Abstract
Purpose of review
This review describes existing evidence addressing the potential modulation of PrEP products, specifically 1% tenofovir (TFV) gel and oral tenofovir-based PrEP, by vaginal dysbiosis and discusses future considerations for delivering novel, long-acting PrEP products to women at high-risk for vaginal dysbiosis and HIV.
Recent findings
We describe results from analyses investigating the modification of PrEP efficacy by vaginal dysbiosis and studies of biological mechanisms that could render PrEP ineffective in the presence of specific microbiota. A secondary analysis from the CAPRISA-004 cohort demonstrated that there is no effect of the 1% TFV gel in the presence of non-Lactobacillus dominant microbiota. Another recent analysis comparing oral tenofovir-based PrEP efficacy among women with and without bacterial vaginosis in the Partners PrEP Study found that oral PrEP efficacy is not modified by bacterial vaginosis. Gardnerella vaginalis, commonly present in women with vaginal dysbiosis, can rapidly metabolize TFV particularly when it is locally applied and thereby prevent TFV integration into cells. Given that vaginal dysbiosis appears to modulate efficacy for 1% TFV gel but not for oral tenofovir-based PrEP, vaginal dysbiosis is potentially less consequential to HIV protection from TFV in the context of systemic drug delivery and high product adherence.
Summary
Vaginal dysbiosis may undermine the efficacy of 1% TFV gel to protect women from HIV but not the efficacy of oral PrEP. Ongoing development of novel ring, injectable, and film-based PrEP products should investigate whether vaginal dysbiosis can reduce efficacy of these products, even in the presence of high adherence.
Keywords: Pre-exposure prophylaxis, efficacy, HIV prevention, vaginal dysbiosis, bacterial vaginosis, women
INTRODUCTION
HIV is a leading cause of morbidity and mortality among women globally, and biomedical HIV prevention products such as pre-exposure prophylaxis (PrEP) are being developed and scaled up to prevent HIV among women (1–6). Daily tenofovir-based oral PrEP is highly efficacious in reducing HIV incidence when adherence levels are high and it is currently being rolled out to populations with substantial HIV risk (7,8). Other PrEP products (e.g. dapivirine, tenofovir alafenamide (TAF), and maraviroc rings, biodegradable tenofovir (TFV) films, cabotegravir injections, TAF or maraviroc pills, TFV implants), are at various stages of the research and development process and are promising, longer-acting HIV prevention options (9■,10■,11■,12). However, it has recently been hypothesized that the efficacy of topically-applied PrEP products may be moderated by vaginosis dysbiosis, a general term indicating that vaginal microbiota are sub-optimal (13■■). This is a significant public health concern given that bacterial vaginosis (a condition diagnosed using microscopy and/or clinical criteria) is common in settings where HIV is highly prevalent and has been shown to significantly influence risk of HIV acquisition through inflammatory pathways (14,15,16■,17,18■,19■■). Specifically, vaginal dysbiosis can result in higher vaginal pH (due to lower concentrations of Lactobacillus species) and increased activated CCR5+ CD4+ T-cells in vaginal mucosa and pro-inflammatory cytokines in cervical secretions (15,16■,18■,19■■,20,21■). Specific bacteria (e.g. Gardnerella vaginalis, Prevotella, Atopobium), and/or microbial diversity may play a significant role in increased HIV risk, with four-fold increases in HIV risk observed among women with non-Lactobacillus dominant genital bacterial communities compared with those with less diverse microbiota (18■,21■). These women need PrEP products that will work effectively in an environment of vaginal dysbiosis (18■,21■).
Results from oral PrEP trials have demonstrated that product adherence is an important moderator of PrEP efficacy (22■,23■,24,25,26). To maximize the impact of PrEP in preventing HIV acquisition among women, PrEP research and delivery programs are developing strategies to improve product adherence. An additional parallel objective is to thoroughly understand whether vaginal microbial communities can impact PrEP efficacy even when product adherence is high. Unanswered questions remain about the biological mechanisms of the potential interaction between vaginal dysbiosis and PrEP efficacy in preventing HIV acquisition and whether this relationship varies by PrEP delivery mechanisms (i.e. oral, topical) and product formulations (i.e. TFV-, dapivirine-, or cabotegravir-based). This article summarizes the existing data from two analyses on the interaction between the vaginal microbiome and estimated PrEP efficacy, provides commentary about hypothesized relationships, and suggests directions for future PrEP development and implementation research in conjunction with vaginal microbiome investigations.
TEXT OF REVIEW
Potential for Vaginal Dysbiosis to Modulate Efficacy of HIV Prevention Products
The CAPRISA-004 randomized controlled trial demonstrated proof-of-concept that topically-applied 1% TFV gel was efficacious in preventing HIV among a sample of 889 HIV-uninfected women in South Africa (27). The overall gel efficacy was 39% (95% CI: 6–60%) in the sample and 54% (95% CI: 4%–80%) among participants with high adherence to the gel BAT24 dosing regimen (defined as one dose within 12 hours before sex, one dose as soon as possible within 12 hours after sex, and no more than two doses in 24 hours) (27). These findings were not replicated in the FACTS 001 or MTN-003/VOICE randomized trials, which also included samples of HIV-uninfected women in sub-Saharan Africa but were undermined by very low product use of 1% TFV gel (23■,28■). Trials of oral tenofovir-based PrEP have found that it is highly efficacious in preventing HIV infection, but oral PrEP efficacy estimates also vary widely by overall adherence to the study product (7,23■,24). The Partners PrEP Study found that oral tenofovir-based PrEP (either as co-formulated emtricitabine (FTC)/tenofovir disoproxil fumarate (TDF) or single agent TDF) had 66% efficacy for FTC/TDF and 71% efficacy for TDF among women (7). Adherence to the daily pills was high in the Partners PrEP Study (approximately 82% of plasma samples from randomly selected participants had detectable TFV levels), which correlated with the high efficacy of oral PrEP seen in this trial (7). Two other studies of oral PrEP (VOICE, FEM-PrEP) conducted among women with lower adherence levels did not find a significant protective PrEP effect (7,23■,24).
While adherence to study product was likely a major contributing factor to limited gel and oral PrEP efficacy in FACTS 001, VOICE, and FEM-PrEP, an additional hypothesis suggests that vaginal dysbiosis may have also contributed to the lack of PrEP efficacy particularly for topical gel. In the trials of 1% TFV gel, approximately 40% of all participants had bacterial vaginosis at enrollment (determined by Nugent score) and researchers have recently speculated that specific bacteria or vaginal microbial diversity may reduce PrEP effectiveness when it is topically, but not orally, administered (13■■,28■,29,30). Two analyses have since been conducted using data from topical gel and oral PrEP trials to explore the potential association between vaginal dysbiosis and PrEP efficacy (Table 1).
TABLE 1.
Summary of analyses examining the effect of vaginal microbiota on PrEP efficacy in HIV-uninfected women
| Study | Site | N | PrEP agent | PrEP adherence | Measurement of vaginal dysbiosis | Overall PrEP efficacy | Efficacy among women with vaginal dysbiosis |
|---|---|---|---|---|---|---|---|
| CAPRISA-004 (13) | South Africa | 688 | 1% TFV gel | 60% of the sample had >50% gel adherence, measured by monthly empty gel applicator returns | Mass spectrometry to identify bacterial proteins at baseline Classified women as Lactobacillus-abundant or non-Lactobacillus-abundant |
39% (95% CI: 6–60%) | 18% (95% CI: −77–63%) among women with non-Lactobacillus-abundant microbiota 61% (95% CI: 11–84%) among women with Lactobacillus-abundant microbiota |
| Partners PrEP Study (31) | Kenya, Uganda | 1470 | Oral FTC/TDF or TDF alone | 82% adherence, measured by detection of tenofovir in plasma in a random sample of participants | Gram stain microscopy to characterize women with BV (Nugent score 7–10), intermediate microbiota (score 4–6), and normal microbiota (score 0–3) at baseline and annual follow-up visits | 70.5% (95% CI: 45.5–84.0%) | 73% (95% CI: 6–92%) efficacy for women with BV 63% (95% CI: −67–92%) efficacy for women with intermediate microbiota 77% (95% CI: 43–90%) efficacy for women with normal microbiota |
BV= bacterial vaginosis; TFV= tenofovir; FTC= emtricitabine; TDF= tenofovir disoproxil fumarate; 95% CI= 95% Confidence Interval
A secondary analysis with 688 women from the CAPRISA-004 study used mass spectrometry-based proteomics to classify women as having either Lactobacillus dominant or non-Lactobacillus dominant vaginal microbiota at baseline (13■■). In women with non-Lactobacillus dominant microbiota at baseline, of which Gardnerella vaginalis was the most common, the 1% TFV gel-based PrEP was not efficacious (efficacy: 18%; 95% CI: −77–63%) (13■■). This is in contrast to women with Lactobacillus dominant microbiota at baseline, where efficacy was 61% (95% CI: 11–84%). Approximately 60% of study participants had >50% gel adherence, as was determined by empty applicator returns, and there were no differences in adherence seen by dominant vaginal microbiota group (13■■). When restricting the sample to women with >50% adherence (n=416), again the gel was efficacious in preventing HIV for women with Lactobacillus dominant microbiota (efficacy: 78%; 95% CI: 29–95%) but did not significantly reduce HIV incidence in the presence of non-Lactobacillus dominated microbiota (efficacy: 26%; 95% CI: −98–73%) (13■■).
A secondary analysis from the Partners PrEP Study, a randomized placebo-controlled PrEP efficacy trial, determined whether vaginal microbiota modified the efficacy of oral tenofovir-based PrEP (either FTC/TDF or single agent TDF) (31■■). For this analysis, women’s follow-up time was classified into subgroups by bacterial vaginosis status using Nugent scoring from microscopy at baseline and annual study visits (31■■). There were no differences in oral PrEP efficacy between periods when women had normal microbiota (Nugent score 0–3), intermediate microbiota (score 4–6), and bacterial vaginosis (score 7–10). Similarly, oral PrEP efficacy was not reduced during periods when women had Gardnerella vaginalis/Bacteroides morphotypes relative to those with Lactobacillus morphotypes (interaction p=0.9) (31■■). These results suggest that vaginal microbiota are unlikely to moderate the efficacy of oral PrEP, which is systemically distributed and has greater TFV detection in plasma than topically applied TFV gel (31■■,32).
Hypothesized Mechanisms for Association between Vaginal Dysbiosis and PrEP Efficacy
Multiple mechanisms could lie at the interface of vaginal microbiota and efficacy of different PrEP products (Figure 1). Current evidence suggests that Gardnerella vaginalis (which is often present in greater quantities among women with non-Lactobacillus dominant microbiota) can degrade TFV, altering its metabolism and ultimately, its availability in tissue (13■■,33■■). Among CAPRISA-004 study participants with >50% gel adherence, TFV detection in cervicovaginal fluid and genital tissue was significantly lower for women with non-Lactobacillus dominant microbiota than for women with Lactobacillus dominant microbiota (13■■). TFV concentrations measured in cervicovaginal lavage samples decreased as quantities of Gardnerella vaginalis increased in the CAPRISA-004 sample, a finding supported by cell culture experiments where TFV concentrations were lower in the presence of Gardnerella vaginalis relative to Lactobacillus cultures (13■■). This TFV metabolism can happen quite rapidly and research has shown reduced tenofovir-diphosphate (TFV-DP, the active form of TFV) levels in cervical tissue within two hours and depleted TFV concentrations in cervicovaginal fluid and plasma after one week when high concentrations of Gardnerella vaginalis are present (13■■,33■■). CAPRISA-004 participants with non-Lactobacillus dominant microbiota also had enhanced cellular membrane transport protein expression, which could have provided another mechanism for rapid TFV metabolism in cervicovaginal tissue for individuals using gel-based PrEP (34■■).
FIGURE 1.
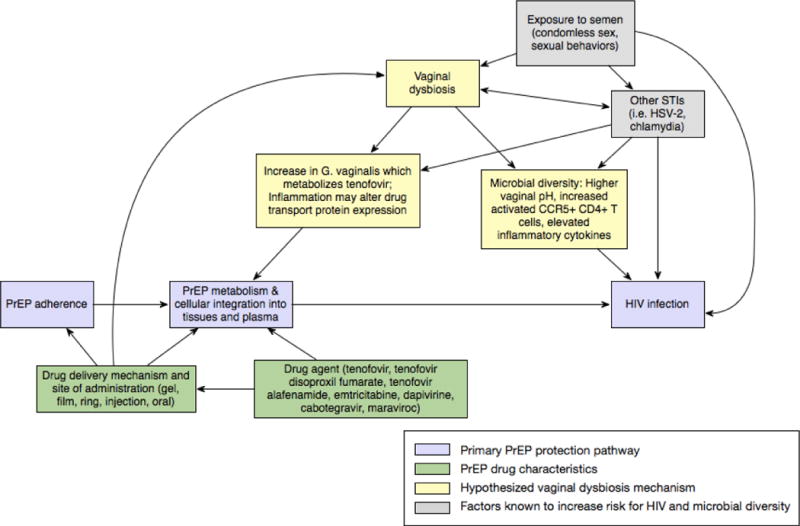
Conceptual model of proposed associations between vaginal microbiota and PrEP efficacy
Pharmacokinetic data have identified key differences between the active study drugs delivered by oral versus topical products which could also help explain associations between vaginal dysbiosis and PrEP efficacy. TFV, contained in 1% TFV gel, is a nucleoside reverse transcriptase that can be measured in plasma and cervicovaginal fluid. When administered as a gel, TFV concentrations in plasma are low and concentrations in fluid reflect levels of drug use (35). Among women in the CAPRISA-004 trial, few HIV seroconverters (14.7%) had TFV concentrations >100 ng/mL in cervicovaginal fluid, a significantly lower number than a comparable subgroup of non-seroconverters (32.8%, p=0.037 for the comparison with seroconverters) (36■). Thus, event-driven TFV gel dosing achieved only limited systemic TFV exposure, but levels of TFV near the application site are correlated with HIV protection (36■,37). With topical gel, the presence of Gardnerella vaginalis could render the gel ineffective, particularly because there are low levels of TFV in plasma to enact systemic protection after locally applied TFV has been depleted.
The molecular structure of TFV, a purine analogue, also renders it particularly vulnerable to rapid metabolism by Gardnerella vaginalis and other vaginal microbiota because it requires intracellular phosphorylation to become activated TFV-DP (13■■,35). Cell cultures that combined TFV, Jurkat cells (HIV T lymphocyte target cells), and Gardnerella vaginalis (along with Lactobacillus spp.) showed that Gardnerella vaginalis is able to metabolize TFV faster than it can be converted to its TFV-DP active form by target cells (13■■). While Prevotella and Mobiluncus species have also been shown to metabolize TFV, they likely cleave it into an inactive adenine metabolite at a slower rate than Gardnerella vaginalis (13■■).
With current formulations of oral PrEP, TFV is delivered as a prodrug (TDF) to improve its bioavailability in the gastrointestinal tract (35). TFV can be detected in plasma for about one week after oral dosing, indicating longer-term systemic availability of TFV than has been seen for gel-based PrEP but lower concentrations in vaginal tissue than gel-based dosing (32,38). Pharmacokinetic studies of oral PrEP have shown that TFV-DP has more difficulty reaching protective concentrations in the female genital tract than in colorectal tissue, and higher levels of adherence (about 6–7 doses/week) are thought to be required to provide effective HIV prevention for people having vaginal exposure to HIV (38,39■). Results from the Partners PrEP Study, VOICE, and FEM-PrEP have shown that oral FTC/TDF and TDF are efficacious with high adherence levels, but oral PrEP is not protective for women with lower adherence to the dosing regimen (7,23■,24). Therefore, daily pill dosing may be unforgiving to missed doses but vaginal microbiota are unlikely to modulate oral PrEP efficacy by metabolizing TFV in the female genital tissue.
Implications for Future PrEP Research and Implementation Programs
Additional research is currently underway to develop, test, and deliver new PrEP products at scale, including vaginal rings, long-acting films and injectables, biodegradable implants, and new oral pill formulations. Studies of these products are well positioned to consider whether vaginal dysbiosis could impact PrEP efficacy even in the context of high product adherence, as these different active drugs and delivery methods will likely have differential vulnerability to modulation by vaginal dysbiosis. For example, the dapivirine intravaginal ring contains 25mg of dapivirine (released over four weeks) that could have different susceptibility to rapid Gardnerella vaginalis metabolism than TFV (9■,10■). However, dapivirine concentrations decrease rapidly in vaginal tissue and cervicovaginal fluid after ring removal, and the drug may have a shorter half-life in tissue than is seen with gel-based TFV (35,40■). If dapivirine is metabolized by Gardnerella vaginalis, this may impact its protective benefits against HIV acquisition. Cabotegravir injections represent another promising PrEP approach and can deliver sustained drug concentrations over a 4–8 week period (35). This PrEP product also provides systemic drug delivery and its efficacy may be less affected by local Gardnerella vaginalis metabolism, as was seen with oral PrEP. Further investigation is warranted.
In addition, unanswered questions remain about whether extended-release TFV-based film products in development (to be topically applied) will be susceptible to reduced efficacy by Gardnerella vaginalis metabolism. Studies of the TFV film will need to assess whether the film delivery mechanism is capable of altering innate HIV immunity through changes to the mucin expression and the quantity of high mannose glycoproteins in the vaginal environment, as has also been suggested for the 1% TFV gel (41). Additionally, the TFV gel was shown to alter local microbial communities in the vagina, making women using these products more susceptible to bacterial dysbiosis and HIV acquisition through inflammatory pathways, and it will be important to explore similar associations for other topically applied HIV prevention products (15,18■,42).
Future studies exploring the relationship between vaginal dysbiosis and PrEP efficacy among women will need to consider several factors that are likely to influence findings, including characteristics of the PrEP drug, adherence to PrEP, and measurement of confounding variables. As has been discussed, PrEP drug type (i.e. TFV, FTC, dapivirine, cabotegravir), drug delivery mechanism (i.e. oral, topical, injectable), active drug concentrations achieved in plasma and cervicovaginal tissue, half-life of drug in target cells, and adherence to the drug product all have the potential to impact PrEP availability in target tissue and drug metabolism by specific vaginal bacteria. In addition, co-infections such as herpes simplex virus-2 (HSV-2) and chlamydia can increase risk of both vaginal dysbiosis and HIV infection and obscure the true relationship between vaginal dysbiosis and PrEP efficacy (16■). Additional strong confounders in the association between vaginal dysbiosis and PrEP efficacy include condomless sex, STI treatment, and use of products for vaginal washing. Observational studies must continue to collect data on these variables in order to accurately interpret results on the association between vaginal dysbiosis and PrEP efficacy.
This review has several limitations which help to highlight directions for future research on the links between vaginal microbiota and PrEP efficacy. Given that this is an emerging research field, only two studies have been conducted to date examining the impact of vaginal dysbiosis on 1% TFV gel and oral PrEP efficacy, and it is difficult to generalize their findings to other study populations and PrEP products. These two studies collected microbiome data infrequently, and only at the baseline visit for the CAPRISA-004 study (13■■,31■■). Studies with more frequent vaginal swab collection could prospectively measure associations between PrEP efficacy and frequently fluctuating microbial communities, while also eliminating concerns about temporality of the relationship between microbiota and HIV incidence. Also, studies that incorporate sensitive measurement techniques such as broad range 16s rRNA gene PCR with pyrosequencing and taxon-directed quantitative PCR probes could quantify the abundance of bacterial species and help to distinguish whether specific bacteria or the overall diversity of bacterial communities are associated with reduced PrEP efficacy. Finally, biomarker data collected from future topical PrEP trials would be able to add to this body of research about the potential mechanism of the association between vaginal dysbiosis and PrEP efficacy.
For PrEP to attain its greatest population impact, it is paramount to optimize adherence. An important priority for the research community is to continue work to develop and implement long-term PrEP methods (i.e. ring, film, and injectable products) that ease the burden of daily adherence and can effectively deliver PrEP drugs to target tissue in any vaginal microbiome environment (43,44). An additional priority is to continue developing innovative implementation strategies to improve daily oral PrEP adherence among women and frame PrEP as a tool to support women’s lives (4,45). Oral PrEP remains a critical biomedical HIV prevention strategy for women with symptomatic or asymptomatic bacterial vaginosis, who are protected from HIV acquisition when adherence to the daily pills is high, and there remains a need to improve oral PrEP access for women regardless of their bacterial vaginosis status (31■■).
CONCLUSION
Recent evidence suggests that vaginal dysbiosis can reduce the efficacy of TFV-based gel PrEP formulations, through altered drug metabolism. However, oral FTC/TDF-based PrEP is not impacted by bacterial vaginosis, with its systemic drug delivery and high drug concentrations in plasma that are not susceptible to metabolism by vaginal microbiota. A priority for research on novel topical biomedical HIV prevention strategies is to explore how vaginal microbiota interact with PrEP products to potentially alter the availability of active drug and product efficacy. For the scale-up of oral PrEP, optimizing delivery approaches that maximize adherence remains critical to achieve population level reductions in HIV incidence.
KEY POINTS.
1% TFV gel with an on-demand dosing schedule is not efficacious in women with non-Lactobacillus dominant vaginal microbiomes but has efficacy estimated at 61% (95% CI 11–84%) in women with Lactobacillius dominant microbiota.
Efficacy of oral PrEP is not modulated by bacterial vaginosis.
The modulation of PrEP efficacy by vaginal dysbiosis may be specific to PrEP drug formulation and delivery mechanism.
In settings with high rates of bacterial vaginosis/vaginal dysbiosis and high HIV burden, efficacy trials of novel PrEP products are well positioned to consider the interaction of vaginal dysbiosis with product efficacy.
Acknowledgments
We are grateful for the dedication of the thousands of women who have participated in PrEP clinical trials and open-label demonstration projects around the world.
Footnotes
Human and Animal Rights
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Compliance with Ethics Guidelines
Conflict of Interest
The authors declare that they have no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published within the last three years, have been highlighted as:
■ important references
■■ very important references
- 1.Adimora AA, Ramirez C, Auerbach JD, Aral SO, Hodder S, Wingood G, et al. Preventing HIV infection in women. J Acquir Immune Defic Syndr. 2013;63(02):S168–73. doi: 10.1097/QAI.0b013e318298a166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramjee G, Daniels B. Women and HIV in sub-Saharan Africa. AIDS Res Ther. 2013;10(1):30. doi: 10.1186/1742-6405-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Underhill K, Operario D, Mimiaga MJ, Skeer MR, Mayer KH. Implementation science of pre-exposure prophylaxis: preparing for public use. Curr HIV/AIDS Rep. 2010;7(4):210–9. doi: 10.1007/s11904-010-0062-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Celum CL, Delany-Moretlwe S, McConnell M, van Rooyen H, Bekker L-G, Kurth A, et al. Rethinking HIV prevention to prepare for oral PrEP implementation for young African women. J Int AIDS Soc. 2015;18(4 Suppl 3):20227. doi: 10.7448/IAS.18.4.20227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marcus JL, Volk JE, Pinder J, Liu AY, Bacon O, Hare CB, et al. Successful implementation of HIV preexposure prophylaxis: lessons learned from three clinical settings. Curr HIV/AIDS Rep. 2016;13(2):116–24. doi: 10.1007/s11904-016-0308-x. [DOI] [PubMed] [Google Scholar]
- 6.Cáceres CF, O’Reilly KR, Mayer KH, Baggaley R. PrEP implementation: moving from trials to policy and practice. J Int AIDS Soc. 2015;18(4 Suppl 3):20222. doi: 10.7448/IAS.18.4.20222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367(5):399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thigpen MC, Kebaabetswe PM, Paxton LA, Smith DK, Rose CE, Segolodi TM, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367(5):423–34. doi: 10.1056/NEJMoa1110711. [DOI] [PubMed] [Google Scholar]
- 9■.Baeten JM, Palanee-Phillips T, Brown ER, Schwartz K, Soto-Torres LE, Govender V, et al. Use of a vaginal ring containing dapivirine for HIV-1 prevention in women. N Engl J Med. 2016;375:2121–2132. doi: 10.1056/NEJMoa1506110. This article presents the primary analysis from the ASPIRE phase 3, randomized, double-blind, placebo-controlled trial of the monthly dapivirine ring. The study demonstrated that the dapivirine ring can prevent HIV acquisition, particularly for women over 21 years of age. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10■.Nel A, van Niekerk N, Kapiga S, Bekker L-G, Gama C, Gill K, et al. Safety and efficacy of a dapivirine vaginal ring for HIV prevention in women. N Engl J Med. 2016;375(22):2133–43. doi: 10.1056/NEJMoa1602046. This article presents the primary analysis from the phase 3, randomized, double-blind, placebo-controlled trial of the monthly dapivirine ring in South Africa and Uganda. The study demonstrated that the dapivirine ring can prevent HIV acquisition and did not find a difference in efficacy by age group. [DOI] [PubMed] [Google Scholar]
- 11■.Schlesinger E, Johengen D, Luecke E, Rothrock G, McGowan I, van der Straten A, et al. A tunable, biodegradable, thin-film polymer device as a long-acting implant delivering tenofovir alafenamide fumarate for HIV pre-exposure prophylaxis. Pharm Res. 2016;33(7):1649–56. doi: 10.1007/s11095-016-1904-6. This article describes the use of a thin-film polymer device (TFPD) as a biodegradable implant for PrEP. The device demonstrated linear drug release for up to 60 or 90 days, depending on desired drug quantity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akil A, Agashe H, Dezzutti CS, Moncla BJ, Hillier SL, Devlin B, et al. Formulation and characterization of polymeric films containing combinations of antiretrovirals (ARVs) for HIV prevention. Pharm Res. 2015;32(2):458–68. doi: 10.1007/s11095-014-1474-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13■■.Klatt NR, Cheu R, Birse K, Zevin AS, Perner M, Noel-Romas L, et al. Vaginal bacteria modify HIV tenofovir microbicide efficacy in African women. Science. 2017;356:938–45. doi: 10.1126/science.aai9383. This article presents findings on associations between vaginal microbiota and tenofovir gel microbicide efficacy in the CAPRISA-004 trial. Gel-based PrEP was not efficacious in women with non-Lactobacillus dominant microbiota, potentially because Gardnerella vaginalis present in these women could metabolize tenofovir. [DOI] [PubMed] [Google Scholar]
- 14.Atashili J, Poole C, Ndumbe PM, Adimora AA, Smith JS. Bacterial vaginosis and HIV acquisition: a meta-analysis of published studies. AIDS. 2008;22(12):1493–501. doi: 10.1097/QAD.0b013e3283021a37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Passmore J-AS, Jaspan HB, Masson L. Genital inflammation, immune activation and risk of sexual HIV acquisition. Curr Opin HIV AIDS. 2016;11(2):156–62. doi: 10.1097/COH.0000000000000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16■.Masese L, Baeten JM, Richardson BA, Bukusi E, John-Stewart G, Graham SM, et al. Changes in the contribution of genital tract infections to HIV acquisition among Kenyan high-risk women from 1993 to 2012. AIDS. 2015;29(9):1077–85. doi: 10.1097/QAD.0000000000000646. This article presents findings on associations between genital tract infections and HIV incidence among women in Kenya during a 20-year follow-up period. The estimated PAR% for bacterial vaginosis and intermediate microbiota was high during the follow-up period. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McClelland RS. CROI. Vol. 2017. Seattle, WA: 2017. Vaginal Microbiome and susceptibility to HIV. Abstract #54. [Google Scholar]
- 18■.Gosmann C, Anahtar MN, Handley SA, Farcasanu M, Abu-Ali G, Bowman BA, et al. Lactobacillus-deficient cervicovaginal bacterial communities are associated with increased HIV acquisition in young South African women. Immunity. 2017;46(1):29–37. doi: 10.1016/j.immuni.2016.12.013. This article presents findings from a prospective cohort study examining associations between genital inflammation and HIV acquisition among South African women. Having diverse vaginal microbiota (not Lactobacillus-dominant) was associated with elevated HIV risk and increased activated CD4+ T cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19■■.Passmore JS, Williams B. AIDS. Vol. 2016. Durban, South Africa: 2016. Role of vaginal microbiota in genital inflammation and enhancing HIV acquisition in women. Abstract #TUSS0604. In a case-control study with women from the CAPRISA-004 trial, HIV-infected cases were found to have an upregulation in inflammatory cytokines prior to HIV acquisition. Specifically, bacterial vaginosis with an abundance of Prevotella bivia was associated with inflammatory cytokine response and increased HIV risk. [Google Scholar]
- 20.Burgener A, McGowan I, Klatt NR. HIV and mucosal barrier interactions: consequences for transmission and pathogenesis. Curr Opin Immunol. 2015;36:22–30. doi: 10.1016/j.coi.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 21■.Anahtar MN, Byrne EH, Doherty KE, Bowman BA, Yamamoto HS, Soumillon M, et al. Cervicovaginal bacteria are a major modulator of host inflammatory responses in the female genital tract. Immunity. 2015;42(5):965–76. doi: 10.1016/j.immuni.2015.04.019. In a prospective cohort study with 146 HIV-negative South African women, high diversity of vaginal microbiota was correlated with genital pro-inflammatory cytokine response. In addition, there were significantly more CCR5+ CD4+ T cells found in the endocervical canal of women with higher cytokine responses (which tended to also be those with the most diverse vaginal flora) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22■.Dimitrov DT, Mâsse BR, Donnell D. PrEP adherence patterns strongly affect individual HIV risk and observed efficacy in randomized clinical trials. J Acquir Immune Defic Syndr. 2016;72(4):444–51. doi: 10.1097/QAI.0000000000000993. This simulation study presents findings on expected PrEP efficacy associated with different levels of oral pill adherence. Pill-taking patterns were found to have a large effect on estimated PrEP efficacy, after comparing model findings with results from PrEP randomized controlled trials. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23■.Marrazzo JM, Ramjee G, Richardson BA, Gomez K, Mgodi N, Nair G, et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2015;372(6):509–18. doi: 10.1056/NEJMoa1402269. This article presents the primary analysis from the VOICE randomized, placebo-controlled trial of oral tenofovir disoproxil fumarate, oral tenofovir-emtricitabine, or 1% tenofovir gel as HIV PrEP. The study did not find oral or gel-based regimens to be efficacious in preventing HIV; however, adherence to the daily regimens was low. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Damme L, Corneli A, Ahmed K, Agot K, Lombaard J, Kapiga S, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012;367(5):411–22. doi: 10.1056/NEJMoa1202614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanscom B, Janes HE, Guarino PD, Huang Y, Brown ER, Chen YQ, et al. Preventing HIV-1 infection in women using oral pre-exposure prophylaxis: a meta-analysis of current evidence. J Acquir Immune Defic Syndr. 2016;73(5):606–8. doi: 10.1097/QAI.0000000000001160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Celum C, Baeten JM. Tenofovir-based pre-exposure prophylaxis for HIV prevention: evolving evidence. Curr Opin Infect Dis. 2012;25(1):51–7. doi: 10.1097/QCO.0b013e32834ef5ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abdool Karim Q, Abdool Karim SS, Frohlich JA, Grobler AC, Baxter C, Mansoor LE, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329(5996):1168–74. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28■.Rees H, Delany-Moretlwe S, Lombard C, Baron D, Panchia R, Myer L. CROI. Vol. 2015. Seattle, WA: 2015. FACTS 001 phase III trial of pericoital tenofovir 1% gel for HIV prevention in women. Abstract #26LB. In the FACTS 001, phase III, multi-center, double-blind, randomized, placebo-controlled trial, 1% tenofovir gel was not found to be efficacious for HIV prevention among South African women. While the gel effectiveness was highest in women who reported product use, overall adherence was low in this cohort. [Google Scholar]
- 29.Friend DR, Kiser PF. Assessment of topical microbicides to prevent HIV-1 transmission: concepts, testing, lessons learned. Antiviral Res. 2013;99(3):391–400. doi: 10.1016/j.antiviral.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 30.Montgomery ET, Mensch B, Musara P, Hartmann M, Woeber K, Etima J, et al. Misreporting of product adherence in the MTN-003/VOICE trial for HIV prevention in Africa: participants’ explanations for dishonesty. AIDS Behav. 2017;21(2):481–91. doi: 10.1007/s10461-016-1609-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31■■.Heffron RA. CROI. Vol. 2017. Seattle, WA: 2017. Oral PrEP is efficacious for HIV prevention among women with abnormal vaginal microbiota. Abstract #85. This work examines the associations between vaginal microbiota and oral PrEP efficacy in the Partners PrEP Study. Oral PrEP efficacy was not significantly different among women with bacterial vaginosis and intermediate microbiota, compared with women who had normal microbiota. [Google Scholar]
- 32.Hendrix CW, Chen BA, Guddera V, Hoesley C, Justman J, Nakabiito C, et al. MTN-001: randomized pharmacokinetic cross-over study comparing tenofovir vaginal gel and oral tablets in vaginal tissue and other compartments. PloS One. 2013;8(1):e55013. doi: 10.1371/journal.pone.0055013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33■■.Hillier SL, Meyn L, Bunge K, Austin M, Moncla BJ, Dezzutti C, et al. CROI. Vol. 2017. Seattle, WA: 2017. Impact of vaginal microbiota on genital tissue and plasma concentrations of tenofovir. Abstract #86LB. In the FAME-04 study of tenofovir gel and film, women with higher concentrations of Gardnerella vaginalis were found to have lower tenofovir drug levels in cervicovaginal fluid and plasma after 7 days. This study highlights the potential for Gardnerella vaginalis to rapidly metabolize PrEP. [Google Scholar]
- 34■■.Zevin AS, Xie IY, Birse K, Arnold K, Romas L, Westmacott G, et al. Microbiome composition and function drives wound-healing impairment in the female genital tract. PLoS Pathog. 2016;12(9):e1005889. doi: 10.1371/journal.ppat.1005889. This article presents findings on epithelial barrier integrity and immune activation among women with Gardnerella vaginalis dominant vaginal microbiota, compared with women with Lactobacillus dominant microbiota. Women with Gardnerella vaginalis dominant microbiota had increased abundance of membrane transport proteins which could influence drug metabolism. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cottrell ML, Srinivas N, Kashuba ADM. Pharmacokinetics of antiretrovirals in mucosal tissue. Expert Opin Drug Metab Toxicol. 2015 Jun;11(6):893–905. doi: 10.1517/17425255.2015.1027682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36■.Kashuba ADM, Gengiah TN, Werner L, Yang K-H, White NR, Karim QA, et al. Genital tenofovir concentrations correlate with protection against HIV infection in the CAPRISA 004 trial: importance of adherence for microbicide effectiveness. J Acquir Immune Defic Syndr. 2015;69(3):264–9. doi: 10.1097/QAI.0000000000000607. This article presents findings from a case-control study with the CAPRISA-004 trial cohort. Significantly fewer HIV-infected cases were found to have cervicovaginal fluid tenofovir concentrations >100 ng/mL compared with uninfected controls. Plasma concentrations were <1 ng/mL in all women receiving the 1% tenofovir gel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mayer KH, Maslankowski LA, Gai F, El-Sadr WM, Justman J, Kwiecien A, et al. Safety and tolerability of tenofovir vaginal gel in abstinent and sexually active HIV-infected and uninfected women. AIDS. 2006;20(4):543–51. doi: 10.1097/01.aids.0000210608.70762.c3. [DOI] [PubMed] [Google Scholar]
- 38.Patterson KB, Prince HA, Kraft E, Jenkins AJ, Shaheen NJ, Rooney JF, et al. Penetration of tenofovir and emtricitabine in mucosal tissues: implications for prevention of HIV-1 transmission. Sci Transl Med. 2011;3(112):112re4. doi: 10.1126/scitranslmed.3003174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39■.Cottrell ML, Yang KH, Prince HMA, Sykes C, White N, Malone S, et al. A translational pharmacology approach to predicting outcomes of preexposure prophylaxis against HIV in men and women using tenofovir disoproxil fumarate with or without emtricitabine. J Infect Dis. 2016;214(1):55–64. doi: 10.1093/infdis/jiw077. This study found that tenofovir diphosphate concentrations were approximately ten-fold higher in colorectal tissue than in the lower female genital tract. Results indicate that a minimum adherence level of approximately 6–7 oral PrEP doses per week are required to protected female genital tissue from HIV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40■.Chen BA, Panther L, Marzinke MA, Hendrix CW, Hoesley CJ, van der Straten A, et al. Phase 1 safety, pharmacokinetics, and pharmacodynamics of dapivirine and maraviroc vaginal rings: a double-blind randomized trial. J Acquir Immune Defic Syndr. 2015;70(3):242–9. doi: 10.1097/QAI.0000000000000702. This article presents findings from a multisite, double-blind, randomized, placebo-controlled trial of dapivirine and maraviroc containing vaginal rings for HIV prevention. Dapivirine concentrations in cervicovaginal fluid and tissue dropped rapidly after ring removal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang L, Koppolu S, Chappell C, Moncla BJ, Hillier SL, Mahal LK. Studying the effects of reproductive hormones and bacterial vaginosis on the glycome of lavage samples from the cervicovaginal cavity. PloS One. 2015;10(5):e0127021. doi: 10.1371/journal.pone.0127021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ravel J, Gajer P, Fu L, Mauck CK, Koenig SSK, Sakamoto J, et al. Twice-daily application of HIV microbicides alter the vaginal microbiota. mBio. 2012;3(6):e00370–12. doi: 10.1128/mBio.00370-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spence P, Bhatia Garg A, Woodsong C, Devin B, Rosenberg Z. Recent work on vaginal rings containing antiviral agents for HIV prevention. Curr Opin HIV AIDS. 2015;10(4):264–70. doi: 10.1097/COH.0000000000000157. [DOI] [PubMed] [Google Scholar]
- 44.Boffito M, Jackson A, Owen A, Becker S. New approaches to antiretroviral drug delivery: challenges and opportunities associated with the use of long-acting injectable agents. Drugs. 2014;74(1):7–13. doi: 10.1007/s40265-013-0163-7. [DOI] [PubMed] [Google Scholar]
- 45.Geary CW, Bukusi EA. Women and ARV-based HIV prevention - challenges and opportunities. J Int AIDS Soc. 2014;17(3 Suppl 2):19356. doi: 10.7448/IAS.17.3.19356. [DOI] [PMC free article] [PubMed] [Google Scholar]


