Abstract
Autophagy is a catabolic cellular process that targets cytosolic material, including mitochondria, to the vacuole or lysosomes for degradation. The selective degradation of mitochondria by autophagy is termed mitophagy. Dysfunctional mitophagy, which leads to the accumulation of damaged mitochondria, has been implicated in Parkinson's disease, cancer, cardiac disease and metabolic disease. In Saccharomyces cerevisiae, mitophagy is initiated by the autophagy receptor Atg32, an outer mitochondrial membrane protein. A lack of structural information for Atg32 has hindered our understanding of the molecular mechanisms of mitophagy initiation. To gain new structural insight into Atg32, we have identified the location of a structured domain within the cytosolic region of Atg32 and completed the backbone and side chain resonance assignments for this domain.
Keywords: autophagy, mitophagy, Atg32, NMR
Biological Context
Macroautophagy (hereafter autophagy) is a cellular process in which cytosolic material is captured in double membrane vesicles, termed autophagosomes (Yin et al. 2016). Completed autophagosomes fuse with the vacuole or lysosomes resulting in the degradation of their cargo. The capture of cargo during autophagy can occur through either a non-selective or a selective mechanism. In selective autophagy, cargos to be degraded are identified by autophagy receptors. These receptors constitute a diverse set of proteins that may be localized in the membrane of the cargo, recognize the cargo directly or recognize ubiquitin moieties on the cargo (Johansen and Lamark 2011). Despite the diversity in these receptors, they all share a common 4 amino acid sequence, termed the Atg8 family interacting motif (AIM). The AIM is recognized by the ubiquitin like proteins Atg8 in Saccharomyces cerevisiae or the LC3 family of proteins in mammals (Noda et al. 2010). During autophagosomal biogenesis, Atg8 becomes conjugated to the expanding autophagosomal membrane and the interaction between Atg8 and the AIM creates a link between the cargo and the membrane.
The degradation of mitochondria by selective autophagy is termed mitophagy. In S. cerevisiae, the primary mitophagy receptor is Atg32, a 529 amino acid single pass outer mitochondrial membrane protein (Kanki et al. 2009). In response to nitrogen starvation, Atg32 identifies mitochondria to be degraded and recruits downstream autophagy proteins, including Atg11, to initiate mitophagy. The only structural information which is currently available for Atg32 is a single crystal structure of Atg8 bound to the AIM of Atg32 (Kondo-Okamoto et al. 2012). As such, much of the molecular mechanisms of mitophagy initiation by Atg32 has remained a mystery. Defects in mitophagy have been observed in Parkinson's disease, cancer and various metabolic disorders. Therefore, gaining insight into the molecular mechanisms of mitophagy initiation will provide new insight into the interplay between mitophagy and disease.
No structured domains have been previously identified in Atg32. To determine if any structured domains are present in Atg32, we screened different constructs within the cytosolic region of Atg32 using Escherichia coli as an expression system. Constructs which expressed and were soluble were checked for the presence of secondary and tertiary structure using circular dichroism and 1D 1H NMR spectroscopy. Constructs were further optimized using limited proteolysis and mass spectrometry. This screening approach led to the identification of a single structured domain in Atg32 comprising residues 200-341. We have completed the backbone and side chain assignments for this newly identified structured domain.
Methods and experiments
Protein Expression and Purification
A construct of Atg32 comprising residues 200-341 was codon optimized for expression in E. coli and subcloned into pET His6 TEV LIC cloning vector (1B) which was a gift from Scott Gradia (Addgene plasmid # 29653). Codon optimized Atg32200-341 pET His6 TEV LIC cloning vector (1B) was transformed into BL21 (DE3) star cells (Invitrogen). Cells were grown in 250 ml of Terrific Broth (TB) in baffled ultrayield flasks at 37°C shaking (220 rpm) to an optical density of approximately 3.0 at 600 nm. Cells were pelleted and the supernatant was removed. Cell pellets were resuspended in M9 minimal media containing either 3 g/L 15N ammonium chloride or 3 g/L 15N ammonium chloride and 10 g/L 13C glucose (Cambridge Isotope Laboratories) for uniform labeling with 15N or 15N and 13C. Cells were subsequently grown at 37°C shaking (220 rpm) until the OD at 600 nm increased by 1.0, which took approximately 1.5 hours. 250 μl of 1 M isopropylthio-β-D-galactoside (IPTG) was added to each 250 ml culture and the cultures were grown for 3 hours at 37°C shaking (220 rpm). Cells were harvested by centrifugation and stored at -80°C.
Frozen cell pellets were resuspended in 50 mM Tris pH 8.0, 500 mM NaCl, 5 mM MgCl2, 0.1% Triton X-100 buffer containing 1 mM PMSF, 10 μg/ml leupeptin and 1 μg/ml pepstatin. Cells were lysed by passing the sample through a French Press (Thermo Electron) 3 times at 4°C. Lysates were cleared by centrifugation at 40,000 × g for 45 minutes at 4°C. The supernatant was applied to 3 ml of TALON resin (Clontech) which was preequilibrated with 50 mM Tris pH 8.0, 500 mM NaCl. Protein was incubated on the resin at 4°C rocking for 30 minutes. The resin was washed with 50 mM Tris pH 8.0, 500 mM NaCl, 2.5 mM imidazole and the protein was eluted in 50 mM Tris pH 8.0, 200 mM NaCl, 200 mM imidazole. TEV protease was added to fractions containing protein and incubated overnight at 4°C to cleave off the hexahistidine tag. Cleaved protein was diluted to a final NaCl concentration of 100 mM and then applied to a 5ml HiTrap SP column (GE Healthcare) preequilibrated in 50 mM Tris pH 8.0, 100 mM NaCl. The protein was eluted using a gradient from 100 mM to 1 M NaCl. Fractions containing protein were pooled and applied to a HiLoad Superdex 75 PG column equilibrated in 20 mM sodium phosphate pH 6.5, 100 mM NaCl, 0.2 mM TCEP. Fractions containing purified protein were pooled and concentrated. The uniformly labeled 15N 13C Atg32200-341 was concentrated to 750 μM.
NMR spectroscopy
All NMR experiments were performed at 308 K on either a Bruker Avance 700 MHz or a Bruker Ascend 850 MHz spectrometer. The sequence-specific backbone assignment was determined using 2D [1H 15N] HSQC, 3D HNCA, 3D HN(CO)CA, 3D HNCO, 3D HN(CA)CO, 3D CBCA(CO)NH, 3D HNCACB and 3D HBHA(CBCACO)NH experiments. Aliphatic side chain assignments were determined using 3D (H)CCH-TOCSY, 3D HC(C)H-COSY, 3D HC(C)H-TOCSY, 3D H(CCCO)NH and 3D (H)CC(CO)NH experiments. Amide side chain assignments were determined using a 3D 15N-resolved NOESY. Methionine epsilon assignments were determined using a 3D 13C-resolved NOESY. For aromatic side chain assignments, a 2D 1H-1H NOESY, 2D 1H-1H TOCSY and 2D 1H-1H DQF-COSY were recorded on a sample dialyzed into 20 mM Sodium Phosphate pH 6.5, 150 mM NaCl and 0.1 mM TCEP made in 100% D2O (Cambridge Isotope Laboratories). 1H chemical shifts were externally referenced to 0 ppm methyl resonance of 2,2-dimethyl-2-silapentane-5-sulfonate (DSS), whereas 13C and 15N chemical shifts were indirectly referenced according to the IUPAC recommendations (Markley et al. 1998). All NMR spectra were processed using Topspin 3.5 (Bruker). Processed spectra were analyzed using CARA (http://cara.nmr.ch/).
Assignment and data deposition
96% of backbone resonance assignments (H, N, CA HA) were completed. Of the 132 peaks (10 prolines) that were expected in the 2D [1H 15N] HSQC only H247, D248, S249 and G291 were absent (Figure 1). These residues are predicted to be in flexible loops and are presumably absent due to exchange. 92% of non-labile 13CH groups were assigned. 15NH side chain moieties of arginines and lysines were not assigned. Secondary structure probabilities were calculated using TALOS-N (Figure 2) (Shen and Bax 2013). Atg32200-341 contains 6 β-strands and 5 α-helices. Chemical shift assignments were deposited in the Biological Magnetic Resonance Data Bank (BMRB) under accession number 27081.
Figure 1.
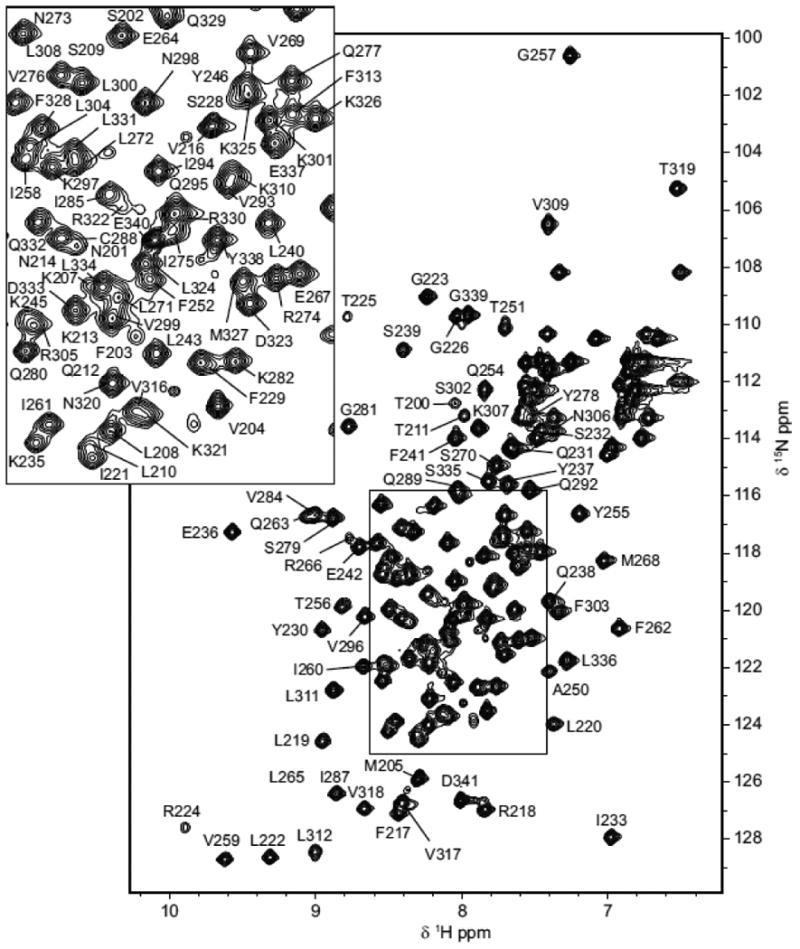
Annotated 2D [1H 15N] HSQC spectrum recorded on a Bruker Avance 700 MHz magnet at 308 K. Residues are labeled with the single letter code and the number at which they appear in the Atg32 sequence. Atg32200-341 contains no tryptophan residues.
Figure 2.
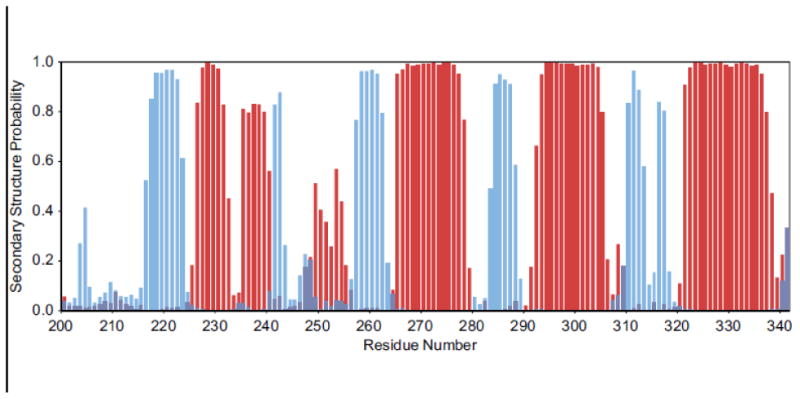
Secondary structure probabilities as calculated using the TALOS-N webserver. Blue bars represent the β-strand probabilities and red bars represent the α-helix probabilities.
Acknowledgments
The authors would like to thank Mandar T. Naik for data collection assistance, Adam H. Barczewski for helpful discussions regarding the assignment and Arminja N. Kettenbach for mass spectrometry analysis of limited proteolysis fragments. This work was supported by a COBRE award from the National Institutes of Health (GM113132).
References
- Johansen T, Lamark T. Selective autophagy mediated by autophagic adapter proteins. Autophagy. 2011;7:279–296. doi: 10.4161/auto.7.3.14487. doi:14487 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanki T, Wang K, Cao Y, Baba M, Klionsky DJ. Atg32 Is a Mitochondrial Protein that Confers Selectivity during Mitophagy. Developmental cell. 2009;17:98–109. doi: 10.1016/j.devcel.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo-Okamoto N, et al. Autophagy-related Protein 32 Acts as Autophagic Degron and Directly Initiates Mitophagy. Journal of Biological Chemistry. 2012;287:10631–10638. doi: 10.1074/jbc.M111.299917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markley JL, et al. Recommendations for the presentation of NMR structures of proteins and nucleic acids. IUPAC-IUBMB-IUPAB Inter-Union Task Group on the Standardization of Data Bases of Protein and Nucleic Acid Structures Determined by NMR Spectroscopy. J Biomol NMR. 1998;12:1–23. doi: 10.1023/a:1008290618449. [DOI] [PubMed] [Google Scholar]
- Noda NN, Ohsumi Y, Inagaki F. Atg8-family interacting motif crucial for selective autophagy. FEBS Lett. 2010;584:1379–1385. doi: 10.1016/j.febslet.2010.01.018. [DOI] [PubMed] [Google Scholar]
- Shen Y, Bax A. Protein backbone and sidechain torsion angles predicted from NMR chemical shifts using artificial neural networks. J Biomol NMR. 2013;56:227–241. doi: 10.1007/s10858-013-9741-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Z, Pascual C, Klionsky DJ. Autophagy: machinery and regulation. Microb Cell. 2016;3:588–596. doi: 10.15698/mic2016.12.546. [DOI] [PMC free article] [PubMed] [Google Scholar]


