Abstract
In the translation of discoveries from the laboratory to the clinic, the track record in developing disease-modifying therapies in neurodegenerative disease is poor. A carefully designed development pipeline built from discoveries in both pre-clinical models and patient populations is necessary to optimize the chances for success. Genetic variation in the leucine-rich repeat kinase two gene (LRRK2) is linked to Parkinson disease (PD) susceptibility. Pathogenic mutations, particularly those in the LRRK2 GTPase (Roc) and COR domains, increase LRRK2 kinase activities in cells and tissues. In some PD models, small molecule LRRK2 kinase inhibitors that block these activities also provide neuroprotection. Herein, the genetic and biochemical evidence that supports the involvement of LRRK2 kinase activity in PD susceptibility is reviewed. Issues related to the definition of a therapeutic window for LRRK2 inhibition and the safety of chronic dosing are discussed. Finally, recommendations are given for a biomarker-guided initial entry of LRRK2 kinase inhibitors in PD patients. Four key areas must be considered for achieving neuroprotection with LRRK2 kinase inhibitors in PD: 1) identification of patient populations most likely to benefit from LRRK2 kinase inhibitors , 2) prioritization of superior LRRK2 small molecule inhibitors based on open disclosures of drug performance, 3) incorporation of biomarkers and empirical measures of LRRK2 kinase inhibition in clinical trials, and 4) utilization of appropriate efficacy measures guided in part by rigorous pre-clinical modeling. Meticulous and rational development decisions can potentially prevent incredibly costly errors and provide the best chances for LRRK2 inhibitors to slow the progression of PD.
Keywords: PARK8, dardarin, ras-of-complex, neurodegeneration, movement disorders, small molecule kinase inhibitors
Graphical Abstract

1. Introduction
In the search for disease-modifying strategies in Parkinson disease (PD) that might slow or halt progression, there are no shortages of plausible targets or theoretical approaches. In the pursuit of a compelling development pipeline for a viable neuroprotection approach, first, the strength of the link between the target and disease pathogenesis and progression must be critically evaluated. Clinical failures are often attributed to the over-estimation of the role of a target in a broad disease population. Second, the likelihood of achieving and measuring the desired manipulation of the target in clinical populations should be estimated. Regardless of the importance of a target in disease, many neuroprotection trials in PD have ended without any definable endpoint. This is typically due to the lack of pharmacodynamic information and confirmation of on-target effects in clinical studies. Finally, there should be a reasonable understanding of the desired effect of the manipulation (e.g., efficacy), firmly defined by reproducible results in both pre-clinical and clinical studies. In the past few decades, the cost of pushing forward in misguided ways in neurodegenerative disease has been huge, with the consumption of very limited resources that could have been used in other fruitful ways.
Herein, the evidence that links the leucine-rich repeat kinase 2 (LRRK2) gene and LRRK2 kinase activity to PD is summarized, as well as initial data from studies involving LRRK2 kinase inhibitors. Several excellent and recent literature reviews provide an overview of the discovery of LRRK2 in PD and biological functions of LRRK2 protein, and these topics are not discussed (Cookson, 2015; Martin et al., 2014; Wallings et al., 2015). Rather, recommendations are provided for critical issues that surround the successful translation of LRRK2 kinase inhibitors, with a focus on biomarker-driven approaches and the selection of appropriate patient populations for clinical studies. Issues related to the safety of LRRK2 kinase inhibitors and the type of clinical benefit that might be expected are also discussed, together with critical knowledge gaps that will need to be filled. In summary, the development of a robust development pipeline seems possible and is needed to convincingly test the hypothesis that LRRK2 kinase inhibitors provide neuroprotection in PD.
2. Genetics of LRRK2-linked PD
The importance of a target in disease pathogenesis and progression is often surmised through human genetics studies, changes to the target in post-mortem tissue, and action in model systems. Although PD is not a heritable condition in most people, there is a significant genetic component and LRRK2 is one of the major genes that underlies this type of risk(Lill et al., 2012; Trinh et al., 2014). With respect to PD susceptibility, genetic variants in LRRK2 can be assigned to three categories. First, mutations that are considered pathogenic (i.e., causative) have large effects on PD risk, for example, lifetime penetrance for PD of 20% or higher. For these large-effect mutations, segregation of patients with the mutations in multiple families proves the mutation is the causative factor. By far the most frequent mutation is the G2019S variant and is among the most prevalent known genetic causes of neurodegeneration(Trinh et al., 2014). Considerable effort has gone into understanding the functional effects of all the pathogenic mutations in LRRK2 as will be discussed.
The second category of LRRK2 variants includes those associated with low-effect on PD risk, where the contribution is an order of magnitude or lower than pathogenic mutations. These variants include those identified in genome-wide association studies. It is difficult to determine whether these genetic variants are functional with respect to disease risk. They may act alone, or they may require synergy with other variants for effects, or they may be non-functional and in disequilibrium with other functional variants. Due to this relative increase in complexity compared to pathogenic mutations, relatively few studies have pursued these variants. The third category of LRRK2 genetic variants in PD includes those in PD cases but with no effect on PD susceptibility. This category includes the clear majority of variants in LRRK2 and involves tens of thousands of common and (mostly) rare coding and non-coding variants. At present, it appears that loss-of-function (LoF) variants (e.g., nonsense polymorphisms that block protein expression) can be included in this third category. In the ExAC Browser Beta database composed of 60,706 unrelated individuals, LoF LRRK2 variants are associated with a ‘constraint metric’ score of null that indicates complete tolerance of loss of function mutations. Presently there is no clear consensus on how any of the second or third category variants may influence LRRK2 kinase activity in cells and tissues.
3. Genetic and biochemical support of a gain-of-function increase in LRRK2 kinase activity in PD susceptibility
As LRRK2 is linked to PD susceptibility through genetics, understanding the functional impact of genetic variants that underlie PD risk will help identify the specific activities that should be prioritized for the development of new therapeutics. LRRK2 is part of an old family of proteins, known as the Ras-of-complex (Roc) family, with homologs in single-celled organisms that share as much as 30% amino-acid homology with LRRK2 in conserved domains like Roc and the COR domain (C-terminal of Roc)(Bosgraaf and Van Haastert, 2003). LRRK2 contains several other domains found in hundreds of other proteins in humans, including the leucine-rich repeat (LRR), ankyrin repeat-like structures in the N-terminal domain, a protein kinase domain, and a WD40-like domain (Figure 1). These domains do not exist in a linear configuration but interact with one another in a complex regulatory cycle(Guaitoli et al., 2016; Liu et al., 2016). Not every Roc family protein contains a kinase domain, indicating that the kinase domain may be dispensable for some conserved functions, whereas the ~350 amino acid COR domain defines the family (Bosgraaf and Van Haastert, 2003). The Roc family (i.e., COR domain containing proteins) can be found in prokaryotes, amoeba, and plants, but no other kinases in humans apart from LRRK1 and LRRK2 contain a COR domain(Bosgraaf and Van Haastert, 2003). The first pathogenic mutation identified in LRRK2, Y1669C, localizes to the COR domain and implicated it for the first time in human disease(Zimprich et al., 2004). Biochemical evidence suggests the COR domain may be important for mediating an active-state dimeric configuration(Rudi et al., 2015). In early genetic studies, the pathogenic mutation R1441C in the Roc GTPase domain was also found, thereby solidifying the Roc-COR connection with PD(Paisan-Ruiz et al., 2004). From a drug development perspective, Ras-family GTPases with similar sequence to LRRK2 Roc have long been implicated as critical proteins in disease, but have been incredibly difficult to pharmacologically modulate in a selective way for therapeutic gain(Scott et al., 2016). The COR domain is a black box with no identified small-molecule binding sites that might render itself to therapeutic modulation. Thus, at the outset, LRRK2 protein was not a great drug target for PD neuroprotection.
Figure 1.
Interpretive model of the kinase-active LRRK2 dimer. The kinase domain harbors the most common LRRK2 pathogenic mutation, G2019S, adjacent to the Roc GTPase domain that harbors the R1441 mutations. The COR domain may be critical for dimer formation and active-state conformations and harbors the Y1699C mutation. The WD40 domain may be responsible for membrane association, and the LRR domain may be important for interacting with other proteins and dimerization. The N-terminal domain does not contain known pathogenic mutations but has constitutive phosphorylation sites (e.g., Ser935) that control interaction with 14-3-3 proteins, Rab proteins, and subcellular localization. This model is interpreted from numerous biochemical and structure studies.
As the kinase domain is not essential for Roc family proteins, the next pair of LRRK2 mutations that localized to the kinase domain, G2019S and I2020T, came as a surprise(Funayama et al., 2005; Kachergus et al., 2005). The prevalence of the G2019S mutation in both sporadic and familial PD in early studies established this variant as a major cause of late-onset PD in some populations. In biochemical studies, the effects of the G2019S mutation have been clearer with respect to kinase-activation than the effects of other mutations(Greggio and Cookson, 2009). Depending on the experimental constraints (source of protein, substrate, co-factors, etc.), the effects of other pathogenic LRRK2 mutations were variable(West, 2015). A consensus awaited the identification of validated LRRK2 kinase substrates and selective LRRK2 kinase inhibitors to first find phosphorylation events dependent on LRRK2 kinase activity in cells and tissue, and then determine the effects of the pathogenic mutations on those substrates.
Presently there are two known LRRK2 kinase substrates. The first acts in cis with respect to LRRK2 kinase activity, pSer-1292 autophosphorylation(Sheng et al., 2012). The second substrate is in trans that includes phosphorylation of some Ras-family Rab GTPases(Steger et al., 2016). These two substrates meet the criteria as bone fide LRRK2 kinase substrates since they decrease in abundance in cells and tissues with LRRK2 kinase inhibition, are increased in cells and tissue in the presence of pathogenic LRRK2 mutations, and LRRK2 can phosphorylate these residues in vitro in kinase assays(West and Cookson, 2016).
Phosphorylated LRRK2 kinase substrates accumulate in the range of ~2 to ~15 fold in the presence of LRRK2 with a pathogenic mutation compared to wild-type LRRK2, in cells and tissues(Sheng et al., 2012; Steger et al., 2016). Ironically, the mutations in the kinase domain appear to have significantly lower effects on substrate phosphorylation than pathogenic mutations in the Roc-COR domains. From these results, it is tempting to conclude that in vitro kinase assays that fail to demonstrate the effects of the Roc-COR mutations lack the dynamic interplay of Roc-COR-mediated regulation of kinase activity that occurs in cells and tissues. The role the phosphorylation events may play in modifying substrate function is not clear but could help to uncover new aspects of PD-linked mechanisms not previously considered.
On a speculative basis, the level of increase in kinase activity afforded by a particular pathogenic variant may modify the penetrance (risk of PD) associated with the mutation (Figure 2). The G2019S mutation in the activation loop of the kinase domain produces a ~3–5 fold effect in some models and clinical samples(Fraser et al., 2016a), and the mutation is well-known to be incompletely penetrant in the Ashkenazi Jewish population (e.g., ~20–30% lifetime risk for PD)(Marder et al., 2015). The Y1699C variant and other ROC variants are nearly fully penetrant(Trinh et al., 2014), with only a few known examples of individuals greater than 65 years of age that do not have PD. These variants appear to have much larger effects on kinase activation in model systems. Ultimately, the best test of a kinase-activation hypothesis for LRRK2 in PD is through clinical studies with LRRK2 kinase inhibitors.
Figure 2.
Pathogenic mutations in LRRK2 are plotted with respect to kinase activation and penetrance. R1628P and G2385R are risk factors for PD in some Asian populations but their relationship to LRRK2 kinase activation is not yet clear.
While the non-coding LRRK2 genetic variants in GWAS studies are well-established risk factors for PD(Lill et al., 2012; Nalls et al., 2014), exome sequencing databases have annotated extremely rare but numerous variants in LRRK2 that collectively may be important in PD risk. In the ExAC Browser database that includes 60,706 unrelated individuals, 152 LRRK2 alleles are annotated with heterozygous loss of function (LoF) mutations (e.g., nonsense mutations). Nonsense mutations that reduce or ablate LRRK2 expression are not enriched in PD patients. Apart from LoF mutations, the frequency of missense mutations that change amino acid sequence in LRRK2 is unremarkable, with 728 observed mutations compared to 708.4 expected. Among these, the pathogenic G2019S mutation was detected in 47 individuals, implicating a population frequency of 0.08%. If this frequency holds true in Caucasian populations, the G2019S mutation would be one of the most frequent known genetic causes (e.g., high odds-ratio for PD susceptibility, e.g., >20–30) of neurodegenerative disease. The aggressive Roc and COR pathogenic mutations are virtually absent in the database, with just one instance of an R1441C mutation.
Within the kinase and Roc-COR domains, abundant rare missense mutations abound and these might be considered for PD risk. In the kinase domain, there are 111 subjects (out of 60,706) annotated with rare coding changes that alter conserved residues in the kinase domain. The Roc-COR domain stretch encompasses 166 rare variants (<0.1%) in 453 individuals. Eight variants are more common (frequencies >0.2%) in the domains but still could be functional. As the LRRK2 field has struggled to functionally characterize the ~seven known pathogenic mutations, and there is no consensus LRRK2 cDNA sequence from which to study these mutations, it seems impossible at present to understand whether any of these rare variants may be functional with respect to LRRK2 kinase activity. Compounding the issue is that different variants may not act alone but in synergy with one another. For example, the G2385R mutation in the WD40 domain may display different properties when in the context of LRRK2 variants found in PD patients with the G2385R mutation. While biochemical studies to explore all the possibilities seem unrealistic, a scalable biomarker assay with good sensitivity and specificity to measure the abundance of LRRK2 kinase substrates may identify particular mutations of larger effect. It is possible that additional genetic studies and large-scale exome analyses coupled with measurements of LRRK2 kinase substrates in biofluids may significantly broaden the number of PD cases with a clear genetic link to LRRK2.
4. Evidence for LRRK2 kinase inhibition in neuroprotection
Studies with LRRK2 kinase inhibitors in models of disease may give clues as to the type of benefit that might be expected in clinical populations. Until recently, LRRK2 small molecule inhibitors suitable for long-term administration have not been available. Most of what is known about LRRK2 inhibition comes from models where LRRK2 expression is reduced (in vitro, like RNAi) or ablated (knockout in vivo). While LRRK2 knockout rodents may not accurately model pharmacological LRRK2 kinase inhibition, there is a reasonable expectation to uncover liabilities associated with targeting LRRK2 as well as possible beneficial action in disease models. Table 1 summarizes studies that involve LRRK2 knockout rodents and LRRK2 kinase inhibitor studies. Protection from LRRK2 knockout and kinase inhibition has been described in models that rely on inflammation as a driving force in dysfunction, for example experimental autoimmune uveitis(Wandu et al., 2015), lipopolysaccharide exposure(Daher et al., 2014), HIV-1 Tat peptide exposure(Puccini et al., 2015), and rhabdomyolysis kidney injury(Boddu et al., 2015). There is some evidence to suggest that inflammation plays a critical role in the rAAV2-WT-hα-synuclein model(Harms et al., 2013; Qin et al., 2016; Van der Perren et al., 2015), and LRRK2 knockouts may be protected from this insult(Daher et al., 2014). However, it is not clear whether LRRK2 knockouts are protected from mutant A53T-hα-synuclein transgenic expression. So far, three different lines of A53T-hα-synuclein have been utilized: first reported, an aggressive fore-brain centric (CAMKII-promoter-driven) model where robust protection in LRRK2 knockouts was observed(Lin et al., 2009). Following that study, crosses of LRRK2 knockout to PrP A53T-hα-synuclein mice did not find protection associated with LRRK2 ablation (Daher et al., 2012). Likewise, LRRK2 knockouts are not protected from the mitochondrial complex I inhibitor MPP that is selectively internalized in DAT-1 expressing neurons(Andres-Mateos et al., 2009). Protection was also not recorded in mice treated with a LRRK2 kinase inhibitor in the MitoPark mice that have neurodegeneration caused by mitochondrial deficits in DAT-1 expressing neurons(Fell et al., 2015). Thus, from these studies it can be deduced that neuroprotection associated with LRRK2 inhibition may be due to reductions in neuroinflammation, potentially related to the damaging effects of wild-type α-synuclein over-expression. In turn, it seems less likely that blocking LRRK2 activity would remedy mitochondria deficits or the expression of toxic A53T α-synuclein. Notably, the A53T mutation in the human α-synuclein gene is localized to a few families world-wide and is associated with a very aggressive form of parkinsonism(Polymeropoulos et al., 1997), so blocking this type of neurotoxicity may not involve the exact same mechanisms as blocking wild-type α-synuclein induced neurodegeneration.
Table 1.
| Model (Publication) | Challenge | Conclusion |
|---|---|---|
| Mouse LRRK2 KO (Andres-Mateos et al., 2009) | MPTP | No effect on dopaminergic neurodegeneration |
| Mouse LRRK2 KO (Lin et al., 2009) | A53T alpha-synuclein transgenic, Tg(tetO-SNCA*A53T)Cai/J x | Reduced alpha-synuclein aggregation, neurodegeneration, and microglia activation |
| Mouse LRRK2 KO (Daher et al., 2012) | A53T alpha-synuclein transgenic, B6.Cg-Tg(Prnp- SNCA*A53T)Mkle/J | No effect on survival, microglial activation or spinal-cord phenotypes |
| Mouse LRRK2 KO (Liu et al., 2011) | Dextran-sulfate sodium | Increased sensitivity to experimental coilits |
| Mouse LRRK2 KO (Wandu et al., 2015) | Experimental Autoimmune Uveitis | Reduced macrophage and adaptive immune response |
| Mouse LRRK2 KO (Puccini et al., 2015) | Intracranial HIV-1 Tat intracranial injection | Reduced inflammation and neurodegeneration |
| Rat LRRK2 KO (Daher et al., 2014) | Intracranial lipopolysaccharide | Reduced dopaminergic neurodegeneration and inflammation |
| Rat LRRK2 KO (Daher et al., 2014) | Intracranial rAAV2/1-hWT-alpha-synuclein | Reduced dopaminergic neurodegeneration |
| Rat LRRK2 KO (Boddu et al., 2015) | Rhabdomyolysis-induced kidney injury | Resistant to acute kidney challenge, HO-1 upregulation |
| MLi-2 LRRK2 inhibition (Fell et al., 2015) | MitoPark (Dat-1/Cre KO of mitochondrial T-FAM) | No effect on dopaminergic neurodegeneration or motor impairment |
| PF-475 LRRK2 inhibition (Daher et al., 2015) | Intracranial rAAV2/1-hWT-alpha-synuclein | Reduced dopaminergic neurodegeneration |
LRRK2 kinase inhibitors that allow for long-term chronic inhibition are just becoming available for testing in animal models. For example, inhibitors MLi-2 and PF-06685360 (otherwise known as PFE360) have improved oral bioavailability and brain permeability and improved kinase selectivity(Baptista et al., 2015; Scott et al., 2017). These compounds have clear advantages over earlier compounds (see Table 2) such as L2-IN-1 derived from a series of ERK5 inhibitors and G02447915 (otherwise known as GNE7915) that were derived from a series of TTK kinase inhibitors(Deng et al., 2011; Fuji et al., 2015). Both TTK and ERK5 have critical functions in many kinds of cells and thus present major off-target liabilities in vivo. Critical off-targets and toxicities would severely impair in vivo experimentation even if the oral bioavailability and brain permeability were improved for second generation inhibitors.
Table 2.
| Generation of Compound (years in usage) |
Example Compound(s) |
Key advancement | Critical Liabilities |
|---|---|---|---|
|
| |||
| Generation “0” (Ca. 2007–2008) | Staurosporine | Demonstration of small molecule LRRK2 inhibition, in vitro utility | No significant selectivity against other kinases |
| Sunitinib | |||
|
| |||
| Generation “1” (Ca. 2009–2011) | L2-IN-1 | Improved selectivity | Poor PK/PD, little or no brain permeability, critical off-targets |
| GSK2578215A | |||
| TAE684 | |||
|
| |||
| Generation “2” (Ca. 2011–2013) | G02447915 | Oral availability and brain permeability | Poor half-life / critical off-targets |
| SRI-29132 | |||
| PF-06447475 | |||
|
| |||
| Generation “3” (Ca. 2014-now) | MLi-2 | Improved potency and optimized dosing strategies | To be determined |
| PF-06685360 | |||
In combining so-called third generation (Table 2) inhibitors with PD models, phenotypes implicated in LRRK2-linked neurodegeneration can be dissected. In late-onset PD, WT α-synuclein forms aggregates in vulnerable dopaminergic neurons in the substantia nigra with progressive and selective degeneration of these neurons. Recently described models in rats and mice that recapitulate these phenotypes may help identify the most efficacious small molecule inhibitors that could be prioritized for further development. However, there are serious concerns with how these types of studies may move forward. Industry has severely scaled back research efforts in neuroscience and neurodegeneration(Kelland, 2011). Further, it has been called into question whether reproducible neuroprotection studies can be successfully performed in the academic environment(Scott et al., 2008). There is hope that new guidelines and implementation of enhanced rigor in design and analysis may remedy some of the issues of past(Landis et al., 2012). Despite calls several years ago for change(Collins and Tabak, 2014), funding bodies still have a very long way to go to insist on earnest replication efforts in the face of an academic environment that typically fails to support such activities.
5. Safety of LRRK2 inhibition
The possibility of achieving a successful therapeutic outcome with LRRK2 kinase inhibitors will greatly diminish if early clinical studies identify insurmountable safety concerns. Complete removal of LRRK2 protein expression is associated with two prominent changes in mice that involve the accumulation of lipids and blood products in the kidney and the accumulation of lamellar bodies in type-II pneumocytes in the lung(Herzig et al., 2011). It is presumed that both phenotypes are forms of dysfunction associated with altered endolysosomal and degradation pathways. However, the phenotypes do not appear to affect either kidney or lung function in young or aged mice or rats. Rats have the same kidney phenotype, albeit more aggressive with changes (e.g., kidney-cortex darkening) detectable shortly after weaning(Boddu et al., 2015). Similar changes may take months to develop in mice(Tong et al., 2012). Also in contrast to mice, changes in the rat LRRK2 knockout lung are less obvious(Baptista et al., 2013). In an acute-kidney toxicity challenge to LRRK2 knockout rats, the darkened kidneys fared better than the controls, perhaps owing to a preconditioning effect and heme-oxygenase 1 upregulation(Boddu et al., 2015). Both lung and kidney changes in LRRK2 knockout rodents are difficult to quantify, in part because they do not result in measurable changes in lung or kidney function. The abnormalities involve a very subtle intracellular phenotype difficult to see, such as the doubling of LAMP-positive (possibly lysosomes) in some uncharacterized subset of cells. In the controlled laboratory environment, both LRRK2 knockout rats and mice live normal life-spans without evidence of toxicities or pathologies. Subtler and more variable phenotypes include mild hypertension, and this may be associated with increased weight, particularly in male rats(Baptista et al., 2013; Boddu et al., 2015; Herzig et al., 2011). None of these phenotypes can be observed in heterozygous knock-down rodents.
Chronic oral dosing of both rats and mice with third-generation LRRK2 kinase inhibitors has been described for the MLi-2 compound, as well as for the PFE475 compound(Daher et al., 2015; Fell et al., 2015). In these studies, dosing that achieved near-continuous brain LRRK2 inhibition (up to 90% inhibition for much of the day) did not produce clear phenotypes associated with the LRRK2 knockout in rodents, and only mild or non-significant effects on reducing total LRRK2 protein. Thus, studies in rodents have so-far been relatively unrevealing in highlighting any specific safety concerns.
In a study involving the second-generation GNE7915-related compounds (Table 2) in non-human primates (NHPs), Fuji et al. identified the lung-phenotype associated with the mouse LRRK2 knockout in NHPs treated with the highest amounts of LRRK2 inhibitors(Fuji et al., 2015). Since the lung phenotype is difficult to quantify, it is not clear whether the NHPs in that study had less or more of a lung phenotype than the mouse LRRK2 knockouts. However, there are several concerns related to a broad interpretation of that study. First, owing to the off-target liability associated with that series of compound, the effects of LRRK2 kinase inhibition cannot be separated from TTK inhibition. Second, the lung changes observed in the NHPs were inappropriately interpreted as pathologies. As there is no disease or deleterious phenotypic changes associated with the unquantified increase of lamellar bodies in the lung, the term pathology to describe the lung changes may not be accurate. Third, the compound series used in those studies shows toxicities in rats of an unspecified nature, precluding any exploration of the compounds in rat models of PD. In general, compounds that fail basic toxicity studies in rats and inhibit critical off-target kinases may not be strong candidates for advancement to safety studies in NHPs because of their limited utility in pre-clinical studies.
More recently there has been additional (third generation, Table 2) inhibitors that have advanced to safety testing in NHPs(Baptista et al., 2015). With the safer, more potent, and more selective MLi2 and PFE360 compounds, the focus has been to measure lung function in the presence of very high exposure levels (>IC95 levels in lung, more than ten-fold exposures above IC50). The rationale is that these high concentrations of more potent compounds may unmask safety liabilities and clarify the lung phenotype in dose escalations and drug washout. The histological analysis of lung could confirm the lamellar body accumulation in type II pneumocytes but only at very high compound concentrations and exposure levels. However, there was no deficit in measures of pulmonary function at the highest doses. Further, the histological lung changes associated with the high-compound exposures reverse and normalize upon cessation of administration of MLi2 or PFE360(Baptista et al., 2015). Kidney deficits or histological abnormalities have not been found in NHPs. While the field awaits the details and formal publications describing these results, no specific safety liabilities have been identified that might halt development at this stage.
PD is not an acute indication and it is difficult to predict whether safety profiles in short duration dosing that involves weeks or months of treatment will reveal issues related to long-term dosing in aged and sick individuals that will involve years and possibly decades of dosing. In consideration of the evidence presented here for LRRK2 function on a systems level, the most likely liability that may go unnoticed in safety studies may be related to dampening specific immunological responses to pro-inflammatory stimuli. This potential liability may be difficult to detect in healthy subjects under controlled conditions, but would be unmasked in larger trials and may cause clinical holds and black box warnings if the physiology underlying the effects are not described and anticipated in advance. Studies that integrate LRRK2 kinase inhibitors in models of autoimmune disease and infectious disease may be helpful to identifying specific liabilities. On the other hand, a better understanding of LRRK2 in the immune system may also lead to repurposing of LRRK2 inhibitors for other indications that involve therapeutic modification of specific immunological responses.
6. Biomarkers for LRRK2 inhibition and therapeutic windows for efficacy
The empirical determination of LRRK2 inhibition in clinical trials will be critical for determining whether LRRK2 small molecules may modify disease course. The development of biomarkers and scalable assays to measure LRRK2 inhibition reliably in patients will optimize chances for neuroprotection. There are several opportunities that have presented themselves in recent years. The discovery of bone fide LRRK2 kinase substrates (e.g, pSer1292-LRRK2 and pRab) that could theoretically be measured in biofluids and cells should ultimately provide a good surrogate measure of LRRK2 kinase activity. Scalable and reliable assays will need to be developed for deployment in safety and intervention studies.
Identification of LRRK2 kinase substrates has occurred relatively recently and there are few studies yet measuring these phosphorylated residues in cells and tissues. Paradoxically, most LRRK2 kinase inhibitor programs are mature in the integration of LRRK2 target engagement measures with respect to pharmacodynamics. This is explained by the discovery that the N-terminal cluster of constitutive phosphorylation sites on LRRK2 that includes pSer910 and pSer935 become dephosphorylated in the presence of a LRRK2 kinase inhibitor(Dzamko et al., 2010). This constitutive phosphorylation is abundant in LRRK2, such that a major fraction of total LRRK2 protein is phosphorylated at these residues(West et al., 2007). The pSer935-specific antibodies are particularly efficacious in part because the phosphorylated site is abundant and the surrounding amino acid sequence has little sequence homology to any other protein. In the presence of a LRRK2 kinase inhibitor bound to LRRK2 in cells and tissue, phosphatase activity, probably PP1(Lobbestael et al., 2013), removes the constitutive phosphorylation and this action is higher than that of other kinases that add phosphorylation. The effect of a loss of constitutive phosphorylation in the presence of a LRRK2 kinase inhibitor is rapid, within minutes of the addition of compounds(Doggett et al., 2012). Curiously, mutations that kill LRRK2 kinase activity do not reduce the abundance of constitutive phosphorylation, and mutations in the kinase domain that increase kinase activity do not increase the abundance of the constitutive phosphorylation. A likely explanation would involve differential 14-3-3 binding to the phospho-sites and conformational changes in the LRRK2 protein due to occupancy of the ATP binding pocket with a non-hydrolysable molecule with much higher affinity than ATP (e.g., 10 nM binding for typical inhibitors versus 10 µM binding affinity for ATP). It is important to note that while the pSer935 site may reflect LRRK2 kinase inhibition, it is an indirect measure of LRRK2 kinase activity and expected phosphorylation of substrates. So far in model systems, pSer935 has mirrored the dephosphorylation of the LRRK2 substrate pSer1292 and pRab (Fraser et al., 2016a; Henry et al., 2015; Steger et al., 2016).
There is a wealth of potential sources of protein that can be exploited for measuring LRRK2 inhibition in the context of a clinical trial. Evaluation of LRRK2 expression via the Illumina mRNA human body map (Figure 3) demonstrates that the bulk of LRRK2 is probably in circulating cells. However, LRRK2 is also expressed in the brain and kidney, and LRRK2 can be measured in cerebral spinal fluid (CSF) in the brain as well as in urine(Fraser et al., 2016a; Fraser et al., 2013; Fraser et al., 2016b). While blood and urine can be procured relatively non-invasively in clinical populations, CSF at face-value may be ideal for measuring LRRK2 inhibition in the brain. Interpretation in the CSF is slightly convoluted in that LRRK2 appears in exosome fractions and the origin and turnover of the microvesicle population in the CSF is almost completely unknown. Further experimentation is clearly warranted in this area to correlate CSF levels with LRRK2 inhibition in brain tissue. In general, a better understanding of the normal biology of biomarkers and substrates useful for measuring LRRK2 kinase inhibition will expedite a successful deployment of a biomarker-guided clinical trial.
Figure 3.
Sources of biomarkers for LRRK2 kinase inhibition in humans. LRRK2 expression (imported from the Illumina RNA Body Map) is highest in circulating cells, and protein expression and phosphorylation can be determined in white-blood cells (PBMCs) and neutrophils and in exosome-fractions purified from cerebral spinal fluid (CSF) and urine. pS935 levels are in indirect measure of LRRK2 kinase inhibition, whereas pS1292 levels and pRab levels are a direct measure of LRRK2 kinase function and known to be increased by LRRK2 pathogenic mutations.
LRRK2 is not an abundant protein and is present in low-nanogram to picogram amounts in typical lysates from tissues and cells, and in the low picogram to femptogram range in small biofluid specimens(Fraser et al., 2013). New scalable ultra-sensitive assays capable of detecting low picogram or femtogram levels of peptide will be needed. If CSF measurements lack feasibility due to low abundance of protein and lack of biomarker reliability, then a viable alternative might be to measure LRRK2 inhibition in blood and urine together with measuring compound levels in CSF. This alternative strategy is indirect and needs extensive additional testing first in rodents and then in NHPs with a particular drug, but may be critical for successful initial implementation of LRRK2 kinase inhibitors. It would not be possible to test the hypothesis that LRRK2 kinase inhibitors might provide neuroprotection without convincing evidence that LRRK2 is inhibited in the brain in response to drug treatment.
An alternative strategy to directly measuring LRRK2 inhibition in the brain might involve the development of a ligand suitable for positron-emission tomography that binds the same LRRK2 ATP pocket as the clinical candidate inhibitor but does so with weaker affinity and/or much lower concentration, with favorable off-rates compared to a clinical candidate LRRK2 inhibitor. With simultaneous dosing of the PET ligand and the LRRK2 inhibitor, the PET signal might diminish in proportion to the LRRK2 inhibitor occupancy of the same binding site. LRRK2 kinase inhibitors with undesirable (quick) turnover and lack of efficacy in pre-clinical models may be suitable for repurposing as PET ligand candidates. However, there are few examples of successful deployment of a competitive PET ligand approach, so the probability that this strategy would be implemented early on seems lower than other approaches.
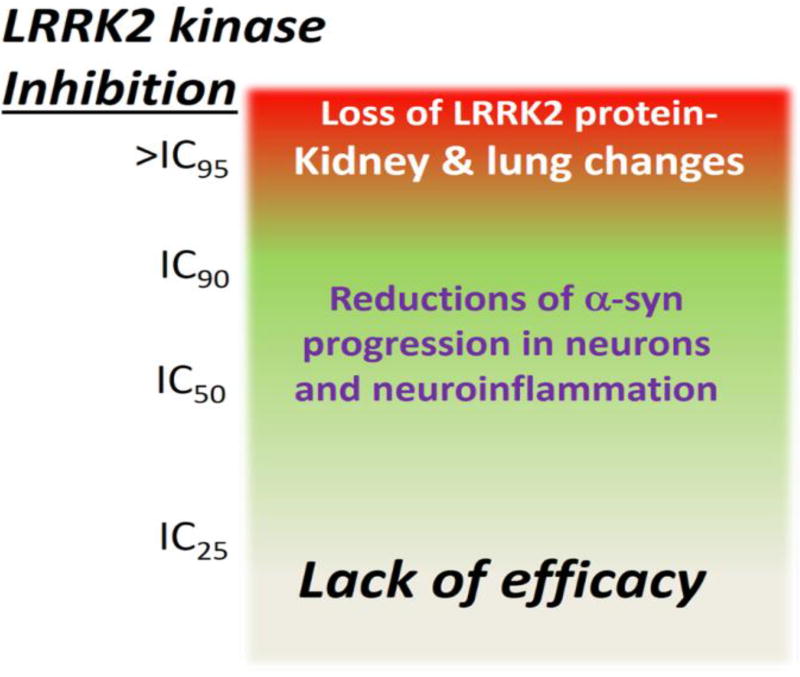
Without a biomarker guided approach for LRRK2 kinase inhibition, failure to observe neuroprotection may be related to insufficient inhibition, for example in blocking only a small percentage of LRRK2 kinase activity in the brain. Conversely, pre-clinical studies in mice, rats, and NHPs are beginning to demonstrate that too much inhibition may result in the loss of total LRRK2 protein and the onset of phenotypes associated with homozygous knockout rodents(Baptista et al., 2015; Fuji et al., 2015). If the therapeutic window of LRRK2 kinase inhibition is small, then neuroprotection and efficacy would be difficult to achieve. Fortunately, the available data supports the notion of a reasonable therapeutic window of opportunity. In rats and mice treated chronically (days and weeks) with doses of inhibitors that do not fully block LRRK2 kinase activity (e.g., 30 mg per kg daily or twice daily with MLi-2 or PFE360 and PFE475), total LRRK2 levels are only slightly reduced(Daher et al., 2015; Fell et al., 2015). NHPs treated with lower levels of inhibitors that still block nearly all the LRRK2 kinase activity did not cause lung abnormalities(Baptista et al., 2015; Fuji et al., 2015). As there appears to be a direct link between the complete or near complete loss of LRRK2 protein and the onset of kidney and lung changes in the rodents and NHPs, the high-end of the therapeutic window would be defined by inhibition levels that do not reduce total LRRK2 protein levels. For defining minimum levels of inhibition, the effects of pathogenic mutations may be informative. As pathogenic mutations cause a relatively modest increase in kinase activity (Figure 1), returning these back to wild-type levels should normalize LRRK2-linked PD activities. Thus, a therapeutic window can be proposed where efficacy would become most likely in blocking at least half or more, but not all, of LRRK2 kinase activity (Figure 4). Additional studies in the pre-clinical space will be critical to support or refine therapeutic windows of efficacy and guide clinical studies accurately.
Figure 4.
Model for a therapeutic window of LRRK2 kinase inhibition for neuroprotection.
7. Development and patient population considerations for LRRK2 kinase inhibitor trials
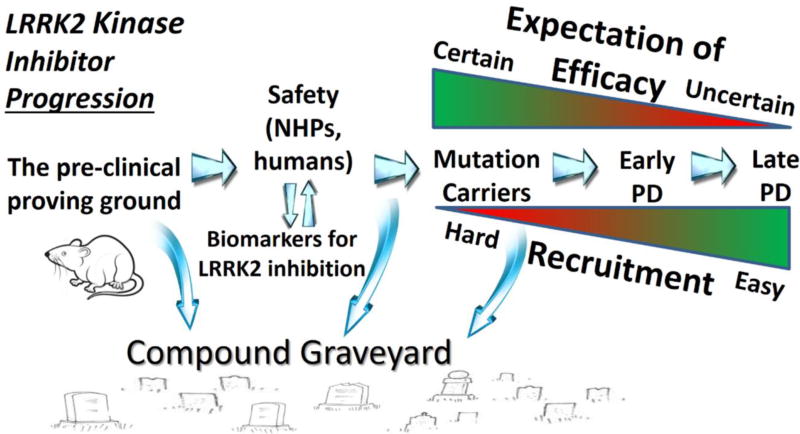
There are numerous challenges associated with identifying the first disease-modifying drug for PD, and LRRK2 kinase inhibitors would not be excluded from most of these challenges. In the context of limited resources and limited initial patient populations, weaker performing compounds in pre-clinical studies should be excluded early from further development in favor of better compounds. Since efficacy will not be determined until later phase clinical trials, a strong package in the front end of development will provide the best chances for neuroprotection. For those compounds determined to have the highest potency, specificity, brain permeability, efficacies in pre-clinical models, and favorable drug properties in NHPs, the first wave of clinical trials will need to include healthy subjects in dose escalation studies. Issues related to the definition of a therapeutic window of neuroprotection and knowledge of variabilities associated with biomarkers for LRRK2 kinase inhibition do not need to be fully resolved in safety and biomarker trials. Rather, these studies will be critical for understanding how to integrate biomarker measures for determination of LRRK2 inhibition in the periphery and the brain. Biomarkers that include measures of LRRK2 substrates or pSer-935 LRRK2 in exosomes from CSF and urine, and these proteins in circulating cells, coupled with measurements of compounds, will help guide interpretation of LRRK2 inhibition in a clinical trial (Figure 3). Biomarker criteria should be incorporated as early as possible for each compound in development (Figure 5), such that compounds that fail to satisfactorily inhibit LRRK2 in clinical populations are blocked from further development. Early trials will also be critical to begin to understand population variability associated with the biomarkers and safety concerns not revealed in pre-clinical studies.
Figure 5.
Model for LRRK2 kinase inhibitor development. Pre-clinical models are informative to the prioritization of compounds for safety studies that include target engagement measures (biomarker driven). Biomarkers will demonstrate the feasibility of a desired level of inhibition related to a therapeutic window of efficacy. Compounds that fail to meet pre-defined criteria should be excluded from further development. Compounds advancing to efficacy trials begin with LRRK2 mutation carriers where the highest expectation of efficacy exists but the hardest level of recruitment. Once a beneficial effect is observed in these patients, the space will open to idiopathic PD. Idiopathic PD subjects should be identified based on relevant genetic and biochemical markers to help stratify PD to those patients most likely to experience benefit from LRRK2 kinase inhibition.
Next, safe doses of a compound that produce satisfactory levels of LRRK2 inhibition in the brain and periphery may be nominated through a process that should be as open as possible in reaching the next (very limited) subject population: LRRK2 mutation carriers. Presently, there are only sufficient preclinical data surrounding pathogenic LRRK2 variants proven by familial segregation to justify these mutation carriers for initial clinical trials(West, 2015). As such, from a regulatory perspective, these mutation carriers with PD should be considered an orphan (rare) disease, with the associated streamlined process for the development of targeted therapies(Reichmann, 2011). Achieving orphan drug designation for LRRK2 kinase inhibitors for carriers of pathogenic LRRK2 mutations may shave years off the development pipeline, thereby expediting progression to idiopathic PD (Figure 5).
Although the recent focus in neurodegenerative disease is to enroll patients with the earliest or even prodromal disease, this will be difficult in LRRK2 mutation carriers. The G2019S mutation is incompletely penetrant, therefore phenoconversion to PD in aged (~55–75 years of age) individuals is low, on the order of ≤1–2% per year. It will not be possible to enroll a large enough population of G2019S carriers diagnosed with PD but not currently on PD-related medications for a LRRK2 kinase inhibitor efficacy trial. More likely would be enrolling mutation carriers with recent disease diagnosis, for example less than seven years past diagnosis, especially as genetic testing for LRRK2 mutations becomes more and more routine. As current therapies have little or no disease modifying effects, the focus on earlier disease is justified from a pathobiological perspective in attempting to rescue neurons from demise versus competing with later stages of disease where fewer neurons remain in some susceptible brain regions.
Efficacies will need to be defined in LRRK2 mutation cohorts for the best chances of clinical trial designs in idiopathic PD (those patients that lack LRRK2 pathogenic mutations). LRRK2 mutation cohorts maximize the effect size of LRRK2 in disease susceptibility and progression, while the cost, as mentioned, is the need to recruit subjects further along in disease. There may be subtle variations of PD progression and disease phenotypes associated with G2019S carriers versus non-carriers(Kestenbaum and Alcalay, 2017), but the strong clinical phenotypic overlap to idiopathic PD suggests the Parkinson’s Progression Markers Initiative (PPMI) and the Parkinson’s disease Biomarker Program (PDBP) should be valuable in identifying group sizes powered to identify disease modification effects in different clinical scales and imaging techniques(Gwinn et al., 2017; Parkinson Progression Marker, 2011; Rosenthal et al., 2016). Earlier safety studies for LRRK2 inhibitors that identify biomarker relationships to compound dosages and maximum tolerated dose in healthy controls may not be fully informative towards identifying dosages of compound needed for the desired level of LRRK2 inhibition in mutation carriers (Figure 4). Further, inclusion of different LRRK2 mutations (e.g., R1441G and Y1699C) together with the more common G2019S mutation may demand additional accommodations in clinical trial design. In favor of multiple dosage groups that are often arbitrarily conceived and reduce power, biomarker-guided adaptive clinical trial designs that adjust dose based on achieving a desired level of LRRK2 inhibition may eliminate ineffective treatment arms from the start. Through this approach, the need for a treatment group near or at the maximum tolerated dose identified in safety studies may also be eliminated, and this may be desirable since complete blockage of LRRK2 kinase activity is associated with total loss of LRRK2 protein and potentially adverse phenotypes (Figure 4). Finally, a Phase II/III adaptive seamless design should also be considered in LRRK2 mutation cohorts to eliminate lead time between stages and expedite additional efficacy trials with competitor compounds and in idiopathic PD.
Idiopathic PD is not a homogenous disease and efforts that fail to incorporate rigorous inclusion criteria such as biomarker-guided enrollment may washout potential disease modification effects from LRRK2 kinase inhibition. Biomarker studies related to LRRK2 kinase activity in idiopathic PD may provide guidance towards inclusion criteria for efficacy studies in idiopathic PD. For example, a proportion of idiopathic PD (10–15% in males and females) demonstrated high pSer1292-LRRK2 levels that were seen in relatively few matched controls(Fraser et al., 2016b). Two recent independent studies demonstrated ~20% of idiopathic PD patients with very high LRRK2 levels in monocytes that were not seen in matched controls(Bliederhaeuser et al., 2016; Cook et al., 2017). Future studies that integrate measures of LRRK2 and LRRK2 kinase substrates in CSF, blood, and urine may confirm that a sub-population of PD exists with very high LRRK2 activity. In the end, this subpopulation of idiopathic patients (i.e., without LRRK2 mutation) may benefit the most from LRRK2 kinase inhibition, even beyond that found in LRRK2 mutation carriers. The stratification of idiopathic PD, for example based on biomarkers, may experience initial but significant push-back from market forces that encourages the inclusion of as large a market as possible early in development. It may take a combination of pressure from regulatory, foundation, and academic forces to help ensure that the best cohorts are identified for clinical trials through precision approaches.
8. Concluding remarks
It has been almost thirteen years since the discovery of mutations in the LRRK2 gene in PD. With respect to targeting LRRK2 kinase activity for neuroprotection in PD, the most salient developments in the last few years include: 1) genome-wide association and exome databases that strengthen the genetic ties between LRRK2 and PD; 2) the development and availability of brain-penetrant and potent small molecule inhibitors (so-called 3rd generation) suitable for long-term dosing in rodents and NHPs, and initial success with these inhibitors in a PD model; 3) emergent safety profiles from rodents and NHPs treated with inhibitors that suggest a path forward to clinical trials; and 4) the discovery of biomarkers in CSF, blood, and urine that have the potential to track LRRK2 kinase inhibition and identify patients most likely to benefit from LRRK2 kinase inhibition. The interest in targeting LRRK2 for neuroprotection in PD has slowly garnered enthusiasm over the last five years in industry, academia, and at a foundation level. While the ingredients all seem in place for a recipe that identifies the first disease-modifying therapy, the devil is in the detail and numerous hurdles remain. Per the track record of clinical trials in neurodegeneration over the last twenty years, the effect of one bad drug or a poor clinical trial design can ripple through the industry to halt other properly designed campaigns. There are no solutions to this problem other than broad engagement early from industry, academia, and patient-advocacy groups to block the progression of weak or underpowered studies.
Herein, a basic scaffold for progression to a successful neuroprotection study is outlined, with a focus on the areas of research that will help speed the process. Chemical lesion models and older genetic models of PD that rely on aggressive phenotypes associated with mutant A53T hα-synuclein expression may not be optimal to find the best LRRK2 kinase inhibitors. Instead, models with high-face validity to disease that might include wild-type α-synuclein provoked neurotoxicity can potentially be harnessed to understand the nature and timeline of neuroprotection afforded by LRRK2 kinase inhibition. Some models of neuroinflammation and related neurodegeneration could also be explored to help find the best molecules. Resources allocated to the standardization of models, including efforts to increase rigor and reliability, and cross-replication of key results (such as efficacy studies in models) will all help ensure that vastly more expensive errors are avoided in the rush to the clinic.
Well-designed safety and toxicity studies incorporating robust biomarkers should be aligned with the continued progression of the compound. Safe compounds with proven effects in reducing LRRK2 phosphorylation and//or the abundance of LRRK2 kinase substrates can be nominated for studies in the precious LRRK2 mutation carrier population. The existence of enough G2019S LRRK2 mutation carriers for targeted efficacy trials is both a blessing and a curse. A blessing in that LRRK2 genetic status with PD diagnosis will clearly define inclusion and the initial population most likely to benefit from LRRK2 kinase inhibition, but a curse in that the numbers of LRRK2 mutation carriers with PD are still low such that only one or a few trials are possible in the coming years. The LRRK2 mutation carriers will be a very coveted proving-ground group of patients, and those inhibitors that show promise can advance to idiopathic PD. Biochemical biomarkers have the best chance to identify patients with evidence of elevated LRRK2 kinase activity that may experience the most benefit from LRRK2 kinase inhibition. Efforts to identify sub-groups of PD based on LRRK2 kinase function may maximize effect sizes that can be detected in existing clinical scales.
In summary, the entirety of the known biology of LRRK2 should be harnessed to help ensure success in the clinic. The definition of timelines of therapeutic benefit, whether inhibitors might be effective early in disease, or can be effective later, and at what level of inhibition, ultimately should feed back into the optimization of future trials. LRRK2 represents one of the only genetic links to neurodegeneration that involves a gain-of-function with an enzyme class proven to be successfully targeted for therapeutic benefit. Thus, with a careful progression that involves collaborative efforts, disclosures of results, and appropriate allocation of resources, there is strong reason to believe that LRRK2 kinase inhibitors will be part of the solution for the treatment of Parkinson disease.
Highlights.
Preclinical studies with LRRK2 small molecule kinase inhibitors are beginning to reveal the type of therapeutic benefit associated with blocking LRRK2 activity.
LRRK2 mutation carriers provide an ideal clinical population for biomarker optimization and initial efficacy trials.
Empirical measurements of LRRK2 kinase inhibition in clinical populations should guide development pipelines and efficacy trials.
Acknowledgments
The Author thanks Professors Roy Alcalay, Corinne Augelli-Szafran, Rita Cowell, Warren Hirst, David Standaert, and Mark Suto for critical review and comments. Some of the opinions expressed here developed from discussions with the LRRK2 Industry Consortium and leadership from the NINDS Office of Translational Research and the Michael J. Fox Foundation for Parkinson’s disease Research. Support for the Author’s work is provided by NIH R01 NS064934, NIH P20 NS092530, NIH U01 NS097028, NIH R21 NS097643, The Michael J. Fox Foundation, and the American Parkinson’s Disease Association.
Abbreviations
- COR
C-terminal of Ras
- CSF
Cerebral Spinal Fluid
- DAT-1
Dopamine transporter-1
- ERK5
Extracellular-signal-regulated kinase 5
- ExAC
Exome Aggregation Consortium
- GTPase
Guanosine Triphosphate Hydrolase
- LoF
Loss of Function
- LRRK2
Leucine-Rich Repeat Kinase 2
- MLi-2
Merck LRRK2 inhibitor 2
- MPP
1-methyl-4-phenylpyridinium
- NHPs
Non-human Primates
- PD
Parkinson Disease
- PET
Positron Emission Tomography
- PFE360
PF-06685360
- PFE475
PF-06447475
- PP1
Protein Phosphatase 1
- PPMI
Parkinson Progression Markers Initiative
- PDBP
Parkinson Disease Biomarker Program
- PrP
Prion Promoter
- rAAV2
Recombinant Adeno-associated Virus Serotype 2
- ROC
Ras-of-Complex
- TTK
TTK Protein Kinase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andres-Mateos E, Mejias R, Sasaki M, Li X, Lin BM, Biskup S, Zhang L, Banerjee R, Thomas B, Yang L, Liu G, Beal MF, Huso DL, Dawson TM, Dawson VL. Unexpected lack of hypersensitivity in LRRK2 knock-out mice to MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:15846–15850. doi: 10.1523/JNEUROSCI.4357-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baptista M, Merchant K, Bharghava S, Bryce D, Ellis M, Estrada A, Fell M, Fuji R, Galatsis P, Hill S, Hirst W, Houle C, Kennedy M, Liu X, Maddess M, Markgraf C, Mei H, Needle E, Steyn S, Yi Z, Yu H, Fiske B, Sherer T. LRRK2 Kinase Inhibitors of Different Structural Classes Induce Abnormal Accumulation of Lamellar Bodies in Type II Pneumocytes in Non-Human Primates but are Reversible and Without Pulmonary Functional Consequences; 45th International Society for Neuroscience Meeting 763.02.2015. [Google Scholar]
- Baptista MA, Dave KD, Frasier MA, Sherer TB, Greeley M, Beck MJ, Varsho JS, Parker GA, Moore C, Churchill MJ, Meshul CK, Fiske BK. Loss of leucine-rich repeat kinase 2 (LRRK2) in rats leads to progressive abnormal phenotypes in peripheral organs. PloS one. 2013;8:e80705. [Google Scholar]
- Bliederhaeuser C, Zondler L, Grozdanov V, Ruf WP, Brenner D, Melrose HL, Bauer P, Ludolph AC, Gillardon F, Kassubek J, Weishaupt JH, Danzer KM. LRRK2 contributes to monocyte dysregulation in Parkinson’s disease. Acta Neuropathol Commun. 2016;4:123. doi: 10.1186/s40478-016-0396-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boddu R, Hull TD, Bolisetty S, Hu X, Moehle MS, Daher JP, Kamal AI, Joseph R, George JF, Agarwal A, Curtis LM, West AB. Leucine-rich repeat kinase 2 deficiency is protective in rhabdomyolysis-induced kidney injury. Human molecular genetics. 2015;24:4078–4093. doi: 10.1093/hmg/ddv147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosgraaf L, Van Haastert PJ. Roc, a Ras/GTPase domain in complex proteins. Biochimica et biophysica acta. 2003;1643:5–10. doi: 10.1016/j.bbamcr.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Collins FS, Tabak LA. Policy: NIH plans to enhance reproducibility. Nature. 2014;505:612–613. doi: 10.1038/505612a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook DA, Kannarkat GT, Cintron AF, Butkovich LM, Fraser KB, Chang J, Grigoryan N, Factor SA, West AB, Boss JM, Tansey MG. LRRK2 levels in immune cells are increased in Parkinson’s disease. npj Parkinson’s Disease. 2017;3:11. doi: 10.1038/s41531-017-0010-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cookson MR. LRRK2 Pathways Leading to Neurodegeneration. Curr Neurol Neurosci Rep. 2015;15:42. doi: 10.1007/s11910-015-0564-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daher JP, Abdelmotilib HA, Hu X, Volpicelli-Daley LA, Moehle MS, Fraser KB, Needle E, Chen Y, Steyn SJ, Galatsis P, Hirst WD, West AB. LRRK2 Pharmacological Inhibition Abates alpha-Synuclein Induced Neurodegeneration. The Journal of biological chemistry. 2015 doi: 10.1074/jbc.M115.660001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daher JP, Pletnikova O, Biskup S, Musso A, Gellhaar S, Galter D, Troncoso JC, Lee MK, Dawson TM, Dawson VL, Moore DJ. Neurodegenerative phenotypes in an A53T alpha-synuclein transgenic mouse model are independent of LRRK2. Human molecular genetics. 2012;21:2420–2431. doi: 10.1093/hmg/dds057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daher JP, Volpicelli-Daley LA, Blackburn JP, Moehle MS, West AB. Abrogation of alpha-synuclein-mediated dopaminergic neurodegeneration in LRRK2-deficient rats. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:9289–9294. doi: 10.1073/pnas.1403215111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X, Dzamko N, Prescott A, Davies P, Liu Q, Yang Q, Lee JD, Patricelli MP, Nomanbhoy TK, Alessi DR, Gray NS. Characterization of a selective inhibitor of the Parkinson’s disease kinase LRRK2. Nature chemical biology. 2011;7:203–205. doi: 10.1038/nchembio.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doggett EA, Zhao J, Mork CN, Hu D, Nichols RJ. Phosphorylation of LRRK2 serines 955 and 973 is disrupted by Parkinson’s disease mutations and LRRK2 pharmacological inhibition. J Neurochem. 2012;120:37–45. doi: 10.1111/j.1471-4159.2011.07537.x. [DOI] [PubMed] [Google Scholar]
- Dzamko N, Deak M, Hentati F, Reith AD, Prescott AR, Alessi DR, Nichols RJ. Inhibition of LRRK2 kinase activity leads to dephosphorylation of Ser(910)/Ser(935), disruption of 14-3-3 binding and altered cytoplasmic localization. The Biochemical journal. 2010;430:405–413. doi: 10.1042/BJ20100784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fell MJ, Mirescu C, Basu K, Cheewatrakoolpong B, DeMong DE, Ellis JM, Hyde LA, Lin Y, Markgraf CG, Mei H, Miller M, Poulet FM, Scott JD, Smith MD, Yin Z, Zhou X, Parker EM, Kennedy ME, Morrow JA. MLi-2, a Potent, Selective, and Centrally Active Compound for Exploring the Therapeutic Potential and Safety of LRRK2 Kinase Inhibition. J Pharmacol Exp Ther. 2015;355:397–409. doi: 10.1124/jpet.115.227587. [DOI] [PubMed] [Google Scholar]
- Fraser KB, Moehle MS, Alcalay RN, West AB, Consortium LC. Urinary LRRK2 phosphorylation predicts parkinsonian phenotypes in G2019S LRRK2 carriers. Neurology. 2016a;86:994–999. doi: 10.1212/WNL.0000000000002436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser KB, Moehle MS, Daher JP, Webber PJ, Williams JY, Stewart CA, Yacoubian TA, Cowell RM, Dokland T, Ye T, Chen D, Siegal GP, Galemmo RA, Tsika E, Moore DJ, Standaert DG, Kojima K, Mobley JA, West AB. LRRK2 secretion in exosomes is regulated by 14-3-3. Human molecular genetics. 2013;22:4988–5000. doi: 10.1093/hmg/ddt346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser KB, Rawlins AB, Clark RG, Alcalay RN, Standaert DG, Liu N, Parkinson’s Disease Biomarker Program, C. West AB. Ser(P)-1292 LRRK2 in urinary exosomes is elevated in idiopathic Parkinson’s disease. Movement disorders : official journal of the Movement Disorder Society. 2016b;31:1543–1550. doi: 10.1002/mds.26686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuji RN, Flagella M, Baca M, Baptista MA, Brodbeck J, Chan BK, Fiske BK, Honigberg L, Jubb AM, Katavolos P, Lee DW, Lewin-Koh SC, Lin T, Liu X, Liu S, Lyssikatos JP, O’Mahony J, Reichelt M, Roose-Girma M, Sheng Z, Sherer T, Smith A, Solon M, Sweeney ZK, Tarrant J, Urkowitz A, Warming S, Yaylaoglu M, Zhang S, Zhu H, Estrada AA, Watts RJ. Effect of selective LRRK2 kinase inhibition on nonhuman primate lung. Science translational medicine. 2015;7 doi: 10.1126/scitranslmed.aaa3634. 273ra215. [DOI] [PubMed] [Google Scholar]
- Funayama M, Hasegawa K, Ohta E, Kawashima N, Komiyama M, Kowa H, Tsuji S, Obata F. An LRRK2 mutation as a cause for the parkinsonism in the original PARK8 family. Annals of neurology. 2005;57:918–921. doi: 10.1002/ana.20484. [DOI] [PubMed] [Google Scholar]
- Greggio E, Cookson MR. Leucine-rich repeat kinase 2 mutations and Parkinson’s disease: three questions. ASN Neuro. 2009;1 doi: 10.1042/AN20090007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guaitoli G, Raimondi F, Gilsbach BK, Gomez-Llorente Y, Deyaert E, Renzi F, Li X, Schaffner A, Jagtap PK, Boldt K, von Zweydorf F, Gotthardt K, Lorimer DD, Yue Z, Burgin A, Janjic N, Sattler M, Versees W, Ueffing M, Ubarretxena-Belandia I, Kortholt A, Gloeckner CJ. Structural model of the dimeric Parkinson’s protein LRRK2 reveals a compact architecture involving distant interdomain contacts. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:E4357–4366. doi: 10.1073/pnas.1523708113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwinn K, David KK, Swanson-Fischer C, Albin R, Hillaire-Clarke CS, Sieber BA, Lungu C, Bowman FD, Alcalay RN, Babcock D, Dawson TM, Dewey RB, Jr, Foroud T, German D, Huang X, Petyuk V, Potashkin JA, Saunders-Pullman R, Sutherland M, Walt DR, West AB, Zhang J, Chen-Plotkin A, Scherzer CR, Vaillancourt DE, Rosenthal LS. Parkinson’s disease biomarkers: perspective from the NINDS Parkinson’s Disease Biomarkers Program. Biomark Med. 2017;11:451–473. doi: 10.2217/bmm-2016-0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms AS, Cao S, Rowse AL, Thome AD, Li X, Mangieri LR, Cron RQ, Shacka JJ, Raman C, Standaert DG. MHCII is required for alpha-synuclein-induced activation of microglia, CD4 T cell proliferation, and dopaminergic neurodegeneration. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:9592–9600. doi: 10.1523/JNEUROSCI.5610-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry AG, Aghamohammadzadeh S, Samaroo H, Chen Y, Mou K, Needle E, Hirst WD. Pathogenic LRRK2 mutations, through increased kinase activity, produce enlarged lysosomes with reduced degradative capacity and increase ATP13A2 expression. Human molecular genetics. 2015;24:6013–6028. doi: 10.1093/hmg/ddv314. [DOI] [PubMed] [Google Scholar]
- Herzig MC, Kolly C, Persohn E, Theil D, Schweizer T, Hafner T, Stemmelen C, Troxler TJ, Schmid P, Danner S, Schnell CR, Mueller M, Kinzel B, Grevot A, Bolognani F, Stirn M, Kuhn RR, Kaupmann K, van der Putten PH, Rovelli G, Shimshek DR. LRRK2 protein levels are determined by kinase function and are crucial for kidney and lung homeostasis in mice. Human molecular genetics. 2011;20:4209–4223. doi: 10.1093/hmg/ddr348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachergus J, Mata IF, Hulihan M, Taylor JP, Lincoln S, Aasly J, Gibson JM, Ross OA, Lynch T, Wiley J, Payami H, Nutt J, Maraganore DM, Czyzewski K, Styczynska M, Wszolek ZK, Farrer MJ, Toft M. Identification of a novel LRRK2 mutation linked to autosomal dominant parkinsonism: evidence of a common founder across European populations. American journal of human genetics. 2005;76:672–680. doi: 10.1086/429256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelland K. Analysis: Neuroscience under threat as Big Pharma backs off. Reuters; London: 2011. [Google Scholar]
- Kestenbaum M, Alcalay RN. Clinical Features of LRRK2 Carriers with Parkinson’s Disease. Adv Neurobiol. 2017;14:31–48. doi: 10.1007/978-3-319-49969-7_2. [DOI] [PubMed] [Google Scholar]
- Landis SC, Amara SG, Asadullah K, Austin CP, Blumenstein R, Bradley EW, Crystal RG, Darnell RB, Ferrante RJ, Fillit H, Finkelstein R, Fisher M, Gendelman HE, Golub RM, Goudreau JL, Gross RA, Gubitz AK, Hesterlee SE, Howells DW, Huguenard J, Kelner K, Koroshetz W, Krainc D, Lazic SE, Levine MS, Macleod MR, McCall JM, Moxley RT, 3rd, Narasimhan K, Noble LJ, Perrin S, Porter JD, Steward O, Unger E, Utz U, Silberberg SD. A call for transparent reporting to optimize the predictive value of preclinical research. Nature. 2012;490:187–191. doi: 10.1038/nature11556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lill CM, Roehr JT, McQueen MB, Kavvoura FK, Bagade S, Schjeide BM, Schjeide LM, Meissner E, Zauft U, Allen NC, Liu T, Schilling M, Anderson KJ, Beecham G, Berg D, Biernacka JM, Brice A, DeStefano AL, Do CB, Eriksson N, Factor SA, Farrer MJ, Foroud T, Gasser T, Hamza T, Hardy JA, Heutink P, Hill-Burns EM, Klein C, Latourelle JC, Maraganore DM, Martin ER, Martinez M, Myers RH, Nalls MA, Pankratz N, Payami H, Satake W, Scott WK, Sharma M, Singleton AB, Stefansson K, Toda T, Tung JY, Vance J, Wood NW, Zabetian CP, Me Genetic Epidemiology of Parkinson’s Disease, C., International Parkinson’s Disease Genomics, C., Parkinson’s Disease, G.C., Wellcome Trust Case Control, C. Young P, Tanzi RE, Khoury MJ, Zipp F, Lehrach H, Ioannidis JP, Bertram L. Comprehensive research synopsis and systematic meta-analyses in Parkinson’s disease genetics: The PDGene database. PLoS genetics. 2012;8:e1002548. doi: 10.1371/journal.pgen.1002548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Parisiadou L, Gu XL, Wang L, Shim H, Sun L, Xie C, Long CX, Yang WJ, Ding J, Chen ZZ, Gallant PE, Tao-Cheng JH, Rudow G, Troncoso JC, Liu Z, Li Z, Cai H. Leucine-rich repeat kinase 2 regulates the progression of neuropathology induced by Parkinson’s-disease-related mutant alpha-synuclein. Neuron. 2009;64:807–827. doi: 10.1016/j.neuron.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Mobley JA, DeLucas LJ, Kahn RA, West AB. LRRK2 autophosphorylation enhances its GTPase activity. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2016;30:336–347. doi: 10.1096/fj.15-277095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Lee J, Krummey S, Lu W, Cai H, Lenardo MJ. The kinase LRRK2 is a regulator of the transcription factor NFAT that modulates the severity of inflammatory bowel disease. Nat Immunol. 2011;12:1063–1070. doi: 10.1038/ni.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobbestael E, Zhao J, Rudenko IN, Beylina A, Gao F, Wetter J, Beullens M, Bollen M, Cookson MR, Baekelandt V, Nichols RJ, Taymans JM. Identification of protein phosphatase 1 as a regulator of the LRRK2 phosphorylation cycle. The Biochemical journal. 2013;456:119–128. doi: 10.1042/BJ20121772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder K, Wang Y, Alcalay RN, Mejia-Santana H, Tang MX, Lee A, Raymond D, Mirelman A, Saunders-Pullman R, Clark L, Ozelius L, Orr-Urtreger A, Giladi N, Bressman S, Consortium LAJ. Age-specific penetrance of LRRK2 G2019S in the Michael J. Fox Ashkenazi Jewish LRRK2 Consortium. Neurology. 2015;85:89–95. doi: 10.1212/WNL.0000000000001708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin I, Kim JW, Dawson VL, Dawson TM. LRRK2 pathobiology in Parkinson’s disease. J Neurochem. 2014;131:554–565. doi: 10.1111/jnc.12949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalls MA, Pankratz N, Lill CM, Do CB, Hernandez DG, Saad M, DeStefano AL, Kara E, Bras J, Sharma M, Schulte C, Keller MF, Arepalli S, Letson C, Edsall C, Stefansson H, Liu X, Pliner H, Lee JH, Cheng R, International Parkinson’s Disease Genomics, C., Parkinson’s Study Group Parkinson’s Research: The Organized, G.I., andMe, GenePd, NeuroGenetics Research, C., Hussman Institute of Human, G., The Ashkenazi Jewish Dataset, I., Cohorts for, H., Aging Research in Genetic, E., North American Brain Expression, C., United Kingdom Brain Expression, C., Greek Parkinson's Disease, C., Alzheimer Genetic Analysis, G. Ikram MA, Ioannidis JP, Hadjigeorgiou GM, Bis JC, Martinez M, Perlmutter JS, Goate A, Marder K, Fiske B, Sutherland M, Xiromerisiou G, Myers RH, Clark LN, Stefansson K, Hardy JA, Heutink P, Chen H, Wood NW, Houlden H, Payami H, Brice A, Scott WK, Gasser T, Bertram L, Eriksson N, Foroud T, Singleton AB. Large-scale meta-analysis of genome-wide association data identifies six new risk loci for Parkinson’s disease. Nature genetics. 2014 doi: 10.1038/ng.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paisan-Ruiz C, Jain S, Evans EW, Gilks WP, Simon J, van der Brug M, Lopez de Munain A, Aparicio S, Gil AM, Khan N, Johnson J, Martinez JR, Nicholl D, Carrera IM, Pena AS, de Silva R, Lees A, Marti-Masso JF, Perez-Tur J, Wood NW, Singleton AB. Cloning of the gene containing mutations that cause PARK8-linked Parkinson’s disease. Neuron. 2004;44:595–600. doi: 10.1016/j.neuron.2004.10.023. [DOI] [PubMed] [Google Scholar]
- Parkinson Progression Marker, I. The Parkinson Progression Marker Initiative (PPMI) Prog Neurobiol. 2011;95:629–635. doi: 10.1016/j.pneurobio.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- Puccini JM, Marker DF, Fitzgerald T, Barbieri J, Kim CS, Miller-Rhodes P, Lu SM, Dewhurst S, Gelbard HA. Leucine-rich repeat kinase 2 modulates neuroinflammation and neurotoxicity in models of human immunodeficiency virus 1-associated neurocognitive disorders. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2015;35:5271–5283. doi: 10.1523/JNEUROSCI.0650-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin H, Buckley JA, Li X, Liu Y, Fox TH, 3rd, Meares GP, Yu H, Yan Z, Harms AS, Li Y, Standaert DG, Benveniste EN. Inhibition of the JAK/STAT Pathway Protects Against alpha-Synuclein-Induced Neuroinflammation and Dopaminergic Neurodegeneration. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2016;36:5144–5159. doi: 10.1523/JNEUROSCI.4658-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichmann JP. NIH: FDA key for speed and safety. Nature. 2011;474:161. doi: 10.1038/474161c. [DOI] [PubMed] [Google Scholar]
- Rosenthal LS, Drake D, Alcalay RN, Babcock D, Bowman FD, Chen-Plotkin A, Dawson TM, Dewey RB, Jr, German DC, Huang X, Landin B, McAuliffe M, Petyuk VA, Scherzer CR, Hillaire-Clarke CS, Sieber BA, Sutherland M, Tarn C, West A, Vaillancourt D, Zhang J, Gwinn K, Consortium P. The NINDS Parkinson’s disease biomarkers program. Movement disorders : official journal of the Movement Disorder Society. 2016;31:915–923. doi: 10.1002/mds.26438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudi K, Ho FY, Gilsbach BK, Pots H, Wittinghofer A, Kortholt A, Klare JP. Conformational heterogeneity of the Roc domains in C. tepidum Roc-COR and implications for human LRRK2 Parkinson mutations. Biosci Rep. 2015;35 doi: 10.1042/BSR20150128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott AJ, Lieu CH, Messersmith WA. Therapeutic Approaches to RAS Mutation. Cancer J. 2016;22:165–174. doi: 10.1097/PPO.0000000000000187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JD, DeMong DE, Greshock TJ, Basu K, Dai X, Harris J, Hruza A, Li SW, Lin SI, Liu H, Macala MK, Hu Z, Mei H, Zhang H, Walsh P, Poirier M, Shi ZC, Xiao L, Agnihotri G, Baptista MA, Columbus J, Fell MJ, Hyde LA, Kuvelkar R, Lin Y, Mirescu C, Morrow JA, Yin Z, Zhang X, Zhou X, Chang RK, Embrey MW, Sanders JM, Tiscia HE, Drolet RE, Kern JT, Sur SM, Renger JJ, Bilodeau MT, Kennedy ME, Parker EM, Stamford AW, Nargund R, McCauley JA, Miller MW. Discovery of a 3-(4-Pyrimidinyl) Indazole (MLi-2), an Orally Available and Selective Leucine-Rich Repeat Kinase 2 (LRRK2) Inhibitor that Reduces Brain Kinase Activity. Journal of medicinal chemistry. 2017;60:2983–2992. doi: 10.1021/acs.jmedchem.7b00045. [DOI] [PubMed] [Google Scholar]
- Scott S, Kranz JE, Cole J, Lincecum JM, Thompson K, Kelly N, Bostrom A, Theodoss J, Al-Nakhala BM, Vieira FG, Ramasubbu J, Heywood JA. Design, power, and interpretation of studies in the standard murine model of ALS. Amyotroph Lateral Scler. 2008;9:4–15. doi: 10.1080/17482960701856300. [DOI] [PubMed] [Google Scholar]
- Sheng Z, Zhang S, Bustos D, Kleinheinz T, Le Pichon CE, Dominguez SL, Solanoy HO, Drummond J, Zhang X, Ding X, Cai F, Song Q, Li X, Yue Z, van der Brug MP, Burdick DJ, Gunzner-Toste J, Chen H, Liu X, Estrada AA, Sweeney ZK, Scearce-Levie K, Moffat JG, Kirkpatrick DS, Zhu H. Ser1292 autophosphorylation is an indicator of LRRK2 kinase activity and contributes to the cellular effects of PD mutations. Science translational medicine. 2012;4 doi: 10.1126/scitranslmed.3004485. 164ra161. [DOI] [PubMed] [Google Scholar]
- Steger M, Tonelli F, Ito G, Davies P, Trost M, Vetter M, Wachter S, Lorentzen E, Duddy G, Wilson S, Baptista MA, Fiske BK, Fell MJ, Morrow JA, Reith AD, Alessi DR, Mann M. Phosphoproteomics reveals that Parkinson’s disease kinase LRRK2 regulates a subset of Rab GTPases. Elife. 2016;5 doi: 10.7554/eLife.12813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Y, Giaime E, Yamaguchi H, Ichimura T, Liu Y, Si H, Cai H, Bonventre JV, Shen J. Loss of leucine-rich repeat kinase 2 causes age-dependent bi-phasic alterations of the autophagy pathway. Mol Neurodegener. 2012;7:2. doi: 10.1186/1750-1326-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinh J, Guella I, Farrer MJ. Disease penetrance of late-onset parkinsonism: a meta-analysis. JAMA Neurol. 2014;71:1535–1539. doi: 10.1001/jamaneurol.2014.1909. [DOI] [PubMed] [Google Scholar]
- Van der Perren A, Macchi F, Toelen J, Carlon MS, Maris M, de Loor H, Kuypers DR, Gijsbers R, Van den Haute C, Debyser Z, Baekelandt V. FK506 reduces neuroinflammation and dopaminergic neurodegeneration in an alpha-synuclein-based rat model for Parkinson’s disease. Neurobiology of aging. 2015;36:1559–1568. doi: 10.1016/j.neurobiolaging.2015.01.014. [DOI] [PubMed] [Google Scholar]
- Wallings R, Manzoni C, Bandopadhyay R. Cellular processes associated with LRRK2 function and dysfunction. FEBS J. 2015;282:2806–2826. doi: 10.1111/febs.13305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandu WS, Tan C, Ogbeifun O, Vistica BP, Shi G, Hinshaw SJ, Xie C, Chen X, Klinman DM, Cai H, Gery I. Leucine-Rich Repeat Kinase 2 (Lrrk2) Deficiency Diminishes the Development of Experimental Autoimmune Uveitis (EAU) and the Adaptive Immune Response. PloS one. 2015;10:e0128906. doi: 10.1371/journal.pone.0128906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West AB. Ten years and counting: moving leucine-rich repeat kinase 2 inhibitors to the clinic. Movement disorders : official journal of the Movement Disorder Society. 2015;30:180–189. doi: 10.1002/mds.26075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West AB, Cookson MR. Identification of bona-fide LRRK2 kinase substrates. Movement disorders : official journal of the Movement Disorder Society. 2016;31:1140–1141. doi: 10.1002/mds.26647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West AB, Moore DJ, Choi C, Andrabi SA, Li X, Dikeman D, Biskup S, Zhang Z, Lim KL, Dawson VL, Dawson TM. Parkinson’s disease-associated mutations in LRRK2 link enhanced GTP-binding and kinase activities to neuronal toxicity. Human molecular genetics. 2007;16:223–232. doi: 10.1093/hmg/ddl471. [DOI] [PubMed] [Google Scholar]
- Zimprich A, Biskup S, Leitner P, Lichtner P, Farrer M, Lincoln S, Kachergus J, Hulihan M, Uitti RJ, Calne DB, Stoessl AJ, Pfeiffer RF, Patenge N, Carbajal IC, Vieregge P, Asmus F, Muller-Myhsok B, Dickson DW, Meitinger T, Strom TM, Wszolek ZK, Gasser T. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron. 2004;44:601–607. doi: 10.1016/j.neuron.2004.11.005. [DOI] [PubMed] [Google Scholar]