Abstract
Purpose
Clinical practice guidelines on chemotherapy-induced peripheral neuropathy (CIPN) use the NCI Common Terminology Criteria for Adverse Events (CTCAE), while recent clinical trials employ a potentially superior measure, the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-CIPN twenty-item scale (QLQ-CIPN20), a patient reported outcome (PRO). Practitioners and researchers lack guidance, how QLQ-CIPN20 results relate to the traditional CTCAE during the serial assessment of patients undergoing chemotherapy.
Methods
Two large CIPN clinical trial datasets (538 patients) pairing QLQ-CIPN20 and CTCAE outcomes were analyzed using a multivariable linear mixed model with QLQ-CIPN20 score as the outcome variable, CTCAE grade as the main effect, and patient as random effect (accounting for internal correlation of serial measures).
Results
The association between QLQ-CIPN20 scores and CTCAE grades was strong (p < 0.0001), whereby patients with higher CTCAE grade had worse QLQ-CIPN20 scores. Some variation of QLQ-CIPN20 scores was observed based on drug, treatment, and cycle. While there was a marked difference in the mean QLQ-CIPN20 scores between CTCAE grades, the ranges of QLQ-CIPN20 scores within each CTCAE grade were large leading to large overlap in CIPN20 scores across CTCAE grades.
Conclusions
A strong positive association of QLQ-CIPN20 scores and CTCAE grade provides evidence of convergent validity as well as practical guidance, how to quantitatively interpret QLQ-CIPN20 scores at the study level in terms of the traditional CTCAE. The present results also highlight an important clinical caveat, specifically, that conversion of QLQ-CIPN20 to a CTCAE may not be reliable at the level of an individual patient.
Keywords: peripheral neuropathy, CTCAE, EORTC QLQ-CIPN20, patient-reported outcome, physician-reported outcome
Introduction
Chemotherapy-induced peripheral neuropathy (CIPN) is a prominent clinical problem. In clinical trials evaluating the prevention and/or treatment of CIPN, the degree of neuropathy has commonly been measured using National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) [1 – 3]. CTCAE was also the primary assessment of CIPN in most studies included in a recent systematic review on CIPN by Mols et al. [4], as well as the recent American Society of Clinical Oncology clinical practice guidelines for the prevention and management of CIPN [5]. However, in recent years, it has been well recognized that the CTCAE method is not an adequate measure to quantify the degree of neuropathy [6 – 11]. The lack of uniformly and clearly defined criteria lead to subjective assignment of CTCAE grades, with an inter-observer agreement of only 46% [6]. Patient reported outcome tools, such as the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire-CIPN twenty-item scale (QLQ-CIPN20), have been shown to be better measures of neuropathy than clinician determined CTCAE grading [12, 13]. For example, the QLQ-CIPN tool has undergone validity testing [21, 23], whereby the CTCAE has not. Furthermore, CTCAE collected in large oncology clinical trials such as those analyzed in this study can be from multiple physicians involved in the longitutidal assessment of a patient, which adds inter-observer variability. Pursuant to this, some clinical trials evaluating CIPN have utilized patient reported outcomes (PRO) alone, without concomitant collection of NCI CTCAE data [14 - 17] while a few others collected both patient-reported as well as clinician-assessed outcomes [3, 8, 18 – 20].
When researchers report QLQ-CIPN20 data, clinicians often ask how these reported scores align with CTCAE grades. To date, this question has been difficult to address because few published studies provide a comparison of the scores obtained from the two. Smith et al. [21], in their paper assessing the reliability and validity of the QLQ-CIPN20, reported a low correlation (n = 203), though statistically significant, between the baseline QLQ-CIPN20 subscales and sensory neuropathy grading using the CTCAE v3.0. Alberti et al. [22] examined the association between the QLQ-CIPN20 sensory subscale and clinician-assessed sensory grading using the NCI – Common Toxicity Criteria (CTC) v2.0, the clinical Total Neuropathy Score (TNSc©), and the modified Inflammatory Neuropathy Cause and Treatment (INCAT) sensory sum score (mISS), reporting a linear trend between the QLQ-CIPN20 sensory subscale and the NCI-CTC sensory grade (n = 281). Both studies evaluated the association between patient-reported and clinician-assessed scores at a single time point (baseline [21] or the first evaluation [22]) and included older versions of the NCI CTCAE grading. More importantly, neither of these studies addressed whether or not a direct comparison exists between QLQ-CIPN20 scores and CTCAE neuropathy grades, while other studies that used both instruments [3, 8, 18 – 20] only reported these outcomes separately.
The current project was developed to investigate the relationship between patient-reported QLQ-CIPN20 scores and physician-reported CTCAE v4.0 grading, and to determine whether it is possible to define ranges of QLQ-CIPN 20 scores associated with different CTCAE neuropathy grades. This secondary analysis includes data from all treatment cycles of two clinical trials conducted by the North Central Cancer Treatment Group (NCCTG), N08CB [19] and N08CA [20]. The NCCTG is now part of the Alliance for Clinical Trials in Oncology (Alliance).
Methods
Clinical trial cohorts
NCCTG N08CB and N08CA were both randomized phase III, double-blind, placebo-controlled trials for prevention of CIPN in which paired data using the QLQ-CIPN20 and the NCI CTCAE were collected at each study visit. The data for both of these measures was collected prior to a subsequent dose of chemotherapy on the appointed day. N08CB was a study of intravenous calcium (Ca) and magnesium (Mg) to prevent oxaliplatin-induced neurotoxicity, where 353 patients with colon cancer were randomly assigned to one of three arms: CaMg before and after oxaliplatin-containing therapy, Ca/Mg before and placebo after, and placebo before and after [19]. Three randomized patients were later found to be ineligible for the trial and were excluded from our analysis hence the analysis of N08CB included data from 350 patients.
N08CA included 185 patients with ovarian, lung, and other cancers receiving a paclitaxel/carboplatin regimen who were randomly assigned to receive glutathione or placebo for prevention of paclitaxel/carboplatin-induced peripheral neuropathy [20]. One hundred and sixty four of these patients were treated every 3 weeks while the remaining patients were treated at either weekly, or every-4-week intervals. To ensure consistency in treatment cycle, the analysis of N08CA was limited to the 164 patients treated every three weeks. Due to differences in diseases, treatment regimens, cycles, and designs between these two studies, CIPN measurement data were analyzed separately. Each participant signed an IRB-approved, protocol-specific informed consent in accordance with federal and institutional guidelines.
CIPN Assessments
Clinician-assessed CIPN was reported as grade 0, 1, 2, 3 or 4 per criteria established in NCI CTCAE v. 4.0 using means that have been established over decades for generating toxicity evaluation by this method. In the N08CB trial, additional standardized questions were used to quantify neurotoxicity associated with chemotherapy (details provided in that publication). The CTCAE was done by clinicians.
Patient assessment of CIPN was obtained using the QLQ-CIPN20 questionnaire, which was completed prior to any chemotherapy on the appointed day. There was no prescription as to whether it was completed before or after clinician judgment of CTCAE scores, although it is not usual to share these scores with patients. This questionnaire contains 20 items on which patients rate their experience for each symptom during the previous week using scores from 1 (not at all) to 4 (very much). The sum score was obtained by adding the scores of items 1 to 19 resulting in a sum score range of 19 to 76, which was termed CIPN20 sum1-19. Item 20 rates male impotence; this is non-informative in female patients and frequently not provided by male patients. As a consequence, this item was excluded in the CIPN20 sum1-19 score in the main analysis. The association between item 20, where available, and CTCAE grading was explored in a separate analysis. Of note, item 19 rates difficulty using the pedals and is only applicable to patients who drive a car. This item can be excluded from the sum scores where the majority of patients do not drive. Since the response rate for this question is higher than 85% in our data, item 19 was included in the sum score for this analysis. In addition to a sum score, the items in CIPN20 have been divided into three subscales. The sensory subscale consists of items 1, 2, 3, 4, 5, 6, 9, 10, and 18; motor: items 7, 8, 11, 12, 13, 14, 15, and 19; and autonomic: items 16, 17, and 20. Four publications provide information regarding the QLQ-CIPN-20's psychometric properties. [8, 21, 22, 23] The published data provide preliminary evidence of its internal consistency and stability reliability, sensitivity, validity (structural, convergent, discriminant), and responsiveness.
Statistical analysis
The primary analysis of this study focuses on the association between CIPN20 sum1 – 19 score and CTCAE grade. Association among the QLQ-CIPN20 subscale scores and CTCAE grades were also evaluated. The following analyses were conducted separately for data from N08CB and N08CA. In a descriptive analysis, the box and whisker plots of the sum1 – 19 score and subscales were constructed by CTCAE grade. The mean and standard error (SE) of QLQ-CIPN20 sum and subscale scores were estimated for each CTCAE grade using data from all occurrences (evaluation cycles) as well as from the first occurrence. Plots of the means and SEs were provided. Data from all occurrences were analyzed using multivariable linear mixed models, with patient as the random effect, to account for potential correlation between multiple observations from the same patient. QLQ-CIPN20 score was the outcome variable. CTCAE grade was included in all models as the main effect. CTCAE is a grading scale with five levels. It is an ordinal variable and was treated as such in this analysis. Note that a correlation coefficient is not an appropriate measure of association between QLQ-CIPN20 (a continuous variable) and CTCAE (an ordinal variable) in this analysis and, hence, was not reported. To adjust for other factors that may impact QLQ-CIPN20 scores and their association with CTCAE, we tested for the effect of cycle and its interaction with CTCAE grade and found that this interaction was not statistically significant. Although, as previously reported, QLQ-CIPN20 scores were not different between treatment arms [19, 20], we tested for an interaction between CTCAE grade and arm to determine whether the association between CTCAE and QLQ-CIPN20 score were different by treatment arm and found that it was not statistically significant. Hence, treatment arm was not included in the final models. Data collection and statistical analyses were conducted by the Alliance Statistics and Data Center. All analyses were based on the study database frozen on January 22, 2013.
Results
QLQ-CIPN20 sum scores corresponding to CTCAE grades 0, 1, 2, and 3 in oxaliplatin treated patients (N08CB)
For N08CB, n=3269 paired CIPN assessments, QLQ-CIPN20/CTCAE, were obtained over the course of multiple patient visits (in 350 patients). Data pairs with missing QLQ-CIPN20 score or missing CTCAE scores were excluded from the analysis. Complete data were available for n=3176 observations and were used for subsequent analysis.
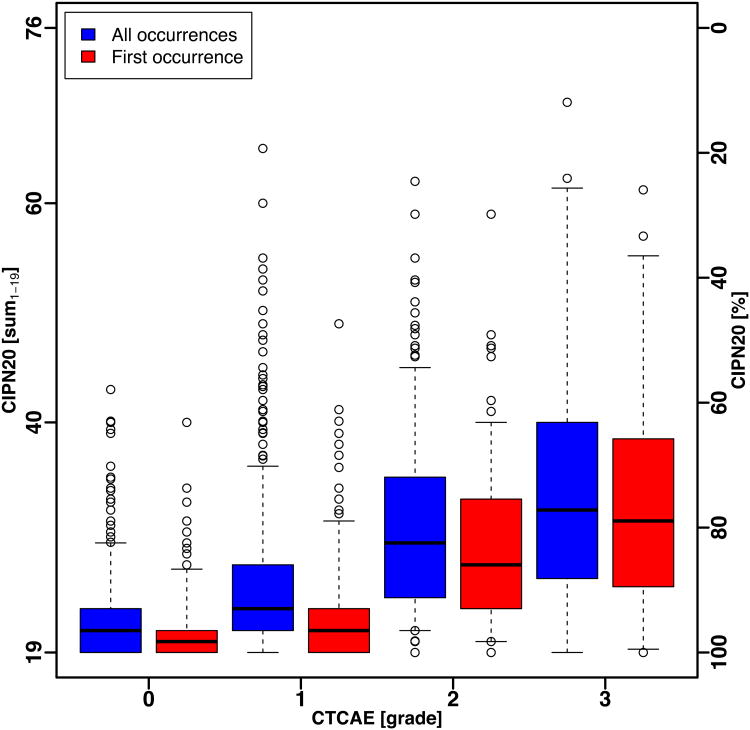
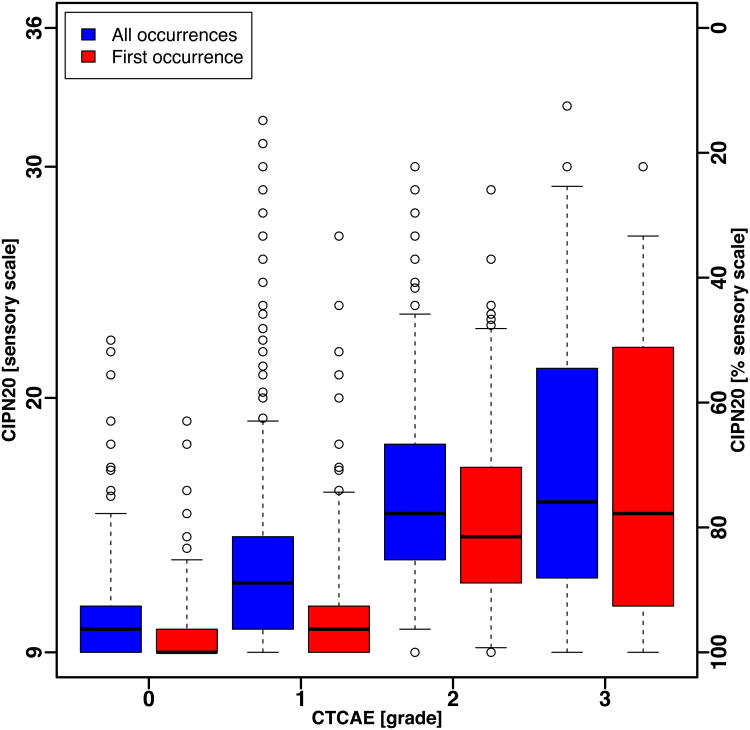
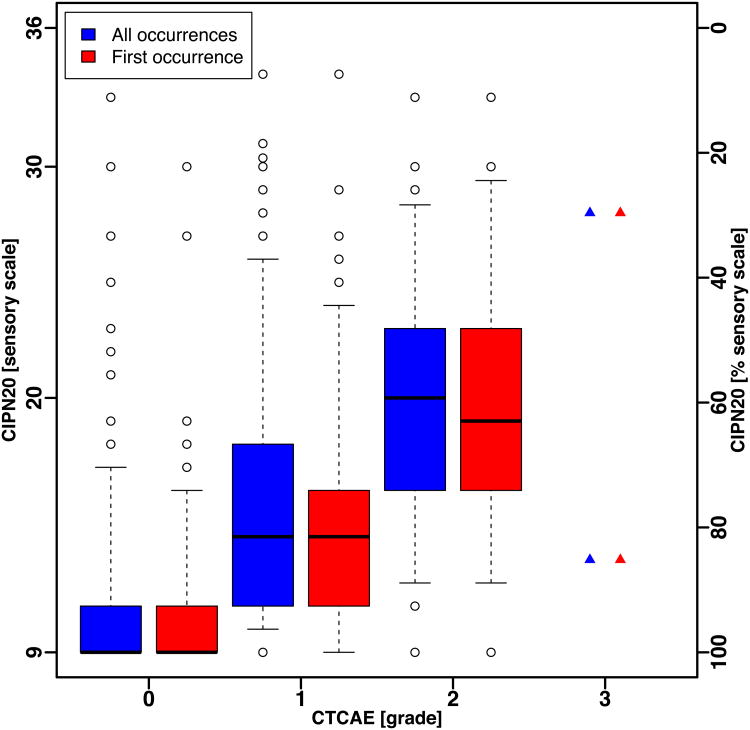
The box and whisker plots of the QLQ-CIPN20 sum score (Fig. 1A) and sensory subscale score (Fig. 1B), for all occurrences and for first occurrence, of patients in N08CB show that the ranges of QLQ-CIPN20 scores by CTCAE grade were large and there was substantial overlap between CTCAE grades. Even when considering only the middle 50% of the scores (the boxes in Fig. 1A and Fig. 1B), there is no clear separation in QLQ-CIPN20 scores between CTCAE grades.
Figure 1.
Box and whisker plot of CIPN20 scores by CTCAE grade for all occurrences and first occurrence in patients treated with oxaliplatin (N08CB). Whiskers show the 5th and 95th percentile of scores.
(A) Sum1 – 19 score.
(B) Sensory subscale.
The means QLQ-CIPN20 sum1-19 scores using all occurrences by CTCAE grade 0, 1, 2, and 3 were 21.6 (SE = 0.12), 24.3 (0.13), 29.9 (0.41), and 33.6 (1.90), respectively. The same result can be expressed on a scale of 0 to 100 (lower scores correspond to worse symptoms, Supplementary Table 1), which represents a simple linear conversion and is provided throughout this report. Expressed on that scale, the results were: 94.9 (0.20), 90.1 (0.24), 79.8 (0.74), and 71.8 (3.67), respectively (Fig. 2A).
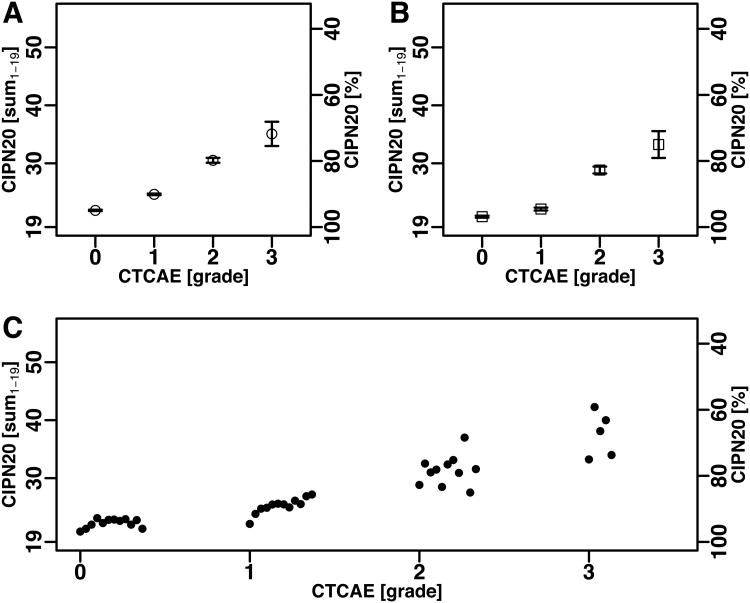
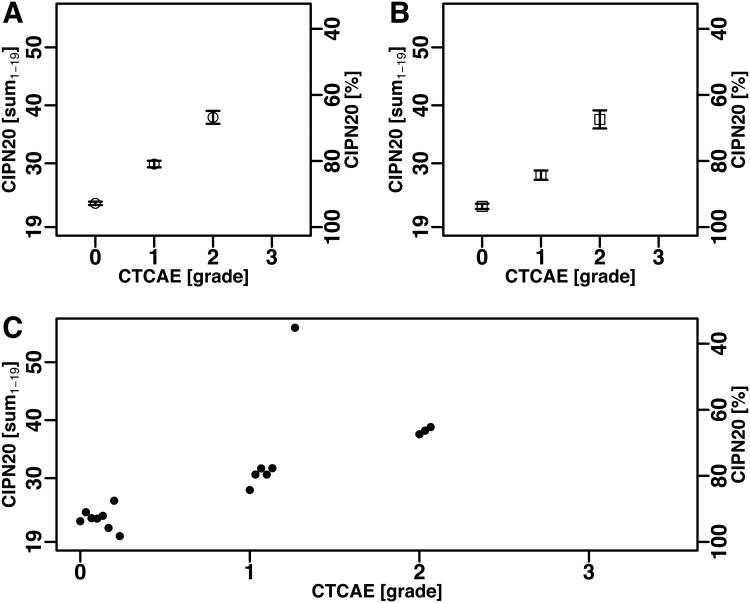
Figure 2.
Mean and mean ± SEM of CIPN20 scores by CTCAE grade in patients treated with oxaliplatin (N08CB).
(A) The CIPN20 sum1-19 for all occurrences.
(B) The CIPN20 sum1-19 at the first occurrence.
(C) The CIPN20 sum1-19 for each CTCAE grade at the first and subsequent occurrences (mean)showing a time dependent trend for grade 1. The time dependent trend observed within grade 1 was significant as discussed in the main text.
Results from the linear mixed model indicate a significant association between QLQ-CIPN20 and CTCAE (p-value < .0001) as well as an association with treatment cycle (p-value < .0001). As expected, after adjusting for cycle, patients with CTCAE grade 3 had the worst QLQ-CIPN20 scores (the highest symptom burden). The mean QLQ-CIPN20 sum score was 8.2 percentage points (95% confidence interval (CI): 7.4– 9.0, p <0.01) lower in patients with grade 3 compared to those with grade 2, 9.3 (95% CI: 8.1– 10.4, p<0.01) lower in grade 2 compared to grade 1, and 3.6 (95% CI: 2.8– 4.4, p <0. 01) lower in grade 1 compared to grade 0 (Supplementary Table 2).
Given the same CTCAE grade, QLQ-CIPN20 scores in later cycles tend to be worse than in earlier cycles. This explains the lower (better) mean QLQ-CIPN20 sum1-19 score for CTCAE grade 1 when only the first evaluation for each patient was considered (Fig. 2B) compared to the mean when all occurrences were included (Fig. 2A). Fig 2C shows the sum score for each CTCAE grade by treatment cycle. Specifically for grade 1, a marked correlation of the QLQ-CIPN20 sum1-19 with cycle was seen (R2 = 0.75, p = 1.8×10-4), which suggests that the QLQ-CIPN20 sum1-19 was a finer-grained assessment method showing gradual symptom worsening over a period, when physicians could not differentiate early or more advanced symptoms within grade 1. Patients in N08CB received one of three treatments. Results did not differ when broken down by treatment arm. Item 20 of the CIPN20, a rating of male impotence that was omitted from the QLQ-CIPN20 sum1-19 score revealed no notable correlation with CTCAE grade at the whole-cohort level.
QLQ-CIPN20 sum scores corresponding to CTCAE grades 0, 1, 2, and 3 in paclitaxel/carboplatin treated patients (N08CA)
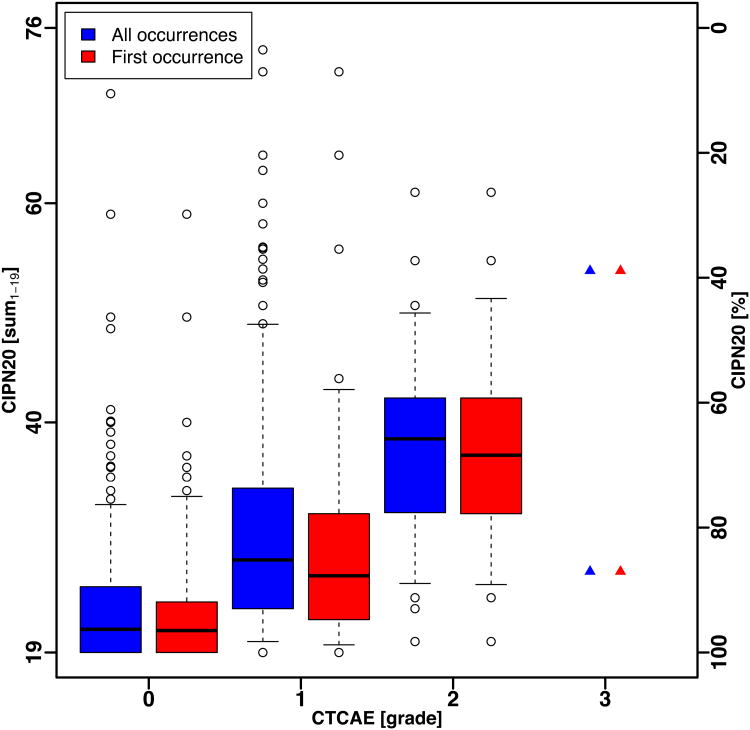
For N08CA, n=746 paired CIPN assessments, QLQ-CIPN20/CTCAE, were obtained over thecourse of multiple patient visits (in 164 patients). Complete data were available for n=709observations (used for subsequent analysis). Similar to the trend observed in N08CB, theranges of QLQ-CIPN20 scores by CTCAE grade in N08CA were large and there wassubstantial overlap between CTCAE grades (Fig. 3A and Fig. 3B). Only two paired assessments with grade 3 were observed. The mean CIPN20 sum1-19 scores in patients with CTCAE grade 0, 1, 2 and 3 were 22.8 (SE = 0.28), 29.4 (0.55), 36.9 (1.08) and 38.0 (13.00), respectively, which on the alternate scale corresponded to 92.8 (0.52), 80.9 (1.00), 66.8 (1.95) and 62.9 (24.07) (Fig. 4A, Supplementary Table 1).
Figure 3.
Box and whisker plot of CIPN20 scores for all occurrences and first occurrence in patients treated with paclitaxel/carboplatin (N08CA). Whiskers show the 5th and 95th percentile of scores.
(A) Sum1 – 19 score.
(B) Sensory subscale.
Figure 4.
Mean and mean ± SEM of CIPN20 scores by CTCAE grade in patients treated with oxaliplatin (N08CA).
(A) The CIPN20 sum1-19 for all occurrences.
(B) The CIPN20 sum1-19 at the first occurrence.
(C) The CIPN20 sum1-19 for each CTCAE grade at the first and subsequent occurrences (mean)showing a time dependent trend for grade 1.
The association between CTCAE and QLQ-CIPN20 from this analysis is consistent with the results shown for N08CB, with a p-value <0.0001. The association with treatment cycle was also significant (p=0.005). Again, patients with grade 3 CTCAE had the worst QLQ-CIPN20 scores. On average, QLQ-CIPN20 sum scores were 3.8 (95% CI: -15.2 – 22.8, p = 0.7) percentage points lower in patients with grade 3 compared to those with grade 2, 13.9 (95% CI: 9.9– 17.8, p <0. 01) lower in grade 2 compared to grade 1, and 10.8 (95% CI: 8.6– 13.0, p =<0. 01) lower in grade 1 compared to grade 0.
Plots of QLQ-CIPN20 sum1 – 19 score including only the first occurrence (Fig 3A and 4B) show similar pattern as all occurrences for CTCAE grades 0. However, QLQ-CIPN20 sum1-19 for CTCAE grades 1 and 2 appears to be lower (better) when only the first occurrence of grade 1 was considered which is consistent with the pattern observed in N08CB. A further depiction of the QLQ-CIPN20 sum1-19 by CTCAE grade by cycle again suggests a correlation between QLQ-CIPN20 scores and treatment cycle for CTCAE grades 1 and 2 (Fig. 4C). This is consistent with the multivariable results indicating an association between cycle and QLQ-CIPN20 scores in this study after adjusting for CTCAE grade. The results did not differ by treatment arm. QLQ-CIPN20 item 20 data were available in too few patients in N08CA to be meaningfully assessed, particularly because many patients in this protocol were women.
Sensory-, motor-, and autonomic EORTC CIPN20 “subscales”
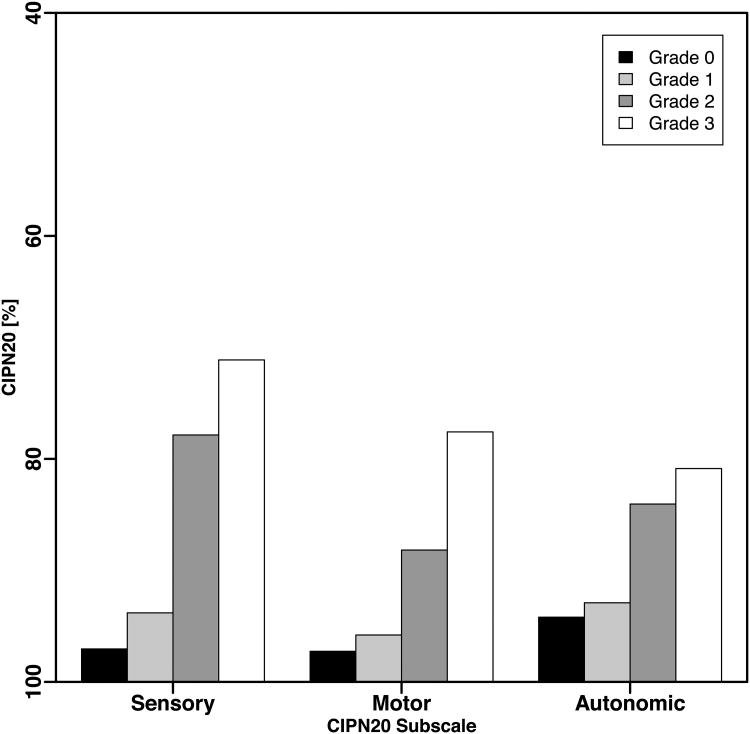
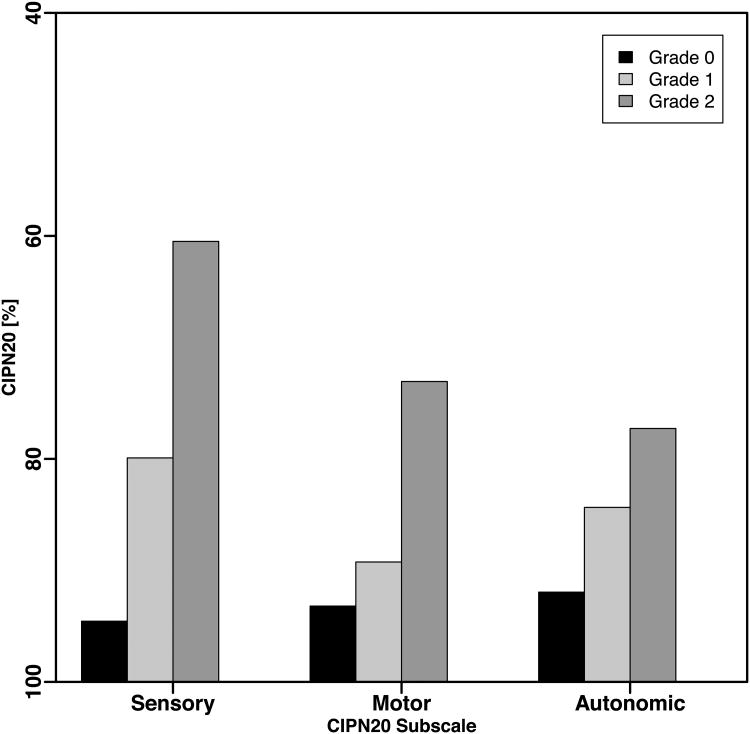
The subscales of the EORTC QLQ-CIPN20, especially the sensory subscale, are often used instead of the QLQ-CIPN20 sum1 - 19 score [3, 16, 20 – 21]. Slightly larger differences between grade 1 vs. 0 and between grade 2 vs. 1 were observed in models assessing association between sensory subscale and CTCAE than was observed in analysis of motor and autonomic subscales (Fig 5A and 5B). This confirms the higher responsiveness of the sensory subscale [8, 21]. However, the overall patterns of association between CTCAE and the subscales of QLQ-CIPN20 were consistent with the results shown for the QLQ-CIPN20 sum1 – 19 score.
Figure 5.
CIPN20 sub-scales. The mean subscale scores were computed for each first occurrence of a CTCAE grade per patient.
(A) N08CB (oxaliplatin).
(B) N08CA (paclitaxel/carboplatin).
Discussion
NCCTG N08CB and N08CA provide a unique opportunity to evaluate the relationship between patient-reported CIPN symptoms (EORTC QLQ-CIPN20) vs. clinician-assessed neuropathy grading (NCI CTCAE). Both were phase III trials with hundreds of patients where QLQ-CIPN20 scores and CTCAE grades were collected concurrently at each of many cycles of treatment, resulting in two large sets of data with paired measures. Data from both studies showed very strong correlation between CIPN20 and CTCAE, suggesting strong convergent validity because both assessment tools, in principle, measure the same symptoms. While this finding is not unexpected, it is, nevertheless, reassuring.
The patient populations from these studies differed in disease, chemotherapy regimens, and CIPN treatments. While the principal findings are similar in both studies, certain specific differences were noted. Specifically, QLQ-CIPN20 scores in paclitaxel/carboplatin-treated patients appeared to be worse (showing more neuropathy burden) for each CTCAE grade than in oxaliplatin-treated patients. Data from both trials showed a consistently strong association between QLQ-CIPN20 score and CTCAE neuropathy grade. While this association has been previously reported by Smith et al. [21] and Alberti et al. [22] with a single observation per patient (i.e., a single time point), the present study extends these findings showing that this association holds over time when data from multiple treatment cycles per patient are available and that this association is independent of treatment cycle.
Our study was based on the largest dataset, n = 3176 and 709 for N08CB and N08CA, respectively, available to date among reports assessing the relationship between patient-reported versus clinician-assessed grading of neuropathy (n = 230 [21] and n = 281 [22]). We found a large range in QLQ-CIPN20 scores within each CTCAE grade, when considering inter-patient variation. Accordingly, we conclude that it is not possible to assign ranges of QLQ-CIPN20 scores that correspond to CTCAE grading levels. This result was not surprising because perfect agreement in each patient would render the QLQ-CIPN-20 redundant. It is more about sensitivity and responsiveness. While both measures are valid (they measure what they are intended to measure – CIPN), the QLQ-CIPN20 questionnaire provides more detailed information, distinguishes more subtle degrees of neuropathy, and is more responsive to change over time. In studies of preventative agents, detection of subtle changes is important. Thus, important information would have been missed, if only the CTCAE - previously the standard - had been recorded in the two trials. An alternative view would be that the overlap in the CIPN20 across CTCAE grades likely reflects the inaccuracy of the CTCAE and the CIPN20 may be more likely to be accurate and sensitive than CTCAE.
QLQ-CIPN20 and CTCAE were very highly correlated at the cohort level (that is the means of QLQ-CIPN20 scores were different across CTCAE grades), a relationship that the present study could describe quantitatively with high precision thanks to the large size of the available cohorts. The data provided may also serve as a critical reference in the planning of future research, supporting that a patient-reported instrument, such as the QLQ-CIPN20, should be used to most accurately measure CIPN in clinical trials designed to do such. The NCI Patient-reported outcome version of the CTCAE, called the PRO-CTCAE, has recently been validated 24. The PRO-CTCAE includes two items asking patients to rate the severity and interference associated with numbness and tingling in their hands and feet. We recommend evaluating the association between QLQ-CIPN20 and PRO-CTCAE in future research.
Supplementary Material
Supplementary Table 1. Mean and standard error of mean (SEM) of CIPN20 % scores for N08CA and N08CB grouped by CTCAE grades.
Supplementary Table 2. Difference and the corresponding 95% confidence interval between the mean CIPN20 % scores for different grades of CTCAE obtained by fitting the linear mixed model and adjusting for cycle for N08CB and N08CA.
Acknowledgments
The authors wish to thank Dr. Cynthia Lynch from Mayo Clinic for accrual contribution to NCCTG N08CA and Dr. Nassim Nabbout from Wichita NCI Community Oncology Research Program for accrual contribution to NCCTG N08CB.
Support: Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under the Award Number UG1CA189823 (to the Alliance for Clinical Trials in Oncology NCORP Grant) and U10CA180790 and by the National Institute Of Nursing Research (NINR) of the National Institutes of Health (NIH) under Award Number R01NR015259 (to A.S.B.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of Interest: The authors report no conflicts of interest.
References
- 1.Kottschade LA, Sloan JA, Mazurczak MA, Johnson DB, Murphy BP, Rowland KM, Smith DA, Berg AR, Stella PJ, Loprinzi CL. The use of Vitamin E for the prevention of chemotherapy-induced peripheral neuropathy: results of a randomized phase III clinical trial. Supportive Care Cancer. 2011;19:1769–1777. doi: 10.1007/s00520-010-1018-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hershman DL, Unger JM, Crew KD, Minasian LM, Awad D, Moinpour CM, Hansen L, Lew DL, Greenlee H, Fehrenbacher L, Wade JL, III, Wong SF, Hortobagyi GN, Meyskens FL, Albain KS. Randomized Double-Blind Placebo-Controlled Trial of Acetyl-L-Carnitine for the Prevention of Taxane-Induced Neuropathy in Women Undergoing Adjuvant Breast Cancer Therapy. J Clin Oncol. 2013;31:2627–2633. doi: 10.1200/JCO.2012.44.8738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zimmerman C, Atherton PJ, Pachman D, Seisler D, Wagner-Johnston N, Dakhil S, Lafky JM, Qin R, Grothey A, Loprinzi C. MC11C4: a pilot randomized, placebo-controlled, double-blind study of venlafaxine to prevent oxaliplatin-induced neuropathy. Support Care Cancer. 2015:1–8. doi: 10.1007/s00520-015-2876-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mols F, Beijers T, Vreugdenhil G, van de Poll-Franse L. Chemotherapy-induced peripheral neuropathy and its association with quality of life: a systematic review. Support Care Cancer. 1014;22:2261–2269. doi: 10.1007/s00520-014-2255-7. [DOI] [PubMed] [Google Scholar]
- 5.Hershman DL, Lacchetti C, Dworkin RH, Smith EML, Bleeker J, Cavaletti G, Chauhan C, Gavin P, Lavino A, Lustberg MB, Paice J, Schneider B, Smith ML, Smith T, Terstriep S, Wagner-Johnston N, Bak K, Loprinzi C. Prevention and Management of Chemotherapy-Induced Peripheral Neuropathy in Survivors of Adult Cancers: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol, JCO. 2014;2013 doi: 10.1200/JCO.2013.54.0914. [DOI] [PubMed] [Google Scholar]
- 6.Postma TJ, Heimans JJ, Muller MJ, Ossenkoppele GJ, Vermorken JB, Aaronson NK. Pitfalls in grading severity of chemotherapy-induced peripheral neuropathy. Ann Oncol. 1998;9:739–744. doi: 10.1023/a:1008344507482. [DOI] [PubMed] [Google Scholar]
- 7.Pachman DR, Barton DL, Watson JC, Loprinzi CL. Chemotherapy-Induced Peripheral Neuropathy: Prevention and Treatment. Clinical Pharmacology & Therapeutics. 2011;90:377–387. doi: 10.1038/clpt.2011.115. [DOI] [PubMed] [Google Scholar]
- 8.Cavaletti G, Cornblath DR, Merkies ISJ, Postma TJ, Rossi E, Frigeni B, Alberti P, et al. The chemotherapy-induced peripheral neuropathy outcome measures standardization study: from consensus to the first validity and reliability findings. Annals of Oncology. 2012;0:1–9. doi: 10.1093/annonc/mds329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilchrist L. Chemotherapy-Induced Peripheral Neuropathy in Pediatric Cancer Patients. Semin Pediatr Neurol. 2012;19:9–17. doi: 10.1016/j.spen.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 10.Visovsky C. Challenges in the Conduct of Research: Chemotherapy-Induced Peripheral Neuropathy. J Adv Pract Oncol. 2013;4:369–371. [PMC free article] [PubMed] [Google Scholar]
- 11.Liu H, Tan AD, Qin R, Sargent DJ, Grothey A, Buckner JC, Schaefer PL, Sloan JA. Comparing and Validating Simple Measures of Patient-Reported Peripheral Neuropathy for Oncology Clinical Trials: NCCTG N0897 (Alliance) A Pooled Analysis of 2440 Patients. SOJ Anesthesiol Pain Manag. 2015;2:1–9. doi: 10.15226/2374-684X/2/2/00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stephens RJ, Hopwood P, Girling DJ, Machin D. Randomized trials with quality of life endpoints: are doctors' ratings of patients' physical symptoms interchangeable with patients' self-ratings? Qual Life Res. 1997;6:225–236. doi: 10.1023/a:1026458604826. [DOI] [PubMed] [Google Scholar]
- 13.Shimozuma K, Ohashi Y, Takeuchi A, Arnishi T, Morita S, Kuroi K, et al. Validation of the Patient Neurotoxicity Questionnaire (PNQ) during taxane chemotherapy in a phase III randomized trial of breast cancer: N-SAC BC 02. Support Care Cancer. 2009;17:1483–1491. doi: 10.1007/s00520-009-0613-7. [DOI] [PubMed] [Google Scholar]
- 14.Pace A, Nisticó C, Cuppone F, Bria E, Galié E, Graziano G, Natoli G, Sperduti I, Jandolo B, Calabretta F, Tomao S, Terzoli E. Peripheral Neurotoxicity of Weekly Paclitaxel Chemotherapy: A Schedule or a Dose Issue? Clinical Breast Cancer. 2007;7:550–554. doi: 10.3816/CBC.2007.n.010. [DOI] [PubMed] [Google Scholar]
- 15.Wenzel LB, Huang HQ, Armstrong DK, Walker JL, Cella D. Health-Related Quality of Life During and After Intraperitoneal Versus Intravenous Chemotherapy for Optimally Debulked Ovarian Cancer: A Gynecologic Oncology Group Study. J Clin Oncol. 2007;25:437–443. doi: 10.1200/JCO.2006.07.3494. [DOI] [PubMed] [Google Scholar]
- 16.Loprinzi CL, Reeves BN, Dakhil SR, Sloan JA, Wolf SL, Burger KN, Kamal A, LeLindqwister NA, Doori GS, Jaslowski AJ, Novotny PJ, Lachance DH. Natural History of Paclitaxel-Associated Acute Pain Syndrome: Prospective Cohort Study NCCTG N08C1. J Clin Oncol. 2011;29:1472–78. doi: 10.1200/JCO.2010.33.0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shinde SS, Seisler D, Soori G, Atherton PJ, Pachman DR, Lafky J, Ruddy KJ, Loprinzi CL. Can pregabalin prevent paclitaxel-associated neuropathy? – An ACCRU pilot trial. Support Care Cancer. 2015:1–7. doi: 10.1007/s00520-015-2807-5. [DOI] [PubMed] [Google Scholar]
- 18.Barton DL, Wos EJ, Qin R, Mattar BI, Green NB, Lanier S, et al. A double-blind, placebo-controlled trial of a topical treatment for chemotherapy-induced peripheral neuropathy: NCCTG trial N06CA. Support Care Cancer. 2011;19:833–841. doi: 10.1007/s00520-010-0911-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loprinzi CL, Qin R, Dakhil SR, Fehrenbacher L, Flynn DA, Atherton P, Seisler D, Qamar R, Lewis GC, Grothey A. Phase III randomized, placebo-controlled, double-blind study of intravenous calcium and magnesium to prevent oxaliplatin-induced sensory neurotoxicity (N08CB/Alliance) J Clin Oncol. 2014;32:997–1005. doi: 10.1200/JCO.2013.52.0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leal AD, Qin R, Atherton PJ, Haluska P, Behrens RJ, Tiber CH, Watanaboonyakhet P, Weiss M, Adams PT, Dockter TJ, Loprinzi CL for the Alliance for Clinical Trials in Oncology. North Central Cancer Treatment Group/Alliance trial N08CA—the use of glutathione for prevention of paclitaxel/carboplatin-induced peripheral neuropathy: A phase 3 randomized, double-blind, placebo-controlled study. Cancer. 2014;120:1890–1897. doi: 10.1002/cncr.28654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith EML, Barton DL, Qin R, Steen PD, Aaronson NK, Loprinzi CL. Assessing patient-reported peripheral neuropathy: the reliability and validity of the European Organization for Research and Treatment of Cancer QLQ-CIPN20 Questionnaire. Qual Life Res. 2013;22:2787–2799. doi: 10.1007/s11136-013-0379-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alberti P, Rossi E, Cornblath DR, Merkies ISJ, Postma TJ, Frigeni B, Bruna J, Velasco R, Argyriou AA, Kalofonos HP, Psimaras D, Ricard D, Pace A, Galiè E, Briani C, Dalla Torre C, Faber CG, Lalisang RI, Boogerd W, Brandsma D, Koeppen S, Hense J, Storey D, Kerrigan S, Schenone A, Fabbri S, Valsecchi MG, Cavaletti G the CI-PeriNomS Group. Physician-assessed and patient-reported outcome measures in chemotherapy-induced sensory peripheral neurotoxicity: two sides of the same coin. Ann Oncol. 2014;25:257–264. doi: 10.1093/annonc/mdt409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Postma TJ, Aaronson NK, Heimans JJ, Muller MJ, Hildebrand JG, Delattre JY, Hoang-Xuan K, Lantéri-Minet M, Grant R, Huddart R, Moynihan C. The development of an EORTC quality of life questionnaire to assess chemotherapy-induced peripheral neuropathy: the QLQ-CIPN20. European Journal of Cancer. 2005;41(8):1135–1139. doi: 10.1016/j.ejca.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 24.Dueck AC, Mendoza TR, Mitchell SA, et al. Validity and Reliability of the US National Cancer Institute's Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE) JAMA Oncol. 2015;1(8):1051–1059. doi: 10.1001/jamaoncol.2015.2639.. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Mean and standard error of mean (SEM) of CIPN20 % scores for N08CA and N08CB grouped by CTCAE grades.
Supplementary Table 2. Difference and the corresponding 95% confidence interval between the mean CIPN20 % scores for different grades of CTCAE obtained by fitting the linear mixed model and adjusting for cycle for N08CB and N08CA.