Abstract
Rationale
Increasing clinical evidence suggests that menthol, a significant flavoring additive in tobacco products, may contribute to smoking and nicotine dependence. Relapse to smoking behavior presents a formidable challenge for the treatment of tobacco addiction. An unresolved issue is whether the mentholation of tobacco products precipitates relapse to tobacco use in abstinent smokers.
Objectives
The present study examined the effects of menthol on the perseverance and relapse of nicotine-seeking behavior in rats.
Methods
Male Sprague-Dawley rats were trained to press a lever for intravenous nicotine self-administration (0.03 mg/kg/infusion) under a fixed-ratio 5 schedule of reinforcement. Each nicotine infusion was signaled by the presentation of a sensory stimulus that was established as a discrete nicotine-conditioned cue. Five minutes prior to the sessions, the rats received an intraperitoneal injection of menthol (0.1 mg/kg) or vehicle. In the subsequent extinction test sessions, nicotine was unavailable with or without menthol and/or the nicotine-conditioned cue. The reinstatement tests were performed the following day after the extinction criterion was met. Menthol was also tested on food-seeking responses. In a subset of nicotine-trained rats, a TRPM8 antagonist RQ-00203078 was given prior to menthol administration.
Results
Continued administration of menthol sustained responses on the previously active and nicotine-reinforced lever in the extinction tests. The re-administration of menthol after extinction reinstated active lever responses. In both the extinction and reinstatement tests, a combination of pre-session menthol administration and cue re-presentation during the session produced a more robust behavioral effect than either menthol or the cue alone. No such effects of menthol was observed in food trained rats. RQ-00203078 did not change menthol effect on nicotine seeking.
Conclusion
These data demonstrated that menthol specifically sustained and reinstated nicotine-seeking behavior and this effect was independent of TRPM8 activity. These findings suggest that menthol in most tobacco products, even not menthol-labeled, may contribute to the perseverance of and relapse to tobacco-seeking behavior.
Keywords: Conditioned stimulus, cue, discriminative stimulus, extinction, food seeking, menthol, nicotine seeking, reinstatement, self-administration, TRPM8
Introduction
Worldwide, tobacco-related diseases have become a major problem, with substantial health and economic consequences (WHO, 2015). In the United States (CDC, 2015), tobacco smoking is a leading preventable cause of premature death, accounting for the loss of 480,000 lives each year. Currently, there are approximately 40 million adult smokers, representing 16.8% of the American adult population. Economic costs that are attributable to tobacco smoking-related diseases are more than $300 billion each year. Although the majority of smokers want to quit smoking and have made attempts to do so, the vast majority (up to 97%) of smokers who try to quit eventually relapse and continue to smoke (Benowitz, 2010; CDC, 2014; Hughes et al., 2008; Shiffman et al., 1998). Even when treated with medications that are currently approved by the U.S. Food and Drug Administration (i.e., nicotine replacement, bupropion, and varenicline), long-term abstinence rates have remained unsatisfactorily low (Aubin et al., 2008; Gonzales et al., 2006; Jorenby et al., 2006; Rose and Behm, 2014; Vogeler et al., 2016). Thus, high rates of relapse present a formidable challenge for successful smoking cessation.
Approximately one-third of smokers in the United States use menthol cigarettes (Caraballo and Asman, 2011; Curtin et al., 2014; Giovino et al., 2004; Giovino, 2010; Pearson et al., 2012; SAMHSA, 2009). Notwithstanding the overall significant progress in reducing tobacco smoking over the last several decades, the use of mentholated cigarettes has become a growing problem, especially among the younger population (Giovino et al., 2015; SAMHSA, 2009; USDHHS, 2014). Increasing evidence has shown a significant impact of menthol on perpetuation of the tobacco epidemic, with increases in both smoking experimentation and regular smoking and a decrease in smoking cessation success (Anderson, 2011a; Anderson, 2011b; Benowitz and Samet, 2011; Delnevo et al., 2011; Delnevo et al., 2015; Fagan et al., 2010; Fagan et al., 2015; Giovino et al., 2015; Tobacco, 2011; TPSAC, 2011). For example, menthol smokers began smoking their first cigarette sooner after waking, inhaled more deeply, and presented heightened nicotine addiction (Ahijevych and Parsley, 1999; Fagan et al., 2010; Hoffman and Simmons, 2011; Hymowitz et al., 1995; Muscat et al., 2009; Okuyemi et al., 2003; Richter et al., 2008). Furthermore, smokers who use menthol tobacco, compared with non-mentholated cigarette smokers, have lower success with smoking cessation and higher rates of relapse (Ahijevych and Garrett, 2010; Besaratinia and Tommasi, 2015; Foulds et al., 2010; Gardiner and Clark, 2010; Levy et al., 2011; Pletcher et al., 2006; Reitzel et al., 2011; Reitzel et al., 2013; Rojewski et al., 2014). Some conflicting observations can be found in the literature (Gardiner and Clark, 2010; Hoffman, 2011), particularly from reports that originated from tobacco companies (e.g., Wang et al., 2010; Werley et al., 2007) that suggest similar cessation outcomes among menthol and nonmenthol smokers. Unfortunately, the issue of whether menthol increases the difficulty of quitting has received little experimental attention.
One hypothesis is that, in addition to directly enhancing the reinforcing actions of nicotine as demonstrated in our previous studies (Biswas et al., 2016), menthol in tobacco products even at a very low amount may readily acquire the properties of interoceptive cues for smoking and nicotine intake. Menthol may become an occasion-setter that is predictive of the presence of nicotine reinforcement. To test this hypothesis, the present study used rat models of nicotine administration and relapse to examine the effects of menthol on the perseverance of operant lever-press responding for nicotine self-administration and the reinstatement of nicotine-seeking behavior after extinction, with an emphasis on interactions between menthol as an occasion setter and discrete nicotine-conditioned cues. This extinction-reinstatement procedure has been widely used to study a variety of drugs of abuse with good face and predictive validities, showing its translational value for our understanding of drug-seeking behavior (Bossert et al., 2013; Epstein et al., 2006; Shaham et al, 2003 for reviews).
Although not menthol-labeled, almost all commercial tobacco products contain certain amount of menthol. In fact, the nonmenthol-labeled cigarettes contains menthol at approximately 1.8 to 73.5 μg/cigarette, which is about 100- to 1000-fold lower than menthol-labeled products (Ai et al., 2016; Farco and Grundmann, 2013; FTC, 2009; Gordon et al., 2011; Hopp, 1993; Wayne and Connolly, 2004). Even though unable to directly change nicotine intake, such low dose of menthol in tobacco products may acquire occasion-setting properties, serving as a discriminative cue for smoking behavior and thus being predictive of nicotine consumption. Therefore, based on our preliminary data showing that menthol at 0.1 – 1 mg/kg but not 0.01 mg/kg produced a discriminative cueing effect on nicotine-seeking behavior, the present study used 0.1 mg/kg menthol to characterize the occasion-setting properties of menthol. This low dose of menthol is to some extent comparable to the menthol intake in heave smokers. As such, the results reported in this study may have relatively more clinical relevance. This study would extend a role of menthol in directly enhancing nicotine reinforcement to an interoceptive cueing role of menthol in maintaining and reinstating nicotine seeking. The results may shed a new light on our fully understanding the contribution of menthol to tobacco addiction. In specific, this study examined the effects of pre-session menthol administration on the perseverance and reinstatement of nicotine-seeking behavior and an interaction of menthol with a discrete nicotine-conditioned cue in these measurements. The effects of menthol on food-seeking behavior were also tested. In addition, to determine whether the classic menthol receptors, i.e., transient receptor potential melastatin 8 (TRPM8) ion channels (Peier et al., 202), are required for the observed effects of menthol, a selective TRPM8 antagonist RQ-00203078 was administered prior to menthol application.
Materials and methods
Subjects
Eighty two male Sprague-Dawley rats (Charles River, Portage, MI, USA), weighing 176–200 g upon arrival, were used. The animals were individually housed in a humidity- and temperature-controlled (21–22°C) colony room on a reverse light/dark cycle (lights on at 8:00 PM, lights off at 8:00 AM). The rats were allowed the first week to acclimate the colony room with free access to laboratory chow. Starting in the second week, the animals were placed on a food-restriction regimen in which a ration of 20 g chow/day was provided to each rats. This feeding regimen allowed the rats to have consistent but low weight gain at approximately 85% of their free-feeding condition. The rats had unlimited access to water throughout the experiments. All of the experimental sessions were conducted during the dark phase at the same time each day (9:00 AM–3:00 PM). The experimental protocol was performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the University of Mississippi Medical Center Institutional Animal Care and Use Committee.
Self-administration apparatus
Experimental sessions were performed in sixteen standard operant conditioning chambers. These chambers were placed inside sound-attenuating, ventilated cubicles (Med Associates, St. Albans, VT, USA). Each chamber was equipped with two retractable response levers on one side panel and a 28-V white light above each lever. A red house light was located on the top center of the opposite panel of the chambers. Intravenous nicotine injections were dispensed by a drug delivery system with a syringe pump (model PHM100-10 rpm, Med Associates). Experimental events and data collection were automatically controlled by a computer and software (Med-PC version IV, Med Associates).
Lever-press training
Starting on the second day of the food-restriction regimen, the food training sessions began. The sessions started with the introduction of one lever and rat responses on the lever was rewarded with the delivery of one food pellet (45 mg). When the rats earned a maximum of 45 food pellets on a fixed-ratio 1 (FR1) schedule, the reinforcement schedule was increased to FR5. The training session ended after the rats earned 45 food pellets on the FR5 schedule. Such training effectively facilitates the learning of operant responding for nicotine self-administration (see below).
Intravenous catheterization surgery
The catheters were constructed of a 15 cm piece of silastic tubing (0.31 mm inner diameter, 0.63 mm outer diameter; Dow Corning, Midland, MI, USA) attached to a 22-gauge stainless-steel guide cannula. The cannula was bent and molded onto a tissue-compatible monofilament polypropylene mesh (Davol, Warwick, RI, USA) with dental cement that became the catheter base. Under isoflurane anesthesia (1–3% in 95% O2 and 5% CO2), the catheter base was anchored beneath the skin at the level of the scapulae. The catheter tubing passed subcutaneously to the ventral lower neck region and was inserted into the right jugular vein (2.5 cm). The rats were allowed at least 7 days to recover from surgery. During the recovery period, the catheters were flushed daily with 0.1 ml of heparinized (30 U/ml) saline containing gentamicin (20 mg/ml) to maintain catheter patency and prevent infection. Thereafter, the catheters were flushed with heparinized saline before and after the experimental sessions. On weekends without experimental session, catheter was flushed once a day.
Nicotine self-administration and conditioning training
The rats received daily 1-h training sessions for intravenous self-administration of nicotine (0.03 mg/kg/infusion, free base). (−)-Nicotine hydrogen tartrate (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in physiological saline. The pH was adjusted to 7.0 ± 0.4 with 1N sodium hydroxide, and the solution was sterilized by filtration through a 0.22 μm syringe filter (Fisher Scientific, Pittsburgh, PA, USA). The rats were placed in the operant conditioning chambers and connected to the intravenous drug infusion system. The sessions began with extension of the two levers and illumination of the red house light. When the rats reached the required number of FR responses on the active lever, an infusion of nicotine was delivered in a volume of 0.1 ml over approximately 1 s, depending on the rat’s body weight. Each nicotine infusion was signaled by the presentation of an auditory/visual stimulus that consisted of a 5-s tone and 20-s illumination of the light above the active lever. This stimulus was discretely conditioned to nicotine self-administration and became a nicotine cue. A 20-s timeout period followed each nicotine infusion, during which time responses were recorded but not reinforced. An FR1 schedule was used for days 1–5, an FR2 for days 6–8 and an FR5 for the remaining days of the experiments. Responses on the inactive lever were recorded but had no programmed consequences. All rats received 25 daily sessions since our previous work has demonstrated successful establishment of stable nicotine self-administration under such a training schedule (Liu et al., 2008; Liu, 2014). Rats met the criterion of ≥ 10 infusions per session with ≤ 20% variation for at least three consecutive sessions.
Five minutes prior to each session, the rats received an intraperitoneal injection of menthol (0.1 mg/kg) or its vehicle (1 ml/kg). (−)-Menthol (cyclohexanol-5-methyl-2-[1-methylethyl]) was purchased from Sigma-Aldrich (St. Louis, MO, USA). It was first dissolved in dimethylsulfoxide (DMSO; St. Louis, MO, USA) and then diluted with deionized water to a final DMSO concentration of 50% (v/v).
Extinction
After completion of the self-administration and conditioning training sessions, the rats were subjected to daily 1-h extinction sessions where nicotine-reinforced lever responding was extinguished by withholding nicotine and its cue. Responses on the active lever resulted in the delivery of saline rather than nicotine, and the cue was not presented. The FR5 schedule and 20 s timeout period were still in effect for saline infusions. The pre-session administration of menthol (or its vehicle) and/or in-session response-contingent presentations of the nicotine cue were scheduled based on the different experimental conditions that are described in detail in Experiment 1 below.
For the reinstatement tests (described below), the extinction sessions were conducted without pre-session menthol administration, nicotine delivery, or nicotine cue presentation. The criterion for extinction was three consecutive sessions, in which the number of responses per session was ≤ 20% of the responses averaged across the last three sessions of the self-administration training phase. All of the rats underwent 10 extinction test sessions because our previous studies showed that nicotine-maintained responding was typically extinguished within 7–10 sessions (Liu et al., 2008; Liu, 2010; Liu, 2014).
Reinstatement
Starting the following day after the rats completed the final extinction session, the reinstatement tests began. In these test sessions, responses on the active lever resulted in a saline infusion on an FR5 schedule. Pre-session administration of menthol or its vehicle and/or in-session presentation of the nicotine cue were scheduled based on the different experimental conditions described below. The test sessions lasted 1 h.
Experiment 1: Effects of menthol and its combination with nicotine cue on the extinction of nicotine-seeking behavior
After completing the nicotine self-administration training phase, the rats were divided into four groups (n = 10/group) for the extinction tests. As described above, nicotine was unavailable to any of the groups in the test sessions. However, one group received neither pre-session menthol (vehicle instead) administration nor in-session cue presentation (vehicle/-); The second group had pre-session menthol administration but no in-session cue presentation (menthol/- or menthol alone); The third group did not get pre-session menthol (vehicle instead) administration while received in-session cue presentation (vehicle/cue or cue alone). The last group received both pre-session menthol administration and in-session cue presentation (menthol/cue).
Experiment 2: Effects of menthol and its combination with nicotine cue on the reinstatement of nicotine-seeking behavior
Twelve rats were used for the reinstatement tests. As described above, these rats received 25 daily nicotine self-administration training sessions with pre-session administration of menthol and then were subjected to 10 daily extinction (saline substitution of nicotine) sessions without pre-session menthol administration or in-session cue presentation. Then, the reinstatement test sessions began. Three test sessions were conducted in the following order: (1) pre-session menthol administration but no in-session cue presentation (menthol alone), (2) no pre-session menthol administration (vehicle instead) but with in-session response-contingent cue presentations (cue alone), and (3) both pre-session menthol administration and in-session cue presentations (menthol+cue). Two extinction sessions were inserted between the reinstatement tests to maintain the extinction baseline before each test session.
Experiment 3: Effects of TRPM8 antagonist on menthol-reinstated nicotine-seeking behavior
Ten rats were used for this test. The rats underwent nicotine self-administration and extinction training exactly like rats described in Experiment 2 above. After completion of the 10 daily extinction sessions, three reinstatement test sessions were performed after an intraperitoneal administration of menthol (0.1 mg/kg) given 5 min prior to the sessions. Two hours before menthol administration, rats were subjected to an intraperitoneal administration of a selective TRPM8 antagonist RQ-00203078 (purchased from Tocris Biosicence, United Kingdom) at 0, 0.3, and 3 mg/kg (dissolved in DMSO and then diluted with deionized water) in a within-subject Latin square design. These dose were selected based on the fact that in rats the ED50 value of RQ-00203078 via oral administration is 0.65 mg/kg and that RQ-00203078 at 3 mg/kg dose via intraperitoneal administration produced a profound TRPM8-blocking effect (Gong and Jasmin, 2017; Ohmi et a., 2014). To guarantee the extinction baseline before each test sessions, two extinction sessions were conducted between test sessions.
Experiment 4: Effects of menthol and its combination with nicotine cue on the reinstatement of nicotine-seeking behavior in menthol-naive rats
A separate group of rats (n = 12) was used for this test. The rats underwent the experimental procedures described in Experiment 2 above, but they did not receive pre-session menthol administration in either the nicotine self-administration or extinction phases. To serve as a control condition for pre-session menthol administration, these animals were subjected to pre-session vehicle administration. After completing the 10 daily extinction sessions, the reinstatement test sessions began, exactly as described for Experiment 2. There were three reinstatement test conditions: (1) menthol alone, (2) cue alone, and (3) menthol+cue. Two extinction sessions were performed before each reinstatement test to maintain the extinction baseline.
Experiment 5: Effects of menthol and its combination with food cue on the reinstatement of food-seeking behavior
Ten rats received 25 daily 1-h food self-administration sessions on an FR5 schedule. As for nicotine tests described above, menthol was intraperitoneally administered 5 min prior to each self-administration sessions. Then, ten daily extinction sessions were performed where neither pre-session menthol administration nor in-session cue presentation was provided. The subsequent three reinstatement test sessions were performed with two extinction sessions in between. The reinstatement conditions were (1) pre-session menthol administration but no in-session cue presentation (menthol alone), (2) no pre-session menthol (vehicle instead) administration but with in-session response-contingent cue presentations (cue alone), and (3) both pre-session menthol administration and in-session cue presentations (menthol+cue).
Statistical analyses
The number of lever responses is expressed as mean ± SEM. The lever response data from the extinction tests were analyzed using repeated-measures analysis of variance (ANOVA), with group (test conditions) as the between-subjects factor and session as the within-subject factor. Similar repeated-measures ANOVAs were used for the inactive lever response data. In the reinstatement tests, extinction baseline data were averaged across the two extinction sessions before each reinstatement test session. The reinstatement test data were analyzed using a one-way repeated-measures ANOVA with test condition as the factor. After overall significance in the ANOVAs, Fisher’s Protected Least Significant Difference (PLSD) post hoc tests were used to verify differences among individual means.
Results
Nicotine self-administration
In the 25 daily 1-h sessions, the rats with pretreatment of menthol administration prior to each session developed steady lever-press responding for intravenous infusions of nicotine. The mean ± SEM number of responses pooled across the final three sessions was 86.4 ± 9.2 on the active and 12.0 ± 4.4 the inactive lever. Correspondingly, these animals self-administered 14.1 ± 2.6 infusions of nicotine at a unit dose of 0.03 mg/kg/infusion. The 12 rats with pre-session vehicle administration (see experiment 4) developed nicotine self-administration at the same rate with responses of 81.1 ± 9.3 on the active lever and 14.5 ± 5.1 on the inactive lever and self-administration of 14.7 ± 3.9 nicotine infusions.
Effects of menthol and its combination with nicotine cue on the extinction of nicotine-seeking behavior
After completing nicotine self-administration and conditioning training with pre-session menthol administration, the extinction tests began. These rats were divided into four groups in a pseudorandom manner, with no difference among groups in the number of responses on the active lever (F3,36 = 0.037, p = 0.99) or inactive lever (F3,36 = 0.041, p = 0.99) or the number of nicotine infusions earned (F3,36 = 0.036, p = 0.99; Table 1). In the subsequent extinction test, the omission of nicotine delivery (saline substitution) resulted in a gradual decrease in responding on the active lever in all rats. However, these animals presented distinct response profiles (Fig. 1, top). An overall two-way repeated-measures ANOVA revealed significant main effects of group (F3,36 = 11.42, p < 0.0001) and session (F9,324 = 40.99, p < 0.0001) but no group × session interaction (F27,324 = 1.05, p = 0.4037). A following Fisher’s PLSD post hoc test showed a significant (p < 0.01) difference of menthol alone, cue alone or menthol/cue groups from the rats under vehicle/- condition. A one-way ANOVA of active lever responses averaged across the final three sessions confirmed a significant effect of group (F3,36 = 5.62, p < 0.01) and post hoc test showed significantly higher levels of responding in the menthol alone (p < 0.05), cue alone (p < 0.01), and menthol/cue (p < 0.001) groups compared with rats under vehicle/- condition (Fig. 1, below). Although the menthol/cue group emitted more responses relative to the menthol alone and cue alone groups, the difference failed to reach statistical significance. Throughout the extinction sessions, the number of responses on the inactive lever remained low and was not significantly different among groups (F3,36 = 1.82, p > 0.05) or across sessions (F9,324 = 0.85, p > 0.05). These data indicate that continued pre-session menthol administration, the response-contingent presentation of nicotine cues, and their combination significantly sustained responding on the active, previously nicotine-reinforced lever, thus delaying the extinction of nicotine-seeking behavior.
Table 1.
Similar profiles of nicotine self-administration behavior among the four groups of rats prior to the extinction test (shown in figure 1). The animals were trained to self-administer nicotine with pre-session menthol administration. The data are expressed as the mean ± SEM across the final three self-administration sessions.
| Extinction Group | Number of responses | Nicotine infusions | Body weight (g) | |
|---|---|---|---|---|
|
| ||||
| Active lever | Inactive lever | |||
| Vehicle/- | 87.6 ± 13.4 | 11.7 ± 3.9 | 14.1 ± 2.0 | 324 ± 31 |
| Menthol/- | 89.1 ± 13.8 | 12.5 ± 4.5 | 15.1 ± 3.5 | 318 ± 26 |
| Vehicle/Cue | 84.2 ± 14.3 | 10.8 ± 3.2 | 13.3 ± 1.7 | 335 ± 36 |
| Menthol/Cue | 88.1 ± 12.3 | 13.3 ± 5.2 | 14.2 ± 2.9 | 329 ± 33 |
Figure 1.
Top: Extinction profiles of responses on the active lever (nicotine-seeking behavior) in different test conditions in rats (n = 10/group). The animals were trained to self-administer nicotine with pre-session menthol administration. SA indicates lever responses averaged across the final three self-administration sessions. Vehicle/- represents the pre-session vehicle without in-session cue presentation condition. Menthol/- represents the pre-session menthol without in-session cue presentation condition. Vehicle/Cue represents the pre-session vehicle with in-session cue presentation condition. Menthol/Cue represents the pre-session menthol with in-session cue presentation condition. For the sake of clarity, the means are presented without the SEM. Below: The mean ± SEM number of active lever responses pooled across the final three extinction sessions. *p < 0.05, **p < 0.01, significant difference from Vehicle/- group.
Effects of menthol and its combination with nicotine cue on the reinstatement of nicotine-seeking behavior
As shown in Fig. 2, in the reinstatement test sessions (n = 12), pre-session re-administration of menthol, response-contingent presentations of the nicotine cue, and the combination of menthol and the cue effectively reinstated the extinguished responding on the active, previously nicotine-reinforced lever. A one-way repeated-measures ANOVA of active lever responses revealed a significant effect of test condition (F3,33 = 14.01, p < 0.0001). Fisher’s PLSD post hoc test confirmed significantly more responses in the menthol alone (p < 0.05), cue alone (p < 0.05), and menthol+cue (p < 0.0001) conditions compared with extinction. The number of active lever responses in the menthol+cue condition was significantly higher than in the menthol alone (p < 0.01) and cue-alone (p < 0.05) conditions. Responses on the inactive lever remained low and indistinguishable from the extinction baseline level (F3,33 = 0.13, p > 0.05, data not shown).
Figure 2.
Lever responses in the reinstatement tests in rats (n = 12) that were trained to self-administer nicotine with pre-session menthol administration. Menthol represents the pre-session menthol without in-session cue presentation condition. Cue represents the pre-session vehicle with in-session cue presentation condition. Menthol+Cue represents the pre-session menthol with in-session cue presentation condition. The data are expressed as the mean ± SEM number of lever responses. *p < 0.05, ****p < 0.0001, significant difference from extinction baseline; ++p < 0.01, significant difference from menthol; ^p < 0.05, significant difference from cue.
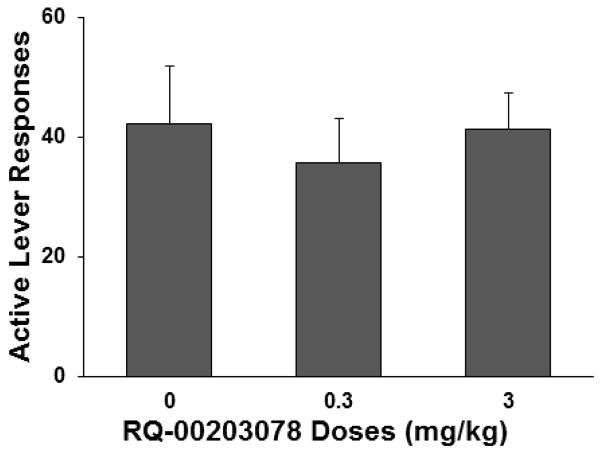
Effects of TRPM8 antagonist on menthol-induced reinstatement of nicotine-seeking behavior
As shown in figure 3, treatment with the TRPM8 antagonist RQ-00203078 prior to menthol (0.1 mg/kg) administration did not alter nicotine-seeking responses that were reinstated by menthol. A one-way repeated-measures ANOVA of active lever responses produced no significant effect of RQ-00203078 treatment (F2,18 = 0.19, p =0.828).
Figure 3.
Effect of TRPM8 antagonist RQ-00203078 on menthol-induced reinstatement of nicotine-seeking responses in the rats (n = 10) trained to self-administer nicotine with pre-session menthol administration. The data are expressed as the mean ± SEM number of lever responses.
Effects of menthol in the reinstatement tests in menthol-naive rats
A separate group of rats (n = 12) underwent the nicotine self-administration/conditioning and extinction phases as described above but without pre-session menthol administration. In the reinstatement tests (Fig. 3), these rats presented cue-induced reinstatement of extinguished active lever responses. However, pre-session menthol administration in these menthol-naive rats did not produce an effect, regardless of cue presentations. The one-way repeated-measures ANOVA of active lever responses revealed a significant effect of test condition (F3,33 = 13.10, p < 0.0001). Fisher’s PLSD post hoc test confirmed a significant difference between the cue alone (p < 0.01) and menthol+cue (p < 0.01) conditions and the extinction and menthol alone conditions. Responses on the inactive lever remained at low levels that were indistinguishable among the different test conditions (F3,33 = 1.13, p > 0.05, data not shown).
Effects of menthol on food-seeking behavior
As shown in figure 5, response-contingent re-presentation of food cue effectively reinstated the extinguished responses on the active lever. However, menthol did not produce an effect either by itself or together with food cue relative to cue alone. After a one-way repeated-measures ANOVA of active lever responses yielding a significant effect of test condition (F3,27 = 6.46, p < 0.01), subsequent post hoc tests found a significant effect of food cue (p < 0.01) but not menthol (p > 0.05).
Figure 5.
Lever responses in the reinstatement tests in the rats (n = 10) that were trained to self-administer food pellets with pre-session administration of menthol. The data are expressed as the mean ± SEM number of lever responses. **p < 0.01, significant difference from extinction or menthol condition.
Discussion
The present study demonstrated an occasion-setting role of menthol in the perseverance and reinstatement of nicotine-seeking behavior. In rats that were trained to self-administer nicotine with pre-session menthol administration, continued menthol administration sustained nicotine seeking under the extinction test condition. After nicotine-reinforced lever responding was extinguished by withholding nicotine delivery without pre-session menthol administration, the resumption of pre-session menthol administration reinstated nicotine-seeking behavior. In both cases, pre-session menthol administration interacted with a discrete nicotine-conditioned cue to produce a more robust effect. Interestingly, the response-reinstating effect of menthol was not changed by a selective TRPM8 antagonist. However, in rats that had self-administered nicotine but did not receive pre-session menthol administration (menthol-naive subjects), menthol administration neither reinstated nicotine-seeking behavior nor interacted with the nicotine cue. These results extended a role of menthol in directly enhancing nicotine reinforcement, as demonstrated in our previous work (Biswas et al., 2016), to an interoceptive cueing effect of menthol on nicotine-seeking behavior. In contrast, menthol did not produce a similar effect in the rats trained to self-administer food with pre-session menthol administration.
This is the first report showing that menthol at a very low dose incapable of directly enhancing nicotine reinforcement can acquire occasion-setting properties and thus set an occasion for nicotine self-administration. In the extinction tests, the continued presence of menthol sustained nicotine-seeking responses relative to the menthol omission counterparts. The finding that menthol acted as an occasion-setter (or a discriminative cue) for nicotine-seeking behavior is in line with the reports demonstrating that the interoceptive state produced by a drug (e.g., nicotine, alcohol, cocaine, and Δ9-tetrahydrocannabinol) functioned as a discriminative stimuli for operant responding for natural rewards, such as food and sex (Troisi, 2003; Troisi and Akins, 2004; Troisi et al., 2010). Using a similar procedure in rats, we observed a similar effect of caffeine in sustaining nicotine-seeking behavior as long as pre-session caffeine administration preceded every session during the self-administration phase (Liu and Jernigan, 2012). The results obtained with such a low dose of menthol may be applicable to most tobacco users but not only menthol-cigarette smokers. Such an interoceptive cueing effect of menthol may have clinical implications. For example, abstinent smokers, particularly those who use mentholated tobacco products, may experience stronger craving for tobacco when they ingest menthol-containing products, such as chewing mint gum or drinking minty drinks. Thus, refraining from exposure to all menthol-containing products during smoking cessation might protect individuals from strong tobacco craving.
Another finding of the present study was that in the reinstatement tests that were conducted after extinction by withholding menthol administration and nicotine delivery and its cue, re-administration of pre-session menthol effectively reinstated extinguished nicotine-seeking behavior. In contrast, the response-reinstating effect of menthol was not observed in rats with a history of self-administering food after pre-session menthol administration. And interestingly, the response-reinstating effect of menthol was observed only in rats that had been trained to self-administer nicotine with pre-session menthol but not in the rats that had received exactly the same nicotine self-administration training but without pre-session menthol. This finding can be explained in the frame of the above discussed occasion-setting effect of menthol and is consistent with previous studies, in which discriminative stimuli effectively reinstated extinguished drug-seeking behavior in animals that were trained to self-administer cocaine, heroin, alcohol, nicotine, and sucrose (Alvarez-Jaimes et al., 2008; Barker et al., 2014; Burbassi and Cervo, 2008; Ciccocioppo et al., 2001; Gracy et al., 2000; Kallupi et al., 2013; Katner et al., 1999; Widholm et al., 2011; Wing and Shoaib, 2008). The present results mirror our previous report that showed that caffeine influences nicotine seeking (Liu and Jernigan, 2012). That study, using a similar protocol in rats, demonstrated a significant role for caffeine in the persistence and reinstatement of nicotine-seeking behavior as long as the animals received caffeine administration prior to every nicotine self-administration training sessions, that is, the interoceptive state that was produced by caffeine acquired the properties of an occasion-setter or discriminative stimulus that was predictive of nicotine availability. These findings are in line with clinical studies showing tobacco craving after exposure to a smoking-related virtual reality environment (occasion-setter) but without the presence of explicit cigarette cues (Conklin et al., 2008).
Another finding of the present study was that in menthol-experienced rats, pre-session menthol administration interacted with the discrete nicotine-conditioned cue to produce a much stronger behavioral effect. The interactive effect of pre-session menthol administration as an occasion setter and discrete nicotine-conditioned cue is consistent with results that were obtained in rats that were trained to self-administer other drugs of abuse (e.g., cocaine and alcohol) or natural rewards (e.g., Cervo et al., 2013; Katner et al., 1999; Moro et al., 2016; Weiss et al., 2001). It could be postulated that pre-session menthol administration produced an interoceptive state (an occasion setter) that signaled the availability of nicotine reward and brought the rats into contact with the lever while the response-contingent presentation of the cue then served as conditioned reinforcement to support responding on the lever. Similar interactive effects of occasion-setting stimuli and discretely conditioned cues in eliciting cigarette craving can be observed in smokers. For example, Paris et al. (2011) reported that a virtual reality environment (i.e., occasion-setting stimuli) and discrete cigarette cues evoked the strongest smoking craving.
In this study, menthol was administered to rats via an intraperitoneal route in order to eliminate the topical and sensory effects of menthol. Together with the negative results obtained from the test using a selective TRPM8 antagonist RQ-00203078, it is confident to conclude that the occasion-setting effect of menthol for nicotine seeking does not involve the classic menthol receptors, the TRPM8 ion channels (Peier et al., 2002) located at the peripheral sensory nerve terminals. In light of increasing evidence showing that menthol can exert its actionn on neurotransmitter receptors such as the nicotinic acetylcholine receptors, r-aminobutyric acid receptors, and serotonin receptors in the central nervous system (Ashoor et al., 2013a; Ashoor et al., 2013b; Lau et al., 2014; Ton et al., 2015), it is suggested that menthol may act directly at central neurotransmitter receptors in the present experimental preparation. Future studies are warranted to elucidate the central-mediated mechanisms via which menthol sustains nicotine-seeking behavior.
In summary, the present results demonstrated that both pre-session menthol administration and in-session cue presentation contributed to the perseverance and recovery of nicotine-seeking behavior in a rat model of nicotine seeking. Interactive actions between menthol and the nicotine cue produced the most robust behaviorally motivating effect. The present study suggests that menthol administration may acquire, via its continual association with nicotine self-administration, the properties of a discriminative stimulus that is predictive of the availability of nicotine reinforcement. Menthol effectively sustained nicotine-seeking behavior in the extinction tests and reinstated extinguished nicotine seeking in the reinstatement tests. These effects of menthol are specific for nicotine seeking and independent of its peripheral and sensory actions. These findings suggest that menthol administration in smokers may set the occasion for nicotine intake and serve as a reminder of tobacco smoking, which would then facilitate nicotine seeking and trigger relapse. Together with other clinical and animal observations that showed that menthol may interact with nicotine to increase nicotine reinforcement (Ahijevych and Garrett, 2004; Ahijevych and Garrett, 2010; Alsharari et al., 2015; Biswas et al., 2016; Wang et al., 2014), these data indicate that menthol may significantly contribute to the initiation, progression, and relapse of tobacco smoking.
Figure 4.
Lever responses in the reinstatement tests in menthol-naive rats (n = 12). Menthol represents the pre-session menthol without in-session cue presentation condition. Cue represents the pre-session vehicle with in-session cue presentation condition. Menthol+Cue represents the pre-session menthol with in-session cue presentation condition. The data are expressed as the mean ± SEM number of lever responses. **p < 0.01, significant difference from extinction or menthol condition.
Acknowledgments
This work was supported by the National Institute on Drug Abuse and Food and Drug Administration Center for Tobacco Products (R01DA037277 to X. Liu). The funding source had no other role other than financial support. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or Food and Drug Administration. The authors would like to thank Thomas Rousselle, Emily Fu, and Haley Nabors for their excellent technical assistance and Dr. Robert Brodell for his strong departmental support.
References
- Agaku IT, King BA, Dube SR. Current cigarette smoking among adults: United States, 2005–2012. Morb Mortal Wkly Rep. 2014;63:29–34. [PMC free article] [PubMed] [Google Scholar]
- Ahijevych K, Parsley LA. Smoke constituent exposure and stage of change in black and white women cigarette smokers. Addict Behav. 1999;24:115–120. doi: 10.1016/s0306-4603(98)00031-8. [DOI] [PubMed] [Google Scholar]
- Ahijevych K, Garrett BE. Menthol pharmacology and its potential impact on cigarette smoking behavior. Nicotine Tob Res. 2004;6(Suppl 1):S17–S28. doi: 10.1080/14622200310001649469. [DOI] [PubMed] [Google Scholar]
- Ahijevych K, Garrett BE. The role of menthol in cigarettes as a reinforcer of smoking behavior. Nicotine Tob Res. 2010;12(Suppl 2):S110–S116. doi: 10.1093/ntr/ntq203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ai J, Taylor KM, Lisko JG, Tran H, Watson CH, Holman MR. Menthol content in US marketed cigarettes. Nicotine Tob Res. 2016;18:1575–1580. doi: 10.1093/ntr/ntv162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsharari SD, King JR, Nordman JC, Muldoon PP, Jackson A, Zhu AZ, Tyndale RF, Kabbani N, Damaj MI. Effects of menthol on nicotine pharmacokinetic, pharmacology and dependence in mice. PLoS One. 2015;10:e0137070. doi: 10.1371/journal.pone.0137070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Jaimes L, Polis I, Parsons LH. Attenuation of cue-induced heroin-seeking behavior by cannabinoid CB1 antagonist infusions into the nucleus accumbens core and prefrontal cortex, but not basolateral amygdala. Neuropsychopharmacology. 2008;33:2483–2493. doi: 10.1038/sj.npp.1301630. [DOI] [PubMed] [Google Scholar]
- Anderson SJ. Marketing of menthol cigarettes and consumer perceptions: a review of tobacco industry documents. Tob Control. 2011a;20(Suppl 2):ii20–ii28. doi: 10.1136/tc.2010.041939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson SJ. Menthol cigarettes and smoking cessation behaviour: a review of tobacco industry documents. Tob Control. 2011b;20(Suppl 2):ii49–ii56. doi: 10.1136/tc.2010.041947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashoor A, Nordman JC, Veltri D, Yang KH, Al Kury L, Shuba Y, Mahgoub M, Howarth FC, Sadek B, Shehu A, Kabbani N, Oz M. Menthol binding and inhibition of alpha7-nicotinic acetylcholine receptors. PLoS One. 2013a;8:e67674. doi: 10.1371/journal.pone.0067674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashoor A, Nordman JC, Veltri D, Yang KH, Shuba Y, Al Kury L, Sadek B, Howarth FC, Shehu A, Kabbani N, Oz M. Menthol inhibits 5-HT3 receptor-mediated currents. J Pharmacol Exp Ther. 2013b;347:398–409. doi: 10.1124/jpet.113.203976. [DOI] [PubMed] [Google Scholar]
- Aubin HJ, Bobak A, Britton JR, Oncken C, Billing CB, Jr, Gong J, Williams KE, Reeves KR. Varenicline versus transdermal nicotine patch for smoking cessation: results from a randomised, open-label trial. Thorax. 2008;63:717–724. doi: 10.1136/thx.2007.090647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ, Bercovicz D, Servilio LC, Simmons SJ, Ma S, Root DH, Pawlak AP, West MO. Rat ultrasonic vocalizations demonstrate that the motivation to contextually reinstate cocaine-seeking behavior does not necessarily involve a hedonic response. Addict Biol. 2014;19:781–790. doi: 10.1111/adb.12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL. Nicotine addiction. N Engl J Med. 2010;362:2295–2303. doi: 10.1056/NEJMra0809890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, Samet JM. The threat of menthol cigarettes to U.S. public health. N Engl J Med. 2011;364:2179–2181. doi: 10.1056/NEJMp1103610. [DOI] [PubMed] [Google Scholar]
- Besaratinia A, Tommasi S. The lingering question of menthol in cigarettes. Cancer Causes Control. 2015;26:165–169. doi: 10.1007/s10552-014-0499-7. [DOI] [PubMed] [Google Scholar]
- Best FW. Effects of some cigarette construction parameters on menthol migration and transfer. Rec Adv Tob Sci. 1993;19:155–201. [Google Scholar]
- Biswas L, Harrison E, Gong Y, Avusula R, Lee J, Zhang M, Rousselle T, Lage J, Liu X. Enhancing effect of menthol on nicotine self-administration in rats. Psychopharmacology. 2016;233:3417–3427. doi: 10.1007/s00213-016-4391-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Marchant NJ, Calu DJ, Shaham Y. The reinstatement model of drug relapse: recent neurobiological findings, emerging research topics, and translational research. Psychopharmacology. 2013;229:453–476. doi: 10.1007/s00213-013-3120-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbassi S, Cervo L. Stimulation of serotonin2C receptors influences cocaine-seeking behavior in response to drug-associated stimuli in rats. Psychopharmacology. 2008;196:15–27. doi: 10.1007/s00213-007-0916-7. [DOI] [PubMed] [Google Scholar]
- Caraballo RS, Asman K. Epidemiology of menthol cigarette use in the United States. Tob Induc Dis. 2011;9(Suppl 1):S1. doi: 10.1186/1617-9625-9-S1-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celebucki CC, Wayne GF, Connolly GN, Pankow JF, Chang EI. Characterization of measured menthol in 48 U.S. cigarette sub-brands. Nicotine Tob Res. 2005;7:523–531. doi: 10.1080/14622200500186270. [DOI] [PubMed] [Google Scholar]
- Cervo L, Di Clemente A, Orru A, Moro F, Cassina C, Pich EM, Corsi M, Gozzi A, Bifone A. Inhibition of glycine transporter-1 reduces cue-induced nicotine-seeking, but does not promote extinction of conditioned nicotine cue responding in the rat. Addict Biol. 2013;18:800–811. doi: 10.1111/adb.12049. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Sanna PP, Weiss F. Cocaine-predictive stimulus induces drug-seeking behavior and neural activation in limbic brain regions after multiple months of abstinence: reversal by D1 antagonists. Proc Natl Acad Sci U S A. 2001;98:1976–1981. doi: 10.1073/pnas.98.4.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin CA, Robin N, Perkins KA, Salkeld RP, McClernon FJ. Proximal versus distal cues to smoke: the effects of environments on smokers’ cue-reactivity. Exp Clin Psychopharmacol. 2008;16:207–214. doi: 10.1037/1064-1297.16.3.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin GM, Sulsky SI, Van Landingham C, Marano KM, Graves MJ, Ogden MW, Swauger JE. Patterns of menthol cigarette use among current smokers, overall and within demographic strata, based on data from four U.S. government surveys. Regul Toxicol Pharmacol. 2014;70:189–196. doi: 10.1016/j.yrtph.2014.06.018. [DOI] [PubMed] [Google Scholar]
- Delnevo CD, Gundersen DA, Hrywna M, Echeverria SE, Steinberg MB. Smoking-cessation prevalence among U.S. smokers of menthol versus non-menthol cigarettes. Am J Prev Med. 2011;41:357–365. doi: 10.1016/j.amepre.2011.06.039. [DOI] [PubMed] [Google Scholar]
- Delnevo CD, Villanti AC, Wackowski OA, Gundersen DA, Giovenco DP. The influence of menthol, e-cigarettes and other tobacco products on young adults’ self-reported changes in past year smoking. Tob Control. 2015;25:571–574. doi: 10.1136/tobaccocontrol-2015-052325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein DH1, Preston KL, Stewart J, Shaham Y. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology. 2006;189:1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan P, Moolchan ET, Hart A, Jr, Rose A, Lawrence D, Shavers VL, Gibson JT. Nicotine dependence and quitting behaviors among menthol and non-menthol smokers with similar consumptive patterns. Addiction. 2010;105(Suppl 1):55–74. doi: 10.1111/j.1360-0443.2010.03190.x. [DOI] [PubMed] [Google Scholar]
- Fagan P, Pohkrel P, Herzog T, Pagano I, Vallone D, Trinidad DR, Sakuma KL, Sterling K, Fryer CS, Moolchan E. Comparisons of three nicotine dependence scales in a multiethnic sample of young adult menthol and non-menthol smokers. Drug Alcohol Depend. 2015;149:203–211. doi: 10.1016/j.drugalcdep.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farco JA, Grundmann O. Menthol: pharmacology of an important naturally medicinal “cool”. Mini Rev Med Chem. 2013;13:124–131. [PubMed] [Google Scholar]
- Foulds J, Hooper MW, Pletcher MJ, Okuyemi KS. Do smokers of menthol cigarettes find it harder to quit smoking? Nicotine Tob Res. 2010;12(Suppl 2):S102–S109. doi: 10.1093/ntr/ntq166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federal Trade Commission. Cigarette report for 2006. Washington DC: Federal Trade Commision; 2009. [Google Scholar]
- Gardiner P, Clark PI. Menthol cigarettes: moving toward a broader definition of harm. Nicotine Tob Res. 2010;12(Suppl 2):S85–S93. doi: 10.1093/ntr/ntq176. [DOI] [PubMed] [Google Scholar]
- Giovino GA. Patterns of and Recent Trends in the Use of Mentholated Cigarettes in the United States. Silver Spring: Federal Drug Administration, Tobacco Product Scientific Advisory Board; 2010. [Google Scholar]
- Giovino GA, Sidney S, Gfroerer JC, O’Malley PM, Allen JA, Richter PA, Cummings KM. Epidemiology of menthol cigarette use. Nicotine Tob Res. 2004;6(Suppl 1):S67–S81. doi: 10.1080/14622203710001649696. [DOI] [PubMed] [Google Scholar]
- Giovino GA, Villanti AC, Mowery PD, Sevilimedu V, Niaura RS, Vallone DM, Abrams DB. Differential trends in cigarette smoking in the USA: is menthol slowing progress? Tob Control. 2015;24:28–37. doi: 10.1136/tobaccocontrol-2013-051159. [DOI] [PubMed] [Google Scholar]
- Gong K, Jasmin L. Sustained morphine administration induces TRPM8-dependent cold hyperalgesia. J Pain. 2017;18:212–221. doi: 10.1016/j.jpain.2016.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, Watsky EJ, Gong J, Williams KE, Reeves KR. Varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA. 2006;296:47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- Gordon SM, Brinkman MC, Meng RQ, Anderson GM, Chuang JC, Kroeger RR, Reyes IL, Clark PI. Effect of cigarette menthol content on mainstream smoke emissions. Chem Res Toxicol. 2011;24:1744–1753. doi: 10.1021/tx200285s. [DOI] [PubMed] [Google Scholar]
- Gracy KN, Dankiewicz LA, Weiss F, Koob GF. Heroin-specific stimuli reinstate operant heroin-seeking behavior in rats after prolonged extinction. Pharmacol Biochem Beh. 2000;65:489–494. doi: 10.1016/s0091-3057(99)00234-8. [DOI] [PubMed] [Google Scholar]
- Hoffman AC. The health effects of menthol cigarettes as compared to non-menthol cigarettes. Tob Induc Dis. 2011;9(Suppl 1):S7. doi: 10.1186/1617-9625-9-S1-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman AC, Simmons D. Menthol cigarette smoking and nicotine dependence. Tob Induc Dis. 2011;9(Suppl 1):S5. doi: 10.1186/1617-9625-9-S1-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopp R. Menthol: its origin, chemistry, physiology and toxicological properties. Rec Adv Tob Sci. 1993;19:3–46. [Google Scholar]
- Hughes JR, Peters EN, Naud S. Relapse to smoking after 1 year of abstinence: a meta-analysis. Addict Behav. 2008;33:1516–1520. doi: 10.1016/j.addbeh.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hymowitz N, Mouton C, Edkholdt H. Menthol cigarette smoking in African Americans and Whites. Tob Control. 1995;4:194–195. [Google Scholar]
- Jamal A, Homa DM, O’Connor E, Babb SD, Caraballo RS, Singh T, Hu SS, King BA. Current cigarette smoking among adults: United States, 2005–2014. Morb Mortal Wkly Rep. 2015;64:1233–1240. doi: 10.15585/mmwr.mm6444a2. [DOI] [PubMed] [Google Scholar]
- Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, Billing CB, Gong J, Reeves KR. Efficacy of varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA. 2006;296:56–63. doi: 10.1001/jama.296.1.56. [erratum: 296:1355] [DOI] [PubMed] [Google Scholar]
- Kalivas PW, McFarland K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology. 2003;168:44–56. doi: 10.1007/s00213-003-1393-2. [DOI] [PubMed] [Google Scholar]
- Kallupi M, de Guglielmo G, Cannella N, Li HW, Calo G, Guerrini R, Ubaldi M, Renger JJ, Uebele VN, Ciccocioppo R. Hypothalamic neuropeptide S receptor blockade decreases discriminative cue-induced reinstatement of cocaine seeking in the rat. Psychopharmacology. 2013;226:347–355. doi: 10.1007/s00213-012-2910-y. [DOI] [PubMed] [Google Scholar]
- Katner SN, Magalong JG, Weiss F. Reinstatement of alcohol-seeking behavior by drug-associated discriminative stimuli after prolonged extinction in the rat. Neuropsychopharmacology. 1999;20:471–479. doi: 10.1016/S0893-133X(98)00084-0. [DOI] [PubMed] [Google Scholar]
- Kreslake JM, Wayne GF, Alpert HR, Koh HK, Connolly GN. Tobacco industry control of menthol in cigarettes and targeting of adolescents and young adults. Am J Public Health. 2008;98:1685–1692. doi: 10.2105/AJPH.2007.125542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau BK, Karim S, Goodchild AK, Vaughan CW, Drew GM. Menthol enhances phasic and tonic GABAA receptor-mediated currents in midbrain periaqueductal grey neurons. Br J Pharmacol. 2014;171:2803–2813. doi: 10.1111/bph.12602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy DT, Blackman K, Tauras J, Chaloupka FJ, Villanti AC, Niaura RS, Vallone DM, Abrams DB. Quit attempts and quit rates among menthol and nonmenthol smokers in the United States. Am J Public Health. 2011;101:1241–1247. doi: 10.2105/AJPH.2011.300178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X. Contribution of drug cue, priming, and stress to reinstatement of nicotine-seeking behavior in a rat model of relapse. In: Egger J, Kalb M, editors. Smoking Relapse: Causes, Prevention, and Recovery. New York: Nova Science Publishers; 2010. pp. 143–163. [Google Scholar]
- Liu X. Effects of blockade of α4β2 and α7 nicotinic acetylcholine receptors on cue-induced reinstatement of nicotine-seeking behaviour in rats. Int J Neuropsychopharmacol. 2014;17:105–116. doi: 10.1017/S1461145713000874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Caggiula AR, Palmatier MI, Donny EC, Sved AF. Cue-induced reinstatement of nicotine-seeking behavior in rats: effect of bupropion, persistence over repeated tests, and its dependence on training dose. Psychopharmacology. 2008;196:365–375. doi: 10.1007/s00213-007-0967-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Jernigan C. Effects of caffeine on persistence and reinstatement of nicotine-seeking behavior in rats: interaction with nicotine-associated cues. Psychopharmacology. 2012;220:541–550. doi: 10.1007/s00213-011-2505-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moro F, Orru A, Marzo CM, Di Clemente A, Cervo L. mGluR2/3 mediates short-term control of nicotine-seeking by acute systemic N-acetylcysteine. Addict Biol. 2016 doi: 10.1111/adb.12443. [DOI] [PubMed] [Google Scholar]
- Muscat JE, Chen G, Knipe A, Stellman SD, Lazarus P, Richie JP., Jr Effects of menthol on tobacco smoke exposure, nicotine dependence, and NNAL glucuronidation. Cancer Epidemiol Biomarkers Prevent. 2009;18:35–41. doi: 10.1158/1055-9965.EPI-08-0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Office of the Surgeon General. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Rockville: Office of the Surgeon General; 2014. [Google Scholar]
- Ohmi M, Shishido Y, Inoue T, Ando K, Fujiuchi A, Yamada A, Watanabe S, Kawamura K. Identification of a novel 2-pyridyl-benzensulfonamide derivative, RQ-00203078, as a selective and orally active TRPM8 antagonist. Bioorg Med Chem Lett. 2014;24:5364–5368. doi: 10.1016/j.bmcl.2014.10.074. [DOI] [PubMed] [Google Scholar]
- Okuyemi KS, Ahluwalia JS, Ebersole-Robinson M, Catley D, Mayo MS, Resnicow K. Does menthol attenuate the effect of bupropion among African American smokers? Addiction. 2003;98:1387–1393. doi: 10.1046/j.1360-0443.2003.00443.x. [DOI] [PubMed] [Google Scholar]
- Paris MM, Carter BL, Traylor AC, Bordnick PS, Day SX, Armsworth MW, Cinciripini PM. Cue reactivity in virtual reality: the role of context. Addict Behav. 2011;36:696–699. doi: 10.1016/j.addbeh.2011.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson JL, Abrams DB, Niaura RS, Richardson A, Vallone DM. A ban on menthol cigarettes: impact on public opinion and smokers’ intention to quit. Am J Public Health. 2012;102:e107–e114. doi: 10.2105/AJPH.2012.300804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108:705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- Pletcher MJ, Hulley BJ, Houston T, Kiefe CI, Benowitz N, Sidney S. Menthol cigarettes, smoking cessation, atherosclerosis, and pulmonary function: the Coronary Artery Risk Development in Young Adults (CARDIA) study. Arch Intern Med. 2006;166:1915–1922. doi: 10.1001/archinte.166.17.1915. [DOI] [PubMed] [Google Scholar]
- Reitzel LR, Li Y, Stewart DW, Cao Y, Wetter DW, Waters AJ, Vidrine JI. Race moderates the effect of menthol cigarette use on short-term smoking abstinence. Nicotine Tob Res. 2013;15:883–889. doi: 10.1093/ntr/nts335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitzel LR, Nguyen N, Cao Y, Vidrine JI, Daza P, Mullen PD, Velasquez MM, Li Y, Cinciripini PM, Cofta-Woerpel L, Wetter DW. Race/ethnicity moderates the effect of prepartum menthol cigarette use on postpartum smoking abstinence. Nicotine Tob Res. 2011;13:1305–1310. doi: 10.1093/ntr/ntr095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter P, Beistle D, Pederson L, O’Hegarty M. Small-group discussions on menthol cigarettes: listening to adult African American smokers in Atlanta, Georgia. Ethn Health. 2008;13:171–182. doi: 10.1080/13557850701784694. [DOI] [PubMed] [Google Scholar]
- Rojewski AM, Toll BA, O’Malley SS. Menthol cigarette use predicts treatment outcomes of weight-concerned smokers. Nicotine Tob Res. 2014;16:115–119. doi: 10.1093/ntr/ntt137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JE, Behm FM. Combination treatment with varenicline and bupropion in an adaptive smoking cessation paradigm. Am J Psychiatry. 2014;171:1199–1205. doi: 10.1176/appi.ajp.2014.13050595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruskin DN, Anand R, LaHoste GJ. Menthol and nicotine oppositely modulate body temperature in the rat. Eur J Pharmacol. 2007;559:161–164. doi: 10.1016/j.ejphar.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology. 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Mason KM, Henningfield JE. Tobacco dependence treatments: review and prospectus. Annu Rev Public Health. 1998;19:335–358. doi: 10.1146/annurev.publhealth.19.1.335. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Use of Menthol Cigarettes. Rockville, MD: Substance Abuse and Mental Health Services Administration, Office of Appied Studies; 2009. [Google Scholar]
- Tobacco Products Scientific Advisory Committee. Menthol Cigarettes and Public Health: Review of the Scientific Evidence and Recommendations. Rockville, MD: Food and Drug Administration; 2011. [Google Scholar]
- Ton HT, Smart AE, Aguilar BL, Olson TT, Kellar KJ, Ahern GP. Menthol Enhances the Desensitization of Human alpha3beta4 Nicotinic Acetylcholine Receptors. Mol Pharmacol. 2015;88:256–264. doi: 10.1124/mol.115.098285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troisi JR., 2nd Nicotine vs. ethanol discrimination: extinction and spontaneous recovery of responding. Integr Physiol Behav Sci. 2003;38:104–123. doi: 10.1007/BF02688829. [DOI] [PubMed] [Google Scholar]
- Troisi JR, 2nd, Akins C. The discriminative stimulus effects of cocaine in a pavlovian sexual approach paradigm in male Japanese quail. Exp Clin Psychopharmacol. 2004;12:237–242. doi: 10.1037/1064-1297.12.4.237. [DOI] [PubMed] [Google Scholar]
- Troisi JR, 2nd, LeMay BJ, Jarbe TU. Transfer of the discriminative stimulus effects of Δ9-THC and nicotine from one operant response to another in rats. Psychopharmacology. 2010;212:171–179. doi: 10.1007/s00213-010-1940-6. [DOI] [PubMed] [Google Scholar]
- Umezu T, Morita M. Evidence for the involvement of dopamine in ambulation promoted by menthol in mice. J Pharmacol Sci. 2003;91:125–135. doi: 10.1254/jphs.91.125. [DOI] [PubMed] [Google Scholar]
- Vogeler T, McClain C, Evoy KE. Combination bupropion SR and varenicline for smoking cessation: a systematic review. Am J Drug Alcohol Abuse. 2016;42:129–139. doi: 10.3109/00952990.2015.1117480. [DOI] [PubMed] [Google Scholar]
- Wang J, Roethig HJ, Appleton S, Werley M, Muhammad-Kah R, Mendes P. The effect of menthol containing cigarettes on adult smokers’ exposure to nicotine and carbon monoxide. Regul Toxicol Pharmacol. 2010;57:24–30. doi: 10.1016/j.yrtph.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Wang T, Wang B, Chen H. Menthol facilitates the intravenous self-administration of nicotine in rats. Front Behav Neurosci. 2014;8:437. doi: 10.3389/fnbeh.2014.00437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne G, Connolly GN. Application, function, and effects of menthol in cigarettes: a servey of tobacco inductry documents. Nicotine Tob Res. 2004;6(suppl 1):S43–S54. doi: 10.1080/14622203310001649513. [DOI] [PubMed] [Google Scholar]
- Weiss F, Martin-Fardon R, Ciccocioppo R, Kerr TM, Smith DL, Ben-Shahar O. Enduring resistance to extinction of cocaine-seeking behavior induced by drug-related cues. Neuropsychopharmacology. 2001;25:361–372. doi: 10.1016/S0893-133X(01)00238-X. [DOI] [PubMed] [Google Scholar]
- Werley MS, Coggins CR, Lee PN. Possible effects on smokers of cigarette mentholation: a review of the evidence relating to key research questions. Regul Toxicol Pharmacol. 2007;47:189–203. doi: 10.1016/j.yrtph.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Widholm JJ1, Gass JT, Cleva RM, Olive MF. The mGluR5 Positive Allosteric Modulator CDPPB Does Not Alter Extinction or Contextual Reinstatement of Methamphetamine-Seeking Behavior in Rats. J Addict Res Ther. 2011;S1:4. doi: 10.4172/2155-6105.S1-004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing VC, Shoaib M. Contextual stimuli modulate extinction and reinstatement in rodents self-administering intravenous nicotine. Psychopharmacology. 2008;200:357–365. doi: 10.1007/s00213-008-1211-y. [DOI] [PubMed] [Google Scholar]
- World Health Organization. 2015 http://apps.who.int/iris/bitstream/10665/178574/1/9789240694606_eng.pdf?ua=1.