Abstract
It is unclear whether black patients with chronic kidney disease (CKD) vs those without CKD who take antihypertensive medication have an increased risk for apparent treatment‐resistant hypertension (aTRH). The authors analyzed 1741 Jackson Heart Study participants without aTRH taking antihypertensive medication at baseline. aTRH was defined as uncontrolled blood pressure while taking three antihypertensive medication classes or taking four or more antihypertensive medication classes, regardless of blood pressure level. CKD was defined as an albumin to creatinine ratio ≥30 mg/g or estimated glomerular filtration rate <60 mL/min/1.73 m2. Over 8 years, 20.1% of participants without CKD and 30.5% with CKD developed aTRH. The multivariable‐adjusted hazard ratio for aTRH comparing participants with CKD vs those without CKD was 1.45 (95% CI, 1.12–1.86). Participants with an albumin to creatinine ratio ≥30 vs <30 mg/g (hazard ratio, 1.44; 95% CI, 1.04–2.00) and estimated glomerular filtration rate of 45 to 59 mL/min/1.73 m2 and <45 vs ≥60mL/min/1.73 m2 (hazard ratio, 1.60 [95% CI, 1.16–2.20] and 2.05 [95% CI, 1.28–3.26], respectively) were more likely to develop aTRH.
Keywords: blood pressure, chronic kidney disease, treatment‐resistant hypertension
1. INTRODUCTION
Apparent treatment‐resistant hypertension (aTRH) is defined as uncontrolled blood pressure (BP; systolic/diastolic BP ≥140/90 mm Hg) with concurrent use of three or more classes of antihypertensive medication, or controlled BP (systolic/diastolic BP <140/90 mm Hg) with use of four or more classes of antihypertensive medication.1 Ideally, one of these antihypertensive medication classes should be a diuretic, and all of the drugs should be prescribed at optimal doses. Most adults with chronic kidney disease (CKD) have hypertension that requires treatment with multiple classes of antihypertensive medication.2 In addition, the prevalence of aTRH is high among adults with CKD.2, 3, 4, 5, 6
CKD is associated with increased salt and water retention, excessive activation of the renin‐angiotensin‐aldosterone system, and overactivation of the sympathetic nervous system.5, 7, 8 In addition, antihypertensive treatment has been associated with smaller reductions in systolic and diastolic BP among adults with lower eGFR and higher albumin to creatinine ratio (ACR), suggesting that more severe CKD may contribute to diminished effectiveness of antihypertensive medication.5, 9 Therefore, it is plausible that adults with CKD have an increased risk for developing aTRH. Determining the association between CKD and aTRH may inform treatment strategies aimed at improving BP control in patients with CKD. This may be particularly useful among black patients, given their high prevalence of both CKD and aTRH and increased risk for cardiovascular outcomes associated with aTRH.6, 10, 11 The goal of the current analysis was to determine whether black adults with CKD have an increased risk for incident aTRH. In addition, we determined risk factors for incident aTRH among participants with CKD. To address these aims, we conducted an analysis of adults who participated in the JHS (Jackson Heart Study).
2. METHODS
2.1. Study participants
JHS is a community‐based observational study of black adults recruited from urban and rural areas of three counties (Hinds, Madison, and Rankin) comprising the Jackson, Mississippi, metropolitan area. Details of the study design and recruitment have been previously published.12, 13, 14 Participants were recruited from the ARIC (Atherosclerosis Risk in Communities) site in Jackson, Mississippi, and a representative sample of tri‐county residents, study volunteers, randomly contacted individuals, and eligible family members of participants in any one of the ARIC, volunteer, or random samples. The final JHS cohort of 5306 black adults 21 years and older was enrolled between 2000 and 2004. The study protocol was approved by the institutional review boards governing research in human subjects at the participating centers and all participants provided written consent.
Participants with hypertension who were taking antihypertensive medication at baseline (n=2462) formed the base population for the current analysis. We excluded 289 participants with prevalent aTRH or for whom aTRH status could not be determined at baseline, and 409 participants who did not attend any follow‐up study visits. Participants who self‐reported end‐stage renal disease (ESRD) at baseline (n=23) were excluded from the analyses. After these exclusion criteria were applied, we included 1741 JHS participants in all analyses.
2.2. Data collection
Of relevance to the current analysis, data were collected during a baseline study visit in 2000–2004 and follow‐up visits in 2005–2008 (visit 2) and 2009–2012 (visit 3). Baseline data were collected during an in‐home interview and a study examination conducted in the JHS clinic after an overnight fast. Information on age, sex, education, cigarette smoking, physical activity, history of myocardial infarction, and self‐reported use of antihypertensive and antidiabetes mellitus medication was collected during the study interview. During the clinic visit, a standardized protocol was followed to measure BP, height, and weight, and collect blood and urine samples. Information was recorded by reviewing the pill bottles for all medications taken within the 2 weeks prior to the clinic visit. Height and weight were measured and used to calculate body mass index. Fasting serum glucose was measured using a glucose oxidase method on a Vitros 950 or 250 analyzer (Ortho Clinical Diagnostics). Glycated hemoglobin was measured using a Tosoh high‐performance liquid chromatography system (Tosoh Corporation). Diabetes mellitus was defined as a glycated hemoglobin level ≥6.5%, a fasting plasma glucose level ≥126 mg/dL, or use of antidiabetes mellitus medication.13
Using specimens collected during the baseline study visit, urinary albumin was measured with the Dade Behring BN II nephelometer (Siemens). Serum and urine creatinine levels were measured using a multi‐point enzymatic spectrophotometric assay on a Vitros 950 Ortho Clinical Diagnostics analyzer. Creatinine values were biochemically calibrated to Cleveland Clinic–equivalent Minnesota Beckman CX3 assay for analysis purposes.15 We measured urinary ACR and estimated glomerular filtration rate (eGFR) was calculated via the Chronic Kidney Disease Epidemiology Collaboration (CKD‐EPI) equation.16 CKD was defined as an ACR ≥30 mg/g or an eGFR <60 mL/min/1.73 m2.
2.3. BP measurement and definition of aTRH
At each visit, BP was measured after a 5‐minute rest on the participant's right arm using one of four cuff sizes selected following measurement of the arm circumference. The average of two measurements taken 1 minute apart was used to define clinic BP. Quality control was assured by technician recertification, procedural checklists, and data review.13, 17 BP was measured using a random‐zero sphygmomanometer (Hawksley & Sons Limited) at visits 1 and 2 and a semiautomatic oscillometric device (Omron HEM‐907XL, Omron Healthcare, Inc) at visits 2 and 3. Among the 4182 JHS participants who attended visit 2 and had their BP measured, 2115 participants were included in a BP comparability substudy, for which BP was assessed simultaneously by a random‐zero sphygmomanometer and the Omron HEM‐907XL device using a Y connector. As described elsewhere,18 the random‐zero BP measurements were calibrated to the semiautomated device using robust regression. When available, BP from the semiautomated device was used. In the current analysis, the calibrated BP measurements were used for 1741 participants at visit 1 and 849 participants at visit 2 who did not have their BP measured using the semiautomatic oscillometric device.
Medication names recorded during the pill bottle review were coded into generic drug names and subsequently grouped into drug classes. One‐pill combinations were classified into multiple medication classes. Antihypertensive medication classes were defined using the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7),19 with updates based on review of medications in each class by study investigators. Medication adherence was defined based on participant self‐report of whether they took each of their prescribed antihypertensive medications in the prior 24 hours. Those who reported not taking one or more of their antihypertensive medications were classified as nonadherent and those who reported taking all of their antihypertensive medications were classified as adherent. Hypertension was defined as a systolic BP ≥140 mm Hg and/or a diastolic BP ≥90 mm Hg and/or self‐reported use of antihypertensive medication. Uncontrolled BP was defined as a systolic BP ≥140 mm Hg and/or a diastolic BP ≥90 mm Hg, and controlled BP was defined as a systolic BP <140 mm Hg and a diastolic BP <90 mm Hg. aTRH was defined as uncontrolled BP with concurrent use of three or more antihypertensive medication classes including a diuretic or use of four or more antihypertensive medication classes including a diuretic with controlled BP.1
2.4. Statistical analysis
We used multiple imputation (n=10 data sets) and chained equations to impute variables with missing data. The number and percentage of participants missing each variable are presented in Table S1. Characteristics of JHS participants at baseline were calculated by CKD status. The statistical significance of differences across groups was calculated using t tests and chi‐square tests, as appropriate. We then calculated the cumulative percentage of participants with and without CKD who developed aTRH at visit 2 and 3. Using interval‐censored Cox regression models, we calculated crude and multivariable‐adjusted hazard ratios for aTRH comparing participants with vs those without CKD. For participants who did not develop aTRH, follow‐up ended at the last study visit they attended. Initial adjustment included age and sex. Full multivariable adjustment included age, sex, education, current smoking, physical activity, body mass index, diabetes mellitus status, and history of myocardial infarction. Next, we calculated the percentage of participants who developed aTRH and crude and multivariable‐adjusted hazard ratios for incident aTRH associated with baseline level of ACR (<10, 10–29, and ≥30 mg/g) and eGFR (≥60, 45–59, and <45 mL/min/1.73 m2), separately. In sensitivity analyses, we conducted the above analyses limited to individuals who were adherent to antihypertensive medication and also conducted a complete case (ie, unimputed) analysis. We calculated multivariable‐adjusted hazard ratios (HRs) for incident aTRH associated with CKD, eGFR, and ACR in subgroups defined by age (<65 vs ≥65 years), sex, diabetes mellitus status, history of myocardial infarction, use of each antihypertensive medication class, and number of classes of antihypertensive medications being taken at baseline. Because of the limited number of participants who developed aTRH in some subgroups, we grouped ACR and eGFR into two categories (ie, ACR <10 vs ≥10 mg/g, consistent with the threshold for normal albuminuria from a spot urine test according to the 2009 National Kidney Foundation and US Food and Drug Administration report on proteinuria as a surrogate outcome in CKD,20 and eGFR ≥60 vs <60 mL/min/1.73 m2) for these analyses. Finally, among participants with CKD, we calculated HRs for incident aTRH associated with each study covariate included in the multivariable model described above. Analyses were conducted using SAS software version 9.4 (SAS Institute Inc).
3. RESULTS
3.1. Study characteristics
Overall, 410 (23.6%) participants included in this analysis had CKD. Participants with CKD vs those without CKD were older and more likely to have less than a high school education, a body mass index ≥30 kg/m2, diabetes mellitus, a history of myocardial infarction, and reduced left ventricular ejection fraction (Table 1). Mean left ventricular mass and systolic BP were higher and mean physical activity score and diastolic BP were lower among participants with CKD vs those without CKD. A higher percentage of participants with CKD vs those without CKD were taking three classes of antihypertensive medication at baseline.
Table 1.
Baseline characteristics of Jackson Heart Study participants with hypertension by CKD status
| Characteristic | No CKD (n=1331) | CKD (n=410) | P value |
|---|---|---|---|
| Age, y | 58.5 (10.1) | 63.0 (11.0) | <.001 |
| Male sex, % | 29.0 | 29.4 | .661 |
| Less than high school education, % | 20.1 | 29.6 | <.001 |
| Current smoking, % | 9.1 | 9.8 | .238 |
| Total physical activity score, exercise unitsa | 8.3 (2.5) | 7.4 (2.5) | <.001 |
| Body mass index ≥30 kg/m2, % | 59.5 | 62.3 | .001 |
| Diabetes mellitus, % | 26.0 | 43.5 | <.001 |
| History of myocardial infarction, % | 4.7 | 10.9 | <.001 |
| Left ventricular ejection fraction <50%, % | 2.1 | 4.4 | <.001 |
| Left ventricular mass, g | 150.3 (42.6) | 164.7 (98.2) | <.001 |
| Systolic blood pressure, mm Hg | 128.7 (14.6) | 132.8 (16.7) | <.001 |
| Diastolic blood pressure, mm Hg | 76.1 (8.3) | 74.8 (9.6) | <.001 |
| Serum creatinine, mg/dL | 1.0 (0.2) | 1.2 (0.4) | <.001 |
| Albumin to creatinine ratio, mg/g | 5.9 (4.2–9.5) | 41.4 (11.8–118.1) | <.001 |
| eGFR, mL/min/1.73 m2 | 84.0 (13.9) | 66.6 (21.6) | <.001 |
| CKD stage, %b | |||
| 1 | 30.0 | 16.4 | <.001 |
| 2 | 70.0 | 33.1 | |
| 3a | 0 | 38.2 | |
| 3b | 0 | 10.1 | |
| 4 | 0 | 2.0 | |
| 5 | 0 | 0.2 | |
| Antihypertensive medication class, % | |||
| ACEI | 34.8 | 38.9 | <.001 |
| Aldosterone antagonist | 1.5 | 1.8 | .134 |
| α‐Blocker | 4.6 | 4.9 | .440 |
| Angiotensin II receptor blocker | 15.0 | 19.7 | <.001 |
| β‐Blocker: cardioselective and nonselective | 18.1 | 20.4 | <.001 |
| β‐Blocker: intrinsic sympathomimetic activity | 0.2 | 0 | .002 |
| Calcium channel blocker | 35.2 | 36.9 | .049 |
| Central‐acting agent | 3.2 | 4.9 | <.001 |
| Combined α‐ and β‐blocker | 0.4 | 1.0 | <.001 |
| Direct vasodilator | 0.3 | 0.5 | .074 |
| Loop diuretic | 6.3 | 10.4 | <.001 |
| Potassium‐sparing diuretic | 14.1 | 13.7 | .470 |
| Thiazide diuretic | 55.5 | 55.6 | .912 |
| No. of antihypertensive medication classes, % | |||
| 1 | 32.9 | 24.6 | <.001 |
| 2 | 47.7 | 46.7 | |
| 3 | 19.4 | 28.7 | |
Values are expressed as mean (SD) or percentage, except for albumin to creatinine ratio, which is presented as median (25th and 75th percentiles).
Abbreviation: ACEI, angiotensin‐converting enzyme inhibitor.
Physical activity score ranges from 1 to 20, with a higher score indicating higher physical activity.
Chronic kidney disease (CKD; defined as an estimated glomerular filtration rate [eGFR] <60 mL/min/1.73 m2 or an albumin to creatinine ratio ≥30 mg/g) classified according to eGFR (mL/min/1.73 m2) as follows: stage 1, ≥90; stage 2, 60–89; stage 3a, 45–59; stage 3b, 30–44; stage 4, 15–29; and stage 5, <15.
3.2. aTRH associated with CKD and levels of ACR and eGFR
Over a median follow‐up of 8.0 years (maximum: 12.2 years), 392 participants developed aTRH. Among study participants with and without CKD, 22.8% and 12.3%, respectively, developed aTRH by visit 2 (median of 4.6 years of follow‐up) (Table 2). By visit 3, 30.5% with CKD and 20.1% without CKD developed aTRH. The crude HR for aTRH comparing those with and those without CKD was 1.73 (95% CI, 1.39–2.16). After full multivariable adjustment, the HR was 1.45 (95% CI, 1.12–1.86). Results were similar when restricted to participants who were adherent to their antihypertensive medications (n=1309) and in the complete case analysis (data not shown).
Table 2.
Percentage of participants who developed aTRH and hazard ratios for incident aTRH hypertension associated with CKD
| No CKD | CKD | |
|---|---|---|
| No. of cases of incident aTRH/No. who attended study visit | ||
| Visit 2 | 151/1229 | 86/377 |
| Visit 2 or visit 3 | 267/1331 | 125/410 |
| Patients who developed aTRH, % | ||
| Visit 2 | 12.3 | 22.8 |
| Visit 2 or visit 3 | 20.1 | 30.5 |
| Hazard ratio (95% CI) for aTRH | ||
| Crude | 1 (reference) | 1.73 (1.39–2.16) |
| Age and sex adjusted | 1 (reference) | 1.58 (1.23–2.03) |
| Multivariable adjusteda | 1 (reference) | 1.45 (1.12–1.86) |
Abbreviations: aTRH, apparent treatment‐resistant hypertension; CKD, chronic kidney disease.
Adjusted for age, sex, less than high school education, current smoking, physical activity, body mass index ≥30 kg/m2, diabetes mellitus, and history of myocardial infarction.
Apparent treatment‐resistant hypertension defined as uncontrolled blood pressure with concurrent use of ≥3 antihypertensive medication classes including a diuretic or use of ≥4 antihypertensive medication classes including a diuretic with controlled blood pressure.
Median time between visits: visit 1 to visit 2, 4.6 years; visit 2 to visit 3, 3.1 years; and visit 1 to visit 3, 8.0 years.
The percentage of participants who developed aTRH increased with higher ACR and lower eGFR (Table 3). The multivariable‐adjusted HR for incident aTRH was 1.54 (95% CI, 1.15‐2.07) and 1.44 (95% CI, 1.04‐2.00) for participants with an ACR 10 to 29 and ≥30 mg/g, respectively, each compared with those with an ACR <10 mg/g. The multivariable‐adjusted HR for incident aTRH was 1.60 (95% CI, 1.16–2.20) and 2.05 (95% CI, 1.28–3.26) for participants with an eGFR 45 to 59 mL/min/1.73 m2 and <45 mL/min/1.73 m2, respectively, each compared with those with an eGFR ≥60 mL/min/1.73 m2. Results were similar when restricted to participants who were adherent to their antihypertensive medications and in a complete case analysis (data not shown). The association between CKD and, separately, ACR ≥10 mg/g and eGFR <60 mL/min/1.73 m2 with incident aTRH was consistent across subgroups (Figure and Figure S1).
Table 3.
Percentage of participants who developed aTRH and hazard ratios for incident aTRH associated with baseline albumin to creatinine ratio and eGFR
| No. of cases of incident aTRH/No. who attended visit 2 or 3 | Participants who developed aTRH at visit 2 or 3, % | Age‐ and sex‐adjusted hazard ratio (95% CI) | Multivariable‐adjusteda hazard ratio (95% CI) | |
|---|---|---|---|---|
| ACR, mg/g | ||||
| <10 | 209/1112 | 18.8 | 1 (reference) | 1 (reference) |
| 10–29 | 105/361 | 29.2 | 1.61 (1.21–2.16) | 1.54 (1.15–2.07) |
| ≥30 | 78/268 | 29.1 | 1.66 (1.22–2.26) | 1.44 (1.04–2.00) |
| eGFR, mL/min/1.73 m2 | ||||
| ≥60 | 321/1534 | 20.9 | 1 (reference) | 1 (reference) |
| 45–59 | 50/157 | 31.8 | 1.53 (1.12–2.09) | 1.60 (1.16–2.20) |
| <45 | 21/50 | 42.2 | 2.45 (1.56–3.86) | 2.05 (1.28–3.26) |
Abbreviations: ACR, albumin to creatinine ratio; aTRH, apparent treatment‐resistant hypertension; eGFR, estimated glomerular filtration rate.
Adjusted for age, sex, less than high school education, current smoking, physical activity, body mass index ≥30 kg/m2, diabetes mellitus, and history of myocardial infarction.
Figure 1.
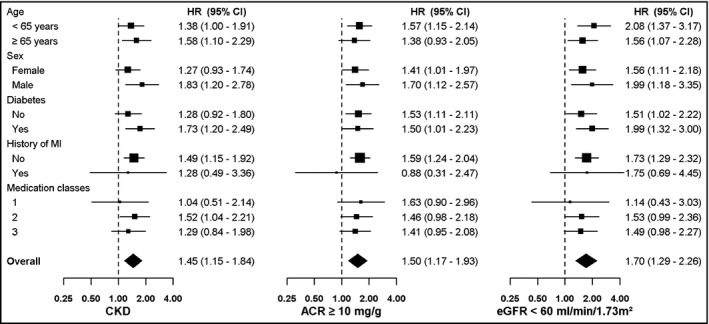
Multivariable‐adjusted hazard ratios (HRs) for incident apparent treatment‐resistant hypertension associated with chronic kidney disease (CKD), albumin to creatinine ratio (ACR), and estimated glomerular filtration rate (eGFR) among participants, in subgroups. CKDis defined as an ACR ≥30 mg/g or an eGFR <60 mL/min/1.73 m2. MI indicates myocardial infarction
3.3. aTRH associated with study covariates
Among participants with CKD, the multivariable‐adjusted HR for incident aTRH was 1.67 (95% CI, 1.10–2.52) for men vs women and 1.66 (95% CI, 1.12–2.48) for participants with vs those without diabetes mellitus (Table 4). In addition, the multivariable‐adjusted HR was 1.70 (95% CI, 1.04–2.79) for participants with vs those without eGFR <60 mL/min/1.73 m2.
Table 4.
Incident aTRH associated with study covariates among participants with CKD (n=410)
| Age‐ and sex‐adjusted hazard ratio (95% CI) | Multivariable‐adjustedahazard ratio (95% CI) | |
|---|---|---|
| Age, per 10 y | 1.11 (0.93–1.33) | 1.08 (0.88–1.34) |
| Male sex | 1.55 (1.04–2.30) | 1.67 (1.10–2.52) |
| Less than high school education | 0.96 (0.62–1.49) | 0.90 (0.58–1.40) |
| Current smoking | 0.98 (0.46–2.08) | 1.08 (0.52–2.26) |
| Total physical activity, per exercise unita | 0.95 (0.87–1.04) | 0.98 (0.90–1.07) |
| Body mass index ≥30 kg/m2 | 1.33 (0.87–2.04) | 1.24 (0.81–1.91) |
| Diabetes mellitus | 1.74 (1.20–2.52) | 1.66 (1.12–2.48) |
| History of myocardial infarction | 0.82 (0.43–1.54) | 0.77 (0.40–1.46) |
| ACR, mg/g | ||
| <10 | 1 (reference) | 1 (reference) |
| ≥10 | 1.06 (0.64–1.77) | 1.23 (0.68–2.22) |
| eGFR, mL/min/1.73 m2 | ||
| ≥60 | 1 (reference) | 1 (reference) |
| <60 | 1.44 (0.94–2.19) | 1.70 (1.04–2.79) |
Abbreviations: ACR, albumin to creatinine ratio; aTRH, apparent treatment‐resistant hypertension; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate.
Adjusted for all variables in the left column.
Physical activity score ranges from 1 to 20, with a higher score indicating higher physical activity.
4. DISCUSSION
In the current study of a large community‐based study of black adults being treated for hypertension, CKD was associated with an increased risk for incident aTRH, which was present after multivariable adjustment. Over 30% of participants with CKD developed aTRH during follow‐up compared with approximately 20% of those without CKD. In addition, higher ACR and lower eGFR values were each associated with an increased risk for aTRH. Among participants with CKD, male sex, diabetes mellitus, and reduced eGFR were associated with higher risk for aTRH.
Prior studies have reported a high prevalence of aTRH among adults with CKD. For example, 40% of participants with hypertension in the CRIC (Chronic Renal Insufficiency Cohort) study5 had aTRH. In the CRIC study, the prevalence of aTRH was higher in participants with lower eGFR. The multivariable‐adjusted odds ratio for aTRH associated with each 5‐mL/min/1.73 m2 lower eGFR was 1.14 (95% CI, 1.10–1.17).5 Older age, male sex, black race, diabetes mellitus, and a higher body mass index were each associated with a higher odds ratio for having aTRH.5 Also, in the REGARDS (Reasons for Geographic and Racial Differences in Stroke) study,6 there was a strong, graded association between higher ACR and lower eGFR with a higher prevalence of aTRH. Male sex, black race, diabetes mellitus, larger waist circumference, history of myocardial infarction or stroke, and lower eGFR and higher ACR levels were each associated with aTRH.6
Laboratory studies demonstrate that chronic elevations in BP promote damage to the renal vasculature, leading to intimal and medial thickening, renal ischemia, and glomerulosclerosis.21, 22 In addition, epidemiologic studies suggest that adults with aTRH have an increased risk for the development of incident CKD. In an analysis of claims data from two health plans within the Cardiovascular Research Network Hypertension Registry, Daugherty and colleagues23 reported that individuals with aTRH were more likely to develop CKD compared with those with nonresistant hypertension (14.5% vs 10.4%). After multivariable adjustment, aTRH was associated with a higher risk of a composite end point including CKD and cardiovascular events (HR, 1.47; 95% CI, 1.33–1.62).23 In addition, among individuals with prevalent CKD, aTRH has been associated with CKD progression.5, 24 In the aforementioned CRIC analysis, aTRH was associated with an increased risk for a composite outcome including 50% reduction in eGFR or ESRD (multivariable‐adjusted HR, 1.28; 95% CI, 1.11–1.46).5
Although there are few data on whether adults with CKD have an increased risk for incident aTRH, this association is biologically plausible. CKD is associated with excessive activation of the renin‐angiotensin‐aldosterone and sympathetic nervous systems, which promote increased sodium retention and increased peripheral resistance.7 In an analysis of the MESA (Multi‐Ethnic Study of Atherosclerosis) study,22 early kidney dysfunction (indicated by serum cystatin C levels) was associated with incident hypertension among participants without clinically apparent kidney or cardiovascular disease. In addition, in a clinic‐based study of adults with uncontrolled BP taking antihypertensive medication at a baseline study visit, an ACR of 30 to 300 mg/g and >300 mg/g were associated with a 5.1 and 10.3 mm Hg smaller systolic BP reduction, respectively, over a median of 5 years of follow‐up when compared with patients with an ACR <30 mg/g.9 Similarly, compared with patients with an eGFR ≥60 mL/min/1.73 m2, those with reduced eGFR (<60 mL/min/1.73 m2) experienced an 8.4‐mm Hg smaller reduction in systolic BP during follow‐up.9 The presence of albuminuria and reduced eGFR also delayed the time to attainment of goal BP.9 These data suggest that individuals with CKD may have attenuated responses to antihypertensive pharmacotherapy, making them more susceptible to developing aTRH.
Blacks are less likely than whites to have impaired kidney function but more likely to develop ESRD.25, 26, 27 For example, in the REGARDS study, the prevalence ratio for eGFR <60 mL/min/1.73 m2 comparing blacks with whites was 0.51 (95% CI, 0.48–0.54).25 However, the United States Renal Data System recently reported that blacks are three times more likely than whites to develop incident ESRD.27 This racial difference was also present in the REGARDS study.26 Data have shown that blacks have a higher prevalence of hypertension compared with whites and are less likely to have controlled BP to <140/90 mm Hg.28 In addition, black patients with CKD are more likely than their white counterparts to have aTRH.6 Along with the findings of the current study, these data emphasize the importance of achieving BP control in black patients with CKD.
Achieving BP control is a major challenge in the management of patients with CKD.29 However, prior studies suggest that, with appropriate interventions, BP goals can be achieved and maintained in populations where BP has historically been difficult to control.30, 31, 32, 33 For example, in AASK (African American Study of Kidney Disease and Hypertension),30 black patients with hypertension and reduced eGFR were randomized to a goal mean arterial pressure (MAP) of either 102 to 107 mm Hg (usual MAP goal) or <92 mm Hg (low MAP goal). In the low MAP goal group, the percentage of participants who achieved a BP <140/90 mm Hg increased from 20% at baseline to 79% by 14 months post‐randomization, while these percentages increased from 22% to 42% in the usual MAP goal group.30 The findings of the current study emphasize the need for intensive clinical management strategies to lower BP early in the course of CKD in an effort to prevent aTRH. This is especially important given that aTRH is associated with increased risk for cardiovascular disease, ESRD, and all‐cause mortality.23, 24, 34, 35
5. STUDY STRENGTHS AND LIMITATIONS
In the current study, misclassification of aTRH was minimized by the use of standardized BP measurements and a pill bottle review to identify the number of antihypertensive medication classes being taken. Other strengths include the large community‐based sample of black adults and the availability of both albuminuria and eGFR measurements. However, the findings of the current study should be considered in the context of certain limitations. Albuminuria and eGFR were assessed at a single time point, making misclassification of CKD status possible. In addition, although highly correlated, JHS used two different methods of measuring BP across study visits, potentially causing misclassification of aTRH status. We also did not have medication dosing information or a validated measure of antihypertensive medication adherence. Some participants may have been nonadherent to therapy or taking an inadequate treatment regimen and not truly treatment resistant.
6. CONCLUSIONS
Data from the current study suggest that black adults with CKD vs those without CKD have an increased risk for developing aTRH. Furthermore, higher ACR and lower eGFR values were each associated with an increased risk for aTRH. Intensive BP monitoring and early therapeutic interventions aimed at preventing the development of aTRH should be a high priority in blacks with CKD.
DISCLOSURES
This manuscript was submitted as an original investigation. All authors have read the manuscript and approve its submission. The manuscript has not been previously published and is not being considered for publication in another journal. The authors have no conflicts of interest to report.
Supporting information
ACKNOWLEDGMENTS
JHS is supported by contracts HHSN268201300046C, HHSN268201300047C, HHSN268201300048C, HHSN268201300049C, and HHSN268201300050C from the National Heart, Lung, and Blood Institute and the National Institute on Minority Health and Health Disparities. Additional support for the current analysis was provided by contracts R01 HL080477 from the National Heart, Lung, and Blood Institute and K24‐HL125704 from the National Institutes of Health. The authors thank the participants and data collection staff of JHS. The views expressed in this article are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the US Department of Health and Human Services.
Tanner RM, Shimbo D, Irvin MR, et al. Chronic kidney disease and incident apparent treatment‐resistant hypertension among blacks: Data from the Jackson Heart Study. J Clin Hypertens. 2017;19:1117–1124. 10.1111/jch.13065
REFERENCES
- 1. Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension. 2008;51:1403‐1419. [DOI] [PubMed] [Google Scholar]
- 2. Muntner P, Anderson A, Charleston J, et al. Hypertension awareness, treatment, and control in adults with CKD: results from the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis. 2010;55:441‐451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Parikh NI, Hwang SJ, Larson MG, Meigs JB, Levy D, Fox CS. Cardiovascular disease risk factors in chronic kidney disease: overall burden and rates of treatment and control. Arch Intern Med. 2006;166:1884‐1891. [DOI] [PubMed] [Google Scholar]
- 4. Coresh J, Wei GL, McQuillan G, et al. Prevalence of high blood pressure and elevated serum creatinine level in the United States: findings from the third National Health and Nutrition Examination Survey (1988‐1994). Arch Intern Med. 2001;161:1207‐1216. [DOI] [PubMed] [Google Scholar]
- 5. Thomas G, Xie D, Chen HY, et al. Prevalence and prognostic significance of apparent treatment resistant hypertension in chronic kidney disease: report from the Chronic Renal Insufficiency Cohort Study. Hypertension. 2016;67:387‐396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tanner RM, Calhoun DA, Bell EK, et al. Prevalence of apparent treatment‐resistant hypertension among individuals with CKD. Clin J Am Soc Nephrol. 2013;8:1583‐1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Julius S. The evidence for a pathophysiologic significance of the sympathetic overactivity in hypertension. Clin Exp Hypertens. 1996;18:305‐321. [DOI] [PubMed] [Google Scholar]
- 8. Campese VM, Mitra N, Sandee D. Hypertension in renal parenchymal disease: why is it so resistant to treatment? Kidney Int. 2006;69:967‐973. [DOI] [PubMed] [Google Scholar]
- 9. Flack JM, Duncan K, Ohmit SE, et al. Influence of albuminuria and glomerular filtration rate on blood pressure response to antihypertensive drug therapy. Vasc Health Risk Manag. 2007;3:1029‐1037. [PMC free article] [PubMed] [Google Scholar]
- 10. Egan BM, Zhao Y, Axon RN, Brzezinski WA, Ferdinand KC. Uncontrolled and apparent treatment resistant hypertension in the United States, 1988 to 2008. Circulation. 2011;124:1046‐1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Muntner P, Davis BR, Cushman WC, et al. Treatment‐resistant hypertension and the incidence of cardiovascular disease and end‐stage renal disease: results from the Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Hypertension. 2014;64:1012‐1021. [DOI] [PubMed] [Google Scholar]
- 12. Sempos CT, Bild DE, Manolio TA. Overview of the Jackson Heart Study: a study of cardiovascular diseases in African American men and women. Am J Med Sci. 1999;317:142‐146. [DOI] [PubMed] [Google Scholar]
- 13. Taylor HA . Jr, Wilson JG, Jones DW, et al. Toward resolution of cardiovascular health disparities in African Americans: design and methods of the Jackson Heart Study. Ethn Dis. 2005;4(suppl 6):S6‐4‐17. [PubMed] [Google Scholar]
- 14. Fuqua SR, Wyatt SB, Andrew ME, et al. Recruiting African‐American research participation in the Jackson Heart Study: methods, response rates, and sample description. Ethn Dis. 2005;4(suppl 6):S6‐S18. [PubMed] [Google Scholar]
- 15. Wang W, Young BA, Fulop T, et al. Effects of serum creatinine calibration on estimated renal function in African Americans: the Jackson Heart Study. Am J Med Sci. 2015;349:379‐384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604‐612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wyatt SB, Akylbekova EL, Wofford MR, et al. Prevalence, awareness, treatment, and control of hypertension in the Jackson Heart Study. Hypertension. 2008;51:650‐656. [DOI] [PubMed] [Google Scholar]
- 18. Abdalla M, Booth JN III, Seals SR, et al. Masked hypertension and incident clinic hypertension among blacks in the Jackson Heart Study. Hypertension. 2016;68:220‐226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206‐1252. [DOI] [PubMed] [Google Scholar]
- 20. Levey AS, Cattran D, Friedman A, et al. Proteinuria as a surrogate outcome in CKD: report of a scientific workshop sponsored by the National Kidney Foundation and the US Food and Drug Administration. Am J Kidney Dis. 2009;54:205‐226. [DOI] [PubMed] [Google Scholar]
- 21. Harvey JM, Howie AJ, Lee SJ, et al. Renal biopsy findings in hypertensive patients with proteinuria. Lancet. 1992;340:1435‐1436. [DOI] [PubMed] [Google Scholar]
- 22. Kestenbaum B, Rudser KD, de Boer IH, et al. Differences in kidney function and incident hypertension: the Multi‐Ethnic Study of Atherosclerosis. Ann Intern Med. 2008;148:501‐508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Daugherty SL, Powers JD, Magid DJ, et al. Incidence and prognosis of resistant hypertension in hypertensive patients. Circulation. 2012;125:1635‐1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tanner RM, Calhoun DA, Bell EK, et al. Incident ESRD and treatment‐resistant hypertension: the reasons for geographic and racial differences in stroke (REGARDS) study. Am J Kidney Dis. 2014;63:781‐788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McClellan W, Warnock DG, McClure L, et al. Racial differences in the prevalence of chronic kidney disease among participants in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Cohort Study. J Am Soc Nephrol. 2006;17:1710‐1715. [DOI] [PubMed] [Google Scholar]
- 26. McClellan WM, Warnock DG, Judd S, et al. Albuminuria and racial disparities in the risk for ESRD. J Am Soc Nephrol. 2011;22:1721‐1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. USRDS . 2015 United States Renal Data System annual data report: epidemiology of kidney disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2015. [Google Scholar]
- 28. Lackland DT. Racial differences in hypertension: implications for high blood pressure management. Am J Med Sci. 2014;348:135‐138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Khosla N, Kalaitzidis R, Bakris GL. The kidney, hypertension, and remaining challenges. Med Clin North Am. 2009;93:697‐715. [DOI] [PubMed] [Google Scholar]
- 30. Wright JT Jr, Agodoa L, Contreras G, et al. Successful blood pressure control in the African American Study of Kidney Disease and Hypertension. Arch Intern Med. 2002;162:1636‐1643. [DOI] [PubMed] [Google Scholar]
- 31. Sarnak MJ, Greene T, Wang X, et al. The effect of a lower target blood pressure on the progression of kidney disease: long‐term follow‐up of the modification of diet in renal disease study. Ann Intern Med. 2005;142:342‐351. [DOI] [PubMed] [Google Scholar]
- 32. Appel LJ, Wright JT Jr, Greene T, et al. Intensive blood‐pressure control in hypertensive chronic kidney disease. N Engl J Med. 2010;363:918‐929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. ACCORD Study Group , Cushman WC, Evans GW, et al. Effects of intensive blood‐pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575‐1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. De Nicola L, Gabbai FB, Agarwal R, et al. Prevalence and prognostic role of resistant hypertension in chronic kidney disease patients. J Am Coll Cardiol. 2013;61:2461‐2467. [DOI] [PubMed] [Google Scholar]
- 35. Kumbhani DJ, Steg PG, Cannon CP, et al. Resistant hypertension: a frequent and ominous finding among hypertensive patients with atherothrombosis. Eur Heart J. 2013;34:1204‐1214. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


