Abstract
Background
Allogeneic hematopoietic cell transplant (HCT) cures many patients, but often with the risk of late effects and impaired quality of life. The value of quantifying patient-reported outcomes (PRO) is increasingly recognized, but routine collection of PROs is uncommon. We evaluated feasibility of prospective PRO collection by an outcomes registry, at multiple time points, in unselected HCT patients transplanted in centers contributing clinical data to the CIBMTR, and correlated those with clinical and demographic data.
Methods
FACT-BMT, SF-36 and PedsQL measures were administered pre-HCT, day 100, 6 and 12 months. Patients were recruited by the transplant center, but post-transplant PRO collection was managed centrally by CIBMTR.
Results
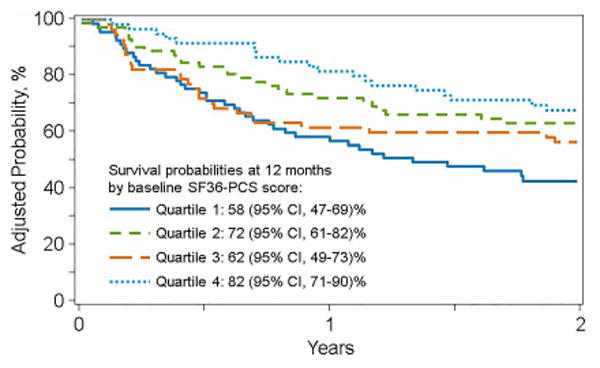
Of 580 eligible patients, 390 (67%) enrolled. Feasibility was shown by high time-specific retention rates (1-year: 176/238, 74%) and participant satisfaction. Factors associated with higher response rate were age >50 (OR 1.58, 95% CI 1.03-2.41, p=0.0355), Caucasian race (OR 4.61, 95% CI 2.66-7.99, p<0.0001) and being married (OR 2.28, 95% CI 1.42-3.65, p=0.0006) in adults; and higher family income in children (OR 4.99, 95% CI 2.12-11.75, p=0.0002). Importantly, pre-HCT PRO scores independently predicted survival after adjusting for patient-, disease- and transplant-related factors. Adjusted probabilities of 1-year survival by increasing quartiles of pre-HCT FACT-BMT and physical component score of SF-36 scores were 56%, 67%, 75%, 76% and 58%, 72%, 62%, 82%, respectively.
Conclusions
A hybrid model of local consent for centralized PRO collection is feasible, and pre-transplant PROs provide critical prognostic information for HCT outcomes.
Keywords: Patient-Reported Outcomes, Transplantation, Survival, SF-36, FACT-BMT
Introduction
While allogeneic hematopoietic cell transplantation (HCT) offers a potential cure to many patients with malignancies or serious blood disorders, a significant risk of late effects and consequent morbidity exists. It is well recognized that patient-reported outcomes (PRO)s show alterations following the procedure compared to baseline. Several studies show an initial deterioration with subsequent improvement in PRO scores using multi-dimensional measures 1,2. Other studies have assessed the transplant related factors which impact PROs, including post-transplant complications which adversely affect health related quality of life (HRQOL)2-6.
Although it is known that baseline PRO data correlates with cancer survival in general7, this relationship is not well studied in the HCT setting. A recent prospective multi-center clinical trial testing an exercise and stress management intervention in HCT patients showed, however, that baseline PROs significantly predict survival post-transplant, even after adjusting for other pre-transplant clinical and demographic factors8. Thus, baseline PRO scores have value for prognostication and individualized counselling of patients.
Despite the value described above, broad systematic efforts to collect PRO data alongside the clinical data collected by transplant outcomes registries, in order to link these in large representative datasets, has not occurred. Doing so would have numerous benefits for conducting research in this area including, amongst others, the availability of detailed clinical outcomes from the registry, a well-defined patient population (with comprehensively collected demographics and pre-transplant characteristics) that is already undergoing extensive medical evaluations, and an existing infrastructure for the collection, collation and analysis of data. In addition, PRO can be used as a variable in multivariate analysis in this setting.
The Center for International Blood and Marrow Transplantation Research (CIBMTR) has been collecting HCT outcomes data worldwide for over 40 years, resulting in a research database including more than 425,000 patients. Information from the database, and the support provided by the CIBMTR Coordinating Center to analyze it, have led to the successful completion of hundreds of studies led by independent investigators across the transplant and wider biomedical community that have significantly guided clinical practice worldwide.
The overall aim of this study was to assess the feasibility of routinely collecting PRO data, using a hybrid model of local consent followed by centralized data collection by the CIBMTR Coordinating Center, in the setting of an established outcomes registry. A second aim was to correlate these data with the registry clinical data and outcomes.
Methods
Patient selection, enrollment and assessment schedule
Seven transplant centers (TCs), of a possible 183, in the United States participated; five enrolled all ages and one each pediatric or adult only. Centers were invited to participate based upon the quality of clinical data submitted to the CIBMTR, interest in survivorship, and willingness to enroll and consent patients for centralized PRO collection. All patients ≥2 years old undergoing an allogeneic HCT at these TCs were eligible. Patients were required to speak English, have access to a telephone and have a valid mailing address in the United States. Of 580 eligible patients, 390 (67%) consented and enrolled. The baseline PRO measures were filled out using paper-and-pencil by patients (and proxies in the case of children) within 30 days before the HCT and returned to the CIBMTR. The measures completed at 100 days, 6 and 12 months post-transplant were administered directly by CIBMTR to the participant by mail. If patients did not return the measure within three weeks of the post-transplant time point, up to three reminder calls were placed after CIBMTR confirmed survival status with the TC.
Forms and PRO measures
CIBMTR study-specific Sociodemographic self-report form
Patients (or proxies) reported sociodemographic information at baseline, including race and ethnicity, marital status, occupation, work status, highest educational grade, health insurance coverage and household income.
PRO measures
Adults completed the Short Form 36 Health Survey (SF-36) and the Functional Assessment of Cancer Therapy – Bone Marrow Transplant (FACT-BMT), the most commonly used measures in HCT studies9. The SF-36 is a 36-item multidimensional measure that assesses patient-reported health and functioning. It takes approximately five minutes to complete. From the SF-36, two summary domains are calculated, the Physical Component Summary (PCS) scale and the Mental Component Summary (MCS) scale. The normal population mean for the PCS and MCS is 50 with a standard deviation of 1010. Higher scores indicate better functioning. The FACT-BMT is a 37-item measure composed of the FACT-G (General) plus a transplant-specific subscale. The FACT-G is comprised of 4 domains, physical (7 items), social (7 items, including sexual satisfaction), emotional (6 items) and functional (7 items, including work, sleep and leisure activities). The transplant-specific module includes 10 scored items, including appetite, appearance, mobility, and fatigue. Higher scores indicate better functioning11. Children (5-18) and proxies (for all children aged 2-18) completed the PedsQL Measurement Model, which integrates generic core scales and disease-specific modules into one measurement system12. The PedsQL is a 23-item measure composed of 4 multidimensional function scales including physical (8 items), social (5 items), emotional (5 items) and school (5 items) which together generate a total summary score. Higher scores indicate better functioning.
Clinical data collection forms
Demographic, disease-specific and transplant-specific data are routinely collected pre-transplant, and outcome data at day 100, 6 months and then annually for 6 years and biannually thereafter lifelong, using CIBMTR's proprietary web-based data collection, FormsNet3.
Post participation satisfaction questionnaire
Patients alive and participating in the study 1-year post transplant were asked to assess their experience with the study and their willingness to receive future contact.
Statistical analysis
For both adults and pediatrics, descriptive statistics were used to summarize patient sample characteristics. Categorical variables were summarized with counts and percentages whilst means and standard deviations were used for continuous variables. The mean and standard deviation of the PRO measures were produced at every time point to describe their change over time. Retention rates are defined as the percentage of patients, remaining alive and enrolled at each time-point, who return their measure(s). Response rate was defined as the proportion of the measures completed out of the total number of measures a patient could have completed. Withdrawal or death, but not relapse, marked the end of the observation window. Binomial regression with the logit link function and allowing for over-dispersion was used to identify factors associated with a higher proportion of forms completed.
For adults, the baseline FACT-BMT and SF-36 quartiles based on the population sample distribution were used for survival analysis and in evaluating HRQOL at one year. A Cox proportional hazards model was used to compare survival of the patients in different baseline quartiles of the HRQOL measures after adjusting for other significant predictors. Proportionality assumptions were tested prior to model development. Backward variable elimination procedures were used to identify significant factors to be included in the final model. Significant covariates were accounted for when calculating adjusted survival probabilities used to illustrate patients' survival experience using graphical tools. Linear regression was used to assess the baseline FACT and SF-36 score effect on the scores from these two measures at 1-year post transplant.
In children, the baseline PedsQL score was used as a binary covariate with established cut-off for ill health12 (69.7 and 65.4 for child self-report and proxy report, respectively). Kaplan-Meier curves were used to estimate survival probabilities for patients above and below the established cut-offs at baseline. A Cox regression model was attempted to predict survival based on the PedsQL baseline score and other covariates. Logistic regression predicting the odds of higher PedsQL score at 1-year post transplant was utilized.
In regression analyses for all ages, covariates considered in the multivariable models included: Patient related (age, gender, race/ethnicity, Karnofsky/Lansky performance and HCT- Comorbidity-Index (HCT-CI)13 scores); disease indication for transplant; treatment-related (conditioning regimen intensity, use of total body irradiation and dosage, stem cell source, donor, use of antithymocyte globulin (ATG)/Campath, year of transplant) and sociodemographic variables (marital status (adults only), education level (adults only), household income and indicator of rural/urban area). A significance level alpha of 0.05 was used throughout, and all tests are two-sided. SAS statistical software (SAS Institute, Cary, NC) was used to perform all statistical analyses.
Results
Patients
Time point retention
390 (67%) of 580 eligible patients were consented and enrolled between August 2011 and September 2013. Reasons that patients did not enroll included: participation in a different PRO study, being too sick, not being interested in participating in research or not being approached in the correct time window. Patients remaining alive and enrolled at 100 days, 6 months and 1 year were sent surveys by postal mail. Of those, 45% of participants returned their survey without a reminder, 30 – 50% (at each time point) required 1 reminder and the remainder received more than 1 reminder. Time point retention rates at 100 days, 6 months and 1 year were 223/323 (69%), 213/281 (75.8%) and 176/238 (74%) respectively. Retention rates in adults were higher at each time point than those in children at 173/238 (73%), 163/202 (81%) and 136/174 (78%) versus 50/85 (59%), 50/79 (63%) and 43/72 (60%), respectively.
Inclusion in analysis (response rate and QOL)
Of 390 patients, 347 (89%) submitted sufficient data for analysis. Forty-three patients were excluded due to: transplant did not occur (n=28); patient withdrew (n= 6); patient returned no measures (n=5) or an unscorable (less than half of the measure completed) baseline FACT-BMT or PedsQL (n=4). A further 7 patients did not return the FACT-BMT or PedsQL at baseline and are not included in the post-transplant analysis. Patient, socio-demographic and transplant data, as well as response rates as defined in the statistical methods, are shown in Tables 1 and 2.
Table 1. Patient, socio-demographic, transplant and PRO measure characteristics of adult patients included in the study.
| Variable | Feasibility Cohort | QOL analysis cohort | |||
|---|---|---|---|---|---|
|
| |||||
| Baseline | 100 day | 6 months | 1 year | ||
| Number of adult patients | 264 | 263 | 171 | 159 | 134 |
| Number of adult patients surviving* | 263 | 227 | 203 | 175 | |
| Median age at transplant (range), years | 55 (19-75) | 55 (19-75) | 55 (19-74) | 55 (19-75) | 54 (19-74) |
| 18-29 | 34 (13) | 34 (13) | 17 (10) | 19 (12) | 11 (8) |
| 30-39 | 24 (9) | 24 (9) | 17 (10) | 13 (8) | 14 (10) |
| 40-49 | 40 (15) | 40 (15) | 23 (13) | 23 (14) | 22 (16) |
| 50-59 | 77 (29) | 77 (29) | 58 (34) | 56 (35) | 47 (35) |
| 60-69 | 79 (30) | 78 (30) | 52 (30) | 43 (27) | 36 (27) |
| 70+ | 10 (4) | 10 (4) | 4 (2) | 5 (3) | 4 (3) |
| Gender | |||||
| Male | 154 (58) | 153 (58) | 97 (57) | 94 (59) | 77 (57) |
| Female | 110 (42) | 110 (42) | 74 (43) | 65 (41) | 57 (43) |
| Race/Ethnicity | |||||
| Caucasian/White | 236 (89) | 235 (89) | 162 (95) | 150 (94) | 125 (93) |
| Black | 12 (5) | 12 (5) | 0 | 2 (1) | 3 (2) |
| Hispanic | 7 (3) | 7 (3) | 4 (2) | 2 (1) | 1 (<1) |
| Asian/Hawaiian/Pacific Islander | 7 (3) | 7 (3) | 3 (2) | 3 (2) | 3 (2) |
| Unknown/Declined | 2 (<1) | 2 (<1) | 2 (1) | 2 (1) | 2 (1) |
| Karnofsky score prior to transplant | |||||
| 90-100% | 158 (60) | 158 (60) | 109 (64) | 101 (64) | 90 (67) |
| ≤ 80% | 106 (40) | 105 (40) | 62 (36) | 58 (36) | 44 (33) |
| HCT-CI | |||||
| 0 | 63 (24) | 63 (24) | 45 (26) | 42 (26) | 37 (28) |
| 1 | 45 (17) | 45 (17) | 33 (19) | 30 (19) | 23 (17) |
| 2 | 48 (18) | 48 (18) | 28 (16) | 23 (14) | 21 (16) |
| 3+ | 101 (38) | 100 (38) | 61 (36) | 61 (38) | 50 (37) |
| Missing | 7 (3) | 7 (3) | 4 (2) | 3 (2) | 3 (2) |
|
| |||||
| Indication for transplant | |||||
| Acute Leukemia | 130 (49) | 129 (49) | 85 (50) | 74 (47) | 62 (46) |
| Chronic Myeloid Leukemia | 19 (7) | 19 (7) | 11 (6) | 12 (8) | 10 (7) |
| Myelodysplasia/Myeloproliferative | 49 (19) | 49 (19) | 32 (19) | 28 (18) | 24 (18) |
| Other Leukemia | 20 (8) | 20 (8) | 15 (9) | 14 (9) | 10 (7) |
| Non Hodgkin Lymphoma | 22 (8) | 22 (8) | 13 (8) | 15 (9) | 14 (10) |
| Hodgkin Disease | 6 (2) | 6 (2) | 3 (2) | 4 (3) | 4 (3) |
| Multiple myeloma/plasma cell leukemia | 7 (3) | 7 (3) | 4 (2) | 4 (3) | 4 (3) |
| Nonmalignant diseases | 11 (4) | 11 (4) | 8 (5) | 8 (5) | 6 (4) |
| Supplemental sociodemographic form returned | |||||
| No | 5 (2) | 5 (2) | 3 (2) | 2 (1) | 3 (2) |
| Yes | 259 (98) | 258 (98) | 168 (98) | 157 (99) | 131 (98) |
| Marital status | |||||
| Married or living with partner | 182 (69) | 181 (69) | 128 (75) | 117 (74) | 104 (78) |
| Single/separated/divorced/widowed | 55 (21) | 55 (21) | 28 (16) | 27 (17) | 19 (14) |
| Missing | 27 (10) | 27 (10) | 15 (9) | 15 (9) | 11 (8) |
| Education level | |||||
| Elementary education | 1 (<1) | 1 (<1) | 0 | 1 (<1) | 1 (<1) |
| Lower secondary education | 7 (3) | 7 (3) | 4 (2) | 5 (3) | 3 (2) |
| Upper secondary education | 57 (22) | 57 (22) | 32 (19) | 29 (18) | 25 (19) |
| Post-secondary, non-tertiary (vocational) | 39 (15) | 39 (15) | 26 (15) | 26 (16) | 19 (14) |
| Tertiary education A - Bachelors/Masters | 101 (38) | 100 (38) | 67 (39) | 63 (40) | 54 (40) |
| Tertiary education B - Associates | 28 (11) | 28 (11) | 22 (13) | 17 (11) | 17 (13) |
| Advanced research qualification | 17 (6) | 17 (6) | 12 (7) | 12 (8) | 9 (7) |
| Missing | 14 (5) | 14 (5) | 8 (5) | 6 (4) | 6 (4) |
|
| |||||
| Household annual income | |||||
| < $60,000 | 52 (20) | 52 (20) | 29 (17) | 28 (18) | 24 (18) |
| ≥ $60,000 | 78 (30) | 77 (29) | 57 (33) | 56 (35) | 42 (31) |
| Missing | 134 (51) | 134 (51) | 85 (50) | 75 (47) | 68 (51) |
| Prior transplant | |||||
| No prior transplant | 230 (87) | 229 (87) | 149 (87) | 140 (88) | 116 (87) |
| Prior autologous HCT | 27 (10) | 27 (10) | 18 (11) | 17 (11) | 16 (12) |
| Prior allogeneic HCT | 7 (3) | 7 (3) | 4 (2) | 2 (1) | 2 (1) |
| Myeloablative conditioning | |||||
| No | 125 (47) | 124 (47) | 86 (50) | 75 (47) | 65 (49) |
| Yes | 139 (53) | 139 (53) | 85 (50) | 84 (53) | 69 (51) |
| Total body irradiation use | |||||
| No | 150 (57) | 149 (57) | 104 (61) | 96 (60) | 77 (57) |
| Yes | 114 (43) | 114 (43) | 67 (39) | 63 (40) | 57 (43) |
| Stem cell source | |||||
| Bone marrow | 38 (14) | 38 (14) | 26 (15) | 22 (14) | 18 (13) |
| Peripheral blood | 197 (75) | 196 (75) | 135 (79) | 128 (81) | 108 (81) |
| Umbilical cord blood | 29 (11) | 29 (11) | 10 (6) | 9 (6) | 8 (6) |
| Donor | |||||
| Matched relative | 104 (39) | 104 (40) | 76 (44) | 72 (45) | 57 (43) |
| Mismatched relative | 4 (2) | 4 (2) | 0 | 0 | 1 (<1) |
| Matched Unrelated | 105 (40) | 104 (40) | 76 (44) | 67 (42) | 59 (44) |
| Mismatched Unrelated | 22 (8) | 22 (8) | 9 (5) | 11 (7) | 9 (7) |
| Cord blood | 29 (11) | 29 (11) | 10 (6) | 9 (6) | 8 (6) |
| ATG/Campath use as part of GVHD prophylaxis | |||||
| No | 197 (75) | 197 (75) | 131 (77) | 121 (76) | 97 (72) |
| Yes | 67 (25) | 66 (25) | 40 (23) | 38 (24) | 37 (28) |
|
| |||||
| Year of transplant | |||||
| 2011 | 25 (9) | 25 (10) | 12 (7) | 12 (8) | 12 (9) |
| 2012 | 185 (70) | 185 (70) | 121 (71) | 113 (71) | 94 (70) |
| 2013 | 54 (20) | 53 (20) | 38 (22) | 34 (21) | 28 (21) |
| Measures completed | |||||
| FACT-BMT and SF-36 | 256 (97) | 256 (97) | 168 (98) | 155 (97) | 129 (96) |
| FACT-BMT only | 7 (3) | 7 (3) | 1 (<1) | 0 | 2 (1) |
| SF-36 only | 0 | 0 | 2 (1) | 4 (3) | 3 (2) |
| Missing baseline FACT-BMT | 1 (<1) | N/A | N/A | N/A | N/A |
| Mean (range) FACT-BMT score | 104 (53-148) | 104 (53-148) | 101 (50-143) | 105 (46-143) | 107 (44-145) |
| < baseline mean score (104) | 131 (50) | 93 (54) | 71 (45) | 58 (43) | |
| ≥ baseline mean score (104) | 132 (50) | 76 (44) | 84 (53) | 73 (54) | |
| Missing FACT-BMT | 1 (<1) | N/A | 2 (1) | 4 (3) | 3 (2) |
| Mean (range) SF-36-PCS | 42 (19-64) | 42 (19-64) | 40 (21-58) | 42 (21-59) | 42 (13-67) |
| < 50 | 193 (73) | 193 (73) | 141 (82) | 120 (75) | 93 (69) |
| ≥ 50 | 63 (24) | 63 (24) | 29 (17) | 39 (25) | 39 (29) |
| Missing SF-36 | 8 (3) | 7 (3) | 1 (<1) | 0 | 2 (1) |
| Mean (range) SF-36-MCS | 49 (19-71) | 49 (19-71) | 50 (17-65) | 50 (20-67) | 52 (15-73) |
| < 50 | 118 (45) | 118 (45) | 64 (37) | 66 (42) | 39 (29) |
| ≥ 50 | 138 (52) | 138 (52) | 106 (62) | 93 (58) | 93 (69) |
| Missing SF-36 | 8 (3) | 7 (3) | 1 (<1) | 0 | 2 (1) |
| Median follow-up of survivors (range), months | 26 (8-38) | 26 (8-38) | 26 (13-38) | 27 (13-38) | 26 (12-38) |
HCT-CI – Hematopoietic cell transplantation Comorbidity Index; GVHD – Graft versus Host Disease; MMF – mycophenolate mofetil; MTX – methotrexate
Includes all adults in this study who were alive in the CIBMTR database at that time point
Table 2. Patient, socio-demographic, transplant and PRO measure characteristics of pediatric patients included in the study.
| Variable | Feasibility cohort | QOL analysis cohort | |||
|---|---|---|---|---|---|
|
| |||||
| Baseline | 100 days | 6 months | 1 year | ||
| Number of children | 83 | 77 | 45 | 46 | 37 |
| Number of children surviving* | 77 | 73 | 70 | 65 | |
| Median age at transplant (range), years | 7 (2-18) | 7 (2-18) | 7 (2-17) | 8 (2-17) | 7 (2-17) |
| 2-4 | 24 (29) | 24 (31) | 14 (31) | 13 (28) | 9 (24) |
| 5-7 | 23 (28) | 21 (27) | 12 (27) | 11 (24) | 12 (32) |
| 8-12 | 20 (24) | 18 (23) | 9 (20) | 10 (22) | 7 (19) |
| 13-18 | 16 (19) | 14 (18) | 10 (22) | 12 (26) | 9 (24) |
| Gender | |||||
| Male | 45 (54) | 42 (55) | 28 (62) | 28 (61) | 21 (57) |
| Female | 38 (46) | 35 (45) | 17 (38) | 18 (39) | 16 (43) |
| Race/Ethnicity | |||||
| Caucasian/White | 67 (81) | 63 (82) | 39 (87) | 39 (85) | 33 (89) |
| Black | 6 (7) | 6 (8) | 3 (7) | 3 (7) | 2 (5) |
| Hispanic | 7 (8) | 5 (6) | 1 (2) | 3 (7) | 1 (3) |
| Asian/Hawaiian/Pacific Islander | 2 (2) | 2 (3) | 1 (2) | 1 (2) | 1 (3) |
| Unknown/Declined | 1 (1) | 1 (1) | 1 (2) | 0 | 0 |
| Lansky score prior to transplant | |||||
| 90-100% | 73 (88) | 68 (88) | 40 (89) | 40 (87) | 32 (86) |
| < 90% | 10 (12) | 9 (12) | 5 (11) | 6 (13) | 5 (14) |
| HCT-CI index | |||||
| 0 | 55 (66) | 52 (68) | 31 (69) | 30 (65) | 26 (70) |
| 1+ | 26 (31) | 23 (30) | 13 (29) | 14 (30) | 11 (30) |
| Missing | 2 (2) | 2 (3) | 1 (2) | 2 (4) | 0 |
| Indication for transplant | |||||
| Acute Myeloid Leukemia | 12 (14) | 11 (14) | 7 (16) | 7 (15) | 4 (11) |
| Acute Lymphoblastic Leukemia | 20 (24) | 17 (22) | 10 (22) | 10 (22) | 10 (27) |
| Chronic Myeloid Leukemia | 1 (1) | 1 (1) | 0 | 1 (2) | 1 (3) |
| Myelodysplasia/Myeloproliferative | 4 (5) | 4 (5) | 2 (4) | 3 (7) | 1 (3) |
| Severe aplastic anemia | 7 (8) | 6 (8) | 3 (7) | 3 (7) | 3 (8) |
| Inherited abnormal erythrocyte diff./function a | 17 (20) | 17 (22) | 12 (27) | 12 (26) | 10 (27) |
| SCID & other immune system disorders b | 11 (13) | 10 (13) | 4 (9) | 4 (9) | 3 (8) |
| Adrenoleukodystophy | 1 (1) | 1 (1) | 0 | 0 | 0 |
| Familial erythrohemophagocytic lymphosis | 9 (11) | 9 (12) | 6 (13) | 5 (11) | 5 (14) |
| Autoimmune enteropathy | 1 (1) | 1 (1) | 1 (2) | 1 (2) | 0 |
| Supplemental sociodemographic form returned | |||||
| No | 5 (6) | 4 (5) | 2 (4) | 3 (7) | 3 (8) |
| Yes | 78 (94) | 73 (95) | 43 (96) | 43 (93) | 34 (92) |
| Household annual income | |||||
| < $60,000 | 36 (43) | 32 (42) | 16 (36) | 15 (33) | 8 (22) |
| ≥ $60,000 | 29 (35) | 29 (38) | 21 (47) | 22 (48) | 20 (54) |
| Missing | 18 (22) | 16 (21) | 8 (18) | 9 (20) | 9 (24) |
| Prior transplant | |||||
| No prior transplant | 77 (93) | 71 (92) | 41 (91) | 43 (93) | 36 (97) |
| Prior allogeneic HCT | 6 (7) | 6 (8) | 4 (9) | 3 (7) | 1 (3) |
| Myeloablative conditioning | |||||
| No | 23 (28) | 22 (29) | 12 (27) | 12 (26) | 10 (27) |
| Yes | 60 (72) | 55 (71) | 33 (73) | 34 (74) | 27 (73) |
| Total body irradiation use | |||||
| No | 53 (64) | 51 (66) | 27 (60) | 30 (65) | 24 (65) |
| Yes | 30 (36) | 26 (34) | 18 (40) | 16 (35) | 13 (35) |
| Stem cell source | |||||
| Bone marrow | 50 (60) | 46 (60) | 27 (60) | 30 (65) | 24 (65) |
| Peripheral blood | 21 (25) | 20 (26) | 13 (29) | 12 (26) | 10 (27) |
| Umbilical cord blood | 12 (14) | 11 (14) | 5 (11) | 4 (9) | 3 (8) |
| Donor | |||||
| Matched relative | 12 (14) | 11 (14) | 6 (13) | 6 (13) | 6 (16) |
| Mismatched relative | 1 (1) | 1 (1) | 0 | 0 | 0 |
| Matched Unrelated | 51 (61) | 47 (61) | 30 (67) | 31 (67) | 24 (65) |
| Mismatched Unrelated | 7 (8) | 7 (9) | 4 (9) | 5 (11) | 4 (11) |
| Cord blood | 12 (14) | 11 (14) | 5 (11) | 4 (9) | 3 (8) |
| ATG/Campath use as part of GVHD prophylaxis | |||||
| No | 33 (40) | 31 (40) | 17 (38) | 17 (37) | 12 (32) |
| Yes | 50 (60) | 46 (60) | 28 (62) | 29 (63) | 25 (68) |
|
| |||||
| Year of transplant | |||||
| 2011 | 9 (11) | 9 (12) | 4 (9) | 6 (13) | 5 (14) |
| 2012 | 55 (66) | 50 (65) | 29 (64) | 29 (63) | 22 (59) |
| 2013 | 19 (23) | 18 (23) | 12 (27) | 11 (24) | 10 (27) |
| PedsQL measures completed | |||||
| Proxy only patients (age<5) | 24 (29) | 24 (31) | 14 (31) | 13 (28) | 9 (24) |
| PedsQL and proxy completed | 49 (59) | 49 (64) | 31 (69) | 33 (72) | 26 (70) |
| Only PedsQL completed | 3 (4) | 3 (4) | 0 | 0 | 1 (3) |
| Only proxy completed | 1 (1) | 1 (1) | 0 | 0 | 1 (3) |
| Missing baseline PedsQL | 6 (7) | N/A | N/A | N/A | N/A |
| Mean (range) PedsQL score | 67 (26-100) | 67 (26-100) | 71 (20-100) | 73 (31-97) | 77 (25-96) |
| Baseline < 69.7 | 28 (34) | 28 (36) | 16 (36) | 14 (30) | 8 (22) |
| ≥ 69.7 | 24 (29) | 24 (31) | 15 (33) | 19 (41) | 19 (51) |
| Missing PedsQL | 7 (8) | 1 (1) | 0 | 0 | 1 (3) |
| N/A – proxy only patients (age<5) | 24 (29) | 24 (31) | 14 (31) | 13 (28) | 9 (24) |
| Mean (range) proxy PedsQL score | 65.5 (10-100) | 65 (10-100) | 64 (14-100) | 71 (24-99) | 73 (25-100) |
| Baseline < 65.4 | 36 (43) | 36 (47) | 22 (49) | 17 (37) | 9 (24) |
| ≥ 65.4 | 38 (46) | 38 (49) | 23 (51) | 29 (63) | 27 (73) |
| Missing proxy PedsQL | 9 (11) | 3 (4) | 0 | 0 | 1 (3) |
| Median follow-up of survivors (range), months | 25 (6-46) | 25 (6-46) | 24 (6-46) | 25 (6-46) | 25 (11-46) |
Inherited abnormalities of erythrocyte differentiation/function includes: Fanconi anemia (n=11), Diamond-Blackfan anemia (n=3), Dyskeratosis congenital (n=2), thalassemia (n=1)
SCID and other immune system disorders includes: Wiskott Aldrich syndrome (n=3), chronic granulomatous disease (n=1), common variable immunodeficiency (n=2), CD40 ligand deficiency (n=2), hyper IgE syndrome (n=1), IPEX (n=1), IPEX-like syndrome (n=1)
HCT-CI – Hematopoietic cell transplantation Comorbidity Index; GVHD – Graft versus Host Disease; MMF – mycophenolate mofetil; MTX – methotrexate
Includes all children in this study who were alive in the CIBMTR database at that time point
Factors associated with response rate in returning PRO measures
In adults, factors significantly associated with higher response rate in the regression model were, age >50 (OR 1.58, 95% CI 1.03-2.41, p=0.0355), Caucasian race (OR 4.61, 95% CI 2.66-7.99, p<0.0001) and being married (OR 2.28, 95% CI 1.42-3.65, p=0.0006). Pre-HCT FACT-BMT, PCS and MCS scores were not significantly associated with response rate.
In pediatric patients, the only factor associated with higher response rate in the regression model was higher family income (OR 4.99, 95% CI 2.12-11.75, p=0.0002). The pre-HCT PedsQL self-report or proxy scores were not significantly associated with response rate.
PRO measures
Adult
The mean FACT-BMT and SF-36 scores are shown in Table 1, with the pattern of change over time shown in Table 3. Patients in the lowest quartile reported improved PRO scores at each post-transplant time point with a significantly higher score at 1-year post transplant compared to baseline. Conversely patients in the higher three quartiles all reported a drop in their day 100 scores, followed by a gradual increase to near baseline for Q2 and Q3 but to below baseline for Q4.
Table 3. FACT and PCS scores over time in each quartile (presented as mean and 95% CI for the mean).
| Baseline FACT and SF-36 PCS Quartile | ||||||||
|---|---|---|---|---|---|---|---|---|
| FACT | SF-36 PCS | |||||||
| Q1 | Q2 | Q3 | Q4 | Q1 | Q2 | Q3 | Q4 | |
| Baseline Quartile Range | 52-89.99 | 90-103.99 | 104-117.99 | >118 | 18-33.99 | 34-41.99 | 42-49.99 | >50 |
| Baseline | 79.3 (76.5- 82.0) |
96.5 (95.5 - 97.5) |
110.2 (109.1-111.2) |
127.4 (125.5 - 129.2) |
30.2 (29.5 - 31.0) |
37.8 (37.2 - 38.4) |
45.3 (44.7 - 45.9) |
55.0 (54.2 - 55.8) |
| 100 days | 91.0 (85.1 - 96.9) |
92.9 (87.2 - 98.5) |
103.3 (98.6-108.0) |
113.7 (109.0-118.3) |
35.2 (32.8 - 37.6) |
37.3 (34.6 - 39.9) |
39.1 (36.0 - 42.2) |
45.3 (43.9 - 48.6) |
| 6 months | 98.1 (90.4 - 105.8) |
92.5 (85.8 - 99.1) |
108.2 (102.3 - 114.1) |
114.8 (109.8- 119.8) |
37.7 (34.2 - 41.2) |
39.4 (36.3 - 42.5) |
43.5 (40.3 - 46.7) |
46.2 (43.7 - 48.7) |
| 12 months | 101.8 (94.4 -109.3) |
96.6 (25.5) (87.4- 105.9) |
108.3 (102.3- 114.3) |
118.1 (112.7-123.5) |
39.0 (34.3 - 43.7) |
39.9 (36.7 - 43.1) |
42.2 (38.8 - 45.6) |
46.5 (42.4 - 50.6) |
The pre-transplant FACT-BMT and PCS were strongly correlated with the pre-transplant KPS (80% of patients in the highest FACT-BMT quartile had KPS>=90 while only 51% in the lowest quartile had a KPS>=90. Similarly, 81% of patients in the highest PCS quartile had KPS >=90 and only 39% in the lowest PCS quartile had a KPS score of >=90). HCT-CI was not correlated with PROs.
Pediatrics
The mean PedsQL scores are shown in Table 2. The overall PedsQL score at baseline was below the cut-off for ill health for the pediatric self-report (67 vs 69.7), but not for the proxy report (65.5 vs 65.4). Children below the cut off for ill health at baseline showed a steady increase in PRO scores at each time point, while those who started with a high score at baseline had an initial drop, with a return to near baseline at 1 year. This pattern was similar for the proxy reports (Table 4).
Table 4. PedsQL scores over time using the cut-off for ill health as a binary (presented as mean and 95% CI for the mean).
| Self-reported PedsQL score at baseline above or below the cut-off for ill-health | Proxy reported PedsQL score at baseline above or below the cut-off for ill-health | |||
|---|---|---|---|---|
| Cut-off for ill health | <69.7 (n=28) | >=69.7 (n=24) | <65.4 (n=36) | >=65.4 (n=38) |
| Baseline | 53.9 (49.5 - 58.3) | 83.0 (79.5 - 86.5) | 48.8 (44.1 - 53.4) | 81.3 (78.0 - 84.6) |
| 100 days | 60.8 (51.5 - 69.9) | 78.1 (70.8 - 85.3) | 52.1 (42.1 - 62.0) | 72.9 (65.9 -79.9) |
| 6 months | 65.2 (56.4 - 74.0) | 78.8 (69.0 - 88.5) | 60.6 (50.9 - 70.4) | 78.2 (71.6 - 84.6) |
| 12 months | 72.3 (61.1 - 83.6) | 80.0 (66.6 - 93.3) | 61.2 (49.4 - 73.0) | 82.2 (76.2 - 88.2) |
Association of baseline PRO measure scores with survival and post-transplant QOL
Adults
Both the pre-HCT FACT-BMT and the PCS were significantly associated with overall survival after adjusting for patient, disease and transplant related factors commonly associated with survival after allogeneic HCT (figures 1a and 1b). The only other factors which retained significance in the model as predictors of lower survival were older patient age (>55 years) and the use of cord blood as a graft source (Supplementary Tables 1 and 2). The MCS at baseline was not associated with overall survival.
Figure 1a. Adjusted Probability of Overall Survival based on the FACT-BMT displayed in quartiles.
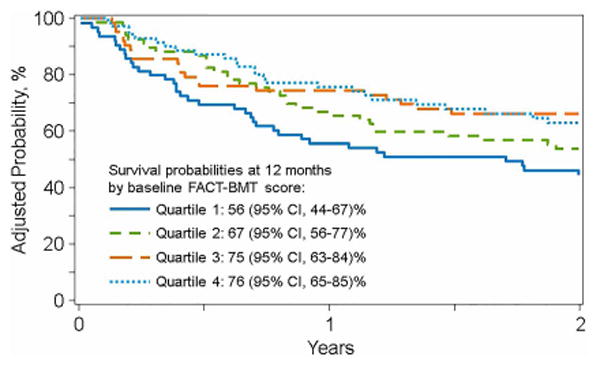
Figure 1b. Adjusted Probability of Overall Survival based on the PCS of the SF-36 displayed in quartiles.
We tested whether a higher baseline PRO score predicted better HRQOL post-transplant. In linear regression models we found that the pre-HCT FACT-BMT and PCS were both associated with the 1-year FACT-BMT or PCS, respectively, after adjusting for other characteristics (data not shown). In addition, the baseline PCS was associated with the FACT-BMT score at 1 year, however the baseline FACT-BMT was not associated with 1 year PCS (data not shown).
Pediatrics
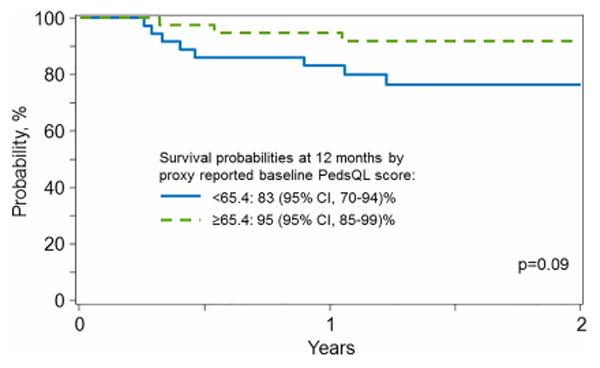
Survival probability estimates at 1 and 2 years for the lower vs higher scores of the baseline PedsQL were 79% and 92% and 69% and 92%, respectively (Figure 1c). Likewise, survival probability estimates at 1 and 2 years for the low vs high scores of the baseline proxy reports were 83% and 95% and 77% and 92%, respectively (Figure 1d). No other covariates were significant in the model. A higher baseline PRO score was associated with a higher score at 1 year, statistically significant for the proxy- but not self-report, (self-report: OR=6.00, 95%CI 0.92-39.18, p=0.0613; proxy report: OR=15.75, 95%CI 1.65-150.12, p=0.0166).
Figure 1c. Probability of Overall Survival based on the PedsQL self-report displayed as a binary covariate.
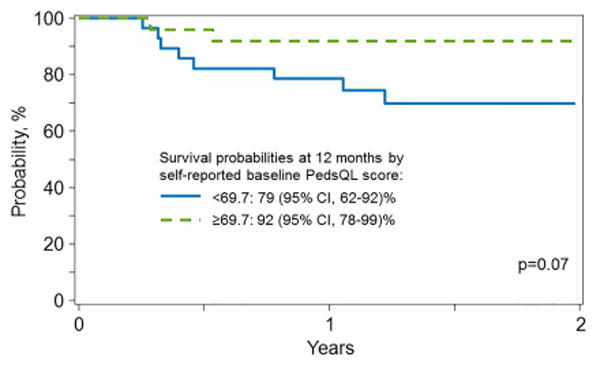
Figure 1d. Probability of Overall Survival based on the PedsQL proxy report displayed as a binary covariate.
Post participation satisfaction questionnaire
Survey results are provided in Table 5. Almost all respondents found the measures easy to complete (89%) and were comfortable doing so (74%). 44% spent 10 minutes or less completing the battery and 79% felt ‘pen and paper’ was convenient. The highest number of respondents (n=94) listed ‘pen and paper’ as their response method of choice, however 46 respondents would prefer either an online website or email questionnaire be used. Patients contacted by phone felt comfortable, but only half of those answering this question would have been willing to be contacted directly by the CIBMTR for study consent and enrollment. Finally, although only 29% reported that completing these measures was helpful to them, 87% reported that they would be willing to complete PRO measures in a routine manner in the future.
Table 5. Responses to post participation patient survey.
| Variable | N (%) |
|---|---|
| Number of patients that completed satisfaction questionnaire | 179 |
| Overall, how easy was it for you to complete the Quality of Life questionnaires? | |
| It was very difficult | 3 (2) |
| It was somewhat difficult | 2 (1) |
| I feel neutral about it | 14 (8) |
| It was somewhat easy | 30 (17) |
| It was very easy | 128 (72) |
| Missing | 2 (1) |
| How convenient did you find filling out the questionnaire by hand (“paper and pencil”)? | |
| It was very convenient | 126 (70) |
| It was somewhat convenient | 17 (9) |
| I feel neutral about it | 13 (7) |
| It was somewhat inconvenient | 6 (3) |
| It was very inconvenient | 13 (7) |
| Missing | 4 (2) |
| Did you have any concerns about the answers you provided on the “paper and pencil” questionnaire being kept private or confidential? | |
| Quite a bit | 4 (2) |
| Somewhat | 10 (6) |
| Not at all | 148 (83) |
| Not sure | 13 (7) |
| Missing | 4 (2) |
| Would you have any concerns about your answers being kept private or confidential if someone were to call you and ask you the questions over the phone? | |
| Quite a bit | 22 (12) |
| Somewhat | 35 (20) |
| Not at all | 96 (54) |
| Not sure | 18 (10) |
| Missing | 8 (4) |
| How much time would you be willing to spend in phone conversation about Quality of Life related survey questions on an intermittent basis? | |
| 30 minutes or more | 8 (4) |
| 20-30 minutes | 10 (6) |
| 10-20 minutes | 26 (15) |
| 5-10 minutes | 72 (40) |
| Fewer than 5 minutes | 51 (28) |
| Missing | 12 (7) |
|
| |
| Would you have any concerns about your answers being kept private or confidential if you were to access the questionnaire online to answer the questions? | |
| Quite a bit | 17 (9) |
| Somewhat | 36 (20) |
| Not at all | 106 (59) |
| Not sure | 15 (8) |
| Missing | 5 (3) |
| How much time would you be willing to spend online answering Quality of Life related survey questions on an intermittent basis? | |
| 30 minutes or more | 11 (6) |
| 20-30 minutes | 16 (9) |
| 10-20 minutes | 56 (31) |
| 5-10 minutes | 63 (35) |
| Fewer than 5 minutes | 23 (13) |
| Missing | 10 (6) |
| Most preferred method for completing questionnaires | |
| Paper and pencil | 94 (53) |
| Phone | 1 (<1) |
| Online website | 26 (15) |
| Emailed | 15 (8) |
| Online website or emailed | 5 (3) |
| Paper and pencil or emailed | 1 (<1) |
| Paper and pencil, online website, or emailed | 2 (1) |
| No method is most preferred | 1 (<1) |
| Missing | 34 (19) |
| Would you have been willing to be contacted 2-3 weeks before the transplant directly by the National Marrow Donor Program (CIBMTR) for enrollment and to complete the questionnaires (rather than being given the materials by your transplant center)? | |
| No | 50 (28) |
| Yes | 49 (27) |
| Missing | 80 (45) |
| In the future, would you be willing to continue to complete Quality of Life surveys? | |
| No | 18 (10) |
| Yes | 156 (87) |
| If “Yes” (to question above), how often would you be willing to complete quality of life surveys? (n=156) | |
| Twice a year | 75 (48) |
| Once a year | 73 (47) |
| Once every two years | 7 (4) |
| Missing | 1 (1) |
| Missing | 5 (3) |
|
| |
| Do you have access to the internet? | |
| No | 9 (5) |
| Yes | 164 (92) |
| Where do you have access to internet? Check all that apply. (n=164) | |
| Home | 160 (98) |
| Work | 57 (35) |
| Smartphone | 84 (51) |
| School | 1 (1) |
| What type of internet speed do you have access to? (n=164) | |
| Dial-up | 5 (3) |
| High speed | 157 (96) |
| Missing | 2 (1) |
| Missing | 6 (3) |
| Do you use Twitter™, or other social networking sites (My Space, Facebook, etc.)? | |
| No | 100 (56) |
| Yes | 71 (40) |
| If “Yes”, would you prefer to receive communication from these sites instead of through phone, mail, or email contact? (n=71) | |
| No | 65 (91) |
| Yes | 4 (6) |
| Missing | 1 (3) |
| Missing | 8 (4) |
| On average, approximately how much time did it take you to complete each questionnaire? | |
| 30 minutes or more | 8 (4) |
| 20-30 minutes | 25 (14) |
| 10-20 minutes | 61 (34) |
| 5-10 minutes | 59 (33) |
| Fewer than 5 minutes | 19 (11) |
| Missing | 7 (4) |
| At any time during the study, did you need assistance from a caregiver to complete the surveys? | |
| No | 160 (89) |
| Yes | 13 (7) |
| If “Yes”, at which time point(s) did you need assistance? Check all that apply. (n=13) | |
| Pre-Transplant | 5 (38) |
| Day 100 | 8 (62) |
| Month 6 | 5 (38) |
| Month 12 | 5 (38) |
| Missing | 6 (3) |
| Did you find providing feedback on your Quality of Life helpful to you in any way? | |
| Yes | 52 (29) |
| No | 51 (28) |
| Maybe | 43 (24) |
| Don't know | 26 (15) |
| Missing | 7 (4) |
| Please rate your comfort level in answering the Quality of Life survey questions. | |
| Uncomfortable | 8 (4) |
| Somewhat uncomfortable | 8 (4) |
| Neutral | 24 (13) |
| Somewhat comfortable | 13 (7) |
| Comfortable | 120 (67) |
| Missing | 6 (3) |
| Would you have been comfortable sharing other information on the questionnaire such as your ability to return to work or school, changes in your position, duties, activity level, disability status, etc.? | |
| No | 18 (10) |
| Yes | 86 (48) |
| Missing | 75 (42) |
| At any time during the study, did you communicate via e-mail or phone with someone at the National Marrow Donor Program (CIBMTR)? | |
| No | 83 (46) |
| Yes | 20 (11) |
| If “Yes”, were you comfortable being contacted by someone other than the transplant center? | |
| No | 3 (2) |
| Yes | 17 (9) |
| Missing | 76 (42) |
| How willing would you be to participate in a study similar to this one in the future? | |
| Very likely | 89 (50) |
| Somewhat likely | 53 (30) |
| Somewhat unlikely | 7 (4) |
| Very unlikely | 11 (6) |
| Not sure | 14 (8) |
| Missing | 5 (3) |
| Would you be willing to participate in a focus group facilitated by CIBMTR with other study participants to further discuss your experience with this study? | |
| No | 54 (30) |
| Yes | 47 (26) |
| Missing | 78 (44) |
Discussion
This study demonstrates that it is feasible to prospectively collect PRO in HCT patients contributing data to an observational database, and that these data add prognostic value beyond that provided by traditional clinical, demographic and transplant factors.
We collected longitudinal PROs in a large proportion of all HCT patients enrolled in the study. Response rates were particularly good, close to 90%, in certain subsets such as older patients, Caucasians and those who were married. This is similar to data from single center studies14,15 showing high retention rates. We also found factors associated with lower response rates. Children (or their proxies), in particular those from low income households, were less likely to return measures16.
Younger and minority participants may respond better to online than postal data collection (http://www.pewsocialtrends.org/2012/04/26/census-bureau-pushes-online-survey-response-option/). In patients who completed our post-participation questionnaire, those indicating a preference for electronic PRO collection had a median age of 30, compared to 53 those who preferred ‘pen and paper’. Others report a high success rate with electronic PRO collection17,18, often with reminders to complete the survey and links directly to the measures provided by email or text. This has the additional benefit of reducing cost to transmit and collect surveys, and decreasing data entry costs and errors. Offering both postal and electronic options in the future may enhance retention.
There was a high degree of satisfaction amongst the participants with the logistical aspects of this study and few had privacy concerns. Few had concerns about direct communication with CIBMTR to collect the measures, which suggests a way to collect data without increasing the burden to TCs. Almost all patients felt that they would like to continue to complete PRO measures, despite the fact that a large proportion felt that doing so was not directly helpful to themselves. Several participants asked to see a compilation of the results, and a comparison of their scores to others. A future goal is to provide real time results to patients19,20, which they could bring to their clinic appointments allowing for intervention if necessary21 and, perhaps, increasing participation for some groups.
A key finding in this study is that pre-transplant scores are significantly associated with both survival and post-transplant self-reported HRQOL. This was found for both the FACT-BMT and the PCS of the SF-36, which individually showed a greater discriminatory value than other more commonly used, physician determined pre-transplant scores (e.g. KPS and HCT-CI). This aligns with the conclusions of a recently published secondary analysis of a prospective randomized clinical trial, BMT CTN 0902 study8. This study showed, in a comparable population including 310 allogeneic transplant recipients, that the pre-HCT PCS was strongly predictive of overall survival, with better survival in each higher quartile similar to that seen in our study. As in our study, the pre-HCT PCS was strongly correlated with the pre-transplant KPS but the latter did not predict survival as an independent factor. A single center study by Hamilton et al22 in 409 allogeneic HCT recipients, showed that the Trial outcome index (TOI) and the physical wellbeing subscales of the FACT-BMT were associated with mortality post-transplant. Our results extend these findings, suggesting that the prognostic value of PRO measures holds true in the ‘real world’ setting of HCT across multiple centers7.
The relatively low number of pediatric patients limits our ability to draw strong conclusions about children. However, the suggestive results support routine PRO collection, as well as further research in this population. A further limitation is that we did not collect all possible post-HCT complications and correlate these with study participation.
In conclusion, these results suggest that a PRO measure pre-HCT may have greater predictive value for mortality than traditionally collected factors, and that collection of such data are feasible in the setting of an outcome registry where analyses of large representative datasets are possible. Incorporating routine pre HCT PRO assessment is critically important to our ability to control for pre-transplant factors when analyzing the contributions of transplant and post-transplant factors to the success of transplantation. It also suggests that routine collection of post-HCT PRO is feasible, which would add to our ability to understand and improve deficits in QOL in HCT survivors.
In order to make routine PRO data collection in HCT a reality, additional work should focus on the following practical questions: what is the most discriminating minimum set of questions to reduce respondent burden but maintain predictive value9? How can PRO data be integrated with clinical data to create the best prognostic index? How can electronic solutions be leveraged to collect and collate PRO directly from patients by a central registry, and matched clinical data while maintaining security and minimizing the burden of data collection. Determining answers to these questions will facilitate integration of routine PRO assessment into the already extensive pre- and post-transplant evaluations that transplant patients undergo.
Supplementary Material
Acknowledgments
We thank the patients, physicians, nurses and research staffs at all participating centers, in particular Drs Giralt, Baker, Wingard, Bolwell, Joshi, Myers, Lazarus, Litzow and Khan.
Funding: CIBMTR is supported by 5U24-CA076518 from NCI, NHLBI, NIAID and 5U10HL069294 from NHLBI, NCI.
CIBMTR Support List: The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement 5U24-CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U10HL069294 from NHLBI and NCI; a contract HHSH250201200016C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-15-1-0848 and N00014-16-1-2020 from the Office of Naval Research; and grants from Alexion; *Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Astellas Pharma US; AstraZeneca; Be the Match Foundation; *Bluebird Bio, Inc.; *Bristol Myers Squibb Oncology; *Celgene Corporation; Cellular Dynamics International, Inc.; *Chimerix, Inc.; Fred Hutchinson Cancer Research Center; Gamida Cell Ltd.; Genentech, Inc.; Genzyme Corporation; *Gilead Sciences, Inc.; Health Research, Inc. Roswell Park Cancer Institute; HistoGenetics, Inc.; Incyte Corporation; Janssen Scientific Affairs, LLC; *Jazz Pharmaceuticals, Inc.; Jeff Gordon Children's Foundation; The Leukemia & Lymphoma Society; Medac, GmbH; MedImmune; The Medical College of Wisconsin; *Merck & Co, Inc.; Mesoblast; MesoScale Diagnostics, Inc.; *Miltenyi Biotec, Inc.; National Marrow Donor Program; Neovii Biotech NA, Inc.; Novartis Pharmaceuticals Corporation; Onyx Pharmaceuticals; Optum Healthcare Solutions, Inc.; Otsuka America Pharmaceutical, Inc.; Otsuka Pharmaceutical Co, Ltd. – Japan; PCORI; Perkin Elmer, Inc.; Pfizer, Inc; *Sanofi US; *Seattle Genetics; *Spectrum Pharmaceuticals, Inc.; St. Baldrick's Foundation; *Sunesis Pharmaceuticals, Inc.; Swedish Orphan Biovitrum, Inc.; Takeda Oncology; Telomere Diagnostics, Inc.; University of Minnesota; and *Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA) or any other agency of the U.S. Government.
Footnotes
Conflicts of Interest: None of the authors have any relevant conflict of interest to disclose
Author Contributions: The study was designed by JDR, AA, RD, SJL, MMH. Study set up, patient contact and data collection was performed by JV, CP, DM, RD, JDR. Data analysis was performed by BES, RB, HRM, RF, JDR and data interpretation by JDR, AA, SJL, MMH, BES, RB, KEF. All authors participated in writing and reviewing the manuscript.
Corporate Members
References
- 1.Bevans MF, Mitchell SA, Barrett JA, et al. Symptom distress predicts long-term health and well-being in allogeneic stem cell transplantation survivors. Biol Blood Marrow Transplant. 2014;20:387–95. doi: 10.1016/j.bbmt.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pidala J, Anasetti C, Jim H. Quality of life after allogeneic hematopoietic cell transplantation. Blood. 2009;114:7–19. doi: 10.1182/blood-2008-10-182592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andrykowski MA, Bishop MM, Hahn EA, et al. Long-term health-related quality of life, growth, and spiritual well-being after hematopoietic stem-cell transplantation. J Clin Oncol. 2005;23:599–608. doi: 10.1200/JCO.2005.03.189. [DOI] [PubMed] [Google Scholar]
- 4.Braamse AM, Gerrits MM, van Meijel B, et al. Predictors of health-related quality of life in patients treated with auto- and allo-SCT for hematological malignancies. Bone Marrow Transplant. 2012;47:757–69. doi: 10.1038/bmt.2011.130. [DOI] [PubMed] [Google Scholar]
- 5.Mosher CE, Redd WH, Rini CM, et al. Physical, psychological, and social sequelae following hematopoietic stem cell transplantation: a review of the literature. Psychooncology. 2009;18:113–27. doi: 10.1002/pon.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wingard JR, Huang IC, Sobocinski KA, et al. Factors associated with self-reported physical and mental health after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2010;16:1682–92. doi: 10.1016/j.bbmt.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Montazeri A. Quality of life data as prognostic indicators of survival in cancer patients: an overview of the literature from 1982 to 2008. Health Qual Life Outcomes. 2009;7:102. doi: 10.1186/1477-7525-7-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wood WA, Le-Rademacher J, Syrjala KL, et al. Patient-reported physical functioning predicts the success of hematopoietic cell transplantation (BMT CTN 0902) Cancer. 2016;122:91–8. doi: 10.1002/cncr.29717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shaw BE, Lee SJ, Horowitz MM, et al. Can we agree on patient-reported outcome measures for assessing hematopoietic cell transplantation patients? A study from the CIBMTR and BMT CTN. Bone Marrow Transplant. :2016. doi: 10.1038/bmt.2016.113. [DOI] [PubMed] [Google Scholar]
- 10.Ware JEK M, Keller SD. SF-36 physical and mental health summary scales: a user's manual. Boston: The Health Institute, New England Medical Center; 1994. [Google Scholar]
- 11.McQuellon RP, Russell GB, Cella DF, et al. Quality of life measurement in bone marrow transplantation: development of the Functional Assessment of Cancer Therapy-Bone Marrow Transplant (FACT-BMT) scale. Bone Marrow Transplant. 1997;19:357–68. doi: 10.1038/sj.bmt.1700672. [DOI] [PubMed] [Google Scholar]
- 12.Varni JW, Limbers C, Burwinkle TM. Literature review: health-related quality of life measurement in pediatric oncology: hearing the voices of the children. J Pediatr Psychol. 2007;32:1151–63. doi: 10.1093/jpepsy/jsm008. [DOI] [PubMed] [Google Scholar]
- 13.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–9. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen MZ, Rozmus CL, Mendoza TR, et al. Symptoms and quality of life in diverse patients undergoing hematopoietic stem cell transplantation. J Pain Symptom Manage. 2012;44:168–80. doi: 10.1016/j.jpainsymman.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta V, Panzarella T, Li L, et al. A prospective study comparing the outcomes and health-related quality of life in adult patients with myeloid malignancies undergoing allogeneic transplantation using myeloablative or reduced-intensity conditioning. Biol Blood Marrow Transplant. 2012;18:113–24. doi: 10.1016/j.bbmt.2011.05.022. [DOI] [PubMed] [Google Scholar]
- 16.Cassedy A, Drotar D, Ittenbach R, et al. The impact of socio-economic status on health related quality of life for children and adolescents with heart disease. Health Qual Life Outcomes. 2013;11:99. doi: 10.1186/1477-7525-11-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bush N, Donaldson G, Moinpour C, et al. Development, feasibility and compliance of a web-based system for very frequent QOL and symptom home self-assessment after hematopoietic stem cell transplantation. Qual Life Res. 2005;14:77–93. doi: 10.1007/s11136-004-2394-2. [DOI] [PubMed] [Google Scholar]
- 18.Wood WA, Deal AM, Abernethy A, et al. Feasibility of frequent patient-reported outcome surveillance in patients undergoing hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2013;19:450–9. doi: 10.1016/j.bbmt.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bantug ET, Coles T, Smith KC, et al. Graphical displays of patient-reported outcomes (PRO) for use in clinical practice: What makes a pro picture worth a thousand words? Patient Educ Couns. 2016;99:483–90. doi: 10.1016/j.pec.2015.10.027. [DOI] [PubMed] [Google Scholar]
- 20.Brundage MD, Smith KC, Little EA, et al. Communicating patient-reported outcome scores using graphic formats: results from a mixed-methods evaluation. Qual Life Res. 2015;24:2457–72. doi: 10.1007/s11136-015-0974-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pidala J, Anasetti C, Jim H. Health-related quality of life following haematopoietic cell transplantation: patient education, evaluation and intervention. Br J Haematol. 2010;148:373–85. doi: 10.1111/j.1365-2141.2009.07992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamilton BK, Law AD, Rybicki L, et al. Prognostic significance of pre-transplant quality of life in allogeneic hematopoietic cell transplantation recipients. Bone Marrow Transplant. 2015;50:1235–40. doi: 10.1038/bmt.2015.122. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


