Abstract
The skin being a protective barrier between external and internal (body) environments has the sensory and adaptive capacity to maintain local and global body homeostasis in response to noxious factors. An important part of the skin response to stress is its ability for melatonin synthesis and subsequent metabolism through the indolic and kynuric pathways. Indeed, melatonin and its metabolites have emerged as indispensable for physiological skin functions and for effective protection of a cutaneous homeostasis from hostile environmental factors. Moreover, they attenuate the pathological processes including carcinogenesis and other hyperproliferative/inflammatory conditions. Interestingly, mitochondria appear to be a central hub of melatonin metabolism in the skin cells. Furthermore, substantial evidence has accumulated on the protective role of the melatonin against ultraviolet radiation and the attendant mitochondrial dysfunction. Melatonin and its metabolites appear to have a modulatory impact on mitochondrion redox and bioenergetic homeostasis, as well as the anti-apoptotic effects. Of note, some metabolites exhibit even greater impact than melatonin alone. Herein, we emphasize that melatonin–mitochondria axis would control integumental functions designed to protect local and perhaps global homeostasis. Given the phylogenetic origin and primordial actions of melatonin, we propose that the melatonin-related mitochondrial functions represent an evolutionary conserved mechanism involved in cellular adaptive response to skin injury and repair.
Keywords: Melatonin, Skin, Mitochondria, Photoprotection, Homeostasis
Introduction to skin functions
The skin together with subcutaneous adipose tissue, defined as the hypodermis, represents the largest body organ (15% of body weight and an average surface of about 2 m2 [1]) with diverse sensory and regulatory functions [2, 3]. The functions of the integument are defined by its anatomic position where it serves not only as the protective barrier between external environment and the body’s internal milieu [4–6], but also is empowered by sensory and adaptive capabilities to react to changing environmental signals to protect and maintain local and global body homeostasis [2, 7]. These functions, crucial for organismal survival, would have been perfected by environmental pressure during evolution of species [6], and some of its elements, perhaps, have been adapted by central neuroendocrine system [7, 8].
Human skin is composed of distinct compartments that include epidermis, dermis with subcutaneous adipose tissue, and adnexal structures [1]. The epidermis represents a self-renewing predominantly keratinocytic structure that during differentiation towards the outer-most surface generates a solid lipid-rich cornified layers [4, 5]. Its important elements are represented by melanocytes that produce and transfer melanin pigment to keratinocytes in an organized fashion to protect skin from harmful action of ultraviolet radiation (UVR) [9]. It must be noted that UVB spectrum of solar radiation is also required for transformation of 7-dehydrocholesterol to vitamin D in the epidermis [10]. Additional components of the epidermis are the immune cells that derive from the bone marrow.
The epidermis is separated from the dermis by the basement membrane, which restricts bidirectional communication between both components. It contains extracellular elements including collagen, elastic fibers, and proteoglycans produced by fibrocytes, which are responsible for cutaneous strength and elasticity. In the deeper levels, there is gradual transition of dermal component into hypodermis (predominantly composed of fat) both of which are providing cushion from the impact stress. The immune cells including lymphocytes, macrophages, mast, and dendritic cells predominantly reside in the dermis, although they can be present in the hypodermis. Their distribution and activities depend on physiological and pathological signals imposed on the skin. Both structures are supplied and connected by vasculature important for cutaneous viability and communication between different elements of the skin and systemic homeostasis as well as for thermoregulation [3]. The hypodermis also plays an important role in energy storage. The adnexa (structures are of the epidermal origin) are located in both the dermis and hypodermis depending on their activity and function. In humans, these include the hair follicles, sebaceous glands, eccrine glands (playing important thermoregulatory functions), and apocrine glands (having predominantly vestigial functions). All cutaneous structures are supplied by a network of somatosensory and autonomic nerve fibers [2]. The former can extend into the upper most layers of the epidermis.
The functions of these different skin compartments are integrated by the skin immune, pigmentary, epidermal, dermal, vascular, and adnexal systems. They further are regulated by local neuroendocrine system through the action of locally produced hormones, neurohormones, neurotransmitters, and cytokines [3]. These are locally organizes along algorithms of hypothalamo-pituitary–adrenal axis [11], hypothalamo-pituitary–thyroid axis [12, 13], catecholaminergic [14], serotoninergic and melatoninergic [15, 16], cholinergic [17, 18], steroidogenic [19], and secosteroidogenic [10, 20, 21], opioidogenic [22, 23], and canabinnoidogenic [24] systems. They use the same mediators as those used by the central neuroendocrine system.
In the context of mitochondrial function in skin cells, local cutaneous melatonin generating and metabolizing system is of particular interest, since they, together with local endocrine system, play crucial roles in protecting epidermal homeostasis against damaging effect of solar radiation (Fig. 1).
Fig. 1.
Skin melatonin–mitochondria axis counteracts the damage inflicted by solar radiation
Melatonin is both synthesized and metabolized in the skin
Melatonin synthesis in the skin
Following Aaron Lerner’s hypothesis that release of melatonin in the skin may lead to pigmentary disorders [25], we used hamster skin to provide evidence that serotonin can be transformed to melatonin in this organ [26] with N-acetylserotonin (NAS) serving as an intermediate of the pathway [26, 27]. This was the first documentation that melatonin can indeed be synthesized in the mammalian skin [26]. The follow-up studies have not only documented that rodent and human skin, as well as normal keratinocytes, melanocytes, and melanoma cells can produce endogenously melatonin, but they also express all the necessary molecular and biochemical elements of the apparatus transforming tryptophan to serotonin with its further metabolism to NAS and melatonin [15, 16, 28–33]. Thus, skin cells do express tryptophan hydroxylase type 1 (TPH1; all resident skin cells) [29–31, 34], TPH2 (melanocytes) [33], arylalkylamine-N-acetyltransferase/serotonin N-acetyltransferase (AANAT/SNAT), and arylamine-N-acetyltransferase (NAT) [16, 28, 31, 32, 34] and hydroxyindole-O-methyltransferase/N-acetylserotonin methyltransferase (HIOMT/NASM) [16, 28, 34]. These findings were confirmed by studies on human and rodent hair follicles [35]. However, the cutaneous biosynthetic pathway for melatonin was defective in the C57BL/6 mouse and used alternative to the AANAT enzyme that acetylated serotonin [34]. Interestingly, in rodent and human skin, serotonin can be acetylated to the NAS by both AANAT/SNAT and arylamine-N-acetyltransferase (NAT) [16, 27, 28, 31, 32]. We also determined melatonin and its metabolites concentrations in the human epidermis using techniques of liquid chromatography–mass spectrometry (LC–MS) [36]. Melatonin levels were at 0.93 ± 0.55 ng/mg protein, which were dependent on race, gender, and age of the donors [36]. Interestingly, the highest melatonin concentration was found in the epidermis from African-American (AA) subjects.
Melatonin metabolism in the skin
The melatonin metabolism in the skin has been discussed extensively in the most recent [37] and previous [15] reviews; therefore, we will be brief and will outline the most important points of the pathway. Originally, we have demonstrated that melatonin produced in the hamster skin is metabolized through the indolic pathway [26], which was similar to the frog skin and retina [38, 39]. The same pathway also appears to operate in human melanoma cells [29]. Studies on human immortalized epidermal keratinocytes (HaCaT) have shown that melatonin is metabolized but both indolic and kynuric pathways, and this metabolism can be stimulated by UVB [40]. Interestingly, exposure of aqueous solution of melatonin to UVB energy leads to its transformation to N 1-acetyl-N 2-formyl-5-methoxykynuramine (AFMK) through defined intermediates of the reaction that is dependent on UVB dose and time of exposure [40]. Again, melatonin metabolism in the skin cells is rapid, which involves both indolic and kynuric pathways, and 6-hydroxymelatonin is the major metabolite in the skin [41]. All metabolites including 6-hydroxymelatonin, 5-methoxytryptamine, 5-methoxytryptophol, AFMK, and N 1-acetyl-5-methoxykynuramine (AMK) [36, 42] and likely 2-hydroxymelatonin [37, 40] are present in the epidermis. All these metabolites potentially can affect mitochondrial functions in the skin cells and consequently the skin phenotype.
Physiological role of melatonin and metabolites in the skin
An overview
The role of melatonin, its precursors, and metabolites in regulation of physiological functions of the skin and its attenuating effects in skin pathology has been extensively discussed in recent reviews [15, 16, 33, 43, 44]. Thus, melatonin can regulate cutaneous adnexal [35, 44–46], pigmentary [9, 16], and barrier [33, 40, 41, 43] functions. It has also oncostatic effects, which has been best illustrated in melanoma cells [47, 48]. Many of the above phenotypic effects exerted by nM or lower concentrations of melatonin are mediated through interaction with membrane bound melatonin receptors type 1 and 2 (MT1 and MT2) [49]. In human skin, MT1 is predominantly expressed in the epidermal compartment, while MT2 is found in the adnexal structures [16, 44]. The putative nuclear receptor for melatonin would require higher concentration of the ligand, the requirement that can be made by its local production or topical application [33, 49]. Here, we must emphasize that retinoic orphan acid receptor A/α (ROR A/α) is not a receptor for melatonin, an error that is frequently repeated by melatonin researchers. It is a receptor for sterols, oxysterols, and secosteroids [33, 50, 51].
The phenotypic effects that would require high local concentration of melatonin or its metabolites would be mediated through its action on mitochondria and/or through activation of the nuclear receptor(s) that still need(s) to be defined. This requirement again can only be met by high local intracellular concentrations of melatonin that could be possible through topical application or its efficient cutaneous production, balanced by a regulated-on site metabolism. It also implicates intra-, auto-, and paracrine mechanisms of melatonin action in skin cells that target mitochondria activities, with secondary phenotypic effects on radioprotection or barrier functions.
Photoprotection
Numerous studies have shown that melatonin and its derivatives can act as anti-oxidants in the skin subjected to ultraviolet [52, 53] or X-ray radiation [54]. Similar effect was observed for keratinocytes, melanocytes, and dermal fibroblasts in culture [33, 55, 56]. Furthermore, melatonin derivative AMK was found to be potent singlet oxygen scavenger [57].
The protective effects of melatonin in the skin cells were investigated by several groups using proliferation assays based on sulforhodamine B (SRB) [58], 3H-thymidine incorporation, morphological, and clonogenic assays [42, 47, 59], as well as lactate dehydrogenase release viability tests [58]. We have reported protective effects of melatonin and its metabolites: 6-hydroxymelatonin, AFMK, N-acetylserotonin, and 5-methoxytryptamine in reducing oxidative cell damage in human skin cells: keratinocytes and melanocytes, elicited by UVB [55, 56]. Pharmacological doses of melatonin and its derivatives protect both type of cells, keratinocytes and melanocytes, from UVB-induced oxidative stress. The formation of intracellular ROS, including nitric monoxide (NO−) and H2O2, in cells exposed to UVB is significantly reduced when cells were pretreated with either melatonin or its metabolites. Interestingly, this process is independent of membrane bound melatonin receptors, as shown in melanocytes [55].
Melatonin and its metabolites also play a role in cellular defense against oxidative damage by promoting glutathione production, as tested of GSH activation or stimulation of genes encoding GSH production, although this effect is more pronounced in keratinocytes than in melanocytes.
The anti-oxidative effects of melatonin have been confirmed earlier [41]. The protective role of melatonin against UVB-induced DNA-base-oxidized intermediate 8-hydroxy-2′-deoxyguanosine (8-OHdG) was also demonstrated in human full-thickness skin histocultured ex vivo [60]. The preincubation with melatonin led to significant reduction in production of 8-OHdG in cells exposed to UV, thus indicating protective melatonin activity against UV-induced stress in skin cells. Melatonin’s free radical scavenging capacity in transformed HaCaT and normal skin keratinocytes exposed to UVB was also previously reported [58].
The mechanism of protection from UVB-induced oxidative stress of melatonin or its metabolites is mediated by the activation of nuclear factor erythroid 2-like 2 (NRF2) and upregulation of NRF2-dependent pathway in both cells, melanocytes and keratinocytes [41, 55]. By stimulating the expression of NRF-2, melatonin and its metabolites also target NRF-2-related enzyme and proteins that further protect cells from UVB and other damaging factors.
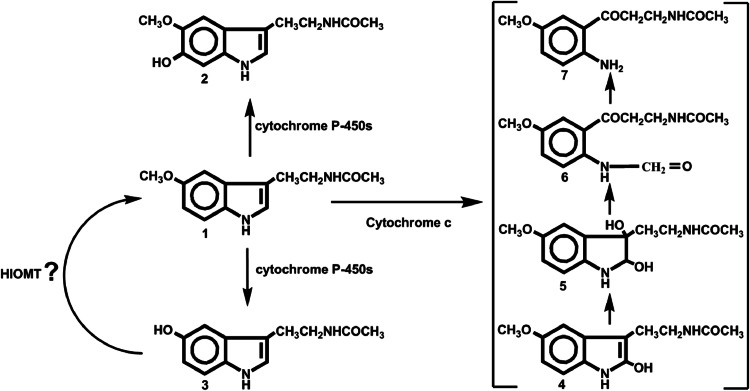
Melatonin and its metabolites exhibit protective actions against UVB-induced cell damage: not only that they reduce the levels of the UVB-induced cell damage, in form of CPD’s or 6-4PP’s, but they also enhance UVB-induced DNA repair in keratinocytes and melanocytes. They stimulate phosphorylation of tumor suppressor protein p53 and enhance nucleotide excision repair (NER) via enhanced interactions between damaged DNA and the NER core factors XPC and XPA. Furthermore, the inhibition of DNA synthesis was observed in human immortalized keratinocytes and melanoma cells treated with melatonin metabolites AFMK and AMK [36, 41, 42], of which production can be stimulated by UVB [40] or which can be the products of melatonin metabolism in the mitochondria (Fig. 2).
Fig. 2.
Melatonin metabolism in mitochondria. 1 melatonin; 2 6-hydroxymelatonin; 3 N-acetylserotonin; 4 2-hydroxymelatonin; 5 2,3-dihydroxymelatonin; 6 N 1-acetyl-N 2-formyl-5-methoxykynuramine; 7 N 1-acetyl-5-methoxykynuramine
It has been demonstrated that melatonin acts as a potent anti-inflammatory and anti-apoptotic factor that attenuates pro-inflammatory cytokines gene expression and the expression of pro-apoptotic proteins in keratinocytes [61]. Melatonin also had an impact on reduction of heat shock Hsp70 protein in UVR-treated human full-thickness skin in organ culture and cultured keratinocytes [61].
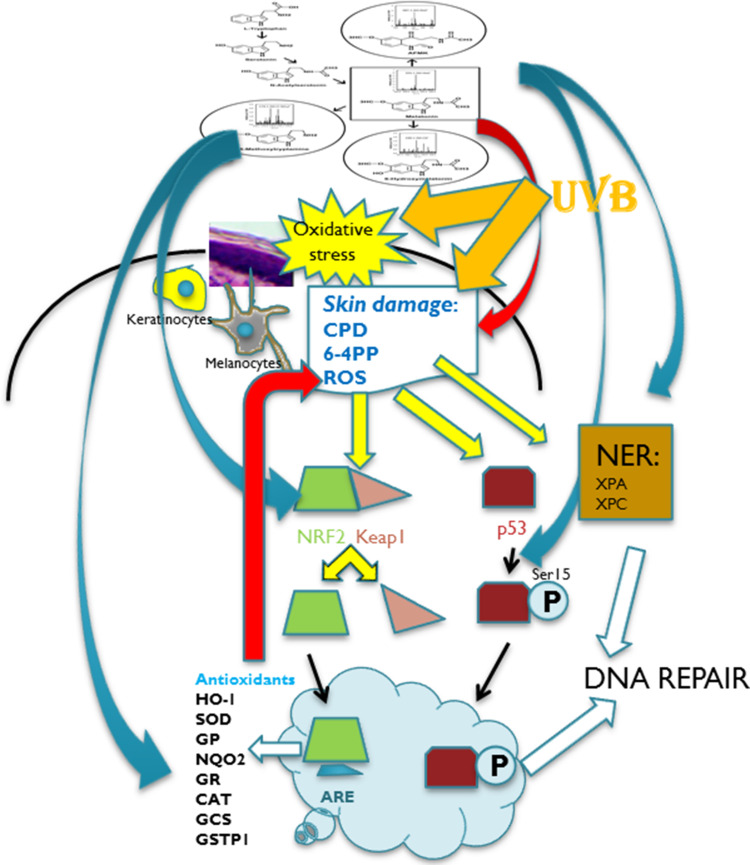
Thus, melatonin and its metabolites act as radioprotectors in the epidermis (Fig. 3). Many of these radioprotective effects would be secondary to melatonin action on the mitochondria or/and mitochondrial melatonin metabolism to biologically active metabolites (Figs. 2, 4, 5).
Fig. 3.
Melatonin and its metabolites act as radioprotectors on epidermal keratinocytes and melanocytes. Local melatoninergic system in skin includes sequential transformation of tryptophan to serotonin and melatonin. NAS is both precursor and metabolite of melatonin. Through indolic pathways, melatonin is hydroxylated to 6(OH)M or metabolized to 5-MT. Through kynuric pathway, melatonin is transformed to AFMK. UVB is absorbed in the epidermis inducing oxidative stress and direct DNA damage. Melatonin and its metabolites inhibit oxidative stress and DNA damage induced by UVB (red arrow). In response to oxidative stress, NRF2 is released from Keap (Kelch-like ECH-associated protein) and translocated to the nucleus. This process is stimulated by melatonin and its metabolites (blue arrow). NRF2 binds to ARE (anti-oxidant response element) and further activates detoxifying enzymes and proteins: melatonin and its metabolites also stimulate the production of anti-oxidants (blue arrow) which further reduces UVB-induced damage to melanocytes and keratinocytes (red arrow). UVB-induced DNA damage to the skin promotes p53 expression, which after phosphorylation accumulates in the nucleus and activates the DNA repair process. Melatonin and its metabolites stimulate phosphorylation of p53 at Ser-15 (blue arrow). Melatonin and its metabolites induce repair of DNA damaged by UVB by enhancing the NER core factors: complementation group C (XPC) and complementation group A (XPA)-DNA interactions (blue arrow). HO-1 heme oxygenase 1, SOD superoxide dismutase, GP glutathione peroxidase, NQO2 quinone reductase 2, GR glutathione reductase, CAT catalase, GCS glutamylcysteine synthetase, GSTP1 glutathione-S-transferase
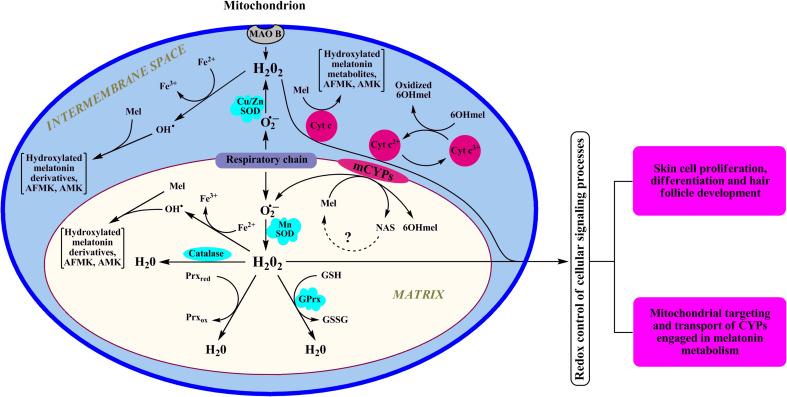
Fig. 4.
Dynamic interaction between melatonin, melatonin metabolites, and mitochondrion redox homeostasis. In mitochondria, cytochrome c is a natural scavenger of H2O2 preventing its accumulation via mechanism linked to reverse electron transfer from succinate to NAD+ [88] or through “alternative electron leak pathway” [89]. The latter mechanism that requires ferrocytochrome is active under physiological conditions. However, if there is a block in electron transfer, ferricytochrome cannot be reduced and cytochrome c would lose its capability to scavenge H2O2. Reduction of ferricytochrome c by 6-hydroxymelatonin (6OHMel) allows use of the electrons removed from the 6-hydroxymelatonin for the detoxication of H2O2. In addition, when electron transport is disrupted, cytochrome c-dependent pseudoperoxidase reaction with melatonin could become dominant [62]. GPrx glutathione peroxidase, Prx peroxiredoxin, Mel melatonin, 6OHmel 6-hydroxymelatonin, MAO B monoamine oxidase B, SOD superoxide dismutase
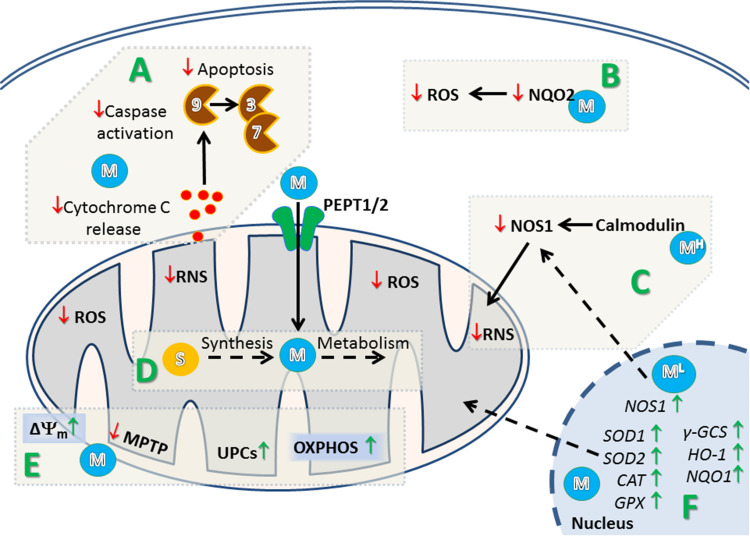
Fig. 5.
Proposed mechanism of regulation of mitochondrial and cellular homeostasis in the skin by melatonin and its metabolites. A Melatonin (M) prevents initiation of mitochondrial pathway of apoptosis through inhibition of cytochrome c leakage from mitochondria and inhibition of activation of caspase 9, 3, and 7. B Melatonin (M) binds and inhibits generation of ROS by quinone reductase 2 (NQO2). C Low concentration of melatonin (1 nM, ML) triggers expression of nitric oxide synthase 1 (NOS1) resulting in elevation of reactive nitrosative species (RNS) and modulation of mitochondrial function. However, at higher concentration (>1 nM), melatonin (MH) interacts with calmodulin what results in inhibition of NOS1 and subsequent decrease in RNS. D Melatonin (M) could be synthesized from serotonin and further metabolized in mitochondria. It was also postulated that melatonin (M) can be transported to mitochondria by peptide transporter 1/2 (PEPT1/2). E Melatonin maintain mitochondrial membrane potential (Δψm) by inhibition of the mitochondrial permeability transition pore (MPTP), and stimulation of uncoupling proteins (UCPs), which results in an increase of oxidative phosphorylation (OXPHOS) and production of ATP. F: Through activation of MT1/2 receptors, melatonin (M) upregulates the expression of anti-oxidant genes in cells subjected to radiation. NOS1 nitric oxide synthase 1
Melatonin–mitochondria axis in the skin
Mitochondrial involvement in melatonin metabolism and synthesis
In mitochondria, melatonin can be metabolized through different pathways, including monooxygenase (the cytochrome P450 dependent pathway) and peroxidase (the kynuric pathway) reactions with the participation of H2O2 (Figs. 2, 4) [37, 40].
The kynuric pathway in mitochondria operates due to pseudoperoxidase activity of cytochrome c and predominantly results in the accumulation of AFMK and its secondary product, AMK [62]. The reaction proceeds through a sequential generation of 2-hydroxymelatonin and 2,3-dihydroxymelatonin as intermediates. Although 2-hydroxymelatonin, AFMK, and AMK are found in the skin cells [36, 37, 40, 42], it is unclear whether they are generated exclusively by cytochrome c-dependent pseudoperoxidase reaction, since these derivatives might also accumulate in the skin due to nonenzymatic processes [15, 40]. However, it is likely that in mitochondria—organelles with own powerful anti-oxidant system, direct scavenging of ROS by melatonin must play a supporting role, at least in attenuating UVR induced oxidative damage.
Interestingly, that H2O2 through activation of protein kinase A- or C-mediated signaling pathways can trigger mitochondrial targeting and transport of cytochromes P450 engaged in melatonin metabolism in mitochondria [63, 64]. Cytochrome P450-mediated 6-hydroxylation and O-demethylation of melatonin in these organelles was first described in the liver [65]; however, it has become clear that these reactions may occur in other tissues, including skin. Several forms of cytochrome P450 (CYP1A, CYP3A, and CYP2E) responsible for the melatonin metabolism in hepatic mitochondria are found in the skin [66] and the production of 6-hydroxymelatonin and N-acetylserotonin was detected in different skin cells [41]. These findings indicate the existence of cutaneous metabolism of melatonin by locally expressed mitochondrial cytochromes P450.
It is clear that there are at least two major pathways of melatonin metabolism in mitochondria [62, 65], each consisting of different types of reactions and playing special physiological roles in skin cells. Pseudoperoxidase oxidation of melatonin by cytochrome c is involved in H2O2-mediated signaling pathways via regulation H2O2 efflux from mitochondria to cytoplasm. Cytochrome P-450-dependent 6-hydroxylation and O-demethylation of melatonin provide mitochondria with target compounds that participate in maintenance of mitochondrial homeostasis. One of them, 6-hydroxymelatonin, prevents effectively oxidative modification of mitochondrial proteins and protects mitochondria from t-BuOOH-induced swelling [33, 67, 68], and the another, N-acetylserotonin, impedes opening of mitochondrial permeability transition pores induced by calcium, phosphate, or neurotoxins [69].
Recent studies on melatonin biosynthesis in mitochondria suggest another intriguing alternative for N-acetylserotonin—its conversion back to melatonin [70]. The probability of such “replenishing reaction” appears to be supported by findings that the mitochondria isolated from oocytes are able to generate melatonin from serotonin [71]. This indicates a presence of the biosynthetic machinery necessary for melatonin synthesis in mitochondria. Indeed, the rate-limiting enzyme of melatonin synthesis, AANAT/SNAT was detected in mitochondria of oocytes [71]. However, the presence of HIOMT or any other enzyme responsible for O-methylation of N-acetylserotonin remains to be defined.
The evidence is accumulating on the reciprocal interactions between intramitochondrial pathways of melatonin metabolism and synthesis, the bioenergetics functions of this organelle, and detoxification processes with final phenotypic effects on the organ level (Fig. 4). Careful studies on these interactions in skin cells represent a realistic and exciting subject for investigations.
Melatonin-dependent regulation of redox homeostasis of the skin cells
Although bioenergetics is a primary function of mitochondria, they also play an essential role in regulating redox signaling. Currently, it is widely accepted that hydrogen peroxide (H2O2) released from mitochondria acts as important regulatory mediator in different signaling pathways and processes, such as cell proliferation, epidermal differentiation, and hair follicle development [72, 73]. Physiological doses of UVB irradiation through the generation of intracellular H2O2 activate epidermal growth factor receptor/extracellular-regulated kinase 1/2 signaling pathway, as well as trigger the phosphorylation and internalization of keratinocyte growth factor receptor in cultured fibroblasts [74, 75]. However, harmful exposure to ultraviolet radiation and other stressors associated with mitochondrial dysfunction may trigger excessive ROS formation, which is typically activating the programmed cell death. Neutralization of dysfunctional cells is an essential process that ensures organized death and removal of several billion cells every day without inducing pathological processes. It is important to note that depletion of basal cells is detrimental for skin repair; thus, specific mechanisms protect cells against excessive mitochondrial damage and ROS formation. In particular, disruption of electron flow, e.g, due to inhibition of ETC complex I or complex III and subsequent escape of electrons, is implicated in superoxide formation (O−·2). Superoxide is typically undergoing disproportionation to H2O2, process greatly enhanced by mitochondrial superoxide dismutase (SOD). These mechanisms prevent harmful production of superoxide-related reactive nitrogen species (RNS) and ensure conversion and removal of lipid membrane permeable H2O2. Notably, H2O2 is relatively week oxidant, unless following metal-dependent production to hydroxyl radical (OH·). Importantly, melatonin is able to directly scavenge highly toxic OH· [76], but does not react with its precursor—H2O2 [77]. Thus, direct scavenging of H2O2 by melatonin has negligible significance, but it is possible that melatonin in mitochondria detoxifies H2O2 due to cytochrome c-dependent pseudoperoxidase reaction, as described below. An important question is, however, whether melatonin concentrations and compartmentation in mitochondria or cytosol are sufficient to diminish the effects of highly reactive species at the site of their production. There is sufficient evidence that cytochrome c, due to its pseudoperoxidase activity, effectively competes with mitochondrial scavenging enzymes (catalase, glutathione peroxidase, and peroxiredoxin III) to control H2O2 levels [78, 79] at the expense of endogenous reductants. Importantly, melatonin oxidation by cytochrome c may exhibit a potential protective mechanism (Figs. 4, 6) [62]. Pseudoperoxidase oxidation of melatonin by cytochrome c is able to compensate for the absence of the conventional H2O2—detoxifying enzymes (catalase, glutathione peroxidase, and peroxiredoxin III) in the intermembranous space and melatonin derivatives (2-hydroxymelatonin, AFMK, and AMK) themselves could further contribute to neutralization of ROS [57]. This makes cytochrome c-mediated kynuric pathway highly effective in reducing the extensive production of ROS occurring in mitochondria under UV irradiation, a function of special importance in the epidermal compartment.
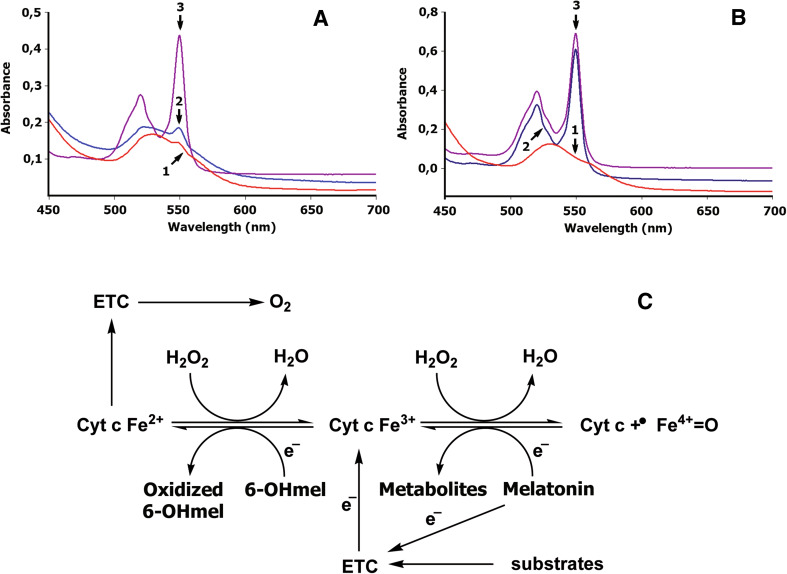
Fig. 6.
Melatonin and 6-hydroxymelatonin as regulators of bioenergetics of mitochondria in physiologic or pathological conditions. Reduction of oxidized cytochrome c by melatonin (a) and 6-hydroxymelatonin (b). Melatonin (a) or 6-hydroxymelatonin (b) was added to 0.025 mM of an oxidized cyt c (trace 1) dissolved in 10 mM Tris–HCl buffer (pH 7.4), to a final concentrations of 0.5 mM (trace 2); sodium dithionite was added to produce total reduction of cyt c (trace 3). The absorbance spectrum was recorded 30 min after addition of melatonin or 6-hydroxymelatonin as described previously [62]. Trace 1 oxidized cytochrome c, trace 2 cytochrome c reduced by melatonin (a) or by 6-hydroxymelatonin (b); trace 3 total reduction of cytochrome c incubated with melatonin (a) or 6-hydroxymelatonin (b) by sodium dithionite. c Melatonin could donate an electron to the Complex I of the ETC in physiologic conditions [90]. Accumulation of oxoferryl cytochrome c (cyt c + ·FeIV = O) induced by high levels of H2O2 could impair the cytochrome c-mediated electron shuttle between complex III and complex IV. Interaction of melatonin with oxoferryl hemoprotein restores the normal redox cycle of cytochrome c, protecting mitochondrial energy homeostasis under oxidative stress. Melatonin metabolite, 6-hydroxymelatonin, effectively reduces oxidized cytochrome c (cyt c Fe3+), thereby supporting electron flux through the respiratory chain, even when cytochrome c is intensively oxidized by high levels of H2O2. Thus, melatonin and 6-hydroxymelatonin interactions with both oxoferryl and ferricytochrome c could play a significant role in bioenergetics of mitochondria in physiologic or pathological conditions
Melatonin and its metabolites as regulators of bioenergetics of mitochondria
It has been suggested that melatonin could donate electrons to the ETC, thus improving mitochondrial respiration and increasing ATP production [80]. At the same time, mitochondrial ETC may be a target not only for melatonin, but also for its metabolites generated in mitochondria [37, 40] (Fig. 6). It appears that AMK like a melatonin exerts effects on electron flux through the respiratory chain [80]. According to our data, 6-hydroxymelatonin in vitro effectively reduces oxidized cytochrome c, thus exhibiting greater reducing potential than melatonin itself (Fig. 6a, b). During mitochondrial respiration, cytochrome c supports electron shuttling between complex III (ubiquinol cytochrome c oxidoreductase) and complex IV (cytochrome c oxidase). Therefore, when an electron is removed from 6-hydroxymelatonin, it becomes available for donation by reduced cytochrome c to complex IV, contributing to mitochondrial energy production (Fig. 6c). This phenomenon ensures electron transfer in the terminal cytochrome c oxidase segment of the ETC, even when the dysfunction occurs in its initial steps, such as in the case of the age-related decline in complex II (succinate:ubiquinone oxidoreductase) activity in human skin fibroblasts [81]. In addition to contribution to mitochondrial bioenergetics, reduction of ferricytochrome c by 6-hydroxymelatonin supports cytochrome c-dependent scavenging of H2O2 (Figs. 4, 6c). In conclusion, we propose that skin protective (direct and indirect) activities of melatonin are dependent on mitochondria through the reciprocal interactions with skin cell homeostasis (Figs. 4, 5).
Anti-apoptotic and protective effects
Keratinocytes need to proliferate to provide an effective epidermal barrier, whereas UV-mediated mitochondrial dysfunction may deplete this pool of cells via intrinsic apoptotic event, which would impair barrier formation [4, 5, 19]. As it has been described above, mitochondria are recognized as a place of synthesis and metabolism of melatonin [70]. Furthermore, melatonin was found to accumulate in mitochondria and the presence of specific melatonin transporter(s) such as PEPT1/2 has been suggested [82]. Consequently, mitochondria were found to be one of the noncanonical targets for melatonin [70].
Melatonin inhibits UV stimulated activation of apoptosis [33, 43, 55, 56, 59, 83]. The inhibition of UV-induced apoptosis was shown by the terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) assay in human HaCaT keratinocytes [59]. Melatonin was also found to inhibit cytochrome c release from mitochondria [58]. In an addition, in keratinocytes subjected to UV, melatonin was shown to decrease activation of Caspases 3, 7, and 9 suggesting inhibition of mitochondrial pathway of apoptosis [83].
Protective activities of melatonin on mitochondria not only relay on direct scavenging of reactive oxygen species, but also include the maintenance of optimal mitochondrial membrane potential (Δψm) [58, 83] as well as cytosolic pH [58]. It has been suggested that protective effects of melatonin on mitochondria are achieved by direct or receptor mediated inhibition of the mitochondrial permeability transition pore (MPTP), and stimulation of uncoupling proteins (UCPs) (see [70] for discussion). However, this hypothesis still remains to be evaluated in the skin cells. Recent studies suggested a complex- and concentration-dependent regulations of mitochondrial homeostasis [84]. At nanomolar and sub-nanomolar concentration, melatonin transiently stimulates the expression of nNOS through activation of MT1 and MT2, which results in NO-mediated modulation of mitochondrial function (inhibition of the oxidative phosphorylation and decrease of mitochondrial membrane potential and ATP synthesis). However, at higher concentrations (>1 nM), melatonin would interact with calmodulin leading to nNOS inhibition [84]. Melatonin and its metabolites 6-OHM, AFMK, AMK, NAS, and 5-MT increased viability of human keratinocytes and melanocytes subjected to UVB irradiation [43, 55, 56, 59]. In addition, increased level of reduced glutathione and decreased levels of nitrate and hydrogen peroxide was observed in keratinocytes pretreated with melatonin metabolites and subsequently irradiated [56]. Accordingly, recent has shown that melatonin at 1 mM concentration counteracts UVB-driven inhibition of ATP production and reduces levels of ROS in the cells after irradiation [85].
About 10 years ago, we have suggested that the sensitivity of skin cell lines as well as melanoma cell lines to melatonin antiproliferative activities may depend on the level of expression of MT1 and MT2 receptors and melatonin-binding quinone reductase NQO2 [43, 47]. Indeed, melatonin was found to upregulate expression of anti-oxidant genes [33, 55, 56]. In the study, based on the dorsal skin flap model, it was shown that melatonin decease amount of ROS as measured by malondialdehyde (MDA) level, and increase level of superoxide dismutase (SOD) and catalase (CAT) [86]. Interestingly, melatonin was found to enhance activities of anti-oxidant enzymes: superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx), as well as increase level of glutathione (GSH) in both normal and diabetic (C2 line) human skin fibroblasts [87]. Melatonin also prevented UVB-driven depletion of anti-oxidative enzyme including SOD1, catalase, and GPx in the human skin ex vivo [60]. It was recently suggested that melatonin could activate second-phase anti-oxidant enzymes (γ-GCS, HO-1, and NQO1) in normal human epidermal keratinocytes subjected to ultraviolet radiation [85].
Interestingly, melatonin is also involved in mitochondrial homoeostasis, including biogenesis, fission and fusion, as well as mitophagy [70]. In addition, dynamics of mitochondrial oscillation resemble and match the melatonin circadian secretory rhythm in pinealocytes and most probably in other melatonin producing cells [70] that may include epidermal cells. Thus, melatonin and its metabolites can regulate skin cell phenotype through their action on mitochondria in a complex manner (Figs. 4, 5, 6).
Conclusions and perspective
The skin, placed at the interphase between external and internal (biological) environments, is a subject for the homeostatic regulation by the local melatonin synthesis and metabolism systems that include regulation of mitochondrial activity in skin cells with attendant phenotypic effects (Figs. 1, 2, 3, 4, 5, 6). The evolutionarily conserved functions of melatonin and its metabolites in protection against oxidative damage and capacity to restore cellular homeostasis and cell integrity are well suited to build the barrier function of the skin. This includes strengthening of the physical epidermal barrier as well as induction of local radioprotective mechanisms enhancing the skin’s ability to counteract or buffer against a damage inflicted by external physical and/or chemical factors or to restore local metabolic homeostasis. In this context, it is of particular interest that these properties are shared by melatonin metabolites some of which are generated by oxidative processes or by direct photochemical transformation induced by UVB. These roles are clearly dependent on local melatonin synthesis and metabolism, since anti-oxidative and radioprotective properties of melatonin and products of its metabolism or photochemical degradation require relatively high concentration of these compounds that cannot be achieved by delivery from central sites of its production including pineal gland. This opens exciting areas for investigation in testing hypothesis whether photochemical degradation induced by UVB represents a photoactivation mechanism that produces AFMK and AMK with a changed biological activity. This could represent an additional and distinct mechanism of photoactivating properties of UVB already well described for the production of vitamin D, all of which were adapted by the integument during evolution to regulate local and systemic homeostasis, where they serve as chemical second messengers of high-energy electromagnetic wavelengths of solar radiation.
We believe that the photoprotective and skin barrier building functions of melatonin and its metabolites are directly or indirectly dependent on mitochondria. These, in addition to mitochondrial metabolism of melatonin, would involve its direct or indirect (via metabolites) action on mitochondrial functions culminating with diverse phenotypic effects. Accepting the 2.5 billion-year-old phylogenetic origin and function of melatonin, the epidermis, placed between external and internal environments, represents a perfect model for evaluating interactions between noxious factors, local melatonin generating and metabolism systems and mitochondria, as regulators of integumental homeostasis with possible systemic consequences. Thus, the epidermal melatonin system may represent a signature/record of the conserved evolutionary function of this molecule and its metabolites as protectors against noxious external and endogenous factors. Melatonin and its metabolites would coordinate mitochondrial interactions with the skin cell to decide whether it survives or enters precisely defined differentiation pathway, necessary for barrier formation, or dies through apoptotic pathways to prevent carcinogenesis.
Acknowledgements
The work was supported by NIH Grants 1R01AR056666-01A2 and 1R01AR071189-01A1 to AS. This paper is dedicated to the memory of Dr. Aaron B. Lerner who trained one of the co-authors (AS).
Footnotes
The paper is dedicated to Aaron B. Lerner who isolated and characterized melatonin.
References
- 1.Bolognia J. Dermatology. 2. Philadelphia: Mosby Elsevier; 2008. [Google Scholar]
- 2.Slominski AT, Zmijewski MA, Skobowiat C, Zbytek B, Slominski RM, Steketee JD. Sensing the environment: regulation of local and global homeostasis by the skin’s neuroendocrine system. Adv Anat Embryol Cell Biol. 2012;212:1–115. doi: 10.1007/978-3-642-19683-6_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Slominski A, Wortsman J. Neuroendocrinology of the skin. Endocr Rev. 2000;21:457–487. doi: 10.1210/edrv.21.5.0410. [DOI] [PubMed] [Google Scholar]
- 4.Elias PM. The skin barrier as an innate immune element. Semin Immunopathol. 2007;29:3–14. doi: 10.1007/s00281-007-0060-9. [DOI] [PubMed] [Google Scholar]
- 5.Feingold KR, Schmuth M, Elias PM. The regulation of permeability barrier homeostasis. J Investig Dermatol. 2007;127:1574–1576. doi: 10.1038/sj.jid.5700774. [DOI] [PubMed] [Google Scholar]
- 6.Elias PM, Menon G, Wetzel BK, Williams JJ. Barrier requirements as the evolutionary “driver” of epidermal pigmentation in humans. Am J Human Biol. 2010;22:526–537. doi: 10.1002/ajhb.21043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Slominski AT, Zmijewski MA, Zbytek B, Tobin DJ, Theoharides TC, Rivier J. Key role of CRF in the skin stress response system. Endocr Rev. 2013;34:827–884. doi: 10.1210/er.2012-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Slominski A. A nervous breakdown in the skin: stress and the epidermal barrier. J Clin Investig. 2007;117:3166–3169. doi: 10.1172/JCI33508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slominski A, Tobin DJ, Shibahara S, Wortsman J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev. 2004;84:1155–1228. doi: 10.1152/physrev.00044.2003. [DOI] [PubMed] [Google Scholar]
- 10.Holick MF. Vitamin D: a millenium perspective. J Cell Biochem. 2003;88:296–307. doi: 10.1002/jcb.10338. [DOI] [PubMed] [Google Scholar]
- 11.Slominski A, Wortsman J, Tuckey RC, Paus R. Differential expression of HPA axis homolog in the skin. Mol Cell Endocrinol. 2007;265–266:143–149. doi: 10.1016/j.mce.2006.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Slominski A, Wortsman J, Kohn L, Ain KB, Venkataraman GM, Pisarchik A, Chung JH, Giuliani C, Thornton M, Slugocki G, Tobin DJ. Expression of hypothalamo-pituitary-thyroid axis related genes in the human skin. J Invest Dermatol. 2002;119:1449–1455. doi: 10.1046/j.1523-1747.2002.19617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Beek N, Bodo E, Kromminga A, Gaspar E, Meyer K, Zmijewski MA, Slominski A, Wenzel BE, Paus R. Thyroid hormones directly alter human hair follicle functions: anagen prolongation and stimulation of both hair matrix keratinocyte proliferation and hair pigmentation. J Clin Endocrinol Metab. 2008;93:4381–4388. doi: 10.1210/jc.2008-0283. [DOI] [PubMed] [Google Scholar]
- 14.Schallreuter KU, Pittelkow MR, Swanson NN, Beazley WD, Korner C, Ehrke C, Buttner G. Altered catecholamine synthesis and degradation in the epidermis of patients with atopic eczema. Arch Dermatol Res. 1997;289:663–666. doi: 10.1007/s004030050258. [DOI] [PubMed] [Google Scholar]
- 15.Slominski A, Tobin DJ, Zmijewski MA, Wortsman J, Paus R. Melatonin in the skin: synthesis, metabolism and functions. Trends Endocrinol Metab. 2008;19:17–24. doi: 10.1016/j.tem.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 16.Slominski A, Wortsman J, Tobin DJ. The cutaneous serotoninergic/melatoninergic system: securing a place under the sun. FASEB J. 2005;19:176–194. doi: 10.1096/fj.04-2079rev. [DOI] [PubMed] [Google Scholar]
- 17.Grando SA. Cholinergic control of epidermal cohesion. Exp Dermatol. 2006;15:265–282. doi: 10.1111/j.0906-6705.2006.00410.x. [DOI] [PubMed] [Google Scholar]
- 18.Grando SA, Pittelkow MR, Schallreuter KU. Adrenergic and cholinergic control in the biology of epidermis: physiological and clinical significance. J Investig Dermatol. 2006;126:1948–1965. doi: 10.1038/sj.jid.5700151. [DOI] [PubMed] [Google Scholar]
- 19.Slominski A, Wortsman J, Paus R, Elias PM, Tobin DJ, Feingold KR. Skin as an endocrine organ: implications for its function. Drug Disc Today Dis Mech. 2008;5:137–144. doi: 10.1016/j.ddmec.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bikle DD. Vitamin D and the skin. J Bone Miner Metab. 2010;28:117–130. doi: 10.1007/s00774-009-0153-8. [DOI] [PubMed] [Google Scholar]
- 21.Slominski AT, Li W, Kim T, Semak I, Wang J, Zjawiony J, Tuckey RC. Novel activities of CYP11A1 and their potential physiological significance. J Steroid Biochem Mol Biol. 2015;151:25–37. doi: 10.1016/j.jsbmb.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Slominski AT, Zmijewski MA, Zbytek B, Brozyna AA, Granese J, Pisarchik A, Szczesniewski A, Tobin DJ. Regulated proenkephalin expression in human skin and cultured skin cells. J Investig Dermatol. 2011;131:613–622. doi: 10.1038/jid.2010.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Slominski A, Wortsman J, Luger T, Paus R, Salomon S. Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiol Rev. 2000;80:979–1020. doi: 10.1152/physrev.2000.80.3.979. [DOI] [PubMed] [Google Scholar]
- 24.Biro T, Toth BI, Hasko G, Paus R, Pacher P. The endocannabinoid system of the skin in health and disease: novel perspectives and therapeutic opportunities. Trends Pharmacol Sci. 2009;30:411–420. doi: 10.1016/j.tips.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lerner AB, Case JD, Mori W, Wright MR. Melatonin in peripheral nerve. Nature. 1959;183:1821. doi: 10.1038/1831821a0. [DOI] [PubMed] [Google Scholar]
- 26.Slominski A, Baker J, Rosano TG, Guisti LW, Ermak G, Grande M, Gaudet SJ. Metabolism of serotonin to N-acetylserotonin, melatonin, and 5-methoxytryptamine in hamster skin culture. J Biol Chem. 1996;271:12281–12286. doi: 10.1074/jbc.271.21.12281. [DOI] [PubMed] [Google Scholar]
- 27.Gaudet SJ, Slominski A, Etminan M, Pruski D, Paus R, Namboordiri MAA. Identification and characterization of two isozymic forms of arylamine N-acetyltransferase in Syrian hamster skin. J Investig Dermatol. 1993;101:660–665. doi: 10.1111/1523-1747.ep12371672. [DOI] [PubMed] [Google Scholar]
- 28.Slominski A, Pisarchik A, Semak I, Sweatman T, Wortsman J, Szczesniewski A, Slugocki G, McNulty J, Kauser S, Tobin DJ, Jing C, Johansson O. Serotoninergic and melatoninergic systems are fully expressed in human skin. FASEB J. 2002;16:896–898. doi: 10.1096/fj.01-0952fje. [DOI] [PubMed] [Google Scholar]
- 29.Slominski A, Semak I, Pisarchik A, Sweatman T, Szczesniewski A, Wortsman J. Conversion of l-tryptophan to serotonin and melatonin in human melanoma cells. FEBS Lett. 2002;511:102–106. doi: 10.1016/S0014-5793(01)03319-1. [DOI] [PubMed] [Google Scholar]
- 30.Slominski A, Pisarchik A, Johansson O, Jing C, Semak I, Slugocki G, Wortsman J. Tryptophan hydroxylase expression in human skin cells. Biochim Biophys Acta. 2003;1639:80–86. doi: 10.1016/S0925-4439(03)00124-8. [DOI] [PubMed] [Google Scholar]
- 31.Slominski A, Pisarchik A, Semak I, Sweatman T, Szczesniewski A, Wortsman J. Serotoninergic system in hamster skin. J Investig Dermatol. 2002;119:934–942. doi: 10.1046/j.1523-1747.2002.00156.x. [DOI] [PubMed] [Google Scholar]
- 32.Semak I, Korik E, Naumova M, Wortsman J, Slominski A. Serotonin metabolism in rat skin: characterization by liquid chromatography-mass spectrometry. Arch Biochem Biophys. 2004;421:61–66. doi: 10.1016/j.abb.2003.08.036. [DOI] [PubMed] [Google Scholar]
- 33.Slominski AT, Kleszczynski K, Semak I, Janjetovic Z, Zmijewski MA, Kim TK, Slominski RM, Reiter RJ, Fischer TW. Local melatoninergic system as the protector of skin integrity. Int J Mol Sci. 2014;15:17705–17732. doi: 10.3390/ijms151017705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Slominski A, Pisarchik A, Semak I, Sweatman T, Wortsman J. Characterization of the serotoninergic system in the C57BL/6 mouse skin. Eur J Biochem. 2003;270:3335–3344. doi: 10.1046/j.1432-1033.2003.03708.x. [DOI] [PubMed] [Google Scholar]
- 35.Kobayashi H, Kromminga A, Dunlop TW, Tychsen B, Conrad F, Suzuki N, Memezawa A, Bettermann A, Aiba S, Carlberg C, Paus R. A role of melatonin in neuroectodermal–mesodermal interactions: the hair follicle synthesizes melatonin and expresses functional melatonin receptors. FASEB J. 2005;19:1710–1712. doi: 10.1096/fj.04-2293fje. [DOI] [PubMed] [Google Scholar]
- 36.Kim TK, Lin Z, Tidwell WJ, Li W, Slominski AT. Melatonin and its metabolites accumulate in the human epidermis in vivo and inhibit proliferation and tyrosinase activity in epidermal melanocytes in vitro. Mol Cell Endocrinol. 2015;404:1–8. doi: 10.1016/j.mce.2014.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Slominski AT, Semak I, Fischer TW, Kim TK, Kleszczynski K, Hardeland R, Reiter RJ. Metabolism of melatonin in the skin: why is it important? Exp Dermatol. 2017;26:563–568. doi: 10.1111/exd.13208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grace MS, Cahill GM, Besharse JC. Melatonin deacetylation: retinal vertebrate class distribution and Xenopus laevis tissue distribution. Brain Res. 1991;559:56–63. doi: 10.1016/0006-8993(91)90286-5. [DOI] [PubMed] [Google Scholar]
- 39.Cahill GM, Besharse JC. Retinal melatonin is metabolized within the eye of Xenopus laevis . Proc Natl Acad Sci USA. 1989;86:1098–1102. doi: 10.1073/pnas.86.3.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fischer TW, Sweatman TW, Semak I, Sayre RM, Wortsman J, Slominski A. Constitutive and UV-induced metabolism of melatonin in keratinocytes and cell-free systems. FASEB J. 2006;20:1564–1566. doi: 10.1096/fj.05-5227fje. [DOI] [PubMed] [Google Scholar]
- 41.Kim TK, Kleszczynski K, Janjetovic Z, Sweatman T, Lin Z, Li W, Reiter RJ, Fischer TW, Slominski AT. Metabolism of melatonin and biological activity of intermediates of melatoninergic pathway in human skin cells. FASEB J. 2013;27:2742–2755. doi: 10.1096/fj.12-224691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim TK, Lin Z, Li W, Reiter RJ, Slominski AT. N1-Acetyl-5-methoxykynuramine (AMK) is produced in the human epidermis and shows antiproliferative effects. Endocrinology. 2015;156:1630–1636. doi: 10.1210/en.2014-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fischer TW, Slominski A, Zmijewski MA, Reiter RJ, Paus R. Melatonin as a major skin protectant: from free radical scavenging to DNA damage repair. Exp Dermatol. 2008;17:713–730. doi: 10.1111/j.1600-0625.2008.00767.x. [DOI] [PubMed] [Google Scholar]
- 44.Fischer TW, Slominski A, Tobin DJ, Paus R. Melatonin and the hair follicle. J Pineal Res. 2008;44:1–15. doi: 10.1111/j.1600-079X.2007.00542.x. [DOI] [PubMed] [Google Scholar]
- 45.Fischer TW, Burmeister G, Schmidt HW, Elsner P. Melatonin increases anagen hair rate in women with androgenetic alopecia or diffuse alopecia: results of a pilot randomized controlled trial. Br J Dermatol. 2004;150:341–345. doi: 10.1111/j.1365-2133.2004.05685.x. [DOI] [PubMed] [Google Scholar]
- 46.Slominski A, Chassalevris N, Mazurkiewicz J, Maurer M, Paus R. Murine skin as a target for melatonin bioregulation. Exp Dermatol. 1994;3:45–50. doi: 10.1111/j.1600-0625.1994.tb00265.x. [DOI] [PubMed] [Google Scholar]
- 47.Fischer TW, Zmijewski MA, Zbytek B, Sweatman TW, Slominski RM, Wortsman J, Slominski A. Oncostatic effects of the indole melatonin and expression of its cytosolic and nuclear receptors in cultured human melanoma cell lines. Int J Oncol. 2006;29:665–672. doi: 10.3892/ijo.29.3.665. [DOI] [PubMed] [Google Scholar]
- 48.Slominski A, Pruski D. Melatonin inhibits proliferation and melanogenesis in rodent melanoma cells. Exp Cell Res. 1993;206:189–294. doi: 10.1006/excr.1993.1137. [DOI] [PubMed] [Google Scholar]
- 49.Slominski RM, Reiter RJ, Schlabritz-Loutsevitch N, Ostrom RS, Slominski AT. Melatonin membrane receptors in peripheral tissues: distribution and functions. Mol Cell Endocrinol. 2012;351:152–166. doi: 10.1016/j.mce.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Slominski AT, Zmijewski MA, Jetten AM. RORalpha is not a receptor for melatonin (response to DOI 10.1002/bies.201600018) Bioessays. 2016;38:1193–1194. doi: 10.1002/bies.201600204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Slominski A, Kim TK, Takeda Y, Janjetovic Z, Brozyna A, Skobowiate C, Wang J, Postlethwite A, Li W, Tuckey R, Jetten A (2014) RORα and ROR γ are expressed in human skin and serve as receptors for endogenously produced noncalcemic 20-hydroxy- and 20,23-dihydroxy-vitamin D. FASEB J 28(7):2775–2789. doi:10.1096/fj.13-242040 [DOI] [PMC free article] [PubMed]
- 52.Bangha E, Elsner P, Kistler GS. Suppression of UV-induced erythema by topical treatment with melatonin (N-acetyl-5-methoxytryptamine). A dose response study. Arch Dermatol Res. 1996;288:522–526. doi: 10.1007/BF02505248. [DOI] [PubMed] [Google Scholar]
- 53.Dreher F, Gabard B, Schwindt DA, Maibach HI. Topical melatonin in combination with vitamins E and C protects skin from ultraviolet-induced erythema: a human study in vivo. Br J Dermatol. 1998;139:332–339. doi: 10.1046/j.1365-2133.1998.02447.x. [DOI] [PubMed] [Google Scholar]
- 54.Hussein MR, Abu-Dief EE, Abd El-Reheem MH, Abd-Elrahman A. Ultrastructural evaluation of the radioprotective effects of melatonin against X-ray-induced skin damage in Albino rats. Int J Exp Pathol. 2005;86:45–55. doi: 10.1111/j.0959-9673.2005.00412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Janjetovic Z, Jarrett SG, Lee EF, Duprey C, Reiter RJ, Slominski AT. Melatonin and its metabolites protect human melanocytes against UVB-induced damage: involvement of NRF2-mediated pathways. Sci Rep. 2017;7:1274. doi: 10.1038/s41598-017-01305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Janjetovic Z, Nahmias ZP, Hanna S, Jarrett SG, Kim TK, Reiter RJ, Slominski AT. Melatonin and its metabolites ameliorate ultraviolet B-induced damage in human epidermal keratinocytes. J Pineal Res. 2014;57:90–102. doi: 10.1111/jpi.12146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schaefer M, Hardeland R. The melatonin metabolite N-acetyl-5-methoxykynuramine is a potent singlet oxygen scavenger. J Pineal Res. 2009;46:49–52. doi: 10.1111/j.1600-079X.2008.00614.x. [DOI] [PubMed] [Google Scholar]
- 58.Kleszczynski K, Tukaj S, Kruse N, Zillikens D, Fischer TW. Melatonin prevents ultraviolet radiation-induced alterations in plasma membrane potential and intracellular pH in human keratinocytes. J Pineal Res. 2013;54:89–99. doi: 10.1111/j.1600-079X.2012.01028.x. [DOI] [PubMed] [Google Scholar]
- 59.Fischer TW, Zbytek B, Sayre RM, Apostolov EO, Basnakian AG, Sweatman TW, Wortsman J, Elsner P, Slominski A. Melatonin increases survival of HaCaT keratinocytes by suppressing UV-induced apoptosis. J Pineal Res. 2006;40:18–26. doi: 10.1111/j.1600-079X.2005.00273.x. [DOI] [PubMed] [Google Scholar]
- 60.Fischer TW, Kleszczynski K, Hardkop LH, Kruse N, Zillikens D. Melatonin enhances antioxidative enzyme gene expression (CAT, GPx, SOD), prevents their UVR-induced depletion, and protects against the formation of DNA damage (8-hydroxy-2′-deoxyguanosine) in ex vivo human skin. J Pineal Res. 2013;54:303–312. doi: 10.1111/jpi.12018. [DOI] [PubMed] [Google Scholar]
- 61.Kleszczynski K, Zwicker S, Tukaj S, Kasperkiewicz M, Zillikens D, Wolf R, Fischer TW. Melatonin compensates silencing of heat shock protein 70 and suppresses ultraviolet radiation-induced inflammation in human skin ex vivo and cultured keratinocytes. J Pineal Res. 2015;58:117–126. doi: 10.1111/jpi.12197. [DOI] [PubMed] [Google Scholar]
- 62.Semak I, Naumova M, Korik E, Terekhovich V, Wortsman J, Slominski A. A novel metabolic pathway of melatonin: oxidation by cytochrome c . Biochemistry. 2005;44:9300–9307. doi: 10.1021/bi050202d. [DOI] [PubMed] [Google Scholar]
- 63.Ahn T, Yun CH. Molecular mechanisms regulating the mitochondrial targeting of microsomal cytochrome P450 enzymes. Curr Drug Metab. 2010;11:830–838. doi: 10.2174/138920010794479655. [DOI] [PubMed] [Google Scholar]
- 64.Konishi H, Tanaka M, Takemura Y, Matsuzaki H, Ono Y, Kikkawa U, Nishizuka Y. Activation of protein kinase C by tyrosine phosphorylation in response to H2O2 . Proc Natl Acad Sci USA. 1997;94:11233–11237. doi: 10.1073/pnas.94.21.11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Semak I, Korik E, Antonova M, Wortsman J, Slominski A. Metabolism of melatonin by cytochrome P450s in rat liver mitochondria and microsomes. J Pineal Res. 2008;45:515–523. doi: 10.1111/j.1600-079X.2008.00630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Baron JM, Wiederholt T, Heise R, Merk HF, Bickers DR. Expression and function of cytochrome p450-dependent enzymes in human skin cells. Curr Med Chem. 2008;15:2258–2264. doi: 10.2174/092986708785747535. [DOI] [PubMed] [Google Scholar]
- 67.Slominski AT, Janjetovic Z, Kim TK, Wasilewski P, Rosas S, Hanna S, Sayre RM, Dowdy JC, Li W, Tuckey RC. Novel non-calcemic secosteroids that are produced by human epidermal keratinocytes protect against solar radiation. J Steroid Biochem Mol Biol. 2015;148:52–63. doi: 10.1016/j.jsbmb.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Slominski A, Kim TK, Zmijewski MA, Janjetovic Z, Li W, Chen J, Kusniatsova EI, Semak I, Postlethwaite A, Miller DD, Zjawiony JK, Tuckey RC. Novel vitamin D photoproducts and their precursors in the skin. Dermatoendocrinology. 2013;5:7–19. doi: 10.4161/derm.23938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bachurin SO, Shevtsova EP, Kireeva EG, Oxenkrug GF, Sablin SO. Mitochondria as a target for neurotoxins and neuroprotective agents. Ann N Y Acad Sci. 2003;993:334–344. doi: 10.1111/j.1749-6632.2003.tb07541.x. [DOI] [PubMed] [Google Scholar]
- 70.Tan DX, Manchester LC, Qin L, Reiter RJ. Melatonin: a mitochondrial targeting molecule involving mitochondrial protection and dynamics. Int J Mol Sci. 2016;17:2124. doi: 10.3390/ijms17122124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.He C, Wang J, Zhang Z, Yang M, Li Y, Tian X, Ma T, Tao J, Zhu K, Song Y, Ji P, Liu G. Mitochondria synthesize melatonin to smeliorate its function and improve mice oocyte’s quality under in vitro conditions. Int J Mol Sci. 2016;17:939. doi: 10.3390/ijms17060939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Marinho HS, Real C, Cyrne L, Soares H, Antunes F. Hydrogen peroxide sensing, signaling and regulation of transcription factors. Redox Biol. 2014;2:535–562. doi: 10.1016/j.redox.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hamanaka RB, Glasauer A, Hoover P, Yang S, Blatt H, Mullen AR, Getsios S, Gottardi CJ, DeBerardinis RJ, Lavker RM, Chandel NS. Mitochondrial reactive oxygen species promote epidermal differentiation and hair follicle development. Sci Signal. 2013;6:ra8. doi: 10.1126/scisignal.2003638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Marchese C, Maresca V, Cardinali G, Belleudi F, Ceccarelli S, Bellocci M, Frati L, Torrisi MR, Picardo M. UVB-induced activation and internalization of keratinocyte growth factor receptor. Oncogene. 2003;22:2422–2431. doi: 10.1038/sj.onc.1206301. [DOI] [PubMed] [Google Scholar]
- 75.Peus D, Vasa RA, Meves A, Pott M, Beyerle A, Squillace K, Pittelkow MR. H2O2 is an important mediator of UVB-induced EGF-receptor phosphorylation in cultured keratinocytes. J Investig Dermatol. 1998;110:966–971. doi: 10.1046/j.1523-1747.1998.00210.x. [DOI] [PubMed] [Google Scholar]
- 76.Poeggeler B, Reiter RJ, Tan DX, Chen LD, Manchester LC. Melatonin, hydroxyl radical-mediated oxidative damage, and aging: a hypothesis. J Pineal Res. 1993;14:151–168. doi: 10.1111/j.1600-079X.1993.tb00498.x. [DOI] [PubMed] [Google Scholar]
- 77.Grzelak A, Macierzynska E, Bartosz G. Melatonin does not react rapidly with hydrogen peroxide. Free Radic Res. 2004;38:1155–1158. doi: 10.1080/10715760412331272486. [DOI] [PubMed] [Google Scholar]
- 78.Kagan VE, Tyurin VA, Jiang J, Tyurina YY, Ritov VB, Amoscato AA, Osipov AN, Belikova NA, Kapralov AA, Kini V, Vlasova II, Zhao Q, Zou M, Di P, Svistunenko DA, Kurnikov IV, Borisenko GG. Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nat Chem Biol. 2005;1:223–232. doi: 10.1038/nchembio727. [DOI] [PubMed] [Google Scholar]
- 79.Venditti P, Napolitano G, Di Meo S. Role of enzymatic and non-enzymatic processes in H2O2 removal by rat liver and heart mitochondria. J Bioenerg Biomembr. 2014;46:83–91. doi: 10.1007/s10863-013-9534-8. [DOI] [PubMed] [Google Scholar]
- 80.Hardeland R, Pandi-Perumal SR. Melatonin, a potent agent in antioxidative defense: actions as a natural food constituent, gastrointestinal factor, drug and prodrug. Nutr Metab (Lond) 2005;2:22. doi: 10.1186/1743-7075-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bowman A, Birch-Machin MA. Age-dependent decrease of mitochondrial complex II activity in human skin fibroblasts. J Investig Dermatol. 2016;136:912–919. doi: 10.1016/j.jid.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 82.Huo X, Wang C, Yu Z, Peng Y, Wang S, Feng S, Zhang S, Tian X, Sun C, Liu K, Deng S, Ma X. Human transporters, PEPT1/2, facilitate melatonin transportation into mitochondria of cancer cells: an implication of the therapeutic potential. J Pineal Res. 2017 doi: 10.1111/jpi.12390. [DOI] [PubMed] [Google Scholar]
- 83.Fischer TW, Zmijewski MA, Wortsman J, Slominski A. Melatonin maintains mitochondrial membrane potential and attenuates activation of initiator (casp-9) and effector caspases (casp-3/casp-7) and PARP in UVR-exposed HaCaT keratinocytes. J Pineal Res. 2008;44:397–407. doi: 10.1111/j.1600-079X.2007.00542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sarti P, Magnifico MC, Altieri F, Mastronicola D, Arese M. New evidence for cross talk between melatonin and mitochondria mediated by a circadian-compatible interaction with nitric oxide. Int J Mol Sci. 2013;14:11259–11276. doi: 10.3390/ijms140611259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kleszczyński K, Zillikens D, Fischer TW. Melatonin enhances mitochondrial ATP synthesis, reduces reactive oxygen species formation, and mediates translocation of the nuclear erythroid 2-related factor 2 resulting in activation of phase-2 antioxidant enzymes (γ-GCS, HO-1, NQO1) in ultraviolet radiation-treated normal human epidermal keratinocytes (NHEK) J Pineal Res. 2016;61:187–197. doi: 10.1111/jpi.12338. [DOI] [PubMed] [Google Scholar]
- 86.Kerem H, Akdemır O, Ates U, Uyanıkgıl Y, Demırel Sezer E, Bılkay U, Turgut M, Sozmen E, Songur E. The effect of melatonin on a dorsal skin flap model. J Investig Surg. 2014;27:57–64. doi: 10.3109/08941939.2013.835892. [DOI] [PubMed] [Google Scholar]
- 87.Kilańczyk E, Bryszewska M. The effect of melatonin on antioxidant enzymes in human diabetic skin fibroblasts. Cell Mol Biol Lett. 2003;8:333–336. [PubMed] [Google Scholar]
- 88.Korshunov SS, Krasnikov BF, Pereverzev MO, Skulachev VP. The antioxidant functions of cytochrome c . FEBS Lett. 1999;462:192–198. doi: 10.1016/S0014-5793(99)01525-2. [DOI] [PubMed] [Google Scholar]
- 89.Zhao Y, Xu JX. The operation of the alternative electron-leak pathways mediated by cytochrome c in mitochondria. Biochem Biophys Res Commun. 2004;317:980–987. doi: 10.1016/j.bbrc.2004.03.144. [DOI] [PubMed] [Google Scholar]
- 90.Acuna-Castroviejo D, Martin M, Macias M, Escames G, Leon J, Khaldy H, Reiter RJ. Melatonin, mitochondria, and cellular bioenergetics. J Pineal Res. 2001;30:65–74. doi: 10.1034/j.1600-079X.2001.300201.x. [DOI] [PubMed] [Google Scholar]