fisher exact test. A two-tailed P value less than .05 was considered statistically significant.
Main outcome measures
MiRNA expression profiles of the CC from obese rats were detected by miRNA microarray analysis. Candidate miRNAs were validated by RT-PCR. Bioinformatics analysis was use to predict the functions of differentially expressed miRNAs. APO-induced penile erection and MAP measurements were used to reflect the function of miR-328a in the penile erectile process.
Results
HFD Can Cause Obesity and Induce ED in Rats
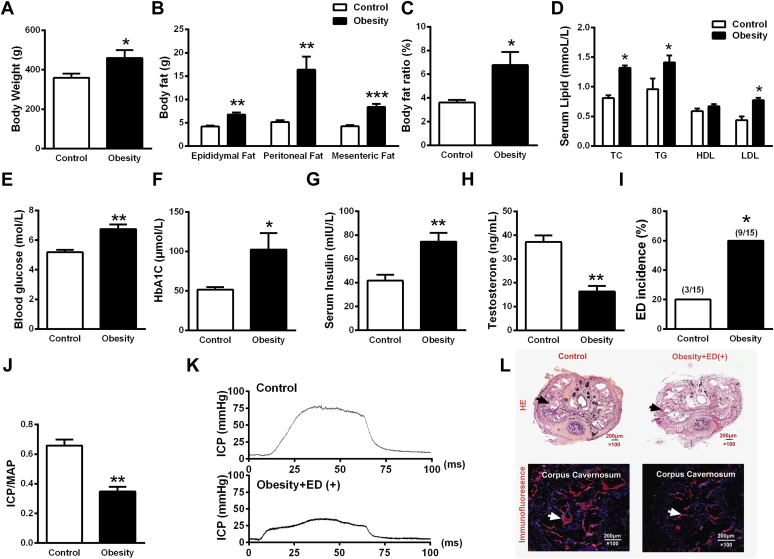
To assess whether the obesity model was successfully established, the body weight of rats was monitored. Rats fed with an HFD for 12 weeks had a greater body weight change than the control group on the base diet (Figure 1A). Epididymal, peritoneal, and mesenteric fat weights and body fat ratio were increased in rats fed the HFD (Figure 1B, C). Blood levels of total cholesterol, low-density lipoprotein cholesterol, and triglyceride also were obviously increased in the HFD group (Figure 1D). All these results indicated that the HFD induced obesity.
Figure 1.
Basic characteristics of rats fed a high-fat diet. Panel A shows body weight (n = 15 per group). Panel B shows body fat (n = 6 per group). Panel C shows the body fat ratio calculated by the ratio of total fat weight to body weight (n = 6 per group). Panel D shows serum lipid contents in blood (n = 6 per group). Panel E shows the content of blood glucose (n = 6 per group). Panel F shows the content of HbA1c (n = 6 per group). Panel G shows the content of testosterone (n = 6 per group). Panel H shows the content of serum insulin (n = 6 per group). Panel I shows the incidence of ED in normal control and obesity plus ED groups (n = 15 per group). Panel J shows erectile function parameters (ICP/MAP ratio) of normal control and obesity plus ED groups (n = 15 per group). Panel K shows representative ICP recordings of normal control and obesity plus ED groups. Panel L shows HE and immunofluorescence histochemistry staining. Arrows represent the corpus cavernosum and smooth muscle (indicated by α-smooth muscle actin) in the corpus cavernosum of each group. *P < .05 vs control; **P < .01 vs control; ***P < .001 vs control for all experiments. ED = erectile dysfunction; HbA1c = hemoglobin A1c; HDL = high-density lipoprotein cholesterol; HE = hematoxylin and eosin; ICP = intracorporal pressure; LDL = low-density lipoprotein cholesterol; MAP = mean arterial pressure; TC = total cholesterol; TG = glycerin trilaurate.
Levels of some risk factors for ED, including fasting blood glucose, hemoglobin A1c, and serum insulin levels, were noticeably increased in the blood of obese rats (Figure 1E–G), whereas the testosterone level was remarkably decreased (Figure 1H). The erectile function of each rat was evaluated by the APO-induced penile erection test. We found that obesity increased the ED incidence from 20.0% to 60.0% (Figure 1I). Cavernous nerve stimulation results showed a significant decrease in the change of the ICP/MAP ratio from baseline to obesity (0.35 ± 0.04) in rats fed the HFD compared with control rats (0.66 ± 0.05; Figure 1J, K). Histologic measurements showed that the structure of the penile and smooth muscle content within the CC of obese rats with ED changed significantly compared with control rats with normal erectile function (Figure 1L). Taken together, these observations confirmed that rats fed the HFD for 12 weeks developed ED.
MiRNAs Were Differentially Expressed in Obesity-Induced ED
The expression of miRNAs in the CC of obese rats with ED and obese rats with normal erectile function was detected by miRNA microarray analysis. Differentially expressed miRNAs were identified as a fold change greater than 1.5. Sixty-eight differentially expressed miRNAs were identified. Among them, 48 miRNAs were unregulated and 20 miRNAs were downregulated in obese rats with ED (Tables 1 and 2). These results indicated that miRNAs could play an important role in the occurrence and development of obesity-induced ED.
Table 1.
Significantly upregulated miRNAs
| ID | Name | Fold change | ID | Name | Fold change |
|---|---|---|---|---|---|
| MIMAT0000874 | rno-miR-200a-3p | 24.03 | MIMAT0000552 | rno-miR-301a-3p | 2.01 |
| MIMAT0003122 | rno-miR-370-3p | 22.88 | MIMAT0001547 | rno-miR-450a-5p | 1.99 |
| MIMAT0000797 | rno-miR-26b-5p | 16.02 | MIMAT0003174 | rno-miR-540-3p | 1.94 |
| MIMAT0003118 | rno-miR-215 | 15.45 | MIMAT0000834 | rno-miR-128-3p | 1.88 |
| MIMAT0000840 | rno-miR-134-5p | 13.96 | MIMAT0000843 | rno-miR-137-3p | 1.85 |
| MIMAT0000783 | rno-miR-10b-5p | 7.25 | MIMAT0000860 | rno-miR-183-5p | 1.84 |
| MIMAT0000848 | rno-miR-142-3p | 5.22 | MIMAT0003162 | rno-miR-1-5p | 1.84 |
| MIMAT0000818 | rno-miR-96-5p | 5.15 | MIMAT0000564 | rno-miR-328a-3p | 1.79 |
| MIMAT0003177 | rno-miR-541-5p | 4.58 | MIMAT0000822 | rno-miR-100-5p | 1.78 |
| MIMAT0000816 | rno-miR-92a-3p | 4.39 | MIMAT0000795 | rno-miR-25-3p | 1.73 |
| MIMAT0000853 | rno-miR-150-5p | 3.97 | MIMAT0000827 | rno-miR-122-5p | 1.72 |
| MIMAT0000879 | rno-miR-206-3p | 3.43 | MIMAT0000824 | rno-miR-103-3p | 1.70 |
| MIMAT0000881 | rno-miR-210-3p | 2.75 | MIMAT0000550 | rno-miR-323-3p | 1.68 |
| MIMAT0000585 | rno-miR-340-3p | 2.71 | MIMAT0000808 | rno-miR-30a-5p | 1.64 |
| MIMAT0000888 | rno-miR-218a-5p | 2.67 | MIMAT0000563 | rno-let-7d-3p | 1.63 |
| MIMAT0003196 | rno-miR-376b-3p | 2.62 | MIMAT0001549 | rno-miR-365-3p | 1.59 |
| MIMAT0000589 | rno-miR-342-3p | 2.53 | MIMAT0000789 | rno-miR-19a-3p | 1.58 |
| MIMAT0000876 | rno-miR-203a-3p | 2.44 | MIMAT0003176 | rno-miR-539-5p | 1.58 |
| MIMAT0000869 | rno-miR-194-5p | 2.39 | MIMAT0000899 | rno-miR-297 | 1.57 |
| MIMAT0003152 | rno-miR-22-5p | 2.33 | MIMAT0003200 | rno-miR-487b-3p | 1.54 |
| MIMAT0000792 | rno-miR-23a-3p | 2.13 | MIMAT0000811 | rno-miR-32-5p | 1.54 |
| MIMAT0000615 | rno-miR-101b-3p | 2.11 | MIMAT0000897 | rno-miR-292-3p | 1.54 |
| MIMAT0000900 | rno-miR-298-5p | 2.10 | MIMAT0000599 | rno-miR-349 | 1.53 |
| MIMAT0000812 | rno-miR-33-5p | 2.02 | MIMAT0000809 | rno-miR-30a-3p | 1.51 |
Table 2.
Significantly downregulated miRNAs
| ID | Name | Fold change | ID | Name | Fold change |
|---|---|---|---|---|---|
| MIMAT0000833 | rno-miR-127-3p | 0.03 | MIMAT0000775 | rno-let-7b-5p | 0.49 |
| MIMAT0000892 | rno-miR-223-3p | 0.18 | MIMAT0003117 | rno-miR-361-5p | 0.49 |
| MIMAT0003204 | rno-miR-409a-5p | 0.19 | MIMAT0000587 | rno-miR-341 | 0.56 |
| MIMAT0000847 | rno-miR-142-5p | 0.23 | MIMAT0003121 | rno-miR-483-3p | 0.57 |
| MIMAT0000828 | rno-miR-124-3p | 0.36 | MIMAT0000787 | rno-miR-18a-5p | 0.58 |
| MIMAT0000821 | rno-miR-99b-5p | 0.38 | MIMAT0000774 | rno-let-7a-5p | 0.58 |
| MIMAT0003115 | rno-miR-207 | 0.40 | MIMAT0000574 | rno-miR-140-3p | 0.60 |
| MIMAT0003201 | rno-miR-382-5p | 0.41 | MIMAT0003113 | rno-miR-489-3p | 0.61 |
| MIMAT0003153 | rno-miR-24-1-5p | 0.42 | MIMAT0003381 | rno-miR-499-5p | 0.61 |
| MIMAT0000813 | rno-miR-34b-5p | 0.46 | MIMAT0000864 | rno-miR-187-3p | 0.62 |
RT-PCR Analysis of miRNA Expression in the CC of Rats
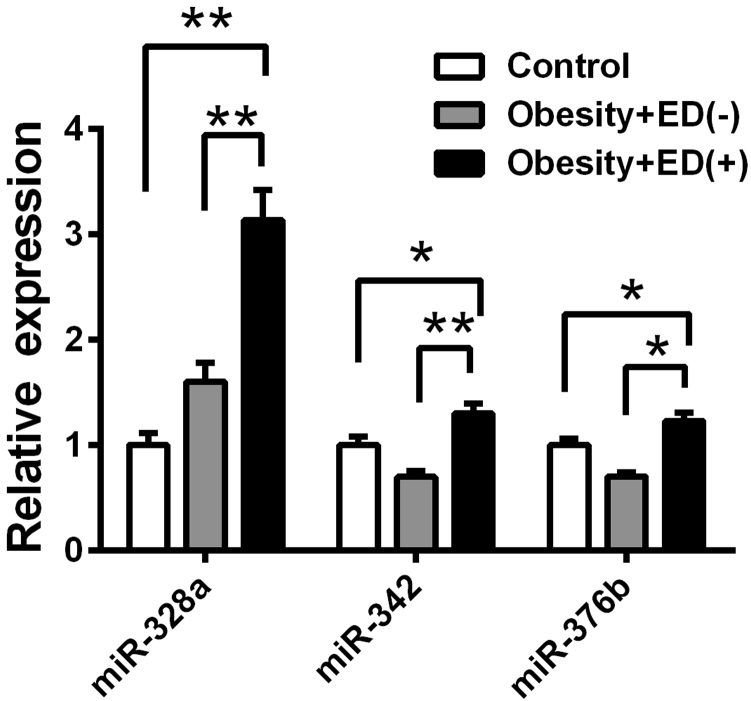
To confirm the miRNA expression in the CC of rats, three potentially functional upregulated miRNAs were chosen for RT-PCR validation from five obese rats with normal erectile function and five obese rats with ED. The expression of miR-328a, miR-342, and miR-376b was upregulated in the CC of obese rats with ED compared with obese rats with normal erectile function and normal control rats, which was consistent with the miRNA microarray analysis results (Figure 2).
Figure 2.
Real-time polymerase chain reaction quantification of three miRNAs in the corpus cavernosum of rats (n = 5). *P < .05; **P < .01 vs obesity ED(−) or control group. ED(−) = without erectile dysfunction; ED(+) = with erectile dysfunction.
Annotation of Potential Targets of Differentially Expressed miRNAs
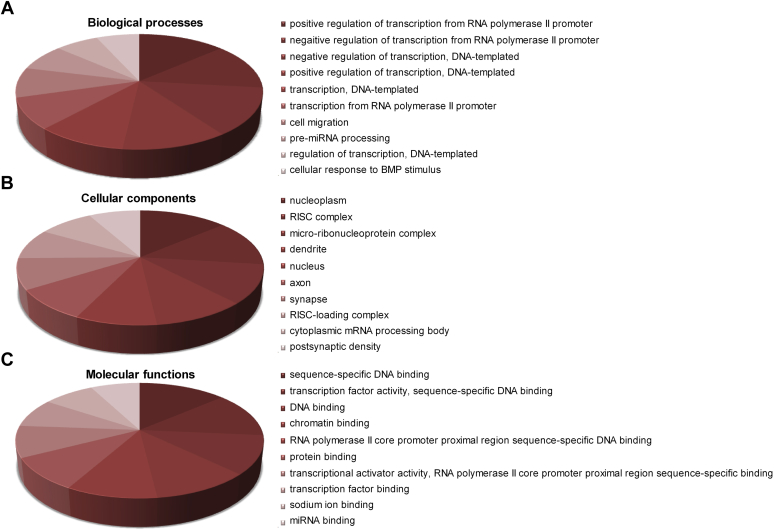
To determine whether dysregulated miRNAs might be causal factors or contributors or merely represent parallel changes in the occurrence of ED, the predicted miRNA target genes were subjected to GO enrichment analysis to identify protein-coding genes regulated by ED-responsive miRNAs. Results showed that differentially expressed genes in 1,303 biological processes, 231 molecular components, and 439 cellular functions were significantly involved in ED-related functions (P < .05). As shown in Figure 3, perhaps the most prominent finding in our study was the association of the clear majority of these genes with pre-miRNA processing; dendrite, axon, and postsynaptic density; and miRNA binding.
Figure 3.
Top 10 enrichment in gene ontology analysis with adjusted P values. Panel A presents biological processes. Panel B presents cellular components. Panel C presents molecular functions. RISC = RNA-inducing silencing complex.
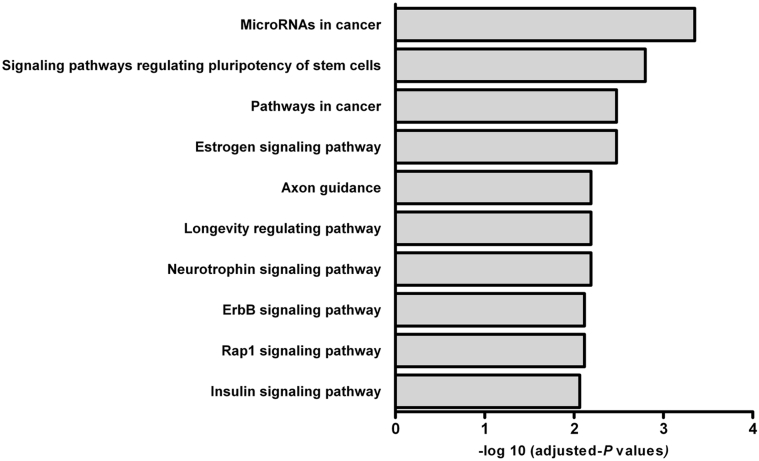
The biological interpretation of the target genes of differential miRNAs was extended using KEGG pathway analysis. Seventy-six different metabolic pathways were found. The top 10 KEGG pathways are shown in Figure 4. These results indicated that several pathways involved in obesity-induced ED, such as miRNAs in cancer, longevity regulating pathway, insulin signaling pathway, axon guide, and neurotrophin signaling pathway, also were activated.
Figure 4.
Top 10 Kyoto Encyclopedia Genes and Genomes pathways with adjusted P values.
Upregulation of miR-328a Facilitates Onset of ED
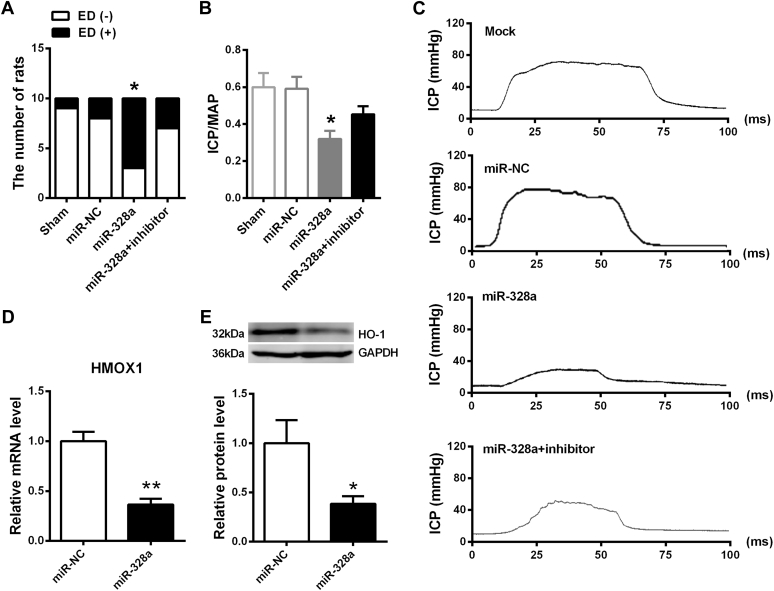
To assess whether miR-328a had any function in the pathogenesis of ED, miR-328a, miR-NC, and miR-328a inhibitor were transfected into the CC of normal rats. Three rats developed an erection and seven developed ED in the miR-328a group, eight rats developed an erection and two developed ED in the miR-NC control group, and nine rats developed an erection and one developed ED in the miR-328a inhibitor control group. These results indicated that miR-328a decreased the erectile response to APO and that the effect of miR-328a on erectile function could be partly reversed by miR-328a inhibitor (Figure 5A). Moreover, control rats displayed normal ICP curves and significant higher ICP/MAP ratios than miR-328a–transfected rats (Figure 5B, C). To confirm the function of miR-328a in the occurrence of ED, we also tested the expression of HMOX1 mRNA and HO-1 protein, which could regulate the signaling mediator of erectile function expression, namely cGMP, in the CC. The results showed that miR-328a overexpression could decrease the expression of HMOX1 mRNA and HO-1 protein (Figure 5D, E).
Figure 5.
Effect of miR-328a on erectile function in rats. Panel A shows the number of ED(−) and ED(+) rats after transfection (n = 10 per group; *P < .05 vs miR-NC). Panel B shows ICP/MAP ratio (n = 10 per group; *P < .05 vs miR-NC). Panel C shows representative ICP tracing response to stimulation of the cavernous nerve (5 V, 10 Hz, and 60-second duration) in rats (n = 10 per group). Panel D shows miR-328a–attenuated HMOX1 mRNA expression (n = 5 per group). Panel E shows miR-328a–attenuated HO-1 protein expression (n = 5 per group; *P < .05; **P < .01 vs miR-NC). ED(−) = without erectile dysfunction; ED(+) = with erectile dysfunction; GAPDH = glyceraldehyde 3-phosphate dehydrogenase; HO-1 = heme oxygenase-1; ICP = intracorporal pressure; MAP = mean arterial pressure; miR-NC = negative control for miR-328a.
Discussion
The World Health Organization data showed that an estimated 35.8 million (2.3%) global disability adjusted life years worldwide are caused by obesity, which lead to adverse metabolic effects on blood pressure, cholesterol, triglycerides, and insulin resistance. Obesity is associated with the high prevalence of ED because of these effects.1, 16 The incidence of obesity will continue to increase with an unhealthy lifestyle. Thus, more and more men could develop obesity-related ED, which affects the quality of their lives. Thus, it is essential to explore effective treatment considering the pathogenesis of obesity-related ED.
Several studies have proved that miRNAs are involved in the pathogenesis of ED. MiRNAs aberrantly expressed in the CC of aging rats with ED include miR-1, miR-200a, miR-203, and miR-206. These four miRNAs might play important roles by regulating the endothelial nitric oxide (NO) synthase, NO, and protein kinase G and the prostaglandin E1 and protein kinase A pathways and thus the development of aging-induced ED in rats.10 Similarly, the alteration of miRNA expression was found in diet-induced vasculogenic ED in mice. Diet-induced ED resulted in a significantly increased expression of miR-1973c and miR-151 and decreased expression of miR-153 and miR-425.11 However, these works did not provide direct evidence that aberrantly expressed miRNAs would cause ED.
In this work, we established a Sprague-Dawley rat model with HFD-induced obesity to evaluate the relation between obesity-related ED and miRNAs. We found that some hazards such as glucose, insulin, and testosterone levels in rats with ED were remarkably changed in the serum of obese rats, although erectile function was decreased in some obese rats; the reason for this observation could be that ED is a complicated neurovascular process and that genetic and psychological factors also are important risk factors for ED. Therefore, we investigated aberrantly expressed miRNAs in the CC of obese rats with ED compared with obese rats with normal erectile function. Sixty-eight miRNAs were differentially expressed in obese rats with ED; 48 were upregulated and 20 were downregulated. In accordance with the study on aging-induced ED, the miRNA microarray analysis showed that miR-1, miR-200a, miR-203, and miR-206 were upregulated in the CC of obese rats with ED. However, let-7b and miR-26b expression patterns in obese rats with ED differed from those in a murine model of diet-induced vasculogenic ED.11 The reason for this observation could be that the grouping method differed in the two works. In the previous study, the control group was composed of healthy mice, which were used to identify aberrantly expressed miRNAs induced by diet-related ED in the reference group; in contrast, in the present study, the control group was composed of obese rats with normal erectile function, which were used to validate aberrantly expressed miRNAs induced by obesity-related ED.
We predicted that miR-328a would target HMOX1, which encodes the HO-1 protein, which increases the expression of cGMP, the signaling mediator of erectile function.17 MiR-342 can regulate multiple angiogenic pathways, including notch, vascular endothelial growth factor, and transforming growth factor-β (TGF-β) signaling, which might be involved in the pathologic process of ED.18 MiR-376 has been reported to target insulin-like growth factor-1 (IGF-1) directly, so the change of miR-376 could involve low serum IGF-1 levels in patients with ED.19 To validate the microarray findings, we selected these three potential functional miRNAs for RT-PCR analysis. Results showed that the expression of miR-328a, miR-376b, and miR-342 was upregulated in the CC of obese rats with ED, which was similar to the miRNA microarray findings. Interestingly, the miR-328a level also was increased in obese rats with normal erectile function but lower than in obese rats with ED, indicating that miR-328a might be involved in obesity-related ED.
The targets of differentially expressed miRNAs were predicted using TargetScan software. GO analysis showed that these predicted targets were involved in 1,303 biological processes, 231 molecular components, and 439 cellular functions. The most common GO categories were related to cell replication, neuroregulation, and miRNA regulation. It has been reported that these categories are related to the pathogenesis of ED.20, 21, 22, 23 KEGG pathway analysis showed 76 statistically remarkable categories. The most enriched pathways, including miRNAs in cancer, longevity regulating pathway, insulin signaling pathway, axon guide, and neurotrophin signaling pathway, also could contribute to the pathogenesis of ED.
To further confirm the accuracy of miRNA array analysis and bioinformatics prediction, the effect of miR-328a on erectile function was evaluated in rats. We found that miR-328a was significantly upregulated in the CC of obese rats with ED. To explore the role of miR-328a in erectile function, miR-328a and miR-NC were transfected into the CC of rats with the normal diet. We found that upregulation of miR-328a impaired erectile function in rats, which could contribute to the downregulation of an important molecule in mediating erectile function (ie, HO-1) in the CC of rats. HO-1 has been reported to generate carbon monoxide, which has a positive effect on soluble guanylate cyclase and cGMP levels in rat vascular endothelial cells and cavernous tissue.24 HO also scavenges reactive oxygen species to prevent NO from reacting with reactive oxygen species and forming peroxynitrite.25 Downregulation of HO-1 gene expression could lead to the decrease in erectile function in diabetes mellitus.
In addition, KEGG results showed that miR-328a is involved in the insulin signaling pathway. IGF-1 is a key regulator in the insulin signaling pathway. It has been proved that miR-328a can regulate hypoxic pulmonary hypertension by targeting the IGF-1 receptor gene.26 In addition, the increased incidence of ED has been associated with diabetes. A decrease in IGF protein expression was observed in rats with streptozotocin-induced diabetes. Direct injection of an adenovirus encoding the IGF-1 gene to the CC reversed impaired erectile function in diabetic rats.27 Furthermore, gene transfer of IGF-1 to the penis could improve erectile capacity in aged rats. This effect was noted by the restoration of the integrity of CC smooth muscle and the modulation of NO-cGMP signaling activity.28
TGF-β is a critical cytokine in the process of fibrosis in the development of aging-related ED.29 Specifically, TGF-β1 is an important molecular involved in a wide range of cellular functions, such as cell cycle regulation, neuronal differentiation, and survival. Moreover, TGF-β1 promotes human hematopoietic progenitors from the quiescence to the cycling state in vitro.30 TGF-β1 also can promote stem cell quiescence and neuronal survival. Recently, miR-328 has been reported to target TGF-β1 and increase cardiac fibrosis in mice.31 These findings suggest that miR-328 might impair stem cell or neuronal survival. CD44 presents a positive expression in adipose-derived stem cells whose high induction rate could guarantee sufficient neuron-like cells. MiR-328 has been found to control zonation morphogenesis by targeting CD44. MiR-328 also has been found to decrease cell adhesion and migration and decrease the formation of capillary structures through the downregulation of CD44 in A431 human epidermoid carcinoma cells.32 These findings strongly suggest that miR-328 could induce ED after cavernous nerve injury.
In light of the previous evidence, it is clear that a delicate balance of mechanisms responsible for maintaining calcium homeostasis is critical to normal erectile function.33 Sarcoplasmic reticulum Ca2+-adenosine triphosphatase (SERCA) has been found to be an important regulator in calcium homeostasis.34 Notably, miR-328 could target SERCA2a directly and increase the intracellular calcium concentration in the pathogenesis of cardiac hypertrophy.35 This evidence implies that miR-328 might govern ED by affecting calcium homeostasis.
There are some limitations to this study. The CC injection could have impaired the tissue to a certain extent, although this treatment did not affect the function of miR-328a in ED. Also, we do not know its psychological effects. Clearly, a new drug delivery strategy should be investigated in future studies.
Conclusions
Our study, for the first time, explored the miRNA profile of the CC of obese rats with ED compared with obese rats with normal erectile function. MiRNA microarray analysis identified 68 differentially expressed miRNAs in the CC of obese rats with ED. Of these, 48 were upregulated and 20 were downregulated. RT-PCR results were consistent with the miRNA microarray findings. Upregulation of miR-328a was found to be responsible for the onset of ED. Determination of changes in miRNAs and related regulatory networks could open exciting avenues to improve the diagnosis and treatment options for ED.
Statement of authorship
Category 1
-
(a)Conception and Design
- Yunlong Bai; Baofeng Yang
-
(b)Acquisition of Data
- Jiaming Ju; Guiyang Li; Juan Xu; Xing Jiang; Peng Zhang; Linchuan Lang; Olga Sadkovaya; Peter V. Glybochko; Wei Zhang
-
(c)Analysis and Interpretation of Data
- Liangshuan Zhang; Yanan Jiang; Juan Xu
Category 2
-
(a)Drafting the Article
- Yunlong Bai; Liangshuan Zhang; Yanan Jiang
-
(b)Revising It for Intellectual Content
- Yunlong Bai; Baofeng Yang
Category 3
-
(a)Final Approval of the Completed Article
- Yunlong Bai; Liangshuan Zhang; Yanan Jiang; Jiaming Ju; Guiyang Li; Juan Xu; Xing Jiang; Peng Zhang; Linchuan Lang; Olga Sadkovaya; Peter V. Glybochko; Wei Zhang; Baofeng Yang
Acknowledgment
The authors would like to thank Drs Zhe Li, Dongmei Gong, Tao Ban, Rong Huo, and Dongfang Gu for their assistance with the supplementary materials.
Footnotes
Conflicts of Interest: Authors declare no conflict of interests.
Funding: This work was supported by the Educational Commission of Heilongjiang Province of China (grant 12511317).
References
- 1.Lavie C.J., McAuley P.A., Church T.S. Obesity and cardiovascular diseases: implications regarding fitness, fatness, and severity in the obesity paradox. J Am Coll Cardiol. 2014;63:1345–1354. doi: 10.1016/j.jacc.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 2.Pinto G., Beltran-Sanchez H. Prospective study of the link between overweight/obesity and diabetes incidence among Mexican older adults: 2001–2012. Salud Publica Mexico. 2015;57(Suppl 1):S15–S21. doi: 10.21149/spm.v57s1.7585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iyengar N.M., Hudis C.A., Dannenberg A.J. Obesity and cancer: local and systemic mechanisms. Annu Rev Med. 2015;66:297–309. doi: 10.1146/annurev-med-050913-022228. [DOI] [PubMed] [Google Scholar]
- 4.Schouten B.W., Bohnen A.M., Groeneveld F.P. Erectile dysfunction in the community: trends over time in incidence, prevalence, GP consultation and medication use—the Krimpen study: trends in ED. J Sex Med. 2010;7:2547–2553. doi: 10.1111/j.1743-6109.2010.01849.x. [DOI] [PubMed] [Google Scholar]
- 5.Kanchan C., Varant K., Leslee S. Diabetes, obesity and erectile dysfunction: field overview and research priorities. J Urol. 2009;182(Suppl):S45–S50. doi: 10.1016/j.juro.2009.07.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loeb S., Folkvaljon Y., Lambe M. Use of phosphodiesterase type 5 inhibitors for erectile dysfunction and risk of malignant melanoma. JAMA. 2015;313:2449–2455. doi: 10.1001/jama.2015.6604. [DOI] [PubMed] [Google Scholar]
- 7.Joshua T., Eric N. MicroRNAs in stress signaling and human disease. Cell. 2012;148:1172–1187. doi: 10.1016/j.cell.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turchinovich A., Cho W.C. The origin, function and diagnostic potential of extracellular microRNA in human body fluids. Front Genet. 2014;5:30. doi: 10.3389/fgene.2014.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li X., Yang W., Lou L. microRNA: a promising diagnostic biomarker and therapeutic target for hepatocellular carcinoma. Digest Dis Sci. 2014;59:1099–1107. doi: 10.1007/s10620-013-3006-1. [DOI] [PubMed] [Google Scholar]
- 10.Pan F., Xu J., Zhang Q. Identification and characterization of the MicroRNA profile in aging rats with erectile dysfunction. J Sex Med. 2014;11:1646–1656. doi: 10.1111/jsm.12500. [DOI] [PubMed] [Google Scholar]
- 11.Barbery C.E., Celigoj F.A., Turner S.D. Alterations in microRNA expression in a murine model of diet-induced vasculogenic erectile dysfunction. J Sex Med. 2015;12:621–630. doi: 10.1111/jsm.12793. [DOI] [PubMed] [Google Scholar]
- 12.Levin B.E., Dunnmeynell A.A. Defense of body weight against chronic caloric restriction in obesity-prone and -resistant rats. Am J Physiol Regul Integr Comp Physiol. 2000;278:R231. doi: 10.1152/ajpregu.2000.278.1.R231. [DOI] [PubMed] [Google Scholar]
- 13.Wang S.R., Ma W.W., Zhao D. Establishment of obesity prone and obesity resistant rats induced by high-fat diet. Chin J Public Health. 2007;23:774–775. [Google Scholar]
- 14.Chen Y., Li S.X., Yao L.S. Valsartan treatment reverses erectile dysfunction in diabetic rats. Int J Impot Res. 2007;19:366–370. doi: 10.1038/sj.ijir.3901534. [DOI] [PubMed] [Google Scholar]
- 15.Bernabe J., Rampin O., Sachs B.D., Giuliano F. Intracavernous pressure during erection in rats: an integrative approach based on telemetric recording. Am J Physiol Regul Integr Comp Physiol. 1999;276:R441–R449. doi: 10.1152/ajpregu.1999.276.2.R441. [DOI] [PubMed] [Google Scholar]
- 16.Corona G., Rastrelli G., Filippi S. Erectile dysfunction and central obesity: an Italian perspective. Asian J Androl. 2014;16:581–591. doi: 10.4103/1008-682X.126386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abdel Aziz M.T., Mostafa T., Atta H. Effect of HO-1 cDNA-liposome complex transfer on erectile signalling of aged rats. Andrologia. 2009;41:176–183. doi: 10.1111/j.1439-0272.2008.00911.x. [DOI] [PubMed] [Google Scholar]
- 18.Yan X.C., Cao J., Liang L. MiR-342-5p is a notch downstream molecule and regulates multiple angiogenic pathways including notch, vascular endothelial growth factor and transforming growth factor β signaling. J Am Heart Assoc. 2016;5:1–15. doi: 10.1161/JAHA.115.003042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liron Z., Roi A., Aviv B. Silencing of a large microRNA cluster on human chromosome 14q32 in melanoma: biological effects of mir-376a and mir-376c on insulin growth factor 1 receptor. Mol Cancer. 2012;11:44. doi: 10.1186/1476-4598-11-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lau D.H., Thompson C.S., Bellringer J.F. Doxazosin and serotonin (5-HT) receptor (1A, 2A, and 4) antagonists inhibit 5-HT–mediated human cavernosal contraction. J Androl. 2006;27:679–685. doi: 10.2164/jandrol.106.000547. [DOI] [PubMed] [Google Scholar]
- 21.Chung H., Jung S.H., Ryu J.K. Isolation and characterization of smooth muscle cells from rat corpus cavernosum tissue for the study of erectile dysfunction. Korean J Urol. 2012;53:556–563. doi: 10.4111/kju.2012.53.8.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He S., Zhang T., Liu Y. Myocardin restores erectile function in diabetic rats: phenotypic modulation of corpus cavernosum smooth muscle cells. Andrologia. 2015;47:303–309. doi: 10.1111/and.12261. [DOI] [PubMed] [Google Scholar]
- 23.Pan F., Qiu X.F., Yu W. MicroRNA-200a is up-regulated in aged rats with erectile dysfunction and could attenuate endothelial function via SIRT1 inhibition. Asian J Androl. 2016;18:74–79. doi: 10.4103/1008-682X.154991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marks G.S., Brien J.F., Nakatsu K. What role does the heme-heme oxygenase-carbon monoxide system play in vasoregulation? Am J Physiol Regul Integr Comp Physiol. 2003;285:R522–R523. doi: 10.1152/ajpregu.00317.2003. [DOI] [PubMed] [Google Scholar]
- 25.Abdel Aziz M.T., Al-Asmar M.F., Mostafa T. Assessment of heme oxygenase-1 (HO-1) activity in the cavernous tissues of sildenafil citrate-treated rats. Asian J Androl. 2007;9:377–381. doi: 10.1111/j.1745-7262.2007.00241.x. [DOI] [PubMed] [Google Scholar]
- 26.Guo L., Qiu Z., Wei L. The microRNA-328 regulates hypoxic pulmonary hypertension by targeting at insulin growth factor 1 receptor and L-type calcium channel-alpha1C. Hypertension. 2012;59:1006–1013. doi: 10.1161/HYPERTENSIONAHA.111.185413. [DOI] [PubMed] [Google Scholar]
- 27.Pu X.Y., Hu L.Q., Wang H.P. Improvement in erectile dysfunction after insulin-like growth factor-1 gene therapy in diabetic rats. Asian J Androl. 2007;9:83–91. doi: 10.1111/j.1745-7262.2007.00215.x. [DOI] [PubMed] [Google Scholar]
- 28.Pu X.Y., Wang X.H., Gao W.C. Insulin-like growth factor-1 restores erectile function in aged rats: modulation the integrity of smooth muscle and nitric oxide-cyclic guanosine monophosphate signaling activity. J Sex Med. 2008;5:1345–1354. doi: 10.1111/j.1743-6109.2008.00817.x. [DOI] [PubMed] [Google Scholar]
- 29.Gonzalez-Cadavid N.F., Rajfer J. Molecular pathophysiology and gene therapy of aging-related erectile dysfunction. Exp Gerontol. 2004;39:1705–1712. doi: 10.1016/j.exger.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 30.Hatzfeld J., Li M.L., Brown E.L. Release of early human hematopoietic progenitors from quiescence by antisense transforming growth factor beta 1 or Rb oligonucleotides. J Exp Med. 1991;174:925–929. doi: 10.1084/jem.174.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Du W., Liang H., Gao X. MicroRNA-328, a potential anti-fibrotic target in cardiac interstitial fibrosis. Cell Physiol Biochem. 2016;39:827–836. doi: 10.1159/000447793. [DOI] [PubMed] [Google Scholar]
- 32.Wang C.H., Lee D.Y., Deng Z. MicroRNA miR-328 regulates zonation morphogenesis by targeting CD44 expression. PLoS One. 2008;3:e2420. doi: 10.1371/journal.pone.0002420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yafi F.A., Jenkins L., Albersen M. Erectile dysfunction. Nat Rev Dis Primers. 2016;2:16003. doi: 10.1038/nrdp.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bai Y., Jones P.P., Guo J. Phospholamban knockout breaks arrhythmogenic Ca2+ waves and suppresses catecholaminergic polymorphic ventricular tachycardia in mice. Circ Res. 2013;113:517–526. doi: 10.1161/CIRCRESAHA.113.301678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li C., Li X., Gao X. MicroRNA-328 as a regulator of cardiac hypertrophy. Int J Cardiol. 2014;173:268–276. doi: 10.1016/j.ijcard.2014.02.035. [DOI] [PubMed] [Google Scholar]