Abstract
Background
Approaches to prostate cancer (PCa) diagnosis and treatment have evolved significantly over past decades. There has been an increasing focus on minimizing overdiagnosis and overtreatment of clinically insignificant PCa. The objective of this study was to evaluate the changes in the diagnostic approach and initial treatment strategy that has evolved over time in an Australian urological private practice.
Materials and methods
Men with newly diagnosed PCa were identified from the private practice electronic and paper medical records from 2005 to 2016 and data was consolidated into six groups of 2-year intervals. Diagnostic strategy was analyzed with particular reference to the use of multiparametric magnetic resonance imaging (mpMRI) scan and 68Ga-prostate specific membrane antigen (PSMA) positron emission tomography/computed tomography (PET/CT) scans. National Comprehensive Cancer Network risk group stratification was correlated with initial treatment strategy and compared over time.
Results
Chart review identified 839 men who had a mean age of 65.8 years. In 2011–2012, prebiopsy mpMRI scan was introduced. Its uptake correlated with a decrease in numbers of men diagnosed with low risk cancer (r = -0.80, P = 0.04) and an increase in numbers of men diagnosed with high-risk cancer (r = 0.90, P = 0.01). The use of 68Ga-PSMA PET/CT was associated with decreasing use of CT and bone scans performed. Open radical prostatectomy had a declining trend particularly when robotic surgery (robotic assisted radical prostatectomy (RARP)) was introduced. Pelvic lymph node dissections performed progressively decreased. An increased use of luteinizing hormone receptor hormone (LHRH) antagonists was seen in favor of LHRH agonists. Whilst use of high dose rate brachytherapy declined, there was an increased use of low dose rate brachytherapy.
Conclusion
Prebiopsy mpMRI has been associated with an increased proportion of newly diagnosed men having clinically significant PCa. Over time, 68Ga-PSMA PET/CT scans, robotic assisted radical prostatectomy (RARP) and LHRH antagonists have increased in use, whilst CT and bone scans, and pelvic lymph node dissections have decreased.
Keywords: Active Surveillance, Magnetic Resonance Imaging, Positron-Emission Tomography, Prostate cancer, Prostatectomy, Radiotherapy
1. Introduction
Prostate cancer (PCa) has been the second commonest cause of Australian male cancer related mortality from 2013 to 2016.1 In light of this worrying statistic, there has been significant beneficial progress in the approaches to diagnosis and treatment of PCa, particularly over the past decade.2, 3, 4
Prostate specific antigen (PSA) testing has been associated with increased early detection of PCa, but any decline in PCa-related mortality has been modest.5 However, PSA testing has been associated with the overdiagnosis of many indolent tumors followed by subsequent overtreatment.6 Transrectal ultrasound-guided biopsies have been the mainstay of PCa diagnosis. The introduction of the prebiopsy multiparametric magnetic resonance imaging (mpMRI) scan can potentially minimize the diagnosis of clinically insignificant disease and improve the detection of clinically relevant disease.7
The introduction of 68Ga-prostate specific membrane antigen (PSMA) positron emission tomography/computed tomography (PET/CT) scans has significantly increased the detection accuracy of metastatic disease compared to the standard imaging protocols of radionuclide bone scans and abdominopelvic CT scans.8
The approach to initial treatment of early stage PCa has changed significantly over recent years. Increasingly, low-risk cancer is being managed conservatively, which has reduced overtreatment of low risk disease. However, an Australian study based in Victoria found that a majority of patients have undergone initial treatment with aggressive interventions such as androgen deprivation therapy (ADT), radical prostatectomy (RP), and radiotherapy, regardless of their risk category of PCa.9
Little data is available that describes the uptake of new technology into urological practice and how it has impacted the management of newly diagnosed PCa patients. The objective of this study is to report on changes in the approach to the diagnosis, investigation, and initial treatment of PCa in an Australian academic specialist prostate private practice over a 12-year period.
2. Materials and methods
We retrospectively reviewed medical records from patients with newly diagnosed PCa from the years 2005 to 2016 inclusive. Their records were identified using a combination of key word searching in the private practice software program and a provided list of men who had biopsy proven PCa from the pathology laboratory utilized by this practice. Men previously diagnosed with PCa and referred for second opinion or continued management were excluded from this study.
The practice introduced a paperless system on September 14, 2011 whereby from this date onwards, patients with newly diagnosed PCa would have their files kept exclusively as electronic records in the practice database. For patients who were diagnosed prior to this date, electronic medical records were used if the patient's paper files had been uploaded into the database. If the complete patient medical record had not been scanned into the database, paper records would be sourced and reviewed. Institutional ethics committee approval was obtained (HREC Project ID: 2017-001).
Data collected prior to prostate biopsy included age, International Prostate Symptom Score (IPSS), digital rectal examination findings, PSA, and mpMRI scans were recorded. The risk categorization for PCa was deduced from the PSA level, local clinical stage, and Gleason score according to current National Comprehensive Cancer Network guidelines. Furthermore, the use and results of extent of disease imaging were recorded.
The patient's initial treatment strategy was classified as active surveillance, watchful waiting, or intervention. Intervention was defined as including ADT, RP, and radiotherapy.
RP was reported as either the open RP (ORP) or RARP, with or without pelvic lymph node dissections (PLND).
Patients who underwent ADT were distinguished as either being treated with luteinizing hormone receptor hormone (LHRH) agonists or LHRH antagonists. There was no inclusion of bilateral orchiectomy as this was never undertaken in this practice.
Prostate radiotherapy included external beam radiation therapy (EBRT) and low dose rate (LDR) brachytherapy or high dose rate (HDR) brachytherapy. It was also reported if the insertion of SpaceOar polyethylene glycol spacing prosthesis and/or fiducial seeds was a component of the treatment.
Data was consolidated into six groups of 2-year time periods. Age and IPSS were presented as means and standard deviations. Due to small numbers of substantial outlier PSA levels for some of the time frames examined, the median PSA and corresponding interquartile ranges were reported. Data in the other categories was counted and summated. Percentages were ascertained for each field of summary data in Table 1, Table 2, relative to the number of patients from the specific year group. For the initial treatment approaches in Table 3, the proportion of the subtype of each therapy was relative to the total number of the certain treatment, for instance the proportions of LHRH agonists and antagonists were relative to the total ADT therapy. The percentage for total treatment strategy was calculated relative to the total number of patients from the particular 2-year interval.
Table 1.
Summary data presenting the investigations for diagnosis of prostate cancer (PCa) for 2005–2016.
| Yr | 2005–2006 | 2007–2008 | 2009–2010 | 2011–2012 | 2013–2014 | 2015–2016 |
|---|---|---|---|---|---|---|
| No. patients | 71 | 160 | 164 | 159 | 143 | 142 |
| Age (± SD) | 67.8 (8.8) | 66.8 (9.4) | 64.6 (9.3) | 63.7 (8.6) | 65.4 (8.3) | 67.9 (8.2) |
| PSA (IQR) | 8.40 (8.90) | 7.12 (6.68) | 6.58 (4.84) | 6.20 (3.75) | 6.40 (5.23) | 6.50 (4.46) |
| IPSS (± SD) | 7 (6) | 10 (8) | 9 (7) | 8 (7) | 7 (6) | 8 (7) |
| T1c (%) | 40 (56.3) | 87 (54.4) | 103 (62.8) | 115 (72.3) | 88 (62.0) | 78 (55.3) |
| ≥ T2 (%) | 31 (43.7) | 72 (45.0) | 61 (37.2) | 44 (27.7) | 54 (38.0) | 63 (44.7) |
| Transperineal biopsy | 1 (1.4) | 1 (0.6) | 1 (0.6) | 0 (0.0) | 2 (1.4) | 3 (2.1) |
| Prebiopsy mpMRI (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 6 (3.8) | 87 (60.8) | 127 (89.4) |
| Low risk (%) | 26 (36.6) | 37 (23.1) | 41 (25.0) | 39 (24.5) | 19 (13.3) | 20 (14.1) |
| Intermediate risk (%) | 29 (40.8) | 86 (53.8) | 87 (53.0) | 74 (46.5) | 79 (55.2) | 72 (50.7) |
| High risk (%) | 16 (22.5) | 36 (22.5) | 36 (22.0) | 46 (28.9) | 45 (31.5) | 49 (34.5) |
IPSS, international prostate symptom score; IQR, interquartile range; mpMRI, multiparametric magnetic resonance imaging; PSA, prostate specific antigen; SD, standard deviation.
Table 2.
The number and proportion of scans done to investigate metastases of prostate cancer from 2005 to 2016 relative to the number of patients in each 2-year group.
| Yr | 2005–2006 | 2007–2008 | 2009–2010 | 2011–2012 | 2013–2014 | 2015–2016 |
|---|---|---|---|---|---|---|
| CT scan (%) | 26 (36.6) | 68 (42.5) | 74 (45.1) | 83 (52.2) | 67 (46.9) | 18 (12.7) |
| Bone scan (%) | 31 (43.7) | 72 (45.0) | 75 (45.7) | 84 (52.8) | 69 (48.3) | 16 (11.3) |
| 68Ga-PSMA PET/CT scan (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.7) | 51 (35.9) |
| Metastases (%) | 1 (1.4) | 0 (0.0) | 2 (1.2) | 5 (3.1) | 7 (4.9) | 4 (2.8) |
68Ga-PSMA PET/CT, (68Ga-prostate specific membrane antigen positron emission tomography/computed tomography); CT, computed tomography.
Table 3.
The number and proportion of patients who underwent a specific initial treatment approach for prostate cancer from 2005 to 2016.
| Yr | 2005–2006 | 2007–2008 | 2009–2010 | 2011–2012 | 2013–2014 | 2015–2016 |
|---|---|---|---|---|---|---|
| Watchful waiting (%) | 4 (5.6) | 9 (5.6) | 3 (1.8) | 2 (1.3) | 1 (0.7) | 3 (2.1) |
| Active surveillance (%) | 10 (14.1) | 31 (19.4) | 40 (24.4) | 51 (32.1) | 35 (24.5) | 32 (22.5) |
| RP | ||||||
| Open RP (%) | 40 (100.0) | 91 (100.0) | 90 (97.8) | 51 (70.8) | 2 (2.4) | 4 (5.1) |
| Robotic RP (%) | 0 (0.0) | 0 (0.0) | 2 (2.2) | 21 (29.2) | 80 (97.6) | 75 (94.9) |
| Total RP (%) | 40 (56.3) | 91 (56.9) | 92 (56.1) | 72 (45.3) | 82 (57.3) | 79 (55.6) |
| PLND (%) | 18 (45.0) | 74 (81.3) | 69 (75.0) | 51 (70.8) | 45 (54.9) | 19 (24.1) |
| no PLND (%) | 22 (55.0) | 17 (18.7) | 23 (25.0) | 21 (29.2) | 37 (45.1) | 60 (75.9) |
| ADT therapy | ||||||
| LHRH agonist (%) | 13 (100.0) | 18 (100.0) | 16 (100.0) | 14 (100.0) | 12 (85.7) | 3 (30.0) |
| LHRH antagonist (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (14.3) | 7 (70.0) |
| Total ADT therapy (%) | 13 (18.3) | 18 (11.2) | 16 (9.8) | 14 (8.8) | 14 (9.8) | 10 (7.0) |
| Radiotherapies | ||||||
| EBRT | 0 (0.0) | 2 (25.0) | 1 (9.1) | 1 (7.1) | 2 (33.3) | 4 (57.1) |
| HDR brachytherapy | 1 (100.0) | 6 (75.0) | 6 (54.5) | 5 (35.7) | 0 (0.0) | 0 (0.0) |
| LDR brachytherapy | 0 (0.0) | 0 (0.0) | 4 (36.4) | 8 (57.1) | 4 (66.7) | 3 (42.9) |
| Total radiotherapies (%) | 1 (1.4) | 8 (5.0) | 11 (6.7) | 14 (8.8) | 6 (4.2) | 7 (4.9) |
| Space-Oar (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 8 (57.1) | 5 (83.3) | 4 (57.1) |
| Fiducial seeds (%) | 0 (0.0) | 6 (75.0) | 2 (18.2) | 10 (71.4) | 5 (83.3) | 5 (71.4) |
ADT, androgen deprivation therapy; EBRT, external beam radiation therapy; HDR, high dose rate; LDR, low dose rate; LHRH, luteinizing hormone receptor hormone; PLND, pelvic lymph node dissection; RP, radical prostatectomy.
To investigate trends and make comparisons between datasets, the Pearson's correlation coefficient (r value) and P value were determined. Statistical significance for any correlation was defined as P < 0.05.
All analyses were executed using Microsoft Excel (Mac 2011 Version 14.6.9, Microsoft Corporation, Redmond, WA, USA) statistical analysis functions.
3. Results
A total of 839 patient records were retrospectively analyzed from 2005 to 2016 inclusive. Baseline data is summarized in Table 1. Over the 12 years, the overall mean age was 65.8 years and mean IPSS was 8 with no apparent change in the variables over the years. In the years 2007 and 2016, two men did not have their local clinical stages determined because they did not undergo a digital rectal examination. One patient did not have a rectum as a result of previous abdominoperineal excision of the rectum. The other man was unable to have the examination performed due to an anal stricture. There was no correlation between the number of biopsies taken and whether transperineal biopsy was performed (r = -0.06, P = 0.90).
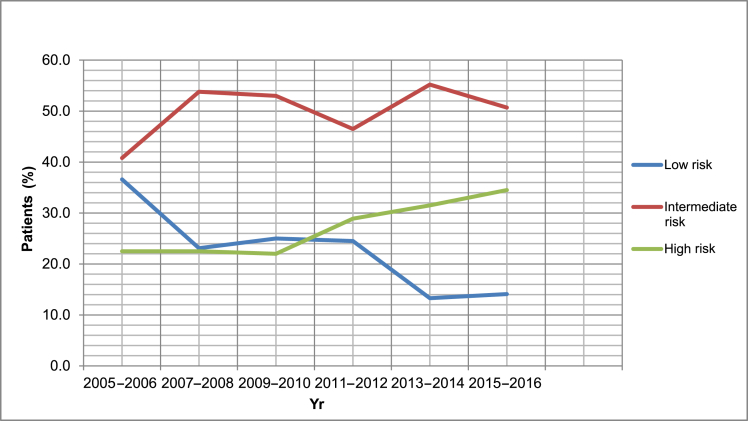
In 2011–2012, the prebiopsy mpMRI was first introduced in the private practice and rapidly increased by 85.6% in use in patient care from 2011 to 2016, as shown in Table 1. This appeared to be associated with a decreasing percentage of men diagnosed with low-risk PCa (r = -0.80, P = 0.04) and an increasing percentage of men diagnosed with high-risk PCa (r = 0.90, P = 0.01) as shown in Fig. 1.
Fig. 1.
Trends in the proportion of patients who were diagnosed with low, intermediate, and high risk prostate cancer (PCa) from the years 2005 to 2016.
Both CT and bone scans were steadily increasing in use (r = 0.93, P = 0.02) from 2005 to 2012, as per Table 2. However, with the introduction of the 68Ga-PET/CT scan in 2013–2014, simultaneously the proportion of CT scans and bone scans decreased by 34.3% and 37% from 2013 to 2016, respectively. From 2005 onwards, 68Ga-PSMA PET/CT scan was strongly associated with a decrease in CT scans (r = -0.93, P < 0.01) and a decrease in bone scans (r = -0.98, P = <0.01). There was no significant correlation between the proportion of men diagnosed with metastases and the proportion of 68Ga-PSMA PET/CT scan (r = 0.17, P = 0.73), CT scan (r = 0.05, P = 0.93), and bone scan (r = -0.02, P = 0.96) reported.
With regards to their initial treatment strategy, 16 patients were lost to follow up and three patients were yet to decide on and receive treatment.
Over time, the proportion of patients diagnosed with low risk PCa was insignificantly correlated with the treatment approach of either watchful waiting (r = 0.65, P = 0.14) or active surveillance (r = -0.44, P = 0.36).
ORP has been decreasing in use from 2007 to 2016; this decrease was particularly significant when RARP was introduced in 2009 (r = -0.88, P = 0.01) and has only increased in utilization since, as shown in Table 3. There has been a general decline in the percentage of RP cases where PLND was performed, which is mirrored in the proportion of procedures where PLND was not performed. However, there is an insignificant correlation between PLND and the total RPs conducted (r = 0.73, P = 0.07).
Regarding ADT, there is a general decreasing trend in the use of LHRH agonists that becomes particularly significant from the years 2013 to 2016, with a 55.7% reduction, during which time LHRH antagonists are introduced and there is an increase in utilization (r = -0.95, P ≤ 0.01).
HDR brachytherapy has been significantly decreasing in use from 2005, when compared with LDR brachytherapy (r = -0.87, P = 0.01). Following 2013, HDR brachytherapy was not selected as treatment by any patients, as shown in Table 3.
Space-Oar (Augemenix, Inc. Bedford, MA, USA) was introduced as a treatment modality from 2011, and from this time onwards, a total of 17 out of 27 patients who were primarily treated with radiotherapy had a Space-Oar implanted. Amongst the LDR brachytherapy, HDR brachytherapy and EBRT cases from 2011, 93.3%, 40%, and 0% had Space-Oars placed, respectively.
Fiducial seed markers were first used in this practice in 2009. From 2009 onwards, a total of 28 out of 46 patients who were primarily treated with radiotherapy had fiducial seeds placed. Amongst the LDR brachytherapy, HDR brachytherapy, and EBRT cases from 2009, 73.7%, 58.8%, and 30% had fiducial seeds placed, respectively.
4. Discussion
The key findings of this study are the changes observed from the introduction of prebiopsy mpMRI scan and 68Ga-PET/CT scan for the diagnosis of PCa.
The mpMRI scan offers more accurate detection in identifying areas of greater likelihood of clinically significant PCa, resulting in increased diagnosis rates.10 This is reflected by the decrease in diagnosis of low risk PCa, and the increase in high-risk PCa. Thus, the mpMRI scan increased the threshold by which low risk cancers were identified, resulting in a reduced detection of low risk disease whilst improving the detection of high-risk PCa.2
It is possible that change in the numbers of men diagnosed with low risk PCa has been influenced by the manner in which Gleason scoring has evolved over time. More recent iterations of the Gleason scoring system has decreased the threshold to label Gleason Grade 3 cancer as being Gleason Grade 4.11 This has an obvious impact on defining low risk PCa. It is therefore a study limitation that the Gleason score of 3+3 back in 2005 might now be reported differently.
Although there has been an increasing incidence of high-risk PCa, this has not impacted upon the incidence of urinary symptomatic disease. Presenting IPSS did not appear to change over the 12 years analyzed, which suggests that more recently diagnosed men are no more symptomatic with urinary symptoms than those diagnosed in previous years.
The PET/CT scan using 68Ga-PSMA ligands has been particularly promising in the imaging evaluation of PCa.4 Retrospective studies have demonstrated that 68Ga-PSMA PET/CT scans have improved the detection of metastatic sites even at low PSA values compared to conventional PET/CT scans using different tracers.12 68Ga-PSMA PET/CT is superior to standard routine imaging in detecting lymph node metastasis,13 and bony metastases.14 This explains our findings where the introduction of the 68Ga-PSMA PET/CT scan resulted in the decreased use of CT and bone scans. Interestingly, there has been an associated decline in the use of PLND as PSMA scans have been adopted. It is yet to be determined whether PSMA scans can reliably assist with a decision to offer or to not offer PLND in conjunction with an RP. This approach reflects the personal bias of the urologist practice examined.
Some of the approaches for the initial treatment of PCa have also undergone changes over the past 12 years, in particular with surgery.
In the Australian public health system, RARP is becoming the dominant approach to radical prostatectomy, with a proven decrease in length of hospital stay and blood infusion rates compared to ORP and laparoscopic surgeries.15 This supports the trend, as observed in this study, of a significant decline in ORP upon the introduction and subsequent increase in the robotic approach. Furthermore, a retrospective study has reported that PLND rates for 2008–2014 were lower than the rates for 2001–2007 across Australia.3 This trend is accurately represented in our findings with an increasing proportion of radical prostatectomies having PLND performed until 2008, then a gradual decline in the procedure post 2008.
The significant pattern in ADT was the rapid increase in the proportion of LHRH antagonist administered, with the simultaneous decline in LHRH agonists used. The only LHRH antagonist available for clinical use has been Degarelix (Ferring Pharmceuticals Pty Ltd, Sydney). Clinical trials have shown that Degarelix induces faster suppression of testosterone and PSA, whilst preventing risk of clinical flare in advanced disease, than LHRH agonists, even though both drugs have the same long-term efficacy in maintaining testosterone suppression.16 There is increasing evidence that an LHRH antagonist may be more appropriate in those men with an increased risk for cardiovascular disease.17
According to an Australian study, since 2007 there was a shift towards a relatively low use of HDR brachytherapy compared to LDR brachytherapy, with a ratio of HDR to LDR brachytherapy of < 0.5 by 2014 across the nation.3 Our data supports this observation with a progressively decreasing trend in the HDR brachytherapy approach and an increasing use of LDR brachytherapy over the 12 years studied. However, a limitation to this finding is the very small numbers of brachytherapy reported in this study. A further limitation of this study is that we do not know for sure if any of the men who underwent EBRT had fiducial seeds or a Space-Oar prosthesis placed by a radiation oncologist. Men referred to undergo definitive EBRT often revert to their primary PCa care being undertaken by the radiation oncologist.
Our study does not demonstrate particular changes in watchful waiting and active surveillance approaches over the past 12 years. Low risk PCa is ideally managed with active surveillance or watchful waiting, but there is no significant correlation between the proportion of low risk cases diagnosed and the percentage of these treatment approaches undertaken. Thus, active surveillance or watchful waiting are not predominantly preferred treatment for low risk cancer, contrary to the findings of an Australian study.8 Over time, there is no significant correlation between the number of diagnosed low risk PCa and the total number of RP (r = 0.31, P = 0.54), the total number of ADT (r = 0.70, P = 0.10), and the total number of radiotherapy (r = 0.67, P = 0.12) reported. Thus, regardless of the proportion of low risk of PCa, there is no change in the preference for aggressive intervention. So, there is no decrease in the selection of aggressive treatment for low risk PCa over the 12 years, which is supported by an Australian study.9 This study further claims that a majority of Victorian patients opt for aggressive intervention regardless of the risk of their PCa.9 Controversially, other research suggests that patients with low risk disease are more likely to be placed on active surveillance if managed in a private practice,18 which perhaps indicates that significance in this treatment approach may be demonstrated when our data is compared with that of a public institution.
The general limitations of our study are that it is retrospective, and the findings are based on a single clinician experience, thus treatment was dictated by a personal bias. However, the strength in our research is due to the low numbers lost to follow up and the large population of male data extracted from a single clinician practice.
In conclusion, over the past 12 years, significant changes have been made to the diagnosis and treatment of PCa. Prebiopsy mpMRI scans have increased the threshold for clinically significant PCa diagnosis. More RRP compared to ORP are performed with a decrease in PLND. There is an increasing use of 68Ga- PSMA PET/CT scan to detect metastatic disease. Degarelix is becoming the more utilized ADT approach compared to LHRH agonists. HDR brachytherapy is less utilized, with LDR brachytherapy increasing in use.
Conflicts of interest
All authors have no conflict of interest to declare.
References
- 1.Australian Institute of Health and Welfare . Australian Institute of Health and Welfare; Canberra: 2016. Australian Cancer Incidence and Mortality (ACIM) books: prostate cancer [Internet]http://www.aihw.gov.au/acim-books/ c2009 [cited 2017, Jan 23]. Available from: [Google Scholar]
- 2.Pokorny M.R., de Rooij M., Duncan E., Schroder R., Parkinson R., Barentsz J. Prospective study of diagnostic accuracy comparing prostate cancer detection by transrectal ultrasound-guided biopsy versus magnetic resonance (MR) imaging with subsequent MR-guided biopsy in men without previous prostate biopsies. Eur Urol. 2014;66:22–29. doi: 10.1016/j.eururo.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Lo J., Papa N., Bolton D.M., Murphy D., Lawrentschuk N. Australian patterns of prostate cancer care: Are they evolving? Prostate Int. 2016;4:20–24. doi: 10.1016/j.prnil.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rauscher I., Maurer T., Fendler W.P., Sommer W.H., Schwaiger M., Eiber M. 68Ga-PSMA ligand PET/CT in patients with prostate cancer: How we review and report. Cancer Imaging. 2016;16:14. doi: 10.1186/s40644-016-0072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cookson M. Prostate cancer: screening and early detection. Cancer Control. 2001;2:133–140. doi: 10.1177/107327480100800203. [DOI] [PubMed] [Google Scholar]
- 6.Sountoulides P., Moutzouris G. Prostate-specific antigen screening, why have the guidelines changed? Expert Rev Anticancer Ther. 2014;14:1277–1281. doi: 10.1586/14737140.2014.971111. [DOI] [PubMed] [Google Scholar]
- 7.Hambrock T., Hoeks C., Hulsbergen-van de Kaa C., Scheenen T., Futterer J., Bouwense S. Prospective assessment of prostate cancer aggressiveness using 3-T diffusion-weighted magnetic resonance imaging-guided biopsies versus a systematic 10-core transrectal ultrasound prostate biopsy cohort. Eur Urol. 2012;61:177–184. doi: 10.1016/j.eururo.2011.08.042. [DOI] [PubMed] [Google Scholar]
- 8.Hossein J. PSMA PET in prostate cancer. J Nucl Med. 2015;56:1131–1132. doi: 10.2967/jnumed.115.157339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evans S.M., Millar J.L., Davis I.D., Murphy D.G., Bolton D.M., Giles G.G. Patterns of care for men diagnosed with prostate cancer in Victoria from 2008 to 2011. Med J Aust. 2013;198:540–545. doi: 10.5694/mja12.11241. [DOI] [PubMed] [Google Scholar]
- 10.Neto J., Parente D. Multiparametric magnetic resonance imaging of the prostate. Magn Reson Imaging Clin N Am. 2013;21:409–426. doi: 10.1016/j.mric.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 11.Epstein J.I., Egevad L., Amin M.B., Delahunt B., Srigley J.R., Humphrey P.A. The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma: definition of grading patterns and proposal for a new grading system. Am J Surg Pathol. 2016;40:244–252. doi: 10.1097/PAS.0000000000000530. [DOI] [PubMed] [Google Scholar]
- 12.Afshar-Oromieh A., Zechmann C.M., Malcher A., Eder M., Eisenhut M., Linhart H.G. Comparison of PET imaging with a (68)Ga-labelled PSMA ligand and (18)F-choline-based PET/CT for the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging. 2014;41:11–20. doi: 10.1007/s00259-013-2525-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maurer T., Gschwend J.E., Rauscher I., Souvatzoglou M., Haller B., Weirich G. Diagnostic efficacy of (68)gallium-PSMA positron emission tomography compared to conventional imaging in lymph node staging of 130 consecutive patients with intermediate to high risk prostate cancer. J Urol. 2016;195:1436–1443. doi: 10.1016/j.juro.2015.12.025. [DOI] [PubMed] [Google Scholar]
- 14.Eiber M., Pyka T., Okamoto S., Rauscher I., Dahlbender M., Tauber R. 68Gallium-HBED-CC-PSMA PET compared to conventional bone scintigraphy for evaluation of bone metastases in prostate cancer patients. Eur Urol Suppl. 2016;15:566. [Google Scholar]
- 15.Basto M., Sathianathen N., Te Marvelde L., Ryan S., Goad J., Lawrentschuk N. Patterns-of-care and health economic analysis of robotic-assisted radical prostatectomy in the Australian public health system. BJU Int. 2016;117:930–939. doi: 10.1111/bju.13317. [DOI] [PubMed] [Google Scholar]
- 16.Rick F., Block N., Schally A. An update on the use of degarelix in the treatment of advanced hormone-dependent prostate cancer. Onco Targets Ther. 2013;16:391–402. doi: 10.2147/OTT.S32426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Albertsen P., Klotz L., Tombal B., Grady J., Olesen T., Nilsson J. Cardiovascular morbidity associated with gonadotropin releasing hormone agonists and an antagonist. Eur Urol. 2014;65:565–573. doi: 10.1016/j.eururo.2013.10.032. [DOI] [PubMed] [Google Scholar]
- 18.Weerakoon M., Papa N., Lawrentschuk N., Evans S., Millar J., Frydenberg M. The current use of active surveillance in an Australian cohort of men: a pattern of analysis from the Victorian Prostate Cancer Registry. BJU Int. 2015;115:50–56. doi: 10.1111/bju.13049. [DOI] [PubMed] [Google Scholar]