Abstract
Introduction
Surrogate decision-makers (“surrogates”) and physicians of incapacitated patients have different views of prognosis and how it should be communicated, but this has not been investigated in neurocritically-ill patients. We examined surrogates’ communication preferences and physicians’ practices during the outcome prognostication for critically-ill traumatic brain injury (ciTBI) patients in two level-1 trauma centers and seven academic medical centers in the U.S.
Methods
We used qualitative content analysis and descriptive statistics of transcribed interviews to identify themes in surrogates (n=16) and physicians (n=20).
Results
The majority of surrogates (82%) preferred numeric estimates describing the patient’s prognosis, as they felt it would increase prognostic certainty, and limit the uncertainty perceived as frustrating. Conversely, 75% of the physicians reported intentionally omitting numeric estimates during prognostication meetings due to low confidence in family members’ abilities to appropriately interpret probabilities, worry about creating false hope, and distrust in the accuracy and data quality of existing TBI outcome models. Physicians felt that these models are for research only and should not be applied to individual patients. Surrogates valued compassion during prognostication discussions, and acceptance of their goals-of-care decision by clinicians. Physicians and surrogates agreed on avoiding false hope.
Conclusion
We identified fundamental differences in the communication preferences of prognostic information between ciTBI patient surrogates and physicians. These findings inform the content of a future decision aid for goals-of-care discussions in ciTBI patients. If validated, these findings may have important implications for improving communication practices in the neuroICU independent of whether a formal decision aid is used.
Keywords: Shared decision making, goals-of-care decisions, decision aid, qualitative research, traumatic brain injury, critical care, surrogate decision-maker
INTRODUCTION
Neurocritically-ill patients are incapacitated and unable to participate in their own medical decision-making. Therefore, patients’ families are enlisted as surrogate decision-makers (“surrogates”). During goals-of-care discussions, surrogates are asked to make decisions about life-sustaining interventions, such as tracheostomy with feeding tube placement, with continuation of care after intensive care unit (ICU) discharge[1,2]. The option to shift to comfort care may also be offered. Making informed decisions about goals-of-care requires surrogates’ understanding of possible long-term outcomes, with or without continuing medical care.
While goals-of-care decisions are life-or-death decisions, they lack standardization and are subject to bias by the prognosticating physicians, who offer certain treatment options at their own discretion[3–5]. Additionally, goals-of-care decisions are emotionally, cognitively, and morally difficult[6]. Due in part to the surrogates’ struggle with these decisions, post-traumatic stress disorder is prevalent in family members of ICU patients at high risk for dying[6,7]. In the setting of critically-ill traumatic brain injury (ciTBI), substantial variability in rates of withdrawal from life-support, both across and within trauma centers, have been recognized[4,3]. Varying levels of confidence and comfort among physicians in providing outcome prognostication may contribute to variability[3]. A shared decision-making (SDM) process for goals-of-care discussions between surrogates and physicians may improve the quality of these discussions by consistently providing evidence-based information and including patient values and preferences, thereby decreasing surrogates’ decision-regret and possibly even rates of post-traumatic stress disorder[8,1,9,2].
Recently, two large professional medical societies highlighted the need for SDM in the ICU to facilitate information exchange, deliberation, and effective decision-making[2], starting with the creation of decision aids (DA)[10,11]. These are disease- and decision-specific SDM tools, created and rated using published guidelines[12]. Currently, no DA exists for goals-of-care decisions in ciTBI patients, or generally in the neuroICU.
The first guideline-recommended step and the objective of this study was to explore key communication preferences and practices by stakeholders (surrogates and physicians) for the outcome prognostication during goals-of-care discussions for ciTBI patients.
METHODS
Between 10/2015 and 8/2016, we conducted a qualitative study of surrogates of ciTBI patients and physicians specialized in ciTBI. The University of Massachusetts Medical School (UMMS) Institutional Review Board approved the study with written consent for in-person interviews, or verbal consent with an approved script for telephone interviews.
Participant recruitment
Using purposive sampling, surrogates were recruited from UMMS (Worcester, MA) and the University of Pittsburg Medical Center ([UPMC], Pittsburg, PA). Both are level-1 trauma centers with independently ongoing prospective observational ciTBI research studies, from which surrogates were recruited (study follow-up period: 12 months). Surrogate study inclusion criteria included: age ≥18 years; English-speaking with willingness to undergo an audio-recorded interview; primary decision-maker for a currently or previously hospitalized ciTBI patient; goals-of-care decision within the past two years. Although participation in either a focus group or an individual interview was offered, all surrogates opted for individual interviews. We attempted to balance surrogate recruitment equally between those who chose “comfort care” versus “continuation of medical care”. However, six surrogates who had made the “comfort care” decision for their loved one declined participation; three stated that they felt “not ready”, “overwhelmed”, “guilty” or fearful that the interview might be “too painful [of an] experience” (Figure 1).
Figure 1. Flow chart of participant enrollment.
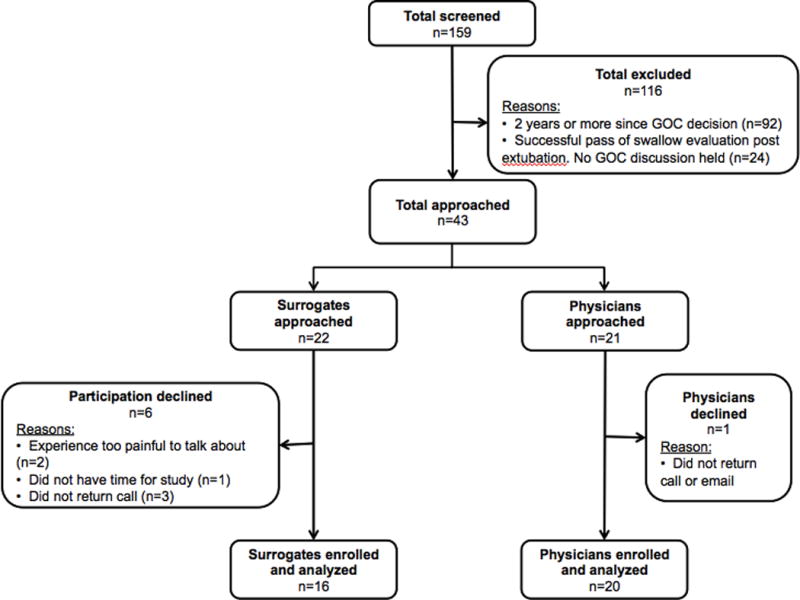
After initial screening, 116 did not meet inclusion criteria and were excluded. A total of 36 participants were enrolled and analyzed (16 surrogates and 20 physicians). Six surrogates and a single physician declined participation.
Physicians were recruited using both purposive and snowball sampling from seven centers representing five geographic regions (Northeast, Mid-Atlantic, South, West, Midwest) and different subspecialities (neurocritical care, neurosurgery, trauma, palliative care). Physician study inclusion criteria were: English-speaking with willingness to undergo an audio-recorded interview; experienced in caring for ciTBI patients; holding goals-of-care discussions with ciTBI surrogates; attending physician ≥2 years after fellowship training. One physician declined participation (Figure 1).
Measurements and Interviews
Baseline participant demographics were obtained using a written survey. Surrogate health literacy and numeracy were measured using the validated Rapid Assessment in Adult Literacy Short Form[13] and the Subjective Numeracy Scale[14]. Two separate semi-structured interview guides with broad, open-ended questions were developed for surrogates and physicians, aimed at eliciting responses regarding the process and preferences of prognosis derivation, as well as the communication of prognosis and uncertainty during ciTBI patient goals-of-care discussions. The interview guides were iteratively refined by input from content and patient-physician communication experts, and tested during two mock interviews. One trained physician interviewer (S.M.) conducted all interviews in person or via telephone. Interviews were digitally audio-recorded and transcribed verbatim by a professional transcription service. Participants received a $25 gift card for their participation.
Coding and analysis
We developed separate coding schemes for surrogates and physicians using parallel deductive and inductive methods. Each initial coding scheme was developed based on the interview guide content by a neurointensivist (S.M.) and an experienced qualitative researcher (K.M.). Two coders (T.Q.;J.M.) cooperatively coded five surrogate and five physician transcripts while making inductive changes to the initial coding schemes when necessary, with review of changes by S.M./K.M. Conceptually similar themes were combined and emerging themes added; conflicts were resolved using a third reviewer. Using these revised coding schemes, coders then independently coded the same three additional transcripts from each cohort, resulting in >80% congruency. All previous and subsequent transcripts were then coded separately using the revised coding schemes, with no further inductive changes required, indicating theme saturation (no new themes emerged; Supplemental Digital Content–Table 1 for detailed qualitative methods). All coding was reviewed by three authors ensuring correct classification. Recruitment was continued to diversify samples and to confirm that indeed no novel information would be obtained through the analysis of several additional transcripts. Theme saturation and feasibility resulted in a final sample size of 16 surrogates and 20 physicians. Qualitative analysis was performed using NVIVO© (QSR International Pty Ltd. [Melbourne, AUS]). Baseline characteristics of the study groups were summarized using descriptive statistics.
RESULTS
Baseline characteristics
The average interview duration was 41 minutes (range 25–61 minutes) for surrogates, and 37 minutes (range 19–65 minutes) for physicians. The baseline characteristics are shown in Table 1. Only 3/16 (19%) of surrogates had initially made the “comfort care” decision for their loved one. However, two surrogates who initially chose “continuation of full medical care” opted for “comfort care” later, at 4 and 12 months after trauma, respectively. This resulted in 31% of surrogates having chosen “comfort care” for their loved one before the interview. Median duration of attending physician practice was 11 years, with half practicing neurocritical care and the others neurosurgery, trauma and palliative care. The geographical distribution of participants is shown in Figure 2.
Table 1.
Baseline characteristics
| Baseline characteristics | Surrogates (n=16) |
Physicians (n=20) |
|---|---|---|
| Age, years (mean ± SD) | 57 ± 12 | 47 ± 8 |
| Female n (%) | 9 (56) | 7 (35) |
| Race n (%) | ||
| Caucasian | 13 (82) | 13 (65) |
| African-American | 2 (12) | 1 (5) |
| Asian | 1 (6) | 6 (30) |
| Ethnicity n (%) | ||
| Non-Latino | 16 (100) | 19 (95) |
| Latino | 0 | 1 (5) |
|
Subjective numeracy scale median (range 1–6) |
4.4 (3.4–6) | |
|
REALM-SF score median (range 0–7) |
7 (6–7) | |
| Highest level of education n (%) | ||
| Graduate/Doctorate degree | 0 | |
| College graduate | 7 (44) | |
| Some college | 4 (25) | |
| 2 years college/Technical school | 4 (25) | |
| High school/GED | 1 (6) | |
| Days from loved one’s TBI to interview [median (range)] | 319 (42–839) | |
|
Surrogate’s GOC decision “Comfort Care” at time of |
5 (31) | |
| Years in practice [median (range)] interview | ||
| Specialty n (%) | 11 (2–40) | |
| Neurocritical Care | 10 (50) | |
| Neurosurgery | 7 (35) | |
| Trauma surgery | 2 (10) | |
| Palliative Care | 1 (5) |
Shown are baseline characteristics for both surrogates and physicians. Abbreviations: SD, standard deviation; REALM-SF, Rapid Estimate of Adult Literacy in Medicine-Short Form; GED, General Educational Development; GOC, goals-of-care.
Figure 2. Geographic location of participants.
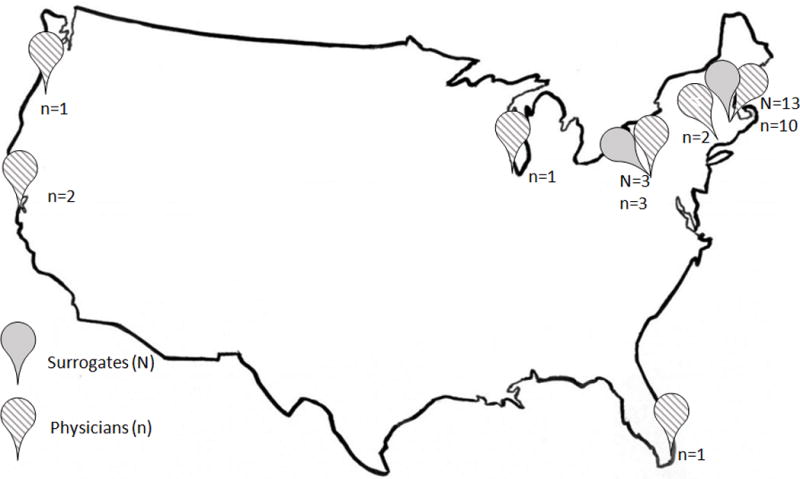
Solid markers indicate surrogates, while striped markers indicate physicians. Surrogates were recruited from two level-one trauma centers (University of Massachusetts Medical School [UMMS] and University of Pittsburgh Medical Center [UPMC]). Physicians were recruited from UMMS, Worcester, MA; UPMC, Pittsburgh, PA; Yale University Medical Center, New Haven, CT; Northwestern University, Chicago, IL; University of Miami Jackson Memorial Hospital, Miami, FL; University of California at San Francisco, CA; Oregon Health Sciences University, Portland, OR.
Major themes
We identified several major themes from the surrogates and physicians regarding communication content, process and style (Figure 3; Supplemental Digital Content–Tables 2 and 3). Some themes represent fundamental contrasts in the communication preferences for prognosis and uncertainty. We found one commonality: avoidance of false hope. Below we describe each theme with a representative quote, including views from surrogates and physicians, thereby highlighting the differences and commonalities between both groups. Additional quotes are shown in Supplemental Digital Content–Tables 2 and 3.
Figure 3. Major themes by surrogates and physicians.
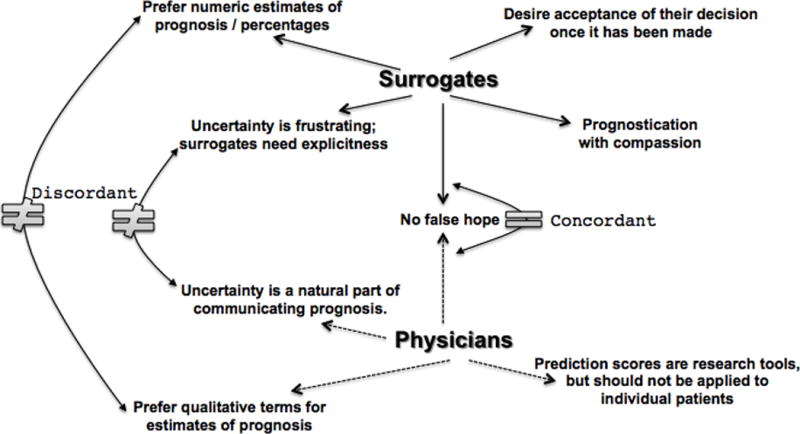
Shown are the major themes identified in the surrogate decision-maker (“surrogates”) interviews (top, solid arrows) and the physician interviews (bottom, dotted arrows). Curved arrows and symbols indicate discordant themes (indicated by “≠”) and concordant themes (indicated by “=”). There was only one concordant theme expressed by both surrogates and physicians: “No false hope”.
Numeric estimates of prognosis
The majority of surrogates (82%) preferred receiving exact numeric estimates from physicians when discussing their loved one’s prognosis. As one surrogate said:
“It’s more clear, it’s more concise, it less confusing; there’s one statement made.”
Only 18% were satisfied with qualitative predictions such as “highly likely” or “unlikely”.
The majority (75%) of physicians, however, stated that they typically omit numeric predictions for a variety of reasons: physicians’ distrust in the accuracy of existing TBI outcome models and the quality of existing research data from which these models were derived, referring to the heterogeneity of TBI with its multiple injury mechanisms. Furthermore, physicians felt that the TBI outcome models were designed to inform research, and were not suitable for application to individual patients:
“I am always very scared when I hear of colleagues trying to use the IMPACT calculator or the CRASH calculator. These things were never designed for clinical decision-making, they were designed to inform clinical trial design.”
An additional prominent reason leading physicians to refrain from providing percentages to families was the concern that families might misinterpret numeric estimates due to stress and over-simplification, thereby creating false hope:
“Even if you give them the 80% chance of mortality, if they’re optimistic, they will latch on to the 20%.”
Finally, one physician feared that numeric estimates, when provided to families with low numeracy, may be “used against” the physicians, without adding further meaning to this term:
“Family members, unless they are very mathematically sophisticated, really don’t interpret these numbers the way that physicians and scientist interpret these numbers. They become simplified and used against you later.”
Uncertainty is frustrating for surrogates, yet for physicians a natural part of communicating prognosis
For most surrogates, the uncertainty surrounding their loved one’s prognosis was frustrating. Some surrogates (19%) believed uncertainty to be an expected or even necessary part of the decision-making process, although they still acknowledged difficulty in dealing with uncertainty. The majority (57%) felt unprepared for the prognostic uncertainty, and continued to struggle with it for many months after making their decision:
“Will she come home? Will she not come home? Will she get better? Will she not? […]Uncertainty makes it the hardest part. And even since we left here, there’s been a lot of moving forward and moving backwards.”
Uncertainty was even more difficult for surrogates when numeric prognostic estimates were not provided. Several surrogates expressed frustration and even distrust with physicians who “refused” to give them numeric estimates:
“I talked to a fourth neurologist and they refused to give me any kind of [prediction] – and it turns out, he just didn’t want to make the decision for me. I’m like, ‘Fine, but give me a percentage.’”
In contrast, physicians had differing approaches to communicating prognostic uncertainty, while understanding the burden it placed on families. One approach adopted by many physicians was limiting the degree of uncertainty as much as possible, albeit without giving specific probabilities or numeric estimates. Intentional vagueness was considered to be unfair to families because, as one physician stated, “it becomes impossible to draw any conclusions from what you’re saying.” Many physicians stressed the importance of recognizing and acknowledging the uncertainty inherent in prognostication, and communicating it to the family as clearly and directly as possible:
“I just own it. I just say I’m not sure[…]Usually I’ll have a hunch, that it is going to go one way or the other, but I readily and openly cop to not being sure and not knowing.”
Physicians felt that being forthcoming about prognostic uncertainty was more calming to families, and that most families were very accepting of the uncertainty once it was stated.
No false hope
For surrogates, the physician’s role in providing hope to families was an important communication aspect. Although it was important to maintain a sense of hope for their loved one, surrogates did not want the physician to provide false hope, preferring instead to make an informed decision based on facts alone:
“I would go elsewhere for hope but what I needed from them were the facts. I was not looking to them for hope but I needed to know the truth.”
Physicians were similarly wary of providing false hope to families. In situations with exceedingly slim changes of favorable recovery, physicians were particularly careful to tailor their discussions in order to prevent families from “holding on” for a very unlikely outcome. Physicians also felt that providing hope early in the hospital course made it much harder for both families to come to terms with an unfavorable outcome.
“I think it’s again important not to be equivocal. There is nothing gained by holding onto false hope. All it does is traumatize everybody more […]. You don’t leave the door even slightly ajar.”
Acceptance of the surrogates’ final decisions
Some surrogates reported that once they had arrived at a goals-of-care decision, members of the clinical team made attempts to convince them that their decision had been hasty or wrong, causing emotional distress:
“But one particular nurse made it clear that his views were not the same as what the doctor had told us and pretty blatantly let each and every one of us know, separately, that we were making a hasty decision. That caused us a lot of additional stress, anxiety, second-guessing. It was one whole night of torture.”
Prognostication with compassion
One strong request made by the majority of surrogates was for physicians to communicate with compassion. Goals-of-care discussions were particularly difficult because the surrogates, and sometimes the physician leading the family meeting, were often unprepared, untrained, and inexperienced. Acknowledging the family’s distress was important to surrogates:
“It was an unfortunate situation, and maybe those doctors should[…]receive some kind of training, but there’s ought to be a protocol for that kind of discussion. […]Not blurt it out. I didn’t take my coat off yet when she said it. It was very difficult.”
DISCUSSION
In this qualitative study we found differences and areas of agreement between the ciTBI surrogates’ communication needs and preferences and physicians’ communication practices. These findings inform the content of a future DA for goals-of-care discussions in ciTBI patients. In addition, these findings may have important implications for improving communication practices in the neuroICU independent of whether a formal DA is used.
Successful implementation of a DA can only occur if its content and presentation of the information is acceptable to and addresses the concerns of all stakeholders (surrogates and physicians)[10,15,16,11,17,18]. The ultimate goal of a ciTBI-specific DA for goals-of-care decisions includes improved knowledge about the disease and its prognosis, goals-of-care-related treatment options (tracheostomy, feeding tube, post-hospital care) with risks and potential outcomes, expressing prognostic uncertainty in a way that surrogates understand, and routinely including patient values and preferences. In DAs concerning other diseases, meeting these goals sets more realistic expectations, helps match patient values to treatments, and reduces decisional conflict and decision passivity among patients or their surrogates[19,8,17,18]. Confirmation of these findings in ciTBI-specific DA is pending.
We found considerable discordance between surrogates and physicians regarding preferences for communicating numeric estimates and the uncertainty of prognosis. These concepts appear closely intertwined; the majority of surrogates requested numeric estimates to reduce uncertainty surrounding the prognosis. While surrogates acknowledged that some degree of uncertainty still remains with numeric estimates, they felt that a percentage would provide more clarity about the prognosis, and would allow them to weigh the risks and benefits of a given choice. The findings in our study reinforce the importance for physicians to admit and explicitly express the inherent prognostic uncertainty for ciTBI patients.
The physician-preferred use of qualitative terms during prognostication has previously been reported in a study of general ICU patients which analyzed 51 audiotaped physician-family conferences about life-support decisions[20]. Only 20% of physicians used quantitative terms in the family conferences, and even fewer (14%) asked whether the family understood the prognostic information. Another study revealed that very few surrogates reported that their beliefs about the patients’ prognoses hinged exclusively on prognostic information provided by physicians [21]. Surrogates also considered patient attributes, including strength of character, life history, appearance and faith when estimating their loved ones’ prognoses [21]. A randomized trial of video-simulated family meetings with 169 surrogates of medical ICU patients, with prognosis conveyed numerically vs. qualitatively, revealed that neither surrogates’ personal estimates nor their understanding of the physician’s prognostication differed between the interventions [22]. This suggests that numeric estimates alone are unlikely to improve a surrogate’s understanding of prognosis, albeit validation of these findings in neuroICU patients is pending.
The International Patient Decision Aids Standards, a framework for high-quality DA’s, recommends the presentation of probabilities in DA’s [12]. The physicians’ preference to omit numeric estimates as documented in our study is discordant to these quality criteria. However, this preference is in line with the caution raised in the literature against the use of prognostic scores in neurological emergencies to scale treatment and provide outcome prognostication [23,24]. Presenting numeric estimates in a future DA for ciTBI could pose a potential implementation barrier.
Our study has several limitations. As is typical in qualitative research, this study was designed to uncover some of the key issues in this area, rather than to produce generalizable results through large numbers of participants and statistical analysis. Future mixed-methods studies are required to describe the prevalence of the views and practices described here, and to elucidate any correlation between factors such as physician specialty, experience-level or injury severity with communication preferences. Despite our efforts in recruiting a balanced number of surrogates making the “comfort care” or “continuation-of-care” decision, a disproportionately small number of our surrogate participants had chosen the former. Reasons for declining participation included that painful memories were too difficult to discuss during the study. Therefore, views and experiences inherent to the withdrawal-of-care decision may have been underrepresented. Another potential limitation of our study was geographical. While we recruited physicians from diverse backgrounds from across the U.S., our surrogate cohort was recruited from two large Level-1 trauma centers in the East of the U.S. with a largely Caucasian patient population. Accordingly, it is possible that surrogate experiences and opinions may differ from those of different racial, ethnic, social and geographical origin and heritage. Additionally, the education and health-literacy level of the surrogate participants was relatively high and may be misrepresenting the surrogate population of many other trauma centers. Within the limitations of the two trauma centers, however, we did our best to recruit surrogates from racial-ethnic minority groups. Given the similarities in the surrogate experiences from both sites, we feel that our results highlight key communication aspects that are unlikely due to specific practices at one center. Finally, we only included surrogates in whom a discussion about tracheostomy had occurred, without exploring communication around prognosis in patients in whom these procedures were not offered.
CONCLUSION
We identified several areas which suggest fundamental differences in the communication preferences of prognostic information between surrogates of ciTBI patients and physicians during goals-of-care discussions. These findings, if validated, may inform the content of a future DA for goals-of-care discussions in ciTBI patients. In addition, these findings may also have important implications for improving communication practices in the neuroICU, independent of the implementation of a formal decision aid.
Supplementary Material
Acknowledgments
Funding:
Funded by NIH/NICHD grant 5K23HD080971 (PI Muehlschlegel). This project was additionally supported by the University of Massachusetts Medical School Center for Clinical and Translational Science which is funded by the NIH Clinical and Translational Science Award to the University of Massachusetts Medical School (UL1TR000161).
Footnotes
Conflicts of Interest:
The authors declare that they have no conflict of interest.
References
- 1.Muehlschlegel S, Shutter L, Col N, Goldberg R. Decision Aids and Shared Decision-Making in Neurocritical Care: An Unmet Need in Our NeuroICUs. Neurocrit Care. 2015;23(1):127–130. doi: 10.1007/s12028-014-0097-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kon AA, Davidson JE, Morrison W, Danis M, White DB, American College of Critical Care M, American Thoracic S Shared Decision Making in ICUs: An American College of Critical Care Medicine and American Thoracic Society Policy Statement. Crit Care Med. 2016;44(1):188–201. doi: 10.1097/CCM.0000000000001396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turgeon AF, Lauzier F, Burns KE, Meade MO, Scales DC, Zarychanski R, Moore L, Zygun DA, McIntyre LA, Kanji S, Hebert PC, Murat V, Pagliarello G, Fergusson DA, Canadian Critical Care Trials G Determination of neurologic prognosis and clinical decision making in adult patients with severe traumatic brain injury: a survey of Canadian intensivists, neurosurgeons, and neurologists. Critical care medicine. 2013;41(4):1086–1093. doi: 10.1097/CCM.0b013e318275d046. [DOI] [PubMed] [Google Scholar]
- 4.Turgeon AF, Lauzier F, Simard JF, Scales DC, Burns KE, Moore L, Zygun DA, Bernard F, Meade MO, Dung TC, Ratnapalan M, Todd S, Harlock J, Fergusson DA. Mortality associated with withdrawal of life-sustaining therapy for patients with severe traumatic brain injury: a Canadian multicentre cohort study. CMAJ. 2011;183(14):1581–1588. doi: 10.1503/cmaj.101786. doi: cmaj.101786 [pii] 10.1503/cmaj.101786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Izzy S, Compton R, Carandang R, Hall W, Muehlschlegel S. Self-fulfilling prophecies through withdrawal of care: do they exist in traumatic brain injury, too? Neurocritical care. 2013;19(3):347–363. doi: 10.1007/s12028-013-9925-z. [DOI] [PubMed] [Google Scholar]
- 6.Anderson WG, Arnold RM, Angus DC, Bryce CL. Posttraumatic stress and complicated grief in family members of patients in the intensive care unit. J Gen Intern Med. 2008;23(11):1871–1876. doi: 10.1007/s11606-008-0770-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McAdam JL, Fontaine DK, White DB, Dracup KA, Puntillo KA. Psychological symptoms of family members of high-risk intensive care unit patients. Am J Crit Care. 2012;21(6):386–393. doi: 10.4037/ajcc2012582. quiz 394. [DOI] [PubMed] [Google Scholar]
- 8.Col N. Communicating Risks and Benefits: An Evidence-Based User’s Guide (FDA) Food and Drug Administration (FDA), US Department of Health and Human Services; Silver Spring, MD: 2011. Chapter 17. Shared Decision Making. [Google Scholar]
- 9.Cox CE, Lewis CL, Hanson LC, Hough CL, Kahn JM, White DB, Song MK, Tulsky JA, Carson SS. Development and pilot testing of a decision aid for surrogates of patients with prolonged mechanical ventilation. Crit Care Med. 2012;40(8):2327–2334. doi: 10.1097/CCM.0b013e3182536a63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.OHRI Ottawa Hospital Research Institute. Decision Aid Development Toolkit. https://decisionaid.ohri.ca/resources.html. Accessed 11/28/2016.
- 11.Ottawa Hospital Research Institute. Decision Aid Implementation Toolkit. 2014 https://decisionaid.ohri.ca/implement.html. Accessed 1/4/2017.
- 12.Elwyn G, O’Connor A, Stacey D, Volk R, Edwards A, Coulter A, Thomson R, Barratt A, Barry M, Bernstein S, Butow P, Clarke A, Entwistle V, Feldman-Stewart D, Holmes-Rovner M, Llewellyn-Thomas H, Moumjid N, Mulley A, Ruland C, Sepucha K, Sykes A, Whelan T, International Patient Decision Aids Standards C Developing a quality criteria framework for patient decision aids: online international Delphi consensus process. BMJ. 2006;333(7565):417. doi: 10.1136/bmj.38926.629329.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arozullah AM, Yarnold PR, Bennett CL, Soltysik RC, Wolf MS, Ferreira RM, Lee SY, Costello S, Shakir A, Denwood C, Bryant FB, Davis T. Development and validation of a short-form, rapid estimate of adult literacy in medicine. Med Care. 2007;45(11):1026–1033. doi: 10.1097/MLR.0b013e3180616c1b. 00005650-200711000-00004 [pii] [DOI] [PubMed] [Google Scholar]
- 14.Fagerlin A, Zikmund-Fisher BJ, Ubel PA, Jankovic A, Derry HA, Smith DM. Measuring numeracy without a math test: development of the Subjective Numeracy Scale. Med Decis Making. 2007;27(5):672–680. doi: 10.1177/0272989X07304449. doi: 0272989X07304449 [pii] 10.1177/0272989X07304449. [DOI] [PubMed] [Google Scholar]
- 15.O’Connor AM, Wennberg JE, Legare F, Llewellyn-Thomas HA, Moulton BW, Sepucha KR, Sodano AG, King JS. Toward the ‘tipping point’: decision aids and informed patient choice. Health Aff (Millwood) 2007;26(3):716–725. doi: 10.1377/hlthaff.26.3.716. [DOI] [PubMed] [Google Scholar]
- 16.Gerteis M, Edgman-Levitan S, Daley J, Delbanco TL. Through the Patient’s Eyes: Understanding and Promoting Patient-Centered Care. (1st) 1993 [Google Scholar]
- 17.Legare F, O’Connor AC, Graham I, Saucier D, Cote L, Cauchon M, Pare L. Supporting patients facing difficult health care decisions: use of the Ottawa Decision Support Framework. Can Fam Physician. 2006;52:476–477. [PMC free article] [PubMed] [Google Scholar]
- 18.Legare F, O’Connor AM, Graham ID, Wells GA, Tremblay S. Impact of the Ottawa Decision Support Framework on the agreement and the difference between patients’ and physicians’ decisional conflict. Med Decis Making. 2006;26(4):373–390. doi: 10.1177/0272989X06290492. [DOI] [PubMed] [Google Scholar]
- 19.Stacey D, Legare F, Col NF, Bennett CL, Barry MJ, Eden KB, Holmes-Rovner M, Llewellyn-Thomas H, Lyddiatt A, Thomson R, Trevena L, Wu JH. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2014;(1):CD001431. doi: 10.1002/14651858.CD001431.pub4. [DOI] [PubMed] [Google Scholar]
- 20.White DB, Engelberg RA, Wenrich MD, Lo B, Curtis JR. The language of prognostication in intensive care units. Med Decis Making. 2010;30(1):76–83. doi: 10.1177/0272989X08317012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boyd EA, Lo B, Evans LR, Malvar G, Apatira L, Luce JM, White DB. It’s not just what the doctor tells me:” factors that influence surrogate decision-makers’ perceptions of prognosis. Crit Care Med. 2010;38(5):1270–1275. doi: 10.1097/CCM.0b013e3181d8a217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee Char SJ, Evans LR, Malvar GL, White DB. A randomized trial of two methods to disclose prognosis to surrogate decision makers in intensive care units. Am J Respir Crit Care Med. 2010;182(7):905–909. doi: 10.1164/rccm.201002-0262OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hemphill JC, 3rd, White DB. Clinical nihilism in neuroemergencies. Emerg Med Clin North Am. 2009;27(1):27–37. doi: 10.1016/j.emc.2008.08.009. doi: S0733-8627(08)00092-8 [pii] 10.1016/j.emc.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Skrobik Y, Kavanagh BP. Scoring systems for the critically ill: use, misuse and abuse. Can J Anaesth. 2006;53(5):432–436. doi: 10.1007/BF03022613. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


