Abstract
Background
The objective of this study was to assess the effects of 25-degree and 30-degree Trendelenburg positions on intraocular pressure (IOP) changes during robot-assisted radical prostatectomy (RARP).
Materials and methods
This prospective study involved a total of 30 consecutive patients undergoing RARP. All participants were randomly divided into two groups: Trendelenburg position with the head down at 25 degrees or 30 degrees. In addition to representative operative outcomes, IOP was measured at six discrete time points; Time 1 (T1): before induction of general anesthesia, patients in a horizontal supine position; T2: after induction of general anesthesia, patients in a horizontal supine position; T3: 1 hour after adopting the Trendelenburg position; T4: 2 hours after adopting the Trendelenburg position; T5: after pneumoperitoneum resolution in the Trendelenburg position; T6: anesthetized before awakening in a supine position.
Results
The total and console operative times, estimated blood loss, and intravenous fluid intake during RARP did not significantly differ between the two groups. While the IOP values measured at the same time points were similar between the two groups, the 25-degree Trendelenburg position significantly attenuated the IOP change from T1 to T3, T4, and T5 compared with those at 30 degrees.
Conclusions
These findings suggest that RARP in the 25-degree Trendelenburg position may reduce the risks of position-related ophthalmic complications without increasing the difficulty of the surgical procedure.
Keywords: Intraocular pressure, Prostate cancer, Robot-assisted radical prostatectomy, Trendelenburg position
1. Introduction
Prostate cancer remains the most common urologic malignancy and the second leading cause of cancer death among men in developed countries.1 Due to the widespread use of prostate-specific antigen (PSA) tests, prostate cancer is increasingly being diagnosed in its initial stages, and radical prostatectomy (RP) is one of the most definitive treatment options for clinically localized disease.2 In recent years, the introduction of robotic technology has markedly revolutionized the surgical management of prostate cancer. Compared with traditional open RP, the advantages of robot-assisted RP (RARP) are: a reduction of the estimated blood loss during surgery, fewer complications, better functional outcomes, and a shorter hospital stay.3
Despite many advantages of RARP, this procedure causes some concerns related to the patient's position during surgery. For example, RARP requires the patient to be placed in the steep Trendelenburg position (head down at 30–45 degrees) with the use of a pneumoperitoneum, which has a significant impact on the circulatory system, leading to limb neuropraxia, facial edema, and several ophthalmic complications.4 Of these, ischemic optic neuropathy is rare, but is one of the most devastating complications mainly due to an increased intraocular pressure (IOP).4, 5, 6, 7 The Trendelenburg position allows better access to the prostate as gravity pulls the abdominal viscera away from the pelvis,8 therefore, it is anticipated that increasing the degree of Trendelenburg tilt will ensure a better surgical view, but it will lead to an increasing IOP. However, to the best of our knowledge, there have been no reports addressing the effect of the difference in angle of the Trendelenburg position on IOP change during RARP.
Considering these findings, we prospectively assessed the effects of 25-degree and 30-degree Trendelenburg positions on several perioperative variables, mainly focusing on IOP change during RARP in one institution.
2. Materials and methods
From April 2013 to November 2013, a total of 30 consecutive men were scheduled for RARP at our institution. The study design was approved by the Research Ethics Committee of our institution and informed consent for performing the present study was obtained from all of the included patients. In this study, one surgeon performed the 30 RARPs in a standard fashion, using the DaVinci system (Intuitive Surgical, Sunnyvale, CA, USA). The original surgical method used for RARP was previously reported by Patel et al.9 Patients with pre-existing glaucoma, retinal vascular diseases, and a history of eye surgery were excluded. The 30 participants were randomly divided into two groups: Trendelenburg position with the head down at 25 degrees and Trendelenburg position with the head down at 30 degrees. During pneumoperitoneum, intraabdominal pressure was maintained at 12 mm Hg, using carbon dioxide for insufflation. Throughout surgery, the anesthesia protocol was standardized for the drugs used; propofol and droperidol were used for sedation, remifentanil and fentanyl were used for analgesia, and rocuronium was used for muscular relaxation. The lungs were mechanically ventilated and we maintained ETCO2 at 30–40 mm Hg. All IOP measurements were performed by the trained ophthalmologists in our institute using a Tono-pen XL handheld tonometer (Medtronic, Jacksonville, FL, USA).
Preoperatively, the patient age, body mass index (BMI), serum PSA at diagnosis, clinical tumor stage, Gleason score at biopsy, and D'Amico risk group10 were recorded. During RARP, IOP was recorded at six discrete time points; Time 1 (T1): before induction of general anesthesia, the patient in a horizontal supine position; T2: after induction of general anesthesia, the patient in a horizontal supine position; T3: 1 hour after adopting the Trendelenburg position with the head down at 25 degrees or 30 degrees; T4: 2 hours after adopting the Trendelenburg position with the head down at 25 degrees or 30 degrees; T5: after pneumoperitoneum resolution in the Trendelenburg position; T6: anesthetized before awakening in a supine position. Other operative parameters including the total and console operative times, estimated blood loss, and intravenous fluid volume were also measured.
All statistical analyses were performed using Statview 5.0 software (Abacus Concepts, Berkeley, CA, USA), and P values < 0.05 were considered significant. Differences in several parameters between the two groups according to the Trendelenburg position were compared using an unpaired t test or the Chi-square test.
3. Results
Table 1 shows the preoperative baseline clinicopathological characteristics of the 30 men analyzed in this study. The median patient age was 66 years (58–75 years) and 65 years (55–77 years) and BMI was 23.0 kg/m2 (19.7–28.0 kg/m2) and 25.0 kg/m2 (19.4–34.4 kg/m2) in the 25-degree and 30-degree Trendelenburg positions, respectively. No significant difference was found between the two groups in the patient age (P = 0.31) or BMI (P = 0.18). Similarly for other parameters representing the tumor characteristics, there was no difference in PSA at the diagnosis (P = 0.69), clinical tumor stage (P = 0.74), Gleason score at biopsy (P = 0.41), or D'Amico risk group (P = 0.20) between the two groups.
Table 1.
Comparison of baseline characteristics between the two groups.
| A: 25-degree Trendelenburg position (n = 15) | B: 30-degree Trendelenburg position (n = 15) | P | |
|---|---|---|---|
| Median age, years (range) | 66 (58–75) | 65 (55–77) | 0.31 |
| Median BMI, Kg/m2 (range) | 23.0 (19.7–28.0) | 25.0 (19.4–34.4) | 0.18 |
| Median PSA, ng/mL (range) | 6.0 (4.1–38.0) | 7.0 (4.3–22.5) | 0.69 |
| Clinical stage, No. (%) | 0.74 | ||
| cT1c | 1 (6.7) | 2 (13.3) | |
| cT2a | 8 (53.3) | 6 (40.0) | |
| cT2b | 2 (13.3) | 4 (26.7) | |
| cT2c | 2 (13.3) | 2 (13.3) | |
| cT3a | 2 (13.3) | 1 (6.7) | |
| Gleason score at biopsy, No. (%) | 0.41 | ||
| 6 | 1 (6.7) | 2 (13.3) | |
| 7 | 10 (66.7) | 12 (80.0) | |
| 8 | 2 (13.3) | 1 (6.7) | |
| 9 | 2 (13.3) | 0 (0.0) | |
| D' Amico risk classification, No. (%) | 0.20 | ||
| Low | 1 (6.7) | 1 (6.7) | |
| Intermediate | 10 (66.7) | 11 (73.3) | |
| High | 4 (26.7) | 3 (20.0) |
BMI, body mass index; PSA, prostate-specific antigen.
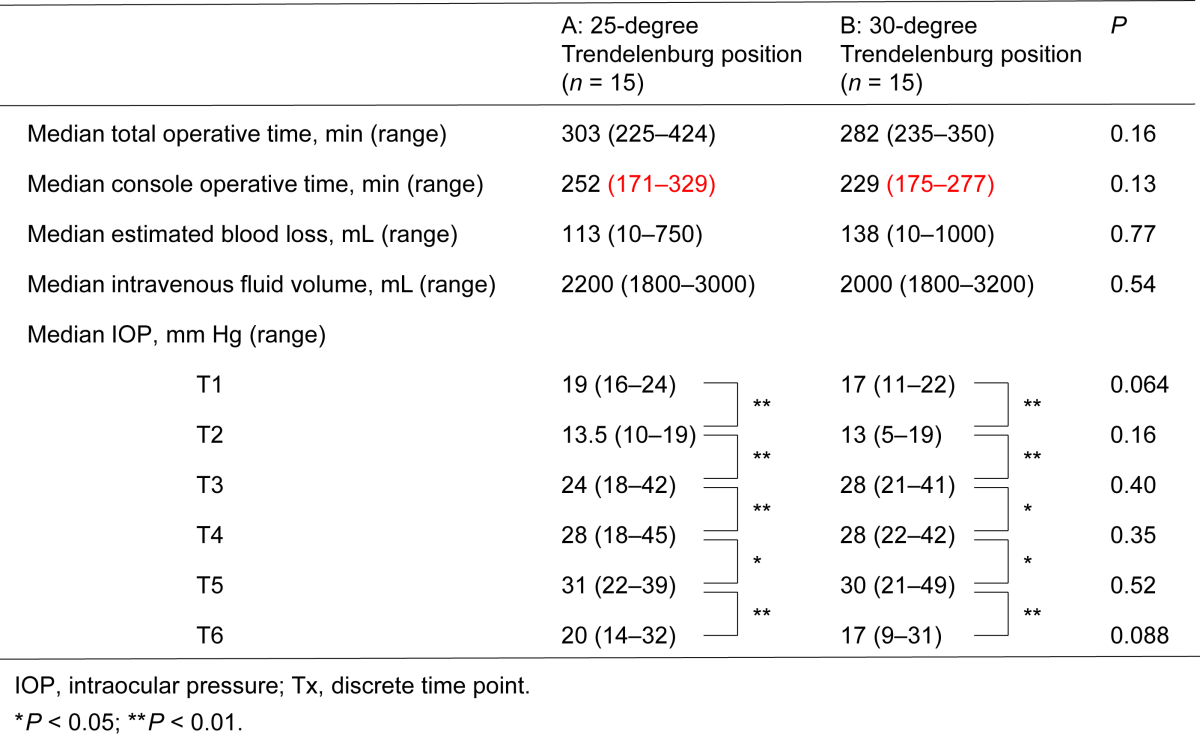
Table 2 lists the effects of the 25-degree and 30-degree Trendelenburg positions on several operative variables. The total (P = 0.16) and console (P = 0.13) operative times, estimated blood loss (P = 0.77), and intravenous fluid intake (P = 0.54) during RARP did not significantly differ between the two groups. No patient received blood transfusion during surgery and there were no complications related with bowel injuries. IOP measurement results in the two groups are also shown in Table 2. Before the induction of general anesthesia, median IOP values were 19 mmHg and 17 mmHg in the 25-degree and 30-degree Trendelenburg positions, respectively. As shown in Table 2, significant time-dependent increases in IOP were observed in both the 25-degree and 30-degree Trendelenburg positions; however, the IOP values measured at the same time points were similar between the two groups.
Table 2.
Effects of 25-degree and 30-degree Trendelenburg positions on several operative variables.
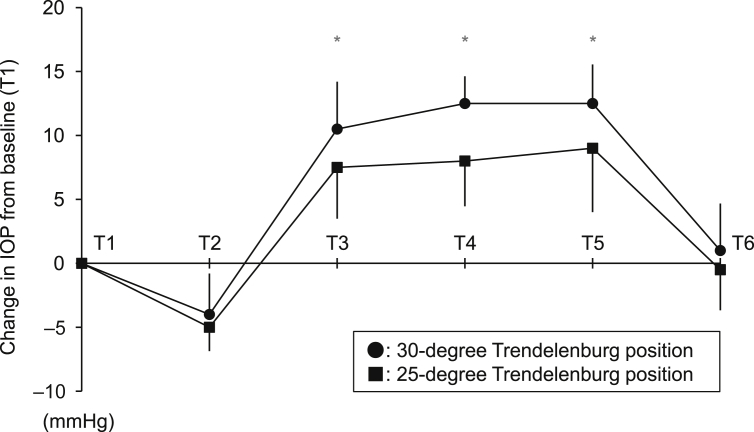
We then evaluated median changes from baseline (T1) IOP in the 25-degree and 30-degree Trendelenburg position groups. There were time-dependent increases in IOP changes from T1 when placed in the Trendelenburg position in the two groups (T3–T5); however, slight IOP changes were observed in the 25-degree Trendelenburg position as compared with those of the 30-degree Trendelenburg position at T3, T4, and T5 (Fig. 1). In this study, no severe ocular complications were found at the final examination.
Fig. 1.
Change in intraocular pressure (IOP) compared with Time 1 (T1). The six discrete time points are as follows: T1: before induction of general anesthesia, the patient in a horizontal supine position; T2: after induction of general anesthesia, the patient in a horizontal supine position; T3: 1 hour after positioning in the Trendelenburg position with the head down at 25 degrees or 30 degrees; T4: 2 hours after positioning in the Trendelenburg position with the head down at 25 degrees or 30 degrees; T5: after pneumoperitoneum resolution in the Trendelenburg position; T6: anesthetized before awakening in a supine position. Bars indicate standard deviation. * Significant difference in IOP change at each time point between the 25-degree and 30-degree Trendelenburg positions (P < 0.05).
4. Discussion
For a long time, open RP has represented the most widely accepted surgical procedure to eradicate clinically localized prostate cancer. As an alternative to traditional RP, laparoscopic RP was first performed by Schuessler et al11 in 1997 and further developed and subsequently refined by Guillonneau and Vallancien12 as minimally invasive surgery. Laparoscopic RP had marked strengths including a smaller incision, less surgical site infection, less blood loss, less postoperative pain, and a shorter hospital stay. However, due to the long learning curve resulting from the loss of haptic feedback, natural hand-eye coordination, and dexterity, laparoscopic surgery has not completely supplanted the open approach.3 Robotic surgery was developed to overcome these limitations of laparoscopic RP, and since its inception in 2001, RARP has rapidly become a predominant procedure for the surgical treatment of localized prostate cancer in the world. Although they lead to fewer overall complications, a quicker convalescence, and improved potency and continence outcomes, complications of these procedures have been described with the dissemination of minimally invasive RPs. Of these, complications relating to the positioning of the patient have been some of the most discussed issues to date, both in laparoscopic RP and RARP.
RARP requires patients to be placed in a steep Trendelenburg position, which is typically defined as a greater than 30-degree tilt of the bed below horizontal, with the head in the lowest position.8 The Trendelenburg position was initially described by a pioneering German surgeon, Freidrich Trendelenburg, in the mid-19th century, and this position has been commonly used to cause the bulk of abdominal viscera to slide toward the diaphragm, providing a more favorable operative field for lower abdominal and pelvic procedures. With the popularization of the Trendelenburg position, many studies have revealed significant negative physiologic effects of this head-down position, particularly when maintained for long periods of time.4 In RARP as well, several complications ranging from mild subcutaneous emphysema to devastating ischemic optic neuropathy related to prolonged exposure to the steep Trendelenburg position have been reported.4, 8
The steep Trendelenburg position during RARP increases IOP,6 and an elevated IOP decreases the perfusion pressure to the optic nerve,13 which can lead to increased risks of ischemic optic neuropathy and visual loss. Despite theoretical posture-related concerns, the impact of steep Trendelenburg on ocular injuries appears to be applicable to a broad range of patients undergoing RARP. For example, Hoshikawa et al7 conducted a prospective study to evaluate the effect on the visual function of RARP patients by increased IOP, and concluded that steep Trendelenburg positioning within 5 hours poses little or no risk from IOP increases in patients without pre-existing ocular disease. Meanwhile, Wen et al4 also reported that rates of ocular complications in nonrobotic RP (0.22%) are comparable with those in RARP (0.17%, P = 0.110), and discussed that visual complications following RP were because of prolonged surgical time and/or excessive blood loss. These findings may suggest that some of the previously described serious ocular consequences during minimally invasive RP did not result from the increased IOP mainly due to the steep Trendelenburg position itself. However, no study has investigated the effect of the difference in angle of the Trendelenburg position on IOP change during RARP along with several operative variables such as the duration of surgery and intraoperative blood loss. Therefore, we conducted a single-center, prospective, randomized controlled study with the aim of evaluating the effects of 25-degree and 30-degree Trendelenburg positions on IOP change along with several representative operative variables.
In this series, a total of 30 participants were randomly divided into two groups: Trendelenburg position with the head down at 25 degrees, and Trendelenburg position with the head down at 30 degrees. On comparing the two groups, there were no significant differences in preoperative baseline characteristics. In addition, we found no significant difference in several operative variables including the total and console operative times, and estimated blood loss between the 25-degree and 30-degree Trendelenburg positions. These outcomes were inconsistent with the anticipated results, because we hypothesized that increasing the degree of Trendelenburg tilt would ensure a better surgical view, leading to a shorter surgical time and reduced blood loss. These findings suggest that the 25-degree Trendelenburg position can offer excellent endoscopic views and surgical outcomes compared with those of the 30-degree Trendelenburg position. The effects of the 25-degree and 30-degree Trendelenburg positions on IOP at six discrete time points were also analyzed. As shown in Table 2, IOP significantly increased time-dependently after Trendelenburg positioning in patients undergoing RARP in both groups (T3–T5). These findings agreed with previous series.6 For example, Awad et al6 suggested that the IOP increase in the steep Trendelenburg position is time-dependent, and on average, an increase of 0.05 mmHg in IOP per minute was observed while patients were in the Trendelenburg position. We then calculated the change in IOP compared with T1, and we found that the 25-degree Trendelenburg position significantly attenuated the elevation of IOP from baseline values in comparison with the 30-degree position. Collectively, despite being an important issue, there is no definitive evidence showing the correlation between the IOP change and postoperative visual function; however, we believe that the 25-degree Trendelenburg position RARP can reduce the risk of catastrophic ophthalmologic complications after RARP without prolonging the operative time and/or increasing blood loss during surgery, as compared with the 30-degree Trendelenburg position.
There are several limitations of the present study. Firstly, this study included a relatively small number of patients and lacked information on major determinants of IOP, such as aqueous humor flow and the central venous pressure; thus, further analysis with larger prostate cancer cohorts evaluating position-related factors obtained during surgery is needed to draw a definitive conclusion. In addition, if the IOP values at a steep Trendelenburg position before and after intraperitoneal insufflation were measured, this would provide a comprehensive explanation of the effect of the difference in the angle of the Trendelenburg position on IOP change during RARP, considering that pneumoperitoneum has been previously shown to increase IOP.14 Secondly, we failed to identify clinical factors significantly influencing IOP at each time point during RARP, hence, it would be of worth to find such factors to avoid potential visual complications following RARP. Thirdly, this study compared the effects of only 25-degree and 30-degree Trendelenburg positions on IOP; therefore, there might be a critical angle, such as 20 degrees, which offers excellent surgical outcomes and mitigates IOP change during RARP. Finally, this study focused on IOP change during RARP alone; however, we should assess other position-related complications such as pharyngeal and laryngeal edema, in order to confirm the safety and feasibility of RARP in the 25-degree Trendelenburg position.
In conclusion, this is the first randomized study to assess the effects of the 25-degree and 30-degree Trendelenburg positions on several perioperative variables, mainly focusing on IOP change during RARP. Representative surgical outcomes in the 25-degree Trendelenburg position were comparable with those at 30 degrees. Furthermore, the 25-degree Trendelenburg position significantly attenuated the elevation of IOP from the baseline in comparison with 30 degrees. Although no ocular complication was observed, these findings suggest that RARP in the 25-degree Trendelenburg position may reduce the risks of position-related ophthalmic complications without increasing the difficulty of the surgical procedure.
Conflicts of interest
All authors declare no conflicts of interest.
Acknowledgments
We thank all the members of the Division of Ophthalmology, Seirei Mikatabara Hospital, Hamamatsu, Japan for IOP measurements and helpful discussion.
References
- 1.Siegel R., Ma J., Zou Z., Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Fitzpatrick J.M. Management of localized prostate cancer in senior adults: The crucial role of comorbidity. BJU Int. 2008;101:16–22. doi: 10.1111/j.1464-410X.2007.07487.x. [DOI] [PubMed] [Google Scholar]
- 3.Sood A., Jeong W., Peabody J.O., Hemal A.K., Menon M. Robot-assisted radical prostatectomy: inching toward gold standard. Urol Clin North Am. 2014;41:473–484. doi: 10.1016/j.ucl.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Wen T., Deibert C.M., Siringo F.S., Spencer B.A. Positioning-related complications of minimally invasive radical prostatectomies. J Endourol. 2014;28:660–667. doi: 10.1089/end.2013.0623. [DOI] [PubMed] [Google Scholar]
- 5.Weber E.D., Colyer M.H., Lesser R.L., Subramanian P.S. Posterior ischemic optic neuropathy after minimally invasive prostatectomy. J Neuroophthalmol. 2007;27:285–287. doi: 10.1097/WNO.0b013e31815b9f67. [DOI] [PubMed] [Google Scholar]
- 6.Awad H., Santilli S., Ohr M., Roth A., Yan W., Fernandez S. The effects of steep Trendelenburg positioning on intraocular pressure during robotic radical prostatectomy. Anesth Analg. 2009;109:473–478. doi: 10.1213/ane.0b013e3181a9098f. [DOI] [PubMed] [Google Scholar]
- 7.Hoshikawa Y., Tsutsumi N., Ohkoshi K., Serizawa S., Hamada M., Inagaki K. The effect of steep Trendelenburg positioning on intraocular pressure and visual function during robotic-assisted radical prostatectomy. Br J Ophthalmol. 2014;98:305–308. doi: 10.1136/bjophthalmol-2013-303536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phong S.V., Koh L.K. Anaesthesia for robotic-assisted radical prostatectomy: considerations for laparoscopy in the Trendelenburg position. Anaesth Intensive Care. 2007;35:281–285. doi: 10.1177/0310057X0703500221. [DOI] [PubMed] [Google Scholar]
- 9.Patel V.R., Thaly R., Shah K. Robotic radical prostatectomy: outcomes of 500 cases. BJU Int. 2007;99:1109–1112. doi: 10.1111/j.1464-410X.2007.06762.x. [DOI] [PubMed] [Google Scholar]
- 10.D'Amico A.V., Whittington R., Malkowicz S.B., Schultz D., Blank K., Broderick G.A. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–974. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 11.Schuessler W., Sculam P., Clayman R., Kavoussi L.R. Laparoscopic radical prostatectomy: initial short-term experience. Urology. 1997;50:854–857. doi: 10.1016/S0090-4295(97)00543-8. [DOI] [PubMed] [Google Scholar]
- 12.Guillonneau B., Vallancien G. Laparoscopic radical prostatectomy: the Montsouris technique. J Urol. 2000;163:1643–1649. doi: 10.1016/s0022-5347(05)67512-x. [DOI] [PubMed] [Google Scholar]
- 13.Walick K.S., Kragh J.E., Ward J.A. Changes in intraocular pressure due to surgical positioning: studying potential risk for postoperative vision loss. Spine. 2007;32:2591–2595. doi: 10.1097/BRS.0b013e318158cc23. [DOI] [PubMed] [Google Scholar]
- 14.Lentschener C., Leveque J.P., Mazoit J.X., Benhamou D. The effect of pneumoperitoneum on intraocular pressure in rabbits with alpha-chymotrypsin- induced glaucoma. Anesth Analg. 1998;86:1283–1288. doi: 10.1097/00000539-199806000-00029. [DOI] [PubMed] [Google Scholar]