Abstract
General anesthetics revolutionized medicine by allowing surgeons to perform more complex and much longer procedures. This widely used class of drugs is essential to patient care, yet their exact molecular mechanism(s) are incompletely understood. One early hypothesis over a century ago proposed that nonspecific interactions of anesthetics with the lipid bilayer lead to changes in neuronal function via effects on membrane properties. This model was supported by the Meyer-Overton correlation between anesthetic potency and lipid solubility and despite more recent evidence for specific protein targets, in particular ion-channels, lipid bilayer-mediated effects of anesthetics is still under debate. We therefore tested a wide range of chemically diverse general anesthetics on lipid bilayer properties using a sensitive and functional gramicidin-based assay. None of the tested anesthetics altered lipid bilayer properties at clinically relevant concentrations. Some anesthetics did affect the bilayer, though only at high supratherapeutic concentrations, which are unlikely relevant for clinical anesthesia. These results suggest that anesthetics directly interact with membrane proteins without altering lipid bilayer properties at clinically relevant concentrations. Voltage-gated Na+ channels are potential anesthetic targets and various isoforms are inhibited by a wide range of volatile anesthetics. They inhibit channel function by reducing peak Na+ current and shifting steady-state inactivation toward more hyperpolarized potentials. Recent advances in crystallography of prokaryotic Na+ channels, which are sensitive to volatile anesthetics, together with molecular dynamics simulations and electrophysiological studies will help identify potential anesthetic interaction sites within the channel protein itself.
Keywords: Isoflurane, gramicidin channel, anesthetic mechanisms, bilayer modification, amphiphiles, NaChBac
Since the first public and successful demonstration of anesthesia using diethyl ether over 170 years ago (Bigelow 1846), scientists have struggled to understand how anesthetic drugs work. For all considerable effort, the mechanism(s) of inhaled anesthetics are not fully understood despite their widespread clinical use. The common volatile anesthetic isoflurane was approved almost 40 years ago, and was added to the WHO Model List of Essential Medicines in 2011 due to its importance in modern medicine (www.who.int). Yet our understanding of the molecular mechanism(s) critical to isoflurane’s anesthetic effects remains limited.
The dramatic effects of anesthetic agents were important in the development of biological thought in the second half of the 19th century. Claude Bernard (1813–1878) developed a unitary vision of life, in which life itself was defined by susceptibility to anesthesia regardless of the species. This also included plants, and his experiments demonstrating the immobilizing effects of ether on the movement of the mimosa plant spiked considerable interest. He concluded that despite the diversity of anesthetic agents, the endpoint –i.e. the anesthetic state– should remain the same (Bernard 1878). The susceptibility to anesthesia of all living beings would separate any effect on non-living objects due to mere physics and chemistry. Rather than challenging Bernard’s unitary paradigm, in the early 20th century Meyer and Overton took a more quantitative approach by focusing on the affinity of anesthetics for lipids. Their experiments lead to the Meyer-Overton correlation between anesthetic potency and lipophilicity (Meyer 1899; Overton 1901). This eventually led to lipid-based hypotheses of anesthetic action, which posit nonspecific effects of anesthetics on lipid bilayer properties that in turn alter membrane (protein) function (Perouansky 2012). The mechanism(s) by which anesthetics might alter membrane function was not specified, however. The proposed mode of action of anesthetics thus differs from the pharmacology of most drugs, which typically alter protein function by binding to specific protein binding sites (Howard et al. 2014). However, membrane proteins, such as ion channels, receptors and transporters, are also subject to regulation by the lipid bilayer (Bienvenüe and Marie 1994; Brown 1994; Lee 2004; Andersen and Koeppe 2007; Marsh 2007), and indirect effects due to drug-induced alterations in protein-lipid bilayer interactions cannot be excluded a priori. Changes in lipid bilayer elasticity or intrinsic lipid curvature can, for example, alter the lipid bilayer contribution to the free energy difference between membrane protein conformations (Lundbaek et al. 2010). This provides a plausible mechanism for anesthetic modulation of membrane protein function through altered lipid bilayer properties (Cantor 1997; Sonner and Cantor 2013).
In this context, it is important that amphiphiles compounds with both hydrophilic and lipophilic properties, including many biologically active compounds tend to be potent modifiers of lipid bilayer mechanical properties (Hwang et al. 2003; Lundbæk et al. 2005; Bruno et al. 2007; Ingólfsson et al. 2007; Rusinova et al. 2011; Howery et al. 2012; Ingólfsson et al. 2014; Rusinova et al. 2015). Indeed, any molecule that partitions into lipid bilayers has the potential to alter lipid bilayer properties –including elasticity (Evans et al. 1995; Zhelev 1998; Bruno et al. 2013)– which in turn can alter the function of membrane proteins embedded in the bilayer.
In this review, we discuss recent studies on the possible role of lipid bilayer modification in anesthetic effects on ion channels, with a specific focus on voltage-gated Na+ channels (Nav). First we describe a highly sensitive asssay to detect changes in lipid bilayer properties; second we discuss the effects of general anesthetics on lipid bilayer properties; third we discuss the effects of inhaled anesthetics on voltage-gated Na+ channels (Nav), a membrane protein known to be inhibited by volatile anesthetics; finally we summarize recent advances in the identification of anesthetic binding sites within prokaryotic ion channels, in particular Nav.
Measuring lipid bilayer properties with gramicidin channels as a molecular force probe
The molecular composition of lipid bilayers is complex; they are composed of a variety of phospholipids and glycolipids, with various fatty acid chains, and cholesterol as major constituents. Polar phospholipids assemble with their hydrophobic hydrocarbon tails oriented toward the center of the bilayer and their hydrophilic head groups facing the aqueous membrane surfaces. Bilayer thickness varies with changes in lipid composition, and is determined in part by the acyl chain length and saturation, and by the presence of cholesterol. The greater the number of cis unsaturated double bonds within the hydrocarbon tails (which increases hydrocarbon chain disorder), the more disordered they are. The more saturated the acyl chains (which increases hydrocarbon chain order) and the higher mole-fractions of sphingomyelin and cholesterol, the more ordered they are. In this energetically favorable bilayer structure, the more disordered lipids can form a liquid disordered (ld) state, whereas the more ordered lipids can form a liquid ordered (lo) state.
Changes in lipid composition profoundly affect the mechanical properties of the bilayer (Evans and Needham 1987), as well as the domain organization (Veatch and Keller 2005b). Membrane proteins are sensitive to changes in lipid bilayer properties, and their function depends on hydrophobic coupling between the integrated membrane protein and bilayer lipids. Further, conformational changes of protein domains that are embedded in the membrane require deformation of the surrounding bilayer (Mouritsen and Bloom 1984; Gruner and Shyamsunder 1991; Andersen et al. 1992; Brown 1994; Lundbæk and Andersen 1994). This bilayer deformation has an energetic cost, known as the bilayer deformation energy , which contributes to the free energy difference between different protein conformations, and therefore ultimately affects protein functions that involve conformation transitions, which are key to gating of ligand- and voltage-gated ion channels (Lundbæk and Andersen 1994; Ashrafuzzaman et al. 2006; Lundbaek et al. 2010).
Membrane lipids appear to form a multi-component bilayer, with domains appearing as temperature decreases (Gray et al. 2015), which suggests that it might be possible to explore how biologically active compounds alter cell membrane properties and membrane protein function using relative simple systems. A simple membrane protein suitable for detecting changes in lipid bilayer properties is the gramicidin ion channel. Gramicidin channels are formed by transmembrane dimerization of monomeric cylindrical (i.e. β6,3-helical) subunits that reside in both leaflets of the bilayer (O’Connell et al. 1990). Importantly, the hydrophobic length (l) of the dimer is usually less than the lipid bilayer thickness (d0) (Fig. 1).
Fig. 1.
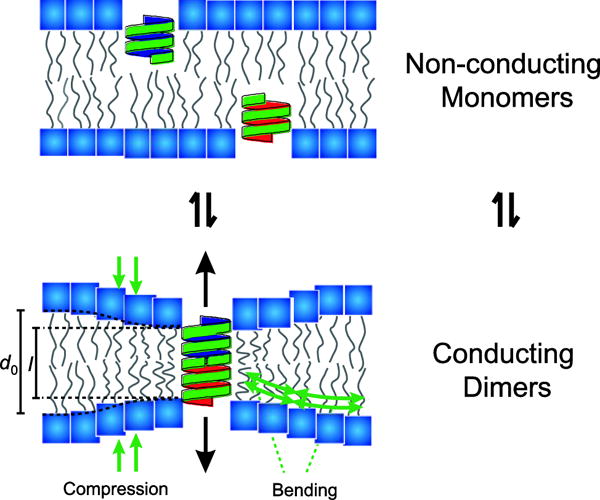
Schematic of gramicidin channels as molecular force probes for sensing changes in bulk lipid bilayer properties. Two non-conducting gramicidin monomers (green cylindrical structures) can dimerize to form a conducting dimer. The thickness of the bilayer (d0) is larger than the length of the conducting dimer (l), therefore a bilayer deformation energy is required to enable this dimerization. Amphiphiles – compounds with both hydrophilic and lipophilic properties – can lead to changes in bulk bilayer properties that can be sensed by gramicidin channels.
Gramicidin channel formation thus requires local deformation (i.e. compression and bending) of the bilayer core in order to match the hydrophobic exterior of the channel (Elliott et al. 1983; Huang 1986; Helfrich and Jakobsson 1990; Lundbæk and Andersen 1999). Therefore, changes in lipid bilayer properties alter the bilayer deformation energy of the monomers and dimers and the bilayer contribution to the free energy of dimerization , where represents intrinsic contributions and refers to . Because contributes to the energetic cost of channel formation, changes in channel appearance rate and lifetime (as reflected by the number of conducting channels) is a measure of bilayer elasticity (Lundbæk et al. 2010).
Gramicidin channels have been used extensively to detect changes in lipid bilayer mechanical properties (Sawyer et al. 1989; Lundbæk and Andersen 1994), including changes in membrane thickness (Elliott et al. 1983), curvature (Lundbaek et al. 1997), tension (Goulian et al. 1998) and elasticity (Lundbæk et al. 2005; Lundbaek et al. 2010; Rusinova et al. 2011). Many small molecule amphiphiles alter lipid bilayer properties that are relevant to the function of ion channels formed by integral membrane proteins (Lundbaek et al. 2010). Gramicidin channels therefore provide a sensitive probe for measuring such changes in lipid bilayer properties relevant to membrane protein function (Lundbæk et al. 1996; Lundbæk et al. 2004; Suchyna et al. 2004; Lundbæk et al. 2005; Artigas et al. 2006; Søgaard et al. 2006; Rusinova et al. 2011; Ingólfsson et al. 2014). Bilayer alterations by amphiphiles also include changes in bilayer deformation energy that come with such conformational changes of membrane proteins such as ion channels. In other words, changes in ion channel function produced by amphiphiles could result not only from binding energy differences to the respective channel states (Monod et al. 1965; Jackson 1989), e.g. the resting and inactivated states or protein conformations I and II (expressed as changes in ), but also from changes in lipid bilayer properties as expressed by changes in .
The contribution of to to reconsideration of how drugs alter membrane protein function. Possible sites of drug interaction with ion channel proteins are shown schematically in Fig. 2. Site 1 represent “simple” channel block. Site 2 is formed solely by the channel protein, and drug binding can lead to inhibition or potentiation of function by altering the free energy difference between various channel states that affect channel gating (due to the changes in that result when the drug binds with different affinity to different channel states). Site 3 is composed of both protein and lipid elements, such that drug binding will alter both and contributions to the total free energy difference between the different channel states. Drug binding to this site is also likely to alter the residual exposure energy , which arises from imperfect hydrophobic matching between adjacent helices and subunits (Mondal et al. 2011). Sites 4 and 5 reflect drug partitioning into in the lipid bilayer at the protein/bilayer boundary (Site 4), where drug binding will alter local lipid packing (reflected in the boundary condition for how the bilayer adapts to a channel-bilayer hydrophobic mismatch (Nielsen et al. 1998)), or in the bulk bilayer (which will result in changes in the bilayer material properties, such as the elastic moduli and intrinsic curvature).
Fig. 2.
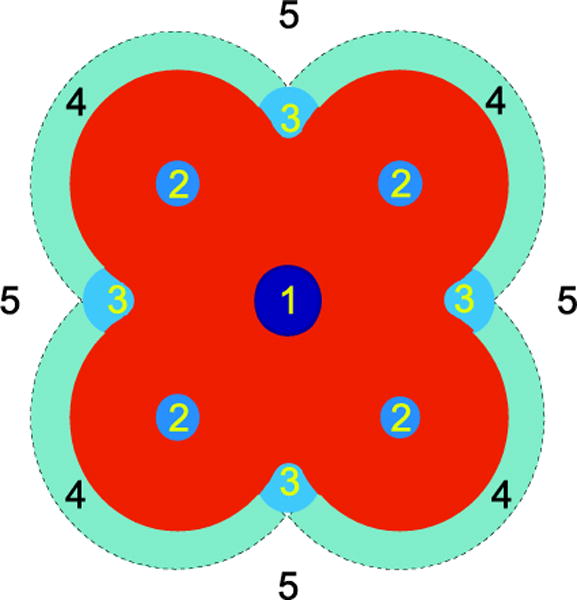
Schematic representation of potential drug-ion channel interaction sites. 1) binding within the pore impeding ion permeation, 2) drug binding sites formed by the channel, 3) specific drug binding sites composed of both the protein and the lipid bilayer, 4) drug accumulation at the protein-bilayer interface, 5) drug partitioning into the lipid bilayer-solution interface. Adapted from (Andersen 2008).
Effects of general anesthetics on lipid bilayer properties
Because many amphiphiles alter both lipid bilayer properties and membrane protein function, it becomes important to determine whether amphiphiles alter function due to direct interactions (binding) with the protein or due to changes in lipid bilayer properties. It is therefore necessary to have tools to explore the relative contributions of the amphiphile effect on the lipid bilayer (indirect) vs. direct membrane protein effects, which often are not immediately evident. To distinguish between these contributions, we tested a variety of general anesthetics, including inhaled ether and alkane anesthetics, intravenous anesthetics and experimental fluorobenzene anesthetics, for their effects on lipid bilayer properties using the gramicidin channel functional assay (Tibbs et al. 2013; Herold et al. 2014; Herold et al. 2017).
We used a gramicidin-channel based fluorescence assay, where large unilamellar vesicles (LUVs) containing gramicidin channels in the bilayer are loaded with a water-soluble fluorophore that is quenchable by the heavy monovalent metal ion Tl+. Upon dimerization of gramicidin channel monomers (Fig. 1), Tl+, which is conducted through gramicidin channels, enters the LUVs, quenching the fluorophore and resulting in decay of the fluorescence signal. The rate of fluorescence decay in the absence or presence of drug can be measured, and changes in the quench rate reflect changes in the gramicidin monomer↔dimer equilibrium. Compounds that facilitate dimerization allow more Tl+ entry, which is reflected in a faster decay of fluorescence (Fig. 3).
Fig. 3.
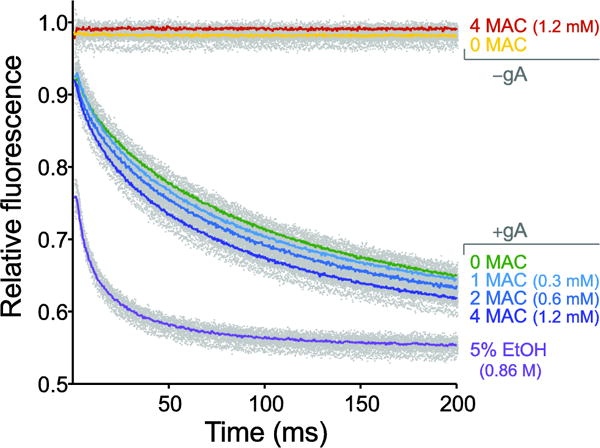
Effects of isoflurane on bulk lipid bilayer properties using the gramicidin based fluorescence assay. Example of normalized fluorescence traces over a 200 ms time course showing three concentrations of isoflurane (expressed as minimum alveolar concentration, MAC, defined as the concentration that prevents movement in response to a painful stimulus in 50% of subjects, comparable to EC50) with ethanol (5% EtOH; ~0.86 M) as a positive control. Gray dots denote results from all experiments (>5 per condition) and solid colored lines denote the average of all experiments for the individual condition. Note the absence of fluorescence decay using vesicles without gramicidin (−gA) and increasing rates of fluorescence decay in gramicidin-containing vesicles (+gA) with increasing concentrations of isoflurane.
At concentrations used in the clinic to produce general anesthesia, none of the anesthetics tested significantly altered lipid bilayer properties as detected by gramicidin channel function (Fig. 4). This finding has the important implication that any effects observed on ion channel function at clinical anesthetic concentrations are not due to changes in lipid bilayer properties (as detected by the gramicidin channel based assay). Certain anesthetics were found to alter bilayer properties at supratherapeutic (toxic) concentrations, such that bilayer perturbations might contribute to the many undesirable off-target effects of anesthetics (data not shown, see (Herold et al. 2017)).
Fig. 4.
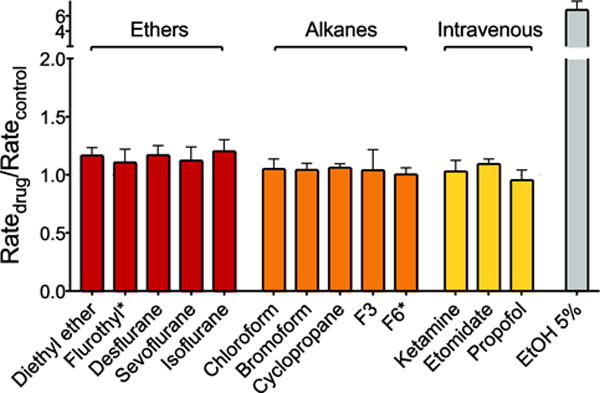
Effects of general anesthetics on lipid bilayer properties using the gramicidin based fluorescence assay. Normalized fluorescence quench rates are displayed for ether anesthetics (red), alkane anesthetics (orange) and intravenous anesthetics (yellow) at the clinical concentration of 1 MAC (or 10 μM for intravenous anesthetics). The value for 5% ethanol (EtOH; ~0.86 M) is shown as a positive control. Flurothyl* and F6* are compounds that do not cause immobility (and therefore are classified as nonanesthetics) that were tested at concentrations predicted to produce anesthesia based on their lipid solubilities. None of the tested anesthetics or nonanesthetics altered bulk lipid bilayer properties as detected by their normalized quench rates (Ratedrug/Ratecontrol), with a value of 1.0 indicating no significant effect on bulk lipid bilayer properties. Data are expressed as mean ± SD, n = 3–5. Adapted from (Herold et al. 2017).
The finding that general anesthetics do not alter lipid bilayer mechanical properties (expressed in ) at clinically relevant concentrations does not exclude more subtle effects, such as altering the domain organization of biological membranes (Gray et al. 2013; Machta et al. 2016). For example, anesthetics might alter the line tension between ld and lo domains, which could alter the lateral organization of ion channels (and other proteins) in the membrane. We therefore also tested anesthetic effects on gramicidin channel function in multi-component bilayers that form coexisting ld and lo domains. Even in this more complex bilayer preparation, there were no effects of representative anesthetics on gramicidin channel function (Herold et al. 2017).
Effects of inhaled anesthetics on voltage-gated Na+ channels
As summarized above, general anesthetics do not alter lipid bilayer properties enough at clinical concentrations to result in indirect effects on membrane protein function. This is a remarkable finding because most hydrophophic/amphiphilic compounds are general modifiers of membrane protein function, and alter membrane protein function at the concentrations at which they alter lipid bilayer properties (Ingólfsson et al. 2014). Therefore, anesthetic effects (at clinical anesthetic concentrations) must result from direct interactions with relevant membrane proteins via interactions with protein binding sites.
Ion channels are membrane proteins that are critical to the regulation of intercellular communication. General anesthetics alter neuronal function by depressing excitatory and enhancing inhibitory synaptic transmission (Rudolph and Antkowiak 2004; Hemmings et al. 2005). In the last two decades, ligand- and voltage-gated ion channels have emerged as the leading candidate targets for the desirable effects of general anesthetics (amnesia, unconsciousness and immobility). For example, most general anesthetics modify the function of -aminobutyric acid (GABA)A receptors (Zimmerman et al. 1994), NMDA-type glutamate (Dickinson et al. 2007; Haseneder et al. 2008), two-pore domain K+ channels (Patel and Honore 2001; Sirois et al. 2002), and/or voltage-gated Na+ (Herold and Hemmings 2012; Herold et al. 2014) and Ca2+ (Study 1994; Nikonorov et al. 1998) channels. Recent evidence indicates an important role for volatile anesthetic effects on presynaptic voltage-gated Na+ channels (Herold and Hemmings 2012).
In the late 1970s reports of the effects of the first clinically used inhaled volatile anesthetic diethyl ether and the halogenated alkane halothane on Na+ currents appeared (Kendig et al. 1979; Bean et al. 1981). Diethyl ether reduced peak Na+ current and shifted steady-state inactivation (h∞) to more hyperpolarized potentials without significant changes on frequency-dependent stimulation or the time-course of recovery from inactivation. The effects of ether can be described within the context of a model originally proposed to describe the effects of the neutrally charged local anesthetic benzocaine (Kendig et al. 1979). However, the effect of benzocaine on the hyperpolarizing shift in the voltage dependence of inactivation was much larger than expected, which was attributed to some degree of open channel block. Unlike with ether, the apparent slowing of recovery from inactivation with benzocaine was thought to be due to slow dissociation of the drug from the channel. At this time, the effects of ether and halothane (Bean et al. 1981) were believed to result from perturbation of the lipid bilayer, though the available evidence did not exclude a direct effect on the channel protein itself. These early studies were performed mostly using nonmammalian preparations such as frog node of Ranvier or crayfish giant axon. In subsequent years, more anesthetics were studied including n-alcohols, methoxyflurane, dichloromethane, chloroform and various hydrocarbon anesthetics (Haydon and Urban 1983c, a, b; Elliott et al. 1985). All anesthetics produced a characteristic reversible hyperpolarizing shift in steady-state (h∞) inactivation, as well as a (not always reversible) reduction in peak Na+ current, resulting in impaired axonal conduction. It was concluded that a substantial increase in membrane thickness after adsorption of anesthetic led to inhibition of Na+ conductance and thus action potential conduction. This was based on earlier studies in which hydrocarbons at similar concentrations as well as short-chain phospholipids increased lipid bilayer thickness, which could ultimately affect channel gating (Fettiplace et al. 1971; White 1977; Dilger 1981; Dilger et al. 1982; Haydon and Urban 1983a). However, other studies concluded that changes in bilayer thickness were too small to account for such a large shift in steady-state inactivation (Franks and Lieb 1979; Elliott et al. 1985). This controversy of whether or not anesthetics sufficiently perturb bilayer properties sustained the search for more specific protein targets.
An early report of the effects of ethanol, ether, halothane and enflurane on mammalian Nav in rodent brain synaptosomes confirmed the findings from nonmammalian species, but a lower concentrations (Harris and Bruno 1985). Molecular cloning enabled the study of anesthetic effects on specific Nav isoforms, and the brain-specific isoform Nav1.2 was one of the first to be found sensitive to clinical concentrations of volatile anesthetics (Rehberg et al. 1996). To date the effects of various volatile anesthetics have been reported for Nav1.4, Nav1.5, Nav1.6, Nav1.7 and Nav1.8 (Ratnakumari and Hemmings 1998; Stadnicka et al. 1999; Ouyang et al. 2003; Shiraishi and Harris 2004; Ouyang and Hemmings 2007; Herold et al. 2009; Ouyang et al. 2009; Yokoyama et al. 2011; Herold et al. 2014; Purtell et al. 2015). Although there are small, agent-specific quantitative differences in their effects, all volatile anesthetics tested inhibit peak Na+ current in a voltage-dependent manner (Fig. 5a), leading to a hyperpolarizing shift in the voltage-dependence of steady-state inactivation (Fig. 5b) consistent with the reports from the 1970s.
Fig. 5.

Isoflurane inhibition of eukaryotic and prokaryotic Nav. a Macroscopic whole-cell Na+ current traces recorded from a mammalian ND7/23 cell endogenously expressing tetrodotoxin-sensitive Nav in the absence (gray discontinued traces) or presence (purple traces) of 0.8 mM isoflurane (~2.5 MAC). From a holding potential (Vh) of −80 mV the alternating stimulation protocols were chosen to test voltage-dependent inhibition. A test pulse (10 ms at 0 mV) to elicit peak Na+ current was preceded by a 300-ms prepulse to either −130 mV (denoted “V0”; left traces) or to a voltage at which approximately half of the channels were in the fast-inactivated state (denoted V½; –70 mV; right traces). b Steady-state inactivation (or Na+ channel availability; h∞) of tetrodotoxin-sensitive Na+ currents (Nav1.4) was tested using a double-pulse protocol with a 30-ms prepulse of from −110 to −20 mV in 10-mV steps, followed by a 25-ms test pulse to −10 mV. Peak Na+ current was normalized (INa/INamax), plotted against prepulse potential, and fitted with a two-state Boltzmann distribution to calculate V½, which was shifted by −10 mV in the presence of 0.8 mM isoflurane. Data are expressed as mean ± SD, n=7. Adapted from (Ouyang et al. 2009). c NaChBac current traces recorded from a transfected HEK293FT cell in the absence (gray discontinued traces) or presence (purple traces) of 0.8 mM isoflurane (~2.5 MAC) using the stimulation protocols depicted from a Vh of either −140 mV or −80 mV to test for voltage-dependent inhibition. The time scale over which NaChBac activates and inactivates is considerably slower than for mammalian Nav (~500 ms vs. 2–3 ms, respectively); the accelerated NaChBac current decay in the presence of isoflurane is also noteworthy. d NaChBac steady-state inactivation was tested from a Vh of −140 mV with 90-s prepulses ranging from −140 to −40 mV followed by a test pulse to −10 mV. V½ was shifted by −16 mV in the presence of 0.8 mM isoflurane. Data expressed as mean ± SD, n = 7–15. Adapted from (Sand et al. 2017).
Thus, there is strong evidence that general anesthetics at clinically relevant concentrations have direct interactions with Nav that do not involve effects mediated via the lipid bilayer (Herold et al. 2014; Herold et al. 2017). The consequence of such direct actions include a shift in the equilibrium from the resting to the inactivated state of the channel. However, many amphiphiles produce similar changes in channel function (Lundbæk et al. 2004; Lundbæk et al. 2005; Rusinova et al. 2011; Ingólfsson et al. 2014), so a role for lipid bilayer mediated effects cannot be completely excluded based solely on these experiments. It was therefore necessary to evaluate anesthetics effects on lipid bilayer properties using a functional assay sensitive to small local changes in lipid properties, like the gramicidin channel assay. Most previous experiments were done at room temperature (22 – 25 C), where there is visible domain separation in giant plasma membrane vesicles (Gray et al. 2015). Thus we cannot exclude effects due to anesthetic-induced changes in lipid domain organization as found by Veatch and colleagues (Gray et al. 2013; Machta et al. 2016). Again, the gramicidin experiments become critical.
Identification of anesthetic binding sites within prokaryotic Nav
The site(s) at which volatile anesthetics bind and alter Nav has not been directly identified, though recent advances in crystallization and molecular dynamics simulations of prokaryotic Nav provide promising avenues of research. Use of bacterial Nav as model proteins for understanding of how volatile anesthetics interact with mammalian Nav is a current focus of research in this area. For example, NaChBac, a prokaryotic Na+ channel (Ren et al. 2001), is sensitive to clinical concentrations of the commonly used volatile anesthetic isoflurane (Ouyang et al. 2007). Similar to mammalian Nav, inhibition of peak Na+ current in NaChBac is voltage-dependent with greater inhibition at more positive (depolarized) holding potentials (Fig. 5c). Inactivation is also shifted in the hyperpolarized direction (Fig. 5d). In contrast to the homologous mammalian Nav that consist of a single ion pore-forming subunit composed of four similar domains, NaChBac channels consist of a homotetramer and lack classic fast-inactivation characteristic of mammalian Nav. An x-ray crystal structure of the prokaryotic Na+ channel NavAb in a closed “pre-activated” conformation (Payandeh et al. 2011) revealed lipophilic fenestrations in the sides of the pore module that were occupied by fatty acyl chains extending into the central cavity. These lipophilic fenestrations of about 8 × 10 Å provide access for pore-blocking drugs to interact with the channel, and are believed to be involved in voltage-dependent block by charged inhibitors like local anesthetics as described by the “modulated receptor hypothesis” (Hille 1977; Payandeh et al. 2011).
Molecular dynamics (MD) flooding simulations using a structural model of NaChBac based on the x-ray crystal structures of homologous channels identified three potential binding sites for isoflurane located near the selectivity filter at the extracellular side, within the S4–S5 linker, as well as in the central cavity, which would disrupt Na+ ion permeation (Raju et al. 2013). The side fenestrations are believed to provide a pathway for hydrophobic drugs like isoflurane to access the pore. Another MD flooding simulation and combined electrophysiology study of NaChBac interactions revealed that sevoflurane, at low (0.2 mM) and high (2.0 mM) concentrations, shifted both the voltage-dependence of activation and inactivation toward more hyperpolarized potentials. However, in contrast to the effects of isoflurane, peak Na+ current was potentiated at the low sevoflurane concentration, and there was an increase in the rate constant for recovery from inactivation (Barber et al. 2014). This acceleration is in contrast to the electrophysiological effects of volatile anesthetics on NaChBac and mammalian Nav, where recovery from inactivation is prolonged (Ouyang et al. 2007; Ouyang et al. 2009; Herold et al. 2014; Purtell et al. 2015). This suggests that sevoflurane acts at multiple binding sites that affect both activation gating, as at the S4–S5 linker or the activation gate, and inactivation gating, through extracellular sites. Slow open-channel block by sevoflurane was postulated based on the observation that high (2 mM, or ~5 MAC) concentrations of sevoflurane accelerated current decay (Barber et al. 2014). Since this effect on channel function occurred at supraclinical sevoflurane concentrations that alter lipid bilayer properties (Herold et al. 2017), bilayer-mediated effects on open channel block cannot be excluded.
In a recent study of isoflurane binding to NaChBac using 19F NMR, isoflurane was proposed to inhibit the channel through an interaction at the base of the selectivity filter and by impeding the pivoting motion at the S4–S5 linker and the hinge controlling gating and inactivation motions of S6 (Kinde et al. 2016). A recent functional study of NaChBac employing electrophysiology and Markov modeling of NaChBac gating revealed that the effects of isoflurane on NaChBac could be described by increases in the rate constants for both channel activation and inactivation, with a dominant effect on inactivation (Sand et al. 2017). Compared to the model of slow open-channel block, this model better described the empirical data, and suggests that isoflurane acts at two distinct binding sites to accelerate both activation and inactivation gating (Sand et al. 2017). The isoflurane binding site at the base of the selectivity filter, identified by NMR (Kinde et al. 2016), is likely responsible for facilitating inactivation.
The results summarized above provide strong evidence that halogenated ether anesthetics alter Nav function primarily through direct interactions with the channel protein. To fully understand how a ligand alters the function of a (membrane) protein, however, it is necessary to have information about how the ligand interacts with the functionally important conformational states –and how they shift the equilibrium between the states. The lipid bilayer can affect the equilibrium distribution associated with any conformational change of the protein, and therefore the kinetics of the transitions between these different states. Detection of changes in this contribution to the free energy difference between individual channel conformations requires a sensitive experimental system similar to the one we have established (Rusinova et al. 2011; Herold et al. 2014; Ingólfsson et al. 2014 2014; Rusinova et al. 2015). Furthermore, experiments done at temperatures and membrane compositions that promote lipid domain separation (Veatch and Keller 2005a; Gray et al. 2015) necessarily requires consideration of how anesthetics alter local lipid (and protein) organization.
Binding sites identified in crystal structures may, or may not, be important for channel modulation, see also (Howard et al. 2014); the important sites are those where the energetics of ligand binding differs between different channel states (Jackson 1989). The recent high-resolution (2.45 Å) crystal structure of the conducting state of prokaryotic NavMs by the Wallace group (Sula et al. 2017) provides a complete, full-length structure (including the voltage-sensing, pore and C-terminal domains) of the open and activated conformation. Taken together with the earlier structures of Nav in various pre-activated states with closed gates (refs 1, 5 and 6 in Sula et al.), this structure provides a framework for further structural and functional studies of anesthetic interactions with voltage-gated Na+ channels.
Acknowledgments
Funding
This work was supported by the German Research Foundation (Deutsche Forschungsgemeinschaft) Grant HE4554/5-1 (K.F.H.), National Institutes of Health Grants GM058055 (to H.C.H.) and GM021347 (to O.S.A.).
Footnotes
Conflicts of Interest
The authors declare that they have no conflicts of interest.
ORCID ID:
KFH: 0000-0002-8614-0578
OSA: 0000-0002-3026-6710
HCH: 0000-0002-6043-5482
References
- Andersen OS. Perspectives on how to drug an ion channel. J Gen Physiol. 2008;131:395–397. doi: 10.1085/jgp.200810012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen OS, Koeppe RE. Bilayer thickness and membrane protein function: an energetic perspective. Annual review of biophysics and biomolecular structure. 2007;36:107–130. doi: 10.1146/annurev.biophys.36.040306.132643. [DOI] [PubMed] [Google Scholar]
- Andersen OS, Sawyer DB, Koeppe R. Modulation of channel function by the host bilayer. Biomembrane Structure and Function. 1992:227–244. [Google Scholar]
- Artigas P, Al’aref SJ, Hobart EA, Diaz LF, Sakaguchi M, Straw S, Andersen OS. 2,3-butanedione monoxime affects cystic fibrosis transmembrane conductance regulator channel function through phosphorylation-dependent and phosphorylation-independent mechanisms: the role of bilayer material properties. Mol Pharmacol. 2006;70:2015–2026. doi: 10.1124/mol.106.026070. [DOI] [PubMed] [Google Scholar]
- Ashrafuzzaman M, Lampson M, Greathouse D, Koeppe R, Ii, Andersen O. Manipulating lipid bilayer material properties using biologically active amphipathic molecules. Journal of Physics: Condensed Matter. 2006;18:S1235. [Google Scholar]
- Barber AF, Carnevale V, Klein ML, Eckenhoff RG, Covarrubias M. Modulation of a voltage-gated Na+ channel by sevoflurane involves multiple sites and distinct mechanisms. Proc Natl Acad Sci U S A. 2014;111:6726–6731. doi: 10.1073/pnas.1405768111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean BP, Shrager P, Goldstein DA. Modification of sodium and potassium channel gating kinetics by ether and halothane. J Gen Physiol. 1981;77:233–253. doi: 10.1085/jgp.77.3.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard C. Leçons sur les Phénoménes de la Vie Communs aux Animaux et aux Végétaus Baillière. Paris 1878 [Google Scholar]
- Bienvenüe A, Marie JS. Chapter 12 - Modulation of Protein Function by Lipids. In: Dick H, editor. Current Topics in Membranes. Vol. 40. Academic Press; 1994. pp. 319–354. [Google Scholar]
- Bigelow HJ. Insensibility during Surgical Operations Produced by Inhalation. The Boston Medical and Surgical Journal. 1846;35:309–317. [Google Scholar]
- Brown MF. Modulation of rhodopsin function by properties of the membrane bilayer. Chem Phys Lipids. 1994;73:159–180. doi: 10.1016/0009-3084(94)90180-5. [DOI] [PubMed] [Google Scholar]
- Bruno MJ, Koeppe RE, Andersen OS. Docosahexaenoic acid alters bilayer elastic properties. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:9638–9643. doi: 10.1073/pnas.0701015104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno MJ, Rusinova R, Gleason NJ, Koeppe RE, 2nd, Andersen OS. Interactions of drugs and amphiphiles with membranes: modulation of lipid bilayer elastic properties by changes in acyl chain unsaturation and protonation. Faraday Discuss. 2013;161:461–480. doi: 10.1039/c2fd20092a. discussion 563–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor RS. The lateral pressure profile in membranes: a physical mechanism of general anesthesia. Biochemistry. 1997;36:2339–2344. doi: 10.1021/bi9627323. [DOI] [PubMed] [Google Scholar]
- Dickinson R, Peterson BK, Banks P, Simillis C, Martin JC, Valenzuela CA, Maze M, Franks NP. Competitive inhibition at the glycine site of the N-methyl-D-aspartate receptor by the anesthetics xenon and isoflurane: evidence from molecular modeling and electrophysiology. Anesthesiology. 2007;107:756–767. doi: 10.1097/01.anes.0000287061.77674.71. [DOI] [PubMed] [Google Scholar]
- Dilger JP. The thickness of monoolein lipid bilayers as determined from reflectance measurements. Biochim Biophys Acta. 1981;645:357–363. doi: 10.1016/0005-2736(81)90208-x. [DOI] [PubMed] [Google Scholar]
- Dilger JP, Fisher LR, Haydon DA. A critical comparison of electrical and optical methods for bilayer thickness determination. Chemistry and Physics of Lipids. 1982;30:159–176. [Google Scholar]
- Elliott JR, Haydon DA, Hendry BM, Needham D. Inactivation of the sodium current in squid giant axons by hydrocarbons. Biophys J. 1985;48:617–622. doi: 10.1016/S0006-3495(85)83817-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott JR, Needham D, Dilger JP, Haydon DA. The effects of bilayer thickness and tension on gramicidin single-channel lifetime. Biochim Biophys Acta. 1983;735:95–103. doi: 10.1016/0005-2736(83)90264-x. [DOI] [PubMed] [Google Scholar]
- Evans E, Needham D. Physical properties of surfactant bilayer membranes: thermal transitions, elasticity, rigidity, cohesion and colloidal interactions. The Journal of Physical Chemistry. 1987;91:4219–4228. [Google Scholar]
- Evans E, Rawicz W, Hofmann A. Lipid bilayer expansion and mechanical disruption in solutions of water-soluble bile acid Falk Symposium. KLUWER ACADEMIC PUBLICATION; 1995. pp. 59–59. [Google Scholar]
- Fettiplace R, Andrews DM, Haydon DA. The thickness, composition and structure of some lipid bilayers and natural membranes. J Membr Biol. 1971;5:277–296. doi: 10.1007/BF01870555. [DOI] [PubMed] [Google Scholar]
- Franks NP, Lieb WR. The structure of lipid bilayers and the effects of general anaesthetics. An x-ray and neutron diffraction study. J Mol Biol. 1979;133:469–500. doi: 10.1016/0022-2836(79)90403-0. [DOI] [PubMed] [Google Scholar]
- Goulian M, Mesquita ON, Fygenson DK, Nielsen C, Andersen OS, Libchaber A. Gramicidin channel kinetics under tension. Biophys J. 1998;74:328–337. doi: 10.1016/S0006-3495(98)77790-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray E, Karslake J, Machta BB, Veatch SL. Liquid general anesthetics lower critical temperatures in plasma membrane vesicles. Biophys J. 2013;105:2751–2759. doi: 10.1016/j.bpj.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray EM, Diaz-Vazquez G, Veatch SL. Growth Conditions and Cell Cycle Phase Modulate Phase Transition Temperatures in RBL-2H3 Derived Plasma Membrane Vesicles. PLoS One. 2015;10:e0137741. doi: 10.1371/journal.pone.0137741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruner SM, Shyamsunder E. Is the mechanism of general anesthesia related to lipid membrane spontaneous curvature? Ann N Y Acad Sci. 1991;625:685–697. doi: 10.1111/j.1749-6632.1991.tb33902.x. [DOI] [PubMed] [Google Scholar]
- Harris RA, Bruno P. Effects of ethanol and other intoxicant-anesthetics on voltage-dependent sodium channels of brain synaptosomes. The Journal of pharmacology and experimental therapeutics. 1985;232:401–406. [PubMed] [Google Scholar]
- Haseneder R, Kratzer S, Kochs E, Eckle VS, Zieglgansberger W, Rammes G. Xenon reduces N-methyl-D-aspartate and alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor-mediated synaptic transmission in the amygdala. Anesthesiology. 2008;109:998–1006. doi: 10.1097/ALN.0b013e31818d6aee. [DOI] [PubMed] [Google Scholar]
- Haydon DA, Urban BW. The action of alcohols and other non-ionic surface active substances on the sodium current of the squid giant axon. The Journal of physiology. 1983a;341:411–427. doi: 10.1113/jphysiol.1983.sp014813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon DA, Urban BW. The action of hydrocarbons and carbon tetrachloride on the sodium current of the squid giant axon. The Journal of physiology. 1983b;338:435–450. doi: 10.1113/jphysiol.1983.sp014682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon DA, Urban BW. The effects of some inhalation anaesthetics on the sodium current of the squid giant axon. The Journal of physiology. 1983c;341:429–439. doi: 10.1113/jphysiol.1983.sp014814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfrich P, Jakobsson E. Calculation of deformation energies and conformations in lipid membranes containing gramicidin channels. Biophys J. 1990;57:1075–1084. doi: 10.1016/S0006-3495(90)82625-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmings HC, Jr, Akabas MH, Goldstein PA, Trudell JR, Orser BA, Harrison NL. Emerging molecular mechanisms of general anesthetic action. Trends in pharmacological sciences. 2005;26:503–510. doi: 10.1016/j.tips.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Herold KF, Hemmings HC., Jr Sodium channels as targets for volatile anesthetics. Frontiers in pharmacology. 2012;3:50. doi: 10.3389/fphar.2012.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold KF, Nau C, Ouyang W, Hemmings HC. Isoflurane inhibits the tetrodotoxin-resistant voltage-gated sodium channel Nav1.8. Anesthesiology. 2009;111:591–599. doi: 10.1097/ALN.0b013e3181af64d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold KF, Sanford RL, Lee W, Andersen OS, Hemmings HC., Jr Clinical concentrations of chemically diverse general anesthetics minimally affect lipid bilayer properties. Proc Natl Acad Sci U S A. 2017 doi: 10.1073/pnas.1611717114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold KF, Sanford RL, Lee W, Schultz MF, Ingolfsson HI, Andersen OS, Hemmings HC., Jr Volatile anesthetics inhibit sodium channels without altering bulk lipid bilayer properties. J Gen Physiol. 2014;144:545–560. doi: 10.1085/jgp.201411172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Local anesthetics: hydrophilic and hydrophobic pathways for the drug-receptor reaction. J Gen Physiol. 1977;69:497–515. doi: 10.1085/jgp.69.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard RJ, Trudell JR, Harris RA. Seeking structural specificity: direct modulation of pentameric ligand-gated ion channels by alcohols and general anesthetics. Pharmacol Rev. 2014;66:396–412. doi: 10.1124/pr.113.007468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howery AE, Elvington S, Abraham SJ, Choi KH, Dworschak-Simpson S, Phillips S, Ryan CM, Sanford RL, Almqvist J, Tran K, Chew TA, Zachariae U, Andersen OS, Whitelegge J, Matulef K, Du Bois J, Maduke MC. A designed inhibitor of a CLC antiporter blocks function through a unique binding mode. Chem Biol. 2012;19:1460–1470. doi: 10.1016/j.chembiol.2012.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HW. Deformation free energy of bilayer membrane and its effect on gramicidin channel lifetime. Biophys J. 1986;50:1061–1070. doi: 10.1016/S0006-3495(86)83550-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang TC, Koeppe RE, 2nd, Andersen OS. Genistein can modulate channel function by a phosphorylation-independent mechanism: importance of hydrophobic mismatch and bilayer mechanics. Biochemistry. 2003;42:13646–13658. doi: 10.1021/bi034887y. [DOI] [PubMed] [Google Scholar]
- Ingólfsson HI, Koeppe RE, 2nd, Andersen OS. Curcumin is a modulator of bilayer material properties. Biochemistry. 2007;46:10384–10391. doi: 10.1021/bi701013n. [DOI] [PubMed] [Google Scholar]
- Ingólfsson HI, Thakur P, Herold KF, Hobart EA, Ramsey NB, Periole X, de Jong DH, Zwama M, Yilmaz D, Hall K, Maretzky T, Hemmings HC, Jr, Blobel C, Marrink SJ, Kocer A, Sack JT, Andersen OS. Phytochemicals perturb membranes and promiscuously alter protein function. ACS chemical biology. 2014;9:1788–1798. doi: 10.1021/cb500086e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson MB. Perfection of a synaptic receptor: kinetics and energetics of the acetylcholine receptor. Proc Natl Acad Sci U S A. 1989;86:2199–2203. doi: 10.1073/pnas.86.7.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendig JJ, Courtney KR, Cohen EN. Anesthetics: molecular correlates of voltage- and frequency-dependent sodium channel block in nerve. The Journal of pharmacology and experimental therapeutics. 1979;210:446–452. [PubMed] [Google Scholar]
- Kinde MN, Bondarenko V, Granata D, Bu W, Grasty KC, Loll PJ, Carnevale V, Klein ML, Eckenhoff RG, Tang P, Xu Y. Fluorine-19 NMR and computational quantification of isoflurane binding to the voltage-gated sodium channel NaChBac. Proc Natl Acad Sci U S A. 2016;113:13762–13767. doi: 10.1073/pnas.1609939113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AG. How lipids affect the activities of integral membrane proteins. Biochim Biophys Acta. 2004;1666:62–87. doi: 10.1016/j.bbamem.2004.05.012. [DOI] [PubMed] [Google Scholar]
- Lundbæk JA, Andersen OS. Lysophospholipids modulate channel function by altering the mechanical properties of lipid bilayers. J Gen Physiol. 1994;104:645–673. doi: 10.1085/jgp.104.4.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundbæk JA, Andersen OS. Spring constants for channel-induced lipid bilayer deformations. Estimates using gramicidin channels. Biophys J. 1999;76:889–895. doi: 10.1016/S0006-3495(99)77252-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundbæk JA, Birn P, Girshman J, Hansen AJ, Andersen OS. Membrane stiffness and channel function. Biochemistry. 1996;35:3825–3830. doi: 10.1021/bi952250b. [DOI] [PubMed] [Google Scholar]
- Lundbæk JA, Birn P, Hansen AJ, Søgaard R, Nielsen C, Girshman J, Bruno MJ, Tape SE, Egebjerg J, Greathouse DV, Mattice GL, Koeppe RE, Andersen OS. Regulation of sodium channel function by bilayer elasticity: the importance of hydrophobic coupling. Effects of Micelle-forming amphiphiles and cholesterol. The Journal of General Physiology. 2004;123:599–621. doi: 10.1085/jgp.200308996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundbæk JA, Birn P, Tape SE, Toombes GES, Søgaard R, Koeppe RE, Gruner SM, Hansen AJ, Andersen OS. Capsaicin regulates voltage-dependent sodium channels by altering lipid bilayer elasticity. Molecular pharmacology. 2005;68:680–689. doi: 10.1124/mol.105.013573. [DOI] [PubMed] [Google Scholar]
- Lundbaek JA, Collingwood SA, Ingolfsson HI, Kapoor R, Andersen OS. Lipid bilayer regulation of membrane protein function: gramicidin channels as molecular force probes. J R Soc Interface. 2010;7:373–395. doi: 10.1098/rsif.2009.0443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundbæk JA, Koeppe RE, 2nd, Andersen OS. Amphiphile regulation of ion channel function by changes in the bilayer spring constant. Proc Natl Acad Sci U S A. 2010;107:15427–15430. doi: 10.1073/pnas.1007455107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundbaek JA, Maer AM, Andersen OS. Lipid bilayer electrostatic energy, curvature stress, and assembly of gramicidin channels. Biochemistry. 1997;36:5695–5701. doi: 10.1021/bi9619841. [DOI] [PubMed] [Google Scholar]
- Machta BB, Gray E, Nouri M, McCarthy NL, Gray EM, Miller AL, Brooks NJ, Veatch SL. Conditions that Stabilize Membrane Domains Also Antagonize n-Alcohol Anesthesia. Biophys J. 2016;111:537–545. doi: 10.1016/j.bpj.2016.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh D. Lateral pressure profile, spontaneous curvature frustration, and the incorporation and conformation of proteins in membranes. Biophys J. 2007;93:3884–3899. doi: 10.1529/biophysj.107.107938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer H. Zur theorie der alkoholnarkose. Archiv fur experimentelle Pathologie und Pharmakologie. 1899;42:109–118. [Google Scholar]
- Mondal S, Khelashvili G, Shan J, Andersen OS, Weinstein H. Quantitative modeling of membrane deformations by multihelical membrane proteins: application to G-protein coupled receptors. Biophys J. 2011;101:2092–2101. doi: 10.1016/j.bpj.2011.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monod J, Wyman J, Changeux JP. On the Nature of Allosteric Transitions: A Plausible Model. J Mol Biol. 1965;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Mouritsen OG, Bloom M. Mattress model of lipid-protein interactions in membranes. Biophys J. 1984;46:141–153. doi: 10.1016/S0006-3495(84)84007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen C, Goulian M, Andersen OS. Energetics of inclusion-induced bilayer deformations. Biophys J. 1998;74:1966–1983. doi: 10.1016/S0006-3495(98)77904-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikonorov IM, Blanck TJ, Recio-Pinto E. The effects of halothane on single human neuronal L-type calcium channels. Anesth Analg. 1998;86:885–895. doi: 10.1097/00000539-199804000-00038. [DOI] [PubMed] [Google Scholar]
- O’Connell AM, Koeppe RE, 2nd, Andersen OS. Kinetics of gramicidin channel formation in lipid bilayers: transmembrane monomer association. Science. 1990;250:1256–1259. doi: 10.1126/science.1700867. [DOI] [PubMed] [Google Scholar]
- Ouyang W, Hemmings H. Isoform-selective effects of isoflurane on voltage-gated Na+ channels. Anesthesiology. 2007;107:91–98. doi: 10.1097/01.anes.0000268390.28362.4a. [DOI] [PubMed] [Google Scholar]
- Ouyang W, Herold KF, Hemmings HC. Comparative effects of halogenated inhaled anesthetics on voltage-gated Na+ channel function. Anesthesiology. 2009;110:582–590. doi: 10.1097/ALN.0b013e318197941e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang W, Jih T, Zhang T, Correa A, Hemmings H. Isoflurane inhibits NaChBac, a prokaryotic voltage-gated sodium channel. The Journal of pharmacology and experimental therapeutics. 2007;322:1076–1083. doi: 10.1124/jpet.107.122929. [DOI] [PubMed] [Google Scholar]
- Ouyang W, Wang G, Hemmings H. Isoflurane and propofol inhibit voltage-gated sodium channels in isolated rat neurohypophysial nerve terminals. Molecular pharmacology. 2003;64:373–381. doi: 10.1124/mol.64.2.373. [DOI] [PubMed] [Google Scholar]
- Overton C. Studien über die Narkose zugleich ein Beitrag zur allgemeinen. Pharmakologie Verlag von Gustav Fischer; Jena: 1901. [Google Scholar]
- Patel AJ, Honore E. Anesthetic-sensitive 2P domain K+ channels. Anesthesiology. 2001;95:1013–1021. doi: 10.1097/00000542-200110000-00034. [DOI] [PubMed] [Google Scholar]
- Payandeh J, Scheuer T, Zheng N, Catterall WA. The crystal structure of a voltage-gated sodium channel. Nature. 2011;475:353–358. doi: 10.1038/nature10238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perouansky M. The quest for a unified model of anesthetic action: a century in Claude Bernard’s shadow. Anesthesiology. 2012;117:465–474. doi: 10.1097/ALN.0b013e318264492e. [DOI] [PubMed] [Google Scholar]
- Purtell K, Gingrich KJ, Ouyang W, Herold KF, Hemmings HC., Jr Activity-dependent depression of neuronal sodium channels by the general anaesthetic isoflurane. Br J Anaesth. 2015;115:112–121. doi: 10.1093/bja/aev203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju SG, Barber AF, LeBard DN, Klein ML, Carnevale V. Exploring volatile general anesthetic binding to a closed membrane-bound bacterial voltage-gated sodium channel via computation. PLoS computational biology. 2013;9:e1003090. doi: 10.1371/journal.pcbi.1003090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratnakumari L, Hemmings H. Inhibition of presynaptic sodium channels by halothane. Anesthesiology. 1998;88:1043–1054. doi: 10.1097/00000542-199804000-00025. [DOI] [PubMed] [Google Scholar]
- Rehberg B, Xiao YH, Duch DS. Central nervous system sodium channels are significantly suppressed at clinical concentrations of volatile anesthetics. Anesthesiology. 1996;84:1223–1233. doi: 10.1097/00000542-199605000-00025. discussion 1227A. [DOI] [PubMed] [Google Scholar]
- Ren D, Navarro B, Xu H, Yue L, Shi Q, Clapham DE. A prokaryotic voltage-gated sodium channel. Science. 2001;294:2372–2375. doi: 10.1126/science.1065635. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Antkowiak B. Molecular and neuronal substrates for general anaesthetics. Nature reviews Neuroscience. 2004;5:709–720. doi: 10.1038/nrn1496. [DOI] [PubMed] [Google Scholar]
- Rusinova R, Herold KF, Sanford RL, Greathouse DV, Hemmings HC, Jr, Andersen OS. Thiazolidinedione insulin sensitizers alter lipid bilayer properties and voltage-dependent sodium channel function: implications for drug discovery. J Gen Physiol. 2011;138:249–270. doi: 10.1085/jgp.201010529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusinova R, Koeppe RE, 2nd, Andersen OS. A general mechanism for drug promiscuity: Studies with amiodarone and other antiarrhythmics. J Gen Physiol. 2015;146:463–475. doi: 10.1085/jgp.201511470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sand RM, Gingrich KJ, Macharadze T, Herold KF, Hemmings HC., Jr Isoflurane modulates activation and inactivation gating of the prokaryotic Na+ channel NaChBac. J Gen Physiol. 2017 doi: 10.1085/jgp.201611600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer DB, Koeppe RE, 2nd, Andersen OS. Induction of conductance heterogeneity in gramicidin channels. Biochemistry. 1989;28:6571–6583. doi: 10.1021/bi00442a007. [DOI] [PubMed] [Google Scholar]
- Shiraishi M, Harris R. Effects of alcohols and anesthetics on recombinant voltage-gated Na+ channels. The Journal of pharmacology and experimental therapeutics. 2004;309:987–994. doi: 10.1124/jpet.103.064063. [DOI] [PubMed] [Google Scholar]
- Sirois JE, Lynch C, 3rd, Bayliss DA. Convergent and reciprocal modulation of a leak K+ current and I(h) by an inhalational anaesthetic and neurotransmitters in rat brainstem motoneurones. The Journal of physiology. 2002;541:717–729. doi: 10.1113/jphysiol.2002.018119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Søgaard R, Werge TM, Bertelsen C, Lundbye C, Madsen KL, Nielsen CH, Lundbæk JA. GABA(A) receptor function is regulated by lipid bilayer elasticity. Biochemistry. 2006;45:13118–13129. doi: 10.1021/bi060734+. [DOI] [PubMed] [Google Scholar]
- Sonner JM, Cantor RS. Molecular mechanisms of drug action: an emerging view. Annu Rev Biophys. 2013;42:143–167. doi: 10.1146/annurev-biophys-083012-130341. [DOI] [PubMed] [Google Scholar]
- Stadnicka A, Kwok WM, Hartmann HA, Bosnjak ZJ. Effects of halothane and isoflurane on fast and slow inactivation of human heart hH1a sodium channels. Anesthesiology. 1999;90:1671–1683. doi: 10.1097/00000542-199906000-00024. [DOI] [PubMed] [Google Scholar]
- Study RE. Isoflurane inhibits multiple voltage-gated calcium currents in hippocampal pyramidal neurons. Anesthesiology. 1994;81:104–116. doi: 10.1097/00000542-199407000-00016. [DOI] [PubMed] [Google Scholar]
- Suchyna TM, Tape SE, Koeppe RE, 2nd, Andersen OS, Sachs F, Gottlieb PA. Bilayer-dependent inhibition of mechanosensitive channels by neuroactive peptide enantiomers. Nature. 2004;430:235–240. doi: 10.1038/nature02743. [DOI] [PubMed] [Google Scholar]
- Sula A, Booker J, Ng LC, Naylor CE, DeCaen PG, Wallace BA. The complete structure of an activated open sodium channel. Nat Commun. 2017;8:14205. doi: 10.1038/ncomms14205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibbs GR, Rowley TJ, Sanford RL, Herold KF, Proekt A, Hemmings HC, Jr, Andersen OS, Goldstein PA, Flood PD. HCN1 channels as targets for anesthetic and nonanesthetic propofol analogs in the amelioration of mechanical and thermal hyperalgesia in a mouse model of neuropathic pain. The Journal of pharmacology and experimental therapeutics. 2013;345:363–373. doi: 10.1124/jpet.113.203620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veatch SL, Keller SL. Miscibility phase diagrams of giant vesicles containing sphingomyelin. Phys Rev Lett. 2005a;94:148101. doi: 10.1103/PhysRevLett.94.148101. [DOI] [PubMed] [Google Scholar]
- Veatch SL, Keller SL. Seeing spots: complex phase behavior in simple membranes. Biochim Biophys Acta. 2005b;1746:172–185. doi: 10.1016/j.bbamcr.2005.06.010. [DOI] [PubMed] [Google Scholar]
- White SH. Studies of the physical chemistry of planar bilayer membranes using high-precision measurements of specific capacitance. Ann N Y Acad Sci. 1977;303:243–265. [PubMed] [Google Scholar]
- Yokoyama T, Minami K, Sudo Y, Horishita T, Ogata J, Yanagita T, Uezono Y. Effects of sevoflurane on voltage-gated sodium channel Na(v)1.8, Na(v)1.7, and Na(v)1.4 expressed in Xenopus oocytes. J Anesth. 2011;25:609–613. doi: 10.1007/s00540-011-1167-7. [DOI] [PubMed] [Google Scholar]
- Zhelev DV. Material property characteristics for lipid bilayers containing lysolipid. Biophys J. 1998;75:321–330. doi: 10.1016/S0006-3495(98)77516-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman SA, Jones MV, Harrison NL. Potentiation of gamma-aminobutyric acidA receptor Cl- current correlates with in vivo anesthetic potency. The Journal of pharmacology and experimental therapeutics. 1994;270:987–991. [PubMed] [Google Scholar]


