Abstract
Purpose
To determine whether bladder neck size is associated with incontinence scores after robot-assisted laparoscopic radical prostatectomy (RALP).
Materials and Methods
Consecutive eligible patients undergoing RALP between July 19 and December 28, 2016, were enrolled into a prospective, longitudinal, observational cohort study. The primary outcome was patient-reported urinary incontinence on the Expanded Prostate Cancer Index Composite (EPIC) scale 6 and 12 weeks post surgery. The relationship between EPIC score for urinary incontinence and bladder neck size was evaluated using multiple regression. Predicted EPIC scores for incontinence were displayed graphically after using restricted cubic splines to model bladder neck size.
Results
In all, 107 patients were enrolled; response rates were 98% and 87% at 6 and 12 weeks, respectively. At 6 and 12 weeks, bladder neck size was not significantly associated with incontinence scores. Comparing the 90th percentile for bladder neck size (18 mm) with the 10th percentile (7 mm) revealed no significant difference in the adjusted EPIC scores for incontinence at 6 weeks (β coefficient, 0.88; 95% CI, −10.92–12.68; P=.88) and at 12 weeks (5.80; 95% CI, −7.36–18.97; P=.39).
Conclusions
These findings question the merit of creating an extremely small bladder neck during RALP. We contend that doing so increases the risk of positive margins at the bladder neck without facilitating early recovery of continence.
Keywords: bladder neck size, patient-reported function, prostate cancer
Introduction
Since the introduction of the anatomic approach to radical prostatectomy (RP) (1), permanent urinary incontinence after RP has become uncommon (2). As a result, increasing focus has been placed on techniques, such as bladder neck preservation (BNP), that may accelerate the recovery of continence after RP (3,4). The underlying hypothesis behind BNP is that the bladder neck conveys passive outlet resistance that may expedite recovery of continence while the external sphincter is still healing (3–5).
Although many studies have demonstrated a beneficial effect of BNP on early continence after RP, including a randomized surgical trial, none have included an objective measure of the quality or extent of BNP (6–10). Similar to the nerve-sparing technique, the quality and extent of BNP undoubtedly vary greatly from patient to patient and are dependent on many factors. The extent of BNP is important to consider because aggressive BNP may also be associated with higher positive margin rates, especially in T3 tumors (11–13). While bladder neck size is not a perfect surrogate for BNP, a study assessing the relationship between measured bladder neck size and postsurgical continence scores would nonetheless inform the necessary extent of BNP during RP.
In this context, we designed a prospective, longitudinal, observational cohort study to test the hypothesis that smaller bladder neck size is associated with improved early continence scores after robot-assisted laparoscopic radical prostatectomy (RALP). If found, a strong relationship between bladder neck size and continence scores would possibly endorse aggressive BNP, provided that the oncologic principles of the operation are not violated. However, if no clear relationship exists, it would suggest that aggressive BNP only increases the risk of positive margins at the bladder neck without benefit for continence.
Materials and Methods
Study Population
Consecutive eligible patients undergoing RALP performed by one surgeon (J.A.S.) at Vanderbilt University Medical Center between July 19, 2016, and December 28, 2016 were enrolled into a prospective, longitudinal, observational cohort study. Patients were excluded if they had previously undergone transurethral resection of the prostate (or equivalent bladder outlet procedure), had previously received radiotherapy for prostate cancer, had any incontinence at baseline, or had a neurogenic bladder. The study was approved by the Vanderbilt University Institutional Review Board (IRB#170615).
Primary Outcome, Primary Exposure, and Covariates
The primary outcome was patient-reported urinary incontinence as measured by the urinary incontinence subscale of the Expanded Prostate Cancer Index Composite (EPIC) questionnaire (14). The surveys were administered 6 and 12 weeks post surgery by telephone by one investigator (M.D.T.) who was blinded to the patients’ clinical information. Patients were called once a day for 5 consecutive days until a survey response was recorded. The EPIC survey instrument was chosen because it has been psychometrically validated for assessing patient-reported urinary function after RP (14). It contains 4 individual items (Supplemental Table 1) that are summarized into one composite numeric score (range, 0–100, with higher scores indicating better function).
Bladder neck size was measured intraoperatively immediately before the vesicourethral anastomosis by inserting a ruler through an assistant port and measuring the largest diameter of the bladder neck in millimeters. Other covariates thought to influence incontinence after surgery were also collected, which included age, body mass index (BMI), preoperative American Urological Association (AUA) symptom score, prostate size as measured by final pathologic analysis, extent of nerve sparing, urethral suspension, and D’Amico risk criteria (15). Urethral suspension was performed for all patients by tacking the dorsal venous complex stitch to the pubic symphysis, as previously described (16). However, this stitch is often cut out during the apical dissection, depending the configuration of the prostate, which makes it a potential confounder.
Sample Size Calculation
The proportion of variation in incontinence that can be explained by bladder neck size is unknown. Therefore, we used established thresholds for effect sizes proposed by Cohen (17) to test the hypothesis that bladder neck size accounts for additional variation in incontinence not accounted for by age, BMI, preoperative AUA symptom score, prostate size, nerve-sparing approach, urethral suspension, and disease risk. Using this convention, a sample size of 107 patients achieves 90% power to detect an R2 of 0.05 (small to moderate effect) (17) attributed to bladder neck size using an F test with a significance level of .05. This is adjusted for the 7 additional covariates listed above with an R2 of 0.50. The power calculation was performed using the powerreg command in Stata 14.1 (StataCorp LLC).
Statistical Analysis
Continuous variables were summarized as median (interquartile range); categorical variables were summarized as count (percentage). The relationship between EPIC domain score for urinary incontinence and bladder neck size was evaluated using multiple regression adjusting for age, BMI, preoperative AUA symptom score, prostate size, nerve sparing, urethral suspension, and disease risk. Because no previous study has evaluated the relationship between bladder neck size and EPIC urinary incontinence scores, we planned to model the relationship nonlinearly using restricted cubic splines with 3 knots (10th, 50th, and 90th percentiles) (18). The predicted EPIC domain scores from the fully adjusted model were graphically displayed after setting all nonfactor variables at the mean and all factor variables at the mode. All statistical analysis was performed using Stata 14.1 (19).
Missing Data
Because a person’s decision to answer (or not answer) a call from an unidentified telephone number was viewed to be unrelated to any item in the study, including the outcome, we considered any missing data to be missing at random. To reduce potential bias from an arbitrary missing-value pattern, we fit the models after multiple imputation by chained equations using 20 imputed data sets. The following variables were used to impute missing values for the primary outcome: bladder neck size, BMI, preoperative AUA symptom score, prostate size, age, use of a urethral suspension stitch, nerve sparing, and D’Amico risk criteria.
Results
We enrolled 107 patients in the study. Baseline clinical and demographic characteristics of the cohort are summarized in Table 1. The median bladder neck size was 13 mm (interquartile range, 10–16 mm). Data were missing for 2 patients at 6 weeks and 14 patients at 12 weeks; thus, response rates were 98% and 87% at 6 and 12 weeks, respectively.
Table 1.
Baseline Clinical and Demographic Characteristics
| Characteristic | Value (N=107)a |
|---|---|
| Age, y | 63.0 (56.0–69.0) |
| Body mass index, kg/m2 | 28.4 (25.2–31.1) |
| Prostate weight, g | 50.2 (40.7–63.4) |
| Bladder neck size, mm | 13.0 (10.0–16.0) |
| AUA symptom score | 8.0 (4.0–15.0) |
| Nerve-sparing approach | |
| None | 54 (50.5) |
| Bilateral | 44 (41.1) |
| Unilateral | 9 (8.4) |
| Urethral suspension stitch used | 51 (47.7) |
| D’Amico risk | |
| High | 19 (17.8) |
| Intermediate | 60 (56.1) |
| Low | 28 (26.2) |
Abbreviation: AUA, American Urological Association.
Values are median (interquartile range) or No. of patients (%).
Incontinence Scores at 6 Weeks
At 6 weeks, bladder neck size was not significantly associated with incontinence scores in the imputed multiple regression model. Comparing the 90th percentile for bladder neck size (18 mm) with the 10th percentile (7 mm) revealed no significant difference in the adjusted EPIC scores for incontinence (β coefficient, 0.88; 95% CI, −10.92–12.68; P=.88) (Table 2). The β coefficient can be interpreted as the predicted difference in EPIC score at 6 weeks between patients with a bladder neck size of 18 mm compared to 7 mm. Graphical display of predicted EPIC domain scores from the fully adjusted model also shows no significant trend (Figure 1).
Table 2.
The Effect of Bladder Neck Size on EPIC Urinary Incontinence Scores at 6 and 12 Weeks
| Variable | Time Post Surgerya
|
|
|---|---|---|
| 6 Weeks | 12 Weeks | |
| Bladder neck sizeb | 0.88 (−10.91–12.68) | 5.80 (−7.63–18.97) |
| BMI | −0.84 (−1.79–0.12) | −0.99 (−2.10–0.12) |
| Preop AUA SS | −1.00c (−1.62–−0.39) | −0.95c (−1.65–−0.26) |
| Prostate volume | 0.17 (−0.06−0.41) | −0.03 (−0.29−0.22) |
| Age | −0.64 (−1.32–0.04) | −1.00d (−1.78−−0.22) |
| No urethral suspension stitch | 4.77 (−4.08–13.62) | 5.79 (−4.17–15.76) |
| Nerve sparing | ||
| None | Ref | Ref |
| Bilateral | 9.28 (−0.86–19.43) | 8.82 (−2.70–20.34) |
| Unilateral | 8.06 (−8.54–24.67) | 2.36 (−14.86–19.57) |
| D’Amico risk criteria | ||
| High | Ref | Ref |
| Intermediate | 3.51 (−8.46–15.48) | −1.18 (−15.25–12.89) |
| Low | 7.50 (−6.51–21.52) | −0.42 (−16.66–15.82) |
| R2 | 0.24 | 0.28 |
Abbreviations: AUA SS, American Urological Association symptom score; BMI, body mass index; EPIC, Expanded Prostate Cancer Index Composite; Ref, reference.
Values are β coefficient (95% CI).
Comparing the 90th percentile with the 10th percentile (18 mm vs 7 mm).
P<.01.
P<.05.
Figure 1.
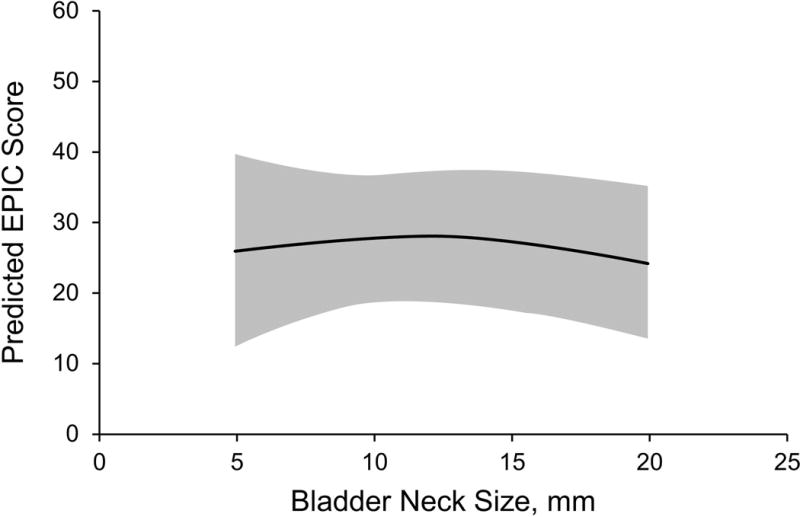
Incontinence Domain at 6 Weeks. Graph shows predicted Expanded Prostate Cancer Index Composite (EPIC) incontinence domain score (95% CI) by bladder neck size.
Incontinence Scores at 12 Weeks
At 12 weeks, bladder neck size was not significantly associated with incontinence scores in the imputed multiple regression model. Comparing the 90th and 10th percentiles for bladder neck size revealed no significant difference in the adjusted EPIC scores for incontinence at 12 weeks (β coefficient, 5.80; 95% CI, −7.36–18.97; P=.39) (Table 2). The β coefficient can be interpreted as the predicted difference in EPIC score at 12 weeks between patients with a bladder neck size of 18 mm compared to 7 mm. Graphical display of predicted EPIC domain scores from the fully adjusted model also shows no significant trend (Figure 2).
Figure 2.
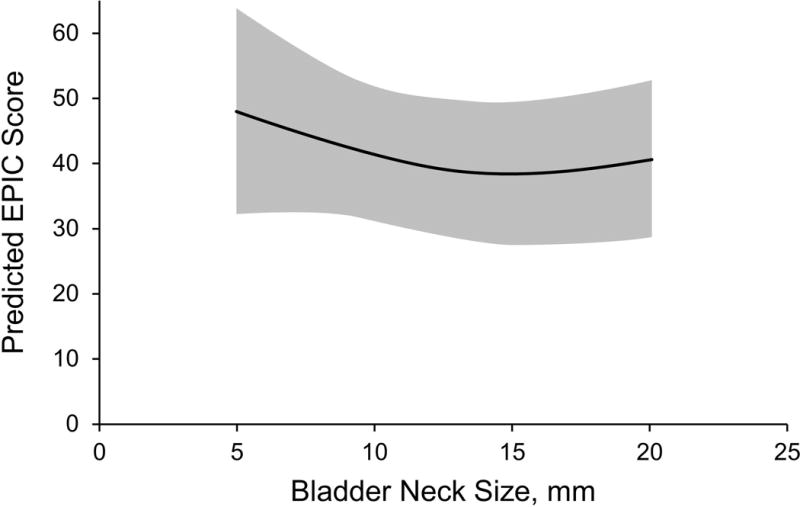
Incontinence Domain at 12 Weeks. Graph shows predicted Expanded Prostate Cancer Index Composite (EPIC) incontinence domain score (95% CI) by bladder neck size.
Bladder Neck Margins
Three patients had a positive margin at the bladder neck (Supplemental Table 2). All 3 patients had a dominant tumor at the base with nonfocal extraprostatic extension. The bladder neck sizes for these 3 patients measured 7 mm, 5 mm, and 15 mm. No bladder neck contractures were noted.
Discussion
The principal finding of this study is that incontinence scores do not vary substantially across a range of bladder neck sizes after BNP. In using a continuous measure for both the predictor and the outcome, we applied a new strategy to uncover any subtle but potentially clinically important effects of varying degrees of BNP. Given the null association in this study, we conclude that efforts to aggressively spare the bladder neck do not substantially improve early continence outcomes after RALP and may put the patient at increased risk for positive margins at the bladder neck.
We would like to emphasize that we are not suggesting that BNP has no effect on incontinence after RP. In fact, enough supportive data have been reported in the open RP and RALP literature to support a causal link between BNP and improved early continence after surgery. In the only completed randomized trial to date, Nyarangi-Dix et al (8) studied 208 men who were randomly assigned to BNP versus bladder neck resection. In the intention-to-treat analysis, BNP was clearly associated with improved continence at 3 and 6 months, findings that are also reproducible in many other analyses (6,7,9). However, our study addresses a different question: namely, the effect of bladder neck size on continence outcomes across a range of bladder neck sizes that would be considered BNP. Before the current study, it was unknown whether efforts to aggressively spare the bladder neck offered any benefit for early recovery of continence, but the data in the current study do not appear to support such a benefit.
The current study strengthens the literature in several key respects. First, it provides an objective measure of the extent of BNP. Although BNP involves more than merely bladder neck size, size is a reasonable surrogate measure for the quality and extent of BNP. Second, it used a psychometrically validated survey instrument to measure postoperative continence after surgery. Prior studies have used a “pads vs no pads per day” definition of incontinence, which may be inadequate for measuring problematic leakage after surgery (6,7,9,10,12,20,21). Third, whereas most previous studies were retrospective (6,7,10,20,22,23), our study had a prospective design with a predefined hypothesis, an objectively measured exposure, a standardized outcome, and a high survey response rate.
Despite these strengths, this study has a few limitations. First, this is a single-surgeon cohort study, which may limit its generalizability. This design, however, was preferable to a multisurgeon or multicenter study in which the relationship between bladder neck size and continence could be confounded by variations in surgical technique. It is worth noting that although trainees participated in the surgical care of these patients, it was done so under the close supervision of the senior author (J.A.S.), which minimized the potential confounding effect of surgical technique on the null association. Second, although the study is prospective and longitudinal, the data are not randomized, which may lead to bias when comparing predicted continence scores over a range of bladder neck sizes. However, a clinical trial may not be feasible given the risk of assigning patients to a larger bladder neck (with the need for reconstructive anastomotic techniques and the greater risk of postoperative urine leak) (6). Third, although the model controls for major predictors of urinary incontinence after surgery, it is possible that residual unmeasured confounding exists. Longer membranous urethral length, for example, has been associated with improved continence outcomes after RALP (24). We did not routinely perform magnetic resonance imaging for all patients and, therefore, we cannot assess the extent to which this may have affected the results. However, because this is a single-surgeon cohort study, membranous urethral length is unlikely to vary significantly and is even more unlikely to vary with bladder neck size, which makes it an unlikely confounder. Fourth, thresholds of continence and incontinence in EPIC domain scores are not firmly established. However, the continuous nature of the instrument was a useful property in the analysis. Last, bladder neck size may not necessarily be a perfect surrogate for the extent of BNP, particularly among inexperienced surgeons. Given the experience of the senior author, however, we expect little variability in bladder neck thickness, which makes it also an unlikely confounder.
Despite these limitations, we believe these findings have important implications for surgeons performing RALP. Whereas BNP undoubtedly improves early continence compared with bladder neck resection after RALP, the current study questions whether efforts to make an extremely small bladder neck have a measurable benefit on early continence outcomes after RALP.
Conclusion
Early incontinence scores after RALP do not vary substantially by bladder neck size. This suggests that efforts to make an extremely small bladder neck during RALP may only increase the risk of positive surgical margins without any measureable improvements in continence outcomes.
Supplementary Material
Acknowledgments
Funding: This work was supported by the National Cancer Institute at the National Institutes of Health (5T32CA106183 to M.D.T.).
Abbreviations
- AUA
American Urological Association
- BMI
body mass index
- BNP
bladder neck preservation
- EPIC
Expanded Prostate Cancer Index Composite
- RALP
robot-assisted laparoscopic radical prostatectomy
- RP
radical prostatectomy
Footnotes
Conflict of interest: None.
Publisher: To expedite proof approval, send proof via email to scipubs@mayo.edu.
References
- 1.Walsh PC. Anatomic radical prostatectomy: evolution of the surgical technique. J Urol. 1998 Dec;160(6 Pt 2):2418–24. doi: 10.1097/00005392-199812020-00010. [DOI] [PubMed] [Google Scholar]
- 2.Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008 Mar 20;358(12):1250–61. doi: 10.1056/NEJMoa074311. [DOI] [PubMed] [Google Scholar]
- 3.Gomez CA, Soloway MS, Civantos F, et al. Bladder neck preservation and its impact on positive surgical margins during radical prostatectomy. Urology. 1993 Dec;42(6):689–93. doi: 10.1016/0090-4295(93)90534-h. [DOI] [PubMed] [Google Scholar]
- 4.Latiff A. Preservation of bladder neck fibers in radical prostatectomy. Urology. 1993 Jun;41(6):566–7. doi: 10.1016/0090-4295(93)90106-k. [DOI] [PubMed] [Google Scholar]
- 5.Malizia AA, Banks DW, Newton NE, et al. Modified radical retropubic prostatectomy: double continence technique [abstract] J Urol. 1989;141:316A. [Google Scholar]
- 6.Friedlander DF, Alemozaffar M, Hevelone ND, et al. Stepwise description and outcomes of bladder neck sparing during robot-assisted laparoscopic radical prostatectomy. J Urol. 2012 Nov;188(5):1754–60. doi: 10.1016/j.juro.2012.07.045. Epub 2012 Sep 19. [DOI] [PubMed] [Google Scholar]
- 7.Deliveliotis C, Protogerou V, Alargof E, et al. Radical prostatectomy: bladder neck preservation and puboprostatic ligament sparing: effects on continence and positive margins. Urology. 2002 Nov;60(5):855–8. doi: 10.1016/s0090-4295(02)01956-8. [DOI] [PubMed] [Google Scholar]
- 8.Nyarangi-Dix JN, Radtke JP, Hadaschik B, et al. Impact of complete bladder neck preservation on urinary continence, quality of life and surgical margins after radical prostatectomy: a randomized, controlled, single blind trial. J Urol. 2013 Mar;189(3):891–8. doi: 10.1016/j.juro.2012.09.082. Epub 2012 Sep 24. [DOI] [PubMed] [Google Scholar]
- 9.Lowe BA. Comparison of bladder neck preservation to bladder neck resection in maintaining postrostatectomy urinary continence. Urology. 1996 Dec;48(6):889–93. doi: 10.1016/s0090-4295(96)00324-x. [DOI] [PubMed] [Google Scholar]
- 10.Gu X, Araki M, Wong C. Continence outcomes after bladder neck preservation during robot-assisted laparoscopic prostatectomy (RALP) Minim Invasive Ther Allied Technol. 2015;24(6):364–71. doi: 10.3109/13645706.2015.1027711. Epub 2015 Mar 22. [DOI] [PubMed] [Google Scholar]
- 11.Marcovich R, Wojno KJ, Wei JT, et al. Bladder neck-sparing modification of radical prostatectomy adversely affects surgical margins in pathologic T3a prostate cancer. Urology. 2000 Jun;55(6):904–8. doi: 10.1016/s0090-4295(00)00451-9. [DOI] [PubMed] [Google Scholar]
- 12.Srougi M, Nesrallah LJ, Kauffmann JR, et al. Urinary continence and pathological outcome after bladder neck preservation during radical retropubic prostatectomy: a randomized prospective trial. J Urol. 2001 Mar;165(3):815–8. [PubMed] [Google Scholar]
- 13.Katz R, Salomon L, Hoznek A, et al. Positive surgical margins in laparoscopic radical prostatectomy: the impact of apical dissection, bladder neck remodeling and nerve preservation. J Urol. 2003 Jun;169(6):2049–52. doi: 10.1097/01.ju.0000065822.15012.b7. [DOI] [PubMed] [Google Scholar]
- 14.Wei JT, Dunn RL, Litwin MS, et al. Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology. 2000 Dec 20;56(6):899–905. doi: 10.1016/s0090-4295(00)00858-x. [DOI] [PubMed] [Google Scholar]
- 15.D’Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998 Sep 16;280(11):969–74. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 16.Patel VR, Coelho RF, Palmer KJ, et al. Periurethral suspension stitch during robot-assisted laparoscopic radical prostatectomy: description of the technique and continence outcomes. Eur Urol. 2009 Sep;56(3):472–8. doi: 10.1016/j.eururo.2009.06.007. Epub 2009 Jun 16. [DOI] [PubMed] [Google Scholar]
- 17.Cohen J. Statistical power analysis for the behavioral sciences. 2nd. Hillsdale (NJ): L. Erlbaum Associates; c1988. p. 567. [Google Scholar]
- 18.Harrell FE., Jr . Regression modeling strategies: with applications to linear models, logistic regression, and survival analysis. New York (NY): Springer; c2001. p. 568. [Google Scholar]
- 19.StataCorp. Stata Statistical Software: Release 14. College Station, (TX): StataCorp LP; c2015. [Google Scholar]
- 20.Poon M, Ruckle H, Bamshad BR, et al. Radical retropubic prostatectomy: bladder neck preservation versus reconstruction. J Urol. 2000 Jan;163(1):194–8. doi: 10.1016/s0022-5347(05)68003-2. [DOI] [PubMed] [Google Scholar]
- 21.Krupski TL, Saigal CS, Litwin MS. Variation in continence and potency by definition. J Urol. 2003 Oct;170(4 Pt 1):1291–4. doi: 10.1097/01.ju.0000085341.63407.46. [DOI] [PubMed] [Google Scholar]
- 22.Choi WW, Freire MP, Soukup JR, et al. Nerve-sparing technique and urinary control after robot-assisted laparoscopic prostatectomy. World J Urol. 2011 Feb;29(1):21–7. doi: 10.1007/s00345-010-0601-z. Epub 2010 Oct 20. [DOI] [PubMed] [Google Scholar]
- 23.Shelfo SW, Obek C, Soloway MS. Update on bladder neck preservation during radical retropubic prostatectomy: impact on pathologic outcome, anastomotic strictures, and continence. Urology. 1998 Jan;51(1):73–8. doi: 10.1016/s0090-4295(97)00463-9. [DOI] [PubMed] [Google Scholar]
- 24.Mungovan SF, Sandhu JS, Akin O, et al. Preoperative membranous urethral length measurement and continence recovery following radical prostatectomy: a systematic review and meta-analysis. Eur Urol. 2017 Mar;71(3):368–378. doi: 10.1016/j.eururo.2016.06.023. Epub 2016 Jul 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


