Abstract
Vaccinology is one of the most important cornerstones in modern medicine, providing better quality of life. The human immune system is composed of innate and adaptive immune processes that interplay when infection occurs. Innate immunity relies on pathogen-associated molecular patterns which are recognized by pathogen recognition receptors localized in antigen presenting cells. After antigen processing and presentation, CD4+ T cell polarization occurs, further leading to B cell and CD8+ activation and humoral and cell-mediated adaptive immune responses. Liposomes are being employed as vaccine technologies and their design is of importance to ensure proper immune responses. Physicochemical parameters like liposome size, charge, lamellarity and bilayer fluidity must be completely understood to ensure optimal vaccine stability and efficacy. Liposomal vaccines can be developed to target specific immune cell types for the induction of certain immune responses. In this review, we will present promising liposomal vaccine approaches for the treatment of important viral, bacterial, fungal and parasitic infections (including tuberculosis, TB). Cationic liposomes are the most studied liposome types due to their enhanced interaction with the negatively charged immune cells. Thus, a special section on the cationic lipid dimethyldioctadecylammonium and TB is also presented.
Keywords: Liposomes, Vaccine formulations, CLR, TLR, Viral infections, Bacterial infections, Parasites
Background
Vaccination is one of the most significant developments in modern science, improving the treatment and controlling the spread of diseases across communities. For formulation scientists and immunologists, there has been an interest in the development of liposomal vaccines for their prophylactic uses in infections (of viral, bacterial, fungal or parasitic origin) [1–3]. To develop safe and effective liposome-based vaccines, scientists should take into consideration several interconnected principles: (1) the design-dependent function of the liposomes (2) the characteristics of liposome-cell interactions when vaccine administration occurs and (3) the specific cell receptor and signaling involved once liposomal vaccines are administered. Each of these principles will affect vaccine efficacy and its potential development from bench to bedside applications. These three principles are also the basis for developing excellent subunit vaccine strategies, which have been of important interest for vaccine scientists for several years [4–7]. To comprehend how the potential liposomal vaccine might work in the host we must understand the immune responses involved in receptor signaling. The field of immunology plays a significant role that will help determine the utilization of vaccinology to the advantage of the patient by developing adequate prophylactic treatment approaches.
Immunologists investigate how our bodies defend themselves from pathogens by determining and describing the signaling mechanisms involved. Basically, when infection occurs, the innate arm of the immune system responds through recognition of distinct molecules on or in pathogens termed ‘pathogen associated molecular patterns (PAMPs)’. These PAMPs differ from host markers and as such are recognized by the first line of defense in the immune system, antigen presenting cells (APCs) such as dendritic cells (DCs), macrophages and neutrophils. The PAMPs are recognized by pattern recognition receptors (PRRs) on the surface and in endosomes of APCs [8–11]. Vertebrates developed the adaptive immune system as a second line of defense that could ‘remember’ pathogens and fight back upon re-challenge. It is composed of cells such as T and B cells that employ de novo synthesized antigen-specific receptors (T- and B cell receptors, TCRs and BCRs). TCR and BCRs are able to recognize pathogen-specific antigens when presented complexed with major histocompatibility complexes (MHC) on the surface of an APC. But polarization of the T or B cells to ensure it acts appropriately against the pathogen, relies on signals provided by the APC (such as co-stimulatory molecules and precise cytokines) in response to the priming by PAMPs. These critical signals lead to differentiation of effector and eventually memory cells that are critical for driving the immune response against future infections with the same pathogen. While innate immune responses are broadly applicable and adaptive immune responses are antigen-specific, the two arms of the immune system work in close concert to mount an effective response to clear the pathogen.
The development of PRRs by the immune system represent a significant advancement in the fight for survival of the host. Therefore, an important question arises to that end: what type of PRRs have been discovered so far and which ligands (or PAMPs) do they tend to recognize? Some PRRs are secreted to the extracellular milieu and participate in pathogen opsonization. However, most PRRs are transmembrane (like C-type lectin and Toll-like receptors; CLRs and TLRs, respectively) or cytosolic (retinoic acid-inducible gene I and nucleotide-binding domain and leucine-rich repeat containing receptors; RLRs and NLRs, respectively). For the purposes of this review, we will discuss the uses of liposomes in vaccines that target CLRs and TLRs. The TLRs identified so far amount to 10 in humans, each one recognizing specific PAMPs from microbial pathogens (Table 1) [10–12]. TLR 10 has been recently described as a modulatory receptor; however, no known ligand has been linked to it [13]. Therefore, further research should be done on TLR 10 to uncover the ligand involved in innate immune responses.
Table 1.
Pathogen-associated molecular patters (PAMPs) recognized by specific TLRs
| Pathogen-associated molecular pattern (PAMP) | Microorganism or classification | TLRs |
|---|---|---|
| Lipoproteins | Bacteria | TLR 1 |
| Peptidoglycan and lipoteichoic acid | Gram positive bacteria | TLR 2 |
| β-glucans | Fungi | |
| dsRNA | Double-stranded and negative-stranded viruses | TLR 3 |
| Lipopolysacharide (LPS) | Gram negative bacteria | TLR 4 |
| Flagelin | Bacteria | TLR 5 |
| Profilin | Toxoplasma gondii | |
| Lipoproteins | Mycoplasma | TLR 6 |
| Imidazoquinolines and ssRNA | Single-stranded viruses | TLR 7 and 8 |
| Unmethylated CpG DNA motifs | Prokaryotic genomes and viral DNA | TLR 9 |
ds double stranded, ss single stranded
Contrary to the transmembrane TLRs, CLRs are classified as soluble or membrane bound. Soluble CLRs (e.g. galectins and collectins) have been reviewed extensively [9, 14]. The mostly studied membrane-associated CLRs are DC-SIGN (DC-specific ICAM3-grabbing non-integrin), Dectin-1 (dendritic cell-associated C-type lectin 1), Dectin-2 (dendritic cell-associated C-type lectin 2), MCL (macrophage C-type lectin) and MINCLE (macrophage-inducible C-type lectin) receptors [8, 9]. These receptors play a significant role in immunomodulatory responses, triggering the differentiation of T-helper cells (TH cells) from naïve CD4+ T cells, through the assistance of an APC. CLRs not only recognize PAMPs but also the damaged-associated molecular patterns (DAMPs) and tumor-associated molecular patterns (TAMPs) from the host [15] during the processes of apoptosis and tumorigenesis, respectively. Glycans from a myriad of pathogens (parasitic, fungal, bacterial or viral) are recognized by CLRs (Table 2); the most common being mannan [16], ManLAM (mannose lipoarabinomannan) [17], mycobacterial cord factor [18], β-1,3-glucans [8] and α-1,2-mannose [8, 19]. In humans, DC-SIGN recognizes both mannan and ManLAM; Dectin-1 recognizes β-1,3-glucans and Dectin-2 recognizes α-1,2-mannose. In mice, mycobacterial cord factor is recognized by MINCLE and currently no glycans have been identified for the human MINCLE. We can observe from the literature review available, there is still more work to be done to determine ligands or PAMPs that interact with specific PRRs. Scientists must pay close attention to interspecies differences in PAMP recognition by PRRs, since we can obtain unwanted immune responses once we study them at the human level. Unwanted immunomodulatory responses can compromise the patient’s outcome from the disease.
Table 2.
PAMPs recognized by specific CLRs
| Pathogen-associated molecular pattern (PAMP) | Microorganism or classification | CLRs |
|---|---|---|
| Mannan | Fungi | DC-SIGN |
| Man-LAM | M. tuberculosis | DC-SIGN and Dectin 2 |
| LeX | Schistosoma mansonii and tissue ligands | DC-SIGN |
| LeY + LPS | Helicobacter pylori | |
| LDNF (SP) | Fasciola hepatica | |
| β-1,3-glucans | Fungi | Dectin 1 |
| gp120 | HIV-1 | DC-SIGN |
| α-1,2-mannose | Fungi | Dectin 2 |
| Glucosyl and mannosyl glycolipids | Malassezia pachydermatis and M. furfur | MINCLE |
| Mycobacterial cord factor | M. bovis BCG and M. tuberculosis |
Le X sialyl-Lewis X tetrasaccharide, Le Y Lewis Y tetrasaccharide, LPS lipopolysaccharide, LDNF fucosylated LacdiNAc
In the vaccine development field, we can identify the importance of collaboration between medicinal chemistry, immunology and formulation science. This article will present and discuss several parameters that play prominent roles in liposomal vaccine development. Liposome size, charge and bilayer composition are some of those parameters to be discussed. Evidence will be presented to the reader for understanding of the mechanisms or effects of such liposome physicochemical characteristics, which impact vaccine development, safety, integrity and efficacy. Furthermore, we present different applications of liposomal vaccine studies that have been published for the treatment of certain infections of distinct etiological origins (viral, bacterial, fungal and parasitic). Finally, the article presents a special section on the development of subunit cationic liposomal vaccines for the prophylactic treatment of tuberculosis infections, one of the most sought-after indications in contemporary vaccinology. The section for tuberculosis vaccine development is focused on DDA-based liposomes; and additional information is available that presents other lipids or phospholipids [20, 21]. The manuscript objective is to present liposomal formulations that are not currently commercially available and inform the scientific community about liposomal formulation for early vaccine development. To date, there are only two commercially approved liposomal vaccines Epaxal and Inflexal, both by Crucell/Berna Biotech. We recommend the reader to further their knowledge in commercially available liposome-based vaccines with another review [22], which discusses the topic extensively.
Liposome design
When designing liposomal vaccines, we should take into consideration certain factors within the liposome structure and its physicochemical properties (Fig. 1). Previous reviews on the topic have discussed the different parameters that could affect the functions and efficacy of liposomes as vaccine agents [23, 24]. Liposomal subunit vaccines are safe, with low reactogenicity, biodegradable and versatile. Reactogenicity refers to the low incidence of expected immune responses, causing symptoms like allergies, fever or pain at injection site among others. This type of vaccine contains antigen(s) (either a protein, lipid, lipopeptide etc.) from the pathogen of interest that is incorporated (depending on the antigen physicochemical nature) in the lipid bilayer or core of the liposome. The liposome will serve as an adjuvant, which potentiates the immune responses of the vaccine, improving its efficacy. Antigen incorporation can be achieved by covalent lipid conjugation (at pre- or post-vesicle formation), non-covalent surface attachment (by antibody–epitopes interactions), encapsulation, electrostatic interactions (with lipids of opposite charge) or surface adsorption. Seminal articles published earlier covered the effects of antigen encapsulation or adsorption on innate immune response differentiation [25–27]. Researchers reported that both incorporation methods dichotomously induced immune responses that enhanced T cell differentiation, when albumin was used as the incorporated model antigen in the liposomal formulations. However, when antigen size and complexity decreases (like in virus- or tumor-derived antigens), a surface adsorption incorporation method will induce better immune responses than encapsulation [28, 29].
Fig. 1.
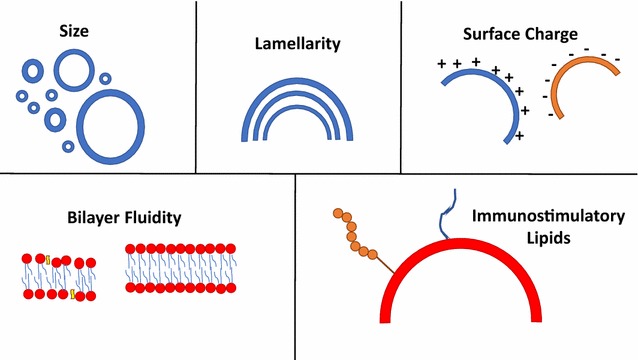
Physicochemical and morphological factors to consider in liposomal vaccine design
Liposomal vesicle size
The factors, or parameters, affecting the function and potential use of a liposome-based vaccine due to their influence in immune responses include liposome size, lamellarity, surface charge, fluidity of the bilayer, formation of lamellar-hexagonal bilayers and the addition of immunostimulatory lipids. Regarding size, it has been previously discussed that larger vesicles (> 2 µm) loaded with a tuberculosis (TB) antigen are likely to induce cell proliferation and low IL-10 induction, contrasting with vesicles around 500 nm that promoted a distinct set of cytokines (IL-1β and IFN-γ) [30]. A study by Brewer et al. presented the effects on immune response differentiation of large (> 225 nm) vs. small (< 155 nm) lipid vesicles [31]. Larger vesicles induced IL-12 cytokine production but smaller vesicles did not. Murine experiments revealed that large vesicles induced TH1 responses due to increased levels of IgG2a and IFN-γ. The smaller particles in this study induced a TH2 immune response due to increase of IL-5 and IgG1 levels. IL-1β triggers a TH17 immune response, whereas IFN-γ induces a TH1 immune response. Therefore, liposome size affects the differentiation of cellular immune responses, rendering this physical parameter a key role in liposomal formulation function.
Vesicle size could be affected by the storage period that the vaccine undergoes and by other environmental parameters. It is well recommended to perform stability studies in different environmental conditions to ensure that liposomal vaccine vesicles do not change over time [32]. Applying several methods during the formulation design and development (like spray drying, sterilization, cryoprotection and PEGylation) will lead to liposomal vesicle stabilization [33–35]. These methods or approaches mentioned earlier avoid unwanted changes in vesicle morphology that could subsequently affect the immune responses of the vaccine. Furthermore, vesicle size stability can be affected by the vesicle lipid composition [36]. An effect observed with unstable lipid vesicles is coalescence, but real-time methods have been developed to study the phenomenon and control stability [37]. Additional work has discussed vesicle stability extensively and we recommend the reader to review the appropriate literature [38–40].
Lamellarity nature of the liposome
The lamellar nature of the liposomal vesicle could also affect the immune system causing differential responses. Beck et al. studied the adjuvanted immune responses to a recombinant HIV protein, CN54 gp140, in small unilamellar (SUV) and large multilamellar vesicles (MLV) [41]. The liposomes were composed of different combinations of monophosphoryl lipid A (MPLA) and lipids (1,2-dimyristoyl-sn-glycero-3-phosphocholine, DMPC; 1,2-dimyristoyl-sn-glycero-3-phospho-(1ʹ-rac-glycerol), DMPG; and cholesterol, Chol), with or without the addition of the saponin QS21. SUVs without QS21 could induce immune responses (characteristic of a TH2 cell-mediated response) to CN54 gp140 protein due to high antibody production, contrasting to MLVs. Adding the saponin QS21 restored immune responses in MLVs (higher IgG1 > IgG2a and IFN-γ titers), stimulating both TH1 and TH2 responses. The saponin did not influence SUVs immune response profile. Shek et al. presented one of the first experiments that compared vesicle lamellarity characteristics against antibody formation enhancement [42]. Liposomes composed of lecithin, dicetyl phosphate and cholesterol were prepared in the presence of bovine serum albumin (BSA). Animals injected with blank liposomes (no BSA) did not generate a significant immune response, as predicted. However, animals injected with BSA-loaded unilamellar vesicles (ULVs) generated strong immune responses compared with multilamellar vesicles (MLVs). Another seminal article presents the co-formulation or adsorption of bovine herpesvirus 1 proteins to large unilamellar (LUVs) and multilamellar (MLVs) liposomes composed of phosphatidylcholine (PC) as the main lipid component [43]. Strong antibody titers were detected in animals injected with LUVs prepared with virus proteins (both adsorbed and co-formulated) and egg PC. Recently, another study demonstrated the effects of the lamellar state for liposomes in subunit vaccines to induce immune responses [44]. SUVs with ovalbumin (OVA) induced greater levels of CD8+ IFN-γ responses against the protein in the spleen. Researchers added TLR3 and nine agonists, enhancing the immune responses in MLVs but not SUVs. Altogether, the studies demonstrate the effect of lamellarity of liposomes in immune responses, being SUVs the preferred state to potentiate innate and adaptive responses which improves vaccine efficacy.
Surface charge
The surface charge of liposomes would be of important consideration for appropriate vaccine design. The overall charge can determine the adsorption or antigen interaction with the liposomes (e.g. anionic antigens will prefer to interact with cationic lipids) which affects antigen loading in the vaccine [18]. Hussain et al. found that replacing the cationic lipid DDA with the neutral lipid distearoyl-sn-glycero-3-phosphocholine (DSPC) decreases the amount of the tuberculosis recombinant antigen H56 from 84 down to 15%. In addition, the function of the vaccine in relation to immune response induction is well documented. Joseph et al. were studying an intranasal influenza vaccine model based on the liposomal formulation of the HN antigen with the polycationic sphingolipid ceramide carbamoyl-spermine (CCS) or other monocationic, neutral and anionic lipids [45]. Neutral and anionic lipid-based formulations were not immunogenic upon intranasal administration in a murine model. However, two out of five monoccationic-based liposomal formulations (containing lipids 1,2-dimyristoyl-3-trimethylammonium-propane, DMTAP; and 1,2-dioleoyl-3-trimethylammonium-propane, DOTAP) induced vigorous local and systemic immune responses (TH1 and TH2 type responses). Researchers compared the monocationic liposomal formulations with the CCS-based liposomal formulation and the only commercially available influenza vaccine, demonstrating the efficacy of the sphingolipid as an immunopotentiator with higher antibody titers and protective immunity for approximately 9 months. Another study with Newcastle disease virus compared the immunization effects in chickens of neutrally- (EPC-Lip), anionically- (PS-Lip) and cationically-charged (SA-Lip) liposomes [46]. Strong humoral responses (local and systemic) were observed in neutral formulations of EPC-Lip, contrasting to the cationic SA-Lip. The anionic formulation mainly composed of phosphatidyl serine (PS-Lip) elicited the higher hemagglutination titers. Recently, Hussain et al. determined that replacing the cationic lipid DDA with the neutral DSPC reduced the TH1-mediated immune response of the formulation [18]. Based on the studies presented above we can conclude that cationic formulations might be the most suitable option to elicit strong immune responses, increasing antibody titers. There is additional information available about cationic lipids, like DDA, which possess significant immunostimulatory and adjuvanting properties [47, 48] and further explanations will be provided ahead.
Bilayer fluidity
The bilayer fluidity, dependent on the lipid gel-liquid crystal transition temperature and its effects on immune responses, is of important interest when designing efficacious vaccines. Many published works have evaluated and described this phenomena, including the earliest work by Yasuda et al. [49]. The effects on immune responses of liposomes prepared with phospholipids of PC with different transition temperatures containing the hapten Dnp-Cap-PE were measured. DMPC, DPPC (1,2-dipalmitoyl-sn-glycero-3-phosphocholine) and DSPC (all with high transition temperatures Tm > 20 °C) were favorable in eliciting antibodies to the hapten, contrasting to results obtained with DOPC (1,2-dioleoyl-sn-glycero-3-phosphocholine), DLPC (1,2-dilauroyl-sn-glycero-3-phosphocholine) and EPC (Tm < 0 °C). Another research team followed a similar experimental design preparing liposomes with low- (DOPC and DLPC, − 20–0 °C), intermediate- (DPPC and sphingomyelin, 25–40 °C) and high-transition temperature (DSPC, > 50 °C) lipids [50]. DMPC, DPPC and sphingomyelin induced immune responses as per a plaque-forming assay, but DSPC was a poor immunogen. Cholesterol was added to the liposomal formulations, inducing significant humoral immune responses. The results from these two groups produced different outcomes: Yasuda et al. [49] presenting that less fluid lipids are better immunogens than fluid phospholipids and van Houte et al. [50] establishing that intermediately fluid phospholipids (which have a phase transition temperature of 25–40 °C) are better immunostimulatory agents. This discrepancy might be attributed to other factors, like particle size and Zeta potential, but such factors were not reported or analyzed to determine their role in these studies.
Additionally, Mazumdar et al. revealed that liposome composition may have an effect on immune responses [51]. Here, researches incorporated a leishmanial antigen (LAg) in liposomes containing DMPC, DPPC or DSPC and described the immunization process and results in a hamster model. No significant delayed hypersensitivity was detected in DMPC- or DPPC-containing lipids, which contrasted with DSPC-containing lipids. Moreover, DSPC-containing lipids protected up to 95% of the hamsters against a leishmanial infection. Recently, Kaur et al. presented results on how cholesterol influences the bilayer fluidity [52]. For instance, a direct correlation of cholesterol and membrane fluidity was observed in DDA:TDB (trehalose dibehenate) liposomal formulations. However, less IgG was detected as cholesterol increased in the system after 12 days of immunization in mice. This effect might be due to the loss of antigen in more fluid (high cholesterol) liposomes as the authors pointed out. The cytokine IFN-γ was at elevated levels when cholesterol was not present in the lipid bilayer. Even with the compelling evidence of how transition temperature and lipid bilayer composition affects immune responses, we can find conflicting results in published data. For example, Hampl et al. found no significant influence of immune response induction based on liposomes containing phospholipids with different transition temperatures [53]. Although the data presented does not follow a clear pattern, the general conclusion is that the more fluid a liposome is the less immune response it will generate when administered in different animal models. Likely behind this phenomenon is that fluidity tends to increase the release of the antigen from the liposomes, affecting antigen presentation and consequently the strength of immune responses. We need to point out that cholesterol also participates as a bilayer stabilizer, increasing rigidity in biological membranes [54]. Therefore, we might consider that the increase in bilayer fluidity by cholesterol observed in the studies discussed above may occur in synthetic bilayers only. Further research must account for such correlation due to the complexities of biological membranes.
Immunostimulatory lipids and liposome deposition
Immunostimulatory lipids might be of value in the vaccine design process. The lipids can act as adjuvants in the vaccine formulation and enhance the immune response we are looking for (innate or adaptive) as previously reported and reviewed [2, 5, 6, 47]. In a study developed by Rao et al., two adjuvants (lipid A and CpG-containing oligodeoxynucleotides, CpG-ODN) were studied in a liposomal formulation based on the HIV envelop protein ogp140 [55]. Both lipid A and CpG-ODN-containing liposomes elicited six and threefold anti-ogp140 antibodies, respectively. Immunization of BALBc mice with the HIV antigen incorporated in lipid A-containing liposomes produced a mixed TH1/TH2 immune response. Combining both adjuvants in the liposomal formulation generated a TH1 immune response. Puangpetch et al. presented the effect of using zwitterionic or cationic lipids in vaccine liposomal formulations [2]. Researchers compared DOTAP (cationic phospholipid) vs. DOPC (neutral phospholid) containing the adjuvant CpG-ODN. DOTAP-based liposomes with adjuvant enhanced the immune response against Burkholderia pseudomallei, which suggest an alternative approach for the treatment of melioidosis. The published data available suggests that certain lipids can induce, or enhance, immune responses. Very important is the fact that cationic liposomes are the ideal model to design effective vaccines due to their immunostimulatory properties.
Finally, the immunostimulation of the vaccine can be affected by the deposition of liposomes at the site of injection which in turn is affected by particle size. Henriksen-Lacey et al. reported this in two separate articles [6, 56]. On Henriksen-Lacey et al. researchers utilized the phospholipid DDA as the building block for the liposomes containing the adjuvant TDB. The antigen for tuberculosis infection, Ag85B-ESAT-6, was co-administered or incorporated to liposomes in mice. Antigen administered alone to mice did not created a depot at the site of injection, causing the antigen to diffuse away from the site and reduce immune responses. The observations directed them to conclude that cationic liposomes made up of DDA promotes depot formation at the site of injection mainly due to size characteristics between antigen-loaded liposomes and antigen administered alone. Later, Henriksen-Lacey et al. [30] studied the effect of liposome composition [DOTAP, DDA or DC-Chol (dimethylaminoethane-carbamoyl-cholesterol)] and the depot formation and antigen distribution. DDA and DC-Chol represented the phospholipids where liposomes induced the migration of monocytes to the site of injection and a significant increase of IFN-γ levels. In general, larger liposomes will form a depot at the site of injection meanwhile smaller liposomes will migrate to lymphoid tissue for antigen presentation and processing.
Liposome-immune cell interactions to improve vaccine effectiveness
Early vaccination strategies included the development of attenuated or inactivated vaccines, which are composed mainly of weakened or dead pathogens, respectively. Currently these kinds of vaccines are facing challenges with variabilities in immune response induction [57, 58]. Modern vaccination strategies are emphasizing the study of subunit vaccines and proving their effectiveness in immune response modulation [59, 60]. Subunit vaccines are characterized by the co-delivery of adjuvants and antigens for immunostimulatory purposes (Fig. 2). The antigen is either a natural or recombinant peptide, protein or molecule derived from the pathogen. The adjuvant will enhance the immune response of the vaccine and potentiate the effect of the antigen during the process. It is very important to know how to present the antigen incorporated in the liposomes to APCs because this will ensure their proper immune cell maturation, antigen presentation and eventual induction of adaptive immune responses as previously reviewed [61, 62]. Cell targeting studies have been investigated and produced significant data and observations of how antigen presentation plays a significant role. Aramaki et al. investigated the utilization of liposomes as carriers for antigens related to gut-associated lymphoid tissue and their uptake by rat Peyer’s patches [63]. Preferential uptake was observed for liposomes in the rat Peyer’s patches than non-patch tissues, specifically for DSPC:PS:Chol liposomes. This suggests that liposome composition affects their uptake. Fluorescently labeled liposomes were internalized by patch tissue in the lower ileum with size (> 374 nm) playing an important direct correlation with uptake. Another report looked at how cationic vesicles composed of DDA could interact with normal and transformed mouse fibroblasts cells [64]. Cell–cell adhesion was observed when DDA concentration was equal or greater to 50 µM. Investigators determined that normal cells were susceptible for DDA vesicles meanwhile transformed cells were resistant to DDA-mediated (> 1 mM) cell death. The interaction with cationic vesicles created a change in cell charge from anionic to cationic, making this system of cationic vesicles ideal for the delivery of negatively charged macromolecules like proteins and DNA.
Fig. 2.
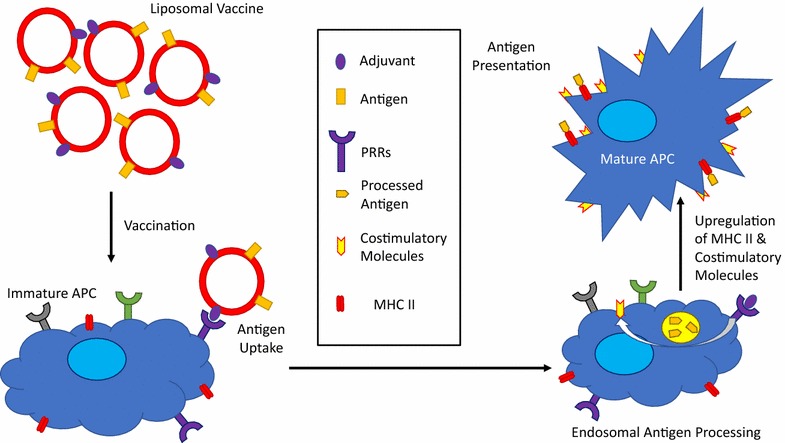
Co-delivery of antigen and adjuvant to APCs in subunit vaccines. Interaction of the adjuvant with the PRR results in upregulation of co-stimulatory molecules necessary for appropriate T cell stimulation
Vaccine interaction with neutrophils, monocytes and APCs
Several studies presented results on how liposome-based vaccines interact with neutrophils and monocytes, key players in inflammation and immune responses. First, Karathanasis et al. used previously characterized and purified peptides to target liposomal nanocarriers to some types of leukocyte cells [65]. Peptides were covalently attached through the carboxyl group of DSPE-PEG by utilizing the crosslinker N,N-dicyclohexylcarbodimide (DCC). Researchers found that targeted liposomes interacted better with monocytes and neutrophils, contrasting with results obtained by non-targeted liposomes. Moreover, the surface density of the peptide directly correlated with liposomes-cell interactions, making this parameter a new way to measure the effectiveness of the interactions and the potential induction of immune responses valuable in subunit vaccine development. Then, Johansen et al. investigated the targeting of monocytes and the delivery of a TLR agonist (TMX-202) using a cationic liposomes-based formulations (mainly POPC (1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine):DOTAP composition) [66]. After an hour incubation, the subset of monocytes targeted by the liposomes were lymphocytes and granulocytes (75–95%). A strong IL-6 and IL-12p40 induction was observed, accompanied by monocyte differentiation to CD14+/DC-SIGN+ DCs. Mainly found in the lymph nodes, lymphocytes include natural killer (NK), T and B cells, which are involved in innate immune responses, cell- and humoral-mediated immunities, respectively. Granulocytes, or polymorphonuclear (PMNs) leukocytes include basophils, neutrophils, mast cells and eosinophils that participate in allergic and inflammatory reactions. The information gathered by the researchers is important because it can determine future treatments and vaccine developments to focus on selected immune responses, depending on the cell or cell types that are being targeted, reducing or avoiding unwanted adverse reactions (e.g. allergies or chronic inflammation).
After interaction with the antigen, cytokines and/or interaction with their milieu, monocytes could differentiate into macrophages or dendritic cells. Both cell types will then migrate to lymph nodes to elicit the corresponding adaptive immune responses. Macrophages and dendritic cells are specialized APCs that reside in the blood stream and help in antigen uptake and antigen presentation to T and B cells, inducing cell-mediated and humoral immune responses, respectively. It is known that antigen-containing liposomes are internalized by pinocytosis in macrophages and then cross presented to CD8+ T cells (specialized T cells that attack and kill tumors), inducing antigen-specific cytotoxic T lymphocytes [67]. This cross-presentation occurs when exogenous antigen is up taken and presented via the class I major histocompatibility complex (MHC I), which classically only happens with endogenous antigens such as those from viruses. To cross-present and elicit a CD8+ T lymphocyte response, the antigen must be delivered to the cytosol APCs. This was studied by Owais et al. in which yeast-derived lipids liposomes and egg PC:Chol liposomes were tested against J774 A1 macrophages and the interactions measured [68]. The fusion rate for yeast lipid liposomes to macrophages was at 40–70%, compared to 1–8% for egg PC:Chol liposomes. Liposome contents were successfully delivered to the cytosol of macrophages. Ovalbumin was used as the model antigen for yeast-derived lipid liposomes and it was found that it elicited a strong CD8+ T cell response. Another group of researchers investigated the uptake mechanisms of liposomes in rat peritoneal macrophages (PM) [69]. Researchers determined that two uptake systems exist because of cholesterol content and size differences of liposomes. For high- (44% molar) and medium-cholesterol (33% molar) content liposomes, the complement receptor-mediated phagocytosis occurs. A complement-independent uptake pathway was suggested for low-cholesterol content liposomes since no inhibition of their internalization rate was observed by the anti-C3 antibody. Therefore, once again, lipid composition is a main player in liposome-cell interactions, potentially affecting the way immune responses develop.
Mannosylated liposomes
Mannosylated liposomes are becoming an alternative method to deliver antigens or antimicrobials to macrophages or DCs [70, 71]. This approach is due to the fact that mannose receptors can be found in these cell types, improving directed-targeting [16]. Other research teams have used cationic lipids like DDA, DOTAP and/or DC-Chol to enhance or potentiate the immune responses due to cell targeting strategies [48, 72, 73]. Korsholm et al. determine that DDA liposomes were minimally internalized by T cells in the mixed splenocyte cultures, contrasting to high uptake rates for APCs (bone marrow dendritic cells) through class II MHC (MHC II), leading to an enhanced OVA presentation. The immune responses described by Varypataki et al. contrasted to Korsholm et al. since DOTAP:DOPC liposomes bearing the peptide SIINFEKL and polyI:C assisted in the delivery of OVA to DCs through MHC I, inducing a CD8+ T cell response.
Understanding and applying innovative ways of cell targeting could improve the discovery pipeline for novel therapeutic agents, avoiding undesirable immune responses that can be detrimental to the patient. These therapies could treat inflammatory diseases [like arthritis or chronic obstructive pulmonary disease (COPD)], infections or cancer. The following sections will focus on how liposome-based vaccines are being utilized for the treatment of infections from viral, bacterial, fungal and parasitic origins. A special section will discuss liposomal vaccines that target the bacterium Mycobacterium tuberculosis to treat TB infections.
Viral infections and liposomes-based vaccines
Viruses are ethiologic agents of different diseases in animals [74] (including humans [75, 76]), plants [77], parasites [78, 79] and bacteria [80, 81]. The basic definition of a virus is a non-living infectious agent that requires living cells for its replication and survival, which allows spreading of the disease. This cell lysis leads to inflammation and tissue damage which are detrimental for the host, enhancing the state of the disease. Additionally, some viruses, like HIV, destroy immune cells hindering effective immune responses when other infections (secondary infections in seropositive patients) occur. Viral infections can be treated by either antiviral therapeutic [82] or by prophylactic vaccine [83] approaches. Here, we will present several examples of vaccine applications based on liposomes for the prophylactic treatment of certain viral infections.
Hepatitis
One of the most significant viruses that affect human health are hepatitis viruses. Hepatitis is an inflammation of the liver tissue caused by the five types of hepatitis viruses (A, B, C, D and E). Hepatitis A and E are spread through contaminated food or water sources. Hepatitis B (HBV) is sexually transmitted or during pregnancy and birth. Both HBV and hepatitis C can be transmitted through blood (needle exchange by IV users) and hepatitis D can only infect people infected with HBV. Two seminal reports investigated novel approaches for the prophylactic treatment of HBV utilizing cationic lipids. Brunel et al. reported the effectiveness of recombinant hepatitis B surface antigen (HBsAg) presentation based on DC-Cholesterol liposomes or aluminum hydroxide (alum) adjuvants in a subcutaneous vaccine model [84]. The DC-Chol-based vaccine elicited antibody (IgG1 and IgG2a) titers in three mice lines (BALB/c, OF1 and B10.M). Compared to the alum-based vaccine, which demonstrated weak immunogenicity, DC-Chol liposomes induced controlled TH1 and TH2 immune responses characterized by normal, but significant levels of cytokines IL-2 and IFN-γ and IL-5, respectively. Controlled immune responses are important to avoid inflammation that could result in tissue damage. The researchers concluded that cationic lipids like DC-Chol could be used as adjuvants, enhancing the immunogenicity of previously non-immunogenic vaccines, specifically in the development of prophylactic vaccines against hepatitis B virus. Another group investigated the use of a transcutaneous vaccine for the treatment of HBV infections [85]. In that report, the vaccine presents some differences from the Brunel et al. article based on the antigen and carrier types. First, cationic transfersomes, a type of liposome, were prepared from DOTMA (1,2-di-O-octadecenyl-3-trimethylammonium propane) phospholipid and sodium deoxycholate (SDC) at different DOTMA weight ratios (75–95% w/w). Second, plasmid DNA encoding the HBsAg gene was loaded to the transfersomes instead of the antigen. The transfersomes were not cytotoxic to HepG2 cells and were stable at different temperatures (4 and 28 °C). Immunization studies included HBsAg DNA-loaded tranfersomes (topical), naked HBsAg DNA (topical and intramuscular) and pure HBsAg (intramuscular) administered to BALB/c mice. Significant levels of anti-HBsAg antibodies and cytokines (IL-2 and IFN-γ) were elicited in topical DNA-loaded transfersomes as compared to intramuscular naked DNA delivery. This antibody and cytokine profile confirmed the induction of TH1 and TH2 immune responses as observed in Brunel et al. Both studies represent great advances in the treatment of HBV infections with novel approaches and administration routes that could applied in the future as preventive vaccines.
Influenza
Influenza is an infectious disease caused by the influenza virus, affecting human health with a pandemic effect in several instances. The virus is divided in three types (A, B and C) and it is spread through the air from coughs and sneezes, therefore the importance for vaccine development studies for the infection. An intranasal (i.n.) liposomal influenza vaccine study was presented by Joseph et al. [45]. The vaccine formulation developed by the group was based in the polycationic sphingolipid N-palmitoyl-d-erythro-sphingosyl-carbamoyl-spermine (or ceramide carbamoyl-spermine, CCS) and was compared with other formulations containing monocationinc phospholipids (DC-Chol, DDA, DSTAP (1,2-stearoyl-3-trimethylammonium-propane), DMTAP and DOTAP). Cholesterol was added to increase liposome fluidity and the lipids DMPC and DMPG were included in neutral and anionic formulations, respectively. All formulations contained the influenza A antigens hemagglutinin and neuraminidase (HN). DMTAP- and DOTAP-based vaccines were the only monocationic lipid formulations to induce strong systemic (serum) and local (lung) TH1 and TH2 responses. Surprisingly, DDA-containing formulations did not induce strong, or significant, local or systemic immune responses. No specific reasons were provided by the team for such results but we can infer that vesicle morphology (multilamellar and oligolamellar cationic formulations), size (1–4 µm diameter) and encapsulation efficiencies (10–90% for non-CCS cationic formulations) would be responsible. The CCS-based vaccine formulation was the only formulation to be at the same or superior level of effectiveness compared to the commercially available vaccine with cholera toxin as the adjuvant. More recently, researchers investigated a triple co-culture model of the human respiratory tract to study the immunostimulatory responses of virosomes and liposomes and their internalization [86]. The epithelial cell line 16HBE was grown with monocyte-derived macrophages (MDMs) and dendritic cells (MDDCs) and exposed to liposomes and virosomes to evaluate the immune responses elicited by the nanocarriers. The virosomes were liposomes prepared with solubilized influenza A/Brisbane/59/2007 H1N1 membrane proteins and included the neutral lipids DOPC and OPPE (1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine, POPE). Liposomes were prepared with previously mentioned neutral lipids and no influenza soluble membrane proteins were added. Virosomes were internalized more efficiently by all cell types in mono- and co-cultures, with APCs like MDMs and MDDCs presenting the highest internalization levels as per flow cytometry and laser scanning microscopy. MDDCs were moderately activated by liposomes and virosomes in monocultures, and inducing elevated levels of cytokine (IL-1β and IL-8) production in co-cultures. Virosomes were internalized at higher levels in epithelial cells in comparison to liposomes.
Respiratory syncytial virus
Another respiratory viral pathogen of interest is the respiratory syncytial virus (RSV). RSV causes respiratory tract infections, specifically lower respiratory tract infections in children and it presents high hospitalization incidence [87]. An i. n. study was developed by Klinguer et al. in which cationic DDA liposomes were mixed with the recombinant fragment of the RSV G protein (BBG2Na) and administered to BALB/c mice [88]. The DDA + BBG2Na liposomal formulation presented significant antibody (IgG and IgA) titers at systemic (serum) and local (nasal) levels. Cytokine production was higher for IL-2 and IFN-γ in cationic liposomes carrying the RSV recombinant antigen, confirming the protection after a viral challenge and the induction of TH1 immune response. These three reports on respiratory viruses and their prophylactic vaccine development confirms the importance of mucosal immunization as it elicits local and systemic B- and T-cell responses [89]. It is also clear that cationic phospholipids like DDA, DC-Chol or DOTAP play a leading role (Table 3) in the immunostimulation of subunit vaccines and more approaches should be investigated. Different administration routes were investigated in the studies for viral infections. However, we cannot verify and compared these administration routes to determine the best alternative due to experimental differences in the studies (liposomal composition and targeted virus). Future experiments should focus their efforts in comparing different administration routes with the same liposomal composition and prophylactic treatment of a particular virus.
Table 3.
Promising vaccine formulations for the treatment of viral infections
| Lipid(s) and sterol used | Virus type | Cell line/animal model used | Administration route | Promising liposome formulation | References |
|---|---|---|---|---|---|
| DOTMA | Hepatitis C | HepG2 cells and BALB/c mice | Transcutaneous | DOTMA | [85] |
| DC-Chol | Hepatitis B | Lymph node cells, BALB/c, OF1 and B10.M mice | Subcutaneous | DC-Chol | [84] |
| DMPC, DMPC/DMPG, DC-Chol/DOPE, DSTAP/Chol, DDA/Chol, DOTAP/Chol, DMTAP/Chol and CCS/Chol | Influenza H3N2 | Splenocytes from BALB/c and C57BL/6 mice | Intranasal | DMTAP/Chol and DOTAP/Chol | [45] |
| DDA | Respiratory Syncytial Virus | BALB/c mice | Intranasal | DDA | [88] |
| DOPC/OPPE (Influenza virosome) and DOPC/OPPE (liposomes) | Influenza | MDMs, MDDCs, 16HBE14o cells, PHNECs and EPCam + cells | N/A | DOPC/OPPE (Influenza virosome) | [86] |
Cell cultures and no vaccination in animal models were employed
N/A not applicable
Bacterial infections and liposomal vaccines
Bacteria can be beneficial for humans, but also detrimental to our health when they harbor pathogenicity traits. Bacteria are divided in Gram positive (+) or negative (−) based on Gram staining, that surveys the peptidoglycan content in the cell wall. Gram (+) bacteria have a positive result in the Gram Stain method, which determines in a qualitative way the presence of the cell wall component: a thick peptidoglycan layer. In contrast, Gram (−) bacteria present a negative result in the stain, indicating a thin peptidoglycan layer, sandwiched between two cell membranes (plasma and outer membranes). Gram (−) bacteria also contain an important immunogenic molecule and pathogenicity factor, the lipopolysaccharide (LPS) [90, 91]. LPS has been used to increase the fusogenicity of cationic liposomes [92]. In that study, researchers developed a carrier system to incorporate LPS into mammalian cell membranes via DOPE (1,2-dioleoyl-sn-glycero-3-phosphoethanolamine):DOTAP (1:1 wt. ratio) liposomes. The team of researchers demonstrated that high LPS concentrations on immortalized fibroblasts generated the activation of macrophages, starting the elimination of LPS-bearing cells.
Additionally, bacteria will have unique cell components, like genetic material, lipids or proteins, that could serve as adjuvants or antigen markers for subunit vaccine development, depending on the molecule chemical characteristics. Li et al. investigated the potential use of cationic (DDA-based) mannosylated liposomes to deliver the model DNA plasmid pGL4.10 (encoding luc2) [19]. The plasmid was protected from nuclease degradation by the liposomes. The cationic mannosylated liposomes showed high uptake and transfection, activating bone marrow DCs (BMDCs). BMDCs activation was characterized by the upregulation of CD80, CD86 and CD40. Another study by Nakanishi et al. studied the effects of positively, negatively and neutrally charged liposomes administered subcutaneously in the immune responses of the fragment A of diphtheria toxin (DTA) and ovalbumin (OVA) [93]. Cationic liposomes were composed of phosphatidylcholine:cholesterol:stearylamine (PC:Chol:SA, 4:5:1 molar ratio), anionic liposomes were composed of PC:Chol:l-α-dimirystoylphosphatidic acid (PC:Chol:DMPA, 4:5:1) and neutral liposomes were composed of PC:Chol (1:1). Positively charged liposomes could induce potent antigen-specific cytotoxic T cell responses. However, DTA-containing cationic liposomes were cytotoxic to macrophages. In contrast, empty cationic liposomes or DTA-loaded anionic and neutral liposomes were not cytotoxic. CD8+ OVA responses were highly induced by positively charged liposomal vaccines, potentially presenting the processed antigen through MHC I. Both research articles presented us the utilization of liposomes to investigate and test the effects on immune responses during vaccination. The immune responses measured varied, but making it clear that cationic liposomes induced the required cells (macrophages and DCs) to obtain the appropriate responses.
The liposomal vaccine studies mentioned above serve as the basis for the following articles which incorporate bacterial antigens for the development of prophylactic vaccines for certain infections. Puangpetch et al. developed a cationic-based liposomal formulations incorporating CpG ODN and to determine the prolongation and mechanisms of the immune responses [2]. The researchers employed the etiologic agent of melioidosis, Burkholderia pseudomalei, as the infection model in BALB/c mice. Cationic and not neutral liposomes administered intramuscularly granted protection against the bacterial challenge study. Prominent levels of IFN-γ were observed 2 days postinfection, but lowered by a CpG ODN-loaded cationic liposome pre-treatment. Neutrophils were not activated by the cationic liposomes with CpG ODN, but macrophages were stimulated by the formulation due to nitric acid production and low intracellular bacterial burden (30 days post vaccination). An additional study involving mannosylated liposomes containing the meningococcal PorA (from Neisseria meningitidis) focused its attention on cell interaction [94]. Anionic (PG- (phosphatidylglycerol) and PS-based) and cationic (DMTAP-based) liposomes were formulated, and one of the anionic formulations was mannosylated (PC:PG:Chol + Man-PE (mannosyl phosphatidylethanolammine). When exposing the formulations to human and murine DCs, researchers observed an increase of liposome-cell interaction in the anionic mannosylated liposomes and cationic liposomes when compared to anionic formulations alone. The result indicated that adding mannosyl moieties to liposomes generated a mannose receptor (MR)-mediated cell interaction. The murine DCs were confirmed to present the markers MHC II+, CD11c+ and CD11b+, meanwhile human DCs presented CD40+, CD1a+ and both MHC I and II. Researchers in the field should address studies that investigate route of administration effects on immune responses. With the studies presented here, it is difficult to determine what best route of administration we should follow for future prophylactic vaccine development. We recommend investigating the optimized formulations in different administration route studies.
The use of cationic liposomes seems of importance for the development of adequate vaccine formulations. The studies presented above demonstrate that by employing cationic phospholipids like DOTAP, DMTAP and DDA, would improve cell interaction levels, allow adequate antigen presentation and induce strong immune responses. These effects will insure that the vaccine will work properly and optimally. For the benefit of the reader, Table 4 presents a summary of the most relevant literature that optimized vaccine formulations for the treatment of bacterial infections. Additionally, previous research on cationic liposomes have discussed the cytotoxicity potential of such formulations. DDA has been determined to be safe as no relevant cytotoxic effects were determined in studies by Hilgers and Snippe and Gall [47, 95]. Only local inflammatory reactions manifested as swelling were observed in mice (when administered alone). Contrasting results are found for DOTAP and DOTMA cationic lipids. DOTAP has been found to be not cytotoxic to macrophages in a study by Jin et al. [96], but Romøren et al. determined that macrophage-derived cell lines were found to be affected by the cationic lipid [97]. The cytotoxic response differences might be due to structural and morphological characteristics in the formulations. Jin et al. employed Tween 20 and tricaprin (part of the solid core) to form solid lipid nanoparticles, meanwhile Romøren et al. just prepared liposomes. Further contradictory information is available for DOTMA, which can be cytotoxic for RAW 264.7 cells at all lipid ratios (DOTMA + DOPE), but not to human umbilical endothelial cells or mouse fibroblasts cells [98]. Kurosaki et al. determined that erythrocytes undergo agglutination and hemolysis when exposed to DOTMA-based liposomes [99]. However, an earlier study by Kurosaki et al. determined lower cytotoxicity for erythrocytes, showing no agglutination and hemolysis, when DOTMA was formulated with N-laurylsarcosine, and Chol, vitamin E and Chol or egg PC and Chol [100]. Future work should include the analysis of lipids alone and formulated with other lipids to elucidate the cytotoxic effects. Additional work should be done for these and other bacterial infections lacking proper prophylactic vaccines, leading to outcome improvement from the infection.
Table 4.
Promising formulations for the treatment of bacterial infections
| Lipid(s) and sterol used | Bacteria or disease | Cell line/animal model used | Administration route | Promising liposome formulation | References |
|---|---|---|---|---|---|
| PC/PG/Chol, PC/PG/Chol/Man-PE, PC/PS/Chol, PC/DMTAP/Chol | Meningitis | Monocyte-derived human DCs and murine bone marrow-derived DCs | N/A | PC/PG/Chol/Man-PE and PC/DMTAP/Chol | [94] |
| PC/Chol/SA, PC/Chol/PA and PC/Chol | Diphteria toxin | BALB/c, C57BL/6 and ddY mice; P815, P13.1 and CD8OVA cells | Subcutaneous | PC/Chol/SA | [93] |
| DOTAP and DOPC | Melioidosis | Neutrophils and splenocytes from BALB/c mice | Intramuscular | DOTAP | [2] |
| DOPE/DOTAP | E. coli | Mouse embryonic fibroblasts (MEF) and RAW 264.7 macrophages | N/A | DOPE/DOTAP | [92] |
| DDA/Chol/Man-C6-Chol and DDA/Chol | E. coli | DC 2.4 cells | N/A | DDA/Chol/Man-C6-Chol | [19] |
Cell cultures and no vaccination in animal models were employed
N/A not applicable
Liposomal-based vaccines in fungal infection treatment
Fungi are eukaryotic organisms that include yeasts, molds and mushrooms. Several yeasts and molds are common pathogenic agents, especially in immunocompromised patients [101, 102]. Antibiotic resistance is being detected, not only in isolates from the USA but also in other developed countries, in different fungal species (like Candida and Aspergillus) [103]. Due to the threat of antifungal resistance, other therapeutic approaches should be investigated, and liposomal vaccines may play a significant role. To the best of our knowledge, the literature review presents liposome-based vaccines for Candida sp. infections but limited information is available for other fungal pathogens. Studies investigating other fungal species with elevated infection prevalence, like Aspergillus, Fusarium, Coccidioidomyces and Zygomycetes sp., have been identified but their treatment approach does not include liposomal vaccines, and we invite further reading on the topic [101, 104, 105]. We will discuss ahead information available for liposomal vaccine development for the prophylactic treatment of Candida sp. infections (Table 5).
Table 5.
Candidiasis treatment with potential liposomal vaccines
| Lipid(s) and sterol used | Fungi or disease | Cell line/animal model used | Administration route | Promising liposome formulation | References |
|---|---|---|---|---|---|
| PC/Chol | C. albicans and C. tropicalis | BALB/cByJ mice | Intravenous | PC/Chol | [106] |
| PC/Chol | BALB/c mice | Intravenous | PC/Chol | [107] | |
| DMPC/DMPG | C. albicans | ICR mice | Subcutaneous | DMPC/DMPG | [108] |
| EPD/DOGS-NTA-Ni | BALB/c mice | Intradermal | EPD/DOGS-NTA-Ni | [109] | |
| ICR mice | Intradermal | [110] | |||
| DDA:MO | Macrophages and BALB/c mice | Subcutaneous | DDA:MO | [111] | |
| BALB/c mice | Subcutaneous | [112] |
The first report dealing with the study and development of a vaccine to treat candidiasis, encapsulated the mannan adhesin portion of Candida albicans [106]. The adhesin protion of the mannan of two C. albicans serotypes (A and B) were incorporated in PC:Chol liposomes (3.2:1 molar ratio). Mice were vaccinated (intravenously) during a period of 5–6 weeks and challenged with C. albicans infection, presenting increasing resistance to disseminated disease. Furthermore, antiserum agglutinins (IgM-type antibodies) from the immunized mice were studied for their humoral protective characteristics against C. tropicalis infection, demonstrating the efficacy of the vaccine. Subsequently, researchers decided to investigate the effectiveness of the monoclonal antibodies obtained from the previous study in a vaginal candidiasis model [107]. Similarly, the vaccine (L-mann) was prepared by the mannan adhesin fraction incorporation into PC:Chol liposomes. Mice were immunized intravenously once a week for 5 weeks prior vaginal inoculation. Vaccinated mice challenged with C. albicans presented lower CFUs (110 ± 38 × 103 CFU/g of vaginal tissue) when compared to non-mannan vaccine approach (240 ± 44 × 103 CFU/g of vaginal tissue). Both article demonstrated the capacity of the vaccine to protect at local or systemic infections of C. albicans.
Further studies investigated zwitterionic and cationic liposomes utilizing ribosomes and recombinant Hsp90 protein as antigens from C. albicans [108–110]. Eckstein et al. prepared anionic DMPC:DMPG liposomes co-lyophilized with RNA obtained from cell lysates of C. albicans cultures or by lipid film formation. Mice protection in a subcutaneous vaccination (60% survival) against the fungal challenge demonstrated the effectiveness of the vaccine prepared by the co-lyophilization method in the presence of C. albicans ribosomes. Subsequently, neutrally charged metalloliposomes with incorporated recombinant Hsp90 (heat shock) protein from C. albicans were developed. Nickel-chelating liposomes were prepared with EPC and 1,2-dioleoyl-sn-glycero-3-[(N-(5-amino-1-carboxypentyl)iminodiacetic acid)succinyl] (nickel salt) (DOGS-NTS-Ni) at molar ratios of 95:5. A non-pyrogenic MDP was used as an adjuvant in the liposomal system and the protein was surface attached by metallochelating bonds. DCs interacted with the liposomes and phagocytosed the nanoparticles in vitro. TH1 and TH2 immune responses were induced after intradermal mice vaccination in comparable levels to the Freund’s complete adjuvant vaccine. Following the previous report, Knotigová et al. employed nickel-chelating liposomes with different MDP-derivatives (norAbuMDP/GMDPs) and tested their adjuvant vaccine potential [110]. Also, Hsp90 from C. albicans was employed as the model antigen. Adaptive and innate immune responses were induced by the developed vaccine systems in intradermal vaccinated rabbits and mice.
Recently, cationic liposomes studies incorporated cell wall surface proteins (CWSPs) of C. albicans and their immunostimulatory properties analyzed during subcutaneous administration [111, 112]. Both studies employed DDA:monooleoylglycerol (MO) at 33:67 molar ratio. Liposomes were not toxic to macrophages and were internalized within 20 min of exposure. In the first study, immunized mice displayed strong humoral- and cell-mediated immune responses. Antibodies were produced against cell wall proteins Cht3p and Xog1p. In the second report, two CWSP-loaded cationic liposomal formulation (ADS1 and ADS2) were tested against disseminated candidiasis. ADS1 immunized mice presented significantly higher levels of C. albicans antibodies, contrasting with the ADS2 formulation. This antibody titer production induced the phagocytosis of the fungus. Elevated levels of the cytokines IL-4, IL-17 and IL-10 were significantly higher than control groups, suggesting TH2, TH17 and anti-inflammatory immune responses, respectively.
Future studies on fungal infections and their prophylactic treatment with vaccines should be performed not only in C. albicans, but also other ethiological agents (e.g. Aspergillus). The literature review revealed a field with potential for growth and development of novel approaches to treat fungal infections, which benefit immunocompromised patients (elderly or HIV-seropositive patients). Additionally, further studies must employ further cationic lipids and their effects on immune response induction. Studies comparing physicochemical properties of liposomes to treat fungal infections must take place to optimize the vaccine strategy and avoid unwanted responses and results. In the studies presented above, the intravenous administration was investigated and revealed affirmative results (~ 60% survival of mice). However, we recommend further studies that compare immunomodulatory responses against mucosal vaccine administration for vaginal candidiasis infections. Similar survival rates were observed for subcutaneous and intradermal administration routes, but future studies should investigate a particular set of liposomes against the different administration to observe if survival rates are affected.
Parasitic infections and liposomal vaccines
Parasitism and malaria
Parasitism is a non-mutual, biological interaction in which the parasite lives inside the host and derives its own nutrients at the host’s expense. Parasites can be classified as macroparasites (visible with the naked eye) like helminths, or microparasites (which are smaller) like viruses, bacteria or protozoa. A textbook example of a parasite is Plasmodium vinckei, causative agent of malaria. In malaria, the parasite (Plasmodium sp.) is transmitted by mosquito bites. The parasite’s sporozoites reside in the liver (in humans), developing into merozoites that infect human red blood cells, initiating the red blood cell cycle. When appropriate, the merozoites will developed into gametocytes that infect more red blood cells that are taken up by mosquitoes during the bites. This initiates the mosquito stages (gametes, ookinetes and oocysts). Oocysts are transmitted to the host, initiating the liver stage.
Postma et al. developed a novel desferrioxamine B (DFO) delivery system based on liposomes to treat malaria [113]. DFO is a siderophore that chelates ferric iron (iron is an vital component of red blood cells). Iron is an important nutrient for P. vinckei as it infects red blood cells. Contrasting to previous liposomal vaccine articles, the researchers investigated the lipid to drug composition and the bilayer fluidity effects on DFO delivery and protection from infection. The lipids were all anionic in charge due to the presence of egg PG. Three different treatments of DFO were analyzed in the study: (1) multiple free DFO subcutaneous injections, (2) intraperitoneal infusion of free DFO and (3) multiple subcutaneous DFO-loaded liposomes injections in C57B1/6J female mice. Parasitemia was suppressed by multiple subcutaneous injections of free DFO before and during infection, but injections prior to infection did not. Suppression of parasitemia and long-term survival was observed for intraperitoneal infusion of free DFO 1 day before infection or by subcutaneous injections of liposomal DFO prior to infection (day-1). Bilayer rigidity was studied by incorporating Chol (intermediate rigidity) and DSPC (high rigidity) in the liposomes, demonstrating that no relationship exists that affect the liposome antimalarial function. However, drug-to-lipid ratio affected the antimalarial activity of the liposomal-based vaccine, suggesting that low drug-to-lipid ratios are the best formulation parameter at combating the infection. When liposomal DFO was administered to mice, a long-term protection against malaria was observed (days 7 and 8, 400 mg/kg/day). This article proves that utilizing a common siderophore (DFO) as an iron chelator in combination with liposomes, improve the therapeutic and prophylactic effects of the vaccine. However, no immune response studies were performed, lacking essential information about the mechanisms of the vaccine when immunization occurs.
A pre-clinical report of the malaria vaccine RTS,S was published by Stewart et al. [114]. The RTS,S/AS02A vaccine utilizes the circumsporozoite protein as antigen. Investigators in this report evaluated the effects of certain adjuvants (AS01B, AS02A, AS05 and AS06) which vary in the concentration of MPL, QS21 or CpG and their formulation delivery system (emulsion vs. formulation). AS01B was the only liposomal formulation in the study. Rhesus macaques were immunized by intramuscular injection with the different RTS,S/adjuvant combinations and specific antibodies, IFN-γ and IL-5 levels were determined after weeks 14 and 34. All regimes were safe and presented elevated antibody titers (except for AS06-containing vaccine formulation). RTS,S/AS01B presented higher levels of IFN-γ at weeks 14 and 34, and the highest IFN-γ to IL-5 ratio when compared to RTS.S/AS02A. Leishmaniasis.
Another parasitic disease is leishmaniasis caused by the parasite Leishmania sp. It is transmitted by sandflies to mammals, transferring metacyclic promastigotes via feeding. The metacyclic promastigotes invade macrophages and granulocytes, developing into amastigotes which multiply by simple division, eventually causing macrophage lysis. Further, amastigotes infect new macrophages or are transferred to the sandflies during feeding. Amastigotes transform into procyclic promastigotes in the gut, maturing into metacyclic promastigotes by simple division. The disease is common in certain regions of Asia, Africa, South and Central America and even southern Europe. The disease is divided into three major syndromes, cutaneous, mucosal or visceral leishmaniasis. Visceral leishmaniasis poses a major risk of death incidence [115].
Three seminal articles provide valuable information regarding the studies and development efforts towards a prophylactic vaccine for leishmanial infections [116–118]. First, Bhowmick et al. presented the immunotherapy effects of leishmanial antigens in liposomes [116]. The researchers correlated the efficacy of soluble leishmanial antigens (SLAs) from Leishmania donovani promastigote membrane incorporated in neutral (lecithin:Chol), negative (lecithin:Chol:PA (phosphatidic acid)) and positively (lecithin:Chol:SA) charged liposomes intraperitoneally administered. L. donovani was eliminated from the liver and spleen when SLAs were present in cationic lipids. IL-4 and IL-10 were downregulated when SLA-cationic liposomes were administered to the mice and the immunomodulatory response presented the TH1 cytokines IFN-γ and IL-12. Subsequently, Banerjee et al. presented two articles which cover the study of cationic stearylamine liposomes for the development of a visceral leishmaniasis vaccine. In the first published article by the team of researchers, amphotericin B (AmB) is used in association with stearylamine (cationic) liposomes as a novel therapeutic approach [117]. When administered to BALBc mice, the leishmanial parasite was eliminated from the liver and spleen. Moreover, when comparing to the conventional liposomal formulation AmBisome, the intravenous administration of AmB-SA-PC liposomes induced the production of IFN-γ from CD8+ and CD4+ T cells. At the same time, the formulation reduced the toxicity effects of the drug by reducing TNF-α levels. In the splenic supernatant culture, IL-10 was downregulated, causing the production of IL-12 and nitric oxide during the AmB-SA-PC liposomes treatment. Also, Banerjee et al. investigated the effects of liposome charge in an antileishmanial assay [118]. Researchers provided evidence of membrane disruption caused by the cationic stearylamine liposomes in promastigotes and amastigotes. No toxicity in murine peritoneal macrophages and human erythrocytes was detected. These studies confirmed the prophylactic effect of SA-PC liposomes against leishmanial infections.
Amoebiasis
Finally, a research paper dealing with parasitic infections present us the incorporation of Entamoeba histolytica Gal/GalNAc lectin LecA antigen in liposomal, emulsion and alum formulations containing synthetic TLR agonists adjuvants [59]. E. histolytica is an anaerobic amoeba and is the etiological agent of amoebiasis, or diarrheal disease commonly transmitted through contaminated water and food sources [119]. The liposome formulation containing a TLR4 and TLR7/8 agonists was selected for further studies due to its ability to induce intestinal IgA, plasma IgG2a/IgG1, IFN-γ and IL-17a. A high mucosal IgA response hinder the ability of parasites to adhere to mammalian cells. The subcutaneous immunization regime success rate reached 55% efficacy.
Parasitic infections are common especially in underdeveloped regions, posing a notable risk for children, adults and the elderly. Further studies should address the use of adjuvants and their immunomodulatory mechanisms when administered in liposomes for vaccine development. Several studies previously discussed optimized certain formulations for vaccine development and we invite the reader to have a look at them (Table 6). Additionally, specific antigens should be employed to induce even more specific immune responses that will enhance the eradication of relevant parasitic diseases like leishmaniasis and amoebiasis. Furthermore, studies should consider administration routes as potential factors that may affect the immunomodulatory responses of vaccines for the treatment of parasitic infections. For instance, toxicity of L. donovani was reduced when administered intravenously and differences in the immune response cytokine profile were detected. These cytokine profiles must need to be addressed, conducting studies with the similar liposomal formulation and administering the vaccines through different routes.
Table 6.
Liposomal vaccine formulations tested in parasitic infection models
| Lipid(s) and sterol used | Parasite | Cell line/animal model used | Administration route | Promising liposome formulation | References |
|---|---|---|---|---|---|
| MPL/QS21 (liposome-based) | Plasmodium falciparum | Rhesus macaques | Intramuscular | MPL/QS21 (liposome-based) | [114] |
| Egg lecithin/Chol, egg lecithin/SA and egg lecithin/PA | Leishmania donovani | BALB/c mice | Intraperitoneal | Lecithin/Chol/SA | [116] |
| PC/Chol, PC/SA, PC/PA and PC/PS | L. donovani | – | Intravenous, N/A | PC/SA | [117, 118] |
| EPC/EPG, EPC/EPG/Chol and DSPC/DPPG/Chol | P. vinckei | Female C57BL/6J mice | Intraperitoneal | EPC/EPG, EPC/EPG/Chol and DSPC/DPPG/Chol | [113] |
Cell cultures and no vaccination in animal models were employed
N/A not applicable
DDA-based cationic liposomal vaccines for the treatment of tuberculosis
The World Health Organization (WHO) estimated 1.8 million deaths in 2015 were related to tuberculosis infections (TBIs) [120]. The global estimate for latent tuberculosis infections (LTBI) was recently determined to be 23% (approximately 1.7 billion persons from the total population) [121]. Because of current tuberculosis (TB) vaccine efficacy variability, inefficiency and waning immunity (like Bacillus Calmette–Guerin, BCG) [57, 58], it is imperative to develop novel vaccines strategies that will improve prophylactic avenues for TBIs and reduce the overall death rate or latency associated with them. One of the principal improvements revealed in the last years is the development of adjuvanted subunit vaccines.
To diminish the detrimental effects of tuberculosis in humans, certain studies have focused on subunit vaccine development based in cationic lipid formulations (Table 7). Adjuvants are required for the development and efficacy of subunit vaccines, potentiating immune responses. Several adjuvants have been studied in the past 20 years including the adjuvanting properties of the lipid DDA [122]. Forty years ago, Snippe et al. investigated the effect of DDA in mice (via an intracutaneous administration) determining that delayed hypersensitivity occurred by the onset of footpad swelling 5 days after vaccination [123]. Subsequently, a study by van Houte et al., where low- (DOPC and DLPC) and high-transition temperature (DSPC) lipids were utilized with DDA as liposome bilayer components, discovered less immunogenicity of the liposomes as DDA concentration decreased [50]. Additionally, an earlier review discussed the immunostimulatory properties of DDA and its uses in different vaccines for veterinary and human infections [47]. We will present the reader with the most significant studies performed that cover the utilization of the cationic lipid DDA in subunit vaccine development.
Table 7.
DDA-based TB vaccine formulation optimization studies
| Lipid(s) and sterol used | Cell line/animal model used | Administration route | Promising liposome formulation | References |
|---|---|---|---|---|
| DDA, DDA/Tween 80, DDD/Span 85, DDA/Tween 80/Span 85, DDA/gelatin, DDA/Chol, DDA/Lecithin, DDA/β-Cyclodextrin and DDA/PLGA | C57BL/6 mice | Subcutaneous | DDA/Chol | [126] |
| DDA, DOTAP, DC-Chol, DOPE/PC and DOPE/PC/PG | Splenocytes from BALB/c and C57BL/6 mice | Subcutaneous | DDA | [125] |
| DDA/DSPC | Splenocytes from C57BL/6 mice | Intramuscular | DDA/DSPC | [18] |
| DDA | Splenocytes from BALB/c mice | Subcutaneous | DDA | [6] |
| DDA | Human/macrophage cell line THP-1 and splenocytes from BALB/c mice | Intramuscular | DDA | [30] |
| DDA | Inguinal lymph nodes or spleens | Subcutaneous | DDA | [128] |
| DDA | C57BL6; spleen and lung lymphocytes | Subcutaneous | DDA | [124] |
| DDA and DDA/Chol | Splenocytes from C57BL/6 mice and THP-1 cells | Intramuscular | DDA | [52] |
| DDA/MMG | Splenocytes from C57BL/6 mice | Subcutaneous | DDA/MMG | [134] |
| DDA | BALB/c mice | Intracutaneous | DDA | [122] |
| DDA | C57BL6 mice | Subcutaneous | DDA | [129] |
| DDA | BALB/c mice | Intracutaneous | DDA | [123] |
| DDA | Splenocytes from BALB/c mice | Intramuscular | DDA | [44] |
| LAM/PC/Chol/stearyl octaarginine | PBMCs | N/A | LAM/PC/Chol/stearyl octaarginine | [17] |
| DDA | DCs | Subcutaneous | DDA | [135] |
Cell cultures and no vaccination in animal models were employed
N/A not applicable
Holten-Andersen et al. mixed the recombinant immunodominant M. tuberculosis antigens (ESAT-6 and Ag85B-ESAT6), DDA and different immunomodulators, analyzing the immune responses against BCG vaccination in mice (subcutaneous administration) [124]. The studied immunomodulators included saponin, calcitriol, β-glucan, n-hexadecane, TDB, muramyl dipeptide (MDP) and monophosphoryl lipid A (MPL). Investigators determined that the combination of the antigens with DDA and TDB generated a strong protective TH1 immune response against the mycobacterium, contrasting with BCG vaccination. In another study, M. bovis BCG lipid extracts were tested for their adjuvant characteristics [125]. BCG lipids were incorporated in DDA-based liposomes and administered subcutaneously to female BALB/c or C57BL/6 mice. BCG lipids coupled with the antigen Ag85B-ESAT-6 fusion protein in cationic liposomes induced significant levels of IFN-γ and relevant antibodies (IgG2A) titers, characteristic of TH1 immune responses. Antigens from other sources (Chlamydia muridarum and tetanus toxoid) were studied and relevant antibodies were detected when administered with the so called mycosomes (BCG lipids + cationic liposomes).
Subsequent studies on cationic liposomes bearing the antigen Ag85B-ESAT-6 (or its modifications) from M. tuberculosis were performed [6, 18, 52, 126]. Henriksen-Laceyet al. incorporated the antigen in DDA or DDA:TDB (8:1 molar ratio) liposomes and administered the vaccine formulation via intramuscular or subcutaneous injections to mice [6]. The antigen did not affect the liposome size, Zeta (ζ) potential or polydispersity index. The cationic lipid formulation was compared with antigen administered alone to mice. Investigators observed the rapid dissemination of the antigen administered alone in mice, contrasting with a depot formation at the injection site when administered in a liposomal formulation (up to 14 days post-vaccination). TDB allowed for the translocation of the liposomes form the site of injection to lymph nodes with the additional effect of monocyte infiltration to the injection site. Another study investigated the effects by which DDA plays a key role as an immunostimulatory lipid [18]. Mice were immunized intramuscularly with the proposed vaccine. Researchers concluded that removing or reducing DDA molar ratio in the liposome bilayer conduced to a reduction in TH1 immune responses against the antigen. Moreover, a team of researchers determined that the addition of cholesterol to the bilayer of DDA:TDB liposomes did not induced strong immune responses suggesting the prominent role of bilayer fluidity [52]. However, the previously mentioned study contrast with results obtained by Liu et al., were the TLR3 ligand Poly I:C was utilized as an adjuvant along with DDA and cholesterol liposomes (DPC liposomes) [126]. Researchers employed the TB fusion protein ESAT-6-Ag85B-MPT64(190-198)-Mtb8.4-Rv2626c (LT70) as the model antigen. DPC liposomes showed stability at size of 400 nm and ζ potential of 40 mV. Strong humoral and cell-mediated immune responses were detected by the production of antigen-specific antibodies after the subcutaneous administration and a markedly protection against a M. tuberculosis infection challenge than the traditional BCG vaccine.
With the advances in recombinant protein antigens from M. tuberculosis, other teams of researchers decided to observe at different antigenic proteins (like OVA) in DDA:TDB liposomes [44, 127], comparing different adjuvants and physicochemical properties in cationic liposomal formulations [30, 128] or investigating the mechanism of protein antigen adsorption [127]. OVA-containing SUVs liposomes composed of DDA:TDB with no TLR ligand showed a higher capacity to induce spleen CD8 IFN-γ responses against the antigen, contrasting to MLVs, administered intramuscularly [44]. Antigen-specific responses were higher on SUVs. Adding TLR3 and TLR9 agonists significantly increased the immune responses on MLVs carrying OVA, but that was not observed in SUVs. The study suggested that liposomes are an excellent delivery vehicle for antigen presentation and vaccine formulation, and we believe that this is the foundation for the subsequent studies based on cationic and neutral lipid formulations. Investigators selected DDA and the synthetic mycobacterial cord factor molecule, TDB, as a suitable model adjuvant (CAF01) for vaccine development [128]. This was based in that mice vaccination (subcutaneous) with CAF01 induced a strong antigen-specific cell- and humoral-mediated responses, contrasting with currently used adjuvants (e.g. alum and MPL). Furthermore, results were strongly supported by the protection from infection when mice were challenged by M. tuberculosis, C. trachomatis or malaria, revealing cell mediated (TB), cell-mediated/humoral (C. trachomatis) and humoral immune responses.
A study showed the significant role that liposome vesicle size plays in the cell-mediated immune responses [30]. Researchers determined that no differences in vaccine (administered intramuscularly) draining from the injection site occurred, but a size-dependent liposome movement (favored by large liposomes) was observed to the popliteal lymph node. Macrophage-like cells internalized liposomes in a size-independent pattern. Size did not affect the antigen-specific antibody response (IgG1/2) of Ag85B-ESAT-6-carrying liposomes, however larger liposomes induced highest cell proliferation and lowest IL-10 levels, which contrasted with smaller vesicles (inducing IFN-γ and IL-1β). Additionally, a study investigated the effects of charge, membrane fluidity and antigen-to-lipid ration on mechanism of protein antigen adsorption [127]. For this purpose, investigators compared cationic (CAF01) and neutral (NAF01) liposomal formulations, mainly composed of DDA or DSPC, respectively. α-Lactalbumin and lysozyme were used as antigen protein models to analyze the parameters. The anionic lactalbumin interacted with cationic liposomes by surface adsorption, and no interaction was observed with zwitterionic liposomes. However, the cationic lysozyme presented no detectable interaction with either type of liposomes. Adsorption of α-lactalbumin generated changes in its tertiary structure, neutralized liposome charge, affected lipid membrane packing (especially for CAF01 liposomes), resulting in a reduction of colloidal stability and liposome aggregation. The CAF01 formulation has completed multiple phase I safety trails in humans to date.
Other studies have focus their attention on cell components, synthetic lipid analogues or mycobacterial lipids as antigens for subunit vaccine development employing DDA as the principal component in the lipid bilayer. Mutant M. bovis BCG ΔmmaA4 strain was formulated in cationic liposomes of DDA:TDB (A4/Adj) and administered subcutaneously [129]. The mutation deletes the mmaA4 gene that encodes a S-adenosylmethionine-dependent methyltransferase involved in mycolic acid biosynthesis in the tubercle bacillus [130]. Immunocompromised mice (TCRδ−/−) immunized with A4/Adj were protected against an infection of M. tuberculosis (2 and 9 months post-vaccination), contrasting with non-adjuvanted mutant and non-vaccinated controls. It is important to note that the immunocompromised mice lack CD4+, CD8+ and NK1.1+ T cells but, due to immunization long-term results, an unconventional T cell population was responsible for the immune responses. Researchers observed CD4− CD8− double negative (DN) T cells and found that the cells accumulated in the lungs of A4/Adj-treated mice, with significant levels of IFN-γ production when comparing to nonvaccinated or nonadjuvanted BCG control test groups. In vitro studies revealed the antimycobacterial properties of DN T cells isolated from adjuvanted BCG-treated mice when compared to whole-spleen cells. The results of this study represent a milestone in medical research since tuberculosis affects dramatically immunocompromised patients, like in HIV infections [131–133].
Synthetic lipid analogues from monomycoloyl glycerol (MMG-1 to 6) from M. tuberculosis has been developed and their supramolecular structure and adjuvant efficacy tested via subcutaneous administration [134]. The analogues displayed longer (MMG-2) or shorter (MMG-3) alkyl chains, or stoichiometry variations of the polar head group (MMG-5) or the hydrophobic moiety (MMG-6). CryoTEM and synchrotron small-angle X-ray (SAX) experiments revealed the supramolecular organization varied from unilamellar and multilamellar (ULVs/MLVs) vesicles in DDA:MMG-1/2/5/6 liposomes to ULVs and hexosomes in DDA:MMG-3. TH1 and TH17 immune responses were induced by DDA:MMG-1/3/6 liposomal formulations in response to a chlamydial antigen, contrasting to different immunostimulatory properties of naked MMG-1 and MMG-6 analogues in vitro. We recommend further studies employing MMG analogues incorporated in liposomes with mycobacterial-derived antigens to assess the efficacy of this chlamydial model. In contrast, another study utilized natural mycobacterial lipids diacylated sulfoglycolipids (Ac2-SGL) and phosphatidyl-myo-inositol dimannosides (PIM2) as antigens in a liposomal vaccine formulated with DDA and TDB as adjuvants [135]. Researchers observed a reduction of bacterial load in the spleen of vaccinated animals via subcutaneous administration, contrasting with the unvaccinated group. The lipid antigen vaccine group showed a remarkable reduction of lung and spleen lesions when compared to the unvaccinated group. Comparison of lipid antigen vaccine with protein antigen vaccine regimes in a guinea pig model revealed no significant differences in the treatments.
From the articles discussed in this section we can count on promising advances in tuberculosis vaccine development. The approaches presented applied recombinant protein antigens, whole-mutant cell and synthetic and natural cell components formulations. Each approach could encounter some drawbacks: mass production of natural and synthetic products from M. tuberculosis and other mycobacteria; liposomal formulation instability and aggregation; unwanted immune responses. Most of the studies discussed above demonstrated that subcutaneous administration could be a successful route of administration and future research should direct efforts to determined other administration routes, like intranasal and intramuscular. By understanding the physicochemical parameters of liposomes and the effects of routes of administration on physiological and immune function requirements, scientists will be able to develop a novel tuberculosis vaccine.
Conclusion
Vaccination represents the major advancement of modern medicine, second in effect only to clean water, in decreasing the spread of detrimental diseases and providing better quality of life. To develop effective and safe vaccines, collaboration between immunologists and formulation scientists must exist. This collaboration will ensure that the vaccine is designed adequately and that the therapy will not cause unwanted immune responses. Also, it is important to understand the basic concepts in immunology (innate vs. adaptive immunity) so we can target the corresponding receptors with the ligands and antigens employed in the vaccine. Many types of cell receptors participate in PAMPs recognition (innate immunity), namely NLRs, STING, RLRs, TLRs and CLRs. Each receptor contributes to significant responses that lead to T helper cell activation and differentiation, with eventual B cell (antibody-mediated) and CD8 T cell-mediated adaptive immune responses.
For liposomal vaccines, we must pay close attention to liposome size, surface charge (ζ potential), morphology (lamellarity) and lipid bilayer fluidity. Size may affect antigen presentation to APCs and consequently immune responses. Likewise, surface charge of the liposome may affect antigen adsorption on the liposome and could affect liposome-cell interactions (cationic liposomes interact better with the anionic membrane of immune cells). MLVs may reduce antigen presentation due to their concentric vesicle morphological nature, contrasting to ULVs which enhances antigen presentation. Antigen leakage and immune response reduction may occur on how the antigen was formulated (adsorption vs. absorption). Absortion is explained by the Tm of the lipids or phospholipids used when developing the vaccine. Lipids with lower Tm tend to be fluid meanwhile lipids with higher Tm would increase bilayer rigidity. During adsorption, the antigen is exposed in the outer layer of the lipid membrane, which could allow easy release or presentation of the antigen. Additionally, we must pay special attention to how liposomes would interact with immune cells and how we can target those cells, enhancing proper immune responses. Adjuvants play a significant role in APCs activation and maturation for eventual T and B cell activation. The adjuvants, properly selected, will interact with their corresponding TLRs or CLRs in a process called targeted cell vaccine delivery. This cell targeted process is also affected by the vaccine route of administration.
We observed in the sections previously discussed key developments in contemporary vaccine research for the treatment of viral, bacterial, fungal and parasitic infections. The studies presented to the reader demonstrated the collaborative and interdisciplinary nature of the field. Cell targeting and adjuvants dominated most of the approaches to develop effective prophylactic subunit vaccines for the treatment of detrimental diseases. In some instances, infection was hindered by the antimicrobial, antiviral, antifungal or antiparasitic activities of the vaccines due to the induction of adequate immune responses (cell- and antibody-mediated), leading to the survival of selected animal models. Additionally, we can conclude that cationic liposomal vaccines are of great interest for future vaccine development due to their enhanced interaction with the negatively charged immune cells. In specific, DDA-based liposomes are being tested in diverse vaccine studies which promise significant advances in the field. Such DDA applications as a building block of liposomal vaccines represent a step forward towards the prophylactic treatment of diverse infections. These developments will improve the current vaccine approaches and will provide better treatments for patients. Additional research efforts must be made towards the development of novel adjuvants that will contribute to the induction of significant immune responses. Both authors read and approved the final manunscript.
Authors’ contributions
LODS and DB developed the concept of the manuscript. LODS reviewed the literature, drafted and wrote the manuscript. DB critically reviewed the manuscript and provided ideas and comments on the draft. Both authors approved the final manuscript.
Acknowledgements
Special thanks to the Formulation Team of the Center for Translational Medicine (CTM) at the University of Montana for their critical review of the manuscript. Special thanks to Dr. Alyson Smith, CTM’s Immunology Director, for her guidance in the immunology-related information discussed in the article.
Competing interests
LODS declares no competing interests. DB is the Chief Operating Officer and member of the Board of Directors, Inimmune, Inc. The authors are entirely responsible for the content of the review and the opinions contained within it.
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
Not applicable.
Funding
This review was written with the support of a NIAID Contract # HHSN272201400050C.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- AmB
amphotericin B
- APCs
antigen presenting cells
- BCG
Bacillus Calmette–Guerin
- BCR
B cell receptor
- BMDCs
bone marrow dendritic cells
- BSA
bovine serum albumin
- CCS
ceramide carbamoyl-spermine
- Chol
cholesterol
- CLR
C-type lectin receptor
- COPD
chronic obstructive pulmonary disease
- CpG-ODN
CpG-containing oligodeoxynucleotides
- CWSPs
cell wall surface proteins
- DAMPs
damaged-associated molecular patterns
- DCs
dendritic cells
- DCC
N,N-dicyclohexylcarbodimide
- DC-Chol
dimethylaminoethane-carbamoyl-cholesterol
- DC-SIGN
DC-specific ICAM3-grabbing non-integrin
- DDA
dimethyldioctadecylammonium
- Dectin-1
dendritic cell-associated C-type lectin 1
- Dectin-2
dendritic cell-associated C-type lectin 2
- DFO or DFB
desferrioxamine B
- DLPC
1,2-dilauroyl-sn-glycero-3-phosphocholine
- DMPC
1,2-dimyristoyl-sn-glycero-3-phosphocholine
- DMPG
1,2-dimyristoyl-sn-glycero-3-phospho-(1ʹ-rac-glycerol)
- DMTAP
1,2-dimyristoyl-3-trimethylammonium-propane
- DOGS-NTS-Ni
1,2-dioleoyl-sn-glycero-3-[(N-(5-amino-1-carboxypentyl)iminodiacetic acid)succinyl] (nickel salt)
- DOPC
1,2-dioleoyl-sn-glycero-3-phosphocholine
- DOPE
1,2-dioleoyl-sn-glycero-3-phosphoethanolamine
- DOTAP
1,2-dioleoyl-3-trimethylammonium-propane
- DOTMA
1,2-di-O-octadecenyl-3-trimethylammonium propane
- DPPC
1,2-dipalmitoyl-sn-glycero-3-phosphocholine
- DSPC
distearoyl-sn-glycero-3-phosphocholine
- DSTAP
1,2-stearoyl-3-trimethylammonium-propane
- DTA
diphtheria toxin
- EPC
egg phosphatidylcholine
- HBsAg
hepatitis B surface antigen
- HBV
hepatitis B virus
- HIV
human immunodeficiency virus
- Hsp90
C. albicans heat shock protein 90
- IFN-γ
gamma interferon
- Ig
immunoglobulin
- IL
interleukins
- i.n.
intranasal
- LAg
leishmanial antigen
- Lip
liposome(s)
- LPS
lipopolysaccharide
- LTBIs
latent tuberculosis infections
- LUVs
large unilamellar vesicles
- ManLAM
mannose lipoarabinomannan
- Man-PE
mannosyl phosphatidylethanolamine
- MCL
macrophage C-type lectin
- MDDCs
monocyte-derived dendritic cells
- MDMs
monocyte-derived macrophages
- MDP
muramyl dipeptide
- MHC I
class I major histocompatibility complex
- MHC II
class II major histocompatibility complex
- MINCLE
macrophage-inducible C-type lectin
- MLVs
multilamellar vesicles
- MMG
monomycoloyl glycerol
- MO
monooleoylglycerol
- MPL or MPLA
monophosphoryl lipid A
- MR
mannose receptor
- NKs
natural killer cells
- NLRs
nucleotide-binding domain and leucine-rich repeat receptor
- OVA
ovabulmin
- PA
phosphatidic acid
- PAMPs
pathogen associated molecular patterns
- PC
phosphatidylcholine
- PG
phosphatidylglycerol
- PMNs
polymorpho nuclear cells
- POPC
1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine
- POPE
1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine
- PRRs
pathogen recognition receptors
- PS
phosphatidylserine
- RLRS
retinoic acid-inducible gene I receptor
- RSV
respiratory syncytial virus
- SA
stearylamine
- SDC
sodium deoxycholate
- SUVs
small unilamellar vesicles
- TAMPs
tumor-associated molecular patters
- TB
tuberculosis
- TBIs
tuberculosis infections
- TCR
T cell receptor
- TDB
trehalose dibehenate
- TH cells
T helper cells
- TLR
toll-like receptor
- TNF-α
tumor necrosis factor alpha
- WHO
World Health Organization
Contributor Information
Luis O. De Serrano, Email: luisdeserrano@hotmail.com
David J. Burkhart, Email: David.Burkhart@mso.umt.edu
References
- 1.Guy B, Pascal N, Francon A, Bonnin A, Gimenez S, Lafay-Vialon E, Trannoy E, Haensler J. Design, characterization and preclinical efficacy of a cationic lipid adjuvant for influenza split vaccine. Vaccine. 2001;19:1794–1805. doi: 10.1016/s0264-410x(00)00386-8. [DOI] [PubMed] [Google Scholar]
- 2.Puangpetch A, Anderson R, Huang YY, Sermswan RW, Chaicumpa W, Sirisinha S, Wongratanacheewin S. Cationic Liposomes extend the immunostimulatory effect of CpG oligodeoxynucleotide against Burkholderia pseudomallei infection in BALB/c mice. Clin Vaccine Immunol. 2012;19:675–683. doi: 10.1128/CVI.05545-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Capron A, Riveau G, Grzych J-M, Boulanger D, Capron M, Pierce R. Development of a vaccine strategy against human and bovine schistosomiasis: background and update. Trop Geogr Med. 1994;46:242–246. [PubMed] [Google Scholar]
- 4.Girard MP, Fruth U, Kieny MP. A review of vaccine research and development: tuberculosis. Vaccine. 2005;23:5725–5731. doi: 10.1016/j.vaccine.2005.07.045. [DOI] [PubMed] [Google Scholar]
- 5.Christensen D, Agger EM, Andreasen LV, Kirby D, Andersen P, Perrie Y. Liposome-based cationic adjuvant formulations (CAF): past, present, and future. J Liposome Res. 2009;19:2–11. doi: 10.1080/08982100902726820. [DOI] [PubMed] [Google Scholar]
- 6.Henriksen-Lacey M, Bramwell VW, Christensen D, Agger EM, Andersen P, Perrie Y. Liposomes based on dimethyldioctadecylammonium promote a depot effect and enhance immunogenicity of soluble antigen. J Control Release. 2010;142:180–186. doi: 10.1016/j.jconrel.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 7.Kallerup RS, Madsen CM, Schioth ML, Franzyk H, Rose F, Christensen D, Korsholm KS, Foged C. Influence of trehalose 6,6ʹ-diester (TDX) chain length on the physicochemical and immunopotentiating properties of DDA/TDX liposomes. Eur J Pharm Biopharm. 2015;90:80–89. doi: 10.1016/j.ejpb.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 8.Geijtenbeek TBH, Gringhuis SI. C-type lectin receptors in the control of T helper cell differentiation. Nat Rev Immunol. 2016;16:433–448. doi: 10.1038/nri.2016.55. [DOI] [PubMed] [Google Scholar]
- 9.Goyal S, Klassert TE, Slevogt H. C-type lectin receptors in tuberculosis: what we know. Med Microbiol Immunol. 2016;205:513–535. doi: 10.1007/s00430-016-0470-1. [DOI] [PubMed] [Google Scholar]
- 10.Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science. 2010;327:291–295. doi: 10.1126/science.1183021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pasare C, Medzhitov R. Toll-like receptors: linking innate and adaptive immunity. Microbes Infect. 2004;6:1382–1387. doi: 10.1016/j.micinf.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 12.Yao C, Oh JH, Lee DH, Bae JS, Jin CL, Park CH, Chung JH. Toll-like receptor family members in skin fibroblasts are functional and have a higher expression compared to skin keratinocytes. Int J Mol Med. 2015;35:1443–1450. doi: 10.3892/ijmm.2015.2146. [DOI] [PubMed] [Google Scholar]
- 13.Oosting M, Cheng SC, Bolscher JM, Vestering-Stenger R, Plantinga TS, Verschueren IC, Arts P, Garritsen A, van Eenennaam H, Sturm P, et al. Human TLR10 is an anti-inflammatory pattern-recognition receptor. Proc Natl Acad Sci USA. 2014;111:E4478–E4484. doi: 10.1073/pnas.1410293111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cagnoni AJ, Saez JMP, Rabinovich GA, Marino KV. Turning-off signaling by siglecs, selectins, and galectins: chemical inhibition of glycan-dependent interactions in cancer. Front Oncol. 2016;6:21. doi: 10.3389/fonc.2016.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dambuza IM, Brown GD. C-type lectins in immunity: recent developments. Curr Opin Immunol. 2015;32:21–27. doi: 10.1016/j.coi.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Opanasopit P, Higuchi Y, Kawakami S, Yamashita F, Nishikawa M, Hashida M. Involvement of serum mannan binding proteins and mannose receptors in uptake of mannosylated liposomes by macrophages. Biochim Biophys Acta Biomem. 2001;1511:134–145. doi: 10.1016/s0005-2736(01)00267-x. [DOI] [PubMed] [Google Scholar]
- 17.Kallert S, Zenk SF, Walther P, Grieshober M, Weil T, Stenger S. Liposomal delivery of lipoarabinomannan triggers Mycobacterium tuberculosis specific T-cells. Tuberculosis. 2015;95:452–462. doi: 10.1016/j.tube.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 18.Hussain MJ, Wilkinson A, Bramwell VW, Christensen D, Perrie Y. Th1 immune responses can be modulated by varying dimethyldioctadecylammonium and distearoyl-sn-glycero-3-phosphocholine content in liposomal adjuvants. J Pharm Pharmacol. 2014;66:358–366. doi: 10.1111/jphp.12173. [DOI] [PubMed] [Google Scholar]
- 19.Li P, Chen S, Jiang Y, Jiang J, Zhang Z, Sun X. Dendritic cell targeted liposomes-protamine-DNA complexes mediated by synthetic mannosylated cholestrol as a potential carrier for DNA vaccine. Nanotechnology. 2013;24:295101. doi: 10.1088/0957-4484/24/29/295101. [DOI] [PubMed] [Google Scholar]
- 20.Christensen D, Korsholm KS, Andersen P, Agger EM. Cationic liposomes as vaccine adjuvants. Expert Rev Vaccines. 2011;10:513–521. doi: 10.1586/erv.11.17. [DOI] [PubMed] [Google Scholar]
- 21.McNeil SE, Perrie Y. Gene delivery using cationic liposomes. Expert Opin Ther Pat. 2006;16:1371–1382. [Google Scholar]
- 22.Bulbake U, Doppalapudi S, Kommineni N, Khan W. Liposomal formulations in clinical use: an updated review. Pharmaceutics. 2017;9:12. doi: 10.3390/pharmaceutics9020012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watson DS, Endsley AN, Huang L. Design considerations for liposomal vaccines: influence of formulation parameters on antibody and cell-mediated immune responses to liposome associated antigens. Vaccine. 2012;30:2256–2272. doi: 10.1016/j.vaccine.2012.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perrie Y, Crofts F, Devitt A, Griffiths HR, Kastner E, Nadella V. Designing liposomal adjuvants for the next generation of vaccines. Adv Drug Deliv Rev. 2016;99:85–96. doi: 10.1016/j.addr.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 25.Shahum E, Therien HM. Immunopotentiation of the humoral response by liposomes—encapsulation versus covalent linkage. Immunology. 1988;65:315–317. [PMC free article] [PubMed] [Google Scholar]
- 26.Shahum E, Therien HM. Liposomal adjuvanticity—effect of encapsulation and surface-linkage on antibody-production and proliferative response. Int J Immunopharmacol. 1995;17:9–20. doi: 10.1016/0192-0561(94)00082-y. [DOI] [PubMed] [Google Scholar]
- 27.Therien HM, Shahum E. Immunopotentiation of the humoral response by liposomes—effect of a T-cell polyclonal activator. Cell Immunol. 1988;116:320–330. doi: 10.1016/0008-8749(88)90234-1. [DOI] [PubMed] [Google Scholar]
- 28.Barnier-Quer C, Elsharkawy A, Romeijn S, Kros A, Jiskoot W. Adjuvant effect of cationic liposomes for subunit influenza vaccine: influence of antigen loading method, cholesterol and immune modulators. Pharmaceutics. 2013;5:392–410. doi: 10.3390/pharmaceutics5030392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Legrue SJ. Carrier and adjuvant properties of liposome-borne tumor-specific antigens. Cancer Immunol Immunother. 1984;17:135–141. doi: 10.1007/BF00200050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henriksen-Lacey M, Devitt A, Perrie Y. The vesicle size of DDA:TDB liposomal adjuvants plays a role in the cell-mediated immune response but has no significant effect on antibody production. J Control Release. 2011;154:131–137. doi: 10.1016/j.jconrel.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 31.Brewer JM, Tetley L, Richmond J, Liew FY, Alexander J. Lipid vesicle size determines the Th1 or Th2 response to entrapped antigen. J Immunol. 1998;161:4000–4007. [PubMed] [Google Scholar]
- 32.Jain S, Indulkar A, Harde H, Agrawal AK. Oral mucosal immunization using glucomannosylated bilosomes. J Biomed Nanotechnol. 2014;10:932–947. doi: 10.1166/jbn.2014.1800. [DOI] [PubMed] [Google Scholar]
- 33.Mohammed AR, Bramwell VW, Coombes AG, Perrie Y. Lyophilisation and sterilisation of liposomal vaccines to produce stable and sterile products. Methods. 2006;40:30–38. doi: 10.1016/j.ymeth.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 34.Ingvarsson PT, Schmidt ST, Christensen D, Larsen NB, Hinrichs WL, Andersen P, Rantanen J, Nielsen HM, Yang M, Foged C. Designing CAF-adjuvanted dry powder vaccines: spray drying preserves the adjuvant activity of CAF01. J Control Release. 2013;167:256–264. doi: 10.1016/j.jconrel.2013.01.031. [DOI] [PubMed] [Google Scholar]
- 35.Bakowsky H, Richter T, Kneuer C, Hoekstra D, Rothe U, Bendas G, Ehrhardt C, Bakowsky U. Adhesion characteristics and stability assessment of lectin-modified liposomes for site-specific drug delivery. Biochim Biophys Acta. 2008;1778:242–249. doi: 10.1016/j.bbamem.2007.09.033. [DOI] [PubMed] [Google Scholar]
- 36.Dunnick JK, Badger RS, Takeda Y, Kriss JP. Vesicle interactions with antibody and peptide hormone: role of vesicle composition. J Nucl Med. 1976;17:1073–1076. [PubMed] [Google Scholar]
- 37.Perrie Y, Ali H, Kirby DJ, Mohammed AU, McNeil SE, Vangala A. Environmental scanning electron microscope imaging of vesicle systems. Methods Mol Biol. 2017;1522:131–143. doi: 10.1007/978-1-4939-6591-5_11. [DOI] [PubMed] [Google Scholar]
- 38.Cohen S, Alonso MJ, Langer R. Novel approaches to controlled-release antigen delivery. Int J Technol Assess Health Care. 1994;10:121–130. doi: 10.1017/s0266462300014045. [DOI] [PubMed] [Google Scholar]
- 39.Chen C, Han D, Cai C, Tang X. An overview of liposome lyophilization and its future potential. J Control Release. 2010;142:299–311. doi: 10.1016/j.jconrel.2009.10.024. [DOI] [PubMed] [Google Scholar]
- 40.Winterhalter M, Lasic DD. Liposome stability and formation: experimental parameters and theories on the size distribution. Chem Phys Lipids. 1993;64:35–43. doi: 10.1016/0009-3084(93)90056-9. [DOI] [PubMed] [Google Scholar]
- 41.Beck Z, Matyas GR, Jalah R, Rao M, Polonis VR, Alving CR. Differential immune responses to HIV-1 envelope protein induced by liposomal adjuvant formulations containing monophosphoryl lipid A with or without QS21. Vaccine. 2015;33:5578–5587. doi: 10.1016/j.vaccine.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 42.Shek PN, Yung BYK, Stanacev NZ. Comparison between multilamellar and unilamellar liposomes in enhancing antibody-formation. Immunology. 1983;49:37–44. [PMC free article] [PubMed] [Google Scholar]
- 43.Hampl J, Stepanek J, Franz J, Svoboda I. Incorporation of Bovine Herpesvirus-1 protein subunits into large unilamellar and multilamellar liposomes. Acta Veterinaria Brno. 1992;61:29–36. [Google Scholar]
- 44.Milicic A, Kaur R, Reyes-Sandoval A, Tang CK, Honeycutt J, Perrie Y, Hill AVS. Small cationic DDA:TDB liposomes as protein vaccine adjuvants obviate the need for TLR agonists in inducing cellular and humoral responses. PLoS ONE. 2012;7:10. doi: 10.1371/journal.pone.0034255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Joseph A, Itskovitz-Cooper N, Samira S, Flasterstein O, Eliyahu H, Simberg D, Goldwaser I, Barenholz Y, Kedar E. A new intranasal influenza vaccine based on a novel polycationic lipid—ceramide carbamoyl-spermine (CCS)-I. Immunogenicity and efficacy studies in mice. Vaccine. 2006;24:3990–4006. doi: 10.1016/j.vaccine.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 46.Tseng LP, Chiou CJ, Deng MC, Lin MH, Pan RN, Huang YY, Liu DZ. Evaluation of encapsulated newcastle disease virus liposomes using various phospholipids administered to improve chicken humoral immunity. J Biomed Mater Res Part B Appl Biomater. 2009;91B:621–625. doi: 10.1002/jbm.b.31437. [DOI] [PubMed] [Google Scholar]
- 47.Hilgers LAT, Snippe H. DDA as an immunological adjuvant. Res Immunol. 1992;143:494–503. doi: 10.1016/0923-2494(92)80060-x. [DOI] [PubMed] [Google Scholar]
- 48.Korsholm KS, Agger EM, Foged C, Christensen D, Dietrich J, Andersen CS, Geisler C, Andersen P. The adjuvant mechanism of cationic dimethyldioctadecylammonium liposomes. Immunology. 2007;121:216–226. doi: 10.1111/j.1365-2567.2007.02560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yasuda T, Dancey GF, Kinsky SC. Immunogenicity of liposomal model membranes in mice—dependence on phospholipid composition. Proc Natl Acad Sci USA. 1977;74:1234–1236. doi: 10.1073/pnas.74.3.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Van Houte AJ, Snippe H, Schmitz MGJ, Willers JMN. Characterization of immunogenic properties of haptenated liposomal model membranes in mice 5 effect of membrane composition on humoral and cellular immunogenicity. Immunology. 1981;44:561–568. [PMC free article] [PubMed] [Google Scholar]
- 51.Mazumdar T, Anam K, Ali N. Influence of phospholipid composition on the adjuvanticity and protective efficacy of liposome-encapsulated Leishmania donovani antigens. J Parasitol. 2005;91:269–274. doi: 10.1645/GE-356R1. [DOI] [PubMed] [Google Scholar]
- 52.Kaur R, Henriksen-Lacey M, Wilkhu J, Devitt A, Christensen D, Perrie Y. Effect of incorporating cholesterol into DDA:TDB liposomal adjuvants on bilayer properties, biodistribution, and immune responses. Mol Pharm. 2014;11:197–207. doi: 10.1021/mp400372j. [DOI] [PubMed] [Google Scholar]
- 53.Hampl J, Franz J, Jordanova K, Stepanek J. Effects of phospholipid-composition on adjuvant efficiency of liposomes. Acta Veterinaria Brno. 1995;64:163–169. [Google Scholar]
- 54.Kummerow FA, Przybylski R, Wasowicz E. Changes in arterial membrane lipid composition may precede growth factor influence in the pathogenesis of atherosclerosis. Artery. 1994;21:63–75. [PubMed] [Google Scholar]
- 55.Rao M, Matyas GR, Vancott TC, Birx DL, Alving CR. Immunostimulatory CpG motifs induce CTL responses to HIV type I oligomeric gp140 envelope protein. Immunol Cell Biol. 2004;82:523–530. doi: 10.1111/j.0818-9641.2004.01283.x. [DOI] [PubMed] [Google Scholar]
- 56.Henriksen-Lacey M, Christensen D, Bramwell VW, Lindenstrom T, Agger EM, Andersen P, Perrie Y. Comparison of the depot effect and immunogenicity of liposomes based on dimethyldioctadecylammonium (DDA), 3 beta Nʹ,Nʹ-dimethylaminoethane)carbomyl cholesterol (DC-Chol), and 1,2-dioleoyl-3-trimethylammonium propane (DOTAP): prolonged liposome retention mediates stronger Th1 responses. Mol Pharm. 2011;8:153–161. doi: 10.1021/mp100208f. [DOI] [PubMed] [Google Scholar]
- 57.Andersen P, Doherty TM. The success and failure of BCG—implications for a novel tuberculosis vaccine. Nat Rev Microbiol. 2005;3:656–662. doi: 10.1038/nrmicro1211. [DOI] [PubMed] [Google Scholar]
- 58.Fine PEM. Variation in protection by BCG—implications of and for heterologous immunity. Lancet. 1995;346:1339–1345. doi: 10.1016/s0140-6736(95)92348-9. [DOI] [PubMed] [Google Scholar]
- 59.Abhyankar MM, Noor Z, Tomai MA, Elyecrog J, Fox CB, Petri WA., Jr Nanoformulation of synergistic TLR ligands to enhance vaccination against Entamoeba histolytica. Vaccine. 2017;35:916–922. doi: 10.1016/j.vaccine.2016.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mohammed AR, Bramwell VW, Kirby DJ, McNeil SE, Perrie Y. Increased potential of a cationic liposome-based delivery system: enhancing stability and sustained immunological activity in pre-clinical development. Eur J Pharm Biopharm. 2010;76:404–412. doi: 10.1016/j.ejpb.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 61.Zhao L, Seth A, Wibowo N, Zhao CX, Mitter N, Yu CZ, Middelberg APJ. Nanoparticle vaccines. Vaccine. 2014;32:327–337. doi: 10.1016/j.vaccine.2013.11.069. [DOI] [PubMed] [Google Scholar]
- 62.Lonez C, Vandenbranden M, Ruysschaert JM. Cationic liposomal lipids: from gene carriers to cell signaling. Prog Lipid Res. 2008;47:340–347. doi: 10.1016/j.plipres.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 63.Aramaki Y, Tomizawa H, Hara T, Yachi K, Kikuchi H, Tsuchiya S. Stability of liposomes in-vitro and their uptake by rat peyer patches following oral-administration. Pharm Res. 1993;10:1228–1231. doi: 10.1023/a:1018936806278. [DOI] [PubMed] [Google Scholar]
- 64.CarmonaRibeiro AM, Ortis F, Schumacher RI, Armelin MCS. Interactions between cationic vesicles and cultured mammalian cells. Langmuir. 1997;13:2215–2218. [Google Scholar]
- 65.Karathanasis E, Geigerman CM, Parkos CA, Chan L, Bellamkonda RV, Jaye DL. Selective targeting of nanocarriers to neutrophils and monocytes. Ann Biomed Eng. 2009;37:1984–1992. doi: 10.1007/s10439-009-9702-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Johansen PT, Zucker D, Parhamifar L, Pourhassan H, Madsen DV, Henriksen JR, Gad M, Barberis A, Maj R, Andresen TL, Jensent SS. Monocyte targeting and activation by cationic liposomes formulated with a TLR7 agonist. Expert Opin Drug Deliv. 2015;12:1045–1058. doi: 10.1517/17425247.2015.1009444. [DOI] [PubMed] [Google Scholar]
- 67.Tanaka Y, Taneichi M, Kasai M, Kakiuchi T, Uchida T. Liposome-coupled antigens are internalized by antigen-presenting cells via pinocytosis and cross-presented to CD8(+) T cells. PLoS ONE. 2010;5:e15225. doi: 10.1371/journal.pone.0015225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Owais M, Gupta CM. Liposome-mediated cytosolic delivery of macromolecules and its possible use in vaccine development. Eur J Biochem. 2000;267:3946–3956. doi: 10.1046/j.1432-1327.2000.01447.x. [DOI] [PubMed] [Google Scholar]
- 69.Huong TM, Harashima H, Kiwada H. Complement dependent and independent liposome uptake by peritoneal macrophages: cholesterol content dependency. Biol Pharm Bull. 1998;21:969–973. doi: 10.1248/bpb.21.969. [DOI] [PubMed] [Google Scholar]
- 70.Song X, Lin Q, Guo L, Fu Y, Han J, Ke H, Sun X, Gong T, Zhang Z. Rifampicin loaded mannosylated cationic nanostructured lipid carriers for alveolar macrophage-specific delivery. Pharm Res. 2015;32:1741–1751. doi: 10.1007/s11095-014-1572-3. [DOI] [PubMed] [Google Scholar]
- 71.White KL, Rades T, Furneaux RH, Tyler PC, Hook S. Mannosylated liposomes as antigen delivery vehicles for targeting to dendritic cells. J Pharm Pharmacol. 2006;58:729–737. doi: 10.1211/jpp.58.6.0003. [DOI] [PubMed] [Google Scholar]
- 72.Varypataki EM, van der Maaden K, Bouwstra J, Ossendorp F, Jiskoot W. cationic liposomes loaded with a synthetic long peptide and poly(I:C): a defined adjuvanted vaccine for induction of antigen-specific T cell cytotoxicity. Aaps J. 2015;17:216–226. doi: 10.1208/s12248-014-9686-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tada R, Hidaka A, Iwase N, Takahashi S, Yamakita Y, Iwata T, Muto S, Sato E, Takayama N, Honjo E, et al. Intranasal immunization with DOTAP cationic liposomes combined with DC-cholesterol induces potent antigen-specific mucosal and systemic immune responses in mice. PLoS ONE. 2015;10:e0139785. doi: 10.1371/journal.pone.0139785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Meiklejohn G, Simpson TW, Stacy IB. experimental infection of domestic animals with Japanese B-encephalitis virus. Proc Soc Exp Biol Med. 1947;65:359–364. doi: 10.3181/00379727-65-15959. [DOI] [PubMed] [Google Scholar]
- 75.Niklasson B, Hakansson C, Lowhagen GB, Jonsson R. Oral mucosal lesions associated with HIV-infection. Swed Dent J. 1986;10:258. [Google Scholar]
- 76.Greenberg HB, Pollard RB, Lutwick LI, Gregory PB, Robinson WS, Merigan TC. Effect of human leukocyte interferon on hepatitis B virus-infection in patients with chronic active hepatitis. N Engl J Med. 1976;295:517–522. doi: 10.1056/NEJM197609022951001. [DOI] [PubMed] [Google Scholar]
- 77.Allard HA. Some properties of the virus of the mosaic disease of tobacco. J Agric Res. 1916;6:649–674. [Google Scholar]
- 78.Brown M, Reed S, Levy JA, Busch M, McKerrow JH. Detection of HIV-1 in entamoeba-histolytica without evidence of transmission to human-cells. Aids. 1991;5:93–96. doi: 10.1097/00002030-199101000-00014. [DOI] [PubMed] [Google Scholar]
- 79.Wang AL, Wang CC. Viruses of parasitic protozoa. Parasitol Today. 1991;7:76–80. doi: 10.1016/0169-4758(91)90198-w. [DOI] [PubMed] [Google Scholar]
- 80.Applemans R, Wagemans J. The bacteriophages of different families. C R Seances Soc Biol Fil. 1922;86:738–739. [Google Scholar]
- 81.Maisin J. The bacteriophages. Arch Int Pharmacodyn Ther. 1922;26:215–245. [Google Scholar]
- 82.Piroth L, Wittkop L, Lacombe K, Rosenthal E, Gilbert C, Miailhes P, Carrieri P, Chas J, Poizot-Martin I, Gervais A, et al. Efficacy and safety of direct-acting antiviral regimens in HIV/HCV-co-infected patients—French ANRS CO13 HEPAVIH cohort. J Hepatol. 2017;67:23–31. doi: 10.1016/j.jhep.2017.02.012. [DOI] [PubMed] [Google Scholar]
- 83.Sil A, Ravi MD, Patnaik BN, Dhingra MS, Dupuy M, Gandhi DJ, Dhaded SM, Dubey AP, Kundu R, Lalwani SK, et al. Effect of prophylactic or therapeutic administration of paracetamol on immune response to DTwP-HepB-Hib combination vaccine in Indian infants. Vaccine. 2017;35:2999–3006. doi: 10.1016/j.vaccine.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 84.Brunel F, Darbouret A, Ronco J. Cationic lipid DC-Chol induces an improved and balanced immunity able to overcome the unresponsiveness to the hepatitis B vaccine. Vaccine. 1999;17:2192–2203. doi: 10.1016/s0264-410x(98)00492-7. [DOI] [PubMed] [Google Scholar]
- 85.Mahor S, Rawat A, Dubey PK, Gupta PN, Khatri K, Goyal AK, Vyas SP. Cationic transfersomes based topical genetic vaccine against hepatitis B. Int J Pharm. 2007;340:13–19. doi: 10.1016/j.ijpharm.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 86.Blom RAM, Erni ST, Krempaska K, Schaerer O, van Dijk RM, Amacker M, Moser C, Hall SRR, von Garnier C, Blank F. A triple co-culture model of the human respiratory tract to study immune-modulatory effects of liposomes and virosomes. PLoS ONE. 2016;11:e0163539. doi: 10.1371/journal.pone.0163539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stein RT, Bont LJ, Zar H, Polack FP, Park C, Claxton A, Borok G, Butylkova Y, Wegzyn C. Respiratory syncytial virus hospitalization and mortality: systematic review and meta-analysis. Pediatr Pulmonol. 2017;52:556–569. doi: 10.1002/ppul.23570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Klinguer C, Beck A, De-Lys P, Bussat MC, Blaecke A, Derouet F, Bonnefoy JY, Nguyen TN, Corvaia N, Velin D. Lipophilic quaternary ammonium salt acts as a mucosal adjuvant when co-administered by the nasal route with vaccine antigens. Vaccine. 2001;19:4236–4244. doi: 10.1016/s0264-410x(01)00156-6. [DOI] [PubMed] [Google Scholar]
- 89.Staats HF, McGhee JR. Application of basic principles of mucosal immunity to vaccine development. In: Kryono H, Ogra PL, McGhee JR (eds) Mucosal Vaccines. San Diego: Academic Press; 1996. p. 17–39. ISBN: 9780124105805.
- 90.Goldberg JB, Pier GB. Pseudomonas aeruginosa lipopolysaccharides and pathogenesis. Trends Microbiol. 1996;4:490–494. doi: 10.1016/s0966-842x(97)82911-3. [DOI] [PubMed] [Google Scholar]
- 91.Ketley JM. Pathogenesis of enteric infection by Campylobacter. Microbiol Uk. 1997;143:5–21. doi: 10.1099/00221287-143-1-5. [DOI] [PubMed] [Google Scholar]
- 92.Naumovska E, Ludwanowski S, Hersch N, Braun T, Merkel R, Hoffmann B, Csiszar A. Plasma membrane functionalization using highly fusogenic immune activator liposomes. Acta Biomater. 2014;10:1403–1411. doi: 10.1016/j.actbio.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 93.Nakanishi T, Kunisawa J, Hayashi A, Tsutsumi Y, Kubo K, Nakagawa S, Nakanishi M, Tanaka K, Mayumi T. Positively charged liposome functions as an efficient immunoadjuvant in inducing cell-mediated immune response to soluble proteins. J Control Release. 1999;61:233–240. doi: 10.1016/s0168-3659(99)00097-8. [DOI] [PubMed] [Google Scholar]
- 94.Foged C, Arigita C, Sundblad A, Jiskoot W, Storm G, Frokjaer S. Interaction of dendritic cells with antigen-containing liposomes: effect of bilayer composition. Vaccine. 2004;22:1903–1913. doi: 10.1016/j.vaccine.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 95.Gall D. The adjuvant activity of aliphatic nitrogenous bases. Immunology. 1966;11:369–386. [PMC free article] [PubMed] [Google Scholar]
- 96.Jin SE, Kim CK. Long-term stable cationic solid lipid nanoparticles for the enhanced intracellular delivery of SMAD3 antisense oligonucleotides in activated murine macrophages. J Pharm Pharm Sci. 2012;15:467–482. doi: 10.18433/j3z312. [DOI] [PubMed] [Google Scholar]
- 97.Romøren K, Thu BJ, Bols NC, Evensen Ø. Transfection efficiency and cytotoxicity of cationic liposomes in salmonid cell lines of hepatocyte and macrophage origin. Biochim Biophys Acta. 2004;1663:127–134. doi: 10.1016/j.bbamem.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 98.Sakurai F, Inoue R, Nishino Y, Okuda A, Matsumoto O, Taga T, Yamashita F, Takakura Y, Hashida M. Effect of DNA/liposome mixing ratio on the physicochemical characteristics, cellular uptake and intracellular trafficking of plasmid DNA/cationic liposome complexes and subsequent gene expression. J Control Release. 2000;66:255–269. doi: 10.1016/s0168-3659(99)00280-1. [DOI] [PubMed] [Google Scholar]
- 99.Kurosaki T, Kitahara T, Fumoto S, Nishida K, Yamamoto K, Nakagawa H, Kodama Y, Higuchi N, Nakamura T, Sasaki H. Chondroitin sulfate capsule system for efficient and secure gene delivery. J Pharm Pharm Sci. 2010;13:351–361. doi: 10.18433/j3gk52. [DOI] [PubMed] [Google Scholar]
- 100.Kurosaki T, Kitahara T, Teshima M, Nishida K, Nakamura J, Nakashima M, To H, Hukuchi H, Hamamoto T, Sasaki H. Exploitation of De Novo helper-lipids for effective gene delivery. J Pharm Pharm Sci. 2008;11:56–67. doi: 10.18433/j31s3b. [DOI] [PubMed] [Google Scholar]
- 101.Lehrnbecher T, Kalkum M, Champer J, Tramsen L, Schmidt S, Klingebiel T. Immunotherapy in invasive fungal infection—focus on invasive aspergillosis. Curr Pharm Des. 2013;19:3689–3712. doi: 10.2174/1381612811319200010. [DOI] [PubMed] [Google Scholar]
- 102.Mathew BP, Nath M. Recent approaches to antifungal therapy for invasive mycoses. ChemMedChem. 2009;4:310–323. doi: 10.1002/cmdc.200800353. [DOI] [PubMed] [Google Scholar]
- 103.Jensen RH. Resistance in human pathogenic yeasts and filamentous fungi: prevalence, underlying molecular mechanisms and link to the use of antifungals in humans and the environment. Dan Med J. 2016;63(10). pii: B5288. [PubMed]
- 104.Hector RF, Rutherford GW, Tsang CA, Erhart LM, McCotter O, Anderson SM, Komatsu K, Tabnak F, Vugia DJ, Yang Y, Galgiani JN. The public health impact of Coccidioidomycosis in Arizona and California. Int J Environ Res Public Health. 2011;8:1150–1173. doi: 10.3390/ijerph8041150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Krishnan-Natesan S, Chandrasekar PH. Current and future therapeutic options in the management of invasive aspergillosis. Drugs. 2008;68:265–282. doi: 10.2165/00003495-200868030-00002. [DOI] [PubMed] [Google Scholar]
- 106.Han YM, Cutler JE. Antibody-response that protects against disseminated candidiasis. Infect Immun. 1995;63:2714–2719. doi: 10.1128/iai.63.7.2714-2719.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Han YM, Morrison RP, Cutler JE. A vaccine and monoclonal antibodies that enhance mouse resistance to Candida albicans vaginal infection. Infect Immun. 1998;66:5771–5776. doi: 10.1128/iai.66.12.5771-5776.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Eckstein M, Barenholz Y, Bar LK, Segal E. Liposomes containing Candida albicans ribosomes as a prophylactic vaccine candidiasis in mice. Vaccine. 1997;15:220–224. doi: 10.1016/s0264-410x(96)00137-5. [DOI] [PubMed] [Google Scholar]
- 109.Masek J, Bartheldyova E, Turanek-Knotigova P, Skrabalova M, Korvasova Z, Plockova J, Koudelka S, Skodova P, Kulich P, Krupka M, et al. Metallochelating liposomes with associated lipophilised norAbuMDP as biocompatible platform for construction of vaccines with recombinant His-tagged antigens: preparation, structural study and immune response towards rHsp90. J Control Release. 2011;151:193–201. doi: 10.1016/j.jconrel.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 110.Knotigova PT, Zyka D, Masek J, Kovalova A, Krupka M, Bartheldyova E, Kulich P, Koudelka S, Lukac R, Kauerova Z, et al. Molecular adjuvants based on nonpyrogenic lipophilic derivatives of norAbuMDP/GMDP formulated in nanoliposomes: stimulation of innate and adaptive immunity. Pharm Res. 2015;32:1186–1199. doi: 10.1007/s11095-014-1516-y. [DOI] [PubMed] [Google Scholar]
- 111.Carneiro C, Correia A, Collins T, Vilanova M, Pais C, Gomes AC, Oliveira ME, Sampaio P. DODAB:monoolein liposomes containing Candida albicans cell wall surface proteins: a novel adjuvant and delivery system. Eur J Pharm Biopharm. 2015;89:190–200. doi: 10.1016/j.ejpb.2014.11.028. [DOI] [PubMed] [Google Scholar]
- 112.Carneiro C, Correia A, Lima T, Vilanova M, Pais C, Gomes AC, Real Oliveira MECD, Sampaio P. Protective effect of antigen delivery using monoolein-based liposomes in experimental hematogenously disseminated candidiasis. Acta Biomater. 2016;39:133–145. doi: 10.1016/j.actbio.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 113.Postma NS, Hermsen CC, Zuidema J, Eling WMC. Plasmodium vinckei: optimization of desferrioxamine B delivery in the treatment of murine malaria. Exp Parasitol. 1998;89:323–330. doi: 10.1006/expr.1998.4282. [DOI] [PubMed] [Google Scholar]
- 114.Stewart VA, McGrath SM, Walsh DS, Davis S, Hess AS, Ware LA, Kester KE, Cummings JF, Burge JR, Voss G, et al. Pre-clinical evaluation of new adjuvant formulations to improve the immunogenicity of the malaria vaccine RTS, S/AS02A. Vaccine. 2006;24:6483–6492. doi: 10.1016/j.vaccine.2006.06.033. [DOI] [PubMed] [Google Scholar]
- 115.Ready PD. Epidemiology of visceral leishmaniasis. Clin Epidemiol. 2014;6:147–154. doi: 10.2147/CLEP.S44267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bhowmick S, Ravindran R, Ali N. Leishmanial antigens in liposomes promote protective immunity and provide immunotherapy against visceral leishmaniasis via polarized Th1 response. Vaccine. 2007;25:6544–6556. doi: 10.1016/j.vaccine.2007.05.042. [DOI] [PubMed] [Google Scholar]
- 117.Banerjee A, De M, Ali N. Complete cure of experimental visceral leishmaniasis with amphotericin B in stearylamine-bearing cationic liposomes involves down-regulation of IL-10 and favorable T cell responses. J Immunol. 2008;181:1386–1398. doi: 10.4049/jimmunol.181.2.1386. [DOI] [PubMed] [Google Scholar]
- 118.Banerjee A, Roychoudhury J, Ali N. Stearylamine-bearing cationic liposomes kill Leishmania parasites through surface exposed negatively charged phosphatidylserine. J Antimicrob Chemother. 2008;61:103–110. doi: 10.1093/jac/dkm396. [DOI] [PubMed] [Google Scholar]
- 119.Kirk MD, Pires SM, Black RE, Caipo M, Crump JA, Devleesschauwer B, Dopfer D, Fazil A, Fischer-Walker CL, Hald T, et al. World Health Organization estimates of the global and regional disease burden of 22 foodborne bacterial, protozoal, and viral diseases, 2010: a data synthesis. Plos Med. 2015;12:21. doi: 10.1371/journal.pmed.1001921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.World Health Organization . Global tuberculosis report 2016. Geneva: World Health Organization; 2016. [Google Scholar]
- 121.Houben R, Dodd PJ. The global burden of latent tuberculosis infection: a re-estimation using mathematical modelling. Plos Med. 2016;13:13. doi: 10.1371/journal.pmed.1002152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tsuruta LR, Quintilio W, Costa MHB, CarmonaRibeiro AM. Interactions between cationic liposomes and an antigenic protein: the physical chemistry of the immunoadjuvant action. J Lipid Res. 1997;38:2003–2011. [PubMed] [Google Scholar]
- 123.Snippe H, Belder M, Willers JMN. Dimethyl dioctadecyl ammonium bromide as adjuvant for delayed-hypersensitivity in mice. Immunology. 1977;33:931–936. [PMC free article] [PubMed] [Google Scholar]
- 124.Holten-Andersen L, Doherty TM, Korsholm KS, Andersen P. Combination of the cationic surfactant dimethyl dioctadecyl ammonium bromide and synthetic mycobacterial cord factor as an efficient adjuvant for tuberculosis subunit vaccines. Infect Immun. 2004;72:1608–1617. doi: 10.1128/IAI.72.3.1608-1617.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rosenkrands I, Agger EM, Olsen AW, Korsholm KS, Andersen CS, Jensen KT, Andersen P. Cationic liposomes containing mycobacterial lipids: a new powerful Th1 adjuvant system. Infect Immun. 2005;73:5817–5826. doi: 10.1128/IAI.73.9.5817-5826.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Liu X, Da Z, Wang Y, Niu H, Li R, Yu H, He S, Guo M, Wang Y, Luo Y, et al. A novel liposome adjuvant DPC mediates Mycobacterium tuberculosis subunit vaccine well to induce cell-mediated immunity and high protective efficacy in mice. Vaccine. 2016;34:1370–1378. doi: 10.1016/j.vaccine.2016.01.049. [DOI] [PubMed] [Google Scholar]
- 127.Hamborg M, Rose F, Jorgensen L, Bjorklund K, Pedersen HB, Christensen D, Foged C. Elucidating the mechanisms of protein antigen adsorption to the CAF/NAF liposomal vaccine adjuvant systems: effect of charge, fluidity and antigen-to-lipid ratio. Biochim Biophys Acta Biomembr. 2014;1838:2001–2010. doi: 10.1016/j.bbamem.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 128.Agger EM, Rosenkrands I, Hansen J, Brahimi K, Vandahl BS, Aagaard C, Werninghaus K, Kirschning C, Lang R, Christensen D, et al. Cationic liposomes formulated with synthetic mycobacterial cordfactor (CAF01): a versatile adjuvant for vaccines with different immunological requirements. PLoS ONE. 2008;3:10. doi: 10.1371/journal.pone.0003116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Derrick SC, Yabe I, Morris S, Cowley S. Induction of unconventional T cells by a mutant Mycobacterium bovis BCG strain formulated in cationic liposomes correlates with protection against Mycobacterium tuberculosis infections of immunocompromised mice. Clin Vaccine Immunol. 2016;23:638–647. doi: 10.1128/CVI.00232-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Boissier F, Bardou F, Guillet VR, Uttenweiler-Joseph S, Daffe M, Quemard A, Mourey L. Further insight into S-adenosylmethionine-dependent methyltransferases—structural characterization of Hma, an enzyme essential for the biosynthesis of oxygenated mycolic acids in Mycobacterium tuberculosis. J Biol Chem. 2006;281:4434–4445. doi: 10.1074/jbc.M510250200. [DOI] [PubMed] [Google Scholar]
- 131.Procop GW. HIV and mycobacteria. Semin Diagn Pathol. 2017;34:332–339. doi: 10.1053/j.semdp.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 132.Perronne C, Zahraoui M, Leport C, Salmon D, Pangon B, Bricaire F, Vilde JL. Tuberculosis in 30 patients with HIV infection. Ann Med Interne. 1988;139:375–376. [Google Scholar]
- 133.Haegi V. Septic tuberculosis in HIV-infection. Schweiz Med Wochenschr. 1987;117:1297–1301. [PubMed] [Google Scholar]
- 134.Martin-Bertelsen B, Korshohn KS, Roces CB, Nielsen MH, Christensen D, Franzyk H, Yaghmur A, Foged C. Nano-self-assemblies based on synthetic analogues of mycobacterial monomycoloyl glycerol and DDA: supramolecular structure and adjuvant efficacy. Mol Pharm. 2016;13:2771–2781. doi: 10.1021/acs.molpharmaceut.6b00368. [DOI] [PubMed] [Google Scholar]
- 135.Larrouy-Maumus G, Layre E, Clark S, Prandi J, Rayner E, Lepore M, de Libero G, Williams A, Puzo G, Gilleron M. Protective efficacy of a lipid antigen vaccine in a guinea pig model of tuberculosis. Vaccine. 2017;35:1395–1402. doi: 10.1016/j.vaccine.2017.01.079. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


