Abstract
Background & Aims
Obesity, kidney disease, and diabetes are common conditions that can affect outcomes of patients with chronic hepatitis C. We aimed to quantify the burden of these comorbid conditions among adults with chronic hepatitis C in the United States and to estimate the risk of death among people with chronic hepatitis C and comorbidities.
Methods
We conducted cross-sectional and prospective analyses of 13,726 participants in the third National Health and Nutrition Examination Survey (NHANES III) and 23,691 participants of NHANES 1999–2012. Serum samples were analyzed for the presence of antibodies to hepatitis C virus (anti-HCV); in samples found to be positive for anti-HCV, we quantified HCV RNA (viral load). Individuals with anti-HCV and detectable HCV RNA were considered to have chronic hepatitis C. Comorbidities were defined using self-reported, physical examination, and laboratory data, as available. We used logistic models and predictive margins to estimate the adjusted prevalence of comorbidities in patients with chronic hepatitis C. We used Poisson regression models to estimate adjusted mortality rates based on chronic hepatitis C status, with or without comorbidities. Cox proportional hazard regression models to estimate adjusted hazards ratios and 95% CIs of all-cause, cardiovascular, and cancer mortality according to chronic hepatitis C status, with and without comorbidities.
Results
Among persons with chronic hepatitis C, the demographic-adjusted prevalence estimate of diabetes was 17.9% (95% CI, 11.2–27.5) and of obesity was 20.9% (95% CI, 12.4–29.5). Overall, 69.6% of persons with chronic hepatitis C had at least 1 major cardiometabolic comorbidity (95% CI, 62.1%–76.2%). Only 38% of adults with chronic hepatitis C reported a diagnosis of liver disease. Chronic hepatitis C was associated with a substantially increased risk of death (hazard ratio, 2.45), especially in the presence of diabetes (hazard ratio, 3.24) or chronic kidney disease (hazard ratio, 4.39).
Conclusion
In an analysis of NHANES data, we found that individuals with chronic hepatitis C have a high burden of major cardiometabolic comorbidities. Diabetes and chronic kidney disease, in particular, are associated with substantial excess mortality in persons with chronic hepatitis C.
Keywords: viral hepatitis, epidemiology, complications, risk factors
Chronic hepatitis C virus (HCV) infection is one of the leading causes of liver-related morbidity and mortality in the U.S. and many other countries1, 2. While the exact prevalence of chronic HCV infection in the US is unknown, estimates from the 2003–2010 National Health and Nutrition Examination Survey (NHANES)–a sample of the civilian non-institutionalized population of the U.S.–indicate that at least ~1% have chronic hepatitis C3. Nonetheless, the burden of HCV is much higher in some subgroups of the population, with estimates ranging from 6% to 35% in male Veterans4 and 10 to 46% among incarcerated individuals5. Updated national data are needed for public health planning, especially in the context of the major recent therapeutic advances for the treatment of HCV infection that have substantially improved prognosis6.
Of particular interest is documenting the full burden and implications of comorbid conditions such as obesity, diabetes and kidney disease among individuals with HCV. According to current clinical guidelines, chronic hepatitis C patients with comorbidities such as HIV or advanced fibrosis have the highest urgency for treatment as their risk for progression is very high7, 8. A number of studies suggest comorbidities such as obesity and diabetes are also associated with increased risk for progression9–11. However, to our knowledge, the current the burden of common co-morbidities among individuals with chronic hepatitis C in the U.S. population is unknown, and their prognostic significance remains unproven. Such data are critical given new screening and treatment guidelines that recommend universal screening among baby-boomers and, from a public health perspective, the need to prioritize patients for treatment in the setting of the high costs and limited resources.
We analyzed the most recent national data from the 1999–2012 NHANES to provide estimates of the prevalence of chronic hepatitis C and the burden of comorbid conditions in individuals infected with chronic hepatitis C in the general U.S. population. We also pooled data from NHANES III (1988–1994) with the continuous NHANES (1999–2010) surveys to comprehensively examine the risk of all-cause and cause-specific death (cardiovascular or cancer) among individuals with chronic hepatitis C alone or in combination with other comorbidities.
Methods
The plan and operation of NHANES can be found in elsewhere12, 13. Briefly, the NHANES are cross-sectional, multistage, stratified, clustered probability samples of the U.S. civilian non-institutionalized population conducted by the National Center for Health Statistics (NCHS), a branch of the Centers for Disease Control (CDC) and Prevention. Individuals participated in an interview conducted at home and also in an extensive physical examination performed at a Mobile Examination Center, which included blood collection.
For the current analyses, we limited our study population to NHANES 1999–2012 participants aged 20 years or older who provided a blood sample and were not missing antibodies to HCV (anti-HCV) and HCV RNA test results. We further excluded individuals with missing data on important covariates, for a total analytical sample of n=23,691. To enhance our sample size for mortality analyses, we also included 13,726 NHANES III (1988–1994) participants aged 20 years or older who were not missing anti-HCV and HCV RNA test results or other important covariates.
The NHANES protocol was reviewed and approved by the Institutional Review Board of the CDC and all participants provided written informed consent.
In the NHANES 1999–2012, serum samples were analyzed for anti-HCV using Ortho HCV enzyme-linked immunosorbent assay (ELISA), version 3.0 (Ortho-Clinical Diagnostics). Specimens that tested positive were then tested using a confirmatory recombinant immunoblot assay (RIBA HCV 3.0 Strip Immunoblot Assay, Chiron). Samples with positive results on both tests are classified as confirmed positive for anti-hepatitis C. Samples that were anti-HCV positive or indeterminate were further tested for HCV RNA using a nucleic acid amplification test for the quantitation of HCV RNA (COBAS AMPLICOR HCV Test [survey years 2005–10], and COBAS AmpliPrep/TaqMan HCV Test, version 2 [years 1999–2004]. Individuals were considered anti-HCV negative if they either had a negative ELISA or a positive ELISA not confirmed by RIBA. Individuals were considered chronically infected if they had detectable HCV RNA.
In the NHANES III, serum samples were tested for antibodies to HCV (anti-HCV) using a second-generation ELISA test (EIA 2.0) and a confirmatory test (HCV MATRIX, Abbott Laboratories). Samples with positive results on the HCV MATRIX were considered positive for anti-hepatitis C. Samples that were anti-HCV positive were further tested for HCV RNA using reverse-transcriptase-polymerase-chain-reaction (RT-PCR) methods.
Additional details regarding methods are described in the online-only data supplement.
Statistical Analyses
We accounted for the complex NHANES sampling design in all analyses and incorporated sampling weights to generate nationally representative estimates. We used the Taylor series (linearization) method to calculate the standard errors.
To estimate the number of persons with chronic hepatitis C or who were anti-HCV positive, we applied our prevalence estimates to the 2010 U.S. Census Population. We used logistic models, adjusting for age, sex and race/ethnicity and used predictive margins to estimate the adjusted prevalence of selected comorbidities by chronic hepatitis C or anti-HCV status.
For the mortality analyses, we pooled data from NHANES 1999–2010 and NHANES III and used Poisson regression models with adjustment for age, age squared, sex, and race/ethnicity, to estimate the adjusted mortality rates and 95% confidence intervals by chronic hepatitis C status with or without selected comorbidities. Hazard ratios and 95% confidence intervals of all-cause, cardiovascular, and cancer mortality according to chronic hepatitis C status, with or without other comorbidity were estimated using Cox proportional hazards regression, with age as the timescale. We used three models: Model 1 included sex, race/ethnicity, and education; Model 2 further adjusted for smoking and alcohol consumption; and Model 3 further adjusted for diabetes, hypertension, albuminuria, and body mass index.
Kaplan-Meier survival curves were used to compare the mortality by key subgroups defined by the following comorbidities: chronic hepatitis C, diabetes, obesity, and any chronic kidney disease. We excluded participants with events or lost to follow up before the age of 45 years of age. All analyses were conducted using Stata/SE, version 13.
Results
During 1999–2012, in the U.S. general, noninstitutionalized, adult population, the overall prevalence of anti-HCV positivity was 1.8% (95% CI 1.6–2.0), representing approximately 4 million (95% CI 3.5–4.4) adults aged 20 or older in 2010. The overall prevalence of chronic hepatitis C was 1.2% (95% CI 1.0–1.4), or 2.6 million (95% CI 2.30–3.03) adults. As shown in Figure 1, there are marked differences in the prevalence of chronic hepatitis C (and anti-HCV positivity) by age, and by birth cohort, and over time there has been a shift in the distribution from younger to old age, consistent with the aging of the “baby boomer” population (i.e. those persons who were born in the years 1945 to 1965). Across survey periods, there was not a significant trend in the prevalence of chronic hepatitis C (online-only Supplemental Figure 1).
Figure 1.
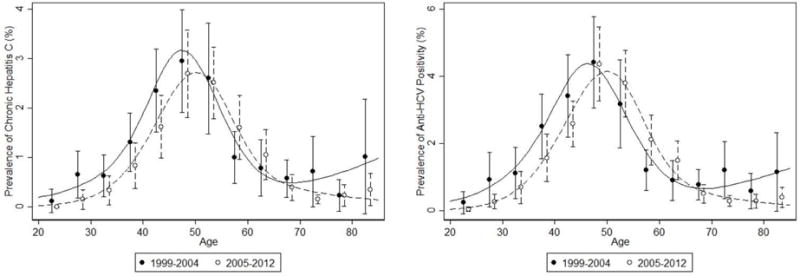
Prevalencea of Chronic Hepatitis C and anti-HCV positivity by Survey Year and age, among the US non-institutionalized adult Population in the U.S., 1999–2012.
aUnadjusted prevalence with underlying plot from a Poisson regression with cubic splines with knots as 25, 35, 45, 55, 65 and 75 years
There was a higher prevalence of chronic hepatitis C among non-Hispanic blacks, men, persons at or below poverty threshold, and persons with less than high school of education (online-only Supplemental Table 1). Individuals with chronic hepatitis C were significantly more likely to report a history of injection drug use (52.4% vs. 2.2%, p-value <0.001) or a history of blood transfusion (24.5% vs. 11.2%, p-value <0.001), as compared to their counterparts without chronic hepatitis C. Additionally, individuals with chronic hepatitis C were more likely to be anti-HBc positive (34.3% vs. 4.8%, p-value <0.001) or HIV positive (1.2% vs 0.4%, p-value= 0.02); however, there was not a statistically significant difference in the prevalence of HBsAg positivity by HCV status (0.7% vs 0.3%, p value=0.19). Persons with chronic hepatitis C were substantially more likely to report current smoking (62.0% vs. 22.2%, p-value <0.001), high current alcohol consumption (61.5% vs. 40.7%) or being a former drinker (16.4% vs. 9.2%, p-value <0.001) (online-only Supplemental Table 2).
Even after accounting for demographic differences, persons with chronic hepatitis C were disproportionally affected by major metabolic, cardiovascular and renal comorbidities (Table 1). Indeed, despite a lower prevalence of obesity (21.7% vs. 33.3%, p-value <0.001) persons with chronic hepatitis C had a higher prevalence of diabetes (16.1% vs. 9.1%, p value=0.02) as compared to persons without chronic hepatitis C. Based on these data, it can be estimated that in 2010, approximately 418,000 U.S. adults (95%CI: 200,000–700,000) had both chronic hepatitis C and diabetes, a similar number of individuals had chronic hepatitis C and albuminuria, and approximately 600,000 (95%CI: 300,000–800,000) had both chronic hepatitis C and obesity. Hypertension, prevalent cardiovascular disease, and albuminuria were all significantly more common in persons with chronic hepatitis C (Table 1). Overall, 69.6% (95% CI: 62.1%–76.2%) of persons with chronic hepatitis C had at least one of the following cardiometabolic and renal comorbidities: diabetes, obesity, hypertension, hypercholesterolemia, reduced kidney function or macroalbuminuria.
Table 1.
Age-,sex-,race/ethnicity-adjusted prevalence estimates (95% CIs) and odds ratios (95% CIs) of selected metabolic, cardiovascular, and renal co-morbid conditions in the US adult population (NHANES 1999–2012) by chronic hepatitis C virus status
| Chronic Hepatitis C | No Chronic Hepatitis C | Odds Ratio (95% CI) | |
|---|---|---|---|
| Diabetesa, % | 16.1 (9.0–23.1) | 9.1 (8.3–9.9) | 2.1 (1.1–3.7) |
| Hypertension, % | 35.2 (30.5–39.8) | 29.0 (28.1–30.0) | 1.4 (1.1–1.8) |
| Congestive Heart Failure, % | 5.3 (2.4–8.2) | 2.3 (2.1–2.5) | 2.5 (1.3–4.8) |
| Stroke, % | 4.2 (2.2–6.2) | 2.7 (2.4–2.9) | 1.6 (1.0–2.8) |
| Coronary Heart Disease, % | 2.1 (0.6–3.6) | 3.4 (3.1–3.6) | 0.6 (0.3–1.3) |
| Heart Attack, % | 3.7 (1.6–5.7) | 3.4 (3.1–3.6) | 1.1 (0.6–2.0) |
| Any cancer, % | 0.1 (0.06–0.13) | 0.1 (0.08–0.09) | 1.2 (0.7–1.8) |
| Non-liver cancer, % | 0.1 (0.06–0.12) | 0.1 (0.08–0.09) | 1.1 (0.7–1.7) |
| Hypolipidemiac, % | |||
| Low total cholesterol | 43.7 (38.6–48.8) | 31.5 (30.8–32.2) | 1.7 (1.4–2.1) |
| Low LDL cholesterolc | 50.5 (38.3–62.8) | 29.3 (28.3–30.4) | 2.5 (1.5–4.1) |
| Low HDL cholesterol | 22.7 (18.1–27.3) | 23.2 (22.3–24.1) | 1.0 (0.7–1.3) |
| Low triglyceridesc | 37.4 (29.1–45.6) | 29.1 (27.9–30.3) | 1.5 (1.0–2.2) |
| Albuminuria >30 mg/g, % | 12.0 (9.0–15.0) | 9.6 (9.1–10.0) | 1.3 (1.0–1.8) |
| eGFR<60 mL/min/1.73m2, % | 8.2 (6.2–10.2) | 6.8 (6.4–7.2) | 1.3 (0.9–2.0) |
| BMI category, % | |||
| Underweight, <18.5 kg/m2 | 2.1 (0.1–4.2) | 1.8 (1.6–1.9) | 0.9 (0.3–2.2) |
| Normal, 18.5-<25 kg/m2 | 41.9 (35.6–48.1) | 30.8 (29.9–31.7) | 1 (reference) |
| Overweight, 25-<30 kg/m2 | 34.2 (29.3–39.2) | 34.2 (33.3–35.0) | 0.7 (0.6–1.0) |
| Obese, ≥30 kg/m2 | 21.7 (16.7–26.7) | 33.3 (32.2–34.2) | 0.5 (0.3–0.7) |
| Anemia, % | 5.6 (3.5–7.6) | 7.4 (6.9–8.0) | 0.7 (0.5–1.1) |
Only among those fasting and in the morning exam.
Lowest sex-specific quartile of each lipid parameter: Total cholesterol <177 mg/dL for men and <180 mg/dL for women; LDL-cholesterol: <100 mg/dL for men and <96 for women; HDL-cholesterol: <38 mg/dL for men and <46 mg/dL for women; triglycerides: <90 mg/dL for men and <79 mg/dL for women.
There were substantial disparities in measures of healthcare access and utilization related to chronic hepatitis C status, with 20.9% of persons with hepatitis C reporting no routine place for health care and 36% reporting no health insurance. Further, individuals with chronic hepatitis C were significantly more likely to have had one or more hospitalizations in the past 12 months and more days with poor physical and mental health compared to those without chronic hepatitis C (online-only Supplemental Table 3).
Only 38% of those with chronic hepatitis C reported physician diagnosis of any liver disease, yet persons with chronic hepatitis C had significantly higher prevalence of liver-related laboratory abnormalities: more than 2/3 of those with chronic hepatitis C had elevated liver enzymes and 14% had elevated APRI (≥1.5), suggesting the presence of significant liver fibrosis (Table 2).
Table 2.
Age-, sex-,race/ethnicity-adjusted prevalence (95% CI) and odds ratios (95% CIs) of liver related abnormalities in the U.S. adult population (NHANES 1999–2012) by chronic hepatitis C virus status
| Chronic Hepatitis C | No Chronic Hepatitis C | Odds ratio (95% CI) | |
|---|---|---|---|
| Awareness of Liver Disease | 37.6 (32.2–43.1) | 2.7 (2.5–2.9) | 23.2 (17.8, 30.1) |
| Low albumina, % | 4.3 (2.1–6.6) | 1.2 (1.0–1.3) | 3.9 (2.2–6.8) |
| Low platelets b, % | 12.6 (5.1–20.2) | 1.1 (1.0–1.3) | 13.0 (6.3–26.7) |
| Elevated ALTc, % | 65.1 (58.5–71.7) | 11.6 (11.2–12.1) | 15.2 (11.2–20.6) |
| Elevated ASTc, % | 65.6 (59.7–71.5) | 8.2 (7.9–8.6) | 21.5 (16.5–28.0) |
| Elevated GGTc, % | 58.6 (52.4–64.7) | 13.5 (12.9–14.0) | 9.4 (7.2–12.2) |
| Intermediate APRI (≥0.5), % | 45.9 (40.7–51.1) | 6.4 (6.0–6.7) | 13.0 (10.4–16.2) |
| Elevated APRI (≥1.5), % | 13.7 (8.6–18.8) | 0.3 (0.2–0.4) | 53.1 (32.7–86.4) |
| Elevated FIB-4 (≥1.3), % | 0.9 (0.1–1.8) | 0.1 (0.1–0.2) | 6.3 (1.9–20.9) |
Low albumin: <3.5 mg/dL;
Low platelets: <100,000 × 103 cells/μL;
Elevated ALT: Male: >41 U/L, Female: >31 U/L; Elevated AST: Male: >37 U/L, Female: >31 U/L; Elevated GGT: Male: >49 U/L, Female: >32 U/L.
There were few individuals who were anti-HCV positive and HCV RNA negative (n=123), compared to these individuals, those with chronic hepatitis C were older, more likely to be male, non-Hispanic Black, at or below poverty threshold, had less than high school of education, more likely to be current smokers, and report high current alcohol consumption. Furthermore, individuals with chronic hepatitis C had higher prevalence of hypertension and liver-related abnormalities, but were less likely to have obesity compared to those with anti-HCV positive and HCV RNA negative results (online-only Supplemental Table 4).
During a median follow up of 8.7 years (range 1–23 years), there were 5,988 deaths (including 1,800 from cardiovascular causes, and 1,367 from cancer) among 36,198 persons with mortality follow up. The incidence rates of death in persons with chronic hepatitis C vs those without were: 16.1 vs 10.7 per 1,000 person years (p<0.01).
Chronic hepatitis C was associated with a significantly increased risk of all-cause mortality (HR 3.47, 95% CI 2.56–4.70) (Table 3). This association was somewhat attenuated but remained elevated after adjustment for sociodemographic characteristics, alcohol consumption and smoking (HR 2.45, 95%CI 1.81–3.31). Further adjustment for comorbidities did not appreciably alter these results. Individuals with chronic hepatitis C were at significantly increased risk of cardiovascular mortality (HR 2.55, 95%CI 1.38–4.71). This association remained elevated even after further adjustment for sociodemographic characteristics, alcohol and smoking and comorbidities (HR 1.86, 95%CI 1.04–3.35).
Table 3.
Hazard Ratios (95%CI) for all-cause, cardiovascular and cancer mortality by chronic hepatitis C status in the U.S. adult population (NHANES III 1988–1994 and NHANES 1999–2012) (follow-up to December 31, 2011)
| Unweighted N | No CHC 35,671 | CHC 527 |
|---|---|---|
| All-Cause Mortality | ||
|
| ||
| Deaths, un-weighted n | 5,873 | 115 |
| Hazard Ratio (95%CI) | ||
| Unadjusted | 1 [Reference] |
3.47 (2.56–4.70) |
| Model 1a | 1 [Reference] |
2.93 (2.15–3.98) |
| Model 2b | 1 [Reference] |
2.45 (1.81–3.31) |
| Model 3c | 1 [Reference] |
2.44 (1.80–3.30) |
|
| ||
| Cardiovascular and cerebrovascular disease mortality | ||
|
| ||
| Deaths, un-weighted n | 1,782 | 18 |
| Hazard Ratio (95%CI) | ||
| Unadjusted | 1 [Reference] |
2.55 (1.38–4.71) |
| Model 1a | 1 [Reference] |
2.18 (1.18–4.03) |
| Model 2b | 1 [Reference] |
1.87 (1.03–3.42) |
| Model 3 c | 1 [Reference] |
1.85 (1.03–3.33) |
|
| ||
| All Cancer mortality | ||
|
| ||
| Deaths, un-weighted n | 1,346 | 21 |
| Hazard Ratio (95%CI) | ||
| Unadjusted | 1 [Reference] |
1.75 (0.90–3.40) |
| Model 1a | 1 [Reference] |
1.46 (0.75–2.85) |
| Model 2b | 1 [Reference] |
1.16 (0.60–2.25) |
| Model 3c | 1 [Reference] |
1.16 (0.60–2.24) |
Model 1: Adjusted for age, age squared, sex, race/ethnicity, education
Model 2: Further adjusted for alcohol consumption (never, low, moderate consumption) and smoking (never, former, current)
Model 3: Further adjusted for diabetes (yes, no), hypertension (yes, no), albuminuria (yes, no), BMI (WHO categories)
In the analyses examining the mortality across key subgroups defined by the presence of comorbidities, the absolute risk of death in persons with chronic hepatitis C was substantially higher among those with diabetes compared to those without diabetes [adjusted mortality rate (per 1000 person-years): 247.8 vs. 139.8]. Among chronic hepatitis C patients with and without chronic kidney disease, the adjusted mortality rates were 210.0 vs 131.5 per 1000 person-years, respectively. In the setting of chronic hepatitis C, both diabetes and chronic kidney disease were associated with substantially reduced survival: median survival was 9 years lower in persons with diabetes and 8.5 years lower in persons with chronic kidney disease (Figure 2 and Online-only Table 5).
Figure 2.
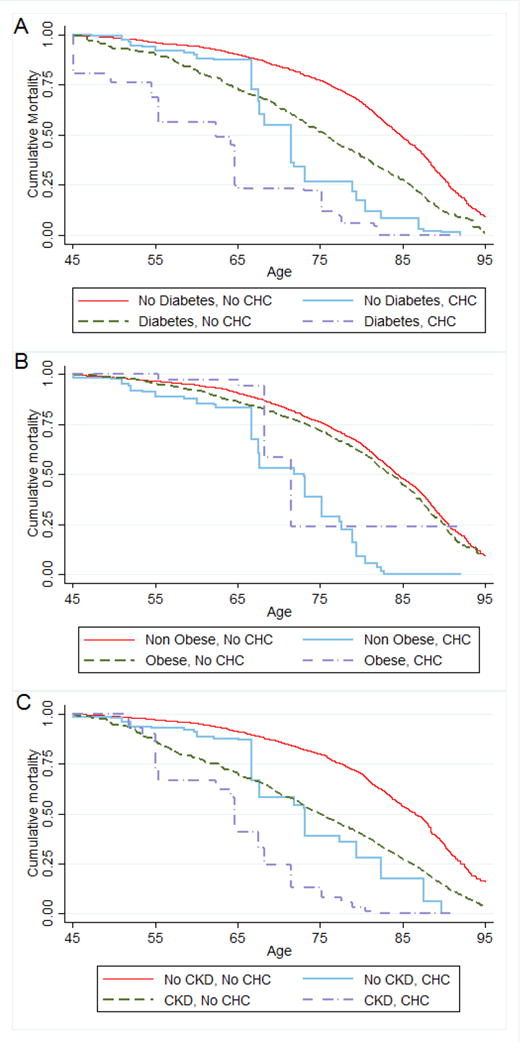
Kaplan-Meier cumulative mortality curves for subgroups defined by comorbidity status: a) Diabetes and Chronic Hepatitis C (CHC), b) Obesity and Chronic Hepatitis C, and c) Any Chronic Kidney Disease (CKD) and Chronic Hepatitis C. U.S. Adult Population NHANES 1988–1994 and 1999–2010
Mortality Follow up Through December of 2011
Discussion
This study provides rigorous quantification of the comorbid status of HCV infected patients in the general U.S. noninstitutionalized population. Overall, 70% of persons with chronic hepatitis C had at least one of the following cardiometabolic and renal comorbidities: diabetes, obesity, hypertension, hypercholesterolemia, reduced kidney function or macroalbuminuria. The high prevalence of obesity and diabetes in persons with chronic hepatitis C is noteworthy and worrisome given the association of diabetes and obesity, with faster progression of liver disease to fibrosis and cirrhosis10, 11, 14–16.
We documented an increased risk of not only total mortality but also cardiovascular mortality among individuals with hepatitis C infection (HR, 2.55). This association was attenuated but remained significant (HR, 1.85) after adjusting for traditional cardiovascular risk factors. To our knowledge, this is a novel finding and further studies are needed to examine the mechanisms and explore specific cardiovascular disease subtypes that might be driving the observed association. Our study demonstrated a substantially increased risk of death among persons with both chronic hepatitis C and diabetes and/or chronic kidney disease in the general population. These results are important given that both chronic kidney disease and diabetes have reached epidemic proportions in the general U.S population and also among those with hepatitis C, particularly in older adults.
Recent randomized clinical trials of direct -acting antivirals (DAA) have demonstrated that more than 90% patients can achieve sustained virological response, however, the vast majority of clinical trials testing the effects of treatments in chronic hepatitis C have included relatively homogeneous populations and have tended to show universal benefit. Indeed, few trials have included substantial numbers of overweight or obese persons or many persons with diabetes. Further, patients with low kidney function have generally been excluded from recent trials17–20. Most of the recent trials have focused on subgroups defined by genotype of the hepatitis C virus, liver fibrosis stage, or parameters of severity of the infection (e.g. viral load); more effort is needed to assess the effectiveness of treatment in patients with common co-morbid conditions.
The association between hepatitis C and diabetes and chronic kidney disease has been reported previously, including prior studies using NHANES data21–28. Previous NHANES studies have also provided valuable information on the overall hepatitis C prevalence (up until 2010)3, 29, 30 and key risk factors3, 29–31. Our study expands on prior work in NHANES on the epidemiology of hepatitis C in the general U.S. population and extend these findings in two major ways: Our study provides the most recent national estimates of the overall burden of chronic hepatitis C and of common comorbid conditions (obesity, diabetes, chronic kidney disease) in the setting of hepatitis C in U.S. adults. We also provide estimates of the risk of total and cause-specific mortality associated with HCV in the presence and absence of these important and common co-morbid conditions, in the general U.S. population, a previous study had reported an increased risk of all-cause mortality but did not include data regarding cardiovascular mortality32.
Our study has certain limitations that should be considered in the interpretation of these results. With the exception of the mortality analyses, our study was cross-sectional and therefore we cannot establish the temporality of the observed associations. In addition, for some conditions, we relied on self-reported data, which is likely to be highly specific for most medial conditions but likely resulted in some degree of under ascertainment. NHANES does not include incarcerated individuals, persons in nursing homes, hospitals, or those who are homeless, all populations who are disproportionally affected by HCV infection5, 33. Thus, our prevalence estimates are likely to be underestimates of the true burden of chronic hepatitis C in the U.S. Combining estimates from non-institutionalized, institutionalized samples and other sources (e.g. Indian reservations), Edlin estimated that at least 3.5 million of US adult are currently infected with HCV34. There were only a very small number of individuals who were anti-HCV positive and HCV RNA negative, preventing us from rigorously examining this subgroup. For individuals with chronic hepatitis C we did not have information on treatment. Lastly, we were not able to examine associations with causes of death other than cancer or cardiovascular disease nor examine specific cardiovascular mortality subtypes.
Strengths of our study include the large and nationally representative sample of civilian noninstitutionalized U.S. adults in NHANES. We were able to examine a comprehensive number of demographic, socioeconomic, health-care related variables and objectively and rigorously measured health conditions. All measurements were obtained by trained personnel using standardized data collection procedures and protocols.
In conclusion, our results highlight the significant burden of co-morbid conditions in patients with chronic hepatitis C and the substantial excess risk of mortality in persons with chronic hepatitis C who also have diabetes and chronic kidney disease, two highly prevalent conditions in the U.S. These findings have important implications for clinical practice and public health planning and should help to inform current controversies regarding prioritization of treatment and public health planning efforts.
Supplementary Material
Acknowledgments
The authors express their appreciation to Usama Bilal, MD, MPH, for his assistance with the preparation of figures and assistance with data analysis.
Funding and support:
Funding for this research was provided by Merck & Co., Inc. (professional service agreement to the National Kidney Foundation) and the National Kidney Foundation (grant to Johns Hopkins Bloomberg School of Public Health). Dr. Lazo was supported by a grant from the Johns Hopkins Institute for Clinical and Translational Research (ICTR) which is funded in part by Grant Number UL1TR001079 from the National Center for Advancing Translational Sciences (NCATS) a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Dr. Selvin was supported by NIH/NIDDK grant K24DK106414. Dr. Thomas was supported by R37DA013806.
Abbreviations
- HCV
Hepatitis C Virus
- HIV
Human Immunodeficiency
- NHANES
National Health and Nutrition Examination Survey
- NCHS
National Center for Health Statistics
- CDC
Centers for Disease Control and Prevention
- Anti-HCV
Hepatitis C antibody
- HCV RNA
HCV viral load
- DAA
Direct-acting antiviral
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
Dr. Nwankwo is an employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., who may own stock and/or hold stock options in the Company.
The other authors have nothing to disclose.
Author’s contributions
Dr. Lazo had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Lazo and Selvin
Acquisition, analysis, or interpretation of the data: All the authors.
Drafting of the manuscript: Lazo
Critical revision of the manuscript for important intellectual content: All the authors
Statistical analysis: Lazo and Daya
Funding: Selvin and Lazo
Supervision: Selvin
References
- 1.Holmberg SD, Spradling PR, Moorman AC, et al. Hepatitis C in the United States. N Engl J Med. 2013;368:1859–61. doi: 10.1056/NEJMp1302973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ly KN, Xing J, Klevens RM, et al. The increasing burden of mortality from viral hepatitis in the United States between 1999 and 2007. Ann Intern Med. 2012;156:271–8. doi: 10.7326/0003-4819-156-4-201202210-00004. [DOI] [PubMed] [Google Scholar]
- 3.Denniston MM, Jiles RB, Drobeniuc J, et al. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014;160:293–300. doi: 10.7326/M13-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beste LA, Ioannou GN. Prevalence and Treatment of Chronic Hepatitis C Virus Infection in the US Department of Veterans Affairs. Epidemiol Rev. 2015;37:131–43. doi: 10.1093/epirev/mxu002. [DOI] [PubMed] [Google Scholar]
- 5.Larney S, Mahowald MK, Scharff N, et al. Epidemiology of hepatitis C virus in Pennsylvania state prisons, 2004–2012: limitations of 1945–1965 birth cohort screening in correctional settings. Am J Public Health. 2014;104:e69–74. doi: 10.2105/AJPH.2014.301943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Innes HA, McDonald SA, Dillon JF, et al. Toward a more complete understanding of the association between a hepatitis C sustained viral response and cause-specific outcomes. Hepatology. 2015;62:355–64. doi: 10.1002/hep.27766. [DOI] [PubMed] [Google Scholar]
- 7.WHO. Guidelines for the Screening, Sare and Treatment of Persons with Hepatitis C Infection. 2014 [PubMed] [Google Scholar]
- 8.IAS-USA AI. Recommendations for Testing, Managing, and Treating Hepatitis C. 2015 [Google Scholar]
- 9.Charlton MR, Pockros PJ, Harrison SA. Impact of obesity on treatment of chronic hepatitis C. Hepatology. 2006;43:1177–86. doi: 10.1002/hep.21239. [DOI] [PubMed] [Google Scholar]
- 10.Negro F, Clement S. Impact of obesity, steatosis and insulin resistance on progression and response to therapy of hepatitis C. J Viral Hepat. 2009;16:681–8. doi: 10.1111/j.1365-2893.2009.01186.x. [DOI] [PubMed] [Google Scholar]
- 11.Petta S, Camma C, Di Marco V, et al. Insulin resistance and diabetes increase fibrosis in the liver of patients with genotype 1 HCV infection. Am J Gastroenterol. 2008;103:1136–44. doi: 10.1111/j.1572-0241.2008.01813.x. [DOI] [PubMed] [Google Scholar]
- 12.Zipf GC,M, Porter KS, et al. National Health and Nutrition Examination Survey: Plan and Operations, 1999–2010. Vol. 1. Vital Health Stat; 2013. [PubMed] [Google Scholar]
- 13.Health USDo, Human Services PHS. Plan and operation of the Third National Health and Nutrition Examination Survey, 1988–1994. Hyattsville, MD: National Center for Health Statistics; 1994. [Google Scholar]
- 14.Asselah T, Rubbia-Brandt L, Marcellin P, et al. Steatosis in chronic hepatitis C: why does it really matter? Gut. 2006;55:123–30. doi: 10.1136/gut.2005.069757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hung CH, Wang JH, Hu TH, et al. Insulin resistance is associated with hepatocellular carcinoma in chronic hepatitis C infection. World J Gastroenterol. 2010;16:2265–71. doi: 10.3748/wjg.v16.i18.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leandro G, Mangia A, Hui J, et al. Relationship between steatosis, inflammation, and fibrosis in chronic hepatitis C: a meta-analysis of individual patient data. Gastroenterology. 2006;130:1636–42. doi: 10.1053/j.gastro.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 17.Afdhal N, Reddy KR, Nelson DR, et al. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med. 2014;370:1483–93. doi: 10.1056/NEJMoa1316366. [DOI] [PubMed] [Google Scholar]
- 18.Afdhal N, Zeuzem S, Kwo P, et al. Ledipasvir and Sofosbuvir for Untreated HCV Genotype 1 Infection. N Engl J Med. 2014;370:1889–98. doi: 10.1056/NEJMoa1402454. [DOI] [PubMed] [Google Scholar]
- 19.Ferenci P, Bernstein D, Lalezari J, et al. ABT-450/r-ombitasvir and dasabuvir with or without ribavirin for HCV. N Engl J Med. 2014;370:1983–92. doi: 10.1056/NEJMoa1402338. [DOI] [PubMed] [Google Scholar]
- 20.Jacobson IM, Dore GJ, Foster GR, et al. Simeprevir with pegylated interferon alfa 2a plus ribavirin in treatment-naive patients with chronic hepatitis C virus genotype 1 infection (QUEST-1): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet. 2014;384:403–13. doi: 10.1016/S0140-6736(14)60494-3. [DOI] [PubMed] [Google Scholar]
- 21.Stepanova M, Lam B, Younossi Y, et al. Association of hepatitis C with insulin resistance and type 2 diabetes in US general population: the impact of the epidemic of obesity. J Viral Hepat. 2012;19:341–5. doi: 10.1111/j.1365-2893.2011.01554.x. [DOI] [PubMed] [Google Scholar]
- 22.Younossi ZM, Stepanova M, Nader F, et al. Associations of chronic hepatitis C with metabolic and cardiac outcomes. Aliment Pharmacol Ther. 2013;37:647–52. doi: 10.1111/apt.12234. [DOI] [PubMed] [Google Scholar]
- 23.Lao XQ, Thompson A, McHutchison JG, et al. Sex and age differences in lipid response to chronic infection with the hepatitis C virus in the United States National Health and Nutrition Examination Surveys. J Viral Hepat. 2011;18:571–9. doi: 10.1111/j.1365-2893.2010.01347.x. [DOI] [PubMed] [Google Scholar]
- 24.Liangpunsakul S, Chalasani N. Relationship between hepatitis C and microalbuminuria: results from the NHANES III. Kidney Int. 2005;67:285–90. doi: 10.1111/j.1523-1755.2005.00080.x. [DOI] [PubMed] [Google Scholar]
- 25.Shaheen M, Echeverry D, Oblad MG, et al. Hepatitis C, metabolic syndrome, and inflammatory markers: results from the Third National Health and Nutrition Examination Survey [NHANES III] Diabetes Res Clin Pract. 2007;75:320–6. doi: 10.1016/j.diabres.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 26.Streiff MB, Mehta S, Thomas DL. Peripheral blood count abnormalities among patients with hepatitis C in the United States. Hepatology. 2002;35:947–52. doi: 10.1053/jhep.2002.32486. [DOI] [PubMed] [Google Scholar]
- 27.Tsui JI, Vittinghoff E, Shlipak MG, et al. Relationship between hepatitis C and chronic kidney disease: results from the Third National Health and Nutrition Examination Survey. J Am Soc Nephrol. 2006;17:1168–74. doi: 10.1681/ASN.2005091006. [DOI] [PubMed] [Google Scholar]
- 28.Mehta SH, Brancati FL, Sulkowski MS, et al. Prevalence of type 2 diabetes mellitus among persons with hepatitis C virus infection in the United States. Ann Intern Med. 2000;133:592–9. doi: 10.7326/0003-4819-133-8-200010170-00009. [DOI] [PubMed] [Google Scholar]
- 29.Armstrong GL, Wasley A, Simard EP, et al. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144:705–14. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- 30.Ditah I, Ditah F, Devaki P, et al. The changing epidemiology of hepatitis C virus infection in the United States: National Health and Nutrition Examination Survey 2001 through 2010. J Hepatol. 2014;60:691–8. doi: 10.1016/j.jhep.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 31.Kuniholm MH, Jung M, Everhart JE, et al. Prevalence of hepatitis C virus infection in US Hispanic/Latino adults: results from the NHANES 2007–2010 and HCHS/SOL studies. J Infect Dis. 2014;209:1585–90. doi: 10.1093/infdis/jit672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.El-Kamary SS, Jhaveri R, Shardell MD. All-cause, liver-related, and non-liver-related mortality among HCV-infected individuals in the general US population. Clin Infect Dis. 2011;53:150–7. doi: 10.1093/cid/cir306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuehn BM. Estimate of new chronic HCV cases lower than expected. Jama. 2014;311:1188–9. doi: 10.1001/jama.2014.2918. [DOI] [PubMed] [Google Scholar]
- 34.Edlin BR, Eckhardt BJ, Shu MA, et al. Toward a more accurate estimate of the prevalence of hepatitis C in the United States. Hepatology. 2015;62:1353–63. doi: 10.1002/hep.27978. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


