This in vitro controlled study assesses whether the helper T cell 1 cytokine interferon-γ inhibits laryngotracheal stenosis–derived fibroblast function in patients undergoing surgical subglottic and tracheal dilation.
Key Points
Question
Can the helper T cell 1 cytokine interferon-γ inhibit laryngotracheal stenosis–derived fibroblasts in vitro?
Findings
Interferon-γ reduced proliferation and collagen expression and production in laryngotracheal stenosis–derived fibroblasts while also reducing the expression of the profibrotic cytokine transforming growth factor β.
Meaning
Immunomodulatory strategies that focus on increasing interferon-γ may be able to attenuate the progression of laryngotracheal stenosis.
Abstract
Importance
Laryngotracheal stenosis (LTS) is a fibroproliferative disorder of the glottis, subglottis, and trachea. In models of fibrosis from other organ systems, the CD4+ T-cell response has been shown to regulate extracellular matrix deposition. Specifically, helper T cell 2 (TH2) promotes fibrosis, whereas TH1 and associated cytokines have been shown to be antifibrotic. However, this antifibrotic effect of the TH1 response has not been demonstrated in LTS.
Objective
To determine whether the TH1 cytokine interferon-γ inhibits the function of LTS-derived fibroblasts in vitro.
Design, Setting, and Participants
This in vitro controlled study included 6 patients with iatrogenic LTS undergoing routine surgical subglottic and tracheal dilation at a single institution. Fibroblasts were isolated from biopsy specimens of laryngotracheal scar and normal-appearing trachea. The presence of fibroblasts was confirmed by an immunohistochemical analysis. Laryngotracheal stenosis–derived fibroblasts were treated with interferon-γ and compared with untreated controls (2 sets of untreated, LTS-derived fibroblasts [media did not contain interferon-γ]) and normal airway fibroblasts (fibroblasts isolated from normal trachea). Data were collected from August 2015 through June 2016.
Interventions
Treatment with interferon-γ, 10 ng/mL.
Main Outcomes and Measures
Cellular proliferation, fibrosis gene expression (using quantitative reverse transcription polymerase chain reaction analysis), soluble collagen, and cellular histologic features were assessed.
Results
Among the 6 patients (6 women; mean [SD] age, 38.3 [17.2] years), LTS-derived fibroblast proliferation was reduced in patients who received interferon-γ treatment compared with untreated controls on days 3 (mean difference, −6515 cells; 95% CI, −10 630 to −2600 cells) to 6 (mean difference, −47 521 cells; 95% CI, −81 285 to −13 757 cells). Interferon-γ treatment reduced collagen types I and III gene expression by 86% and 68%, respectively, and resulted in lower total collagen production (10.94 vs 14.89 μg/mL). In addition, interferon-γ treatment resulted in a 32% reduction in expression of transforming growth factor β in LTS-derived fibroblasts.
Conclusions and Relevance
Interferon-γ reduced proliferation, soluble collagen production, and collagen expression in LTS-derived fibroblasts while also reducing the expression of the profibrotic cytokine transforming growth factor β. These findings suggest that therapeutics aimed at increasing interferon-γ and the TH1 response could attenuate LTS.
Introduction
Laryngotracheal stenosis (LTS) is a critical narrowing of the glottis, subglottis, and/or trachea secondary to the development of pathologic fibrosis or scar. Laryngotracheal stenosis is most commonly a result of postintubation injury but can also be autoimmune related, radiation induced, or idiopathic. The multiple consequences of LTS include communication handicap and airway obstruction, a potentially life-threatening complication if not managed appropriately. The contemporary management of LTS is primarily surgical and includes serial dilation, tracheal or cricotracheal resection, laryngotracheoplasty, and/or permanent tracheostomy. Medical therapies available for the management of LTS are limited, reflecting a need for improved understanding of disease pathogenesis.
The pathologic fibrosis observed in LTS has been attributed to aberrant wound healing and unregulated tissue remodeling; however, the molecular and immunologic mechanisms of this process remain to be fully elucidated. Fibroblasts, which are the main effector cell in fibrosis, have been demonstrated to be hypermetabolic and display a profibrotic phenotype in LTS. Although aberrant fibroblast function is ultimately responsible for the deposition of excessive collagen in LTS, the phenotypic changes observed in these fibroblasts has been proposed to be mediated by an adaptive immune mechanism. In a murine model of subglottic stenosis, aberrant wound healing and the development of stenosis was mediated by circulating lymphocytes. Furthermore, CD4+ T-cell–related cytokines have been shown to be elevated in biopsy samples from human LTS scar, thus implicating a potential role for this specific arm of T-cell immunity in the pathogenesis of LTS.
The role of the CD4+ T-cell immune response in fibrosis has been more clearly defined in other fibrotic diseases. Specifically, the differentiation of CD4+ T cells into a helper T cell 1 (TH1) or TH2 lineage has been shown to regulate fibrosis. In idiopathic pulmonary fibrosis (IPF), a fibroproliferative disease characterized by deposition of excessive collagen in the interstitial space, levels of the TH1 cytokine interferon-γ (INF-γ) have been shown to be significantly reduced, whereas levels of the TH2-related cytokines interleukin 4 (IL-4) and IL-13 are elevated. In addition, when CD4+ cells are skewed toward the TH2 phenotype, increased expression of profibrotic genes not observed with the TH1 phenotype is noted. In systemic sclerosis, a diffuse fibrotic disease affecting the skin and organs, upregulated TH2 cytokines have been demonstrated. Alternatively, studies have shown that increasing the TH1 response can mediate an antifibrotic effect and, in a model of IPF, has been shown to suppress collagen production and subsequent fibrosis. Cumulatively, these studies indicate that the deposition of excessive collagen, the hallmark of fibrotic disease, may share a common immunologic mechanism by which an imbalance between the TH1 and TH2 responses promotes pathologic fibrosis. Given that LTS is a fibroproliferative disorder, therapy aimed at increasing the TH1 response or that of its related cytokines may be an effective strategy for inhibiting the propagation of disease. However, immune stimulation has not been explored in LTS, and whether the antifibrotic effects of the TH1 axis can be mediated through direct cytokine signaling alone remains unclear.
The critical role of fibroblast proliferation and collagen deposition in the development of LTS and the known hyperfunctional phenotype of LTS-derived fibroblasts makes them an ideal cell line for in vitro analysis. With use of LTS-derived fibroblasts, the influence of cytokine stimulation on LTS-related fibrosis can be quantified in vitro. The goal of this investigation was to determine whether treatment with the TH1 cytokine INF-γ can inhibit LTS-derived fibroblast function. Using an in vitro system of fibroblast culture, we assessed the association of INF-γ with fibroblast proliferation, histologic features, collagen production, and expression of fibrosis-related genes. This study provides an improved understanding of immunologic regulation of the fibroblasts responsible for LTS and potential therapeutic information about TH1-based immunotherapy in LTS.
Methods
Fibroblast Isolation and Culture
Biopsy specimens of human laryngotracheal scar and normal-appearing areas of the airway were procured during routine surgical dilation of subglottic and tracheal stenosis in 6 patients with iatrogenic LTS. All specimens used in this study were incidentally obtained from female patients with iatrogenic LTS, and sex was not a selection criterion. All patients had a history of prolonged intubation (eTable in the Supplement). Control groups in this study consisted of fibroblasts isolated from normal-appearing airway (normal airway fibroblasts) and fibroblasts isolated from areas of laryngotracheal scar (LTS fibroblasts). The experimental group consisted of LTS fibroblasts treated with INF-γ. This study was approved by the institutional review board of Johns Hopkins University. All patients provided written informed consent.
Data were collected from August 2015 through June 2016. Fresh tissue specimens were processed and fibroblasts were isolated and grown in fibroblast growth medium (Dulbecco modified Eagle medium; Gibco) supplemented with 10% fetal bovine serum (HyClone; Thermo Scientific), 100 U/mL penicillin (Gibco), 100 μg/mL streptomycin (Gibco), and nonessential amino acids (Gibco) at 37°C in a 5% carbon dioxide–humidified atmosphere as previously described. Fibroblasts were characterized in vitro using immunohistochemical staining. Fibroblasts were identified by positive staining with a monoclonal rat antibody specific for an unknown antigen present in fibroblasts called ER-TR7 (Santa Cruz Biotechnology Inc) and negative staining against the epithelial markers pan cytokeratin and AE1/AE3 using a monoclonal mouse antihuman pan cytokeratin antibody (Becton Dickenson) and a monoclonal mouse anti-AE1/AE3 antibody (Ventana Medical Systems). In addition, negative staining for the vascular endothelial marker ETS-related gene (ERG) was confirmed using a monoclonal mouse anti-ERG vascular antibody (Biocare Medical) and for the muscle-specific marker desmin using a monoclonal mouse antihuman desmin antibody (Agilent Technologies). Fibroblasts were limited to passages 2, 3, and 4 for all experiments in this study. All studies were conducted in triplicate except cell proliferation, which was conducted in duplicate.
Cell Proliferation by Cell Count
Once confluent, adherent LTS-derived fibroblasts and normal airway fibroblasts underwent trypsinization, and 5000 cells were passaged into each well of a 6-well plate (Falcon; BD Biosciences). Cells were grown under the following conditions: (1) control in fibroblast growth media or (2) experimental in fibroblast growth media supplemented with 10 ng/mL of INF-γ (Abcam), resulting in a specific activity of 200 U/mL, a previously described dose correlating with minimum treatment concentrations of INF-γ in vivo. Media was aspirated and replaced with the same conditions 1 day after initial seeding. Cells were harvested from 1 well each day for 6 days; a mean total cell count was determined for each condition on each day using a hemocytometer. All proliferation studies were conducted in duplicate as previously described.
Cell Staining and Histologic Evaluation
The LTS-derived fibroblasts were cultured in control and experimental medium conditions for 3 days on microscope glass coverslips pretreated with a 0.1% gelatin solution. After 3 days, all glass coverslips were fixed with 10% formalin and stained with Masson trichrome. Microscopic images of the stained sections were obtained with a microscope (AX10; Zeiss) at 20 × magnification.
Gene Expression Analysis by Quantitative Real-Time Polymerase Chain Reaction
The LTS-derived and normal airway fibroblasts were seeded in 6-well culture plates (Falcon; BD Biosciences) with 250 000 cells per well and were grown in control and experimental medium conditions for 72 hours. Cells were harvested and RNA was extracted using a specialized kit (RNeasy Micro Kit; Qiagen) and then quantified using a spectrophotometer (NanoDrop 2000; Thermo Scientific). We used RNA for the creation of complementary DNA, and gene expression was quantified using quantitative real-time polymerase chain reaction (RT-PCR) as previously described. The cycle threshold value (CT) of the genes of interest were normalized against β-actin (ΔCT) for all samples and then compared with expression in normal airway fibroblasts (ΔΔCT). Gene expression is presented as the relative fold change calculated by 2−ΔΔCT. All samples were investigated in biological triplicate. Gene expression was quantified for the fibrosis-related genes collagen type I (COL1 [NCBI Entrez Gene 1277]), collagen type III (COL3 [NCBI Entrez Gene 1281]), fibronectin 1 (FN1 [NCBI Entrez Gene 2335]), α-smooth muscle actin (ACTA2 [NCBI Entrez Gene 59]), and matrix melloproteinase 1 (MMP1 [NCBI Entrez Gene 4312]). In addition, gene expression of the profibrotic cytokine transforming growth factor β (TGFB1 [NCBI Entrez Gene 7040]) and TGF-β receptor 1 (TGFBR1 [NCBI Entrez Gene 7046]) was assessed.
Assay for Soluble Collagen Quantification
The LTS-derived and normal airway fibroblasts were seeded in 6-well plates at a concentration of 250 000 cells per well in control media. After 24 hours, media were aspirated and replaced with control or experimental media. Cells were incubated for 96 hours, and soluble collagen content was quantified using a commercial collagen dye–binding assay (Sircol; Biocolor Ltd). Before quantification, collagen in the extracted media was concentrated using the concentration reagent. Concentrated collagen was mixed with the sirius red dye reagent for 30 minutes and then centrifuged at 12 000g. The remaining dye was aspirated, and the collagen-dye complex was washed. Collagen-bound dye was released in alkali reagent. Spectrophotometric measurement was determined at 555 nm using a microplate reader (Synergy2; BioTek), and total soluble collagen was quantified based on a standard curve.
Statistical Analysis
Results are displayed as means and 95% CIs of the mean difference. Differences between treatment and control groups were compared using a nonparametric Wilcoxon signed rank test. A 2-sided P < .05 was considered statistically significant. Data analysis was performed using Prism GraphPad statistical software.
Results
LTS Fibroblast Proliferation
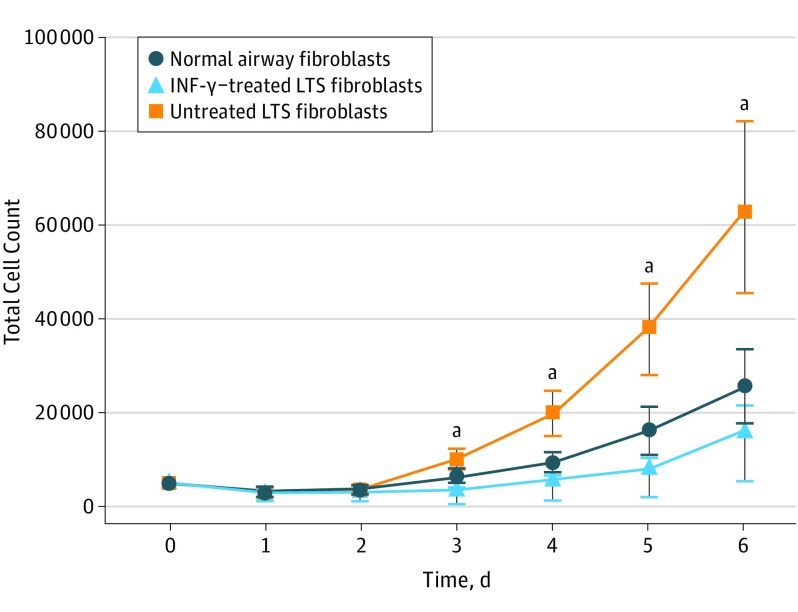
Of the 6 participants (6 women; mean [SD] age, 38.3 [17.2] years), fibroblasts proliferated in control and experimental media. The addition of INF-γ to the growth media resulted in decreased total cell counts for all 6 patient samples. Collectively, fibroblasts cultured in media treated with 10 ng/mL of INF-γ had a significantly lower mean cell count on days 3 (mean difference, −6515; 95% CI, −10 630 to −2600), 4 (mean difference, −14 182; 95% CI, −22 860 to −5505), 5 (mean difference, −29 766; 95% CI, −47 550 to −11 981), and 6 (mean difference, −47 521; 95% CI, −81 285 to −13 757) compared with untreated LTS-derived fibroblasts (Figure 1).
Figure 1. Inhibition of Laryngotracheal Stenosis (LTS)–Derived Fibroblast Proliferation.
Interferon-γ (INF-γ) significantly reduced the proliferation of LTS-derived fibroblasts when compared with untreated fibroblasts on days 3 (mean difference, −6515; 95% CI, −10 630 to −2600), 4 (mean difference, −14 182; 95% CI, −22 860 to −5505), 5 (mean difference, −29 766; 95% CI, −47 550 to −11 981), and 6 (−47 521; 95% CI, −81 285 to −13 757) in all 6 patient samples. Error bars indicate 95% CIs.
aP ≤ .01 compared with untreated fibroblasts.
Collagen Expression and Deposition in LTS Fibroblasts
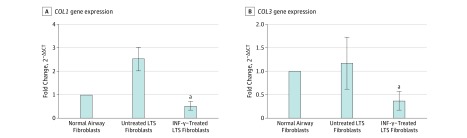
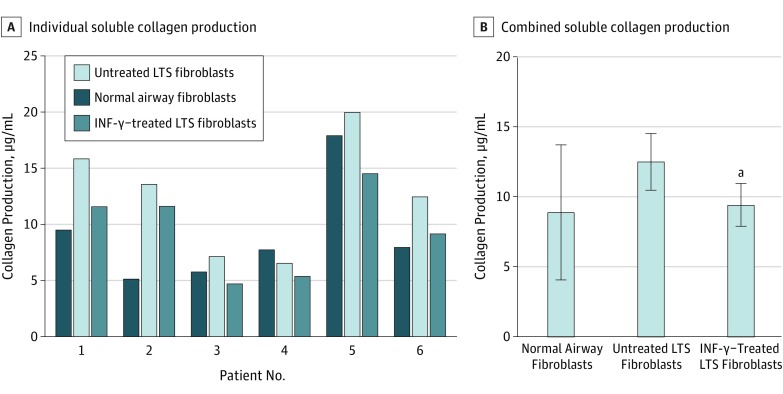
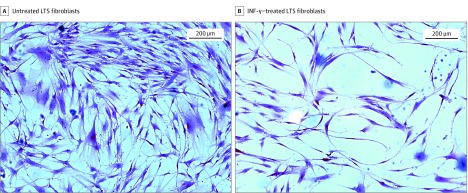
Gene expression analysis using quantitative RT-PCR demonstrated a reduction in COL1 and COL3 gene expression in INF-γ–treated, LTS-derived fibroblasts from all 6 patient samples. Overall, we found a 5.51-fold reduction in COL1 expression (95% CI, 3.31-9.15) (86% reduction) and a 3.07-fold reduction in COL3 expression (95% CI, 1.99-4.72) (68% reduction) in LTS-derived fibroblasts treated with INF-γ compared with untreated LTS-derived fibroblasts (Figure 2). In addition, INF-γ treatment reduced soluble collagen production in all 6 LTS-derived fibroblast cell lines (Figure 3A). When compared as a group, this change resulted in a significant reduction in soluble collagen production in LTS-derived fibroblasts treated with INF-γ compared with untreated LTS fibroblasts (10.94 vs 14.89 μg/mL; mean difference, 3.950 μg/mL; 95% CI, −4.753 to −1.419 μg/mL) (Figure 3B). With use of the Masson trichrome stain, which stains collagen blue, in vitro collagen deposition by LTS-derived fibroblasts grown in control and INF-γ–treated media was demonstrated (Figure 4).
Figure 2. Inhibition of Collagen Types I and III Gene Expression in Laryngotracheal Stenosis (LTS)–Derived Fibroblasts.
Gene expression is presented as the relative fold change calculated as the cycle threshold (CT) normalized against β-actin and compared with the expression in normal airway fibroblasts (2−ΔΔCT). Interferon-γ reduced gene expression 5.51-fold in COL1 (95% CI, 3.31-9.15) and 3.07-fold in COL3 (95% CI, 1.99-4.72) in LTS-derived fibroblasts compared with untreated fibroblasts in all 6 patient samples. Gene expression was normalized to normal airway fibroblasts. Error bars indicate 95% CIs. INF-γ indicates interferon-γ.
aP = .03.
Figure 3. Reduction of Total Collagen Production in Laryngotracheal Stenosis (LTS)–Derived Fibroblasts.
A, All 6 patient samples of LTS fibroblasts demonstrated reduced collagen production after interferon-γ (INF-γ) treatment. B, When compared as a group, INF-γ–treated fibroblasts had a significant reduction in the total amount of soluble collagen compared with untreated fibroblasts (10.94 vs 14.89 μg/mL; mean difference, 3.950 μg/mL; 95% CI, −4.753 to −1.419 μg/mL).
aP = .03 compared with untreated fibroblasts.
Figure 4. Fibroblast Morphologic Analysis.
Untreated laryngotracheal stenosis (LTS)–derived fibroblasts demonstrate confluent fibroblasts with strong collagen staining (dark blue) compared with proliferation and collagen staining of LTS-derived fibroblasts treated with interferon-γ (INF-γ).
TGF-β Expression in LTS Fibroblasts
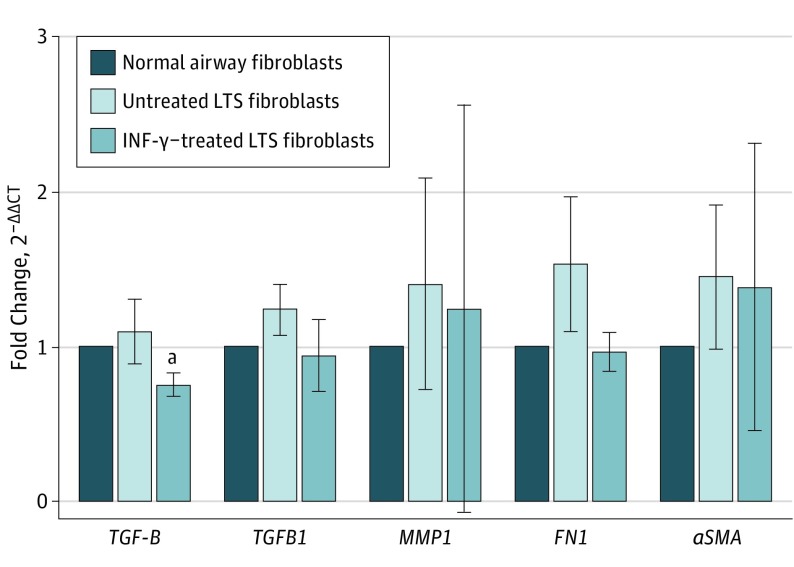
Quantitative RT-PCR analysis demonstrated a 32% reduction in TGFB1 gene expression in all 6 patients (fold change, 0.68; 95% CI, 0.49-0.96) and a 24% reduction in TGFBR1 gene expression (fold change, 0.76; 95% CI, 0.42-1.37) in INF-γ–treated LTS fibroblasts compared with controls (Figure 5). In addition, we found a 37% reduction in the expression of FN1 when LTS-derived fibroblasts were treated with INF-γ (fold change, 0.63; 95% CI, 0.34-1.16) and a minimal 6% reduction in ACTA2 expression (fold change, 0.94; 95% CI, 0.36-2.53) after treatment (Figure 5).
Figure 5. Gene Expression Analysis of Fibrosis-Related Genes of Interest.
Gene expression analysis of the profibrotic cytokine transforming growth factor β (TGFB1) revealed a significant reduction in laryngotracheal stenosis (LTS)–derived fibroblasts treated with interferon-γ (INF-γ) compared with untreated fibroblasts in all 6 patient samples. Gene expression is described in the legend of Figure 2. There was no significant difference in the gene expression of TGFB1, MMP1, FN1, or ACTA2. Gene expression is described in the legend of Figure 2. Error bars indicate 95% CIs.
aP = .03 compared with untreated fibroblasts.
Discussion
Laryngotracheal stenosis is a chronic and debilitating fibroproliferative disease characterized by luminal narrowing of the airway secondary to a chronic inflammatory response that promotes increased fibroblast activity, collagen deposition, and the formation of pathologic scar. The specific nature of this profibrotic inflammatory response may be related to an anomalous CD4+ T-cell response. In our study, we revealed the potent antifibrotic effects of the TH1 cytokine INF-γ on LTS-derived fibroblasts by demonstrating significant reductions in fibroblast proliferation, fibrosis-related gene expression, and collagen production after INF-γ treatment. These results indicate that the fibroblasts associated with LTS scar formation can be inhibited by INF-γ signaling.
In the present study, INF-γ was used as an in vitro surrogate for assessing the effects of TH1 signaling on LTS-derived fibroblasts, the primary effector cell in LTS. Although this in vitro system only captures the effects of 1 cytokine and does not reflect the complex cell-cell signaling network involved in an in vivo system, it provides a concise assessment of the association of the primary TH1 cytokine with the fibroblasts responsible for potentiating fibrosis and scar formation in LTS. Given the central role of INF-γ in the TH1 response, the reduction in fibroblast activity demonstrated in this study indicates a potential antifibrotic role for TH1 cells in LTS. In addition, these results are consistent with other reports investigating the effects of stimulating the TH1 response in fibroproliferative disease and indicate that the fibrosis associated with LTS may be regulated by similar immunologic mechanisms. Although the molecular mechanism for INF-γ’s inhibition of LTS-derived fibroblast function was not directly assessed in our study, previous studies have demonstrated that INF-γ downregulates signaling of the profibrotic cytokine IL-4. On the basis of these findings, the fibrosis that hallmarks LTS might be inhibited by modulating the TH1 response.
The TH1 and TH2 response in fibrosis is more clearly defined in other fibrotic diseases. In fibroproliferative diseases of the skin, kidney, and lung, an accentuated TH2 CD4+ T-cell response promotes pathologic fibrosis. Specifically, the dysregulated CD4+ T-cell response in fibrosis is characterized by decreased concentrations of the TH1 cytokine INF-γ and increases in the TH2-related cytokines IL-4 and IL-13. In these diseases, increasing concentrations of the TH1 cytokine INF-γ reduced the profibrotic effects of IL-4 and IL-13. In a model of IPF, the administration of an intranasal vaccinia vaccine has demonstrated the ability to stimulate a robust TH1 response and subsequently reduce the development of pathologic fibrosis through increased INF-γ signaling. Given these findings, treatment strategies focused on shifting the balancing of TH1 and TH2 response in fibrosis have become a topic of interest in organ fibrosis. Because of the antifibrotic effect of INF-γ on LTS-derived fibroblasts in the present study, treatment strategies focused on increasing INF-γ through TH1 stimulation, similar to the vaccinia vaccine, could be beneficial in attenuating the fibrosis associated with LTS. Moreover, similar strategies have been investigated in other proliferative diseases of the larynx. In recurrent respiratory papillomatosis, a dysregulated CD4+ T-cell response characterized by a defective TH1 and predominant TH2 response has been demonstrated. In recurrent respiratory papillomatosis, the inability to mount an effective TH1 response has been hypothesized to promote recurrence and papilloma growth. Therefore, treatment strategies, including the use of intralesional mumps and bacillus Calmette-Guerin vaccination that stimulate the TH1 response, have been explored and may represent potential therapies in LTS.
In the present study, INF-γ manifests as a cytokine capable of inhibiting collagen production and mitigating the development of fibrosis. However, despite these robust antifibrotic effects of INF-γ, it is unlikely to be a suitable systemic therapeutic for LTS secondary to a host of poorly tolerated constitutional symptoms, including fever, chills, myalgia, and vomiting. In addition, the systemic use of INF-γ in the treatment of IPF failed to demonstrate a survival benefit. Alternatively, therapies that aim to increase INF-γ through immunomodulation of the CD4+ T-cell response with a focus on increasing TH1 T-cell populations may prove to be more beneficial. As previously mentioned, the use of vaccine-based immunotherapy can induce a TH1 response and in IPF, its use demonstrated the potential to reverse the progression of fibrosis and improve disease status. Immunomodulatory strategies such as this that increase the TH1 response, have not been explored in LTS but, based on the findings in this study, may be beneficial. In addition, although this particular strategy focuses on stimulating the TH1 response, other strategies focused on reducing the TH2 response may also prove to be effective but have not yet been explored.
The present study also demonstrated that TGFB1 gene expression in LTS fibroblasts was significantly reduced with INF-γ treatment. Transforming growth factor β is a profibrotic cytokine secreted by numerous cell types, including fibroblasts, and is associated with increased collagen deposition and fibrosis. In LTS, increased TGF-β has been identified in biopsy specimens of stenosis, justifying preclinical attempts at therapeutic inhibition of TGF-β. However, the ubiquitous nature of TGF-β and likely the need to inhibit the activated form have limited the clinical application of TGF-β inhibition. However, the reduction of TGFB1 expression in this study may indicate that TGF-β is a surrogate marker for fibrosis. In addition, the antifibrotic effects of INF-γ treatment on LTS-derived fibroblasts may be mediated through inhibition of TGFB1 gene expression because previous in vitro studies have demonstrated that INF-γ was unable to antagonize TGF-β directly. Although these inferences represent interesting explanations for the interactions of INF-γ and TGF-β, additional studies are needed to delineate a relationship.
Limitations
Although this study demonstrates an antifibrotic effect for INF-γ on human LTS-derived fibroblasts, it has several limitations. The effects demonstrated in isolated cell culture in this in vitro study may not be reflective of the effects in vivo. In addition, disease mechanisms in LTS and its underlying immunopathologic features are not completely understood, and some inferences are being made based on more clearly defined fibrotic diseases, which may not reflect the true pathogenesis of LTS. Finally, the heterogeneity of LTS in regard to its severity and etiology may represent variable phenotypes and mechanisms of pathogenesis. Given these differences, immunologic signaling pathways in fibroblast-associated idiopathic LTS may be different from those in iatrogenic LTS and therefore may not demonstrate the same response to INF-γ treatment. Nonetheless, the antifibrotic effect of INF-γ in this study is encouraging and provides some evidence that the TH1 response may be able to attenuate iatrogenic LTS pathogenesis; stimulation of this response could represent an effective treatment strategy for LTS.
Conclusions
Interferon-γ inhibited LTS-derived fibroblast proliferation, gene expression, and function in vitro. In addition, the observed antifibrotic effects of INF-γ may be mediated through a reduction in TGFB1 gene expression. The inhibitory effect of INF-γ on LTS-derived fibroblasts and its role in the TH1 response suggest that shifting the TH1-TH2 balance may represent a potential immunologic strategy for disease modulation in LTS. Future in vivo studies are needed to delineate this immunologic mechanism in LTS so that targeted medical therapies can be investigated.
eTable. Patient Demographics
References
- 1.Gelbard A, Francis DO, Sandulache VC, Simmons JC, Donovan DT, Ongkasuwan J. Causes and consequences of adult laryngotracheal stenosis. Laryngoscope. 2015;125(5):1137-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gadkaree SK, Pandian V, Best S, et al. Laryngotracheal stenosis: risk factors for tracheostomy dependence and dilation interval. Otolaryngol Head Neck Surg. 2017;156(2):321-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hillel AT, Karatayli-Ozgursoy S, Benke JR, et al. Voice quality in laryngotracheal stenosis: impact of dilation and level of stenosis. Ann Otol Rhinol Laryngol. 2015;124(5):413-418. [DOI] [PubMed] [Google Scholar]
- 4.Herrington HC, Weber SM, Andersen PE. Modern management of laryngotracheal stenosis. Laryngoscope. 2006;116(9):1553-1557. [DOI] [PubMed] [Google Scholar]
- 5.Liu IY, Mendelsohn AH, Ching H, Long J, Chhetri DK, Berke GS. Staged laryngotracheoplasty in adult laryngotracheal stenosis: predictors of long-term decannulation. JAMA Otolaryngol Head Neck Surg. 2015;141(3):211-218. [DOI] [PubMed] [Google Scholar]
- 6.Minnigerode B, Richter HG. Pathophysiology of subglottic tracheal stenosis in childhood. Prog Pediatr Surg. 1987;21:1-7. [DOI] [PubMed] [Google Scholar]
- 7.Namba DR, Ma G, Samad I, et al. Rapamycin inhibits human laryngotracheal stenosis–derived fibroblast proliferation, metabolism, and function in vitro. Otolaryngol Head Neck Surg. 2015;152(5):881-888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma G, Samad I, Motz K, et al. Metabolic variations in normal and fibrotic human laryngotracheal-derived fibroblasts: a Warburg-like effect. Laryngoscope. 2017;127(3):E107-E113. doi: 10.1002/lary.26254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghosh A, Malaisrie N, Leahy KP, et al. Cellular adaptive inflammation mediates airway granulation in a murine model of subglottic stenosis. Otolaryngol Head Neck Surg. 2011;144(6):927-933. [DOI] [PubMed] [Google Scholar]
- 10.Hillel AT, Samad I, Ma G, et al. Dysregulated macrophages are present in bleomycin-induced murine laryngotracheal stenosis. Otolaryngol Head Neck Surg. 2015;153(2):244-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haft S, Lee JY, Ghosh A, et al. Inflammatory protein expression in human subglottic stenosis tissue mirrors that in a murine model. Ann Otol Rhinol Laryngol. 2014;123(1):65-70. [DOI] [PubMed] [Google Scholar]
- 12.Collins SL, Chan-Li Y, Hallowell RW, Powell JD, Horton MR. Pulmonary vaccination as a novel treatment for lung fibrosis. PLoS One. 2012;7(2):e31299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tapmeier TT, Fearn A, Brown K, et al. Pivotal role of CD4+ T cells in renal fibrosis following ureteric obstruction. Kidney Int. 2010;78(4):351-362. [DOI] [PubMed] [Google Scholar]
- 14.Wallace VA, Kondo S, Kono T, et al. A role for CD4+ T cells in the pathogenesis of skin fibrosis in tight skin mice. Eur J Immunol. 1994;24(6):1463-1466. [DOI] [PubMed] [Google Scholar]
- 15.Emura M, Nagai S, Takeuchi M, Kitaichi M, Izumi T. In vitro production of B cell growth factor and B cell differentiation factor by peripheral blood mononuclear cells and bronchoalveolar lavage T lymphocytes from patients with idiopathic pulmonary fibrosis. Clin Exp Immunol. 1990;82(1):133-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park S-W, Ahn M-H, Jang HK, et al. Interleukin-13 and its receptors in idiopathic interstitial pneumonia: clinical implications for lung function. J Korean Med Sci. 2009;24(4):614-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoffmann KF, McCarty TC, Segal DH, et al. Disease fingerprinting with cDNA microarrays reveals distinct gene expression profiles in lethal type 1 and type 2 cytokine-mediated inflammatory reactions. FASEB J. 2001;15(13):2545-2547. [DOI] [PubMed] [Google Scholar]
- 18.Hasegawa M, Sato S, Nagaoka T, Fujimoto M, Takehara K. Serum levels of tumor necrosis factor and interleukin-13 are elevated in patients with localized scleroderma. Dermatology. 2003;207(2):141-147. [DOI] [PubMed] [Google Scholar]
- 19.Narayanan AS, Whithey J, Souza A, Raghu G. Effect of γ-interferon on collagen synthesis by normal and fibrotic human lung fibroblasts. Chest. 1992;101(5):1326-1331. [DOI] [PubMed] [Google Scholar]
- 20.Serpier H, Gillery P, Salmon-Ehr V, et al. Antagonistic effects of interferon-γ and interleukin-4 on fibroblast cultures. J Invest Dermatol. 1997;109(2):158-162. [DOI] [PubMed] [Google Scholar]
- 21.Okada T, Sugie I, Aisaka K. Effects of gamma-interferon on collagen and histamine content in bleomycin-induced lung fibrosis in rats. Lymphokine Cytokine Res. 1993;12(2):87-91. [PubMed] [Google Scholar]
- 22.Wynn TA. Fibrotic disease and the TH1/TH2 paradigm. Nat Rev Immunol. 2004;4(8):583-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wall L, Burke F, Barton C, Smyth J, Balkwill F. IFN-γ induces apoptosis in ovarian cancer cells in vivo and in vitro. Clin Cancer Res. 2003;9(7):2487-2496. [PubMed] [Google Scholar]
- 24.Collins SL, Chan-Li Y, Oh M, et al. Vaccinia vaccine–based immunotherapy arrests and reverses established pulmonary fibrosis. JCI Insight. 2016;1(4):e83116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeVoti JA, Steinberg BM, Rosenthal DW, et al. Failure of gamma interferon but not interleukin-10 expression in response to human papillomavirus type 11 E6 protein in respiratory papillomatosis. Clin Diagn Lab Immunol. 2004;11(3):538-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeVoti JA, Rosenthal DW, Wu R, Abramson AL, Steinberg BM, Bonagura VR. Immune dysregulation and tumor-associated gene changes in recurrent respiratory papillomatosis: a paired microarray analysis. Mol Med. 2008;14(9-10):608-617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pashley NRT. Can mumps vaccine induce remission in recurrent respiratory papilloma? Arch Otolaryngol Head Neck Surg. 2002;128(7):783-786. [DOI] [PubMed] [Google Scholar]
- 28.Vetskova EK, Muhtarova MN, Avramov TI, Stefanova TR, Chalakov IJ, Nikolova MH. Immunomodulatory effects of BCG in patients with recurrent respiratory papillomatosis. Folia Med (Plovdiv). 2013;55(1):49-54. [DOI] [PubMed] [Google Scholar]
- 29.King TE Jr, Albera C, Bradford WZ, et al. ; INSPIRE Study Group . Effect of interferon gamma-1b on survival in patients with idiopathic pulmonary fibrosis (INSPIRE): a multicentre, randomised, placebo-controlled trial. Lancet. 2009;374(9685):222-228. [DOI] [PubMed] [Google Scholar]
- 30.Ziesche R, Hofbauer E, Wittmann K, Petkov V, Block LH. A preliminary study of long-term treatment with interferon gamma-1b and low-dose prednisolone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 1999;341(17):1264-1269. [DOI] [PubMed] [Google Scholar]
- 31.Raghu G, Brown KK, Bradford WZ, et al. ; Idiopathic Pulmonary Fibrosis Study Group . A placebo-controlled trial of interferon gamma-1b in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2004;350(2):125-133. [DOI] [PubMed] [Google Scholar]
- 32.Antoniou KM, Nicholson AG, Dimadi M, et al. Long-term clinical effects of interferon gamma-1b and colchicine in idiopathic pulmonary fibrosis. Eur Respir J. 2006;28(3):496-504. [DOI] [PubMed] [Google Scholar]
- 33.Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214(2):199-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scioscia KA, Miller F, April MM, Gruber BL. Growth factors in subglottic stenosis. Ann Otol Rhinol Laryngol. 1996;105(12):936-943. [DOI] [PubMed] [Google Scholar]
- 35.Simpson CB, White S, McGuff HS. Anti-transforming growth factor beta as a treatment for laryngotracheal stenosis in a canine model. Laryngoscope. 2008;118(3):546-551. [DOI] [PubMed] [Google Scholar]
- 36.Dillard DG, Gal AA, Roman-Rodriguez J, White S, Jacobs IN. Transforming growth factor and neutralizing antibodies in subglottic stenosis. Ann Otol Rhinol Laryngol. 2001;110(5, pt 1):393-400. [DOI] [PubMed] [Google Scholar]
- 37.Connolly EC, Freimuth J, Akhurst RJ. Complexities of TGF-β targeted cancer therapy. Int J Biol Sci. 2012;8(7):964-978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hasegawa T, Nakao A, Sumiyoshi K, Tsuboi R, Ogawa H. IFN-γ fails to antagonize fibrotic effect of TGF-β on keloid-derived dermal fibroblasts. J Dermatol Sci. 2003;32(1):19-24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Patient Demographics