Abstract
Background
Older adults after hip fracture are at increased risk of being prescribed potentially inappropriate medications (PIM), and may be particularly vulnerable to their adverse effects.
Objective
To examine the association of PIM use with time to full functional recovery within one year of hip fracture repair.
Design
Secondary analysis of a prospective longitudinal study.
Setting
Eight St. Louis, Missouri hospitals.
Participants
Older adults (n = 477) aged 60 years or older who had surgical repair of a hip fracture free of delirium, dementia, or depression at baseline.
Measurements
Drugs at baseline were categorized using the American Geriatrics Society 2012 Beers criteria. The outcome was the Functional Recovery Scale (FRS) total score measured at four time points during a 12-month period of observation. Cox proportional hazards models examined time to 95% recovery of function (‘full recovery’), adjusting for demographics, cognition, depression, medical comorbidity, pre-fracture functioning, and pain as covariates.
Results
PIM use was common following hip fracture, with 51% of participants prescribed at least one PIM and 17.4% prescribed two or more PIM. PIM use was significantly associated with longer time to achieve full recovery with a hazard ratio (HR) of 0.69 (95% CI: 0.52–0.92; p = 0.012) and this association was stronger for two or more PIM compared to one PIM (HR = 0.60; 95% CI 0.40–0.90; p = 0.014).
Conclusion
PIM use was associated with longer time to full functional recovery in older adults who underwent surgery for a hip fracture, particularly in those using two or more PIM at baseline.
1. Introduction
A hip fracture is a watershed moment for older adults with the potential for significant impact on future disability, loss of independence and risk of mortality [1, 2]. Poor short-term functional recovery is associated with a poor long-term prognosis [3]. At least one-third of patients who suffer a hip fracture do not regain pre-fracture function; those who do recover take an average of six months to do so [4]. The failure to functionally recover threatens the independence of older adults [5]. Achievement of full functional recovery after hip fracture has important implications for the quality of life of older adults [6].
The use of potentially inappropriate medication (PIM) may be a modifiable risk factor for poor recovery after hip fracture. Following hip fracture, older adults are particularly vulnerable to the adverse effects of PIM, with risks of delirium, recurrent falls, repeat hip fracture, and mortality [7–10]. They may also exhibit psychiatric symptoms and distress, and are thus at increased risk of being newly prescribed PIM post-fracture [11]. While patients with hip fracture are both a high-risk population for being prescribed PIM and more likely to suffer adverse outcomes with the use of these drugs, no study has yet examined the impact of PIM use on the achievement of functional recovery after hip fracture.
In this secondary analysis of a prospective longitudinal hip fracture study, we examined the association of PIM with time to full functional recovery after hip fracture. The study excluded patients with delirium, dementia, or major depression at baseline: all of these excluded conditions are strongly associated with poor outcomes and are also associated with the use of PIM. As a result, the study sample represents a population with a good probability of achieving recovery. We hypothesized that the use of PIM would be associated a longer time to achieve full recovery in function after hip fracture.
2. Methods
2.1 Setting and sample
Participants were older adults after hip fracture recruited from eight hospitals in St. Louis, MO between 2008–2012, with full details of recruitment and assessment as previously described [12, 13]. Procedures were approved by the institutional review boards at Washington University School of Medicine in St. Louis, MO, and at the eight participating area hospitals. Participants provided written informed consent prior to undergoing study procedures. All procedures were in compliance with the ethics principles for human experimentation stated in the Declaration of Helsinki.
Inclusion criteria were age 60 years or older and a primary diagnosis of hip fracture with surgical repair. Exclusion criteria were non-ambulatory status prior to the fracture, cognitive impairment at the time of the fracture consistent with a diagnosis of dementia (assessed by chart review and brief bedside assessment), delirium (by observation, chart review and completion of delirium rating scale), major depressive episode at the time of fracture (based on baseline Structured Clinical Interview for Diagnosis and Statistical Manual of Medical Disorders-IV[14]), metastatic cancer, interferon treatment, severe sensory impairment, non-English speaking, and inability to consent or cooperate with study protocol. For this study, we excluded seven individuals who had missing information about medications at baseline; this resulted in 477 participants for statistical analyses.
Analyses in this study are based on variables collected at baseline (within 2–14 days after surgical hip fracture repair) and at weeks 4, 12, 26, and 52 following baseline.
2.2 Measures
Medication use at baseline was recorded from the patient’s chart. PIM was classified according to the American Geriatrics Society 2012 Beers Criteria [15], which were created through literature review and expert consensus. In the current study, PIM was defined using the Beers Criteria’s: i) ‘disease-independent recommendations’ [15] and ii) specific recommendations for older adults with a history of falls or fractures.
Functional recovery was measured with the Functional Recovery Scale score [FRS;16]. Patients were asked to rate how much help they needed with basic and instrumental activities of daily living and mobility on a scale of 0 (cannot do at all) to 4 (no help needed). The total FRS is a score out of 100 (optimal function). At the baseline visit, the patient was asked to rate their immediate pre-fracture function; post-fracture function was measured at weeks 4, 12, 26, and 52. For the purpose of this study, full recovery was defined as achieving 95% of pre-fracture function. This threshold is consistent with previous literature [17], accommodates both the risk of inflation of pre-fracture function due to a retrospective assessment, and an expected decline in function that would normally be observed over a one-year time frame in older adults [16].
Other variables that may influence functional recovery were included as covariates. Severity of depressive symptoms was measured with the Montgomery Asberg Depression Rating Scale at weeks 4, 12, 26, and 52 (MADRS). At the baseline visit, pre-fracture severity of depressive symptoms was rated retrospectively for the week prior to fracture using the MADRS. The Duke Social Support Index (DSSI) instrumental support sub-scale at baseline assessed the amount of help received from a support network with higher scores representing more support. The Cumulative Illness Rating Scale for Geriatrics (CIRS-G) measured cumulative medical burden at the time of the fracture, including conditions that arose during the hospitalization. Cognitive function was assessed at baseline by the Short Blessed Test (SBT), with higher scores indicating more cognitive impairment. Pain at all time points was measured using a numerical rating scale from 0 (no pain) to 10 (worst pain).
2.3 Data analysis
Time-to-event curves were constructed using Kaplan-Meier methods. A Cox proportional hazards model examined time to full recovery during the 12-month period of observation. As a sensitivity analysis, we repeated the survival analysis dividing the cohort by the number of PIM (0, 1, or >1) to which they were exposed. We selected a broad range of variables a priori for inclusion in the model based on variables relevant to exposure to PIM use or functional outcomes after hip fracture. The following variables were included as fixed effect covariates: age, sex, marital status, race, education, pre-fracture FRS, pre-fracture MADRS, baseline SBT score, baseline CIRS-G score, baseline pain, smoking status, drinking status, social support and total number of baseline medications (excluding PIM). Time was subject to interval censoring. Pain and depression were included as time-varying covariates. We used a backwards elimination technique, with variables not contributing to the prediction of time to recovery being sequentially removed from the model. Statistical analyses were completed using R version 3.2.3 (R Foundation for Statistical Computing, Vienna, Austria).
3. Results
3.1 Description of Baseline PIM Use
Fifty one percent of the participant group was prescribed at least one PIM and 17.4% was prescribed two or more PIM at baseline. The most commonly used PIM were sedative/hypnotics (26.4% of participants), antidepressants (19.1%), and medications with anticholinergic properties (15.3%). Table 1 lists the baseline demographic and clinical characteristics of PIM users and non-users.
Table 1.
Baseline characteristics of PIM users compared to PIM non-users.
| PIM user | Non-user | p-Value | |
|---|---|---|---|
| n=233 | n=244 | ||
| Female | 160 (68.7) | 200 (82.0) | 0.001a |
| Age (y) | 78.5 ± 8.4 | 78.4 ± 9.1 | 0.92 |
| Race: | |||
| White/Caucasian | 235 (96.3) | 212 (91.0) | 0.022a |
| Other | 9 (3.7) | 21 (9.0) | |
| Education: | |||
| Elementary school | 16 (6.6) | 14 (6.0) | 0.092a |
| High school | 120 (49.2) | 93 (39.9) | |
| Bachelors degree | 75 (30.7) | 80 (34.3) | |
| Graduate degree | 14 (5.7) | 25 (10.7) | |
| Marital status: | |||
| Married | 86 (35.2) | 94 (40.3) | 0.067a |
| Never married | 11 (4.5) | 21 (9.0) | |
| Separated/Divorced | 26 (10.7) | 26 (11.1) | |
| Widowed | 121 (49.6) | 92 (39.5) | |
| Smoking status: | |||
| Never | 106 (43.4) | 86 (36.9) | 0.325a |
| Past | 109 (44.7) | 118 (50.6) | |
| Current | 28 (11.5) | 29 (12.4) | |
| >7 alcoholic drinks weekly | 81 (33.2) | 91 (39.0) | 0.215a |
| Total number of medications | 6.1 ± 3.4 | 5.0 ± 3.2 | <0.001 |
| CIRS-G | 13.6 ± 3.8 | 11.7 ± 3.3 | <0.001 |
| FRS (pre-fracture) | 94.5 ± 7.8 | 97.1 ± 5.9 | <0.001 |
| Pain score | 3.6 ± 2.9 | 2.9 ± 2.7 | 0.0055 |
| SBT | 4.8 ± 3.4 | 4.3 ± 3.2 | 0.092 |
| MADRS | 3.9 ± 4.8 | 2.6 ± 3.6 | 0.0013 |
| DSSI | 9.9 ± 2.0 | 9.9 ± 2.1 | 0.66 |
PIM: Potentially Inappropriate Medications; CIRS-G: Cumulative Illness Rating Scale for Geriatrics; FRS: Functional Recovery Score; SBT: Short Blessed Test; MADRS: Montgomery Asberg Depression Rating Scale; DSSI: Duke Social Support Index (Instrumental)
Values are mean ± SD or n (%). T-test unless otherwise indicated.
Fischer’s exact test
3.2 Association of Baseline PIM Use with Time to Full Functional Recovery
Overall, 48.6% (95% CI: 44.1–53.2%) of all participants achieved full functional recovery, defined as achieving at least 95% of pre-fracture function. As shown in the Kaplan-Meir time to event curve (Figure 1a), 43.9% (95% CI: 37.6–50.3%) of the PIM user group achieved full functional recovery, compared to 53.7% (95% CI: 47.0–60.2%) of non-PIM users, for a crude hazard ratio of 0.69 (95% CI: 0.53–0.91; p = 0.008).
Figure 1. Kaplan-Meir survival curves demonstrating time to 95% hip fracture recovery a) In PIM users vs non-PIM users and b) By number of PIM.
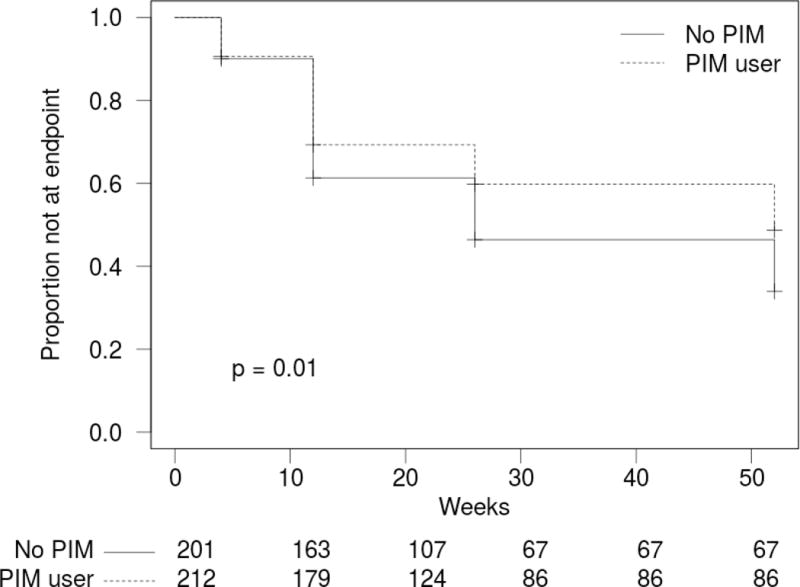
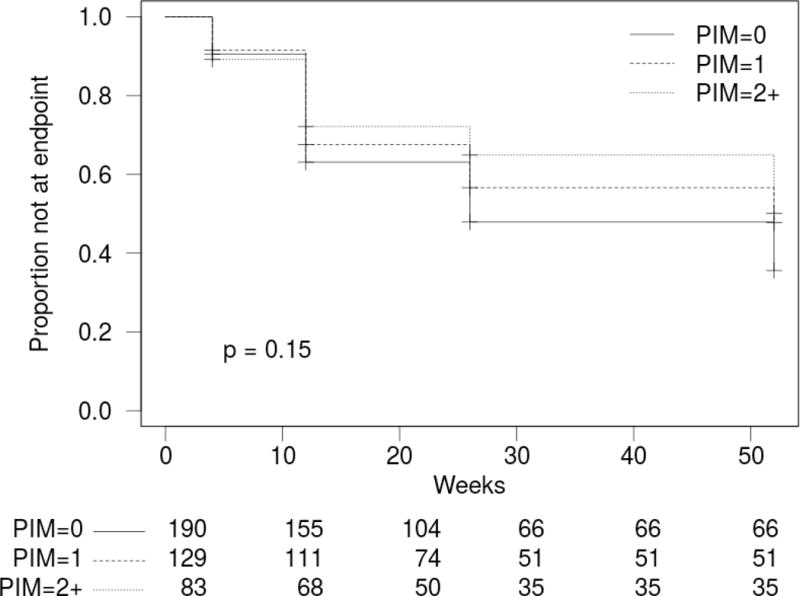
PIM: Potentially Inappropriate Medication
Using Cox proportional hazards modeling, we investigated the effect of PIM use on time to full functional recovery while controlling for age, sex, marital status, race, education, pre-fracture FRS, pre-fracture MADRS, baseline SBT score, baseline CIRS-G score, baseline pain, smoking status, drinking status, social support and total number of baseline medications. After a backwards removal of variables not contributing to the model, age, race, cognition, medical comorbidities, baseline pain and pre-fracture FRS remained along with PIM group membership (Table 2). Use of PIM was associated with significantly longer time to full recovery with an adjusted hazard ratio of 0.69 (95% CI: 0.52–0.92; p = 0.012). In other words, PIM users were 31% (95% CI: 8% to 48%) less likely than non-PIM users to achieve full recovery at any given time within 12 months of hip fracture. When the group was divided by the number of PIM at baseline, two or more PIM (Hazard ratio (HR) = 0.60; 95% CI 0.40–0.90; p = 0.014), but not one PIM (HR = 0.77; 95% CI: 0.55–1.06; p = 0.11), was associated with a statistically significantly longer time to recovery compared to no PIM. In other words, subjects who used two or more PIM were 40% (95% CI: 10% to 60%) less likely to achieve full recovery than those receiving no PIM.
Table 2.
Cox proportional hazards model of time to full recoverya after hip fracture.
| coef | exp(coef) | se(coef) | z | Pr(>|z|) | |
|---|---|---|---|---|---|
| Baseline PIM user | −0.371 | 0.690 | 0.147 | −2.52 | 0.012 |
| Age | −0.047 | 0.954 | 0.008 | −5.67 | 0.000 |
| Race (Other) | −0.755 | 0.470 | 0.310 | −2.43 | 0.015 |
| CIRS-G | −0.056 | 0.946 | 0.022 | −2.59 | 0.010 |
| Baseline pain | −0.092 | 0.913 | 0.028 | −3.32 | 0.001 |
| Pre-fracture FRS | −0.045 | 0.956 | 0.009 | −4.81 | 0.000 |
PIM: Potentially Inappropriate Medication; CIRS-G: Cumulative Illness Rating Scale for Geriatrics; FRS: Functional Recovery Scale.
Full recovery is defined as recovering at least 95% of pre-fracture FRS score
4. Discussion
In this study, we present two important findings: 1) the high prevalence of PIM use after hip fracture and 2) the association between PIM use and time to full functional recovery after hip fracture. The frequency (51%) of PIM use in this sample of patients with hip fracture corresponds with previously reported PIM rates in the United States in older hospitalized patients (58.4%;[19]) and older community populations (42.6%; [20]). Our cohort had more sedative-hypnotic use, but less antidepressant use than a recent cohort of community-dwelling Medicare beneficiaries experiencing a fragility fracture [21].
We found an association between PIM use and increased time to functional recovery after a hip fracture, as hypothesized. This finding was most pronounced in persons taking two or more PIM, and was independent of other variables known to impact on functional recovery. Although PIM use was associated with less chance of achieving full functional recovery by the end of the 12-month observation period, almost half of the study group nevertheless achieved full recovery, reflecting the overall recovery potential of a post-hip fracture group once those with dementia and delirium are excluded.
This is the first study to consider the effect of PIM prescribing on long-term functional outcomes after hip fracture. One study found relationship between anticholinergic use for short-term functional outcomes on an orthogeriatric rehab unit, although the effect was quite small [22]. Prior studies have examined the relationship between medication use and hip fracture mortality. For example, anticholinergic risk score predicts three-month mortality after hip fracture [9]. Another retrospective cohort study found that inappropriate prescribing increased three-year risk of mortality post-fracture by 28%, as measured by the screening tool of older people’s prescriptions (STOPP) and screening tool to alert to right treatment (START), [10]. A recent study identified that fall-related medications and polypharmacy were both associated with mortality after hip fracture [23]. Each of these studies examined an older and much more cognitively impaired cohort than is included in this study.
A unique strength of this study is that the hip fracture sample excluded those individuals at highest risk of poor outcomes (those with dementia, delirium or depression], removing important confounders in the interaction between PIM use and functional recovery. Confounding is a significant risk in retrospective or observational studies of PIM use. PIM use is a marker of medical complexity and psychological distress, and is correlated with polypharmacy, older age, and greater medical burden [24], all of which are associated with worse functional outcomes in older adults. We know that depressive symptoms, cognitive function, and pain have been independently linked to poorer functional outcomes after hip fracture [12, 25–27], but there are few studies of PIM use and functional outcomes that have controlled for these variables [28, 29] as we have done here. Another strength of this study is that the outcome assessments and clinical covariates were collected prospectively and through validated assessment tools, allowing inclusion of a broad number of demographic and clinical variables.
In this study, we demonstrate that, irrespective of dementia, delirium and depression, and independent of other well-known contributors to poor hip fracture outcomes such as depressive symptoms, cognition and pain, PIM use is associated with a longer time to achieve recovery. Our findings suggest that there may be an opportunity to improve long-term recovery after hip fracture through use of prescribing interventions that reduce PIM use. Unfortunately, to date there is little evidence that interventions to reduce PIM prescribing improve functional outcomes [30]. The challenge of effecting change in the prescribing of inappropriate medication was highlighted by a recent study that found that the prevalence of falls-promoting medications does not change post-fracture, with the small number of medication discontinuations balanced by the initiation of falls-promoting medication [21].
A limitation to the interpretation of this study is that PIM use was measured only at baseline, i.e. at the end of the patient’s hospitalization for hip fracture. While we don’t know whether patients continued to be prescribed these medications in the 12 months post-hip fracture, previous studies have found that hospitalization tends to increase the prevalence of medications classed as PIM rather than decrease them [11, 21, 31] and PIM are rarely stopped after a fracture [21]. Studies designed to systematically reduce the use of PIM during hospitalization have shown that intensive intervention is required to effect even a small change in prevalence of PIM use [32]. Thus it is unlikely there would have been a significant reduction in PIM use during the 12-month post-hospitalization period.
5. Conclusion
PIM use was associated with a lower probability of achieving full functional recovery in a 12-month period after hip fracture, especially in those using two or more PIM at baseline. This finding could have implications for decision-making around post-hip fracture prescribing, particularly with regards to prescribing of multiple PIM, and the need to identify therapies that support rather than hinder recovery.
Key points.
The prescribing of potentially inappropriate medication (PIM) is common in the period after a hip fracture.
PIM use is independently associated with longer time to full functional recovery after a hip fracture
The association between PIM use and longer functional recovery after hip fracture is most pronounced in persons prescribed two or more PIM.
Acknowledgments
Funding
Eric Lenze was supported in this work by the National Institute of Mental Health [grant number R01MH074596] and the Taylor Family Institute for Innovative Psychiatric Research. Andrea Iaboni was supported by a University Health Network Psychiatric Consultants Research Grant. No other sources of funding were used to assist in the conduct of this study or the preparation of this article.
EJL receives grant/research support from Takeda, Lundbeck, and Janssen, as well as foundations (Barnes Jewish foundation, Taylor Family Institute for Innovative Psychiatric Research). AJF currently receives grant support from the U.S. National Institutes of Health, the Canadian Institutes of Health Research, Brain Canada, the Ontario Brain Institute, and Lundbeck, and within the past three years has received honoraria from Pfizer Canada.
Footnotes
Compliance with Ethical Standards
Conflicts of Interest
Andrea Iaboni, Kerri Rawson, and Craig Burkett have no conflicts to report.
Ethical Approval
Procedures were approved by the institutional review boards at Washington University School of Medicine and the eight participating hospitals.
Contributor Information
Andrea Iaboni, Department of Psychiatry, University of Toronto, and Toronto Rehabilitation Institute, University Health Network, Toronto, ON, Canada.
Kerri Rawson, Department of Psychiatry, Washington University School of Medicine, St. Louis, MO, USA.
Craig Burkett, Department of Statistical Sciences, University of Toronto, Toronto, ON, Canada.
Eric J Lenze, Healthy Mind Lab, Department of Psychiatry, Washington University School of Medicine, St. Louis, MO, USA.
Alastair Flint, Department of Psychiatry, University of Toronto, and University Health Network, Toronto, ON, Canada.
References
- 1.Bentler SE, Liu L, Obrizan M, et al. The aftermath of hip fracture: discharge placement, functional status change, and mortality. Am J Epidemiol. 2009;170(10):1290–9. doi: 10.1093/aje/kwp266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haentjens P, Magaziner J, Colón-Emeric CS, et al. Meta-analysis: Excess mortality after hip fracture among older women and men. Ann Intern Med. 2010;152(6):380–90. doi: 10.1059/0003-4819-152-6-201003160-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boonen S, Autier P, Barette M, et al. Functional outcome and quality of life following hip fracture in elderly women: A prospective controlled study. Osteoporos Int. 2004;15(2):87–94. doi: 10.1007/s00198-003-1515-z. [DOI] [PubMed] [Google Scholar]
- 4.Bertram M, Norman R, Kemp L, et al. Review of the long-term disability associated with hip fractures. Inj Prev. 2011;17(6):365–70. doi: 10.1136/ip.2010.029579. [DOI] [PubMed] [Google Scholar]
- 5.Guralnik JM, Ferrucci L, Simonsick EM, et al. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995 Mar 2;332(9):556–61. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hallberg I, Bachrach-Lindström M, Hammerby S, et al. Health-related quality of life after vertebral or hip fracture: A seven-year follow-up study. BMC Musculoskelet Disord. 2009;10(1) doi: 10.1186/1471-2474-10-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lloyd BD, Williamson DA, Singh NA, et al. Recurrent and injurious falls in the year following hip fracture: a prospective study of incidence and risk factors from the Sarcopenia and Hip Fracture study. J Gerontol A Biol Sci Med Sci. 2009;64(5):599–609. doi: 10.1093/gerona/glp003. [DOI] [PubMed] [Google Scholar]
- 8.Lee HB, Mears SC, Rosenberg PB, et al. Predisposing factors for postoperative delirium after hip fracture repair in individuals with and without dementia. J Am Geriatr Soc. 2011;59(12):2306–13. doi: 10.1111/j.1532-5415.2011.03725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mangoni AA, van Munster BC, Woodman RJ, et al. Measures of anticholinergic drug exposure, serum anticholinergic activity, and all-cause postdischarge mortality in older hospitalized patients with hip fractures. Am J Geriatr Psychiatry. 2013;21(8):785–93. doi: 10.1016/j.jagp.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 10.Gosch M, Wortz M, Nicholas JA, et al. Inappropriate prescribing as a predictor for long-term mortality after hip fracture. Gerontology. 2014;60(2):114–22. doi: 10.1159/000355327. [DOI] [PubMed] [Google Scholar]
- 11.Iaboni A, Fischer HD, Diong C, et al. Initiation of antidepressant medication after hip fracture in community-dwelling elderly. Am J Geriatr Psychiatry. 2015;23(10):1007–15. doi: 10.1016/j.jagp.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Cristancho P, Lenze EJ, Avidan MS, et al. Trajectories of depressive symptoms after hip fracture. Psychol Med. 2016 May;46(7):1413–25. doi: 10.1017/S0033291715002974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rawson KS, Dixon D, Nowotny P, et al. Association of functional polymorphisms from brain-derived neurotrophic factor and serotonin-related genes with depressive symptoms after a medical stressor in older adults. PLoS One. 2015;10(3):e0120685. doi: 10.1371/journal.pone.0120685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I), Clinician Version, Administration Booklet. American Psychiatric Pub; 2012. [Google Scholar]
- 15.Fick D, Semla T, Beizer J, et al. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60(4):616–31. doi: 10.1111/j.1532-5415.2012.03923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zuckerman JD, Koval KJ, Aharonoff GB, et al. A functional recovery score for elderly hip fracture patients: I. Development. J Orthop Trauma. 2000 Jan;14(1):20–5. doi: 10.1097/00005131-200001000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Munin MC, Seligman K, Dew MA, et al. Effect of rehabilitation site on functional recovery after hip fracture. Arch Phys Med Rehabil. 2005 Mar;86(3):367–72. doi: 10.1016/j.apmr.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 18.Montgomery SA, Asberg M. New depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–9. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 19.Tosato M, Landi F, Martone AM, et al. Potentially inappropriate drug use among hospitalised older adults: Results from the CRIME study. Age and Ageing. 2014;43(6):767–73. doi: 10.1093/ageing/afu029. [DOI] [PubMed] [Google Scholar]
- 20.Davidoff AJ, Miller GE, Sarpong EM, et al. Prevalence of potentially inappropriate medication use in older adults using the 2012 Beers criteria. J Am Geriatr Soc. 2015 Mar;63(3):486–500. doi: 10.1111/jgs.13320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Munson JC, Bynum JW, Bell J, et al. Patterns of prescription drug use before and after fragility fracture. JAMA Intern Med. 2016;176(10):1531–8. doi: 10.1001/jamainternmed.2016.4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koshoedo S, Soiza RL, Purkayastha R, et al. Anticholinergic drugs and functional outcomes in older patients undergoing orthopaedic rehabilitation. Am J Geriatr Pharmacother. 2012;10(4):251–257. doi: 10.1016/j.amjopharm.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 23.Kragh Ekstam A, Elmstahl S. Do fall-risk-increasing drugs have an impact on mortality in older hip fracture patients? A population-based cohort study. Clin Interv Aging. 2016;11:489–96. doi: 10.2147/CIA.S101832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gallagher P, Lang PO, Cherubini A, et al. Prevalence of potentially inappropriate prescribing in an acutely ill population of older patients admitted to six European hospitals. Eur J Clin Pharmacol. 2011 Nov;67(11):1175–88. doi: 10.1007/s00228-011-1061-0. [DOI] [PubMed] [Google Scholar]
- 25.Feng L, Scherer SC, Tan BY, et al. Comorbid cognitive impairment and depression is a significant predictor of poor outcomes in hip fracture rehabilitation. Int Psychogeriatr. 2010;22(2):246–53. doi: 10.1017/S1041610209991487. [DOI] [PubMed] [Google Scholar]
- 26.Givens JL, Sanft TB, Marcantonio ER. Functional recovery after hip fracture: the combined effects of depressive symptoms, cognitive impairment, and delirium. J Am Geriatr Soc. 2008;56(6):1075–9. doi: 10.1111/j.1532-5415.2008.01711.x. [DOI] [PubMed] [Google Scholar]
- 27.Morrison RS, Magaziner J, McLaughlin MA, et al. The impact of post-operative pain on outcomes following hip fracture. Pain. 2003;103(3):303–11. doi: 10.1016/S0304-3959(02)00458-X. [DOI] [PubMed] [Google Scholar]
- 28.Zuckerman IH, Langenberg P, Baumgarten M, et al. Inappropriate drug use and risk of transition to nursing homes among community-dwelling older adults. Med Care. 2006 Aug;44(8):722–30. doi: 10.1097/01.mlr.0000215849.15769.be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hanlon JT, Fillenbaum GG, Kuchibhatla M, et al. Impact of inappropriate drug use on mortality and functional status in representative community dwelling elders. Med Care. 2002 Feb;40(2):166–76. doi: 10.1097/00005650-200202000-00011. [DOI] [PubMed] [Google Scholar]
- 30.Cooper JA, Cadogan CA, Patterson SM, et al. Interventions to improve the appropriate use of polypharmacy in older people: a Cochrane systematic review. BMJ Open. 2015;5(12):e009235. doi: 10.1136/bmjopen-2015-009235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kragh A, Elmstahl S, Atroshi I. Older adults’ medication use 6 months before and after hip fracture: a population-based cohort study. J Am Geriatr Soc. 2011;59(5):863–8. doi: 10.1111/j.1532-5415.2011.03372.x. [DOI] [PubMed] [Google Scholar]
- 32.Walsh KA, O’Riordan D, Kearney PM, et al. Improving the appropriateness of prescribing in older patients: a systematic review and meta-analysis of pharmacists’ interventions in secondary care. Age Ageing. 2016 Mar;45(2):201–9. doi: 10.1093/ageing/afv190. [DOI] [PubMed] [Google Scholar]


