Abstract
Deoxyhypusine synthase (DHS) catalyzes the post-translational modification of eukaryotic translation factor 5A (eIF5A) by the polyamine, spermidine, that converts one specific lysine residue to deoxyhypusine [Nε-4-aminobutyl(lysine)], which is subsequently hydroxylated to hypusine [Nε-4-amino-2-hydroxybutyl(lysine)]. Hypusine synthesis represents the most critical function of polyamine. As eIF5A has been implicated in various human diseases, identification of specific inhibitors of hypusine modification is of vital importance. DHS catalyzes a complex reaction that occurs in two stages, first, the NAD-dependent cleavage of spermidine to form an enzyme-butylimine intermediate and enzyme-bound NADH, and second, the transfer of the butylimine moiety from the enzyme intermediate to the eIF5A precursor and subsequent reduction of the eIF5A-butylimine intermediate by enzyme-bound NADH to form deoxyhypusine [Nε-4-aminobutyl(lysine)]. Our data demonstrate that there is a measurable release of enzyme-bound NADH in the absence of eIF5A precursor and that the DHS activity can be determined by coupling the first phase reaction with the NADH-Glo assay in which the generation of luminescence is dependent on NADH derived from the DHS partial reaction. The conventional DHS assay that measures the incorporation of radioactivity from [1,8- 3H]spermidine into the eIF5A precursor in the complete reaction cannot be readily adapted for high throughput screening (HTS). In contrast, the non-radioactive DHS/NADH-Glo coupled assay is highly specific, sensitive and reproducible and could be configured for HTS of small molecule libraries for the identification of new inhibitors of DHS. Furthermore, the coupled assay provides new insights into the dynamics of the DHS reaction especially regarding the fate of NADH.
Keywords: eIF5A, hypusine, deoxyhypusine synthase, enzyme assay, post-translational modification, polyamine
Introduction
The eukaryotic translation initiation factor 5A (eIF5A)1 contains a unique amino acid, hypusine [Nε-4-amino-2-hydroxybutyl(lysine)], a lysine derivative that is modified by the polyamine, spermidine (for reviews see Park 2006; Wolff and Park 2015). Hypusine occurs only in this protein and is essential for eIF5A activity. The polyamines, putrescine, spermidine and spermine, are natural organic polycations that are ubiquitous and vital for eukaryotic cell growth and survival (see reviews Pegg and Casero 2011; Pegg 2016). Of numerous proposed functions of polyamines in living organisms, the requirement of spermidine for hypusine formation is the clearly defined and important function of polyamines in eukaryotic cell proliferation (Chattopadhyay et al. 2008).
Hypusine is formed post-translationally by two consecutive enzymatic reactions (Scheme 1). First, deoxyhypusine synthase (DHS, EC 2.5.1.46) catalyzes the transfer of the 4-aminobutyl moiety of spermidine to a specific lysine residue of the eIF5A precursor to form the deoxyhypusine [Nε-4-aminobutyl(lysine)] residue (Joe et al. 1995). This intermediate is subsequently hydroxylated at C2 of the butylamine side chain by deoxyhypusine hydroxylase (DOHH, EC 1.14.99.29) (Park et al. 2006) to complete hypusine synthesis and maturation of eIF5A. Both DHS and DOHH are exclusive to eIF5A modification; thus, hypusine synthesis in eIF5A represents the most specific protein modification known to date.
Scheme 1. Posttranslational modification of eIF5A by two enzymatic steps.
Hypusine is formed in the eIF5A precursor by two consecutive reactions, catalyzed by deoxyhypusine synthase and deoxyhypusine hydroxylase. eIF5A(Lys), eIF5A precursor; eIF5A(Dhp), eIF5A intermediate containing deoxyhypusine; eIF5A(Hpu), mature eIF5A containing hypusine.
eIF5A is an essential protein required for protein synthesis and cell proliferation (Dever et al. 2014; Mathews and Hershey 2015; Park et al. 2010; Rossi et al. 2014) and DHS and DOHH genes are also essential in mammalian embryonic development, as evidenced from gene knockout studies in the mouse (Nishimura et al. 2012; Sievert et al. 2014). eIF5A and its modification enzymes are highly conserved in all eukaryotes, attesting to their fundamental and vital role. Furthermore, the eIF5A isoforms (eIF5A-1 and eIF5A-2), as well as DHS and DOHH, have been associated with various human pathological conditions, including HIV-1 infection, diabetes, tumorigenesis and metastasis (see reviews (Mathews and Hershey 2015; Nakanishi and Cleveland 2016)). In view of the highly specific nature of the hypusine modification and the implication of eIF5A in various human diseases, the hypusine biosynthetic enzymes present novel potential targets for intervention. Although eIF5A has been proposed to be an elongation factor that relieves ribosome stalling at polyproline stretches (Dever et al. 2014; Gutierrez et al. 2013; Rossi et al. 2014), its true physiological function is not fully understood.
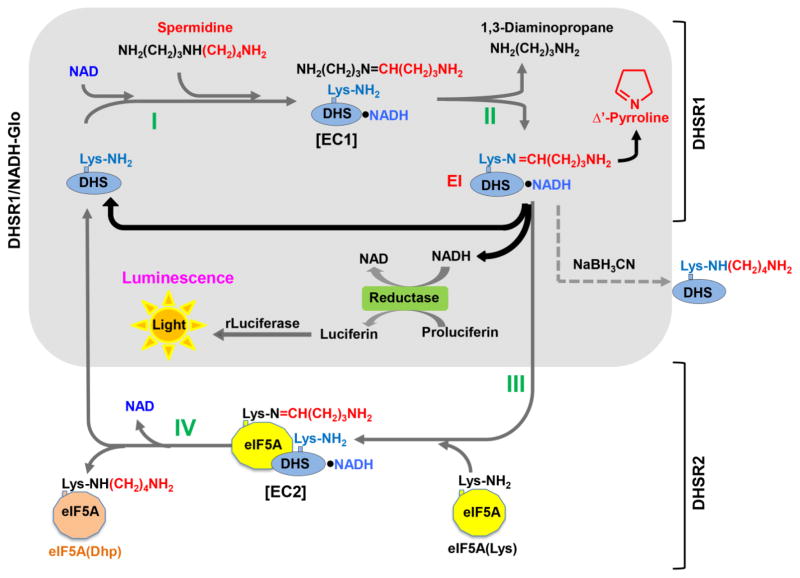
From a series of mechanism studies (Wolff et al. 1990; Wolff et al. 1997; Wolff et al. 2000), the DHS complete reaction (DHSRC) has been proposed to occur in four steps (Scheme 2): I) the NAD-dependent dehydrogenation of spermidine with the generation of NADH bound to the enzyme, II) cleavage of dehydrospermidine and formation of an enzyme-butylimine intermediate (EI) at the active site lysine with the release of 1,3-diaminopropane, III) transimination from the enzyme intermediate to an ε-amino group of a specific lysine residue of the eIF5A precursor to form an eIF5A-butylimine intermediate and IV) reduction of the eIF5A intermediate to the deoxyhypusine form by the enzyme-bound NADH generated in step I. In the absence of eIF5A precursor (eIF5A(Lys)), however, DHS still can catalyze an abortive, partial reaction (steps I and II above, designated as DHSR1 in Scheme 2), resulting in the formation of the enzyme-butylimine intermediate and enzyme-bound NADH. The existence of the enzyme-butylimine intermediate was verified by trapping it into a stable adduct by reduction of the partial reaction mixture (containing radiolabeled spermidine) with NaBH3CN (Wolff et al. 1997). After the reduction, radiolabeled deoxyhypusine was identified at Lys 329 of the human enzyme, indicating the key catalytic role for this residue in the DHS reaction.
Scheme 2. A proposed mechanism of the DHS reaction and coupling of the DHS partial reaction with the NADH-Glo reaction.
The DHS complete reaction (DHSRC) consists of two stages DHSR1 and DHSR2. DHSR1 involves two steps: I) NAD-dependent dehydrogenation of spermidine to form an enzyme/dehydrospermidine/NADH complex (EC1), II) cleavage of dehydrospermidine to form an enzyme butylimine intermediate (EI) with bound NADH and the release of 1,3-diaminopropane. DHSR2 involves two steps: III) butylimine transfer from EI to eIF5A precursor to form eIF5A butylimine intermediate/DHS/NADH complex (EC2), IV) reduction of eIF5A butylimine intermediate by enzyme-bound NADH to form deoxyhypusine. The major findings of this study including the release of NADH from EI and its usage in NADH-Glo reaction, and regeneration of free enzyme for cycling of the spermidine cleavage (DHSR1) are indicated by solid black arrows. The DHSR1/NADH-Glo coupled reaction in which enzyme bound NADH is released and used for reduction of proluciferin to luciferin to generate luminescence is shown as a shaded block. The enzyme intermediate has been validated by reduction with NABH3CN. EC1, putative enzyme complex 1; EI, enzyme intermediate; EC2, putative enzyme complex 2.
In our previous study, enzyme-bound NADH generated from the DHS partial reaction (DHSR1) was detected by fluorescence measurements with excitation peaks at 285 and 351 nm and emission peak at 441 nm, which were distinct from free NADH fluorescence (excitation at 260 nm and 341 nm and emission at 463 nm) (Wolff et al. 2000). The enzyme-bound NADH fluorescence rapidly declined upon addition of the eIF5A precursor to the partial reaction mixture indicating a rapid consumption of enzyme-bound NADH for reduction of the eIF5A-butylimine intermediate (Scheme 2, step IV). As the ratio of the enzyme-bound NADH to enzyme monomer was estimated to be 0.88 (close to 1), it was also assumed that the enzyme remains largely as the enzyme intermediate (EI, Scheme 2) under the partial reaction conditions (at RT). Thus, it was not known whether NADH generated in the DHSR1 could be dissociated from the enzyme to be detected by an independent coupling reaction that depends on free NADH.
DHS is a tetrameric enzyme consisting of four identical subunits of ~41 kDa. The crystal structures of the human enzyme in complex with NAD (Liao et al. 1998) and in complex with NAD and an inhibitor, N1-monoguanyl-1,7-diaminoheptane (GC7) (Umland et al. 2004), revealed four active sites formed at the interface of dimers. As the enzyme is inactive as a monomer, compounds that disrupt tetramer formation, as well as those that interfere with the binding of NAD, spermidine or eIF5A precursor to the enzyme would inhibit the DHS reaction (DHSR). Many compounds structurally related to spermidine were tested as inhibitors of DHS (Jakus et al. 1993). Of these, GC7 is the most potent inhibitor in vitro and also in cultured mammalian cells (Park et al. 1994). Although this inhibitor exerts strong inhibition of eIF5A hypusine modification and proliferation in mammalian cells, its cellular effects may not be limited to inhibition of DHS. No comprehensive screening studies in a search for specific inhibitors of the hypusine biosynthetic enzymes have been reported, due to a lack of a DHS assay suitable for high throughput screening (HTS).
Therefore, we devised a new method utilizing the NADH generated from the DHS partial reaction by coupling it with the NADH-Glo assay in which a NADH-dependent reductase converts proluciferin to luciferin and luminescence is generated by Renilla luciferase. Our current data demonstrate that NADH formed in the DHS partial reaction dissociates from the enzyme to a significant extent and can be quantified by the NADH-Glo reaction, thus offering a new, non-radioactive method to measure the DHS activity in biological samples. Moreover, the DHS/NADH-Glo coupled assay should be readily amenable to a HTS format.
Materials and Methods
Materials
[1,8-3H]Spermidine (36 Ci/mmol) was purchased from PerkinElmer/NEN. NAD(P)H-Glo detection system was purchased form Promega Corp (Madison WI). Pre-weighed NADH and NAD vials and Corning 96-well white plates (CSL3912) were purchased from Sigma Chemical Company (St. Louis, MO). Recombinant eIF5A precursor, eIF5A(Lys) was purified as described previously (Joe and Park 1994; Park et al. 2011). His-tagged human deoxyhypusine synthase was produced in E. coli, BL21/DE3 cells transformed with p24b-hDHS vector and the recombinant enzyme was purified using a Ni-NTA resin (Qiagen). The inhibitor of deoxyhypusine synthase, GC7 was synthesized as reported previously (Jakus et al. 1993)
Methods
NADH-Glo assay
The assay was performed using NAD(P)H-Glo Detection System (Promega) following the manufacturer’s instructions. Briefly, the Luciferin detection reagent was reconstituted by the addition of 10 ml of reconstitution buffer to a bottle of lyophilized powder (~70 mg) of Renilla luciferase and ATP, to make a 1 x luciferin detection reagent and stored frozen at −20 °C in 1 ml aliquots. Reductase and the reductase substrate, proluciferin, were added (5.5 μl each to 1 ml of luciferin detection reagent) to make the final NADH-Glo reagent and this mixture was used immediately. As a source of NADH, NADH standard solutions (0.5–20 μM in 50 μl) in Glycine-NaOH buffer (pH 8.0–9.5) were mixed with 50 μl of the NADH-Glo reagent in Eppendorf tubes or in a 96 well white plate. The mixtures were incubated for 15 min to 1 h at RT and luminescence was measured using a Biotek Synergy microplate reader. All the luminescence assays were performed in duplicate.
Deoxyhypusine synthesis assay
The DHS complete reaction (DHSRC) was carried out as described previously (Wolff et al. 2011) with the indicated levels of spermidine, NAD, eIF5A precursor and the enzyme. Typical reaction mixtures (20 μl) contained 0.01–0.2 μg His-hDHS, 20 μg of carrier BSA, 100 μM NAD, 3.6 μCi (0.1 nmol) of [1,8-3H]spermidine plus unlabeled spermidine (0.3 nmol) to make final spermidine concentration to 20 μM, and 3.4 μg (10 μM) of eIF5A precursor in 0.1 M Glycine-NaOH buffer (pH 9.0), unless indicated otherwise. The reaction mixture was incubated at RT or 37 °C for the indicated period, 500 μg carrier BSA was added and the reaction was stopped by the addition of 1 ml of ice cold 10 % TCA containing putrescine, spermidine and spermine (1 mM each). After a 20-min incubation on ice, the TCA precipitated proteins were separated by centrifugation at 10,000 × g. After removal of the TCA supernatant, the TCA precipitates were washed by resuspension in 1 ml of 10 % TCA containing 1 mM polyamines and by removal of the TCA supernatant following centrifugation. After repeated washing of the TCA precipitates (3 times) to remove all non-covalently bound radioactive spermidine, the precipitates were dissolved in 0.1 ml of 0.15 N NaOH and radioactivity was measured using a Beckman Liquid Scintillation counter. The radioactivity in the washed TCA precipitates of a reaction mixture not containing the enzyme was used as a negative control and this radioactivity was subtracted from all the assay values. The radioactivity (dpm) incorporated into the eIF5A precursor was converted to pmol after estimation of the specific radioactivity of deoxyhypusine (half of that in [1,8-3H]spermidine).
Deoxyhypusine synthase partial reaction (DHSR1) and coupling of DHS reaction with NADH-Glo reaction
The DHS partial reaction mixture composition was the same as that for the DHS complete reaction mixture except that only non-labeled spermidine was added and the eIF5A precursor was omitted. In a typical DHSR1 assay, a mixture containing 20 μM spermidine, 100 μM NAD in 50 μl of Glycine-NaOH buffer (pH 9.0) and enzyme (0.5 μg), were incubated at 37 °C for 2 h. These concentrations of spermidine and NAD were chosen, since the DHSR1 reached close to a maximum rate at these levels (data not shown), while consuming <10 % of the substrate or the cofactor during the course of the reactions.
In most experiments, the DHSR1/NADH-Glo coupled assays were performed as a two-step reaction. DHS partial reaction mixtures (100 μl) were first incubated to generate NADH. After the initial DHSR1 incubation period, the mixtures were either heated at 65 °C for 10 min to release all enzyme-bound NADH, or not heated (kept at RT for 10 min). Then each sample was divided into two wells (50 μl per well of a white 96 well plate) and an equal volume (50 μl) of the NADH-Glo reagent was added. The plate was incubated at RT for 15–60 min on a rocking platform before the measurement of luminescence using a Biotek Synergy microplate reader. Two or more consecutive luminescence readings were made for each plate. Coupling of the complete DHS reaction with NADH-Glo was carried out in the same way using DHSRC mixtures. Since there was some day-to-day variation in NADH-Glo assay signals, a NADH-Glo standard assay was performed using freshly prepared NADH solutions (0.5–5 μM) in parallel to prepare a calibration curve for each set of experiments and the NADH generated from the DHSR was estimated based on the standard curve. The one-step DHSR1/NADH-Glo coupled assay was carried out by mixing equal volumes of DHSR1 mixture with NADH-Glo reagent before incubation.
Results
We previously reported that the DHS partial reaction (Scheme 2, DHSR1) produces an enzyme-butylimine intermediate, along with enzyme-bound NADH, when the enzyme is incubated with spermidine and the cofactor NAD without the eIF5A precursor (Wolff et al. 1997; Wolff et al. 2000). Identification of 1,3-diaminopropane and Δ1-pyrroline upon treatment of a DHSR1 mixture with TCA (Wolff et al. 1990), suggested uncoupling of this first phase of DHS reaction from the latter phase of deoxyhypusine synthesis (Scheme 2, DHSR2). However, it was not clear to what extent the enzyme-bound NADH and the enzyme-bound butylimine moiety could be released (spontaneously, without TCA treatment) as free NADH and Δ1-pyrroline, respectively, for the enzyme to proceed with a new cycle of the partial reaction. Coupling the DHSR1 with the NADH-Glo reaction was undertaken to address these questions and to develop a new DHS assay adaptable for HTS.
Compatibility of DHS and NADH-Glo assay conditions
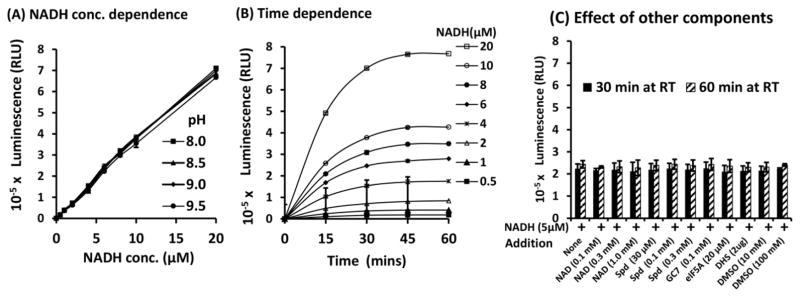
Since the DHSR1/NADH-Glo coupled reaction involves three enzymes, DHS, proluciferin reductase and Renilla luciferase, a successful coupling should accommodate all three reactions. We wondered whether the DHSR mixture would interfere with the NADH-Glo reaction. A typical deoxyhypusine synthesis reaction, using a low concentration (~ 5 μM) of radiolabeled spermidine, is sharply dependent on the pH (optimum at pH 9.0–9.5, Glycine-NaOH buffer) (Lee and Park 2000; Lee et al. 1999). This may be the case partly because only the diprotonated form of spermidine (with an uncharged secondary amino group) acts as a substrate for DHS and the percentage of this form is low at neutral pH, but increases with higher pH (Lee et al. 1999). In order to see if the high pH of the DHSR buffer (Glycine-NaOH) would affect the NADH-Glo reaction, NADH solutions (0.5, 1, 2, 4, 6, 8, 10 and 20 μM) in 0.1 M Gly-NaOH buffer of increasing pH (pH 8.0, 8.5, 9.0, and 9.5) were mixed with an equal volume of the NADH-Glo reagent and incubated at RT. The luminescence increased linearly with the concentration of NADH (Fig. 1A) and at almost identical rates for the NADH solutions at pH 8.0 – 9.5. The lack of effect of high pH (pH >8) of the NADH solutions on the luminescence may be due to the strong buffering capacity of the NADH-Glo assay buffer. The NADH-Glo reaction was rapid, reaching near the maximum luminescence by 30 min at RT (Fig. 1B).
Figure 1. NADH-Glo reaction.
Dependence on (A) the concentration of NADH, (B) incubation time and (C) effects of other components. A, NADH (0.5–20 μM) solutions in 0.1 M Gly-NaOH buffers of different pH (8.0, 8.5, 9.0 and 9.5) was reacted with an equal volume of NADH-Glo reagent at RT for 30 min and luminescence was measured in duplicate samples. B. Luminescence measurements of samples in A in pH 9.0 buffer were made after 15, 30, 45 and 60 min of NADH-Glo assay. C. The components of the DHSR and DMSO, were added individually to NADH solutions (5 μM in 0.1 M Gly-NaOH buffer pH 9.0) at the final concentrations indicated and reacted with NADH-Glo reagent. After 30 and 60 min incubation at RT, luminescence was measured. The experiments were performed in duplicate. RLU, relative light unit
The possibility of interference of the NADH-Glo assay by individual components of the DHSR mixture was tested. This luminescence assay is specific for NADH and NAD (1 mM) yielded no luminescence (not shown). Addition of NAD (up to 1 mM) to NADH solution did not increase luminescence (Fig. 1C). The addition of other components of the DHSR, i.e., spermidine (30,100 and 300 μM), DHS inhibitor GC7 (100 μM), eIF5A precursor (20 μM), or DHS (2 μg), individually to the NADH-Glo assay mixture did not result in any luminescence (not shown), nor did they inhibit the NADH-derived luminescence (Fig. 1C). These findings indicate that the DHSR mixture (pH 9.0–9.5) can be directly mixed with the NADH-Glo reagent for coupling of the two reactions. Since most small molecule library compounds are solubilized in DMSO, the effects of DMSO were also checked and no/little inhibition was observed at 10–100 mM DMSO (Fig. 1C).
Detection of free NADH in the DHS partial mixture by coupling with NADH-Glo assay; the pH dependence of the DHS partial and the complete reactions
We then measured the DHS partial reaction by coupling with the NADH-Glo assay (Fig. 2A) and compared the pH dependence of the DHSR1/NADH-Glo reaction (Fig. 2A) and the deoxyhypusine synthase complete reaction (DHSRC) (Fig. 2B) under parallel conditions at two levels of the enzyme. The DHSR1/NADH-Glo coupled assay was carried out in two steps in which the DHSR1 mixtures were first incubated at 37 °C for 2 h, then mixed with an equal volume of the NADH-Glo reagent and incubated for 30 min at RT. Robust luminescence signals, dependent on the enzyme amount and pH were observed (Fig. 2A), indicating that NADH generated from DHSR1 is released from the enzyme and is successfully utilized for the NADH-Glo assay (Scheme 2). Apparently, the luminescence was not due to a NADH contaminant in the purified enzyme, as the incubation of the enzyme alone, or the DHSR mixture without spermidine or NAD, with the NADH-Glo reagent did not yield any luminescence (data not shown). Both DHSR1/NADH-Glo and DHSRC assays appeared to be optimum around pH 9.0 and this pH was chosen for the rest of the study. The two reactions displayed similar pH dependence at pH 8.5–9.5, but a clear difference was noted at pH 8.0. Substantial luminescence was observed in the DHSR1/NADH-Glo mixture at pH 8 (>30% of the maximum value at pH 9.0), an indication that the partial reaction occurred to a considerable extent. Yet, there was no/little formation of DHSRC product at this pH (Fig. 2B). The lack of deoxyhypusine formation at pH 8 (Fig. 2B) could be due to instability of the enzyme intermediate or ineffective transfer of the butylimine moiety from the enzyme intermediate to eIF5A(Lys) at this pH (Scheme 2, step III).
Figure 2. pH dependence of the DHS partial reaction coupled with the NADH-Glo assay (DHSR1/NADH-Glo) (A) and the DHS complete reaction (DHSRC) (B).
A, DHS partial reaction was performed at two levels of the enzyme (0.35 and 1.0 μg/50 μl) in 0.1 M Gly-NaOH buffer at pH, 8.0, 8.5, 9.0 or 9.5 containing 20 μM spermidine, and 100 μM NAD. After 2 h incubation at 37 °C, an equal volume of NADH-Glo reagent was added and incubated for 30 min at RT, before the luminescence measurements. RLU, relative light unit. B, Deoxyhypusine synthesis was measured at two levels of enzyme (0.03 and 0.1 μg/20 μl) in a reaction mixture containing 30 μM eIF5A precursor, radioactive spermidine (20 μM, radiolabeled plus unlabeled spermidine) and 100 μM NAD in 0.1 M Gly-NaOH buffer at pH 8.0, 8.5, 9.0 or 9.5. After 2 h incubation at 37 °C, the radioactivity incorporated into eIF5A was measured after TCA precipitation as described under Methods. The experiment was repeated with similar results and a representative experiment done in duplicate is shown.
Estimation of free NADH generated in the DHS partial and complete reactions
The amounts of NADH generated from the DHS partial and complete reactions were estimated under various conditions. The luminescence from the DHSR1/NADH-Glo (Fig. 3A) and the DHSRC/NADH-Glo (Fig. 3B) two-step coupled assays was measured at increasing levels of the enzyme (0.5–3 μg in 50 μl), using DHSR mixtures incubated either at RT (dashed lines) or 37 °C (solid lines) for 2 h. The NADH-derived luminescence increased with the enzyme amount.
Figure 3. NADH-dependent luminescence in DHSR1/NADH-Glo assay (A) and DHSRC/NADH-Glo assay (B).
A, Two sets of DHSR1 mixtures containing 0.5, 1.0, 2.0 or 3.0 μg (per 50 μl) of enzyme, 20 μM spermidine and 100 μM NAD in 0.1M Gly-NaOH buffer (pH 9.0) were incubated at 37 °C (solid lines) or RT (25 °C, dashed lines) for 2 h. One set was heated at 65 °C for 10 min (circles) while the other set was left at RT (triangles). Equal volume of NADH-Glo reagent was added to each sample and luminescence was measured after 30 min incubation at RT. B, The assays were performed as in A, using the DHSRC mixture that contained 10 μM eIF5A in addition to NAD, spermidine and the enzyme. The experiment was repeated with similar results and a representative experiment (in duplicate) is shown.
In order to determine the amount of NADH bound to the enzyme (Scheme 2) during the coupled assay, one set of DHSR samples was heated at 65 °C for 10 min to ensure the release of all enzyme-bound NADH. When the DHSR mixtures were heated, cooled and then mixed with NADH-Glo reagent, notable increases in luminescence were observed (Fig. 3, circles vs triangles). The increased luminescence (Fig. 3, vertical solid arrows), reflects the level of the enzyme-bound NADH, likely in the form of the enzyme intermediate (EI) (Scheme 2). Based on this difference, the enzyme-bound NADH was estimated to be approximately 70 pmol, close to that of the enzyme added (3 μg, 75 pmol monomer), suggesting that most of the DHS enzyme was in the NADH-bound form, consistent with our previous report (Wolff et al. 2000). The total amount of NADH generated in the DHSR1 mixture during the 2 h incubation at 37 °C (heated DHSR1 samples, Fig. 3A solid line and solid circle), was estimated to be approximately 450 pmol per 3 μg (75 pmol monomer) of the enzyme, leading to an estimate of the NADH: DHS ratio of 6. This finding indicates that the DHS partial reaction cycled independently of eIF5A modification, releasing free NADH, albeit at a slow rate. Luminescence signals were lower but substantial for the parallel DHSR1 mixtures incubated at RT and coupled with NADH-Glo (Fig. 3A dashed lines, ~ 25% of their counterparts at 37 °C).
To our surprise, luminescence signals were also produced when the DHS complete reaction was coupled with NADH-Glo assay (27–36% of those from DHSR1/NADH-Glo assay counterparts) (Fig. 3B). Thus, a partial dissociation of enzyme-bound NADH must occur even during the DHS complete reaction cycle, albeit to a lesser extent than in the partial reaction. Furthermore, increases in luminescence were also observed in the heated DHSRC samples coupled with NADH-Glo assay and the magnitude of these luminescence increases were comparable to those from the DHSR1 counterparts (compare the vertical solid arrows at 3 μg enzyme, Fig. 3A and 3B), suggesting accumulation of enzyme intermediate in the complete DHS reaction mixture as well as in the partial reaction mixture.
Identification of enzyme-butylimine intermediate in the DHS partial and complete reaction mixtures
In order to validate the accumulation of the DHS enzyme intermediate in the complete reaction mixture, DHSR mixtures containing [1,8-3H]spermidine were treated with NaBH3CN to trap the enzyme butylimine intermediate into a stable enzyme adduct (Fig. 4). The NaBH3CN reduction was carried out for both partial and the complete DHSR mixtures after 3, 10, 20 and 30 min incubation at 37 °C. Radiolabeled DHS was detected in the NaBH3CN-treated samples of DHSR1 (Fig. 4A, lanes 2–5), and also of DHSRC (Fig. 4A, lanes 7–10) but not in the unreduced sample (Fig. 4A, lane 1 and lane 6). This labeling of the enzyme in the DHSRC mixture confirms the accumulation of the enzyme butylimine intermediate during the complete reaction, although DHS labeling was somewhat weaker in DHSRC than in DHSR1. As anticipated, strong labeling of deoxyhypusine in eIF5A occurred in the DHSRC mixture, regardless of NaBH3CN treatment (Fig. 4A, lanes 6–10). There were no significant changes in the labeling of the enzyme with increased incubation time (3–30 min) of DHSR1 or DHSRC mixtures, suggesting a rapid formation of the enzyme intermediate and its relatively steady level during the DHSR incubation. The labeling of DHS by NaBH3CN reduction offers concrete evidence for the accumulation of the enzyme butylimine intermediate (EI) during the DHS complete reaction as well as the partial reaction (Fig. 4). This notion is consistent with the notable luminescence increases in the DHSRC/NADH-Glo samples upon heating of DHSRC (Fig. 3B, vertical solid arrow).
Figure 4. Identification of the enzyme-butylimine intermediate in the DHSR1 and DHSRC mixtures by reduction with NaBH3CN.
(A) fluorogram and (B) Coomassie blue staining. The DHSR1 mixture contained 10 μg enzyme, 20 μCi (0.56 nmol) of [1,8-3H]spermidine plus 1.44 nmol of unlabeled spermidine, 100 μg BSA and 100 μM NAD in 100 μl of 0.1M Gly-NaOH buffer (pH 9.0). DHSRC mixture contained 20 μg of eIF5A precursor in addition to all the components of DHSR1. After 3, 10, 20, and 30 min at 37 °C, 20 μl of the reaction mixture was removed and treated with 1 μl of NaBN3CN (0.1 M) three times at 5 min intervals on ice. Additional carrier BSA (100 μg) was added and the mixtures were precipitated with 10 % TCA containing nonlabeled putrescine, spermidine and spermine (1 mM each). The TCA precipitates were washed two times and dissolved in 50 μl of SDS sample buffer. After SDS-PAGE, staining and destaining, the gel was treated with Amplify for 30 min, dried and exposed to Xray film at −80 °C. The experiment was repeated with similar results and a representative experiment is shown.
Comparison of the rates of DHS complete reaction and the partial reaction
The rates of the DHS partial and complete reactions were compared under parallel conditions using low levels of enzyme (0.05–0.25 μg in 50 μl), at 1 and 2 h of incubation (Fig. 5). The deoxyhypusine formation was estimated to be approximately 45.6 pmol per pmol of enzyme (upon 2 h incubation at 37 °C, Fig. 5C), whereas NADH production was ~5 pmol per pmol of enzyme (Fig. 5A) under the same pH and concentrations of spermidine and NAD. The production of free NADH from DHS partial reaction is quite slow compared to that of deoxyhypusine synthesis in the complete reaction (Fig. 5). The nine-fold higher rates of the complete reaction is expected, since coupling of the DHSR1 with DHSR2 (owing to a strong affinity between DHS and eIF5A(Lys)) would accelerate the partial reaction. This is not the case for DHSR1/NADH-Glo assay where there is no affinity between DHS (EI) and the enzymes of the NADH-Glo assay. The two reactions also exhibited different temperature dependence. Whereas the DHSRC was fairly effective at RT with production of deoxyhypusine at 60 % of that at 37 °C (Fig. 5C and 5D), NADH generated from DHSR1 at RT was only ~ 17 % of that produced at 37 °C (Fig. 5A and 5B).
Figure 5. Comparison of DHS partial and complete reaction rates at 37 °C (A, C) and RT (B, D).
DHSR1 was performed at 37 °C or at RT as in Fig. 3A, but using lower levels of the enzyme (0.05, 0.1 and 0.25 μg in 50 μl). NADH-Glo reagent was added after 1 or 2 h incubation of DHSR1. The luminescence (RLU) values (left Y-axis) were converted to pmol of NADH produced per 50 μl reaction mixture (indicated on the right Y-axis). The DHSRC mixture contained the same components as the DHSR1, except that a mixture of radioactive spermidine and cold spermidine was used and the eIF5A precursor was added at 30 μM. The pmol of deoxyhypusine formed per 50 μl DHSRC was estimated as indicated on the Y-axis. The experiment was repeated with similar results and a representative experiment (in duplicate) is shown.
Inhibitory effects of GC7 and Comparison of one-step and two-step DHSR1/NADH-Glo assays
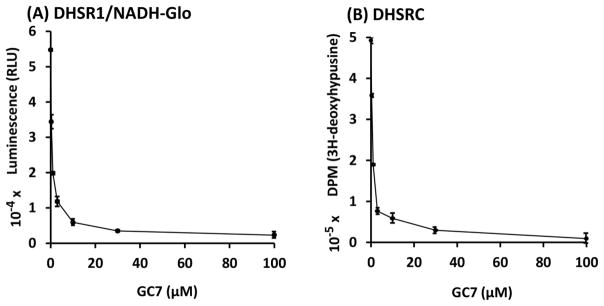
The effects of the known inhibitor of DHS, GC7, on the DHSR1/NADH-Glo and DHSRC assays, were examined in order to compare the sensitivities of the two assays in inhibitor screening. GC7 (0.3–100 μM) caused a sharp decrease in luminescence in DHSR1/NADH-Glo samples with an IC50 of < 1 μM (Fig. 6A). The inhibition of the DHSR1/NADH-Glo reaction by GC7 was nearly as effective as that of the complete reaction (Fig. 6B).
Figure 6. The effects of GC7 on the DHSR1/NADH-Glo (A) and DHSRC (B) assays.
The inhibitory effect of GC7 was tested at 0.3, 1, 3, 10, 30 and 100 μM in the DHS partial reaction and the complete reaction. A, DHSR1 mixtures contained NAD (100 μM), spermidine (20 μM), 0.5 μg of enzyme and indicated concentrations of GC7. After incubation of DHSR1 mixtures at 37 °C for 2 h, NADH-Glo reagent was added and incubated for 30 min at RT before luminescence measurements. B, DHSRC assay was performed in 20 μl, under parallel conditions as DHSR1 except that the eIF5A precursor (3.4 μg), spermidine (3.6 μCi (0.1 nmol) of [1,8-3H]spermidine and 0.3 nmol of unlabeled spermidine) and the enzyme (0.1 μg) were used for each sample. The experiment was repeated with similar results and a representative experiment (in duplicate) is shown.
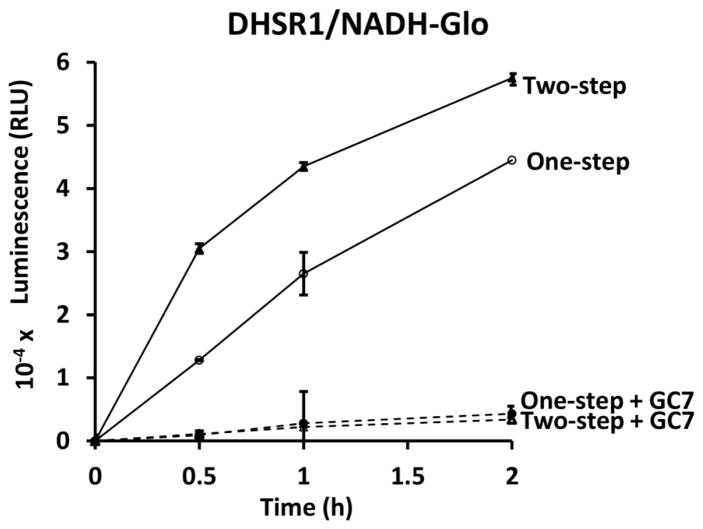
As there was no indication of interference in the NADH-Glo assays by DHSR components (Fig. 1), the DHSR1 mixture and the NADH-Glo reagent were combined at time zero and the time course of luminescence of the one-step assay was compared with that of the parallel two-step DHSR1/NADH-Glo assay (Fig. 7). The NADH derived luminescence signals in the one-step assay were somewhat lower than those of the two-step assay (80 % at 2 h) (Fig. 7), probably due to the two-fold dilution of the DHSR components (enzyme, NAD and spermidine) in the one-step assay by the addition of an equal volume of the NADH-Glo reagents at time zero. There appears to be no significant interference in the NADH-Glo assay by DHSR components and vice versa. GC7 exerted almost complete inhibition at 20 μM in both one-step and two-step assays (Fig. 7). These findings suggest that the DHSR1/NADH-Glo coupled reaction can be flexibly adapted either as a one-step or two-step assay for optimization in an HTS format.
Figure 7. Comparison of one-step and two-step DHSR1/NADH-Glo assay.
For the two-step assay, two sets of DHSR1 mixtures containing 3.0 μg of the enzyme and 20 μM spermidine and 100 μM NAD in 300 μl (duplicates of 50 μl at each of three time points) were prepared. GC7 (20 μM) was added to one set. After 0.5, 1 and 2 h incubation at 37 °C, 0.1 ml samples (duplicates of 50 μl) were removed and stored frozen until the final samples were collected. Then, the DHSR1 mixture was mixed with an equal volume of NADH-Glo reagent and luminescence was measured after 30 min incubation at RT. In parallel, two sets of DHSR1 mixtures (one without and the other with GC7 (20 μM)) were mixed with NADH-Glo reagent at time zero and the one-step coupled assay mixtures were incubated at 37 °C for 0.5, 1.0 and 2.0 h. Luminescence was measured after an additional 0. 5 h incubation at RT. The experiment was repeated with similar results and a representative experiment (in duplicate with error bars) is shown.
Discussion
In the current study, we have developed a new non-radioactive assay for deoxyhypusine synthase that can be configured for the HTS format. Our data demonstrate, for the first time, that NADH produced at the first step of the DHS reaction slowly dissociates from the enzyme and can be quantified by a NAD(P)H-dependent luminescence assay as a measure of DHS activity (Scheme 2, solid black arrow). The DHS partial reaction, DHSR1, is compatible with the NADH-Glo reaction and there seems to be little interference between the two assays. Furthermore, the DHS enzyme, spermidine, and NAD, as well as NADH, are relatively stable in the DHS assay buffer allowing the accumulation of NADH over a prolonged incubation period. Although the coupled assay was mainly conducted in two steps in this study, a single step assay also works (Fig 7), resulting in a time-dependent increase in luminescence over the period of 2 h. These findings suggest that the DHSR1/NADH-Glo coupled assay is adaptable for a HTS of small molecule libraries for identification of new inhibitors of DHS. Luminescence measurements of the coupled DHSR1/NADH-Glo assay in a multi-well format (96, 384 or 1584 wells) would offer convenience and accuracy in handling a wide range of concentrations of substrate, cofactor and inhibitors in high throughput screening.
The production of free NADH from DHS partial reaction is quite slow compared to that of deoxyhypusine synthesis in the DHS complete reaction (Fig. 5). Nonetheless, this slow production of free NADH is offset by a sensitive detection method for NADH using the NADH-Glo assay, which can detect NADH at sub μM levels (0.01–1 μM). The DHSR1/NADH-Glo coupled assay has advantages over the conventional DHS assay in that it does not involve radioactivity and is versatile for the HTS format. The conventional DHS assay, which measures the incorporation of radioactivity from [1,8-3H]spermidine into the eIF5A precursor as radioactive deoxyhypusine, is cumbersome, involves multiple manual steps and is thereby prone to experimental errors. Even a simplified version of the assay, the measurement of the radioactivity in the TCA-precipitated proteins, still involves several steps (Wolff et al. 2011). Although a radioactive DHS assay, that involves binding of the radiolabeled eIF5A on a 96 well filtration plate and counting of the filter-associated radioactivity after washing, was proposed for HTS (Sommer et al. 2004), it is not practical for screening a large number of compounds from multiple libraries. In addition to its potential application for HTS, the NADH-Glo assay offers an alternative, non-radioactive method to measure the DHS activity in biological samples, after removal of low molecular weight fractions containing NADH by using a desalting column.
Our current investigation using freed NADH as a probe provides new insights into the dynamic nature of the DHS reaction while substantiating the proposed mechanism (Scheme 2) involving an enzyme butylimine intermediate with bound NADH. In the previous studies (Wolff et al. 1990; Wolff et al. 1997; Wolff et al. 2000), the DHSRC was postulated to be a tightly coupled reaction in which NADH generated at step I remains bound to the enzyme to be used at step IV (Scheme 2). Clearly, the most efficient use of the NADH formed in DHSR1 is to provide the hydride ion to reduce the eIF5A butylimine intermediate to complete deoxyhypusine synthesis in DHSR2 (step IV, Scheme 2). Tangible evidence for the enzyme-bound NADH was obtained from the NADH fluorescence study (Wolff et al. 2000). In this work, the enzyme-bound NADH fluorescence declined rapidly upon addition of eIF5A precursor indicating the direct reutilization of the hydride ion of enzyme-bound NADH in deoxyhypusine synthesis. No clear evidence for freed NADH could be obtained, however, since the fluorescence peak corresponding to free NADH is close to that of the enzyme-bound NADH that displays 15 folds higher flurorescence intensity than that of free NADH (Wolff et al. 2000). Thus, it was assumed that the DHS partial reaction is virtually stalled in the absence of eIF5A precursor with little release of NADH. On the contrary, our current data reveals that enzyme-bound NADH is released to a measurable level during the DHS partial reaction and even during the DHS complete reaction (Fig. 3). Although previous study (Wolff et al. 1990) suggested that spermidine cleavage could be uncoupled from eIF5A modification in the absence of eIF5A precursor, there was no accurate estimation of the rate of DHS partial reaction. Coupling with the NADH-Glo assay has offered a convenient method to measure the rate of the DHS partial reaction and the DHS activity. The accumulation of free NADH to a level several folds higher than the enzyme in the DHSR1 (Fig. 3A, Fig. 5A) clearly indicates that the enzyme intermediate (EI) is converted back to a free enzyme upon cleavage of butylimine to Δ1-pyrroline concomitant with release of NADH, and that the freed enzyme can engage in multiple cycles of the spermidine cleavage reaction (Scheme 2, solid black arrow).
NADH intrinsic fluorescence itself has been employed as a detection signal in HTS (Napper and Sivendran 2011). However, the NADH fluorescence detection is not nearly as sensitive as the NADH-Glo assay where NADH signals are amplified as luciferin luminescence. Furthermore, natural fluorescence from a vast number of small molecule compounds would interfere with the NADH fluorescence-based measurements. The DHSR1/NADH-Glo coupled assay has an advantage, as little/no interference in luminescence is expected from the library of compounds. An alternative NAD(P)H detection method was reported by coupling of the isocitrate dehydrogenase 1 (IDH1) to a NAD(P)H-dependent diaphorase reaction which converts resazurin to resorufin, a compound that fluoresces at 585 nm (Candeias et al. 1998; Davis et al. 2016). Likewise, DHSR1 might be coupled with a diaphorase reaction for HTS, although it is not yet known which coupled assay would work better.
eIF5A, DHS and DOHH have been implicated in various human pathological conditions including cancer (Mathews and Hershey 2015; Nakanishi and Cleveland 2016), diabetes (Maier et al. 2010), and retroviral infections (Hoque et al. 2009; Olsen and Connor 2017; Saxena et al. 2016). Especially, eIF5A-2, the isoform that is not normally expressed but is increased in certain cancer cells or tissues (Clement et al. 2006), has been associated with cancer cell migration, metastasis and poor prognosis of several cancers including ovarian, gastric, non-small cell lung carcinoma and bladder cancer (see reviews (Park et al. 2014; Wang et al. 2013). A number of studies have been performed to target hypusine modification using chemical inhibitors or siRNAs against DHS, or DOHH. The DHS inhibitor, GC7, inhibits the growth of many cancer cells alone or in combination therapy with a chemotherapeutic agent and inhibits melanoma tumor growth in mouse (Jasiulionis et al. 2007). However, GC7 is not totally specific, and other unknown side effects and toxicity in animal model precludes its use in human clinical trials. Two inhibitors of DOHH, cyclopirox (CPX) and deferriprone (DFP), the approved drugs used for treatment of thalassemia, inhibit certain cancer cell growth (Shen and Huang 2016) and also inhibit retroviral infections including HIV-1 (Caceres et al. 2016; Hoque et al. 2009; Saxena et al. 2016). Yet, the specificity of these inhibitors is in question and other cellular targets cannot be ruled out. Thus, it is imperative to identify and develop new, specific inhibitors of the hypusine biosynthetic enzymes, applicable to various human diseases. To this end, our new DHSR1/NADH-Glo coupled assay should pave the way for HTS of multiple small molecule libraries.
Acknowledgments
Funding
The research was supported by the intramural program of the National Institute of Dental and Craniofacial Research, National Institutes of Health.
Abbreviations list
- eIF5A
eukaryotic initiation factor 5A
- DHS
deoxyhypusine synthase
- DOHH
deoxyhypusine hydroxylase
- HTS
high-throughput screening
- GC7
N1-guanyl-1.7-diaminoheptane
- DHSR
deoxyhypusine synthase reaction
- DHSRC
deoxyhypusine synthase complete reaction
- DHSR1
deoxyhypusine synthase partial reaction (first phase)
- DHSR2
the second phase of DHS reaction
- DHSR1/NADH-Glo assay
DHSR1 coupled with NADH-Glo assay
- DHSRC/NADH-Glo assay
DHSRC coupled with NADH-Glo assay
- RT
room temperature
Footnotes
Compliance with ethical standard
Conflict of interest
The authors declare that there is no conflict of interest associated with the manuscript.
Author contributions
MHP conceived the idea for the project, designed and conducted experiments and wrote the manuscript. AM and SM conducted experiments and performed data analyses. ECW took part in the experiments, the interpretation of experimental data and the preparation of the manuscript.
References
- Caceres CJ, Angulo J, Contreras N, et al. Targeting deoxyhypusine hydroxylase activity impairs cap-independent translation initiation driven by the 5′untranslated region of the HIV-1, HTLV-1, and MMTV mRNAs. Antiviral Res. 2016;134:192–206. doi: 10.1016/j.antiviral.2016.09.006. [DOI] [PubMed] [Google Scholar]
- Candeias LP, MacFarlane DPS, McWhinnie SLW, et al. The catalysed NADH reduction of resazurin to resorufin. J Chem Soc Perk T. 1998;2:2333–2334. [Google Scholar]
- Chattopadhyay MK, Park MH, Tabor H. Hypusine modification for growth is the major function of spermidine in Saccharomyces cerevisiae polyamine auxotrophs grown in limiting spermidine. Proc Natl Acad Sci U S A. 2008;105:6554–6559. doi: 10.1073/pnas.0710970105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement PM, Johansson HE, Wolff EC, et al. Differential expression of eIF5A-1 and eIF5A-2 in human cancer cells. FEBS J. 2006;273:1102–1114. doi: 10.1111/j.1742-4658.2006.05135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MI, Shen M, Simeonov A, et al. Diaphorase Coupling Protocols for Red-Shifting Dehydrogenase Assays. Assay Drug Dev Technol. 2016;14:207–212. doi: 10.1089/adt.2016.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dever TE, Gutierrez E, Shin BS. The hypusine-containing translation factor eIF5A. Crit Rev Biochem Mol Biol. 2014;49:413–425. doi: 10.3109/10409238.2014.939608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez E, Shin BS, Woolstenhulme CJ, et al. eIF5A promotes translation of polyproline motifs. Mol Cell. 2013;51:35–45. doi: 10.1016/j.molcel.2013.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoque M, Hanauske-Abel HM, Palumbo P, et al. Inhibition of HIV-1 gene expression by Ciclopirox and Deferiprone, drugs that prevent hypusination of eukaryotic initiation factor 5A. Retrovirology. 2009;6:90. doi: 10.1186/1742-4690-6-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakus J, Wolff EC, Park MH, et al. Features of the spermidine-binding site of deoxyhypusine synthase as derived from inhibition studies. Effective inhibition by bis- and mono-guanylated diamines and polyamines. J Biol Chem. 1993;268:13151–13159. [PubMed] [Google Scholar]
- Jasiulionis MG, Luchessi AD, Moreira AG, et al. Inhibition of eukaryotic translation initiation factor 5A (eIF5A) hypusination impairs melanoma growth. Cell Biochem Funct. 2007;25:109–114. doi: 10.1002/cbf.1351. [DOI] [PubMed] [Google Scholar]
- Joe YA, Park MH. Structural features of the eIF-5A precursor required for posttranslational synthesis of deoxyhypusine. J Biol Chem. 1994;269:25916–25921. [PubMed] [Google Scholar]
- Joe YA, Wolff EC, Park MH. Cloning and expression of human deoxyhypusine synthase cDNA. Structure-function studies with the recombinant enzyme and mutant proteins. J Biol Chem. 1995;270:22386–22392. doi: 10.1074/jbc.270.38.22386. [DOI] [PubMed] [Google Scholar]
- Lee CH, Park MH. Human deoxyhypusine synthase: interrelationship between binding of NAD and substrates. Biochem J. 2000;352(Pt 3):851–857. [PMC free article] [PubMed] [Google Scholar]
- Lee YB, Joe YA, Wolff EC, et al. Complex formation between deoxyhypusine synthase and its protein substrate, the eukaryotic translation initiation factor 5A (eIF5A) precursor. Biochem J. 1999;340(Pt 1):273–281. [PMC free article] [PubMed] [Google Scholar]
- Liao DI, Wolff EC, Park MH, et al. Crystal structure of the NAD complex of human deoxyhypusine synthase: an enzyme with a ball-and-chain mechanism for blocking the active site. Structure. 1998;6:23–32. doi: 10.1016/s0969-2126(98)00004-5. [DOI] [PubMed] [Google Scholar]
- Maier B, Ogihara T, Trace AP, et al. The unique hypusine modification of eIF5A promotes islet beta cell inflammation and dysfunction in mice. J Clin Invest. 2010;120:2156–2170. doi: 10.1172/JCI38924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews MB, Hershey JW. The translation factor eIF5A and human cancer. Biochim Biophys Acta. 2015;1849:836–844. doi: 10.1016/j.bbagrm.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi S, Cleveland JL. Targeting the polyamine-hypusine circuit for the prevention and treatment of cancer. Amino Acids. 2016;48:2353–2362. doi: 10.1007/s00726-016-2275-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napper AD, Sivendran S. Miniaturized High-Throughput Fluorescent Assay for Conversion of NAD(P)H to NAD(P) Curr Protoc Chem Biol. 2011;3 doi: 10.1002/9780470559277.ch100155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura K, Lee SB, Park JH, et al. Essential role of eIF5A-1 and deoxyhypusine synthase in mouse embryonic development. Amino Acids. 2012;42:703–710. doi: 10.1007/s00726-011-0986-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen ME, Connor JH. Hypusination of eIF5A as a Target for Antiviral Therapy. DNA and Cell Biol. 2017;36:1–4. doi: 10.1089/dna.2016.3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, Aravind L, Wolff EC, et al. Molecular cloning, expression, and structural prediction of deoxyhypusine hydroxylase: a HEAT-repeat-containing metalloenzyme. Proc Natl Acad Sci U S A. 2006;103:51–56. doi: 10.1073/pnas.0509348102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, Dias CA, Lee SB, et al. Production of active recombinant eIF5A: reconstitution in E. coli of eukaryotic hypusine modification of eIF5A by its coexpression with modifying enzymes. Protein Eng Des Sel. 2011;24:301–309. doi: 10.1093/protein/gzq110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MH, Wolff EC, Lee YB, et al. Antiproliferative effects of inhibitors of deoxyhypusine synthase. Inhibition of growth of Chinese hamster ovary cells by guanyl diamines. J Biol Chem. 1994;269:27827–27832. [PubMed] [Google Scholar]
- Park MH. The post-translational synthesis of a polyamine-derived amino acid, hypusine, in the eukaryotic translation initiation factor 5A (eIF5A) J Biochem. 2006;139:161–169. doi: 10.1093/jb/mvj034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MH, Nishimura K, Zanelli CF, et al. Functional significance of eIF5A and its hypusine modification in eukaryotes. Amino Acids. 2010;38:491–500. doi: 10.1007/s00726-009-0408-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MH, Mandal S, Mandal A, et al. eIF5A. In: Parsyan A, editor. Translation and Its Regulation in Cancer Biology and Medicine. Netherlands: Springer Netherlands; 2014. pp. 223–232. [DOI] [Google Scholar]
- Pegg AE, Casero RA., Jr Current status of the polyamine research field. Methods Mol Biol. 2011;720:3–35. doi: 10.1007/978-1-61779-034-8_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg AE. Functions of Polyamines in Mammals. J Biol Chem. 2016;291:14904–14912. doi: 10.1074/jbc.R116.731661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi D, Kuroshu R, Zanelli CF, et al. eIF5A and EF-P: two unique translation factors are now traveling the same road. Wiley Interdiscip Rev RNA. 2014;5:209–222. doi: 10.1002/wrna.1211. [DOI] [PubMed] [Google Scholar]
- Saxena D, Spino M, Tricta F, et al. Drug-Based Lead Discovery: The Novel Ablative Antiretroviral Profile of Deferiprone in HIV-1-Infected Cells and in HIV-Infected Treatment-Naive Subjects of a Double-Blind, Placebo-Controlled, Randomized Exploratory Trial. PLoS One. 2016;11:e0154842. doi: 10.1371/journal.pone.0154842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen T, Huang S. Repositioning the Old Fungicide Ciclopirox for New Medical Uses. Curr Pharm Des. 2016;22:4443–4450. doi: 10.2174/1381612822666160530151209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sievert H, Pallmann N, Miller KK, et al. A novel mouse model for inhibition of DOHH-mediated hypusine modification reveals a crucial function in embryonic development, proliferation and oncogenic transformation. Dis Model Mech. 2014;7:963–976. doi: 10.1242/dmm.014449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer MN, Bevec D, Klebl B, et al. Screening assay for the identification of deoxyhypusine synthase inhibitors. J Biomol Screen. 2004;9:434–438. doi: 10.1177/1087057104264031. [DOI] [PubMed] [Google Scholar]
- Umland TC, Wolff EC, Park MH, et al. A new crystal structure of deoxyhypusine synthase reveals the configuration of the active enzyme and of an enzyme. NAD. inhibitor ternary complex. J Biol Chem. 2004;279:28697–28705. doi: 10.1074/jbc.M404095200. [DOI] [PubMed] [Google Scholar]
- Wang FW, Guan XY, Xie D. Roles of eukaryotic initiation factor 5A2 in human cancer. Int J Biol Sci. 2013;9:1013–1020. doi: 10.7150/ijbs.7191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff EC, Park MH, Folk JE. Cleavage of spermidine as the first step in deoxyhypusine synthesis. The role of NAD. J Biol Chem. 1990;265:4793–4799. [PubMed] [Google Scholar]
- Wolff EC, Folk JE, Park MH. Enzyme-substrate intermediate formation at lysine 329 of human deoxyhypusine synthase. J Biol Chem. 1997;272:15865–15871. doi: 10.1074/jbc.272.25.15865. [DOI] [PubMed] [Google Scholar]
- Wolff EC, Wolff J, Park MH. Deoxyhypusine synthase generates and uses bound NADH in a transient hydride transfer mechanism. J Biol Chem. 2000;275:9170–9177. doi: 10.1074/jbc.275.13.9170. [DOI] [PubMed] [Google Scholar]
- Wolff EC, Lee SB, Park MH. Assay of deoxyhypusine synthase activity. Methods Mol Biol. 2011;720:195–205. doi: 10.1007/978-1-61779-034-8_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff EC, Park MH. Role of the Polyamine Spermidine as a Precursor for Hypusine Modification in eIF5A. In: Kusano TS, Japan H, editors. Polyamines. Springer; Japan: 2015. pp. 121–129. [Google Scholar]