Abstract
The vascular access is the lifeline for the hemodialysis patient. In the United States, the Fistula First Breakthrough Initiative (FFBI) has been influential in improving use of arteriovenous fistulas (AVF) in prevalent hemodialysis patients. Currently, prevalent AVF rates are near the goal of 66% set forth by the original FFBI. However, central venous catheter (CVC) rates remain very high in the United States in patients initiating hemodialysis, nearly exceeding 80%. A new direction of the of the FFBI has focused on strategies to reduce CVC use, and subsequently the FFBI has now been renamed the “Fistula First-Catheter Last Initiative”. However, an AVF may not be the best vascular access in all hemodialysis patients, and arteriovenous grafts (AVG) and central venous catheters (CVC) may be appropriate and the best access for a subset of hemodialysis patients. Unfortunately, there still remains very little emphasis within vascular access initiatives and guidelines directed towards evaluation of the individual patient context, specifically patients with poor long-term prognoses and short life expectancies, patients with multiple comorbidities, patients who are more likely to die than reach end stage renal disease (ESRD), and patients of elderly age with impaired physical and cognitive function. Given the complexity of medical and social issues in advanced CKD and ESRD patients, planning, selection, and placement of the most appropriate vascular access are ideally managed within a multidisciplinary setting and requires consideration of several factors including national vascular access guidelines. Thus, the evolution of the FFBI should underscore the need for multidisciplinary health teams with a major emphasis placed on “the right access for the right patient” and improving the patient’s overall quality of life.
Keywords: Hemodialysis Vascular Access, Arteriovenous Fistula, Arteriovenous Graft, Hemodialysis Catheter, Fistula First
Introduction
The Fistula First Breakthrough Initiative (FFBI) was a program initiated in the United States in 2003 as the National Vascular Access Improvement Initiative (NVAII) in response to the extremely low use of arteriovenous fistulas (AVF) in the United States hemodialysis patients [1, 2]. This project was initiated in collaboration with the Centers for Medicare & Medicaid Services (CMS), the End Stage Renal Disease (ESRD) Networks, and the entire renal community. The NVAII was renamed the FFBI in 2005 when the emphasis of this national project shifted towards increasing use of AVFs in the United States. The primary goals of the FFBI were to: (1) increase use of AVFs for hemodialysis patients, (2) collect, analyze, and disseminate information of AVF use in the United States, (3) and ultimately exceed the Kidney Disease Outcomes Quality Initiative (K/DOQI) Clinical Practice Guidelines of 50% AVF use in incident and 40% AVF use in prevalent hemodialysis patients [3, 4]. The original FFBI continuously evolved due to early achievement of these initial benchmarks. By 2005 the initial K/DOQI benchmark of 40% prevalent use was already achieved and consequently, the FFBI AVF target was increased in 2009 to a new goal of 66% AVFs in prevalent hemodialysis patients[1, 5]. More recently, due to the continued high rates of incident and prevalent dialysis catheter use, the original focus of the FFBI has now transformed to “Fistula First-Catheter Last” Workgroup Coalition [1]. The purpose of this article is to review the history of the FFBI, the impact of the FFBI on vascular access care in the United States, current efforts of the FFBI to address contemporary barriers in vascular access management, and the future of the FFBI.
Historical Perspective of the Fistula First Initiative
Early Years of Vascular Access Care in the United States
The first arteriovenous access in the United States was placed by Belding Scribner in 1960 [6] and first autologous AVF by Michael Brescia in 1966 [7]. Since that time technological advances in dialysis technology, expansion of Medicare payments for the ESRD program, and a more liberal expansion of dialysis eligibility (e.g. inclusion of patients with diabetes, elderly patients, vascular disease, etc.) resulted in the rapid growth in the ESRD population in the United States. Furthermore, the ability to deliver adequate dialysis therapy with prosthetic conduits, such polytetrafluoroethylene grafts (AVG) [8–10], and central venous catheters (CVC) in the upper extremity system [11–13] also expanded the pool of dialysis patients eligible for treatment. The number of surgical vascular accesses created each year has continued to increase with 500,000 created in 2007 [14]. However, the cumulative effect of the increase in hemodialysis patients in the U.S. has been the proportion of autologous AVFs use decreased and CVCs and AVGs increased in the late 1990’s and early 2000’s. Consequently, this also accounted for increased procedures for vascular access failures and hospitalizations for vascular access complications, and resulted in greater morbidity and mortality due to vascular access dysfunction [15–17]. In the 1990’s vascular access complications totaled over $1 billion dollars annually in the United States accounting for 14% of the entire ESRD budget [17].
The Birth of the Fistula First Breakthrough Initiative
In 2003, CMS and the ESRD networks in collaboration implemented a National Vascular Access Improvement Initiative called the Fistula First Breakthrough Initiative (FFBI). The goal of the FFBI was to collect, analyze, and provide educational tools to improve AVF use in the U.S [3]. The initial target goal of the FFBI was to achieve or exceed 40% AVF use in prevalent hemodialysis patients and 50% in incident hemodialysis patients, outlined by the 2001 K/DOQI vascular access guidelines [18], respectively, in U.S. hemodialysis patients. After attaining the target of 40% prevalent AVF use in August 2005, 10 months ahead of the projected target date, CMS established a new quality goal of 66% AVF use by 2009. The overall rationale for changing the AVF target was the observation that successful AVF use was much higher in Europe and Asia, where AVFs rate ranged from 60–90% [5, 19].
Fistula First Outcomes Dashboard
Another major goal of the FFBI was the creation of a registry to track real-time data in order to provide continuous quality improvement (CQI) [1]. This registry was created to report monthly ESRD network data to continuously track vascular access use (e.g. AVF, AVG, and CVC), report AVF trending data, and to make adjustments within the FFBI to efficiently and effectively execute the CQI process. The reported vascular access data was reported on the Fistula First website, as part the Fistula First Outcomes Dashboard [20].
Impact of the FFBI on Vascular Access Care
Landmark Increase in AVF Use in the United States
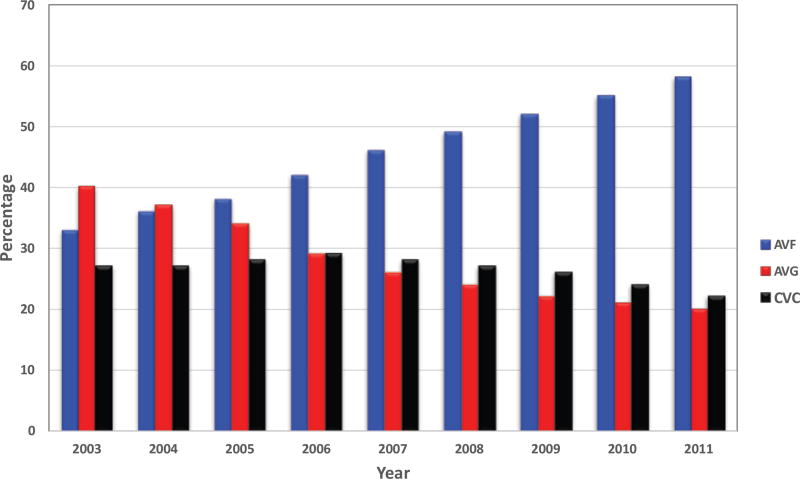
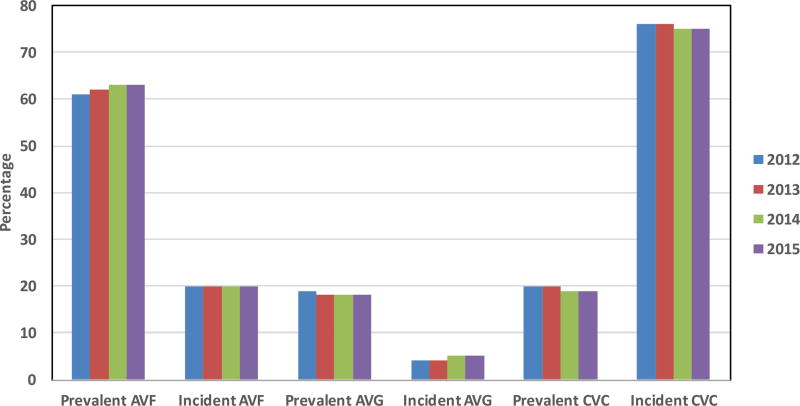
The FFBI has had a substantial positive impact in improving vascular access outcomes in the United States. In 1998, the prevalent AVF use in the U.S. was 26% [21]. Following implementation of the FFBI, from 2003 to 2010 the prevalent AVF use rate increased from 33 to 55% with a reduction of arteriovenous graft (AVG) use [1] (Figure 1). Recent data from the FFBI has reported that AVF use in the United States was 63% in December 2015 (Figure 2) [20]. This dramatic improvement in prevalent AVF rates was in part due to the “11 Change Concepts for Increasing Arteriovenous Fistula Use” (Table 1), which provided a defined process and infrastructure to achieve the AVF targets [20, 22]. These concepts have focused on fundamental topics that are critical to achieving a successfully functional AVF including [20, 22]: (1) CQI review of vascular access, (2) timely referral to nephrologists for evaluation of chronic kidney disease, (3) early referral to a vascular access surgeon for AVF evaluation and timely placement, (4) surgeon selection with full range of surgical techniques and approaches, placement of secondary AVFs, (5) AVF cannulation training, monitoring and maintenance of AVF after creation to ensure successful maturation and function, (6) education for caregivers, family, and patients, and (7) review of outcomes to guide clinical practice. In recent years the FFBI Concept Changes have expanded to include [22] modifying hospital systems to detect chronic kidney disease (CKD) and promote AVF planning and placement while hospitalized and focus more efforts to address and support quality of life issues in hemodialysis patients (Table 1).
Figure 1. Trends in Prevalent Vascular Access use since Initiation of the Fistula First Initiative.
AVF prevalence has steadily increased while AVG use has steadily decreased. CVC use has remained consistently over 20% during this same time period. Data obtained and adapted from the original Fistula First Dashboard, www.fistulafirst.com. Fistula First dashboard now located at: http://esrdncc.org/ffcl/for-ffcl-professionals/. All data of prevalent AVF rates (AVFs currently in use for dialysis), prevalent AVG rates, and prevalent CVC rates reported is adapted from January data except 2003, where data is from July, the first month prevalent data was available that year.
Figure 2. Recent Trends in Incident and Prevalent Vascular Access Use from 2012–2015.
AVF and CVC prevalent use have remained largely unchanged at 63% and 18%, respectively. Low incident AVF use and high incident CVC use have also remained largely unchanged. All data is reported from December of the calendar year. Data adapted from Fistula First Catheter Last (FFCL) Dashboard: http://esrdncc.org/ffcl/for-ffcl-professionals/. Last assessed July 13, 2016.
Table 1. Fistula First Breakthrough Initiative Change Concepts.
There are currently 13 change concepts with the first 11 representing the original concepts of the Fistula First Initiative. Adapted from: http://esrdncc.org/ffcl/change-concepts/; last assessed July 13, 2016
|
FFBI and the Dialysis Catheter Problem
While the 2006 K/DOQI vascular access guidelines also set a CVC target of <10% [18], this was not one of the main focuses of the FFBI [20], although the CVC reduction has been continually addressed throughout the initiative. As discussed in previous sections, the prevalent AVF use has dramatically improved likely due to reductions in AVG use. However, prevalent CVC use has remained relatively unchanged [20]. Prevalent CVC rates have remained in the mid-20% range since the FFBI. From 2003–2011 prevalent CVC use has ranged from 22–28% [1] (Figure 1). The consequences of CVC are very significant. CVC use has been associated with greater risks of morbidity related to infectious complications [23–25], hospitalization [26], CVC dysfunction [27], central venous stenosis [28, 29], and mortality [30, 31]. One potential explanation for the lack of improvement in CVC rates is that as more AVFs are placed, the CVC is serving as a bridge access until the AVF is suitable for cannulation. Moreover, AVF maturation failure in the United States has been reported to be as high as 60% from a large multicenter study [32]. Delays in AVF maturation (and AVF maturation failures) will necessarily delay conversion of CVC to AVF in patients due to interventions to promote AVF maturation.
Addressing the CVC Problem: Fistula First Catheter Last Initiative
In recent years, due to recognition that CVC use has remained very high, the FFBI has shifted greater focus to CVC reduction. As a result, the initiative has been renamed to the “Fistula First-Catheter Last Initiative” [33]. Recent data from December 2015 shows that the prevalent CVC rate is 19.0% in the United States [20] (Figure 2). However, prevalent CVC use greater than 90 days in hemodialysis patients has improved to 11.0% [20]. One potential strategy to improve prevalent catheter rates is to improve incident AVF use. This strategy would require improving the processes of care for AVF evaluation and placement in advanced CKD patients who will progress to ESRD. At present, the incident CVC use was 75% from the FFBI dashboard from 2015 [20].
Fistula First Breakthrough Initiative and Addressing Contemporary Barriers in Vascular Access Management
Right Access for the Right Patient
Although the FFBI has demonstrated that creation of AVFs is possible in the majority of hemodialysis patients, it largely fails to consider patients in whom the risks of AVF may outweigh the risks, thus, not incorporating a “patient-centered” approach. In fact, the 2006 K/DOQI vascular access guidelines workgroup recognized that “the fistula first at all costs may not be the most cost-effective or optimal approach for each individual patient” [2, 18]. Moreover, the K/DOQI vascular access workgroup also emphasized that overall goal for the hemodialysis patient should be a functional AVF, not placement of AVF in patients with poor likelihood of maturation or usability. Workgroup members from both K/DOQI and FFBI have been quoted that the focus of these guidelines and initiatives should be on “individualizing patient care, because it is about what is best for the patient” [34, 35]. At present the CMS-mandated QIP rewards centers that have high AVF prevalence and penalizes centers with high CVC prevalence [33]. The current model does not take into consideration case-based adjustment for patients with greater comorbidities and clinical risk factors, thus, essentially emphasizing a philosophy of “one-size-fits-all” for vascular access [33]. Moving towards a more patient-centered approach will require a greater focus on the subset of patients who will likely not realize the benefits of AVF placement including elderly ESRD patients, patients with poor vasculature (e.g. calcification and small arteries and veins), patients with slowly progressive CKD who more likely to die than progress to ESRD, and those patients with poor overall health and prognosis and limited life expectancy. Furthermore, a greater emphasis should be placed on patient quality of life, comfort, and satisfaction versus solely on AVF targets and clinical outcomes.
Elderly Patients and Vascular Access Creation
The elderly represents one of the largest segments of hemodialysis patients in the United States. From the 2014 USRDS report, patients ≥75 years comprise 26% and 22% of all incident and prevalent hemodialysis patients, respectively [36]. The prevalence per million of hemodialysis patients continues to increase in all age groups but the overall magnitude of increase from 2000 to 2012 remains greatest in the ≥75 age group, a 34% increase, compared to a 23% increase in patients age 65–74 and 19% increase in patients age 45–64[36]. These trends are also present in countries outside of the United States. In Canada, the number of incident ESRD patients ≥75 years has doubled between 1996 and 2005, while the number of incident patients with ESRD aged 20–64 decreased [37, 38]. In the United Kingdom, from 2005 to 2008, the number of patients ≥65 years has increased by 29% compared to 16% in those aged 18–65 [38, 39]. The challenge in the elderly population in respect to vascular access decisions is that due to the large number of comorbidities and frailty of these patients, the beneficial aspects of AVF placement may never be realized. This is most clearly reflected in the high mortality rate (30–50%) in the first year of dialysis [40, 41]. Thus, in the elderly ESRD hemodialysis patient population some key questions include: (1) Is the AVF or the AVG the more appropriate vascular access?, (2) Is a CVC appropriate in elderly hemodialysis patients with high comorbidities and limited lifespan?, and (3) Should there be a greater focus on patients preferences and quality of life?
There have been several recent studies that have demonstrated no mortality benefit of an AVF versus AVG in the elderly hemodialysis population. Using the USRDS database, Desilva et al found that in hemodialysis patients ≥67 years of age, there was not a significant mortality difference between those patients with a AVG as the first access placed and those patients with a AVF [42]. However, they clearly found that the overall survival was significantly worse in the catheter groups versus AVF or AVG group [42]. Yuo et al have shown that patients initiating hemodialysis with a CVC have similar survival after AVF and AVG creation[43]. In patients older than 80 years with albumin levels >4.0 g/dL, AVF creation is associated with higher mortality compared with AVG creation [43]. This may likely be due to earlier removal of CVC in patients with AVG compared to AVF [44]. In regards to vascular access patency, in elderly patients, increasing age (>65) has been associated with increased AVF maturation failure (odds ratio 2.23; 95% confidence interval 1.25–3.96)[45] and in patients age >70 vs ≤70 inferior 12 month primary (35% vs 67%, respectively) and secondary AVF patency (36% vs 67%, respectively)[46]. Furthermore, a meta-analysis of 13 observation studies, which included a total of 1,841 patients, showed increased AVF maturation failure and overall reduction in AVF patency of radiocephalic and brachiocephalic AVFs in elderly patients (>65 years of age) versus younger patients (<65 years of age) [47].
Decision-making processes for vascular access in ESRD patients, particularly elderly patients, should not solely be made based on morbidity and mortality data. Some patients favor a more “day to day” and “wait and see” approach and often times prefer the convenience of a CVC, because CVCs allow for avoidance of needles during cannulation, better physical appearance, and less bleeding [48–52]. In fact, Quinn et al. in a recent publication have reported that among elderly hemodialysis patients, satisfaction was greatest among elderly patients using a CVC [50].
Patient Vascular Anatomy and Vascular Access Creation
A patient’s vascular anatomy is an important prerequisite and determinant in whether a AVF should or can be created. The 2006 KDOQI Vascular Access guidelines [18] and FFBI [20] recommend vessel mapping as an important component of the AVF evaluation process before all permanent vascular access placement. Pre-operative vascular mapping has been shown to increase the proportion of AVF created and patients dialyzing with functional AVFs [53, 54]. Several studies have suggested to use a 2.0- to 2.5-mm vein diameter threshold for successful creation of AVF [54–56]. The 2006 K/DOQI Vascular Access Guidelines recommend duplex ultrasound as the preferred method for preoperative vascular mapping[18]. However, given the high AVF maturation failure rates, in the United States [32], an increase in the elderly ESRD population, and patients with greater comorbidities and chronic conditions initiating hemodialysis, a more patient-centered may be warranted within the scope of FFBI. This would include consideration of all vascular access types, including CVCs. This patient-centered approach, in addition to vascular anatomy, needs to include patient preferences, attributes, life expectancy, and quality of life.
Slow Chronic Kidney Disease Progression and Vascular Access Placement
While the FFBI has made extraordinary efforts in improving prevalent AVF rates, one of the remaining challenges is to increase AVF and decrease CVC use in patients initiating hemodialysis. Currently, in the United States AVF use at dialysis initiation remains approximately 20% and CVC use 80%, and this trend has remained largely unchanged over the last 5–10 years [36] (Figure 2). The low incident AVF rates likely impact the ability to improve prevalent AVF rates further. One potential strategy to increase incident AVF rates is earlier placement of AVF in advanced CKD. Hakim et al [57] have advocated a ‘30-20-10’ GFR criteria: (1) referral to nephrology for kidney replacement therapy education and preparation at 30 ml/min/1.73m2(2) referral to a vascular access surgeon for vascular access placement at 20 ml/min/1.73m2, and (3) hemodialysis initiation at 10 ml/min/1.73m2. The Canadian Society of Nephrology recommends placement of AVF when eGFR is between 15–20 ml/min/1.73m2 [58], the Japanese Society for Dialysis Therapy recommends AVF placement when creatinine clearance is between 10–20/ml/min [59], and the European Best Practices Guidelines recommends vascular access placement when eGFR reaches < 30ml/min/1.73m2 [60].
Earlier placement of pre-ESRD AVF may have unintended consequences which are worthy of consideration. Accurately predicting an individual patient’s rate of decline of kidney function is very challenging, particularly in the elderly population. While elderly patients have a high prevalence of CKD, this population of patients have been shown to have a slower decline of kidney function, lower incidence of progression to ESRD, and higher mortality rate [61–64]. Thus, the elderly population may constitute a group of CKD patients that will never use a vascular access placed before the initiation of hemodialysis. Recent studies have reported that up to two-thirds of elderly patients who undergo AVF placement die before their AVF was ever used for hemodialysis because either they did not initiate hemodialysis or successfully mature [46]. O’Hare et al [62], using a theoretical model in a Veterans Affairs population, demonstrated that in older patients who undergo AVF placement, the ratio of unnecessary to necessary permanent access surgeries, at different levels of eGFR, was always greater than in younger patients. Moreover, in their study cohort, if all the patients had been referred for permanent access placement at cohort entry, the ratio of unnecessary to necessary procedures after 2 years of follow-up would have been 5:1 for patients aged 85–100 years but only 0.5:1 for those aged 18–44 years [62]. These findings are significant because unnecessary surgeries and procedures are costly, carry a risk to patients with very little benefit, and can impact the overall quality of life of a patient.
Given the difficulties of predicting kidney disease progression and the need for hemodialysis, one pragmatic approach, particularly in the elderly population, would be to delay permanent access placement closer to the time of ESRD and place an AVG or initiate hemodialysis with a CVC and evaluate the patient’s overall response to dialysis initiation and subsequently place an AVG or AVF. AVGs in pre-dialysis patients placed closer to time of hemodialysis initiation has been shown to be an effective CVC sparing strategy [65]. Furthermore, patients initiating dialysis with a CVC and later having AVF or AVG creation have been shown to have similar mortality rates [43].
Quality of Life
While the AVF is the preferred vascular access type in hemodialysis patients due to the lowest morbidity and mortality compared to a dialysis catheter, having an understanding about the patient perspective on perceived advantages of different types of vascular accesses may help provide better patient-centered care and individualize access selection. It is well documented that hemodialysis patients have worse health-related quality of life (HRQOL) compared to the general population [66]. Moreover, HRQOL has been reported to predict mortality in hemodialysis patients [67]. Thus, the vascular access type placed and utilized may play a substantial role in HRQOL.
Quinn et al. have developed a vascular access questionnaire to assess patient-reported views regarding vascular access use [50]. While symptom score was similar in patients dialyzing with AVF vs catheter, they reported that patients using AVFs were more likely to experience pain, bleeding, bruising, swelling, and disturbed by their appearance of their access [50]. However, elderly patients reported lower symptom scores with catheters vs. AVFs [50]. Afsar et el have reported that patients dialyzing with a AVF or AVG vs CVC may have better perceived HRQOL, however, there was no association with vascular access type and depression [68]. Finally, Kosa et al have developed a short-form vascular access questionnaire (SF-VAQ) focused on vascular access-related quality of life [69]. This SF-VAQ was administered to 132 hemodialysis patients (35 AVF and 14 AVG) and demonstrated that patients with AVF had the highest SF-VAQ scores [69]. Thus, quality of life may be better in patients dialyzing with AVF or AVG, but symptom scores may be better in elderly patients with CVCs. Future studies need to validate these quality of life instruments in larger interventional studies in vascular access and in the context of randomized clinical trials.
Fistula First Breakthrough Initiative: The Next Steps
Improving Permanent Access Placement in Advanced Chronic Kidney Disease Patients
It will be very difficult to increase prevalence of AVF use further without improving incident AVF rates. At present the incident AVF rates in the United States remains very low at around 20% (Figure 2) [36]. One major challenge is that even among patients with adequate pre-ESRD nephrology care (documented care by a nephrologist >12 months prior to dialysis initiation), over 50% of these patients initiate dialysis with a CVC [70]. These results emphasize the challenges that nephrologists face when caring for patients with advanced CKD in regards to timing of vascular access referral. The major barriers faced at this stage in CKD care include patient denial of severity of kidney disease, lack of resources available to provide patient vascular access education and planning, inefficient and ineffective processes of care for evaluation and surgical referral for vascular access, and loss of patient follow-up [71].
Recent efforts, driven by the Renal Physician Association (RPA) in partnership with major stakeholders such as large dialysis organizations, has initiated a vascular access initiative to emphasize the role of the nephrologist in leading system change and engaging other major stakeholders (e.g. hospitals, Quality Improvement Organizations (QIOs) and vascular access surgeons) [71]. These collaborations have resulted in development of tools to address and improve ESRD vascular access care such as: (1) template letters to hospital CEOs, QIOs, and vascular access surgeons, (2) a detailed description of the role of nephrologist and dialysis providers within this initiative, and (3) guidance pertaining to patient education and surgical vascular access management for patients with advanced CKD [71]. Moreover, recently Medicare has also approved funding for patients with stage IV CKD education. This has provided a powerful resource for FFBI to implement pre-dialysis education and for the RPA to emphasize upon nephrologists to establish or refer patients to educational programs for preparation for dialysis and vascular access selection and surgery [71].
Despite the recent efforts by major stakeholders to address many of the barriers impacting pre-ESRD vascular access care, a major unmet need which needs to be resolved first before improvement in outcomes can occur is addressing the problem of fragmentation of pre-ESRD care. The main challenge is to develop a uniform process for referring patients to a nephrologist for CKD evaluation and subsequently a surgeon for vascular evaluation. One future solution to addressing fragmented care is development and utilization of a multidisciplinary care approach and multidisciplinary nephrology clinics.
Multidisciplinary Approach to Vascular Access Care
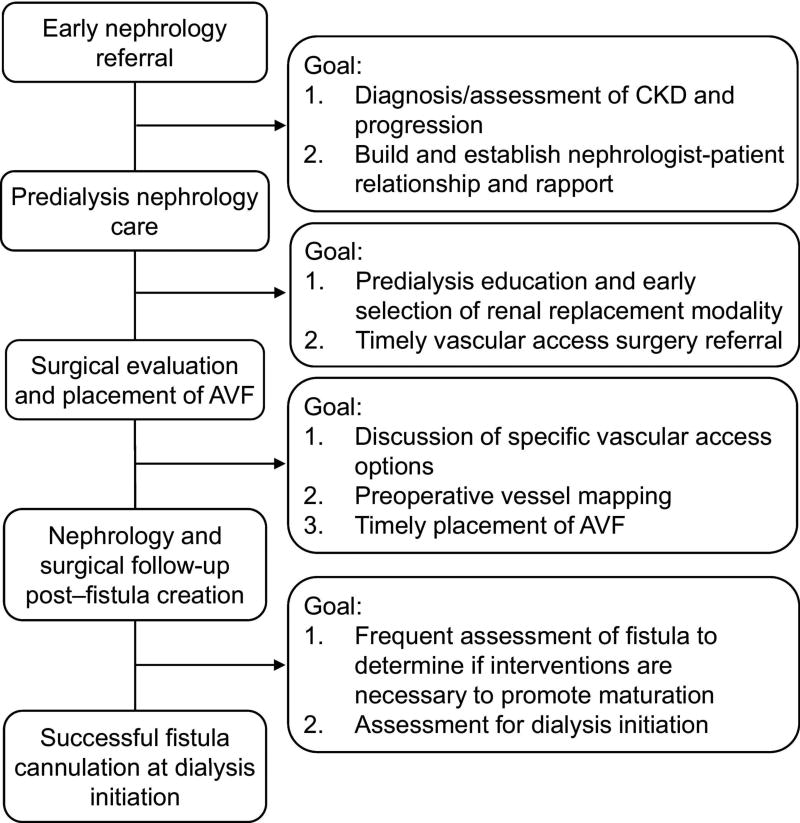
In order to successfully achieve a functional AVF, particularly in advanced CKD patients prior to initiating hemodialysis, several major processes need to occur [72–75] (Figure 3): (1) early referral to a nephrologist from a primary care provider for chronic kidney disease evaluation and management, (2) timely discussion with the CKD patient regarding future kidney replacement modalities, (3) referral to a vascular access surgeon for vascular access discussion and placement, and (4) close follow-up of maturing AVF with possible interventions for non-maturing AVFs. To achieve all of these processes requires a multidisciplinary approach involving collaboration between primary care physicians, nephrologists, vascular access surgeons, interventionalists, dialysis nurses, and vascular access coordinators. However, the nephrologist should play a central role in coordinating care for the advanced CKD and hemodialysis patient and function as the “Captain of the Ship”.
Figure 3. Process of Care Model to Achieve Successful AVF Use in Advanced Chronic Kidney Disease and Hemodialysis Patients.
All these individual processes will need to be met to achieve a functional AVF for cannulation. Any failure to achieve any of these processes will consign the patient to dialysis with a CVC. Adapted from reference 73 (Lee et al, Am J Kidney Dis. 2011 Jun;57(6):814–7) with permission from Elsevier.
The primary care physician must serve an active and vigilant role in screening for CKD and timely referral to a nephrologist for CKD evaluation and treatment. For the advanced CKD patient, late referral to a nephrologist negatively impacts timely placement of a vascular access [76].
The nephrologist must effectively serve as the leader of the vascular team, and assume responsibility and accountability in the efforts to educate hemodialysis patients about vascular access planning and timely placement of permanent vascular access. The role of the nephrologist in vascular access care includes [22]: (1) oversight of the multidisciplinary CQI team, (2) responsibility for ensuring educational and patient care related to vascular access in the dialysis facility, (3) ensuring selection and referrals to dedicated and experienced vascular access surgeons and interventionalists, and (4) ensuring coordinated care between the vascular access coordinator and dialysis nurse and dialysis unit.
Vascular access surgeries are being performed in advanced CKD and ESRD patients with many comorbidities, especially vascular-related comorbidities. Selection of surgeons who are experienced in creating all types of AVF (e.g. forearm, upper arm, and transpositions) and AVG (e.g. loop, straight, and immediate-use AVGs) is crucial for providing comprehensive surgical care for these complex dialysis patients. Furthermore, the quality of surgical training in vascular access procedures is critical for type of vascular access placement and successful development. Data from the Dialysis Outcomes and Practice Patterns Study from 12 international countries reported that Risk of primary AVF failure was 34% lower when performed by surgeons who created ≥25 (vs. <25) AVF during surgical training period [77]. Moreover, the most significant predictors of AVF versus AVG placement in hemodialysis patients was the number of AVFs placed during training and degree of emphasis on vascular access creation during training [77]. Recently, the FFBI has provided a number of workshops focused on surgeon education and training in vascular access and collaborated with the Society for Vascular Surgery to develop vascular access quality measures [78]. However, moving forward, a more concerted effort will need to be undertaken by major stakeholders (e.g. FFBI, dialysis organizations, Society of Vascular Access Surgery, National Kidney Foundation, American Society of Diagnostic and Interventional Nephrology, etc.) to increase the priority status of surgical vascular access care in the United States.
Given the high rate of vascular access dysfunction (e.g. AVF maturation failure, AVG stenosis, and CVC dysfunction) the interventionalist (interventional radiologist and/or interventional nephrologist) plays a critical role in maintenance and restoration of a patient’s dialysis access. Vascular access interventions commonly employed by interventionalist include vascular access education, vascular mapping, percutaneous balloon angioplasty, thrombectomy, intravascular coil and stent insertion, and tunneled hemodialysis catheter-related procedures. Interventional nephrology, a subspecialty of nephrology, may be the ideal specialty to provide interventional services, as nephrologists have an in depth perspective on kidney disease and dialysis access [79–81]. In the last 20 years interventional nephrologists have demonstrated an ability to provide effective, safe, and economical interventional vascular access management care to the dialysis patient population [82]. The challenge that remains in the future for the interventionalist is providing wider access of services, particularly to smaller rural communities, and coordinating services between vascular access surgeons, nephrologists, vascular access coordinators, and dialysis nurses.
The role of the dialysis nurse is critical in the care of the hemodialysis patient. The dialysis nurse has the unique position of being able to care for the hemodialysis patient thrice weekly during treatment. Thus, the dialysis nurse has the ability to examine and monitor both maturing accesses for development and detect problems in current vascular accesses that may result in failure of a vascular access. Moreover, the dialysis nurse plays a crucial role in cannulation of a vascular access. Complications related to cannulation, such as infiltration, have been reported to increase the risk of AVF thrombosis and CVC dependence. One major area of future improvement in the area of dialysis nursing in the United States will need to be the staffing of more experienced nurses in the dialysis unit. Unlike Japan and other European countries, in dialysis facilities in the United States, the ratio of Registered Nurse (RN) to patients is often 1:12 [74]. Moreover, a recent publication by Yoder et al., using data from the 2009 CMS ESRD Annual Facility Survey, reported that the ratios of RNs and licensed practical nurses to patients were 35% (p<0.001) and 42% (p<0.001) lower in for-profit facilities than those in nonprofit facilities, respectively, but the patient care technician-to-patient ratio was 16% (p<0.001) higher in for-profit facilities than those in nonprofit facilities [83]. Given that the vascular access is the hemodialysis patient’s lifeline, the major stakeholders in dialysis access care should focus more resources and efforts on quality assurance and performance improvement initiatives that maximize licensed nurse-staffing levels in hemodialysis facilities.
The vascular access coordinator is essential for organizing care for the hemodialysis patient. Close coordination of care among nephrologists, surgeons, interventionalists, the dialysis staff, and the patient is required to optimize vascular access outcomes, and can be expedited and overseen by having a dedicated access coordinator to streamline the process [84]. Polkinghorne et al reported substantial improvements, following incorporation of a vascular access coordinator , in AVF use and reduction in CVC use at dialysis initiation [85]. Incident AVF rates in their center increased from 56% to 75% and CVC rates decreased from 40% to 25% [85]. Dwyer et al. have reported that after the implementation of a comprehensive access program at their center led by a vascular access coordinator, the prevalent AVF rate increased from 50% to 65% [86]. Thus, every dialysis program should strongly consider incorporating a vascular access coordinator to facilitate and integrate a multidisciplinary approach to vascular access care.
Right Access for the Right Patient
While the AVF remains the gold standard for the majority of hemodialysis patients and CVC should be the least preferred vascular access in most circumstances, vascular access guidelines and quality initiatives do not adequately address or acknowledge the trade-offs involved in managing elderly patients, patients with multiple chronic comorbidities, patients with previously failed AVFs with prolonged catheter dependence, patients with limited life expectancy, or consider the value that the patient places in his/her vascular access in the context of quality of life. The goal and challenge for the multidisciplinary team of caregivers for dialysis patients is to assist patients with vascular access decision making based on an individualized assessment of risks and benefits and quality of life, while also factoring in the values, beliefs, and patient preferences [87]. In order for this paradigm change to be possible, our current outcome metrics, which focus on strict targets of incident and prevalent AVF, AVG, and CVC use, must allow for metrics to also evaluate patient preferences and quality of life [87].
Conclusion
The FFBI has largely been a very successful CQI project in the United States to address vascular access processes of care issues that has resulted in increased AVF prevalence. This initiative has been successful primarily through efforts to improve vascular access education, develop “change concepts” that provide a roadmap to implement vascular access recommendations and improve vascular access outcomes, and efforts to improve overall access to vascular access care. Due to the continued high rates of CVCs in patients initiating hemodialysis, there has been a recent shift in the in the FFBI to a “Fistula First-Catheter Last” approach. While recognizing the importance of achieving vascular access benchmarks, in the future, the vascular access community and stakeholders need to include a more patient-centered approach (“Right Access for the Right Patient”) that incorporates life expectancy, unnecessary surgical and interventional procedures, and emphasizes overall improvement in patient quality of life.
Acknowledgments
Funding:
Dr. Lee is supported by an American Society of Nephrology Carl W. Gottschalk Scholar Grant, University of Alabama at Birmingham Nephrology Research Center Anderson Innovation Award, University of Alabama at Birmingham Center for Clinical and Translational Science Multidisciplinary Pilot Award (1UL1TR001417-01), grant 1R43DK109789-01 from National Institutes of Diabetes, Digestive and Kidney Diseases (NIDDK) and grant 1I01BX003387-01A1 from a Veterans Affairs Merit Award.
Footnotes
Compliance with Ethical Standards
Ethical approval:
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of Interest:
Dr. Lee is a consultant for Proteon Therapeutics and Merck. Dr. Lee serves as a counselor for the American Society of Diagnostic and Interventional Nephrology and serves as a member of the National Kidney Foundation Kidney Disease Outcome Quality Initiative (K/DOQI) Guideline workgroup.
References
- 1. [Assessed May 19, 2011];Fistula First National Access Improvements Initiative. Available at: http://www.fistulafirst.org/
- 2.Lok CE. Fistula first initiative: advantages and pitfalls. Clinical journal of the American Society of Nephrology : CJASN. 2007;2(5):1043–53. doi: 10.2215/CJN.01080307. [DOI] [PubMed] [Google Scholar]
- 3.Lynch JR, et al. Achieving the goal of the Fistula First breakthrough initiative for prevalent maintenance hemodialysis patients. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2011;57(1):78–89. doi: 10.1053/j.ajkd.2010.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Kidney Foundation: DOQI Clinical Practice Guidelines for Vascular Access: Update 2000. Am J Kidney Dis. 2001;37(1):S137–S181. doi: 10.1016/s0272-6386(01)70007-8. [DOI] [PubMed] [Google Scholar]
- 5.Spergel LM. Has the Fistula First Breakthrough Initiative caused an increase in catheter prevalence? Semin Dial. 2008;21(6):550–2. doi: 10.1111/j.1525-139X.2008.00501.x. [DOI] [PubMed] [Google Scholar]
- 6.Quinton W, Dillard D, Scribner BH. Cannulation of blood vessels for prolonged hemodialysis. Trans Am Soc Artif Intern Organs. 1960;6:104–13. [PubMed] [Google Scholar]
- 7.Brescia MJ, et al. Chronic hemodialysis using venipuncture and a surgically created arteriovenous fistula. N Engl J Med. 1966;275(20):1089–92. doi: 10.1056/NEJM196611172752002. [DOI] [PubMed] [Google Scholar]
- 8.Jacob ET, Shapira Z, Boner G. Dacron arterio-venous interposition graft: an access to circulation in patients on chronic hemodialysis. Angiology. 1977;28(1):31–5. doi: 10.1177/000331977702800105. [DOI] [PubMed] [Google Scholar]
- 9.Johnson JM, Goldfarb D, Baker LD., Jr Expanded polytetrafluoroethylene as a small artery replacement. A preliminary report. Am J Surg. 1976;132(6):723–7. doi: 10.1016/0002-9610(76)90444-x. [DOI] [PubMed] [Google Scholar]
- 10.Baker LD, Jr, Johnson JM, Goldfarb D. Expanded polytetrafluoroethylene (PTFE) subcutaneous arteriovenous conduit: an improved vascular access for chronic hemodialysis. Trans Am Soc Artif Intern Organs. 1976;22:382–7. [PubMed] [Google Scholar]
- 11.Schwab SJ, et al. Prospective evaluation of a Dacron cuffed hemodialysis catheter for prolonged use. American journal of kidney diseases : the official journal of the National Kidney Foundation. 1988;11(2):166–9. doi: 10.1016/s0272-6386(88)80206-3. [DOI] [PubMed] [Google Scholar]
- 12.Moss AH, et al. Use of a silicone catheter with a Dacron cuff for dialysis short-term vascular access. Am J Kidney Dis. 1988;12(6):492–8. doi: 10.1016/s0272-6386(88)80100-8. [DOI] [PubMed] [Google Scholar]
- 13.Shusterman NH, Kloss K, Mullen JL. Successful use of double-lumen, silicone rubber catheters for permanent hemodialysis access. Kidney Int. 1989;35(3):887–90. doi: 10.1038/ki.1989.69. [DOI] [PubMed] [Google Scholar]
- 14.Gomes A, Schmidt R, Wish J. Re-envisioning Fistula First in a patient-centered culture. Clin J Am Soc Nephrol. 2013;8(10):1791–7. doi: 10.2215/CJN.03140313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.NKF-DOQI clinical practice guidelines for vascular access. National Kidney Foundation-Dialysis Outcomes Quality Initiative. Am J Kidney Dis. 1997;30(4 Suppl 3):S150–91. [PubMed] [Google Scholar]
- 16.Feldman HI, et al. Hemodialysis vascular access morbidity in the United States. Kidney Int. 1993;43(5):1091–6. doi: 10.1038/ki.1993.153. [DOI] [PubMed] [Google Scholar]
- 17.Feldman HI, Kobrin S, Wasserstein A. Hemodialysis vascular access morbidity. J Am Soc Nephrol. 1996;7(4):523–35. doi: 10.1681/ASN.V74523. [DOI] [PubMed] [Google Scholar]
- 18.Clinical Practice Guidelines for Vascular Access. Am J Kidney Dis. 2006;48:S176–S273. doi: 10.1053/j.ajkd.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 19.Ethier J, et al. Vascular access use and outcomes: an international perspective from the Dialysis Outcomes and Practice Patterns Study. Nephrol Dial Transplant. 2008;23(10):3219–26. doi: 10.1093/ndt/gfn261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. [Accessed December 9, 2016];Fistula First Catheter Last Initiative. Available at: http://esrdncc.org/ffcl/
- 21.Kinney R. 2005 Annual Report: ESRD Clinical Performance Measures Project. American Journal of Kidney Diseases. 2006;48:S1–S105. doi: 10.1053/j.ajkd.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 22.Vassalotti JA, et al. Fistula first breakthrough initiative: targeting catheter last in fistula first. Semin Dial. 2012;25(3):303–10. doi: 10.1111/j.1525-139X.2012.01069.x. [DOI] [PubMed] [Google Scholar]
- 23.Lacson E, Jr, et al. Change in vascular access and hospitalization risk in long-term hemodialysis patients. Clin J Am Soc Nephrol. 2010;5(11):1996–2003. doi: 10.2215/CJN.08961209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bradbury BD, et al. Conversion of vascular access type among incident hemodialysis patients: description and association with mortality. Am J Kidney Dis. 2009;53(5):804–14. doi: 10.1053/j.ajkd.2008.11.031. [DOI] [PubMed] [Google Scholar]
- 25.Allon M, et al. Effect of change in vascular access on patient mortality in hemodialysis patients. Am J Kidney Dis. 2006;47(3):469–77. doi: 10.1053/j.ajkd.2005.11.023. [DOI] [PubMed] [Google Scholar]
- 26.Collins AJ, et al. US Renal Data System 2010 Annual Data Report. Am J Kidney Dis. 2011;57(1 Suppl 1):A8, e1–526. doi: 10.1053/j.ajkd.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 27.Chan MR. Hemodialysis central venous catheter dysfunction. Semin Dial. 2008;21(6):516–21. doi: 10.1111/j.1525-139X.2008.00495.x. [DOI] [PubMed] [Google Scholar]
- 28.Agarwal AK. Central vein stenosis. Am J Kidney Dis. 2013;61(6):1001–15. doi: 10.1053/j.ajkd.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 29.Agarwal AK, Haddad NJ, Khabiri H. How should symptomatic central vein stenosis be managed in hemodialysis patients? Semin Dial. 2014;27(3):278–81. doi: 10.1111/sdi.12205. [DOI] [PubMed] [Google Scholar]
- 30.Dhingra RK, et al. Type of vascular access and mortality in U.S. hemodialysis patients. Kidney Int. 2001;60(4):1443–51. doi: 10.1046/j.1523-1755.2001.00947.x. [DOI] [PubMed] [Google Scholar]
- 31.Astor BC, et al. Type of vascular access and survival among incident hemodialysis patients: the Choices for Healthy Outcomes in Caring for ESRD (CHOICE) Study. J Am Soc Nephrol. 2005;16(5):1449–55. doi: 10.1681/ASN.2004090748. [DOI] [PubMed] [Google Scholar]
- 32.Dember LM, et al. Effect of Clopidogrel on Early Failure of Arteriovenous Fistulas for Hemodialysis: A Randomized Controlled Trial. JAMA. 2008;299(18):2164–2171. doi: 10.1001/jama.299.18.2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kalloo S, Blake PG, Wish J. A Patient-Centered Approach to Hemodialysis Vascular Access in the Era of Fistula First. Semin Dial. 2016;29(2):148–57. doi: 10.1111/sdi.12465. [DOI] [PubMed] [Google Scholar]
- 34.Dinwiddie L. "Eligibility" is key word in Fistula First Breakthrough Initiative in determining fistula use. Nephrol News Issues. 2006;20(9):39–40. [PubMed] [Google Scholar]
- 35.Lok CE. Fistula first initiative: advantages and pitfalls. Clin J Am Soc Nephrol. 2007;2(5):1043–53. doi: 10.2215/CJN.01080307. [DOI] [PubMed] [Google Scholar]
- 36.Saran R, et al. US Renal Data System 2014 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis. 2015;66(1 Suppl 1):Svii, S1–305. doi: 10.1053/j.ajkd.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prakash S, O'Hare AM. Interaction of aging and chronic kidney disease. Semin Nephrol. 2009;29(5):497–503. doi: 10.1016/j.semnephrol.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vachharajani TJ, et al. Elderly patients with CKD--dilemmas in dialysis therapy and vascular access. Nat Rev Nephrol. 2014;10(2):116–22. doi: 10.1038/nrneph.2013.256. [DOI] [PubMed] [Google Scholar]
- 39.Brown EA, Johansson L. Epidemiology and management of end-stage renal disease in the elderly. Nat Rev Nephrol. 2011;7(10):591–8. doi: 10.1038/nrneph.2011.113. [DOI] [PubMed] [Google Scholar]
- 40.Kurella M, et al. Octogenarians and nonagenarians starting dialysis in the United States. Ann Intern Med. 2007;146(3):177–83. doi: 10.7326/0003-4819-146-3-200702060-00006. [DOI] [PubMed] [Google Scholar]
- 41.Canaud B, et al. Clinical practices and outcomes in elderly hemodialysis patients: results from the Dialysis Outcomes and Practice Patterns Study (DOPPS) Clin J Am Soc Nephrol. 2011;6(7):1651–62. doi: 10.2215/CJN.03530410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Desilva RN, et al. Fistula first is not always the best strategy for the elderly. J Am Soc Nephrol. 2013;24(8):1297–304. doi: 10.1681/ASN.2012060632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yuo TH, et al. Patients started on hemodialysis with tunneled dialysis catheter have similar survival after arteriovenous fistula and arteriovenous graft creation. J Vasc Surg. 2015;62(6):1590–7. e2. doi: 10.1016/j.jvs.2015.07.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leake AE, et al. Arteriovenous grafts are associated with earlier catheter removal and fewer catheter days in the United States Renal Data System population. J Vasc Surg. 2015;62(1):123–7. doi: 10.1016/j.jvs.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 45.Lok CE, et al. Risk equation determining unsuccessful cannulation events and failure to maturation in arteriovenous fistulas (REDUCE FTM I) J Am Soc Nephrol. 2006;17(11):3204–12. doi: 10.1681/ASN.2006030190. [DOI] [PubMed] [Google Scholar]
- 46.Richardson AI, 2nd, et al. Should fistulas really be first in the elderly patient? The journal of vascular access. 2009;10(3):199–202. doi: 10.1177/112972980901000311. [DOI] [PubMed] [Google Scholar]
- 47.Lazarides MK, et al. A meta-analysis of dialysis access outcome in elderly patients. J Vasc Surg. 2007;45(2):420–426. doi: 10.1016/j.jvs.2006.10.035. [DOI] [PubMed] [Google Scholar]
- 48.Bay WH, Van Cleef S, Owens M. The hemodialysis access: preferences and concerns of patients, dialysis nurses and technicians, and physicians. Am J Nephrol. 1998;18(5):379–83. doi: 10.1159/000013380. [DOI] [PubMed] [Google Scholar]
- 49.Chaudhry M, et al. Seeing eye to eye: the key to reducing catheter use. The journal of vascular access. 2011;12(2):120–6. doi: 10.5301/jva.2011.6390. [DOI] [PubMed] [Google Scholar]
- 50.Quinn RR, et al. The Vascular Access Questionnaire: assessing patient-reported views of vascular access. J Vasc Access. 2008;9(2):122–8. [PubMed] [Google Scholar]
- 51.Xi W, et al. Patient attitudes towards the arteriovenous fistula: a qualitative study on vascular access decision making. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2011 doi: 10.1093/ndt/gfr055. [DOI] [PubMed] [Google Scholar]
- 52.Moist LM, et al. Optimal hemodialysis vascular access in the elderly patient. Semin Dial. 2012;25(6):640–8. doi: 10.1111/sdi.12037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Robbin ML, et al. US vascular mapping before hemodialysis access placement. Radiology. 2000;217(1):83–8. doi: 10.1148/radiology.217.1.r00oc2883. [DOI] [PubMed] [Google Scholar]
- 54.Allon M, et al. Effect of preoperative sonographic mapping on vascular access outcomes in hemodialysis patients. Kidney Int. 2001;60(5):2013–20. doi: 10.1046/j.1523-1755.2001.00031.x. [DOI] [PubMed] [Google Scholar]
- 55.Gibson KD, et al. Assessment of a policy to reduce placement of prosthetic hemodialysis access. Kidney Int. 2001;59(6):2335–45. doi: 10.1046/j.1523-1755.2001.00751.x. [DOI] [PubMed] [Google Scholar]
- 56.Silva MB, Jr, et al. A strategy for increasing use of autogenous hemodialysis access procedures: impact of preoperative noninvasive evaluation. J Vasc Surg. 1998;27(2):302–7. doi: 10.1016/s0741-5214(98)70360-x. discussion 307–8. [DOI] [PubMed] [Google Scholar]
- 57.Hakim RM, Himmelfarb J. Hemodialysis access failure: a call to action-revisited. Kidney Int. 2009 doi: 10.1038/ki.2009.318. [DOI] [PubMed] [Google Scholar]
- 58.Canadian Society of, N. Report of the canadian society of nephrology vascular access working group. Semin Dial. 2012;25(1):22–5. doi: 10.1111/j.1525-139X.2011.01009.x. [DOI] [PubMed] [Google Scholar]
- 59.Ohira S, et al. 2005 Japanese Society for Dialysis Therapy guidelines for vascular access construction and repair for chronic hemodialysis. Ther Apher Dial. 2006;10(5):449–62. doi: 10.1111/j.1744-9987.2006.00410.x. [DOI] [PubMed] [Google Scholar]
- 60.Tordoir J, et al. EBPG on Vascular Access. Nephrol Dial Transplant. 2007;22(Suppl 2):ii88–117. doi: 10.1093/ndt/gfm021. [DOI] [PubMed] [Google Scholar]
- 61.Hemmelgarn BR, et al. Progression of kidney dysfunction in the community-dwelling elderly. Kidney Int. 2006;69(12):2155–61. doi: 10.1038/sj.ki.5000270. [DOI] [PubMed] [Google Scholar]
- 62.O'Hare AM, et al. When to refer patients with chronic kidney disease for vascular access surgery: should age be a consideration? Kidney Int. 2007;71(6):555–61. doi: 10.1038/sj.ki.5002078. [DOI] [PubMed] [Google Scholar]
- 63.O'Hare AM, et al. Age affects outcomes in chronic kidney disease. J Am Soc Nephrol. 2007;18(10):2758–65. doi: 10.1681/ASN.2007040422. [DOI] [PubMed] [Google Scholar]
- 64.Shechter SM, Skandari MR, Zalunardo N. Timing of arteriovenous fistula creation in patients With CKD: a decision analysis. Am J Kidney Dis. 2014;63(1):95–103. doi: 10.1053/j.ajkd.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 65.Shingarev R, et al. Arteriovenous graft placement in predialysis patients: a potential catheter-sparing strategy. Am J Kidney Dis. 2011;58(2):243–7. doi: 10.1053/j.ajkd.2011.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chan R, et al. The effects of kidney-disease-related loss on long-term dialysis patients' depression and quality of life: positive affect as a mediator. Clin J Am Soc Nephrol. 2009;4(1):160–7. doi: 10.2215/CJN.01520308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kalantar-Zadeh K, et al. Association among SF36 quality of life measures and nutrition, hospitalization, and mortality in hemodialysis. J Am Soc Nephrol. 2001;12(12):2797–806. doi: 10.1681/ASN.V12122797. [DOI] [PubMed] [Google Scholar]
- 68.Afsar B, et al. Vascular access type, health-related quality of life, and depression in hemodialysis patients: a preliminary report. J Vasc Access. 2012;13(2):215–20. doi: 10.5301/jva.5000032. [DOI] [PubMed] [Google Scholar]
- 69.Kosa SD, Bhola C, Lok CE. Measuring patient satisfaction with vascular access: vascular access questionnaire development and reliability testing. J Vasc Access. 2015;16(3):200–5. doi: 10.5301/jva.5000339. [DOI] [PubMed] [Google Scholar]
- 70.Collins AJ, et al. US Renal Data System 2012 Annual Data Report. Am J Kidney Dis. 2013;61(1 Suppl 1):A7, e1–476. doi: 10.1053/j.ajkd.2012.11.031. [DOI] [PubMed] [Google Scholar]
- 71.Wish JB. Vascular access for dialysis in the United States: progress, hurdles, controversies, and the future. Semin Dial. 2010;23(6):614–8. doi: 10.1111/j.1525-139X.2010.00797.x. [DOI] [PubMed] [Google Scholar]
- 72.Lee T, Roy-Chaudhury P, Thakar CV. Improving incident fistula rates: a process of care issue. Am J Kidney Dis. 2011;57(6):814–7. doi: 10.1053/j.ajkd.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Allon M, et al. A multidisciplinary approach to hemodialysis access: prospective evaluation. Kidney Int. 1998;53(2):473–9. doi: 10.1046/j.1523-1755.1998.00761.x. [DOI] [PubMed] [Google Scholar]
- 74.Allon M. Fistula first: recent progress and ongoing challenges. Am J Kidney Dis. 2011;57(1):3–6. doi: 10.1053/j.ajkd.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Allon M, Robbin ML. Increasing arteriovenous fistulas in hemodialysis patients: problems and solutions. Kidney Int. 2002;62(4):1109–24. doi: 10.1111/j.1523-1755.2002.kid551.x. [DOI] [PubMed] [Google Scholar]
- 76.Astor BC, et al. Timing of nephrologist referral and arteriovenous access use: the CHOICE Study. Am J Kidney Dis. 2001;38(3):494–501. doi: 10.1053/ajkd.2001.26833. [DOI] [PubMed] [Google Scholar]
- 77.Saran R, et al. Enhanced training in vascular access creation predicts arteriovenous fistula placement and patency in hemodialysis patients: results from the Dialysis Outcomes and Practice Patterns Study. Ann Surg. 2008;247(5):885–91. doi: 10.1097/SLA.0b013e31816c4044. [DOI] [PubMed] [Google Scholar]
- 78.Sidawy AN, et al. The Society for Vascular Surgery: clinical practice guidelines for the surgical placement and maintenance of arteriovenous hemodialysis access. J Vasc Surg. 2008;48(5 Suppl):2S–25S. doi: 10.1016/j.jvs.2008.08.042. [DOI] [PubMed] [Google Scholar]
- 79.Beathard GA, Litchfield T I. Physician Operators Forum of Rms Lifeline. Effectiveness and safety of dialysis vascular access procedures performed by interventional nephrologists. Kidney Int. 2004;66(4):1622–32. doi: 10.1111/j.1523-1755.2004.00928.x. [DOI] [PubMed] [Google Scholar]
- 80.Asif A, et al. Interventional nephrology: from episodic to coordinated vascular access care. J Nephrol. 2007;20(4):399–405. [PubMed] [Google Scholar]
- 81.Vachharajani TJ, et al. Dialysis vascular access management by interventional nephrology programs at University Medical Centers in the United States. Semin Dial. 2011;24(5):564–9. doi: 10.1111/j.1525-139X.2011.00985.x. [DOI] [PubMed] [Google Scholar]
- 82.Beathard GA. Role of interventional nephrology in the multidisciplinary approach to hemodialysis vascular access care. Kidney Res Clin Pract. 2015;34(3):125–31. doi: 10.1016/j.krcp.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yoder LA, et al. Patient care staffing levels and facility characteristics in U.S. hemodialysis facilities. Am J Kidney Dis. 2013;62(6):1130–40. doi: 10.1053/j.ajkd.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Allon M. Current management of vascular access. Clin J Am Soc Nephrol. 2007;2(4):786–800. doi: 10.2215/CJN.00860207. [DOI] [PubMed] [Google Scholar]
- 85.Polkinghorne KR, Seneviratne M, Kerr PG. Effect of a vascular access nurse coordinator to reduce central venous catheter use in incident hemodialysis patients: a quality improvement report. Am J Kidney Dis. 2009;53(1):99–106. doi: 10.1053/j.ajkd.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 86.Dwyer A, et al. A vascular access coordinator improves the prevalent fistula rate. Semin Dial. 2012;25(2):239–43. doi: 10.1111/j.1525-139X.2011.00961.x. [DOI] [PubMed] [Google Scholar]
- 87.Moist LM, Al-Jaishi AA. Preparation of the Dialysis Access in Stages 4 and 5 CKD. Adv Chronic Kidney Dis. 2016;23(4):270–5. doi: 10.1053/j.ackd.2016.04.001. [DOI] [PubMed] [Google Scholar]