Abstract
The centromere directs chromosome segregation and genetic inheritance but is not itself heritable in a canonical, DNA-based manner. In most species, centromeres are epigenetically defined by the presence of a histone H3 variant CENP-A (Centromere Protein A), independent of underlying DNA sequence. Therefore, centromere inheritance depends on maintaining the CENP-A nucleosome mark across generations. Experiments in cycling somatic cells have led to a model in which centromere identity is maintained by a cell cycle-coupled CENP-A chromatin assembly pathway. However, the processes of animal gametogenesis pose unique challenges to centromere inheritance because of the extended cell cycle arrest and the massive genome reorganization in the female and male germline respectively. Here, we review our current understanding of germline centromere inheritance and highlight outstanding questions.
Keywords: Germline, centromere, inheritance, CENP-A
Introduction
The Epigenetic Nature of Centromere Identity
Centromeres direct chromosome segregation and therefore must be inherited with each chromosome through every cell cycle. Most eukaryotic models of centromere inheritance propose that propagation of chromatin-assembled CENP-A nucleosomes allows for epigenetic inheritance of centromere identity, independent of underlying DNA sequence (Allshire and Karpen 2008; Black and Cleveland 2011). However, centromeres are typically associated with characteristic DNA sequences. The first centromere ever isolated came from budding yeast (S. cerevisiae), where plasmids containing a centromeric DNA sequence persisted for multiple cell cycles and segregated normally in meiosis (Clarke and Carbon 1980). Indeed, in budding yeast a 125 bp DNA sequence is necessary and sufficient for centromere specification (Fitzgerald-Hayes et al. 1982; Panzeri and Philippsen 1982; Bloom and Carbon 1982; Saunders et al. 1988), an example of a ‘point’ centromere (Pluta et al. 1995). Centromere DNA is highly diverged and is typically composed of repetitive or a mixture of repetitive and non-repetitive sequences (Locke et al. 2003; Piras et al. 2010; Shang et al. 2010), termed ‘regional’ centromeres. Regional centromeres in humans, for example, contain up to 5 Mb of 171 bp long alpha-satellite repeats (Waye and Willard 1987). Centromeres can also extend to the entire chromosome length in the case of holocentromeres, which have arisen independently multiple times during the evolution of plants and animals (Mola and Papeschi 2006), for example in C. elegans, some protozoans, some insects, green algae, and certain plants (Guerra et al. 2010; Melters et al. 2013).
The first evidence for epigenetic specification of centromeres came from the analysis of human patient samples, which revealed the inactivation of one centromere of a dicentric chromosome (Earnshaw and Migeon 1985) and the formation of neocentromeres (Choo 1997). Neocentromeres are ectopic centromeres on complex DNA sequences (i.e. not repetitive DNA) that have arisen in rare cases when a chromosome fragment is removed from its natural centromere by chromosome rearrangement, or in even rarer cases when CENP-A nucleosomes migrate from their original location in repetitive centromeric chromatin DNA to a new location on the chromosome that does not contain any DNA repeats (Choo 1997; Depinet et al. 1997; Barry et al. 1999; Scott and Sullivan 2014). Such neocentromeres were discovered in various organisms (Marshall et al. 2008; Guerra et al. 2010; Burrack and Berman 2012; Scott and Sullivan 2014) and can be inherited in mitosis and meiosis through at least three generations (Tyler-Smith et al. 1999; Amor et al. 2004). Further, it does not appear that DNA at neocentromeres must evolve to become more repetitive in order to maintain centromeres (Barry et al. 1999). These observations suggest that the typical centromere DNA sequences are neither necessary nor sufficient for centromere specification and that centromere inheritance is epigenetic and conferred by the presence of CENP-A nucleosomes. In fact, targeting to non-centromeric chromatin containing a lac operator array via fusion of the lac repressor protein to CENP-A or its chaperone HJURP is sufficient for the formation of functional centromeres in flies and human, and can recruit other kinetochore proteins such as CENP-C and HEC1 (Mendiburo et al. 2011; Barnhart et al. 2011). Notable exceptions to the requirement for CENP-A are kinetoplastids that do not possess CENP-A protein and rely instead on a set of unconserved centromere proteins (Lowell and Cross 2004; Berriman et al. 2005; Akiyoshi and Gull 2014) and at least two conserved outer kinetochore components, NUF2 and NDC80 (D’Archivio and Wickstead 2016), but it is still unclear how the kinetochore assembly site is determined in these species (Akiyoshi and Gull 2014). However, taken together, most studies support the centrality of CENP-A nucleosomes in specifying and inheriting the centromere through multiple generations in most organisms.
The Challenge of Inheriting Centromeres through the Germline
Accurate and quantitative inheritance of genetic information is achieved by replicating DNA and then partitioning it equally into daughter cells. DNA is a stable molecule, which enables long term storage of genetic information without decay. Because centromeres are specified epigenetically in most species by the presence of CENP-A nucleosomes, centromere inheritance through the germline requires the maintenance of these nucleosomes through gametogenesis. Mechanisms of CENP-A chromatin assembly and propagation are well established in somatic cells and have been extensively reviewed elsewhere (Erhardt et al. 2008; Falk and Black 2012; De Rop et al. 2012; Chen and Mellone 2016). In short, existing CENP-A is partitioned equally between sisters during DNA replication in S-phase and then replenished in G1, upon exit from mitosis (Schuh et al. 2007; Jansen et al. 2007; Lagana et al. 2010). Both male and female germlines pose challenges for this inheritance pathway. A common feature of oocyte development is an arrest phase, which is most dramatic in mammals where prophase I can last for months (mice) or decades (humans) without detectable assembly of new CENP-A chromatin (Smoak et al. 2016) (Fig.1a, b). In spermatogenesis nearly all histones are replaced by very small arginine-rich basic proteins called protamines during the chromatin-to-nucleoprotamine transition to compact DNA (Fig.1c) (Gaucher et al. 2010; Rathke et al. 2014; Bao and Bedford 2016). CENP-A nucleosomes are maintained through both the prophase I arrest in mammalian oogenesis and the widespread histone replacement in spermatogenesis, but the underlying mechanisms remain unclear.
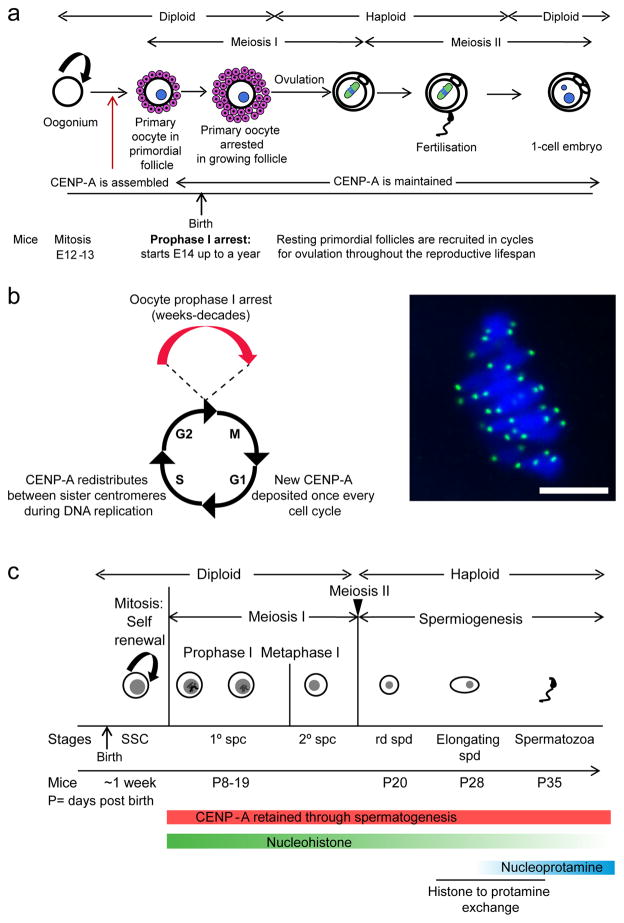
Fig. 1. Assembly and retention of CENP-A nucleosomes in mammalian gametogenesis.
a) Schematic showing mammalian oogenesis: Fetal oogonium cells proliferate to form primary oocytes which enter meiosis I and are arrested in prophase I prior to birth. These primary oocytes represent the entire ovarian reserve from which oocytes are ovulated during the reproductive lifespan of the female. The duration of the prophase I arrest varies (~months in mice and years in humans). Meiotic resumption occurs when a primary oocyte enters meiosis II to form the secondary oocyte which is then ovulated and arrests in metaphase II until fertilization. CENP-A nucleosomes are assembled prior to prophase I arrest and remains at centromeres for this entire process. b) Centromere inheritance is tightly coupled to the cell cycle in cycling somatic cells. CENP-A nucleosomes are evenly partitioned between sister centromeres during S-phase, effectively diluting the amount at each centromere by 50%. In most systems studied, CENP-A is replenished only once per cell cycle, in a strictly controlled manner. Oocytes arrest in prophase I for weeks to decades in mammals, and it is unclear how CENP-A nucleosomes persist at the centromere during this time. An image of a wild type mouse oocyte stained for CENP-A (green) and DNA (blue) at metaphase I. CENP-A loaded prior to meiosis is maintained even in aged oocytes, indicating that CENP-A nucleosomes demonstrate an unusual stability in the germline compared to canonical H3 nucleosomes (Smoak et al. 2016). c) Schematic showing the first wave of spermatogenesis in mammals. Spermatogenic stem cells (SSC) undergo proliferation to generate more precursors and primary spermatocytes that are committed to meiosis. Primary spermatocytes undergo meiosis I and form secondary spermatocytes. These complete meiosis II to form haploid round spermatids (rd spd), beginning the haploid phase of spermatogenesis called spermiogenesis. Histone-to-protamine exchange initiates ~28 days post birth in mice, and most histones except CENP-A nucleosomes are replaced by protamines in mature sperm. Sperm is continually produced by waves of spermatogenesis during the lifespan of the organism.
Although CENP-A is essential for defining centromeric chromatin in most eukaryotes, there are four known insect lineages that have independently transitioned from monocentricity to holocentricity (Drinnenberg et al. 2014). This transition has led to the genomic loss of CENP-A and CENP-C while outer kinetochore proteins NDC80 or MIS12 remained (Drinnenberg et al. 2014). These holocentric insects therefore have a centromere not defined by CENP-A, thus differing from other eukaryotes studied to date. In C. elegans, a holocentric nematode, CENP-A is present in mitotic cell cycles but not during gametogenesis (Monen et al. 2005), and different mechanisms designate microtubule attachment sites and coordinate the two step loss of cohesion required for faithful segregation of chromosomes during meiosis (Nabeshima et al. 2005; Monen et al. 2005; Cabral et al. 2014). However, CENP-A is reloaded onto chromatin where there is no germline transcription and CENP-A nucleosomes are required for mitotic divisions, indicating that although CENP-A may be dispensable for chromosome segregation in the germline, CENP-A chromatin is required for somatic cell cycles (Monen et al. 2005; Gassmann et al. 2012).
Germline CENP-A assembly: deviations from mitotic cells
Several lines of evidence suggest that germline CENP-A chromatin assembly does not follow the somatic cell model. In contrast to G1 CENP-A chromatin assembly in mitotic cell cycles, biphasic deposition is observed in the male germline, for example in prophase I and at exit from MII in Drosophila, and during pre-meiotic G2 and interkinesis in higher plants (Raychaudhuri et al. 2012; Dunleavy et al. 2012; Schubert et al. 2014) (Fig. 2). However, similar to somatic cells, nascent CENP-A chromatin assembly in the germline occurs when CDK activity is expected to be low, which is known to promote CENP-A assembly in somatic cells (Silva et al. 2012). Drosophila CENP-A (CID) nucleosomes are assembled throughout prophase of meiosis I in oocytes (Raychaudhuri et al. 2012; Dunleavy et al. 2012). However in mouse oocytes, there is no evidence of a CENP-A chromatin assembly pathway during prophase I or later in meiosis, and nascent CENP-A chromatin assembly likely occurs only during pre-meiotic G1 (Smoak et al. 2016). Taken together, the available evidence suggests that CENP-A chromatin assembly follows a pattern of a single deposition phase in the fruit fly and mouse female germline, and deposits in a biphasic pattern in fruit fly male germline and plants. Nascent CENP-A chromatin assembly still remains to be investigated in the male germline in mammals. Although the reason behind these differences is unclear, it does appear that CENP-A chromatin assembly has been differentially adapted in the germline, compared to somatic cells, possibly to account for varied challenges in gametogenesis.
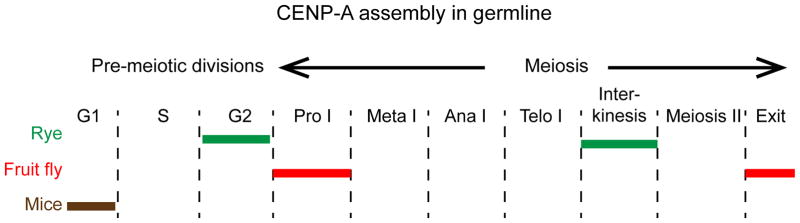
Fig. 2. Timing of CENP-A deposition in the germline in different species.
Schematic shows the stages of germline divisions with CENP-A loading in different organisms indicated with colored bars. In the monocotyledonous plant rye, CENP-A shows biphasic centromere chromatin assembly in G2 and interkinesis (Schubert et al. 2014). In fruit flies CENP-A (CID) gradually assembles at centromeres in prophase I (Raychaudhuri et al. 2012; Dunleavy et al. 2012), and males have a second loading phase similar to plants after exit from meiosis II. In mouse, CENP-A centromere chromatin assembly in the female germline likely occurs pre-meiotically, and the same population of CENP-A lasts through both meiotic divisions (Smoak et al. 2016).
Quantitative inheritance of CENP-A nucleosomes is another challenge for the germline, so that centromere chromatin is maintained at consistent levels across generations. Assembly of CENP-A chromatin could be template dependent, where existing CENP-A nucleosomes dictate the deposition of an equal number of new CENP-A nucleosomes. Alternatively a fixed number of CENP-A nucleosomes could assemble independent of the initial template size. To distinguish between these models in the Drosophila male germline, cid mutant flies were rescued with a Cid-GFP transgene, and Cid-GFP protein was reduced to ~33% by RNAi in sperm using a germline specific promoter (Raychaudhuri et al. 2012). These flies were crossed to Cid-GFP females to restore cid expression in the progeny. Cid-GFP levels at centromeres were reduced to ~72% in the embryos, wing imaginal discs and mature sperm in the progeny (Raychaudhuri et al. 2012). This result is consistent with template dependent centromere inheritance given that only one parent was reduced. This model also makes two additional predictions: (1) the Y chromosome should remain at 33%, similar to the male parent, and (2) paternally inherited centromeres should have less Cid-GFP than maternally inherited centromeres. However, analysis of spermatocytes showed reduction to 75% on the Y, similar to other chromosomes, and did not reveal differences between homologous autosomes to any greater extent than in controls (Raychaudhuri et al. 2012). Overall, there is some evidence for a template dependent model, but mechanisms of quantitative centromere inheritance remain unclear, and have not been investigated in mammals where there are differences in meiotic CENP-A chromatin assembly compared to Drosophila.
Assembly of CENP-A chromatin requires specific chaperones (HJURP in human, Scm3 in budding yeast and CAL1 in Drosophila) to differentiate this low abundance variant from bulk histone H3 (Mizuguchi et al. 2007; Stoler et al. 2007; Camahort et al. 2007; Foltz et al. 2009; Dunleavy et al. 2012). Contribution of such chaperones to germline transmission of CENP-A has not been studied in vertebrates, but Drosophila CAL1 is required for CENP-A assembly both in spermatocytes and oocytes (Raychaudhuri et al. 2012; Dunleavy et al. 2012; Kwenda et al. 2016). Thus, the requirement for a chaperone is similar in somatic and germline cell cycles.
Centromere inheritance: Spermatogenesis
Retention of CENP-A through spermatogenesis, while other histones are almost completely removed (Fig. 1c), played an important role historically. CENP-A was initially identified, along with CENP-B and CENP-C, using the sera of patients with CREST syndrome (Earnshaw and Rothfield 1985; Valdivia and Brinkley 1985; Earnshaw 2015). The first evidence that CENP-A was a specialized histone was its co-purification with core histones H3 and H4 (Palmer et al. 1987). The subsequent purification of CENP-A protein to homogeneity took advantage of the observation that it survives the chromatin-to-nucleoprotamine transition in bull sperm, despite the removal of nearly all other histones (Fig. 3b, c) (Palmer et al. 1990). Some canonical nucleosomes survive the transition and remain chromatin bound, but the function of these remaining histones at locations throughout the genome and whether their retention is part of a regulatory mechanism in early embryogenesis, or simply random evasion of the protamine-exchange machinery, is still debated (Hammoud et al. 2009; Brykczynska et al. 2010; Meyer-Ficca et al. 2013; Erkek et al. 2013; van de Werken et al. 2014; Samans et al. 2014). Immunoblotting of acid soluble proteins showed that CENP-A levels in bull sperm nuclei (Palmer et al. 1990) were comparable to calf thymus nuclei or human tissue culture nuclei relative to DNA, suggesting that CENP-A is completely retained through spermiogenesis (Fig. 3d). The high concentration of CENP-A nucleosomes in mature sperm relative to other histones facilitated CENP-A purification and partial sequencing through fragmentation and degradation. This analysis showed homology to histone H3, leading to the first proposal that it was a histone H3 variant (Fig. 3a; Palmer et al. 1991). Indeed, its subsequent cloning revealed that CENP-A contains a histone fold domain most similar to histone H3 (Sullivan et al. 1994).
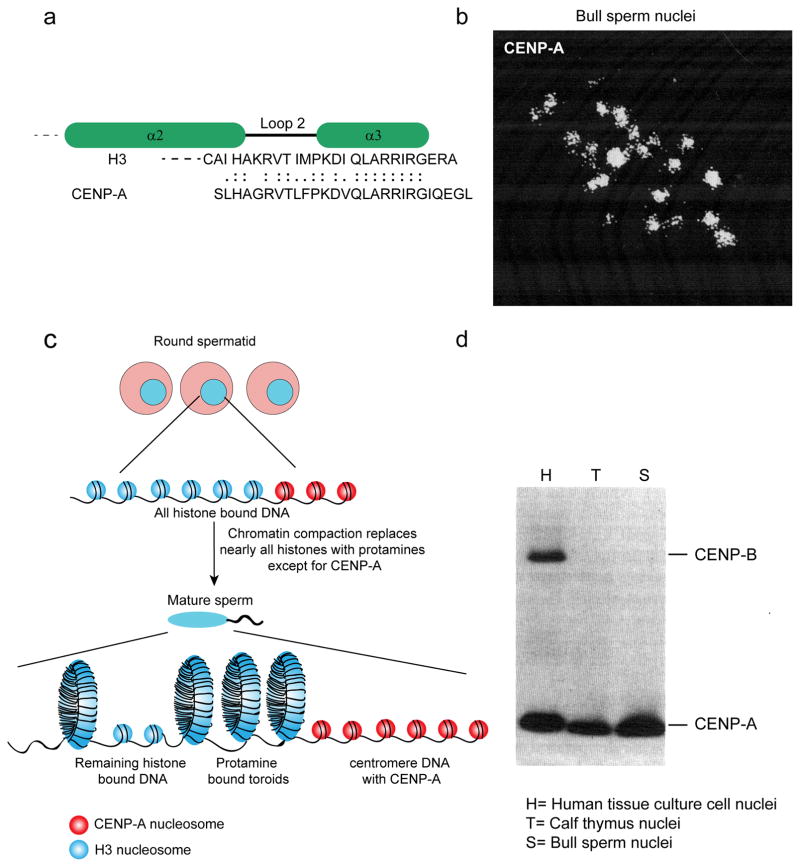
Fig. 3. CENP-A retention in sperm allowed for purification of CENP-A and its identification as a histone.
a) Sequence alignment showing homology of 27 amino acid residues of CENP-A and H3. The sequence of these 27 amino acids was obtained using a gas phase sequenator to sequence peptides derived from digestion of CENP-A purified from bull sperm (Palmer et al. 1991). These peptides showed >50% sequence identity to bovine H3, most strikingly at the C-terminus across loop 2 and the α3 helix as shown. Identical residues are indicated by double dots; conservative substitutions by single dots. b) Immunofluorescence visualization of bull sperm nuclei using anticentromere serum revealed punctate foci of CENP-A (Palmer et al. 1990) c) During the transition from round spermatid to elongating/condensing spermatids, there is a drastic reorganization of the sperm genome. Nearly all canonical histones are replaced by protamines to form toroidal DNA structures, except for centromeric CENP-A bound DNA (adapted from Schagdarsurengin et al. 2012). d) The original immunoblot analysis using anti-centromere antibodies (ACA) isolated from patients with CREST syndrome compared nuclei isolated from human tissues culture cells, calf thymus cells, and bull sperm (Palmer et al. 1990). Nuclei from mature bull sperm contain similar amounts of CENP-A as other cell types. This result confirms that the foci shown in panel B are centromeres and suggests that CENP-A is quantitatively retained through spermatogenesis, unlike other histones. CENP-B was not detected in either whole calf thymus or bull sperm with this serum.
There are still several unanswered questions which stem from these early studies on CENP-A retention in the male germline. How does sperm deal with both protamine and CENP-A nucleosome bound chromatin? Protamines allow chromatin to adopt a flatter, toroidal shape as opposed to nucleosome wrapped chromatin, so it is likely that chromatin architecture is different at the centromere than at other chromatin loci in mature sperm. After fertilization, sperm chromatin decondenses, protamines are replaced by histones, and other centromere proteins are imported into the pronucleus from the ooplasm (McLay and Clarke 2003). In round spermatids, centromeric and peri-centromeric chromatin cluster together to form the chromocenter (Zalensky et al. 1993; Zalensky et al. 1995; Gurevitch et al. 2001). Whether specific chromosome arrangements are functionally necessary in sperm is unclear. These early studies also emphasize the key question of how CENP-A nucleosomes are preferentially retained in sperm chromatin while most other canonical histones are lost, for which we put forth a hypothesis later in this review.
Centromere inheritance: Oogenesis
Mammalian oogenesis presents a challenge for centromere inheritance in the female germline because of the extended prophase I arrest, which can last for a period of a few weeks to decades depending on species. In mouse, CENP-A is stably retained at centromeres during the arrest, with no detectable new loading for the reproductive lifespan of the animal. Mice with an oocyte specific conditional knockout of CENP-A early in the prophase I arrest are fully fertile with wild type levels of CENP-A at oocyte centromeres. These results indicate that in the absence of new CENP-A synthesis, centromeric chromatin assembled prior to meiotic entry is sufficient for centromere function more than 1 year later and transmission to the next generation (Smoak et al. 2016, Fig 4, 3). This stability of CENP-A nucleosomes does not extend to other histones, as deposition of histone H3.3 during prophase I is required for normal chromatin structure and oocyte survival in mouse (Nashun et al. 2015; Tang et al. 2015). Mechanisms of centromere inheritance vary, however, in other organisms. For example, Drosophila oocytes have a meiotic prophase I loading pathway (Dunleavy et al. 2012; Kwenda et al. 2016), and in holocentric C. elegans centromere inheritance through the female germline is completely CENP-A independent (Fig. 4, 1) (Monen et al. 2005; Gassmann et al. 2012).
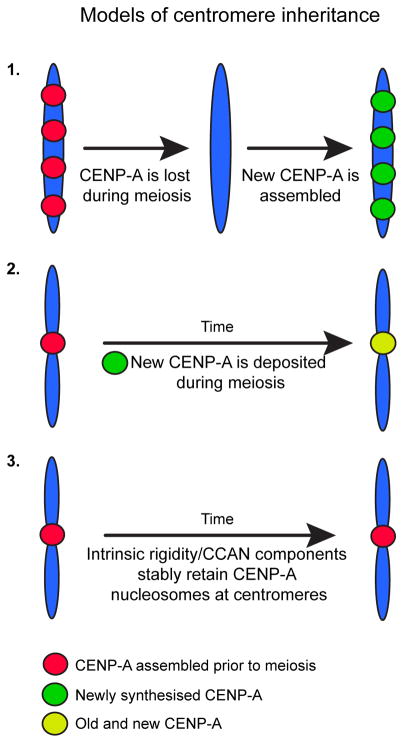
Fig. 4. Models for centromere inheritance through the germline.
Three models have been proposed to explain centromere inheritance in the female germline. Model 1 is that centromere inheritance is CENP-A independent, as shown in C. elegans (Monen et al. 2005). Model 2 invokes nascent CENP-A chromatin assembly to maintain centromeres (Dunleavy et al. 2012). Model 3 states that centromere inheritance depends on the intrinsic rigidity of CENP-A nucleosomes and interactions with other CCAN components, which maintains these nucleosomes at the centromere throughout oogenesis (Sekulic et al. 2010; Falk et al. 2015; Smoak et al. 2016).
Models for CENP-A stability in the germline
Mechanisms underlying the remarkable retention of CENP-A through both spermatogenesis and oogenesis are unknown. We propose that the intrinsic structural rigidity of CENP-A nucleosomes maintains them at centromeres through gametogenesis. Solution biophysical and high-resolution structural analyses of CENP-A revealed intrinsic differences in the internal dynamics of the (CENP-A/H4)2 tetramer compared to the (H3/H4)2 tetramer (Black et al. 2004; Sekulic et al. 2010). The interface of CENP-A with its partner histone, H4, contains hydrophobic stitches that lend conformational rigidity to CENP-A nucleosomes (Sekulic et al. 2010). This rigidity may create a stable nucleosome that survives both prophase I arrest and histone replacement. Indeed, mutation of the six amino acid residues that generate the stitches, to the counterpart residues found in conventional histone H3, greatly reduces its accumulation at centromeres in somatic cells while not affecting interactions with HJURP (Bassett et al 2012). The importance of the specific hydrophobic residues at the CENP-A/H4 or H3/H4 interface is also borne out by studies on the testis specific histone H3.5, which forms an unstable nucleosome attributable to a the presence of a Leu103 residue instead of the phenylalanine usually present in canonical H3 or CENP-A at the corresponding contact site with H4 (Tachiwana et al. 2010; Schenk et al. 2011; Urahama et al. 2016).
Non-histone centromere proteins also contribute extrinsically to CENP-A retention. CENP-A nucleosomes recruit the constitutive centromere associated network (CCAN), a collection of ~16 proteins that localize to the centromere throughout the cell-cycle and direct kinetochore assembly during cell division (Cheeseman and Desai 2008; Perpelescu and Fukagawa 2011; Hori et al. 2012). Two of these proteins, CENP-C and CENP-N, contact CENP-A nucleosomes directly (Carroll et al. 2009; Guse et al. 2011; Kato et al. 2013), and CENP-C can reshape CENP-A nucleosomes and plays an important role in retaining CENP-A at the centromere (Falk et al. 2015; Falk et al. 2016). Indeed, a single point mutation of the nucleosome interaction surface of CENP-C retains binding to CENP-A but eliminates structural stability and hinders its ability to retain CENP-A nucleosomes at the centromere (Guo et al. 2017). CENP-C and CENP-S have also been shown to be important for resisting unfolding of centromeric chromatin in low ionic strength solutions (Vargiu et al. 2017). In addition, flies with impaired CENP-C function have reduced CENP-A at centromeres in spermatids, indicating an extrinsic mechanism for maintaining CENP-A during early meiosis in males (Kwenda et al. 2016). However, since CENP-C protein was not detected in Drosophila or Xenopus sperm (Milks et al. 2009; Raychaudhuri et al. 2012), the mechanism of retaining CENP-A nucleosomes through the genome wide histone-protamine exchange in sperm is still unclear. CENP-N crosslinks CENP-A to nucleosomal DNA and also contributes strongly to CENP-A stability at centromeres (Guo et al. 2017), and it is not yet clear whether or not it is present on sperm chromatin.
In addition there are meiosis specific proteins that ensure that sister kinetochores are co-oriented in meiosis I. In mice for example, the protein MEIKIN is required for co-orientation and present on chromosomes through the prophase arrest in oocytes (Kim et al. 2015), but its contribution to stabilizing CENP-A needs further investigation.
Conclusion
Much progress has been made towards understanding how centromere identity is maintained and transmitted through somatic cell cycles. However, many gaps exist in our understanding of whether and how these processes are different in the germline. The role of CENP-A nucleosomes in maintaining centromere identity is further demonstrated by the induction of haploids in plants by altered centromeric function (Ravi et al. 2011). In a cross between a wild type plant and an Arabidopsis cenh3 mutant complemented by a chimeric CENP-ACENH3 transgene, chromosomes from the mutant parent are lost in the progeny (Ravi and Chan 2010; Ravi et al. 2014). Loss of CENP-ACENH3 also precedes chromosome elimination in interspecific barley crosses, supporting the idea that variation in centromeric histones results in interspecific incompatibility and haploid induction (Sanei et al. 2011).
Inherent structural features of CENP-A nucleosomes may contribute to their retention in both the male and female germlines. Thus, there may exist a simple unified mechanism for centromere inheritance. Going forward it will be important to investigate the significance of the extraordinary stability exhibited by CENP-A nucleosomes in the germline as loss of these nucleosomes in aged oocytes where CENP-A stability is somehow compromised may cause aneuploidy. A parallel situation might be where the loss of cohesins during the extended prophase I arrest in mammalian oocytes can lead to age associated aneuploidy (Chiang et al. 2010; Lister et al. 2010; Chiang et al. 2011; Chiang et al. 2012), and the rate of cohesin loss is almost certainly influenced by the genetics of the individual. In addition, the amount of CENP-A at a centromere can influence which chromosome from a homologous pair is destined for the egg versus the polar body during the asymmetric division in MI (Chmátal et al. 2014; Iwata-Otsubo et al. 2017, in press), and it is not yet clear how CENP-A retention (or lack of it) could influence the ability of a chromosome to “drive” in female meiosis. Taken together, the implications for future centromere studies in the germline are broad, with their impact to be felt in areas as diverse as human reproductive biology, molecular mechanisms of epigenetic processes, and eukaryotic evolution.
Acknowledgments
We acknowledge the members of the Black and Lampson labs and Richard M. Schultz for helpful comments on the manuscript. We thank R. Margolis (Sanford-Burnham Institute) for allowing us to reproduce data from published papers. We apologize to any authors whose work we were unable to cite.
Footnotes
Compliance with ethical standards:
Conflict of Interest: Writing of this review was supported by National Institutes of Health grant to HD058730 (to M.A.L., and B.E.B.). The authors declare that they have no conflict of interest.
Ethical approval: All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
References
- Akiyoshi B, Gull K. Discovery of unconventional kinetochores in kinetoplastids. Cell. 2014;156:1247–58. doi: 10.1016/j.cell.2014.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allshire RC, Karpen GH. Epigenetic regulation of centromeric chromatin: old dogs, new tricks? Nat Rev Genet. 2008;9:923–937. doi: 10.1038/nrg2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amor DJ, Bentley K, Ryan J, et al. Human centromere repositioning “in progress. Proc Natl Acad Sci. 2004;101:6542–6547. doi: 10.1073/pnas.0308637101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao J, Bedford MT. Epigenetic regulation of the histone-to-protamine transition during spermiogenesis. Reproduction. 2016;151:R55–70. doi: 10.1530/REP-15-0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnhart MC, Kuich PHJL, Stellfox ME, et al. HJURP is a CENP-A chromatin assembly factor sufficient to form a functional de novo kinetochore. J Cell Biol. 2011;194:229–43. doi: 10.1083/jcb.201012017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry AE, Howman EV, Cancilla MR, et al. Sequence analysis of an 80 kb human neocentromere. Hum Mol Genet. 1999;8:217–227. doi: 10.1093/hmg/8.2.217. [DOI] [PubMed] [Google Scholar]
- Berriman M, Ghedin E, Hertz-Fowler C, et al. The genome of the African trypanosome Trypanosoma brucei. Science. 2005;309:416–22. doi: 10.1126/science.1112642. [DOI] [PubMed] [Google Scholar]
- Black BE, Cleveland DW. Epigenetic centromere propagation and the nature of CENP-A nucleosomes. Cell. 2011;144:471–479. doi: 10.1016/j.cell.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black BE, Foltz DR, Chakravarthy S, et al. Structural determinants for generating centromeric chromatin. Nature. 2004;430:578–582. doi: 10.1038/nature02766. [DOI] [PubMed] [Google Scholar]
- Bloom KS, Carbon J. Yeast centromere DNA is in a unique and highly ordered structure in chromosomes and small circular minichromosomes. Cell. 1982;29:305–17. doi: 10.1016/0092-8674(82)90147-7. [DOI] [PubMed] [Google Scholar]
- Brykczynska U, Hisano M, Erkek S, et al. Repressive and active histone methylation mark distinct promoters in human and mouse spermatozoa. Nat Struct Mol Biol. 2010;17:679–87. doi: 10.1038/nsmb.1821. [DOI] [PubMed] [Google Scholar]
- Burrack LS, Berman J. Neocentromeres and epigenetically inherited features of centromeres. Chromosom Res. 2012;20:607–619. doi: 10.1007/s10577-012-9296-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral G, Marques A, Schubert V, et al. Chiasmatic and achiasmatic inverted meiosis of plants with holocentric chromosomes. Nat Commun. 2014;5:5070. doi: 10.1038/ncomms6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camahort R, Li B, Florens L, et al. Scm3 is essential to recruit the histone h3 variant cse4 to centromeres and to maintain a functional kinetochore. Mol Cell. 2007;26:853–65. doi: 10.1016/j.molcel.2007.05.013. [DOI] [PubMed] [Google Scholar]
- Carroll CW, Silva MCC, Godek KM, et al. Centromere assembly requires the direct recognition of CENP-A nucleosomes by CENP-N. Nat Cell Biol. 2009;11:896–902. doi: 10.1038/ncb1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeseman IM, Desai A. Molecular architecture of the kinetochore–microtubule interface. Nat Rev Mol Cell Biol. 2008;9:33–46. doi: 10.1038/nrm2310. [DOI] [PubMed] [Google Scholar]
- Chen C-C, Mellone BG. Chromatin assembly: Journey to the CENter of the chromosome. J Cell Biol. 2016;214:13–24. doi: 10.1083/jcb.201605005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang T, Duncan FE, Schindler K, et al. Evidence that weakened centromere cohesion is a leading cause of age-related aneuploidy in oocytes. Curr Biol. 2010;20:1522–8. doi: 10.1016/j.cub.2010.06.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang T, Schultz RM, Lampson MA. Age-dependent susceptibility of chromosome cohesion to premature separase activation in mouse oocytes. Biol Reprod. 2011;85:1279–83. doi: 10.1095/biolreprod.111.094094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang T, Schultz RM, Lampson MA. Meiotic origins of maternal age-related aneuploidy. Biol Reprod. 2012;86:1–7. doi: 10.1095/biolreprod.111.094367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chmátal L, Gabriel SI, Mitsainas GP, et al. Centromere strength provides the cell biological basis for meiotic drive and karyotype evolution in mice. Curr Biol. 2014;24:2295–300. doi: 10.1016/j.cub.2014.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo KH. Centromere DNA dynamics: latent centromeres and neocentromere formation. Am J Hum Genet. 1997;61:1225–1233. doi: 10.1086/301657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke L, Carbon J. Isolation of a yeast centromere and construction of functional small circular chromosomes. Nature. 1980;287:504–9. doi: 10.1038/287504a0. [DOI] [PubMed] [Google Scholar]
- D’Archivio S, Wickstead B. Trypanosome outer kinetochore proteins suggest conservation of chromosome segregation machinery across eukaryotes. J Cell Biol. 2016 doi: 10.1083/jcb.201608043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rop V, Padeganeh A, Maddox PS. CENP-A: the key player behind centromere identity, propagation, and kinetochore assembly. Chromosoma. 2012;121:527–538. doi: 10.1007/s00412-012-0386-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depinet TW, Zackowski JL, Earnshaw WC, et al. Characterization of neo-centromeres in marker chromosomes lacking detectable alpha-satellite DNA. Hum Mol Genet. 1997;6:1195–204. doi: 10.1093/hmg/6.8.1195. [DOI] [PubMed] [Google Scholar]
- Drinnenberg IA, deYoung D, Henikoff S, Malik HS. Recurrent loss of CenH3 is associated with independent transitions to holocentricity in insects. Elife. 2014 doi: 10.7554/eLife.03676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunleavy EM, Beier NL, Gorgescu W, et al. The cell cycle timing of centromeric chromatin assembly in Drosophila meiosis is distinct from mitosis yet requires CAL1 and CENP-C. PLoS Biol. 2012;10:1–16. doi: 10.1371/journal.pbio.1001460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnshaw WC. Discovering centromere proteins: from cold white hands to the A, B, C of CENPs. Nat Rev Mol Cell Biol. 2015;16:443–449. doi: 10.1038/nrm4001. [DOI] [PubMed] [Google Scholar]
- Earnshaw WC, Migeon BR. Three related centromere proteins are absent from the inactive centromere of a stable isodicentric chromosome. Chromosoma. 1985;92:290–6. doi: 10.1007/BF00329812. [DOI] [PubMed] [Google Scholar]
- Earnshaw WC, Rothfield N. Identification of a family of human centromere proteins using autoimmune sera from patients with scleroderma. Chromosoma. 1985;91:313–21. doi: 10.1007/BF00328227. [DOI] [PubMed] [Google Scholar]
- Erhardt S, Mellone BG, Betts CM, et al. Genome-wide analysis reveals a cell cycle-dependent mechanism controlling centromere propagation. J Cell Biol. 2008;183:805–18. doi: 10.1083/jcb.200806038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erkek S, Hisano M, Liang C-Y, et al. Molecular determinants of nucleosome retention at CpG-rich sequences in mouse spermatozoa. Nat Struct Mol Biol. 2013;20:868–75. doi: 10.1038/nsmb.2599. [DOI] [PubMed] [Google Scholar]
- Falk SJ, Black BE. Centromeric chromatin and the pathway that drives its propagation. Biochim Biophys Acta. 2012;1819:313–21. doi: 10.1016/j.bbagrm.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk SJ, Guo LY, Sekulic N, et al. Chromosomes. CENP-C reshapes and stabilizes CENP-A nucleosomes at the centromere. Science. 2015;348:699–703. doi: 10.1126/science.1259308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk SJ, Lee J, Sekulic N, et al. CENP-C directs a structural transition of CENP-A nucleosomes mainly through sliding of DNA gyres. Nat Struct Mol Biol. 2016;23:204–208. doi: 10.1038/nsmb.3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher AM, Al-Gazali L, Pramathan T, et al. Centromeric inactivation in a dicentric human Y;21 translocation chromosome. Chromosoma. 1997;106:199–206. doi: 10.1007/s004120050240. [DOI] [PubMed] [Google Scholar]
- Fitzgerald-Hayes M, Clarke L, Carbon J. Nucleotide sequence comparisons and functional analysis of yeast centromere DNAs. Cell. 1982;29:235–44. doi: 10.1016/0092-8674(82)90108-8. [DOI] [PubMed] [Google Scholar]
- Foltz DR, Jansen LET, Bailey AO, et al. Centromere-specific assembly of CENP-a nucleosomes is mediated by HJURP. Cell. 2009;137:472–84. doi: 10.1016/j.cell.2009.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassmann R, Rechtsteiner A, Yuen KW, et al. An inverse relationship to germline transcription defines centromeric chromatin in C. elegans. Nature. 2012;484:534–537. doi: 10.1038/nature10973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaucher J, Reynoird N, Montellier E, et al. From meiosis to postmeiotic events: The secrets of histone disappearance. FEBS J. 2010;277:599–604. doi: 10.1111/j.1742-4658.2009.07504.x. [DOI] [PubMed] [Google Scholar]
- Guerra M, Cabral G, Cuacos M, et al. Neocentrics and Holokinetics (Holocentrics): Chromosomes out of the Centromeric Rules. Cytogenet Genome Res. 2010;129:82–96. doi: 10.1159/000314289. [DOI] [PubMed] [Google Scholar]
- Guo LY, Allu PK, Zandarashvili L, et al. Centromeres are maintained by fastening CENP-A to DNA and directing an arginine anchor-dependent nucleosome transition. Nat Commun. 2017;8:15775. doi: 10.1038/ncomms15775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevitch M, Amiel A, Ben-Zion M, et al. Acrocentric centromere organization within the chromocenter of the human sperm nucleus. Mol Reprod Dev. 2001;60:507–516. doi: 10.1002/mrd.1116. [DOI] [PubMed] [Google Scholar]
- Guse A, Carroll CW, Moree B, et al. In vitro centromere and kinetochore assembly on defined chromatin templates. Nature. 2011;477:354–8. doi: 10.1038/nature10379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammoud SS, Nix DA, Zhang H, et al. Distinctive chromatin in human sperm packages genes for embryo development. Nature. 2009;460:473–8. doi: 10.1038/nature08162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori T, Shang W-H, Takeuchi K, Fukagawa T. The CCAN recruits CENP-A to the centromere and forms the structural core for kinetochore assembly. J Cell Biol. 2012;200:45–60. doi: 10.1083/jcb.201210106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata-Otsubo A, Dawicki-McKenna JM, Akera T, et al. Expanded satellite repeats amplify a discrete CENP-A nucleosome assembly site on chromosomes that drive in female meiosis. Current Biology. 2017 Jul 27; doi: 10.1016/j.cub.2017.06.069. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen LET, Black BE, Foltz DR, Cleveland DW. Propagation of centromeric chromatin requires exit from mitosis. J Cell Biol. 2007;176:795–805. doi: 10.1083/jcb.200701066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato H, Jiang J, Zhou B-R, et al. A conserved mechanism for centromeric nucleosome recognition by centromere protein CENP-C. Science. 2013;340:1110–3. doi: 10.1126/science.1235532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Ishiguro K, Nambu A, et al. Meikin is a conserved regulator of meiosis-I-specific kinetochore function. Nature. 2015;517:466–71. doi: 10.1038/nature14097. [DOI] [PubMed] [Google Scholar]
- Kwenda L, Collins CM, Dattoli AA, Dunleavy EM. Nucleolar activity and CENP-C regulate CENP-A and CAL1 availability for centromere assembly in meiosis. Development. 2016;143:1400–12. doi: 10.1242/dev.130625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagana A, Dorn JF, De Rop V, et al. A small GTPase molecular switch regulates epigenetic centromere maintenance by stabilizing newly incorporated CENP-A. Nat Cell Biol. 2010;12:1186–93. doi: 10.1038/ncb2129. [DOI] [PubMed] [Google Scholar]
- Lister LM, Kouznetsova A, Hyslop LA, et al. Age-related meiotic segregation errors in mammalian oocytes are preceded by depletion of cohesin and Sgo2. Curr Biol. 2010;20:1511–21. doi: 10.1016/j.cub.2010.08.023. [DOI] [PubMed] [Google Scholar]
- Locke DP, Segraves R, Carbone L, et al. Large-scale variation among human and great ape genomes determined by array comparative genomic hybridization. Genome Res. 2003;13:347–57. doi: 10.1101/gr.1003303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowell JE, Cross GAM. A variant histone H3 is enriched at telomeres in Trypanosoma brucei. J Cell Sci. 2004;117:5937–47. doi: 10.1242/jcs.01515. [DOI] [PubMed] [Google Scholar]
- Marshall OJ, Chueh AC, Wong LH, Choo KHA. Neocentromeres: new insights into centromere structure, disease development, and karyotype evolution. Am J Hum Genet. 2008;82:261–82. doi: 10.1016/j.ajhg.2007.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLay DW, Clarke HJ. Remodelling the paternal chromatin at fertilization in mammals. Reproduction. 2003;125:625–33. doi: 10.1530/rep.0.1250625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melters DP, Bradnam KR, Young HA, et al. Comparative analysis of tandem repeats from hundreds of species reveals unique insights into centromere evolution. Genome Biol. 2013;14:R10. doi: 10.1186/gb-2013-14-1-r10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendiburo MJ, Padeken J, Fulop S, et al. Drosophila CENH3 Is Sufficient for Centromere Formation. Science (80- ) 2011;334:686–690. doi: 10.1126/science.1206880. [DOI] [PubMed] [Google Scholar]
- Meyer-Ficca ML, Lonchar JD, Ihara M, et al. Alteration of poly(ADP-ribose) metabolism affects murine sperm nuclear architecture by impairing pericentric heterochromatin condensation. Chromosoma. 2013;122:319–335. doi: 10.1007/s00412-013-0416-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milks KJ, Moree B, Straight AF. Dissection of CENP-C– directed Centromere and Kinetochore Assembly. Mol Biol Cell. 2009;20:4246–4255. doi: 10.1091/mbc.E09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuguchi G, Xiao H, Wisniewski J, et al. Nonhistone Scm3 and histones CenH3-H4 assemble the core of centromere-specific nucleosomes. Cell. 2007;129:1153–64. doi: 10.1016/j.cell.2007.04.026. [DOI] [PubMed] [Google Scholar]
- Mola LM, Papeschi AG. Holokinetic chromosomes at a glance. BAG - J Basic Appl Genet. 2006;17:17–33. [Google Scholar]
- Monen J, Maddox PS, Hyndman F, et al. Differential role of CENP-A in the segregation of holocentric C. elegans chromosomes during meiosis and mitosis. Nat Cell Biol. 2005;7:1248–55. doi: 10.1038/ncb1331. [DOI] [PubMed] [Google Scholar]
- Nabeshima K, Villeneuve AM, Colaiácovo MP. Crossing over is coupled to late meiotic prophase bivalent differentiation through asymmetric disassembly of the SC. J Cell Biol. 2005;168:683–9. doi: 10.1083/jcb.200410144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashun B, Hill PWS, Smallwood SA, et al. Continuous histone replacement by Hira is essential for normal transcriptional regulation and de novo DNA methylation during mouse oogenesis. Mol Cell. 2015;60:611–625. doi: 10.1016/j.molcel.2015.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer DK, Day KO, Le Trongt H, et al. Purification of the centromere-specific protein CENP-A and demonstration that it is a distinctive histone. Proc Natl Acad Sci U S A. 1991;88:3734–3738. doi: 10.1073/pnas.88.9.3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer DK, O’Day K, Margolis RL. The centromere specific histone CENP-A is selectively retained in discrete foci in mammalian sperm nuclei. Chromosoma. 1990;100:32–6. doi: 10.1007/BF00337600. [DOI] [PubMed] [Google Scholar]
- Palmer DK, O’Day K, Wener MH, et al. A 17-kD centromere protein (CENP-A) copurifies with nucleosome core particles and with histones. J Cell Biol. 1987;104:805–15. doi: 10.1083/jcb.104.4.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panzeri L, Philippsen P. Centromeric DNA from chromosome VI in Saccharomyces cerevisiae strains. EMBO J. 1982;1:1605–11. doi: 10.1002/j.1460-2075.1982.tb01362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perpelescu M, Fukagawa T. The ABCs of CENPs. Chromosoma. 2011;120:425–446. doi: 10.1007/s00412-011-0330-0. [DOI] [PubMed] [Google Scholar]
- Piras FM, Nergadze SG, Magnani E, et al. Uncoupling of Satellite DNA and Centromeric Function in the Genus Equus. PLoS Genet. 2010;6:e1000845. doi: 10.1371/journal.pgen.1000845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluta AF, Mackay AM, Ainsztein AM, et al. The centromere: hub of chromosomal activities. Science. 1995;270:1591–4. doi: 10.1126/science.270.5242.1591. [DOI] [PubMed] [Google Scholar]
- Rathke C, Baarends WM, Awe S, Renkawitz-Pohl R. Chromatin dynamics during spermiogenesis. Biochim Biophys Acta. 2014;1839:155–68. doi: 10.1016/j.bbagrm.2013.08.004. [DOI] [PubMed] [Google Scholar]
- Ravi M, Chan SWL. Haploid plants produced by centromere-mediated genome elimination. Nature. 2010;464:615–8. doi: 10.1038/nature08842. [DOI] [PubMed] [Google Scholar]
- Ravi M, Marimuthu MPA, Tan EH, et al. A haploid genetics toolbox for Arabidopsis thaliana. Nat Commun. 2014;5:5334. doi: 10.1038/ncomms6334. [DOI] [PubMed] [Google Scholar]
- Ravi M, Shibata F, Ramahi JS, et al. Meiosis-specific loading of the centromere-specific histone CENH3 in Arabidopsis thaliana. PLoS Genet. 2011;7:e1002121. doi: 10.1371/journal.pgen.1002121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raychaudhuri N, Dubruille R, Orsi GA, et al. Transgenerational Propagation and Quantitative Maintenance of Paternal Centromeres Depends on Cid/Cenp-A Presence in Drosophila Sperm. PLoS Biol. 2012 doi: 10.1371/journal.pbio.1001434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samans B, Yang Y, Krebs S, et al. Uniformity of nucleosome preservation pattern in mammalian sperm and Its connection to repetitive DNA elements. Dev Cell. 2014;30:23–35. doi: 10.1016/j.devcel.2014.05.023. [DOI] [PubMed] [Google Scholar]
- Sanei M, Pickering R, Kumke K, et al. Loss of centromeric histone H3 (CENH3) from centromeres precedes uniparental chromosome elimination in interspecific barley hybrids. Proc Natl Acad Sci U S A. 2011;108:E498–505. doi: 10.1073/pnas.1103190108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders M, Fitzgerald-Hayes M, Bloom K. Chromatin structure of altered yeast centromeres. Proc Natl Acad Sci U S A. 1988;85:175–9. doi: 10.1073/pnas.85.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schagdarsurengin U, Paradowska A, Steger K. Analysing the sperm epigenome: roles in early embryogenesis and assisted reproduction. Nat Rev Urol. 2012;9:609. doi: 10.1038/nrurol.2012.183. [DOI] [PubMed] [Google Scholar]
- Schenk R, Jenke A, Zilbauer M, et al. H3.5 is a novel hominid-specific histone H3 variant that is specifically expressed in the seminiferous tubules of human testes. Chromosoma. 2011;120:275–85. doi: 10.1007/s00412-011-0310-4. [DOI] [PubMed] [Google Scholar]
- Schubert V, Lermontova I, Schubert I. Loading of the centromeric histone H3 variant during meiosis-how does it differ from mitosis? Chromosoma. 2014;123:491–7. doi: 10.1007/s00412-014-0466-9. [DOI] [PubMed] [Google Scholar]
- Schuh M, Lehner CF, Heidmann S. Incorporation of Drosophila CID/CENP-A and CENP-C into centromeres during early embryonic anaphase. Curr Biol. 2007;17:237–43. doi: 10.1016/j.cub.2006.11.051. [DOI] [PubMed] [Google Scholar]
- Scott KC, Sullivan BA. Neocentromeres: a place for everything and everything in its place. Trends Genet. 2014;30:66–74. doi: 10.1016/j.tig.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekulic N, Bassett EA, Rogers DJ, Black BE. The structure of (CENP-A–H4)2 reveals physical features that mark centromeres. Nature. 2010;467:347–351. doi: 10.1038/nature09323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang W-H, Hori T, Toyoda A, et al. Chickens possess centromeres with both extended tandem repeats and short non-tandem-repetitive sequences. Genome Res. 2010;20:1219–1228. doi: 10.1101/gr.106245.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MCC, Bodor DL, Stellfox ME, et al. Cdk activity couples epigenetic centromere inheritance to cell cycle progression. Dev Cell. 2012;22:52–63. doi: 10.1016/j.devcel.2011.10.014. [DOI] [PubMed] [Google Scholar]
- Smoak EM, Stein P, Schultz RM, et al. Long-Term Retention of CENP-A Nucleosomes in Mammalian Oocytes Underpins Transgenerational Inheritance of Centromere Identity. Curr Biol. 2016 doi: 10.1016/j.cub.2016.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoler S, Rogers K, Weitze S, et al. Scm3, an essential Saccharomyces cerevisiae centromere protein required for G2/M progression and Cse4 localization. Proc Natl Acad Sci U S A. 2007;104:10571–6. doi: 10.1073/pnas.0703178104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan KF, Hechenberger M, Masri K. Human CENP-A contains a histone H3 related histone fold domain that is required for targeting to the centromere. J Cell Biol. 1994;127:581–592. doi: 10.1083/jcb.127.3.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachiwana H, Kagawa W, Osakabe A, et al. Structural basis of instability of the nucleosome containing a testis-specific histone variant, human H3T. Proc Natl Acad Sci U S A. 2010;107:10454–9. doi: 10.1073/pnas.1003064107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang MCW, Jacobs SA, Mattiske DM, et al. Contribution of the two genes encoding histone variant h3.3 to viability and fertility in mice. PLoS Genet. 2015;11:e1004964. doi: 10.1371/journal.pgen.1004964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler-Smith C, Gimelli G, Giglio S, et al. Transmission of a Fully Functional Human Neocentromere through Three Generations. Am J Hum Genet. 1999;64:1440–1444. doi: 10.1086/302380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urahama T, Harada A, Maehara K, et al. Histone H3.5 forms an unstable nucleosome and accumulates around transcription start sites in human testis. Epigenetics Chromatin. 2016;9:2. doi: 10.1186/s13072-016-0051-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdivia MM, Brinkley BR. Fractionation and initial characterization of the kinetochore from mammalian metaphase chromosomes. J Cell Biol. 1985;101:1124–34. doi: 10.1083/jcb.101.3.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Werken C, van de Werken C, van der Heijden GW, et al. Paternal heterochromatin formation in human embryos is H3K9/HP1 directed and primed by sperm-derived histone modifications. Nat Commun. 2014;5:5868. doi: 10.1038/ncomms6868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargiu G, Makarov AA, Allan J, et al. Stepwise unfolding supports a subunit model for vertebrate kinetochores. Proc Natl Acad Sci U S A. 2017;114:3133–3138. doi: 10.1073/pnas.1614145114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waye JS, Willard HF. Nucleotide sequence heterogeneity of alpha satellite repetitive DNA: A survey of alphoid sequences from different human chromosomes. Nucleic Acids Res. 1987;15:7549–7569. doi: 10.1093/nar/15.18.7549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalensky AO, Allen MJ, Kobayashi A, et al. Well-defined genome architecture in the human sperm nucleus. Chromosoma. 1995;103:577–90. doi: 10.1007/BF00357684. [DOI] [PubMed] [Google Scholar]
- Zalensky AO, Breneman JW, Zalenskaya IA, et al. Organization of centromeres in the decondensed nuclei of mature human sperm. Chromosoma. 1993;102:509–18. doi: 10.1007/BF00368344. [DOI] [PubMed] [Google Scholar]