Abstract
Background
HIV-positive men who have sex with men (MSM) are at disproportionately high risk for anal cancer. There is no definitive approach for management of high-grade squamous intraepithelial lesions (HSIL), the anal cancer precursor, and evidence suggests that posttreatment adjuvant quadrivalent HPV (qHPV) vaccination improves HSIL treatment effectiveness. Our objective was to evaluate the optimal HSIL management strategy considering the clinical effectiveness and cost-effectiveness, and to identify optimal age to initiate HSIL management.
Methods
We constructed a decision-analytic model of the natural history of anal carcinoma and HSIL management strategies in 27 years or older HIV-positive MSM. The model was informed by the SEER-Medicare database and published studies. Outcomes included lifetime cost, life expectancy, quality-adjusted life expectancy, cumulative risk of cancer and cancer-related deaths, and cost-effectiveness from a societal perspective.
Results
‘Active monitoring’ was the most effective approach in patients younger than 29, thereafter ‘HSIL treatment plus adjuvant qHPV vaccination’ became most effective. When considering the cost-effectiveness [i.e., the incremental cost-effectiveness ratio (ICER)<$100 000/QALY], ‘do nothing’ was cost-effective until age 38, and ‘HSIL treatment plus adjuvant qHPV vaccination’ beyond age 38 (95% C.I. 34–43). The ICER decreased as age at HSIL management increased. Outcomes were sensitive to the rate of HSIL regression or progression and cost of high-resolution anoscopy and biopsy.
Conclusions
Management of HSIL in HIV-positive MSM aged 38 years or above using treatment plus adjuvant qHPV vaccination is likely to be cost-effective. Conservative approach of no treatment is likely to be cost-effective in younger patients.
Keywords: Anal cancer, high-grade squamous intraepithelial lesion, Precursor, Management, Cost-effectiveness, Treatment, Human Papillomavirus, Human papillomavirus vaccine
INTRODUCTION
HIV-positive men who have sex with men (MSM) are at disproportionately high risk for anal cancer. The incidence of anal cancer in HIV-positive MSM is approximately 80 times higher than the incidence in men from the rest of the general population.1–3 Persistent infection with human papillomavirus (HPV) has been associated with the development of high-grade squamous intraepithelial lesions (HSIL), which is a precursor of anal cancer.4 The incidence of HSIL among HIV-positive MSM (12.8 cases per 1000 person-months in 2011) has increased by 12% between 2000 and 2009,5–9 and the prevalence of HSIL among HIV-positive MSM is almost 50%.10–13
Treatment for HSIL is likely to prevent progression to invasive cancer;14 however, the best management of these lesions remains highly controversial.15–17 In recent years, HRA-directed ablation with laser, cryotherapy, infrared coagulator, or electrocautery has been evolving into a standard approach for the management of HSIL.18–21 Studies have shown that ablation is more effective than no treatment in clearing HSIL; however, the rate of HSIL recurrence after initial treatment is extremely high, with approximately 60% to 70% of HIV-positive MSM experiencing recurrence within 1 year of treatment.19, 21–23 Risk of recurrence increases with each additional lesion treated,14 and despite treatment and routine follow-up, patients are at risk of progressing to cancer.14, 23 An alternative potential approach to managing HSIL is ablative treatment along with adjuvant or therapeutic quadrivalent human papillomavirus (qHPV) vaccination (i.e., vaccination after treatment for HSIL). Recent studies suggest that posttreatment adjuvant qHPV vaccination improves treatment effectiveness by decreasing the risk of HSIL recurrences by approximately 50%, and is cost-saving compared with treatment alone.24–26
If not treated, HSIL can naturally regress.27 Some suggest that HSIL treatment should be delayed and patients be followed until the development of anal cancer because patients in addition to having frequent recurrences, may experience postoperative complications such as anal stenosis and fecal incontinence and thus the risk of HSIL treatment may outweigh the benefit.16, 17 This approach for HSIL management is known as ‘active monitoring’ or ‘watchful waiting’,17, 28 which consists of patients being followed periodically using digital anorectal examination (DARE) and high-resolution anoscopy (HRA) for early detection of anal cancer, and undergo biopsy of visible lesions.
In this study, our first objective was to find the optimal strategy to manage HSIL in HIV-positive MSM considering clinical effectiveness and cost effectiveness of the following approaches: do nothing, active-monitoring, HSIL treatment alone, or HSIL treatment plus adjuvant qHPV vaccination. Our second objective was to identify optimal age to initiate HSIL management.
METHODS
We developed a Markov-based state-transition model to simulate clinical course of HIV-positive MSM diagnosed with HSIL. The model was developed assuming a U.S. societal perspective over the time horizon of patients’ lifetimes using annual cycles. For a detailed model description, see online Supplemental Material Section A.
Baseline population
The baseline population consisted of a cohort of 40-year-old (range 27–60 years) HIV-positive MSM with first-time HSIL diagnosis in the U.S. The base case age of 40 years was chosen because the median age at HSIL diagnosis in prior studies was 40.22, 29, 30 We categorized patients based on their CD4 cell count distribution (i.e., CD4>500, CD4 200–500, or CD4<200).
Comparators for clinical and economic evaluation
Do nothing
Given that there is no uniform standard of care for HSIL, we simulated a “do nothing” strategy as one potential option. The rationale for including “do nothing” was that not all patients progress to anal cancer, and progression can take several years; therefore, it might be reasonable to delay treatment until patients develop anal cancer. Diagnosis of invasive cancer was based on symptoms (typically bleeding, anal mass or anal pain), and the distribution of the stages of diagnosis was based on the SEER database.31, 32 These patients subsequently would receive treatment for invasive cancer.
Active monitoring or watchful waiting
An alternative strategy is “active monitoring” or “watchful waiting”. We simulated active monitoring of patients using DARE and HRA-guided biopsy for early detection of cancer.28 Patients could progress to invasive cancer, and their stage of diagnosis was based on Berry et al.33 These patients subsequently would receive treatment for cancer.
Treatment for HSIL
The third strategy was defined by the immediate treatment of HSIL. After initial diagnosis and treatment, these patients were monitored annually using DARE and HRA for subsequent risk of HSIL recurrence or cancer. Long-term HSIL treatment effectiveness data in terms of risk of recurrence after treatment for initial and subsequent HSIL were obtained from the literature (online Supplemental Material Table A.3).14, 21–23 We also incorporated loss to follow-up during the annual screening and treatment for subsequent lesions.22 Patients lost during the follow-up were at risk of progressing to anal cancer.
Treatment plus adjuvant qHPV vaccination
Finally, we simulated the long-term effectiveness of providing HSIL treatment plus adjuvant qHPV vaccination. Patients who receive adjuvant qHPV vaccination were less likely to experience HSIL recurrence.26 Vaccine effectiveness data were obtained from the literature (online Supplemental Material Table A.3).24–26 Detailed description of all comparators and clinical data is included in online Supplemental Material Section A.
Natural history and health state transitions
Annual disease transitions occurred at two levels: 1) within health states for HIV, and 2) within health states for anal disease. Anal carcinogenesis (i.e., probabilities of anal disease progression or regression) was conditional on receipt of form of care for HSIL, patient’s HIV status, and receipt of antiretroviral therapy.
Disease progression from HSIL to anal cancer in patients not receiving treatment for HSIL or who were actively monitored was calibrated using a separate natural history model of anal carcinogenesis that used age-specific incidence of anal cancer in HIV-positive MSM (online Supplemental Material Section B) as a calibration target. For patients receiving treatment for HSIL and subsequent HSIL recurrences, disease progression to invasive cancer was based on an estimation by Pineda et al.23 HSIL regression data were obtained from the literature.27 Because natural history data on disease progression after natural regression were not available, we used the available data on initial disease transition (online Supplemental Material Table A.2).5, 34, 35 Patients during their lifetime might die of HIV-related illness, anal cancer, or other unrelated causes.32, 36
Costs and health-related quality of life
Our model included the costs of HIV-related care (by CD4 count), anal cancer treatment (by stage), HSIL treatment, HRA and biopsy, and adjuvant qHPV vaccination (online Supplemental Material Table A.4).32, 37–39 We converted all costs to US 2016 dollars using consumer price indices for medical care.40
To account for differential quality of life in different health states, we assigned health-related quality of life weights (utilities) to each health state using published data. Utilities were assigned based on HIV status, anal disease status, and age.34, 41
Analysis and model outcomes
We followed the recommendations of the U.S. Panel on Cost-Effectiveness in Health and Medicine for conducting our analysis.42 For each strategy we simulated the life expectancy, quality-adjusted life years (QALYs), and total lifetime cost. Future costs and QALYs were reported in terms of the net present value using an annual discount rate of 3%.43 We presented outcomes for all HIV-positive MSM, as well as for the subgroups stratified by patient HIV status. Comparative assessment was conducted and the results were presented in the form of incremental cost-effectiveness ratios (ICERs), decrease in lifetime risk of anal cancer, and decrease in the lifetime anal cancer mortality risk. To determine the cost-effectiveness of strategies by age, we estimated outcomes by age at the initiation of HSIL management, starting from age 27 onwards. We used commonly recommended societal willingness-to-pay (WTP) threshold of $100 000/QALY to determine the cost-effectiveness of strategies.44
We performed comprehensive sensitivity analyses on all model parameters to evaluate robustness of the outcomes. Deterministic (one-way and two-way) sensitivity analyses was conducted by varying input model parameters. Finally, probabilistic sensitivity analysis was conducted to assess decision uncertainty and the outcomes were presented using cost-effectiveness acceptability curves (CEAC).
RESULTS
Model validation
We cross-validated our model by comparing our calibrated progression rates from HSIL to anal cancer with those reported by previously published studies.34, 45, 46 Model-calibrated HSIL to anal cancer progression rate was within the plausible range of the reported progression and followed a similar trends (i.e., rate of progression increases with increase in age).
We also compared model-predicted cumulative risk of HSIL to the cumulative risk reported by Burgos et al.47 The predicted natural history HSIL risk was within the 95% confidence intervals (CIs) of the reported risk. For detailed model validation, see online Supplemental Material Section C.
Clinical outcomes
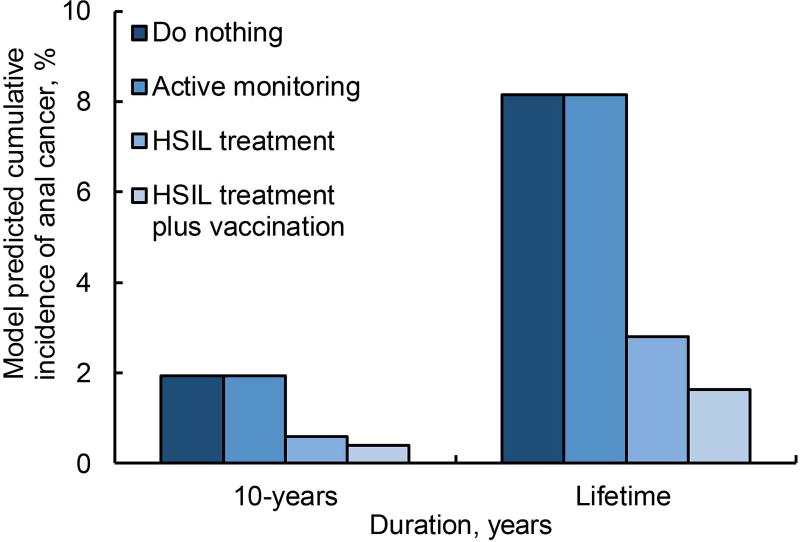
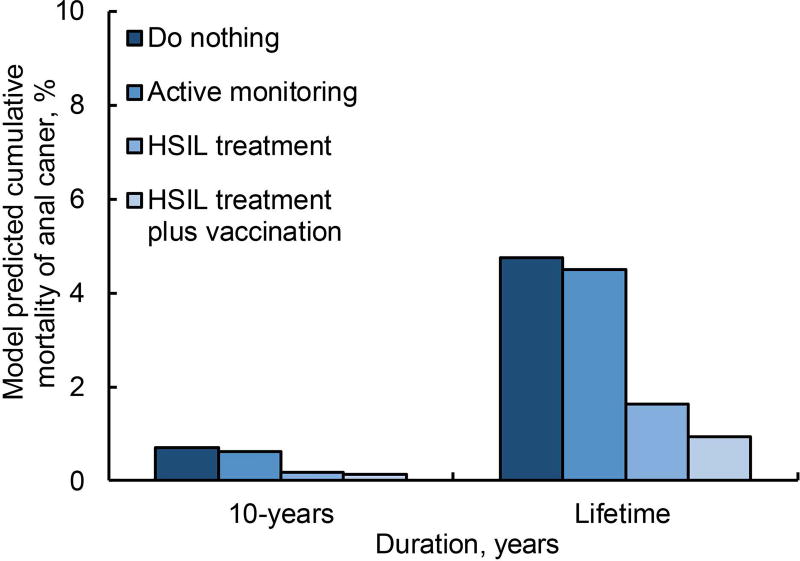
For a 40-year-old HIV-positive MSM, compared with do nothing, treatment decreased the lifetime incidence of anal cancer and mortality by 66%, and treatment plus adjuvant qHPV vaccination decreases lifetime incidence and mortality by 80% (Figure 1a and 1b). Active monitoring did not reduce cumulative incidence of anal cancer; however, early detection attributed to active monitoring could decrease lifetime mortality risk by 6%.
Figure 1. Percentage cumulative incidence of anal cancer and percentage deaths attributed to anal cancer in 40-year-old MSM.
a depicts cumulative risk of anal cancer in a 40-year old male, i.e., considering a base case, for the strategies—(i) do nothing, (ii) active monitoring, (iii) HSIL treatment, and (iv) HSIL treatment plus adjuvant qHPV vaccination—represented in the order of the color intensity from darker to lighter. The outcomes are reported over a period of 10 years and over an average patient’s lifetime.
b depicts cumulative risk of death attributed to anal cancer in a 40-year old male, i.e., considering a base case, for the strategies—(i) do nothing, (ii) active monitoring, (iii) HSIL treatment, and (iv) HSIL treatment plus adjuvant qHPV vaccination—represented in the order of the color intensity from darker to lighter. The outcomes are reported over a period of 10 years and over an average patient’s lifetime.
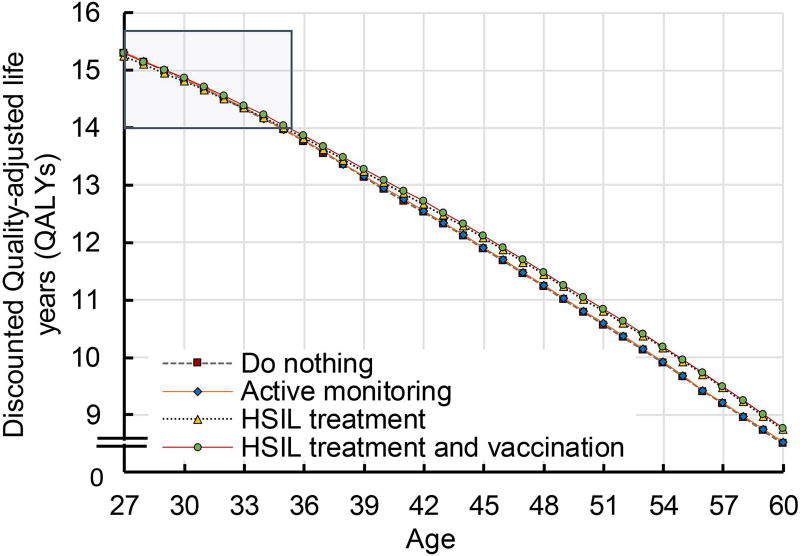
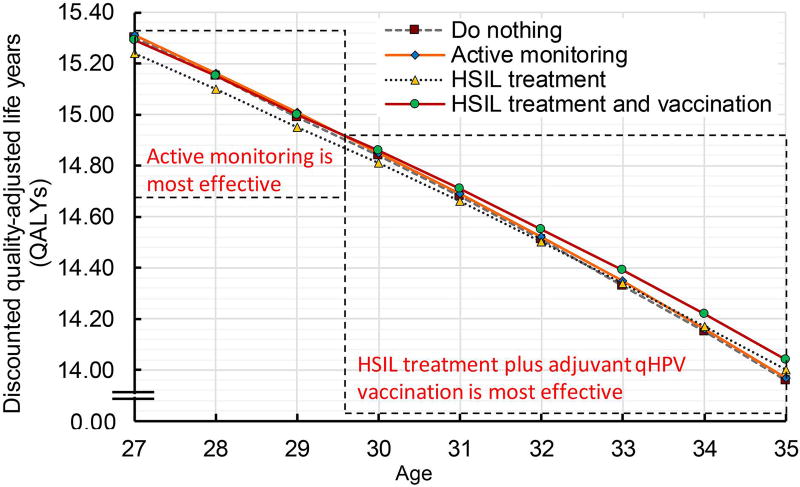
We further evaluated the influence of age on optimal HSIL management strategy. We found that active monitoring was the most effective HSIL management strategy in patients up to age 29 (i.e., it was associated with highest quality-adjusted life expectancy) (Figure 2a and 2b). From age 30 onwards, treatment and qHPV vaccination was the most effective strategy.
Figure 2. Clinical effectiveness by age.
a shows quality-adjusted life expectancy by HSIL management strategies and age. The strategies (i) do nothing, (ii) active monitoring, (iii) HSIL treatment, and (iv) HSIL treatment plus adjuvant qHPV vaccination are represented by the lines dotted grey, dashed orange, dotted blue, and dashed red. The QALYs reported using an annual discount rate of 3%.
b shows quality-adjusted life expectancy by HSIL management modalities in patients 27–35 years of age (magnified version of Figure 2a). The strategies (i) do nothing, (ii) active monitoring, (iii) HSIL treatment, and (iv) HSIL treatment plus adjuvant qHPV vaccination are represented by the lines dotted grey, dashed orange, dotted blue, and dashed red. The QALYs reported using an annual discount rate of 3%.
Cost-effectiveness analysis
For a 40-year old HIV-positive MSM, treatment plus adjuvant qHPV vaccination was the most cost-effective strategy with the resulting ICER of $73 367 per QALY (Table 1). Active monitoring and treatment for HSIL were dominated by treatment plus adjuvant qHPV vaccination strategy and were eliminated.48
Table 1.
Lifetime clinical effectiveness and cost-effectiveness of management of anal intraepithelial lesions
| Variable | Lifetime Cost, $ |
Total average life expectancy, y* |
QALYs | ICER, $ per QALY |
Cumulative decrease in cancer incidence, % |
Cumulative decrease in cancer mortality, % |
|---|---|---|---|---|---|---|
| HSIL diagnosis age of 40 years (base case) | ||||||
| Do nothing | 367 768 | 27.94 | 12.93 | – | – | – |
| Active monitoring/watchful waiting | 372 636 | 27.99 | 12.95 | Weakly Dominated† | 0% | 6% |
| Treat HSIL | 379 044 | 28.14 | 13.05 | Weakly Dominated† | 66% | 66% |
| Treat HSIL+ adjuvant qHPV vaccination | 379 440 | 28.24 | 13.09 | 73 367 | 80% | 80% |
|
| ||||||
| HSIL diagnosis age of 30 years | ||||||
| Treat HSIL | 416 213 | 33.65 | 14.81 | Dominated‡ | 54% | 53% |
| Do nothing | 405 473 | 33.87 | 14.84 | – | – | – |
| Active monitoring/watchful waiting | 411 207 | 33.91 | 14.85 | 491 300 | 0% | 6% |
| Treat HSIL+ adjuvant qHPV vaccination | 416 759 | 33.79 | 14.86 | 790 972 | 75% | 75% |
|
| ||||||
| HSIL diagnosis age of 50 years | ||||||
| Do nothing | 320 811 | 22.03 | 10.79 | – | – | – |
| Active monitoring/watchful waiting | 324 623 | 22.09 | 10.81 | Weakly Dominated† | 0% | 7% |
| Treat HSIL | 331 110 | 22.45 | 11.01 | Weakly Dominated† | 74% | 75% |
| Treat HSIL+ adjuvant qHPV vaccination | 331 304 | 22.51 | 11.05 | 40 597 | 84% | 85% |
|
| ||||||
| HSIL diagnosis age of 60 years | ||||||
| Do nothing | 266 186 | 16.47 | 8.50 | – | – | – |
| Active monitoring/watchful waiting | 268 876 | 16.52 | 8.52 | Weakly Dominated† | 0% | 8% |
| Treat HSIL | 273 805 | 16.87 | 8.74 | Dominated‡ | 79% | 80% |
| Treat HSIL+ adjuvant qHPV vaccination | 273 828 | 16.90 | 8.77 | 27 968 | 87% | 87% |
Abbreviations: QALYs, quality-adjusted life years; ICER, incremental cost-effectiveness ratio; HSIL, high-grade squamous intraepithelial lesion; qHPV, quadrivalent human papillomavirus.
Base case: analysis was conducted considering cohort start age of 40 years and all model parameters at their base value.
Undiscounted and unadjusted for quality of life.
Strategy is both less effective and more costly than a linear combination of two other strategies with which it is mutually exclusive.
Strategy is more costly and less effective than the next alternative.
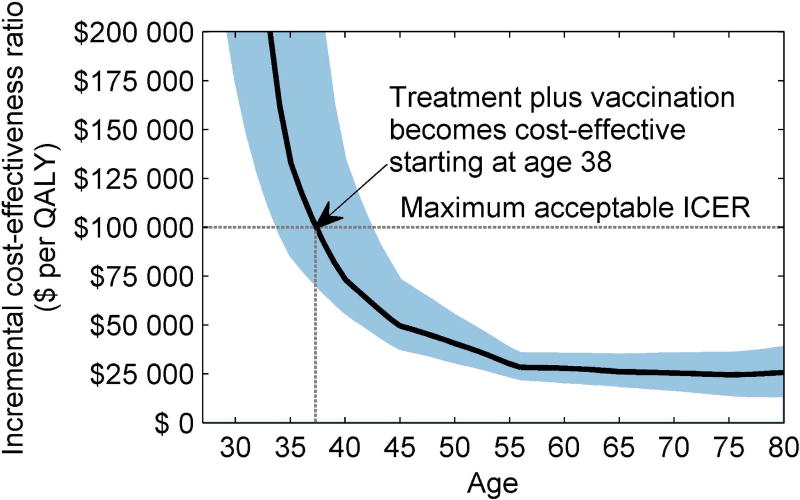
When we estimated the cost-effectiveness by age, we found that treatment plus adjuvant qHPV vaccination was cost-effective in patients 38 years or older (95% CI 34–43) (Figure 3). In patients 37 years or younger, do nothing was cost-effective.
Figure 3. One-way sensitivity analysis on age at HSIL diagnosis.
Figure shows the incremental cost-effectiveness ratio (95% CI) by HSIL age at diagnosis. The x-axis represents age at HSIL diagnosis and the y-axis represents willingness-to-pay threshold. Willingness-to-pay threshold of $100 000/QALY is considered economically acceptable.
Sensitivity analysis
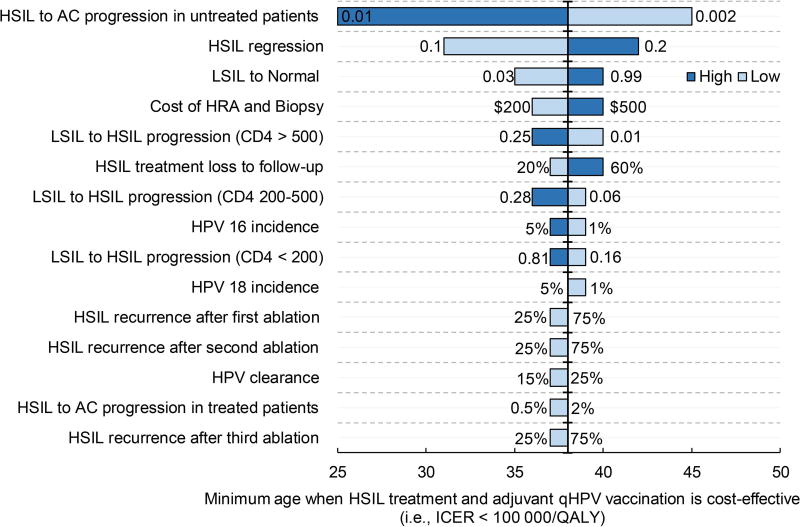
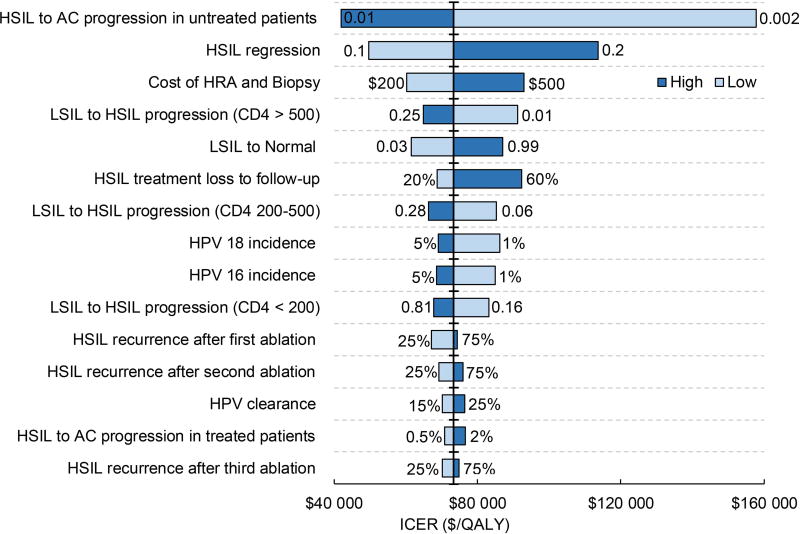
In order to evaluate the sensitivity of results to age, we identified the top 15 model parameters in the order of their impact on the minimum age at which treatment plus adjuvant qHPV vaccination becomes cost-effective (Figure 4a) and the base case ICER (Figure 4b). The outcomes were most sensitive to HSIL progression and regression in untreated patients. If age-adjusted HSIL to anal cancer progression is 0.01 (base case of 0.0035 or 1 out of 286 per year), management of HSIL using treatment plus qHPV vaccination was cost-effective irrespective of the age of patients. Similarly, when age-adjusted HSIL regression was 10% (base case 15%), then treatment plus qHPV vaccination was cost-effective starting at age 31 years. The base case ICERs for these values were $42 054/QALY and $49 616/QALY, respectively.
Figure 4. Tornado diagrams displaying the top 15 model parameters in the order of their impact on HSIL age at diagnosis and their impact on ICER.
a depicts the top 15 model parameters in the order of their impact on age. The horizontal axis represents the age at HSIL diagnosis. The parameters are arrayed along the vertical line, which represents the minimum HSIL age at diagnosis of 38 years when treatment plus adjuvant qHPV vaccination becomes cost-effective. Bars are arranged in the ascending order of their bar width (degree of uncertainty). The longest bar represents the parameter generating the widest uncertainty.
b depicts the top 15 model parameters in the order of their impact on cost-effectiveness. The horizontal axis represents the incremental cost-effectiveness ratio (the ratio of incremental cost and incremental effectiveness comparing undominated strategies). The parameters are arrayed along the vertical line, which represents the outcome point of base case ICER of $73 367/QALY. Bars are arranged in the ascending order of their bar width (degree of uncertainty). The longest bar represents the parameter generating the widest uncertainty.
AC, anal cancer; HRA, high-resolution anoscopy
The ICER was not sensitive to HSIL treatment effectiveness and vaccine efficacy as it did not exceed $100 000/QALY even when high recurrence rate and worst-case adjuvant qHPV vaccination efficacy was assumed (online Supplemental Material Table D.1). Additional one-way sensitivity analysis on natural history and cost parameters is presented in online Supplemental Material Table D.2. The ICER changed marginally for the subgroup of patients presented with CD4>500, CD4 200–500, or CD4<200. For all subgroups, treatment plus adjuvant qHPV vaccination remained cost-effective.
Two-way sensitivity analysis demonstrated that the cost-effective strategy changed with the change in combination of HSIL to anal cancer progression in untreated (base case 0.0035) and treated (base case 0.0012) patients (online Supplemental Material Figure D.1). For example, when values for HSIL to anal cancer progression in untreated and treated patients were 0.001 and 0.003, respectively, ‘do nothing’ was cost-effective.
The probabilistic sensitivity analysis showed that in 40-year HIV-positive MSM adjuvant qHPV vaccination was cost-effective with 73% probability at the threshold of $100 000/QALY; alternatively, do nothing was cost-effective with 27% probability (online Supplemental Material Figure D.2). In HIV-positive MSM at the age of 30, 50, and 60, the probabilities that HSIL treatment plus adjuvant qHPV vaccination was cost-effective were 18%, 99.5%, and 100%, respectively (online Supplemental Material Figure D.3).
DISCUSSION
Our comparative clinical and cost-effectiveness analysis shows that active monitoring but not treating until invasive cancer is identified is likely to be the most effective HSIL management strategy in HIV-positive MSM 29 years or younger. In patients older than 30 years, treatment plus adjuvant qHPV vaccination is potentially most effective. However, when considering the cost-effectiveness of interventions, we found that ‘do nothing’ was likely to be cost-effective until age 38, and HSIL treatment along with adjuvant qHPV vaccination was potentially most cost-effective in patients age 38 years (95% CI 34–43) or older, considering a commonly used WTP threshold of $100 000/QALY. It was neither effective nor cost-effective to treat 29 years or younger patients owing to their lower likelihood of developing anal cancer and the increased natural regression of HSIL among the younger patients. This finding is consistent with the rationale that the incidence of anal cancer increases with increased age;3, 49 therefore, the benefits of treatment might be higher in older patients.
In a manner similar to cervical disease in women, the regression of anal HSIL has been shown to be associated with age. Tong et al27, 50 found that among HIV-positive MSM, the regression rate in men over the age of 35 was significantly lower than for those younger than 35 (p=0.048). Thus, the findings from our study support a conservative approach of active monitoring in men under age 35, and would represent an approach comparable to management of cervical HSIL among young immune competent women.51, 52 For immune competent women between age 21 and 25, minimum evaluation and management of abnormal cervical cytology is recommended.51, 52 Our findings could inform the development of similar guidelines for anal HSIL management for preventing ‘overtreatment’ in younger HIV-positive MSM.
Unlike management of cervical HSIL, where detected cervical abnormalities by regular screening can be effectively managed using procedures such as loop electrosurgery excision procedure (LEEP),53 complete ablative management of anal HSIL is difficult and is associated with high recurrence and a higher likelihood of adverse events.22, 54 Therefore, regular anal cytology based screening protocols for detection of these lesions would be unreasonable without identifying a definitive and cost-effective management strategy for HSIL.11, 54–56
Surgical and ablative anal HSIL therapies are not without significant risk. They can be associated not only with additional cost but also temporary morbidity including pain, rectal bleeding and discharge. Long-term complications are rare but can include anal stenosis, fistula and fissure formation, and discomfort with alteration in sexual satisfaction. Therefore, before recommending treatment, it is crucial for clinicians to consider the risk of progression to invasive cancer, treatment duration, treatment-related adverse effects, the likelihood that a patient will comply with and adhere to treatment for subsequent lesions, patient preference, and the cost of care.
Several obstacles exist to developing HSIL management guidelines. For example, compared with cervical colposcopy, HRA along with HSIL management is difficult and involves challenges including uneven topography; obscuring of lesions due to anal warts, hemorrhoids, folds, stool or muscle; lesions may also be located at the base of folds and anal glands.57 Thus, in order to adequately screen for and manage HSIL lesions, clinicians often need additional training above and beyond those specified by even sub-specialty medical boards. Our analysis suggests that treating HSIL in HIV-positive MSM is cost-effective; however, at this time the number of clinicians with adequate experience to diagnose and manage HSIL in HIV-positive individuals in the U.S. remains inadequate. Therefore, it is imperative to take educational efforts to train clinicians the in requisite procedures.
The National Cancer Institute–supported AIDS Malignancy Consortium has recently launched a phase III clinical trial (Anal Cancer/HSIL Outcomes Research or ANCHOR study) to compare the outcomes of active monitoring via regular exams to treatment for HSIL. The ANCHOR study will hopefully fill in some of the gaps related to the natural history of HSIL in HIV infected individuals, however, ensuring cost-effective approaches when implementing HSIL screening and management recommendations and guidelines remain of utmost importance in this day and age of rising health care costs.
Our study is not without limitations. The data including HSIL treatment effectiveness, adjuvant qHPV vaccination effectiveness, and HSIL natural history, though represent from the best available evidence, were obtained from studies that were not randomized and did not directly compare the HSIL management strategies. We addressed this limitation by conducting an extensive sensitivity analysis in which we found that the ICER and optimal age when treatment plus vaccination becomes effective or cost-effective was most sensitive to HSIL to anal cancer progression and HSIL regression among untreated patients. Though observation studies and the Study of Prevention of Anal Cancer (SPANC) trial have reported HSIL regression,27, 58 HSIL to anal cancer progression rate remains unobserved. Given the outcomes were most sensitive to HSIL progression rate, which remains the area of greatest uncertainty, our findings should be interpreted within the context of this limitation. Furthermore, in our model calibration, we found that the progression is likely to increase with increases in age. Future research should be prioritized to assess age-specific HSIL progression and regression, which remains the area of greatest uncertainty. Finally, the objective of our study was to estimate cost-effectiveness for US healthcare system. Based on the cost of HSIL care, anal cancer care, and HIV care and based on the outcomes (for example, recurrence rate, survival, etc.), the cost-effectiveness will vary for other health care systems (e.g., Canada, UK, and Australia).
The Advisory Committee of the Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) recommends HPV vaccination for primary prevention of anal cancer in males aged 11 through 26 years. HPV vaccination is not licensed for males aged older than 26 years. No data on HPV vaccination efficacy exists for primary prevention in males aged older than 26 years. We considered posttreatment adjuvant qHPV vaccination as a comparator in these men. This is because there is early data suggesting that men over age 26 with anal HSIL may have decreased risk of recurrent HSIL when given adjuvant qHPV vaccine at the time of HSIL treatment without adding any side effects. Others have reported similar evidence for decreasing recurrent cervical intraepithelial neoplasia, vaginal intraepithelial neoplasia, and respiratory and laryngeal papillomatosis.59–66 Posttreatment adjuvant vaccination is not completely accepted into clinical practice because of the lack of randomized clinical trial (RCT) data. Furthermore, it is not understood whether the vaccine prevents recurrence of initially treated lesion or it halts new HSIL development. Due to these uncertainties, it might be several more years before data from RCTs might inform this practice for prevention of anal cancer; however, implementation of such a strategy sooner rather than later might present an important cancer prevention opportunity.
Finally, our results demonstrate that age can be used as an important predictor for the determination of individuals who might benefit more from treatment versus those who may not. Emerging data show that persistence of HPV16 or any high-risk HPV (hrHPV) might play a role in the lack of HSIL clearance and subsequent progression to anal cancer in HIV-infected individuals.58 As this association is established, age-specific risk-stratified outcomes of HSIL management must be determined. Likewise, smoking might play a role in the determination of an algorithm for HSIL management.67 Therefore, the role of smoking on anal cancer natural history and treatment algorithms needs to be evaluated.
Conclusion
In conclusion, our study is first to demonstrate that younger HIV-positive MSM might benefit from a conservative approach to HSIL management. Furthermore, posttreatment adjuvant qHPV vaccination is likely to be most cost-effective strategy for managing HSIL in patients 38 years or older. Future research needs to be prioritized to determine the age-specific natural history of anal cancer.
Supplementary Material
Acknowledgments
Funding
The study was funded in part by a fellowship for Ashish A. Deshmukh supported by a grant from The University of Texas MD Anderson Cancer Center, Janice Davis Gordon Memorial Postdoctoral Fellowship in Colorectal Cancer Prevention; the National Institutes of Health through The University of Texas MD Anderson Cancer Center Support Grant [CA016672]; and the National Cancer Institute (R01 CA163103; Principal Investigator: Chiao).
The authors wish to thank Jessica P. Hwang, MD, MPH and Bei Hu, MD both from The University of Texas MD Anderson Cancer Center for constructive comments, Jason Ong, PhD, MMed, MBBS, FRACGP from the Monash University Faculty of Medicine, Nursing and Health Sciences for analytic suggestions, and Michael Worley for editorial contributions that enhanced quality of the manuscript.
SEG received personal fees from Merck & Co. Inc., grants and personal fees from Medtronic Inc., outside the submitted work. TW received grant support (paid to Weill Cornell Medicine) from Bristol-Myers Squibb, Gilead Sciences, and Glaxo Smith Kline/ViiV Healthcare. JC received personal fees from Merck & Co. Inc., grants and personal fees from Gilead Sciences, outside the submitted work.
Footnotes
Conflicts of interest and disclosure: There are no conflicts of interest to report for AAD, EYC, SBC, EAS, AGN, and XW.
Author contributions: AAD, EYC, EAS, SBC, and JC conceived the study. AAD, EYC, SBC, EAS, SEG, AGN, TW, and JC designed the study. AAD and XW did the analysis. AAD, EYC, SBC, EAS, SEG, AGN, TW, and JC interpreted the results. AAD, EYC, and JC wrote the initial draft of the manuscript. AAD, EYC, SBC, EAS, SEG, AGN, TW, XW, and JC critically revised the manuscript and approved the final version.
References
- 1.Piketty C, Selinger-Leneman H, Bouvier AM, et al. Incidence of HIV-related anal cancer remains increased despite long-term combined antiretroviral treatment: results from the french hospital database on HIV. J Clin Oncol. 2012;30(35):4360–6. doi: 10.1200/JCO.2012.44.5486. [DOI] [PubMed] [Google Scholar]
- 2.Daling JR, Weiss NS, Hislop TG, et al. Sexual practices, sexually transmitted diseases, and the incidence of anal cancer. N Engl J Med. 1987;317(16):973–7. doi: 10.1056/NEJM198710153171601. [DOI] [PubMed] [Google Scholar]
- 3.Silverberg MJ, Lau B, Justice AC, et al. Risk of anal cancer in HIV-infected and HIV-uninfected individuals in North America. Clin Infect Dis. 2012;54(7):1026–34. doi: 10.1093/cid/cir1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.zur Hausen H. Papillomaviruses causing cancer: evasion from host-cell control in early events in carcinogenesis. J Natl Cancer Inst. 2000;92(9):690–8. doi: 10.1093/jnci/92.9.690. [DOI] [PubMed] [Google Scholar]
- 5.Machalek DA, Poynten M, Jin F, et al. Anal human papillomavirus infection and associated neoplastic lesions in men who have sex with men: a systematic review and meta-analysis. Lancet Oncol. 2012;13(5):487–500. doi: 10.1016/S1470-2045(12)70080-3. [DOI] [PubMed] [Google Scholar]
- 6.de Pokomandy A, Rouleau D, Ghattas G, et al. HAART and progression to high-grade anal intraepithelial neoplasia in men who have sex with men and are infected with HIV. Clin Infect Dis. 2011;52(9):1174–81. doi: 10.1093/cid/cir064. [DOI] [PubMed] [Google Scholar]
- 7.Palefsky JM, Holly EA, Ralston ML, Jay N, Berry JM, Darragh TM. High incidence of anal high-grade squamous intra-epithelial lesions among HIV-positive and HIV-negative homosexual and bisexual men. AIDS. 1998;12(5):495–503. doi: 10.1097/00002030-199805000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Phanuphak N, Teeratakulpisarn N, Triratanachat S, et al. High prevalence and incidence of high-grade anal intraepithelial neoplasia among young Thai men who have sex with men with and without HIV. AIDS. 2013;27(11):1753–62. doi: 10.1097/QAD.0b013e328360a509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simard EP, Watson M, Saraiya M, Clarke CA, Palefsky JM, Jemal A. Trends in the occurrence of high-grade anal intraepithelial neoplasia in San Francisco: 2000–2009. Cancer. 2013;119(19):3539–45. doi: 10.1002/cncr.28252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palefsky JM, Holly EA, Efirdc JT, et al. Anal intraepithelial neoplasia in the highly active antiretroviral therapy era among HIV-positive men who have sex with men. AIDS. 2005;19(13):1407–14. doi: 10.1097/01.aids.0000181012.62385.4a. [DOI] [PubMed] [Google Scholar]
- 11.Severini A. Anal intraepithelial neoplasia in men living with HIV in the era of highly active antiretroviral therapy. Clin Infect Dis. 2011;52(9):1182–3. doi: 10.1093/cid/cir070. [DOI] [PubMed] [Google Scholar]
- 12.Piketty C, Darragh TM, Da Costa M, et al. High prevalence of anal human papillomavirus infection and anal cancer precursors among HIV-infected persons in the absence of anal intercourse. Ann Intern Med. 2003;138(6):453–9. doi: 10.7326/0003-4819-138-6-200303180-00008. [DOI] [PubMed] [Google Scholar]
- 13.Sobhani I, Vuagnat A, Walker F, et al. Prevalence of high-grade dysplasia and cancer in the anal canal in human papillomavirus-infected individuals. Gastroenterology. 2001;120(4):857–66. doi: 10.1053/gast.2001.22446. [DOI] [PubMed] [Google Scholar]
- 14.Goldstone SE, Johnstone AA, Moshier EL. Long-term outcome of ablation of anal high-grade squamous intraepithelial lesions: recurrence and incidence of cancer. Dis Colon Rectum. 2014;57(3):316–23. doi: 10.1097/DCR.0000000000000058. [DOI] [PubMed] [Google Scholar]
- 15.Pineda CE, Welton ML. Controversies in the management of anal high-grade squamous intraepithelial lesions. Minerva Chir. 2008;63(5):389–99. [PubMed] [Google Scholar]
- 16.Weis SE. Current treatment options for management of anal intraepithelial neoplasia. Onco Targets Ther. 2013;6:651–65. doi: 10.2147/OTT.S38217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orchard M, Roman A, Parvaiz AC. Anal intraepithelial neoplasia--is treatment better than observation? Int J Surg. 2013;11(6):438–41. doi: 10.1016/j.ijsu.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 18.Palefsky JM. Anal cancer prevention in HIV-positive men and women. Curr Opin Oncol. 2009;21(5):433–8. doi: 10.1097/CCO.0b013e32832f511a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pineda CE, Welton ML. Management of anal squamous intraepithelial lesions. Clin Colon Rectal Surg. 2009;22(2):94–101. doi: 10.1055/s-0029-1223840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Macaya A, Munoz-Santos C, Balaguer A, Barbera MJ. Interventions for anal canal intraepithelial neoplasia. Cochrane Database Syst Rev. 2012;12:CD009244. doi: 10.1002/14651858.CD009244.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richel O, de Vries HJ, van Noesel CJ, Dijkgraaf MG, Prins JM. Comparison of imiquimod, topical fluorouracil, and electrocautery for the treatment of anal intraepithelial neoplasia in HIV-positive men who have sex with men: an open-label, randomised controlled trial. Lancet Oncol. 2013;14(4):346–53. doi: 10.1016/S1470-2045(13)70067-6. [DOI] [PubMed] [Google Scholar]
- 22.Goldstone RN, Goldstone AB, Russ J, Goldstone SE. Long-term follow-up of infrared coagulator ablation of anal high-grade dysplasia in men who have sex with men. Dis Colon Rectum. 2011;54(10):1284–92. doi: 10.1097/DCR.0b013e318227833e. [DOI] [PubMed] [Google Scholar]
- 23.Pineda CE, Berry JM, Jay N, Palefsky JM, Welton ML. High-resolution anoscopy targeted surgical destruction of anal high-grade squamous intraepithelial lesions: a ten-year experience. Dis Colon Rectum. 2008;51(6):829–35. doi: 10.1007/s10350-008-9233-4. discussion 35-7. [DOI] [PubMed] [Google Scholar]
- 24.Deshmukh AA, Chiao EY, Das P, Cantor SB. Clinical effectiveness and cost-effectiveness of quadrivalent human papillomavirus vaccination in HIV-negative men who have sex with men to prevent recurrent high-grade anal intraepithelial neoplasia. Vaccine. 2014;32(51):6941–7. doi: 10.1016/j.vaccine.2014.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deshmukh AA, Chhatwal J, Chiao EY, Nyitray AG, Das P, Cantor SB. Long-term outcomes of adding HPV vaccine to the anal intraepithelial neoplasia treatment regimen in HIV-positive men who have sex with men. Clin Infect Dis. 2015;61(10):1527–35. doi: 10.1093/cid/civ628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Swedish KA, Factor SH, Goldstone SE. Prevention of recurrent high-grade anal neoplasia with quadrivalent human papillomavirus vaccination of men who have sex with men: a nonconcurrent cohort study. Clin Infect Dis. 2012;54(7):891–8. doi: 10.1093/cid/cir1036. [DOI] [PubMed] [Google Scholar]
- 27.Tong WW, Jin F, McHugh LC, et al. Progression to and spontaneous regression of high-grade anal squamous intraepithelial lesions in HIV-infected and uninfected men. AIDS. 2013;27(14):2233–43. doi: 10.1097/QAD.0b013e3283633111. [DOI] [PubMed] [Google Scholar]
- 28.Tanum G, Tveit K, Karlsen KO. Diagnosis of anal carcinoma--doctor's finger still the best? Oncology. 1991;48(5):383–6. doi: 10.1159/000226964. [DOI] [PubMed] [Google Scholar]
- 29.Gaisa M, Sigel K, Hand J, Goldstone S. High rates of anal dysplasia in HIV-infected men who have sex with men, women, and heterosexual men. AIDS. 2014;28(2):215–22. doi: 10.1097/QAD.0000000000000062. [DOI] [PubMed] [Google Scholar]
- 30.Crawshaw BP, Russ AJ, Stein SL, et al. High-resolution anoscopy or expectant management for anal intraepithelial neoplasia for the prevention of anal cancer: is there really a difference? Dis Colon Rectum. 2015;58(1):53–9. doi: 10.1097/DCR.0000000000000267. [DOI] [PubMed] [Google Scholar]
- 31.Pitts R, Goldstone SE, Gaisa M, et al. Prognosis of anal carcinoma in HIV infected persons in the antiretroviral-era. The Conference on Retroviruses and Opportunistic Infections (CROI); Seattle, Washington. 2015. [Google Scholar]
- 32.Deshmukh AA, Zhao H, Franzini L, et al. Total Lifetime and Cancer-related Costs for Elderly Patients Diagnosed With Anal Cancer in the United States. Am J Clin Oncol. 2015 doi: 10.1097/COC.0000000000000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berry JM, Jay N, Cranston RD, et al. Progression of anal high-grade squamous intraepithelial lesions to invasive anal cancer among HIV-infected men who have sex with men. Int J Cancer. 2014;134(5):1147–55. doi: 10.1002/ijc.28431. [DOI] [PubMed] [Google Scholar]
- 34.Czoski-Murray C, Karnon J, Jones R, Smith K, Kinghorn G. Cost-effectiveness of screening high-risk HIV-positive men who have sex with men (MSM) and HIV-positive women for anal cancer. Health Technol Assess. 2010;14(53):iii–iv. ix–x, 1–101. doi: 10.3310/hta14530. [DOI] [PubMed] [Google Scholar]
- 35.Hernandez AL, Efird JT, Holly EA, Berry JM, Jay N, Palefsky JM. Incidence of and risk factors for type-specific anal human papillomavirus infection among HIV-positive MSM. AIDS. 2014;28(9):1341–9. doi: 10.1097/QAD.0000000000000254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Phillips AN, Gazzard B, Gilson R, et al. Rate of AIDS diseases or death in HIV-infected antiretroviral therapy-naive individuals with high CD4 cell count. AIDS. 2007;21(13):1717–21. doi: 10.1097/QAD.0b013e32827038bf. [DOI] [PubMed] [Google Scholar]
- 37.Bozzette SA, Joyce G, McCaffrey DF, et al. Expenditures for the care of HIV-infected patients in the era of highly active antiretroviral therapy. N Engl J Med. 2001;344(11):817–23. doi: 10.1056/NEJM200103153441107. [DOI] [PubMed] [Google Scholar]
- 38.U.S. Department of Health and Human Services. Medicare Physician Fee Schedule (MFS) Available from URL: https://www.cms.gov/apps/physician-fee-schedule/overview.aspx.
- 39.U.S. Department of Health and Human Services. Outpatient Prospective Payment System (OPPS) 2016 [Google Scholar]
- 40.U.S. Department of Labor Bureau of Labor Statistic. Consumer Price Index Data from 1913 to 2016. Washington, DC: United States; 2016. [Google Scholar]
- 41.Hanmer J, Lawrence WF, Anderson JP, Kaplan RM, Fryback DG. Report of nationally representative values for the noninstitutionalized US adult population for 7 health-related quality-of-life scores. Med Decis Making. 2006;26(4):391–400. doi: 10.1177/0272989X06290497. [DOI] [PubMed] [Google Scholar]
- 42.Sanders GD, Neumann PJ, Basu A, et al. Recommendations for Conduct, Methodological Practices, and Reporting of Cost-effectiveness Analyses: Second Panel on Cost-Effectiveness in Health and Medicine. JAMA. 2016;316(10):1093–103. doi: 10.1001/jama.2016.12195. [DOI] [PubMed] [Google Scholar]
- 43.Siegel JE, Weinstein MC, Russell LB, Gold MR. Recommendations for reporting cost-effectiveness analyses. Panel on Cost-Effectiveness in Health and Medicine. JAMA. 1996;276(16):1339–41. doi: 10.1001/jama.276.16.1339. [DOI] [PubMed] [Google Scholar]
- 44.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness--the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371(9):796–7. doi: 10.1056/NEJMp1405158. [DOI] [PubMed] [Google Scholar]
- 45.Goldie SJ, Kuntz KM, Weinstein MC, Freedberg KA, Welton ML, Palefsky JM. The clinical effectiveness and cost-effectiveness of screening for anal squamous intraepithelial lesions in homosexual and bisexual HIV-positive men. JAMA. 1999;281(19):1822–9. doi: 10.1001/jama.281.19.1822. [DOI] [PubMed] [Google Scholar]
- 46.Karnon J, Jones R, Czoski-Murray C, Smith KJ. Cost-utility analysis of screening high-risk groups for anal cancer. J Public Health (Oxf) 2008;30(3):293–304. doi: 10.1093/pubmed/fdn045. [DOI] [PubMed] [Google Scholar]
- 47.Burgos J, Curran A, Tallada N, et al. Risk of progression to high-grade anal intraepithelial neoplasia in HIV-infected MSM. AIDS. 2015;29(6):695–702. doi: 10.1097/QAD.0000000000000603. [DOI] [PubMed] [Google Scholar]
- 48.Cantor SB. Cost-effectiveness analysis, extended dominance, and ethics: a quantitative assessment. Med Decis Making. 1994;14(3):259–65. doi: 10.1177/0272989X9401400308. [DOI] [PubMed] [Google Scholar]
- 49.Shiels MS, Kreimer AR, Coghill AE, Darragh TM, Devesa SS. Anal Cancer Incidence in the United States, 1977–2011: Distinct Patterns by Histology and Behavior. Cancer Epidemiol Biomarkers Prev. 2015;24(10):1548–56. doi: 10.1158/1055-9965.EPI-15-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tong WW, Hillman RJ, Kelleher AD, Grulich AE, Carr A. Anal intraepithelial neoplasia and squamous cell carcinoma in HIV-infected adults. HIV Med. 2014;15(2):65–76. doi: 10.1111/hiv.12080. [DOI] [PubMed] [Google Scholar]
- 51.Massad LS, Einstein MH, Huh WK, et al. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. Obstet Gynecol. 2013;121(4):829–46. doi: 10.1097/AOG.0b013e3182883a34. [DOI] [PubMed] [Google Scholar]
- 52.American College of O, Gynecologists. Practice Bulletin No. 140: management of abnormal cervical cancer screening test results and cervical cancer precursors. Obstet Gynecol. 2013;122(6):1338–67. doi: 10.1097/01.AOG.0000438960.31355.9e. [DOI] [PubMed] [Google Scholar]
- 53.McCredie MR, Sharples KJ, Paul C, et al. Natural history of cervical neoplasia and risk of invasive cancer in women with cervical intraepithelial neoplasia 3: a retrospective cohort study. Lancet Oncol. 2008;9(5):425–34. doi: 10.1016/S1470-2045(08)70103-7. [DOI] [PubMed] [Google Scholar]
- 54.Fakoya A, Lamba H, Mackie N, et al. British HIV Association, BASHH and FSRH guidelines for the management of the sexual and reproductive health of people living with HIV infection 2008. HIV Med. 2008;9(9):681–720. doi: 10.1111/j.1468-1293.2008.00634.x. [DOI] [PubMed] [Google Scholar]
- 55.Asboe D, Aitken C, Boffito M, et al. British HIV Association guidelines for the routine investigation and monitoring of adult HIV-1-infected individuals 2011. HIV Med. 2012;13(1):1–44. doi: 10.1111/j.1468-1293.2011.00971.x. [DOI] [PubMed] [Google Scholar]
- 56.Critchlow CW, Surawicz CM, Holmes KK, et al. Prospective study of high grade anal squamous intraepithelial neoplasia in a cohort of homosexual men: influence of HIV infection, immunosuppression and human papillomavirus infection. AIDS. 1995;9(11):1255–62. doi: 10.1097/00002030-199511000-00007. [DOI] [PubMed] [Google Scholar]
- 57.Palefsky JM. Practising high-resolution anoscopy. Sex Health. 2012;9(6):580–6. doi: 10.1071/SH12045. [DOI] [PubMed] [Google Scholar]
- 58.Grulich A. Natural history of anal HPV infection and HSIL and implications for screening and treatment: Data from SPANC study International Anal Neoplasia Society. San Francisco, California: 2016. [Google Scholar]
- 59.Joura EA, Garland SM, Paavonen J, et al. Effect of the human papillomavirus (HPV) quadrivalent vaccine in a subgroup of women with cervical and vulvar disease: retrospective pooled analysis of trial data. BMJ. 2012;344:e1401. doi: 10.1136/bmj.e1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kang WD, Choi HS, Kim SM. Is vaccination with quadrivalent HPV vaccine after loop electrosurgical excision procedure effective in preventing recurrence in patients with high-grade cervical intraepithelial neoplasia (CIN2-3)? Gynecol Oncol. 2013;130(2):264–8. doi: 10.1016/j.ygyno.2013.04.050. [DOI] [PubMed] [Google Scholar]
- 61.Cyrus N, Blechman AB, Leboeuf M, et al. Effect of Quadrivalent Human Papillomavirus Vaccination on Oral Squamous Cell Papillomas. JAMA Dermatol. 2015;151(12):1359–63. doi: 10.1001/jamadermatol.2015.2805. [DOI] [PubMed] [Google Scholar]
- 62.Swedish KA, Goldstone SE. Prevention of anal condyloma with quadrivalent human papillomavirus vaccination of older men who have sex with men. PLoS One. 2014;9(4):e93393. doi: 10.1371/journal.pone.0093393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ghelardi A, Bay P, Tonetti A, Ragusa A, Azienda U. Speranza study: preliminary results of HPV vaccination after loop electrosurgical excision procedure for cervical intraepithelial neoplasia: Toscana nord ovest (Italy) 2016 [Google Scholar]
- 64.Garland SM, Paavonen J, Jaisamrarn U, et al. Prior human papillomavirus-16/18 AS04-adjuvanted vaccination prevents recurrent high grade cervical intraepithelial neoplasia after definitive surgical therapy: Post-hoc analysis from a randomized controlled trial. Int J Cancer. 2016;139(12):2812–26. doi: 10.1002/ijc.30391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hermann JS, Weckx LY, Monteiro Nurmberger J, Santos Junior GF, Campos Pignatari AC, Nagata Pignatari SS. Effectiveness of the human papillomavirus (types 6, 11, 16, and 18) vaccine in the treatment of children with recurrent respiratory papillomatosis. Int J Pediatr Otorhinolaryngol. 2016;83:94–8. doi: 10.1016/j.ijporl.2016.01.032. [DOI] [PubMed] [Google Scholar]
- 66.Madeleine MM, Johnson LG, Doody DR, Tipton ER, Carter JJ, Galloway DA. Natural Antibodies to Human Papillomavirus 16 and Recurrence of Vulvar High-Grade Intraepithelial Neoplasia (VIN3) J Low Genit Tract Dis. 2016;20(3):257–60. doi: 10.1097/LGT.0000000000000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bertisch B, Franceschi S, Lise M, et al. Risk factors for anal cancer in persons infected with HIV: a nested case-control study in the Swiss HIV Cohort Study. Am J Epidemiol. 2013;178(6):877–84. doi: 10.1093/aje/kwt153. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.