Abstract
Mass drug administration (MDA) is the current strategy for interrupting the transmission of lymphatic filariasis (LF) infection and control of the disease in endemic areas. However, subject noncompliance has resulted in the presence of several ‘transmission hotspots’ in the endemic regions threatening the reemergence of LF. This situation is further complicated by the fact that the drugs used in MDA are not effective against adult LF worms, a major concern for the control strategy. Thus, there is clearly a need for an effective and sustainable approach to control LF. Prophylactic vaccine combined with targeted treatment of infected patients and vector control is suggested as a more sustainable strategy to eliminate LF infection from endemic regions. A multivalent vaccine (rBmHAT) developed in our laboratory confered about 90% protection in rodents. However, when we tested the rBmHAT vaccine along with alum in rhesus macaques only about 40% protection was achieved and the immune response obtained was Th2 biased. In an attempt to improve the vaccine, in this study we tested two vaccine antigens (rBmHAT and rBmHAX) along with two adjuvant formulations [alum+GLA (AL019) and mannosylated chitosan (MCA)] in a mouse model. Our results show that rBmHAT is a better vaccine antigen than rBmHAX. Combination of rBmHAT with AL019 or MCA adjuvants gave 94% and 88% protection respectively against challenge infections. Immunized animals developed antigen specific memory T cells that secreted significant levels of IL-4, IFN-γ and IL-17 suggesting the generation of a balanced Th1/Th2 responses following immunization. A major advantage of MCA adjuvant is that the vaccine booster doses can be administered orally. These studies thus showed that rBmHAT is a better vaccine antigen and can be given in combination with AL019 or MCA adjuvant to obtain excellent results.
Keywords: TLR-4 agonist, MCA, alum, vaccine, adjuvant, lymphatic filariasis, Brugia malayi
Introduction
Lymphatic filariasis (LF) is a mosquito transmitted tropical parasitic infection caused mainly by three species of filarial parasites, Wuchereria bancrofti, Brugia malayi and B. timori. According to the World Health Organization (WHO) currently about 73 countries are considered endemic for LF (WHO 2007). The Global Programme to Eliminate Lymphatic Filariasis (GPELF) was launched in 2000 to eliminate LF by 2020 from the endemic regions using annual mass drug administration (MDA) as a preventive chemotherapy strategy (WHO 2007; WHO 2016). This approach has significantly reduced the incidence of LF in several countries. In fact, China and the Republic of Korea have already declared eliminating LF from these regions in 2007 and 2008 (WHO 2007). However, majority of the endemic regions still face significant roadblocks in stopping the transmission and elimination of LF. Treatment with drugs alone is not effective as a prophylaxis against LF. There is a need for a more sustained approach such as a prophylactic vaccination to stop transmission and eliminate LF from the endemic areas (Dakshinamoorthy et al. 2013c; Jambulingam et al. 2016; Harris et al. 2017). Our laboratory and others have identified several potential vaccine antigens that are shown to confer significant protection against challenge infections in experimental animals (Samykutty et al. 2010; Dakshinamoorthy et al. 2013b, Dakshinamoorthy et al. 2013c; Arumugam et al. 2014). One of our recent trials in non-human primates using a trivalent fusion protein vaccine (rBmHAT) showed that approximately 40% protection could be achieved in vaccinated animals against a challenge infection (Dakshinamoorthy et al. 2012). In these studies we used alum as an adjuvant and the immune responses were predominantly biased towards IgG1/IL-4. However, in naturally immune endemic normal individuals and in immunized rodents the protective immune responses were correlated with balanced Th1/Th2 responses (Dakshinamoorthy et al. 2013a; Dakshinamoorthy et al. 2013c; Dakshinamoorthy et al. 2014). Therefore, there is a need to improve the current vaccine formulation so that a balanced Th1/Th2 response can be achieved following immunization with enhanced protection.
Adjuvants play an important role in enhancing the potency of an antigen and polarizing the immune responses to Th1 or Th2 (Di Pasquale et al. 2015). Among these, alum is the most commonly used adjuvant in the human and veterinary vaccines (Gupta 1998; Marrack et al. 2009). Alum is known to polarize the immune response to a Th2 bias (Marrach et al. 2009). Several other adjuvants such as the monophosphoryl lipid A (Casella and Mitchell 2008), imidazoquinolines (Steinhagen et al. 2010), poly(I:C) (Tewari et al. 2010), CpG motifs (Mohan et al. 2013) and glucopyranosyl lipid A (Coler et al. 2011) are also extensively tested. These adjuvants recognize specific pattern recognizing receptors on immune cells activating both innate and adaptive immune responses against the vaccine antigen (Schnare et al. 2001). Adjuvants containing TLR4 agonist can promote both Th1 and Th2 biased responses towards vaccine antigens (Bortolatto et al. 2008; Didierlaurent et al. 2009; Fox et al. 2010; Arias et al. 2012; Goulopoulou et al. 2016). One of our recent studies showed that including alum plus a synthetic TLR-4 as an adjuvant for rBmHATαc promoted a Th1/Th2 biased response (Dakshinamoorthy et al. 2013a). Similarly, mannosylated chitosan adjuvant (MCA) is successfully used as an adjuvant to target the mannose receptors on macrophages to stimulate a Th1 biased immune response against the vaccine antigens (Jiang et al. 2008; Carroll et al. 2016). In this study we attempted to evaluate two different adjuvants; alum + GLA (GLA-SE is an emulsion that is not adsorbed to alum, AL019) obtained from Infectious Disease Research Institute (IDRI) and MCA obtained from Pacific GeneTech Inc for their ability to promote Th1/Th2 biased response following vaccination with rBmHAT in mice and determine if a higher percentage of protection can be achieved following a challenge infection with B. malayi third stage larvae (L3). The second aspect of the study was to use a different vaccine antigen, rBmHAX. The only difference here is that we replaced the tetraspanin large extracellular loop (TSP) sequence in the rBmHAT with thoredoxin peroxide-2 (TPX-2) to make the rBmHAX multivalent construct. The rationale for this switch is based on our previous publication, where we show that TPX-2 is a potent inducer of Th1 responses (Gnanasekar et al. 2004; Anand et al. 2008) and the percent of protection conferred with rBmTPX-2 is better than rBmTSP (Anand et al. 2008; Dakshinamoorthy et al. 2013b). In the present study we evaluated the vaccine potential of rBmHAX along with AL019 and MCA adjuvants.
Material and methods
Animals and parasites
B. malayi infective third stage larvae (L3) were obtained from the NIAID/NIH Filariasis Research Reagent Resource Centre (University of Georgia, Athens, GA) and Balb/c mice were purchased from Taconic biosciences (Hudson, NY).
Adjuvants
Alum plus synthetic TLR4 agonist GLA (AL019) was purchased from Infectious Disease Research Institute, Seattle, WA and Mannosylated Chitosan (MCA) was a gift from Pacific GeneTech, Hong Kong.
Construction of multivalent gene sequence
Multivalent gene sequences of bmhat (consisting of bmhsp12.6, bmalt-2 and bmtsp) and bmhax (consisting of bmhap12.6, bmalt-2 and bmtpx2) were constructed at Genscript (Piscataway, NJ) using published gene sequences (Dakshinamoorthy et al. 2013c; Dakshinamoorthy et al. 2014).
Cloning, expression and purification of recombinant multivalent fusion proteins
Genscript supplied the sequences in pUC57 vector. The genes were amplified using forward CGGGATCCATGGAAGAAAAGGTAGTG and reverse CGGAATTCTCAATCTTTTTGAGATGAAT primers for BmHAT and forward CGGGATCCATGGAAGAAAAGGTAGTG and reverse CCCGAATTCTTAATGTTTCTCAAAATATGCTTT primers for BmHAX with restriction sites for BamHI and EcoRI. The PCR amplified products were cloned into pRSETA expression vector, transformed into competent BL21 (DE3) E. coli cells for expression of the recombinant proteins with 6X histidine tag as described previously (Dakshinamoorthy et al. 2013c). Recombinant fusion proteins were purified using immobilized metal affinity Ni+ charged sepharose column (GE Healthcare Life Sciences, Pittsburg, PA) and eluted with 50 mM–300 mM imidazole. Endotoxin in the final purified protein preparation was removed using an endotoxin removal column (Thermo Fisher Scientific, Rockford, IL). The expression and purity of recombinant proteins were checked in 12% SDS PAGE gel and western blot using anti-his antibodies (Qiagen, Valencia, CA). Protein concentration was determined using a Bradford reagent (Thermo Fisher Scientific).
Immunization of Balb/c mice
Six weeks old male Balb/c mice were randomly divided into six groups with five mice per group. Two groups of mice were immunized three times at two weeks’ interval with 15 μg of purified rBmHAT plus 10 μg of one of the adjuvant formulation (AL019 or MCA) and two groups of mice were immunized with 15 μg of rBmHAX plus 10 μg of one of the adjuvant formulation (AL019 or MCA). Two groups of mice served as AL019 or MCA controls. All immunizations with AL019 adjuvants were given s/c route. However, for immunization with MCA, the first immunization was given s/c and booster second and third immunizations were given by oral gavage (Instech, Plymouth meeting, PA). Blood samples were collected by submandibular bleeding (Golde et al. 2005) on day -2, 0, 14, 28 and 42. Sera was separated and stored at −80°C.
Titer of antigen-specific IgG
Titer of antigen specific IgG antibodies was determined by an indirect ELISA as described previously (Dakshinamoorthy et al. 2013c). IgG antibodies in diluted sera samples (1:100, 1:500, 1:1,000, 1:2,000, 1:4,000 and 1:8,000) were detected using biotin-labeled goat anti mouse IgG (BioLegend, San Diego, CA) and color developed using streptavidin conjugated horse radish peroxidase (HRP) and 1-step Ultra TMB-ELISA substrate (Thermo Fisher Scientific). The reaction was stopped using 0.16M H2SO4 and optical density at 450 nm was read in a BioTek Synergy2 ELISA reader.
Levels of antigen-specific antibody isotypes in the sera of mice
Levels of antigen specific IgG1, IgG2a, IgG2b, IgG3, IgE, IgM and IgA antibodies against rBmHAT or rBmHAX were determined in the sera of mice using an indirect ELISA as described above using respective isotype-specific HRP labeled antibodies.
Analysis of vaccine-induced protection in mice
Vaccine-induced protection was determined by surgically implanting a micropore chamber containing 20 B. malayi L3 into the peritoneal cavity of mouse as described previously (Abraham et al. 1989; Dakshinamoorthy et al. 2013a). 72h after implanting, contents of each chamber were examined using a light microscope at 400X for larval viability as described previously (Joseph and Ramaswamy 2013). Larvae that appeared transparent and straight with no movement were counted as dead. Live larvae were active, coiled and translucent. Percentage protection was calculated using the formula: (Number of dead parasites/Number of recovered parasites) X 100.
Splenocyte proliferation and flow cytometric analysis
Two weeks after the last immunization, spleens were collected and single cell suspension was prepared. Cells at a concentration of 1×106/ml were incubated with 5mM CFSE (BioLegend) in the dark for 20 minutes at 37°C. After washing the cells were stimulated for five days with 1μg/ml of the respective antigen. Cells treated with concanavalin-A or media alone remained as controls. Following incubation cells were stained with APC labeled anti-CD3 antibody (BioLegend) and the proliferating T cell populations were determined on a BD FACScalibur flow cytometer and data analyzed using cell quest software v6.1.2.
Flow cytometric analysis for cell surface markers
1×106/ml of splenocytes stimulated with 1 μg/ml of respective antigens (rBmHAT or rBmHAX), ConA or media alone were stained with combinations of CD3-APC/CCR7-FITC/CD62LPE and analyzed on a BD FACScalibur flow cytometer after gating the cells for CD3. Subpopulation of cells that are double positive for CCR7 and CD62L were counted as central memory T cells (TCM) and cells double negative for CCR7 and CD62L were counted as effector memory T cells (TEM). Data was analyzed using cell quest software v6.1.2.
Secreted levels of cytokines in the culture supernatants of antigen-stimulated splenocytes
1×106 splenocytes in 1 ml were stimulated with 1μg of rBmHAT or rBmHAX for 72h at 37°C. Culture supernatants were collected and secreted levels of IFNγ, IL-2, IL-4, IL-10 and IL-17 were determined using a Cytokine Bead Array (BD Biosciences) as described previously (Dakshinamoorthy et al. 2013a).
Statistical analysis
GraphPad Prism version 7.0 (GraphPad Software, San Diego, CA) was used to analyze the data. Comparison between two individual data points was made using Student’s t-test. For multiple comparisons, one way ANOVA was used along with the Tukey-Kramer and/or Dunnet’s post-test wherever appropriate. For cytokine analysis two-way ANOVA was used with Bonferronipost test. A probability (P) value of <0.05 was considered statistically significant.
Results
Cloning and expression of multivalent fusion proteins
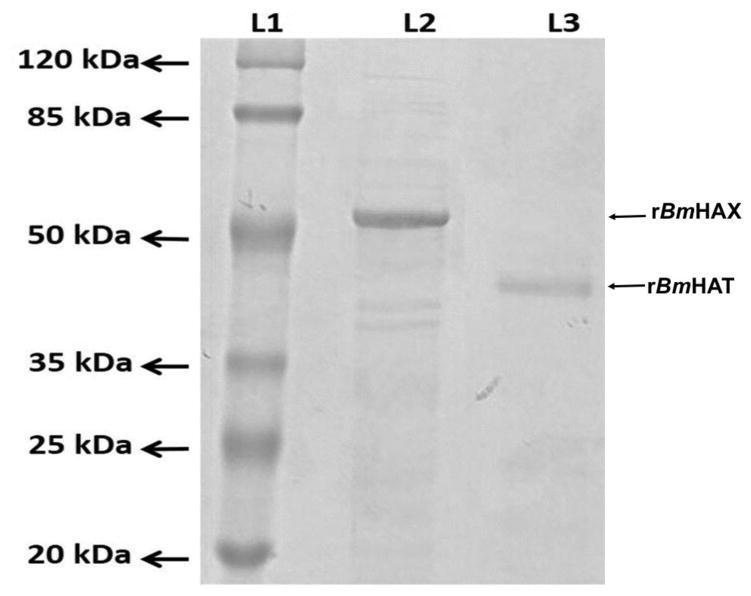
Purified recombinant proteins separated on an SDS PAGE gel showed a prominent band at 39 kDa for rBmHAT and a prominent band at 52 kDa for rBmHAX (Fig 1). Endotoxin levels in the final purified preparations were <10 EU/μg of the vaccine protein.
Figure 1. Expression of rBmHAX and rBmHAT.
The multivalent genes bmhax (1320 bp) and bmhat (957 bp) were amplified and cloned into the expression vector pRSETA and successfully expressed in E. coli BL21 (DE3). Both the expressed proteins were purified by immobilized metal affinity chromatography (IMAC) and purity confirmed by western blot analysis using anti-His antibodies. Purified rBmHAX and rBmHAT were separated on a 12% SDS PAGE gel. Lane 1- Molecular weight marker, Lane 2- rBmHAX (a prominent band at 52 kDa) and Lane 3 - rBmHAT (a prominent band at 39 kDa).
Immunization with rBmHAT along with AL019 or MCA gave the highest level of protection against challenge infections
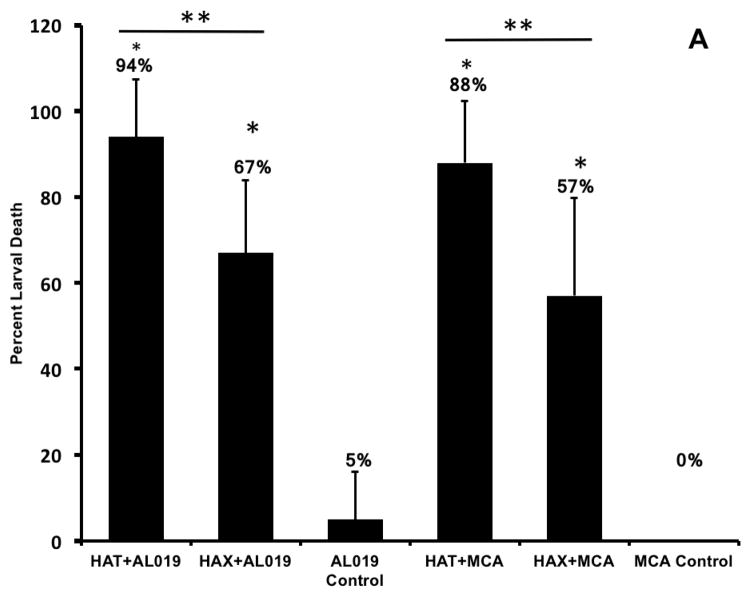
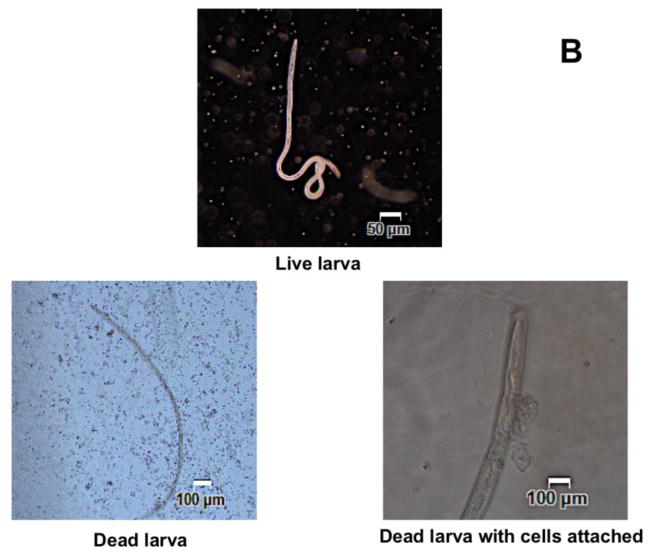
In vivo challenge experiments showed that compared to AL019 adjuvant controls that had only 5±11.2% larval death, mice immunized with rBmHAT plus AL019 showed 94±13.4% (p<0.0001) larval death and mice immunized with rBmHAX plus AL019 showed 67.5±17% (p<0.0001) larval death (Fig 2a). Comparison between rBmHAT and rBmHAX immunization showed that significantly higher protection (p<0.0236) was observed in rBmHAT immunized animals (Fig 2a). Most of the dead larva had cells attached to their surface (Fig 2b). The number of cells attached to the larval surface varied, some L3 had very few cells attached to the anterior or posterior end, whereas, some L3 were totally covered with the cells. In this study, we did not evaluate the type of cells that were attached to the L3s.
Figure 2. Percent protection in vaccinated animals was calculated by determining the percent larval death.
Figure 2A. Approximately 20 live B. malayi L3s sealed in a micropore chamber was surgically implanted into the peritoneal cavity of mice. 72 hrs after implanting the chambers were removed and the number of live and dead larvae was counted and percent larval death determined. Compared to the controls, there was significant larval death in vaccinated animals (Fig 2A). The highest percent of larval death (expressed as protection) was observed in mice immunized with rBmHAT. Comparison between rBmHAT and rBmHAX immunization plus AL019 adjuvant showed that significantly higher protection (p<0.0236) was observed in rBmHAT + AL019 immunized animals. Similarly, larval death in rBmHAT plus MCA immunized mice was significantly higher (p<0.0332) compared to those in rBmHAX plus MCA immunized mice. Figure 2B. Several cells were found attached to the dead larvae in the vaccinated animals (bottom panel). However, no cells were found attached to the live larvae collected from control animals. Magnification bars are indicated in each photomicrograph. n=10, statistically significant *p<0.0001 and **p<0.0006.
Similarly, when MCA was used as the adjuvant for rBmHAT or rBmHAX immunizations, 87.88±14.42% (p<0.0001) and 55.5±22.8% (p<0.0006) larval death respectively were observed compared to MCA adjuvant controls where no larval death occurred (0±0%). Larval death in rBmHAT plus MCA immunized mice was significantly high (p<0.0332) compared to those in rBmHAX plus MCA immunized mice. These results thus show that rBmHAT is a better vaccine antigen than rBmHAX. AL019 was found to be a better adjuvant for rBmHAT immunizations in mice. The most interesting finding in this study was the significant protection that we observed when MCA was used as the adjuvant along with rBmHAT, clearly showing that oral booster doses of MCA adjuvanated rBmHAT were immunogenic.
Immune correlates of protection after immunization
Mice immunized with rBmHAT or rBmHAX developed high titer of antigen-specific IgG antibodies
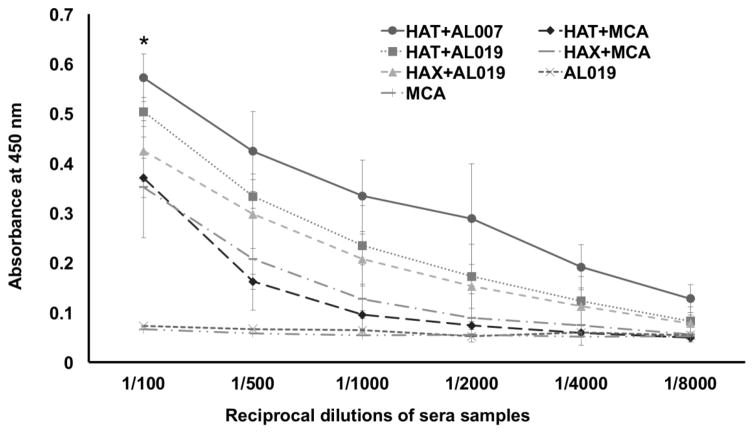
Titer of IgG antibodies against each of the recombinant antigens was determined in the sera of immunized mice using an indirect ELISA. Results showed that high titer of antigen-specific IgG antibodies were generated in all vaccinated mice irrespective of the adjuvant used. AL019 adjuvanated rBmHAT and AL019 adjuvanated rBmHAX gave the highest IgG titer of 1:8,000 (Fig 3). Despite the fact that booster doses of MCA adjuvanated rBmHAT or MCA adjuvanated rBmHAX were given orally, these mice also developed significant titer (1:4,000) of antigen-specific IgG antibodies. Antigen-specific IgG titer was significantly high (p<0.0318) in rBmHAT plus AL019 immunized animals compared to the other vaccinated groups. These findings thus suggested that any of the two adjuvants (AL019 or MCA) could be used along with the recombinant antigens to trigger significant antigen-specific IgG antibody titers in mice.
Figure 3. Titer of antigen-specific IgG antibodies in the sera of immunized mice.
An indirect ELISA was used to determine the titer of anti-rBmHAT and anti-rBmHAX IgG antibodies in the sera of immunized animals. Pre-immune sera and sera from control mice were used as the baseline controls in these assays. Results showed that vaccination with rBmHAT and rBmHAX induced high titer of IgG antibodies. AL019 adjuvant appeared to be slightly better adjuvant than MCA in promoting antigen-specific IgG responses. It is interesting to note that the booster doses of MCA adjuvanated rBmHAT and rBmHAX were given orally, yet comparable titers of IgG antibodies were generated in immunized mice. n=10 *significant p<0.01.
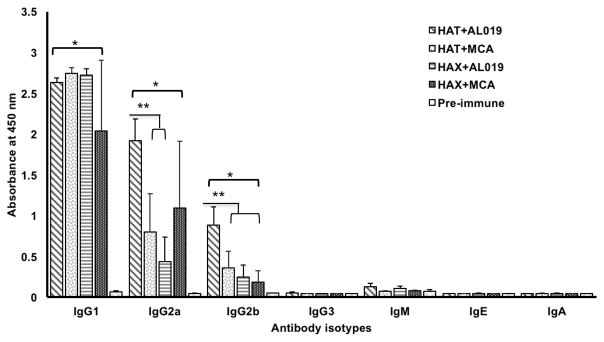
Antigen-specific IgG1, IgG2a, IgG2b antibodies were elevated in the sera of vaccinated mice
To determine the pattern of humoral immune response generated following vaccination, we determined the levels of antigen-specific IgG1, IgG2a, IgG2b, IgG3, IgM, IgE and IgA antibodies in the sera of mice vaccinated with rBmHAT or rBmHAX in combination with different adjuvant formulations. Our results showed that compared to preimmune sera samples, antigen-specific IgG1 (p<0.0001), IgG2a (p<0.0001) and IgG2b (p<0.0138) antibodies were significantly high in the sera of all vaccinated animals (Fig 4). Levels of IgG1 did not show any significant differences between the vaccinated groups (Fig 4). However, levels of IgG2a (p<0.0016) and IgG2b (p<0.0016) was significantly high in rBmHAT plus AL019 immunized animals compared to rBmHAT plus MCA immunized animals suggesting that AL019 may be a slightly better compared to MCA in promoting a balanced Th1/Th2 humoral response to rBmHAT in mice. Nevertheless, both the adjuvants can clearly promote a balanced Th1/Th2 response in mice. Levels of IgM, IgE and IgA antibodies were not significantly different from the controls in all vaccinated animals (Fig 4).
Figure 4. Levels of antigen-specific antibody isotypes.
in the sera of immunized mice were determined using an indirect ELISA. Results show that significant levels of antigen-specific IgG1>IgG2a>IgG2b antibodies (in that order of abundance) were present in the sera of all immunized mice compared to the adjuvant controls. Levels of IgG1 were not significant between the vaccinated groups. However, levels of IgG2a and IgG2b were significantly high in rBmHAT plus AL019 immunized animals compared to rBmHAT plus MCA immunized animals. The most predominant antibody isotypes were IgG1, IgG2a and IgG2b suggesting that a balanced Th1/Th2 response was generated following immunization with the antigens. Levels of IgE, IgA and IgM were not significantly different from the controls. n=10. *Statistically significant p<0.0001, ** p<0.0016.
Spleen cells from vaccinated animals showed antigen specific recall response
Spleen cells from all vaccinated animals proliferated significantly (P<0.001) in response to the antigen stimulation compared to the adjuvant control groups. Spleen cells from the vaccinated group (rBmHAT+AL019, rBmHAT+MCA, rBmHAX+AL019 and rBmHAX+MCA) divided three times as indicated by 4 clear CFSE peaks in all the vaccinated mice compared to the unstimulated and adjuvant controls, which had only 1 CFSE peak (data not shown). These results suggest that antigen-responding cells were present in the spleen of vaccinated mice.
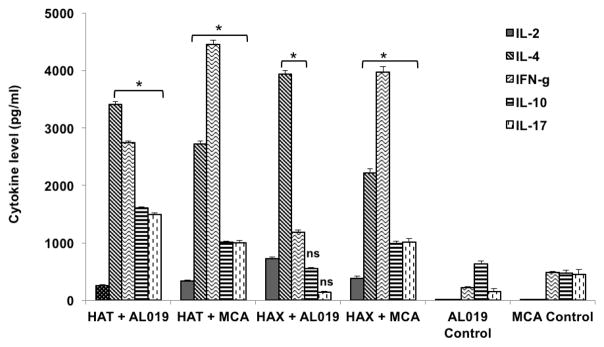
Antigen-responding spleen cells of vaccinated animals predominantly secreted both Th1 and Th2 cytokines
Secreted levels of cytokines in the culture supernatants of antigen-stimulated spleen cells were measured using a cytokine bead array. Our results show that cells from all vaccinated animals secreted significantly high (p<0.0001) levels of IL-2, IL-4, IL-10, IL-17 and IFN-γ compared to controls (Fig 5). Cells from rBmHAT plus AL019 secreted significant (p<0.0001) levels of IL-4, IFN-γ, IL-10 and IL-17. Cells from rBmHAX plus AL019 immunized mice predominantly secreted IL-4 and levels of IL-10 and IL-17 were not significant compared to the controls. MCA was more efficient in promoting IFN-γ secreting cells irrespective of the antigen used. These results confirmed our results from the antibody responses that both AL019 and MCA can promote balanced Th1/Th2 cytokine responses to vaccine antigens in immunized mice.
Figure 5. Levels of secreted cytokines in the culture supernatants of spleen cells.
stimulated with respective vaccine antigens. 1 × 106 spleen cells were stimulated with 1 μg/ml of rBmHAT or rBmHAX for 72 hrs at 37°C. Levels of secreted cytokines in the culture supernatants were determined using a BD cytokine bead array. Results show that spleen cells from vaccinated mice secreted significantly high levels of both IL-4 and IFN-γ compared to respective AL019 or MCA controls. MCA was more efficient in promoting IFN-γ secreting cells irrespective of the antigen used. However, cells from mice immunized with rBmHAX plus AL019 predominantly secreted IL-4. Levels of secreted IL-17 and IL-10 were also high in the culture supernatants of spleen cells from rBmHAT (with AL019 or MCA) and rBmHAX plus MCA vaccinated animals. These results suggested that spleen cells from both rBmHAT and rBmHAX vaccinated animals secreted cytokines with a balanced Th1/Th2 cytokine pattern irrespective of the adjuvants used. n=5, *Significant p<0.0001 compared to adjuvant controls. ns. not significant.
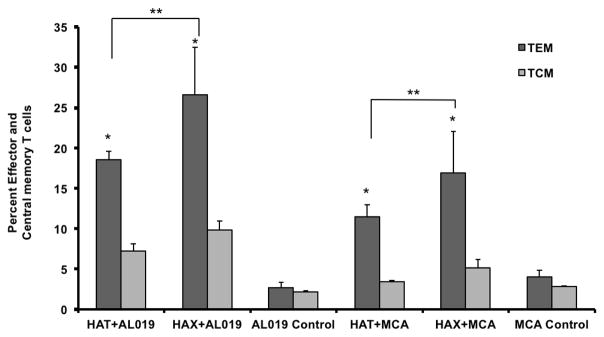
Immunization with rBmHAT generated TEM cells in the spleen
Spleen cells were incubated with 1 μg/ml of rBmHAT or rBmHAX for 5 days at 37°C. Following incubation the cells were stained with CD3/CCR7/CD62L and evaluated in a flow cytometer. Cells were first gated for CD3 and the CCR7+ and CD62L+ sub population of cells within the CD3 was determined. Majority of the CD3 cells were CD62Llow and CCR7low suggesting effector memory T cells. Compared to AL019 or MCA controls, rBmHAT and rBmHAX immunized animals had significantly high (p<0.0001) number of TEM and TCM cells. rBmHAT immunized animals had significantly high number of TEM (p<0.0061) and TCM (p<0.0061) cells compared to rBmHAX immunized animals in their spleen (Fig 6).
Figure 6. Memory T cell populations.
in the spleen of mice vaccinated with rBmHAT or rBmHAX. Isolated spleen cell were stimulated with respective antigen for five days and stained with CD3, CD62L and CCR7 and was evaluated by flow cytometry. CD3+CD62L-CCR7- cell population were counted as effector memory T cells and CD3+ CD62L+CCR7+ cells were counted as central memory T cells. Significant numbers of TEM and TCM T cell population were present in the spleen of vaccinated animals. rBmHAT immunized animals had slightly higher number of memory cells than rBmHAX immunized animals. n=5. *Statistically significant (p<0.001) compared to controls, **Significant (p<0.0.0061) between the vaccinated groups.
Discussion
An effective prophylactic vaccine against LF can support the effort towards control and total elimination of LF from a community (Ramaswamy 2016). Subjects living in an endemic region can develop natural immunity and carry protective antibodies against the infective stage of LF. These naturally immune individuals are called ‘Endemic Normals (EN)” (Day 1991). Using a phage-display based cDNA expression library of the parasite, sera of EN subjects were screened to identify several potential vaccine antigens that recognized the protective antibodies (Gnanasekar et al. 2004). Subsequent evaluation of these antigens especially as a multivalent formulation (rBmHAT) gave the highest (92%) rate of protection in rodent models (Thirugnanam et al. 2007; Samykutty et al. 2010; Kalyanasundaram and Balumuri 2011; Dakshinamoorthy et al. 2013a; Dakshinamoorthy et al. 2013c; Arumugam et al. 2014). However, the same vaccine with alum adjuvant gave only about 40% protection in rhesus macaques (Dakshinamoorthy et al. 2014) and the immune response generated was predominantly a Th2 biased with little or no Th1 response. Thus, there is a critical need to improve the vaccine formulation. Results presented in this study show that inclusion of alum + GLA (AL019) or MCA as an adjuvant for rBmHAT promoted balanced Th1/Th2 responses and significantly improved the vaccine-induced protection.
We were able to express and purify both rBmHAT and rBmHAX with minimal endotoxin contamination. Following immunization, both the antigens elicited significant levels of IgG antibodies irrespective of the adjuvant used for immunization. Vaccine antigen plus AL019 was given subcutaneously. However, for immunizations with MCA adjuvant, only the first dose of immunization was given subcutaneously. The rest of the booster doses were given orally. Despite giving the booster immunization as an oral dose, comparable levels of IgG antibody titer was generated in these vaccinated animals. These findings were similar to those observed by Carroll et al. (2016), who also demonstrated induction of significant cellular immunity and type 1 interferons following the use of MCA as an adjuvant in mice. Ability to deliver the vaccine orally is potentially a major advantage of using MCA as adjuvant especially in clinical setting where the vaccine booster doses can be given orally to children. Further studies are needed to determine if all the immunization doses with MCA adjuvant can be given orally and still achieve the high IgG antibody titer. Protection studies showed that rBmHAT is a better vaccine antigen than rBmHAX (94% vs 67%). Compared to our previous studies, inclusion of AL019 as an adjuvant for rBmHAT immunization was found to be slightly better (94% vs 92% protection) (Samykutty et al. 2010, Dakshinamoorthy et al. 2013a; Dakshinamoorthy et al. 2013c). We did not include alum adjuvant group in this study. Nevertheless, nearly all our previous studies used alum as an adjuvant for rBmHAT and consistently we observed approximately 94% protection against challenge infection in the mouse model. The present study showed that protection obtained with the rBmHAT + AL019 formulation was comparable or slightly better to the rBmHAT + Alum formulation. As far as the vaccine-induced immune responses, rBmHAT + AL019 were considerably better in inducing a balanced Th1/Th2 responses compared to our previously reported (Dakshinamoorthy et al. 2014) vaccine-induced immune responses with rBmHAT + Alum. One of our earlier vaccination trails in the mouse model suggested that significant protection can be achieved in the mouse model with the vaccine protein alone and no adjuvant (Dakshinamoorthy et al. 2014). However, when we tested the protein alone formulation in the rhesus macaque, all five animals became positive (unpublished data) indicating that the protein alone formulation is not protective in non-human primates. Given these findings, we decided not to pursue the ‘no adjuvant’ formulation for further vaccine development.
Several of our previous studies showed that the mechanism of parasite killing in rBmHAT vaccinated animals involve antibody dependent cell mediated cytotoxicity (ADCC) mechanism. Thus, both cells and antibodies are critical for the killing of larvae. In this study also we observed numerous cells attached to the surface of the dead larvae confirming our previous observations (Dakshinamoorthy et al. 2013c; Dakshinamoorthy et al. 2014). In this study we did not analyze the larvae bound cell population, however, one of our ongoing studies show that the bound cells are largely macrophages and produce activation products such as myeloperoxidase. Larvae incubated in sera samples from control animals had no cells attached to them.
Analyses of the antigen-specific antibody responses generated following vaccination with rBmHAT showed that IgG1, IgG2a and IgG2b isotype were predominant suggesting a balanced Th1 and Th2 responses. These findings were further confirmed when the cytokine responses of spleen cells were analyzed. Spleen cells from vaccinated animals secreted significant amounts of IL-4 and IFN-γ in response to rBmHAT stimulation. This observation further confirmed the generation of a balanced Th1/Th2 response to the antigens in the vaccinated animals. Increases in IL-17 have been shown to be critical for the vaccine-induced protection in several systems (Lin et al. 2010, Habets et al. 2016). Thus, an increase in the levels of secreted IL-17 in the culture supernatants of spleen cells from vaccinated animals suggests a role for IL-17 secreting cells in the vaccine-induced protection to rBmHAT. The spleen T cell population from vaccinated animals also contained both effector memory and central memory T cells phenotypes. Taken together these findings suggest that significant humoral and cellular protective immune responses were generated against the vaccine antigens. Our studies also confirmed that AL019 and MCA adjuvants can promote a balanced Th1/Th2 response to vaccine antigens in mice. Our previous vaccination trials using rBmHAT in macaque gave only ~40% protection (Dakshnamoorthy et al. 2014). These poor results were attributed to lack of induction of Th1 responses following vaccination. The fact that both AL019 and MCA can promote balanced Th1/Th2 responses suggests that any of these two adjuvants can be tested in the macaque model to improve the vaccine-induced protection to rBmHAT. Previous studies showed that vaccination using rBmHAT was safe in the macaque model and the monkeys did not develop any IgE responses to rBmHAT Dakshinamoorthy et al. 2014). In the current vaccination trial also there was no IgE responses to the vaccine antigens in the mice when given along with AL019 or MCA adjuvant.
In conclusion, our present study showed that including AL019 or MCA as adjuvant along with rBmHAT vaccination significantly improved the rate of protection and the immune responses generated were a balanced Th1/Th2 response. Further studies are planned to test the rBmHAT plus AL019 or MCA adjuvant in the macaque model.
Acknowledgments
We wish to acknowledge the support of the NIH/NIAID Filariasis research reagent resource center, College of Veterinary Medicine, University of Georgia, Athens, GA under NIAID (supply contract AI#30022) for providing B. malayi third stage infective larvae (L3). We are also grateful to Infectious Diseases Research Institute, Seattle, WA and Pacific GeneTech, Hong Kong for providing AL019 and MCA adjuvants.
Funding
This study was supported by the National Institute of Health (NIH), MD, USA (grant number- AI-116441).
Footnotes
Conflict of interest
There are no conflicts of interest for any of the authors.
Ethical approval: Use of animal in this study was approved by the Animal Care Committee of the University of Illinois, Rockford. The study followed the National Institutes of Health guidelines for the care and use of Laboratory animals.
Ethical approval: All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted. Use of animal in this study was approved by the Animal Care Committee of the University of Illinois, Rockford. The study followed the National Institutes of Health guidelines for the care and use of Laboratory animals. This article does not contain any studies with human participants performed by any of the authors.
References
- Abraham D, Grieve RB, Holy JM, Christensen BM. Immunity to larval Brugia malayi in BALB/c mice: protective immunity and inhibition of larval development. Am J Trop Med Hyg. 1989;40:598–604. doi: 10.4269/ajtmh.1989.40.598. [DOI] [PubMed] [Google Scholar]
- Anand SB, Murugan V, Prabhu PR, Anandharaman V, Reddy MV, Kaliraj P. Comparison of immunogenicity, protective efficacy of single and cocktail DNA vaccine of Brugia malayi abundant larval transcript (ALT-2) and thioredoxin peroxidase (TPX) in mice. Acta Trop. 2008;107:106–112. doi: 10.1016/j.actatropica.2008.04.018. Epub 2008 May 1. [DOI] [PubMed] [Google Scholar]
- Arias MA, Van Roey GA, Tregoning JS, Moutaftsi M, Coler RN, Windish HP, Reed SG, Carter D, Shattock RJ. Glucopyranosyl Lipid Adjuvant (GLA), a Synthetic TLR4 Agonist, Promotes Potent Systemic and Mucosal Responses to Intranasal Immunization with HIVgp140. PLoS ONE. 2012;7:e41144. doi: 10.1371/journal.pone.0041144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arumugam S, Wei J, Ward D, Abraham D, Lustigman S, Zhan B, Klei TR. Vaccination with a genetically modified Brugia malayi cysteine protease inhibitor-2 reduces adult parasite numbers and affects the fertility of female worms following a subcutaneous challenge of Mongolian gerbils (Meriones unguiculatus) with B. malayi infective larvae. Int J Parasitol. 2014;44:675–679. doi: 10.1016/j.ijpara.2014.05.003. Epub 2014 Jun 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolatto J1, Borducchi E, Rodriguez D, Keller AC, Faquim-Mauro E, Bortoluci KR, Mucida D, Gomes E, Christ A, Schnyder-Candrian S, Schnyder B, Ryffel B, Russo M. Toll-like receptor 4 agonists adsorbed to aluminium hydroxide adjuvant attenuate ovalbumin-specific allergic airway disease: role of MyD88 adaptor molecule and interleukin-12/interferon-gamma axis. Clin Exp Allergy. 2008;38:1668–1679. doi: 10.1111/j.1365-2222.2008.03036.x. Epub 2008 Jun 25. [DOI] [PubMed] [Google Scholar]
- Carroll EC, Jin L, Mori A, Muñoz-Wolf N, Oleszycka E, Moran HB, Mansouri S, McEntee CP, Lambe E, Agger EM, Andersen P, Cunningham C, Hertzog P, Fitzgerald KA, Bowie AG, Lavelle EC. The Vaccine Adjuvant Chitosan Promotes Cellular Immunity via DNA Sensor cGAS-STING-Dependent Induction of Type I Interferons. Immunity. 2016;44:597–608. doi: 10.1016/j.immuni.2016.02.004. Epub 2016 Mar 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casella CR, Mitchell TC. Putting endotoxin to work for us: monophosphoryl lipid A as a safe and effective vaccine adjuvant. Cell Mol Life Sci. 2008;65:3231–3240. doi: 10.1007/s00018-008-8228-6. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coler RN, Bertholet S, Moutaftsi M, Guderian JA, Windish HP, Baldwin SL, Laughlin EM, Duthie MS, Fox CB, Carter D, Friede M, Vedvick TS, Reed SG. Development and Characterization of Synthetic Glucopyranosyl Lipid Adjuvant System as a Vaccine Adjuvant. PLoS One. 2011;6:e16333. doi: 10.1371/journal.pone.0016333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakshinamoorthy G, Samykutty AK, Munirathinam G, Shinde GB, Nutman T, Reddy MV, Kalyanasundaram R. Biochemical Characterization and Evaluation of a Brugia malayi Small Heat Shock Protein as a Vaccine against Lymphatic Filariasis. PLoS One. 2012;7:e34077. doi: 10.1371/journal.pone.0034077. Epub 2012 Apr 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakshinamoorthy G, Kalyanasundaram R. Evaluating the efficacy of rBmHATalphac as a multivalent vaccine against lymphatic filariasis in experimental animals and optimizing the adjuvant formulation. Vaccine. 2013a;32:19–25. doi: 10.1016/j.vaccine.2013.10.083. Epub 2013 Nov 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakshinamoorthy G, Munirathinam G, Stoicescu K, Reddy MV, Kalyanasundaram R. Large Extracellular Loop of Tetraspanin as a potential vaccine candidate for filariasis. PLoS One. 2013b;8:e77394. doi: 10.1371/journal.pone.0077394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakshinamoorthy G, Samykutty AK, Munirathinam G, Reddy MV, Kalyanasundaram R. Multivalent fusion protein vaccine for lymphatic filariasis. Vaccine. 2013c;31:1616–1622. doi: 10.1016/j.vaccine.2012.09.055. Epub 2012 Oct 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakshinamoorthy G, von Gegerfelt A, Andersen H, Lewis M, Kalyanasundaram R. Evaluation of a multivalent vaccine against lymphatic filariasis in rhesus macaque model. PLoS One. 2014;9:e112982. doi: 10.1371/journal.pone.0112982. eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day KP. The endemic normal in lymphatic filariasis: A static concept. Parasitol Today. 1991;7:341–343. doi: 10.1016/0169-4758(91)90215-a. [DOI] [PubMed] [Google Scholar]
- Di Pasquale A, Preiss S, Tavares Da Silva F, Garçon N. Vaccine Adjuvants: from 1920 to 2015 and Beyond. Vaccines (Basel) 2015;3:320–343. doi: 10.3390/vaccines3020320. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didierlaurent AM, Morel S, Lockman L, Giannini SL, Bisteau M, Carlsen H, Kielland A, Vosters O, Vanderheyde N, Schiavetti F, Larocque D, Van Mechelen M, Garçon N. AS04, an Aluminum Salt- and TLR4 Agonist-Based Adjuvant System, Induces a Transient Localized Innate Immune Response Leading to Enhanced Adaptive Immunity. J Immunol. 2009;183:6186–6197. doi: 10.4049/jimmunol.0901474. Epub 2009 Oct 28. [DOI] [PubMed] [Google Scholar]
- Fox CB, Friede M, Reed SG, Ireton GC. Synthetic and natural TLR4 agonists as safe and effective vaccine adjuvants. Subcell Biochem. 2010;53:303–321. doi: 10.1007/978-90-481-9078-2_14. [DOI] [PubMed] [Google Scholar]
- Gnanasekar M, Rao KV, He YX, Mishra PK, Nutman TB, Kaliraj P, Ramaswamy K. Novel phage display-based subtractive screening to identify vaccine candidates of Brugia malayi. Infect Immun. 2004;72:4707–4715. doi: 10.1128/IAI.72.8.4707-4715.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golde WT, Gollobin P, Rodriguez LL. A rapid, simple, and humane method for submandibular bleeding of mice using a lancet. Lab Animal. 2005;34:39–43. doi: 10.1038/laban1005-39. [DOI] [PubMed] [Google Scholar]
- Goulopoulou S, McCarthy CG, Webb RC. Toll-like Receptors in the Vascular System: Sensing the Dangers Within. Pharmacol Rev. 2016;68:142–167. doi: 10.1124/pr.114.010090. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta RK. Aluminum compounds as vaccine adjuvants. Adv Drug Deliv Rev. 1998;32:155–172. doi: 10.1016/s0169-409x(98)00008-8. [DOI] [PubMed] [Google Scholar]
- Habets MN, van Selm S, van Opzeeland FJ, Simonetti E, Hermans PW, de Jonge MI, Diavatopoulos DA. Role of antibodies and IL17-mediated immunity in protection against pneumococcal otitis media. Vaccine. 2016;34:5968–5974. doi: 10.1016/j.vaccine.2016.09.057. Epub 2016 Oct 19. [DOI] [PubMed] [Google Scholar]
- Harris JR, Wiegand RE. Detecting infection hotspots: Modeling the surveillance challenge for elimination of lymphatic filariasis. PLoS Negl Trop Dis. 2017;11:e0005610. doi: 10.1371/journal.pntd.0005610. eCollection 2017 May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jambulingam P, Subramanian S, de Vlas SJ, Vinubala C, Stolk WA. Mathematical modelling of lymphatic filariasis elimination programmes in India: required duration of mass drug administration and post-treatment level of infection indicators. Parasit Vectors. 2016;9:501. doi: 10.1186/s13071-016-1768-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang HL, Kang ML, Quan JS, Kang SG, Akaike T, Yoo HS, Cho CS. The potential of mannosylated chitosan microspheres to target macrophage mannose receptors in an adjuvant-delivery system for intranasal immunization. Biomaterials. 2008;29:1931–1939. doi: 10.1016/j.biomaterials.2007.12.025. Epub 2008 Jan 25. [DOI] [PubMed] [Google Scholar]
- Joseph SK, Ramaswamy K. Single multivalent vaccination boosted by trickle larval infection confers protection against experimental lymphatic filariasis. Vaccine. 2013;31:3320–3326. doi: 10.1016/j.vaccine.2013.05.077. Epub 2013 Jun 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyanasundaram R, Balumuri P. Multivalent vaccine formulation with BmVAL-1 and BmALT-2 confer significant protection against challenge infections with Brugia malayi in mice and jirds. Res Rep Trop Med. 2011;2011:45–56. doi: 10.2147/RRTM.S13679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Slight SR, Khader SA. Th17 cytokines and vaccine-induced immunity. Semin Immunopathol. 2010;32:79–90. doi: 10.1007/s00281-009-0191-2. Epub 2010 Jan 30. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrack P, McKee AS, Munks MW. Towards an understanding of the adjuvant action of aluminum. Nat Rev Immunol. 2009;9:287–293. doi: 10.1038/nri2510. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan T, Verma P, Rao DN. Novel adjuvants & delivery vehicles for vaccines development: A road ahead. Indian J Med Res. 2013;138:779–795. [PMC free article] [PubMed] [Google Scholar]
- Ramaswamy K. Lymphatic Filariasis: Current Status of Elimination using Chemotherapy and the Need for a Vaccine. In: Saxena AK, editor. Top Med Chem. 1. Springer International Publishing; Switzerland: 2016. pp. 1–28. [DOI] [Google Scholar]
- Samykutty A, Dakshinamoorthy G, Kalyanasundaram R. Multivalent Vaccine for Lymphatic Filariasis. Procedia Vaccinol. 2010;3:12–18. doi: 10.1016/j.provac.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnare M, Barton GM, Holt AC, Takeda K, Akira S, Medzhitov R. Toll-like receptors control activation of adaptive immune responses. Nat Immunol. 2001;2:947–950. doi: 10.1038/ni712. [DOI] [PubMed] [Google Scholar]
- Steinhagen F, Kinjo T, Bode C, Klinman DM. TLR-Based Immune Adjuvants. Vaccine. 2011;29:3341–3355. doi: 10.1016/j.vaccine.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari K, Flynn BJ, Boscardin SB, Kastenmueller K, Salazar AM, Anderson CA, Soundarapandian V, Ahumada A, Keler T, Hoffman SL, Nussenzweig MC, Steinman RM, Seder RA. Poly(I:C) is an effective adjuvant for antibody and multi-functional CD4+ T cell responses to Plasmodium falciparum circumsporozoite protein (CSP) and αDEC-CSP in Non Human Primates. Vaccine. 2010;28:7256–7266. doi: 10.1016/j.vaccine.2010.08.098. Epub 2010 Sep 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirugnanam S, Pandiaraja P, Ramaswamy K, Murugan V, Gnanasekar M, Nandakumar K, Reddy MV, Kaliraj P. Brugia malayi: comparison of protective immune responses induced by Bm-alt-2 DNA, recombinant Bm-ALT-2 protein and prime-boost vaccine regimens in a jird model. Exp Parasitol. 2007;116:483–491. doi: 10.1016/j.exppara.2007.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. [accessed 06.09.17]; http://www.who.int/lymphatic_filariasis/global_progress/en/
- WHO. Global programme to eliminate lymphatic filariasis: progress report, 2015. Wkly Epidemiol Rec. 2016 Sep 30;91(39):441–455. [PubMed] [Google Scholar]