Abstract
Over ninety percent of head and neck cancers overexpress the epidermal growth factor receptor (EGFR). In diverse tumor types, EGFR overexpression has been associated with poorer prognosis and outcomes. Therapies targeting EGFR include monoclonal antibodies, tyrosine kinase inhibitors, phosphatidylinositol 3-kinase (PI3K) inhibitors, and antisense gene therapy. Few EGFR-targeted therapeutics are approved for clinical use. The monoclonal antibody cetuximab is Food and Drug Administration (FDA) approved EGFR-targeted therapy, yet has exhibited modest benefit in clinical trials. The humanized monoclonal antibody nimotuzumab is also approved for head and neck cancers in Cuba, Argentina, Colombia, Peru, India, Ukraine, Ivory Coast and Gabon in addition to nasopharyngeal cancers in China. Few other EGFR-targeted therapeutics for head and neck cancers have led to as significant responses as seen in lung carcinomas, for instance. Recent genome sequencing of head and neck tumors has helped identify patient subgroups with improved response to EGFR inhibitors, for example cetuximab in patients with the KRAS-variant and the tyrosine kinase inhibitor erlotinib for tumors harboring MAPK1E322K mutations. Genome sequencing has furthermore broadened our understanding of dysregulated pathways, holding the potential to enhance the benefit derived from therapies targeting EGFR.
Keywords: EGFR, head and neck SCC, genomics, cetuximab
1. Introduction
Stanley Cohen’s discovery of epidermal growth factor (EGF) was awarded the 1986 Nobel Prize, heralding the development of EGFR-targeted therapeutics [1]. In diverse tumor types including head and neck, bladder, ovarian and cervical cancers, EGFR overexpression has been associated with poorer prognosis and outcomes [2–4]. In 2004, the FDA initially approved the monoclonal antibody cetuximab for metastatic colorectal cancer. Its use was expanded to head and neck squamous cell carcinomas (HNSCC) in 2006. Cetuximab remains the only EGFR-directed treatment FDA-approved for head and neck cancers. Here, we review EGFR-targeted therapies and highlight insights from recent genomic research relevant to head and neck cancers.
2. Receptor Pathway and Function
2.1 EGFR Structure
EGFR, also called HER1 or ErbB1, was the first member of the ErbB family of tyrosine kinase receptors discovered [5]. This family also includes HER2/neu (ErbB2), HER3 (ErbB3), and HER4 (ErbB4). The 170 kDa EGFR receptor spans the membrane once and contains extracellular, transmembrane, and intracellular regions. The extracellular component is comprised of 4 domains. Domains I and III are leucine rich and structurally similar to domains found in the insulin receptor [6], a cell surface receptor known to share downstream signaling pathways with EGFR [7,8]. Domains II and IV are cysteine rich and similar to laminin [9]. The intracellular region harbors the intrinsic tyrosine kinase activity of EGFR. Existing in both closed monomer and open dimer conformations [10], EGFR is composed of twenty percent carbohydrates, with N-linked glycosylation affecting receptor structure and stability; increased glycosylation stabilizes and drives the equilibrium towards the extended conformation [4,11,12].
2.2 EGFR Pathway
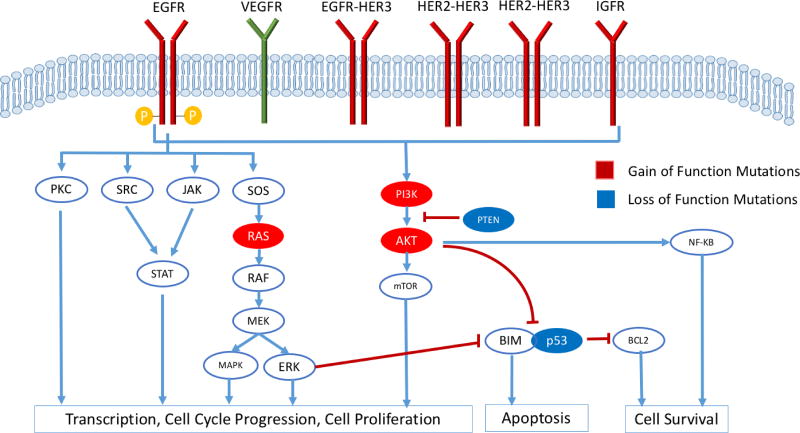
Epidermal growth factor (EGF), transforming growth factor-alpha (TGF-α), amphiregulin, heparin-binding EGFR, and betacellulin are among the ligands which bind to domains I and III of EGFR. Subsequent exposure of domain II results in receptor dimerization via disulfide bonds. After dimerization at the cell surface, autophosphorylation of tyrosine residues in the cytoplasmic region provides docking sites for signal transducers, including proteins such as Ras, to bind and initiate intracellular signaling cascades and gene transcription [4,13]. Downstream signaling cascades of EGFR can be broadly divided into the following pathways: RAS/RAF/MEK/MAPK/ERK, phosphatidylinositol 3-kinase (PI3K) and Akt, protein kinase C (PKC), Src, and the JAK/STAT pathways (Figure 1) [14]. These extensively studied signaling cascades influence gene expression, proliferation, angiogenesis, apoptosis inhibition, cell motility, metastasis, adhesion, and angiogenesis [4,15].
Figure 1.
Epidermal growth factor receptor downstream signaling pathways include RAS/RAF/MEK/MAPK/ERK, phosphatidylinositol 3-kinase(PI3K) and Akt, protein kinase C (PKC), Src, and the JAK/STAT pathways. Subsequent signaling cascades influence gene expression, proliferation, angiogenesis, apoptosis inhibition, cell motility, metastasis, adhesion, and angiogenesis.
2.3 EGFR Function in Normal Physiology and Cancer
Indisputably, EGFR possesses a critical role in development and differentiation, particularly in epithelial and glial cells. Highly expressed in the basal layer of the epidermis and the outer root sheath of hair cells, EGFR influences migration and differentiation of keratinocytes and hair follicle development. Mouse models expressing mutant EGFR develop papillomas and squamous cell carcinomas (SCC) [16]. In neurons, EGFR regulates migration and neurodegeneration, with mutations leading to glioma-like tumors in murine models [16]. Furthermore, in lung tissue, EGFR influences maturation of type II pneumocytes; following lung damage these cells proliferate into type 1 pneumocytes, and replace damaged tissue.
In head and neck cancers, EGFR is overexpressed in over 90% of tumors and correlates with poorer outcomes [17,18]. In tissue from 91 HNSCC patients, tumor EGFR level was a statistically significant predictor of disease-free survival (DFS) (p=0.0001) along with tumor site and TGF-α level [17]. In the large phase III RTOG 9003 trial evaluating radiation regimens, retrospective subset analysis of 155 patients reinforced the correlation between EGFR expression and decreased overall survival (OS) along with increased local-regional relapse [19]. In addition to overall increased expression, EGFR copy number was associated with a 91% (20/22 patients) 5-year mortality compared to 29% (30/102 patients) in patients with a normal copy number [20]. Similar associations exist for breast, lung, and other tumor types [4,21,22].
3. EGFR Targeted Therapies
Until the development of targeted therapeutics, chemotherapy for head and neck cancers was predominated by non-specific inhibitors of cellular division and proliferation. FDA-approved therapies included cisplatin, methotrexate, 5-fluorouracil (5-FU), bleomycin, and docetaxel, all of which produced clinical response rates ranging from 20–40% [22]. Common side effects included dysphagia, odynophagia, nausea, vomiting, and hematologic suppression [22,23]. EGFR-targeted therapies approved and under-development include monoclonal antibodies (Table I), tyrosine kinase inhibitors (Table II), PI3K inhibitors, and antisense gene therapy.
Table I.
EGFR-targeted Monoclonal Antibodies
| Compound | Company | Description | Approval and Clinical Indications |
|---|---|---|---|
| Cetuximab Erbitux (IMC-C225) | ImClone Systems Incorporated Bristol-Myers Squibb Eli Lily Merck KGaA | Chimeric, murine antibody and human IgG1 | 2004: FDA approval for metastatic colorectal |
| 2006: FDA approval for use in combination with XRT for locally or regionally advanced HNSCC or as monotherapy for platinum refractory, recurrent, or metastatic HNSCC | |||
| 2009: FDA approval for KRAS wild type colorectal cancer | |||
| 2011: FDA approval for use as first-line treatment in combination with platinum based chemotherapeutics and 5-FU for recurrent local-regional or metastatic HNSCC | |||
| Panitumumab Vectibix (ABX-EGF) | Amgen Takeda | Humanized mAb | 2006: FDA approval for metastatic CRC |
| 2007: European Medicines Agency approval for use in combination with FOLFIRI chemotherapy for metastatic colon cancer | |||
| 2008: Health Canada approval for refractory EGFR-expressive metastatic CRC with wild type KRAS | |||
| 2014: FDA approval in combination with FOLFOX for first line treatment of wild type KRAS CRC | |||
| Nimotuzumab | YM Biosciences | Humanized mAb | 2006: Approval for HNSCC in India |
| 2008: Approval in combination with XRT for NPC in China | |||
| Phase II and III studies for cancers including HNSCC, esophageal, gastric, CRC, and gliomas | |||
| Zalutumumab Genmab | Genmab MATOS Pharma | Human IgG1 | Phase I, II, and III for HNSCC, NSCLC, and CRC |
| Duligotuzumab | Roche | Humanized dual EGFR/HER3 mAb | Phase I and II studies in HNSCC |
CRC, colorectal cancer. FDA, Food and Drug Administration. FOLFOX, a chemotherapy combination of leucovorin, fluorouracil, and oxaliplatin. HNSCC, head and neck squamous cell carcinoma. NPC, nasopharyngeal carcinoma. XRT, radiotherapy.
Table II.
EGFR-targeted Tyrosine Kinase Inhibitors
| Compound | Company | Description | Approval and Clinical Indications |
|---|---|---|---|
| Gefitinib Iressa (ZD1839) | AstraZeneca Pharmaceuticals | Reversible binding EGFR specific Oral medicine | 2003: advanced or metastatic NSCLC |
| Erlotinib Tarceva (OSI-774) | Genentech Astellas | Reversible binding EGFR specific | 2004: locally advanced or metastatic NSCLC; approved in combination with gemcitabine for locally advanced or metastatic pancreatic cancer |
| Lapatinib Tykerb | GlaxoSmithKline | Reversible binding Inhibition of HER2/neu and EGFR | 2007- in combination for breast cancer patient on capecitabine |
| 2010- in combination with an aromatase inhibitor for HER2 and hormone receptor positive metastatic breast cancer | |||
| Afatinib | Boehringer Ingelheim Pharmaceuticals | Irreversible Pan-ErbB binding | 2013: first-line treatment of metastatic NSCLC with EGFR exon 19 deletions or exon 21 (L858R) substitutions |
| Dasatinib (Sprycel) | Bristol-Myers Squibb | c-Scr kinases; thought to interfere with nuclear localization and of EGFR (Raju 2012) | 2006: adult chromosome-positive chronic myelogenous leukemia (CP-CML) for which imatinib was ineffective |
| 2010: newly diagnosed CP-CML | |||
| Dacomitinib | Pfizer | Irreversible Pan-ErbB binding | Phase I, II and III trials for cancers including HNSCC, NSCLC, and glioblastoma multiforme |
| ASP8273 | Astellas Pharma | Irreversible binding Affinity higher for EGFR activating and T790M mutations compared to wild type | Phase I, II, and III trials in NSCLC and solid malignancies |
3.1 Monoclonal Antibodies
In 2006, cetuximab was the first targeted treatment for head and neck cancers approved by the FDA (Table I). A chimeric murine antibody linked to human IgG, cetuximab was approved in combination with radiation (XRT) in locally advanced (LA) disease, as a single agent for recurrent or metastatic HNSCC after failure of platinum therapies, and in combination with 5-FU and platinum based therapies for first-line recurrent or metastatic HNSCC [14]. In addition to inhibiting ligand binding, alternative mechanisms of action involve initiating receptor endocytosis, activating antibody-dependent cell-mediated cytotoxicity (ADCC), and inhibiting repair of radiation-induced damage [23,24].
In clinical care, cetuximab improved patient outcomes when combined with radiotherapy (Table III). Randomized, phase III, multicenter trials assessing the addition of cetuximab to radiotherapy noted increased local-regional control and increased median OS from 29.3 months (95% CI 20.6–41.4) to 49.0 months (95% CI 32.8–69.5) [25,26]. Importantly, patients experienced unchanged rates of treatment-related toxicities. However, higher grade of acneiform rash, a common side effect, was associated with improved OS and thought indicative of an inflammatory response [26].
Table III.
Select Clinical Trials of EGFR Therapeutics
| Lead Author (Year Published) |
Phase | Study | Patient and Disease Demographics
|
Outcomes | |||
|---|---|---|---|---|---|---|---|
| Number | Stage | Tumor Characteristics | EGFR expression (% of total patients) |
||||
| Bonner et al. (2006) | III | High-dose XRT with and without cetuximab | 424 | III or IV | Nonmetastatic, measurable SCC | 79 |
|
| Oropharynx (56%), hypopharynx (17%), larynx (27%) | |||||||
| Bonner et al. (2010) | III | High-dose XRT with and without cetuximab | 424 | III or IV | Nonmetastatic, measurable SCC | 79 |
|
| Update on Bonner et al. (2006) study | Oropharynx (56%), hypopharynx (17%), larynx (27%) | ||||||
| Vermorken et al. (2007) | II | Treatment with cetuximab and subsequent combination of cetuximab and platinum therapy in the setting of disease progression | 103 | III or IV | Recurrent or metastatic SCC | 97 |
|
| Pharynx (38%), larynx (20%), paranasal sinuses (3%); other (39%) | |||||||
| Open-label, no control arm | Progression after a 2-6 cycles of platinum-based therapy | ||||||
| Vermorken et al. (2008) | III | Platinum-based therapy and fluorouracil with and without cetuximab | 442 | NR | Metastatic or local-regionally recurrent SCC | 92 |
|
| Oral cavity (20%), oropharynx (34%), hypopharynx (14%), larynx (25%), other (7%) | |||||||
| Ang et al. (2014) | III | Cisplatin-based C-XRT with and without cetuximab | 891 | III or IV | Oropharynx (70%), hypopharynx (7%), larynx (23%) | NR |
|
| Weidhass et al. (2016) | III | Cisplatin-based C-XRT with and without cetuximab | 413 | III or IV | Oropharynx (72%), hypopharynx/larynx (28%) 17% KRAS-variant (70/413) | NR |
|
| Patients subcategorized by KRAS mutation | |||||||
| Mesia et al. (2015) | II | Cisplatin-based C-XRT compared to a dose-reduced cisplatin-based C-XRT with panitumumab | 150 | III or IV | Locally advanced SCC | NR |
|
| CONCERT-1 | Oral cavity (9%), oropharynx (53%), hypopharynx (19%), larynx (18%) | ||||||
| Fayette et al. (2016) | II | Duligotuzumab compared to cetuximab following progressing on/after cisplatin-based chemotherapy | 121 | III or IV | Recurrent or metastatic SCC | NR |
|
| MEHGAN study | Oral cavity (29%), oropharynx (30%), hypopharynx (10%), larynx (16%), unspecified (10%), unknown (6%) | ||||||
| Martins et al. (2013) | II | Cisplatin and XRT with and without erlotinib Randomized | 204 | III or IV | Locally advanced SCC | 4/90 samples assessed had EGFR amplification |
|
| Oral cavity (7%), oropharynx (67%), hypopharynx (6%), larynx (18%), nasopharynx (1%), other (1%) | |||||||
| Argiris et al. (2013) | III | Docetaxel with or without gefitinib | 270 | NR | Recurrent or metastatic SCC | NR |
|
| Randomized | Oral cavity (22%), oropharynx (33%), larynx (26%), multiple (5%), other (14%) | ||||||
| Kim et al. (2015) | II | Dacomitinib monotherapy | 48 | NR | Local-regionally recurrent or metastatic SCC | NR |
|
| Progression on or intolerance to platinum therapy | |||||||
| Oral cavity (37%), oropharynx (23%), hypopharynx(17%), larynx (19%), maxillary sinus (4%) | |||||||
| Machiels et al. (2015) | III | Afatinib or methotrexate as a second-line therapy following prior platinum-based therapy and disease progression | 483 | NR | Recurrent or metastatic SCC | NR |
|
| Progression after or on platinum-based therapy | |||||||
| Oral cavity (28%), oropharynx (32%), hypopharynx (19%), larynx (21%) | |||||||
| Harrington et al. (2015) | III | Adjuvant C-XRT with lapatinib or placebo followed by 1 year of lapatinib or placebo | 688 | II, III, IVA | Surgical margin <5mm or ECE | 70 (IHC 3+) |
|
| Oral cavity (41%), oropharynx (19%), hypopharynx(13%), larynx (23%), multiple sites (4%) | |||||||
| Soulières et al. (2017) | II | Buparlisib, oral pan-PI3K inhibitor, or placebo with paclitaxel as second-line therapy after progression with platinum-based treatment | 158 | NR | Recurrent or metastatic SCC | NR |
|
| Progression after or on platinum-based therapy | |||||||
| BERIL-1 | Oral cavity (29%), oropharynx (28%), hypopharynx (18%), larynx (16%), nasopharynx (3%), other/unknown (6%) | ||||||
C-XRT, chemoradiotherapy. ECE, extracapsular extension. HR, hazard ratio. NR, not recorded. OS, overall survival. PFS, progression-free survival. SCC, squamous cell carcinoma. XRT, radiotherapy.
Cetuximab also conferred additional benefit in combination with chemotherapy (Table III). In a phase II multicenter study, patients with recurrent or metastatic HNSCC were started on cetuximab therapy; cisplatin was subsequently added following disease progression. Of the 103 patients, 46% benefited from cetuximab with either disease control or stabilization with a mean time to progression of 70 days [27]. Similarly, in a phase III trial, addition of cetuximab to platinum-based and 5-FU therapies increased median OS from 7.4 months to 10.1 months and progression-free survival (PFS) from 3.3 months to 5.6 months [28]. Though the improvements observed were modest, these trials prompted FDA approval for cetuximab in combination with XRT for locally or regionally advanced HNSCC or as monotherapy for platinum refractory, recurrent, or metastatic HNSCC in 2006. The latter trial, of note, led to expansion of cetuximab from treatment of only platinum-refractory to any untreated recurrent or metastatic tumors. While addition of cetuximab to radiotherapy or chemotherapy increased survival, the addition of cetuximab to both radiotherapy and cisplatin in combination did not amplify clinical benefit [29].
Ongoing research and development are focused more on fully humanized EGFR-targeted antibodies (Table I). Panitumumab, FDA approved for colorectal cancers, has led to modest outcomes for HNSCC. In the phase III randomized SPECTRUM trial, OS was not significantly improved for patients with late stage disease randomized to cisplatin and 5-FU with or without panitumumab; PFS was modestly increased from 4.6 months to 5.8 months [30]. Similarly, in the CONCERT-1 phase II trial, the addition of panitumumab to cisplatin-based therapy for late-stage HNSCC did not improve two-year local-regional control though led to increased rates of grade 3 and 4 side effects [31]. Ongoing trials are assessing the role of panitumumab in adjuvant treatment (NCT00798655). Zalutumumab has a decreased immunogenic profile with lower risk of hypersensitivity; however, OS was not significantly improved following treatment for patients with incurable HNSCC [32]. Ongoing trials will assess the role of zalutumumab in curative chemoradiation (C-XRT) (NCT00496652). Finally, nimotuzumab is an antibody which requires bivalent binding to EGFR and thus selectively binds to cells with higher EGFR expression. Clinical trials showed improved clinical response rates when nimotuzumab was added to XRT (59.5% versus 34.2%) [33]. Rash was rarely detected and increased EGFR expression correlated with improved survival [33,34]. Nimotuzumab is approved for HNSCC in countries including Cuba, Argentina, Colombia, Peru, India, Ukraine, Ivory Coast and Gabon. In China, nimotuzumab is administered in combination with radiation for nasopharyngeal carcinomas. It is still being assessed in clinical trials in the United States.
To amplify the therapeutic response of targeting EGFR, duligotuzumab was developed to target both EGFR and HER3. However, a phase II trial showed no significant improve in PFS nor OS when compared to cetuximab (Table III) [35].
3.2 Tyrosine Kinase Inhibitors
Tyrosine kinase inhibitors (TKI) target the intracellular catalytic domain of receptor tyrosine kinases (Table II). Reversible binding TKIs, including gefitinib and erlotinib, were initially approved for non-small cell lung cancer (NSCLC) but have yet to enhance outcomes for HNSCC. Irreversible binding TKIs, which were subsequently developed and include afatinib, appear clinically promising.
Gefitinib and erlotinib were approved for NSCLC in 2003 and 2004, respectively. In a randomized phase II trial of 204 late stage HNSCC patients, the addition of erlotinib to cisplatin and XRT did not confer additional tumor response or patient survival [36]. Gefitinib also did not improve survival or outcomes in a phase III randomized trial of 270 metastatic or recurrent HNSCC patients [37]. For comparison, in NSCLC, these reversible binding TKIs exhibit RECIST (Response Evaluation Criteria in Solid Tumors) response rates of 55% to 75% for patients harboring an EGFR tyrosine kinase domain mutation [38,39].
A new generation of TKIs with multiple targets and irreversible binding have shown clinical potential in HNSCC. Afatinib, an irreversible inhibitor of EGFR, HER2, and HER4 kinases exhibited comparable outcomes to cetuximab. In a randomized, phase II study assessing afatinib versus cetuximab for treatment of recurrent or metastatic HNSCC in 124 patients, median OS was 35.9 weeks with afatinib and 47.1 weeks for cetuximab (p = 0.78) [40]. Following treatment failure in each arm, patients were transferred to the other treatment arm, during which disease control was 38.9% with afatinib and 18.8% with cetuximab. In light of these promising results, a phase III trial involving 483 patients following treatment failure on platinum-based therapy noted improved PFS with use of afatinib (median 2.6 months) compared to methotrexate (median 1.7 months) for second-line treatment (hazard ratio (HR) 0.80, 95% CI 0.65–0.98, p=0.03) [41].
Dacomitinib, another irreversible multi-targeted TKI, and lapatinib, an oral reversible inhibitor of EGFR and HER2, have exhibited limited effects in early studies [42–46].
3.3 Phosphatidylinositol 3-kinase (PI3K) Inhibitors
PI3K mutations are prevalent in head and neck cancers, noted in 34% of HPV negative HNSCC and 56% of HPV positive samples [46,47]. Buparlisib is an oral, pan-PI3K inhibitor noted to modestly improve PFS in recurrent and metastatic head and neck cancer patients (Table III). In a phase II trial of 158 patients assessing buparlisib as a second-line therapy following progression on platinum-based chemotherapy, buparlisib improved median PFS to 4.6 months with buparlisib and paclitaxel compared to 3.5 months with placebo and paclitaxel (HR 0.65, 95% CI 0.45–0.95) [49]. Of note, 46% of patients were previously treated with EGFR-targeted therapy. Future studies in varying patient populations may elicit more marked improvements in survival.
3.4 Antisense Gene Therapy
Antisense therapy centers on inhibiting messenger RNA (mRNA) by binding complementary, engineered nucleic acids. This is thought to lead to inhibition of transcription, splicing, and mRNA modification. An additional mechanism described is RNase H-mediated cleavage [50].
EGFR-targeted antisense therapy has completed early phase clinical testing. In a phase I trial of 17 HNSCC patients, antisense DNA targeting EGFR was directly injected into patients’ tumors. Seven patients demonstrated either stable or clinically responsive disease noted by decreased tumor volume [51]. A phase I/II trial combining EGFR antisense with radiation and cetuximab was recently completed (NCT01592721). Future research will also need to address systemic activity of EGFR-targeted antisense activity.
4. Insights from Genomic Research
Despite the widespread overexpression of EGFR in cancers, cetuximab treatment leads to only a modest response in HNSCC [52]. As a novel tool, genome sequencing has restructured our understanding of dysregulated pathways and provided deeper insight into EGFR-targeted therapies.
Given the broad landscape of mutations in HNSCC, mutations in four major classes of proteins/pathways have been identified: 1) mitogenic pathways (PI3K/mTOR), 2) differentiation and NOTCH pathways, 3) regulators of cell cycle proliferation through p16 and cyclin D1, and 4) regulators of apoptosis, including p53, whose loss of function is found almost universally in smoking-related HNSCC (Table IV) [47]. Whole-exome sequencing of 151 head and neck tumor samples revealed that, aside from p53, the PI3K pathway, which promotes mitogenic signaling, was the most commonly mutated pathway, with mutations occurring in 30.5% of samples [47,48]. Additional sequencing efforts discovered novel mutations in NOTCH1, functioning as a tumor suppressor gene [53,54].
Table IV.
Frequency of mutations in commonly deregulated head and neck cancer pathways
| Cell Cycle | RTK/RAS/PI(3)K | Cell Death | Differentiation | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
||||||||
| Gene | HPV − | HPV + | Gene | HPV − | HPV + | Gene | HPV − | HPV + | Gene | HPV − | HPV + |
|
|
|
|
|
||||||||
| Pathway | 96 | 100 | Pathway | 62 | 61 | Pathway | 44 | 31 | Pathway | 64 | 44 |
| CCND1 | 31 | 3 | EFGR | 15 | 6 | CASP8 | 11 | 3 | NOTCH | 26 | 17 |
| CDK6 | 8 | 0 | ERBB2 | 4 | 3 | TP53 | 84 | 3 | TP63 | 19 | 28 |
| CDKN2A | 57 | 0 | FGFR1 | 10 | 0 | FAT1 | 32 | 3 | |||
| RB1 | 4 | 6 | FGFR3 | 2 | 11 | ||||||
| E2F1 | 2 | 19 | IGF1R | 4 | 0 | ||||||
| MYC | 14 | 3 | HRAS | 5 | 0 | ||||||
| TP53 | 84 | 3 | PIK3CA | 34 | 56 | Activated Gene | |||||
| PTEN | 12 | 6 | Inactivation Gene | ||||||||
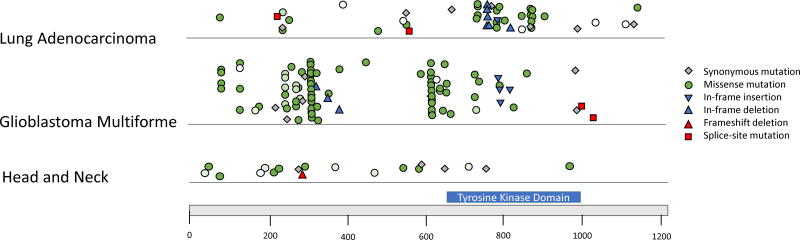
Sequencing of HNSCC tumors has not identified recurrent EGFR driving mutations. In contrast to NSCLC in which EGFR mutations are clustered in exons 18–21, the region encoding the tyrosine kinase domain, EGFR mutations in head and neck cancers appear more dispersed across the gene (Figure 2) [54]. Chang et al. (2016) assessed 11,119 human tumor samples and 41 types of cancers to create an algorithm identifying frequently mutated residues; hot spots were noted in HRAS and PIK3CA in head and neck cancers but not in EGFR [55]. Perhaps lack of recurrent EGFR mutations contributes to the limited effects of TKIs and cetuximab in HNSCC. In contrast, TKIs for the treatment of NSCLC which harbor tyrosine kinase domain mutations exhibit RECIST response rates of 55% to 75% [39].
Figure 2. EGFR Mutation Patterns.
Mutation patterns in EGFR across tumor types. EGFR mutations appear recurrent and localized in lung cancer and glioblastoma multiforme, in contrast to the pattern seen in head and neck carcinomas. Missense mutations, represented by circles, are colored by degree of conservation of base pair; dark green is conserved and white is not conserved [51].
With the lack of driving mutations and the global upregulation of EGFR, the vast landscape of mutations implicates co-activation of additional pathways. Notably the KRAS-variant germline and MAPK1E322K mutation were highlighted in recent literature. Patients harboring a germline mutation in the micro-RNA binding site of KRAS have poorer overall survival [57]. Surprisingly, in a phase III trial in which cetuximab did not confer benefit when added to chemoradiation in unselected HNSCC patients [29], patients with the KRAS-variant (70 of 413 patients tested) had increased OS in the first two years following treatment with cetuximab (HR 0.19; 95% CI, 0.04–0.86; P = 0.03) [57]. This improvement in survival from cetuximab was not seen for wild-type KRAS patients. In KRAS-variant patients, TFG-β1 was found to be upregulated; this cytokine has been implicated in suppressing antitumor immunity through regulatory T-cell induction [58]. Authors of this study proposed that through ADCC and improved dendritic cell priming of cytotoxic T lymphocytes, cetuximab bolstered the antitumor immunity otherwise inhibited in KRAS-variant patients [57].
In addition to the KRAS-variant, genomic sequencing revealed that tumor samples from a patient with a MAPK1E322K mutation were exquisitely sensitive to EGFR TKIs. In a window-of-opportunity clinical trial, a patient with a stage IVA tongue carcinoma who received a 13-day course of erlotinib experienced remarkable disease reduction from initial clinical T1N2c disease with bulky lymphadenopathy to pathological T1N0 disease. Following surgery, the patient has remained disease-free for more than 4 years without additional treatment [59]. No EGFR mutation was identified in this patient. However, the patient’s MAPK1E322K mutation was studied in in vitro and in vivo models and found to be associated with upregulation of amphiregulin and stimulation of an autocrine feedback loop involving EGFR, ERK, and amphiregulin. Remarkably, upregulated amphiregulin increased tumor sensitivity to erlotinib, an effect emphasized by the loss of erlotinib sensitivity following amphiregulin knockdown in MAPK1E322K models [60].
Improved response to EGFR inhibitors (cetuximab in HNSCC tumors with the KRAS-variant and erlotinib in HNSCCs harboring MAPK1E322K mutations) emphasizes the importance of patient selection for EGFR-targeted therapies. These studies suggest that genomic sequencing will further elicit predictive biomarkers of EGFR therapeutic response and deepen our understanding of EGFR-related cellular dysfunction that can be exploited in the clinic.
In summary, the clinical benefit of EGFR-targeted therapies in head and neck tumors has been more modest than expected given the near universal upregulation of EGFR. No dominant EGFR driver mutation has been discovered in HNSCC as in NSCLC, and KRAS mutations do not clearly indicate endogenous cetuximab resistance as they have in colon cancer. Most HNSCC cohorts sequenced to date have been performed on primary tumors without accompanying information on cetuximab treatment and clinical outcome. The coexistence of multiple deregulated pathways, in the absence of driver EGFR mutations, strongly supports the co-activation of alternative signaling pathways as a mechanism of de novo or acquired cetuximab resistance. As with KRAS-variant tumors and MAPK1E322K mutations, opportunities to exploit these pathways may lead to improved patient selection and therapeutic strategies.
5. Conclusion
Cetuximab remains the only FDA approved EGFR-targeted therapy for HNSCC and provides improved survival in a subset of patients when used in combination with chemotherapy or radiation. However, long-term survival rates for head and neck cancers have remained unchanged despite increased use of EGFR-targeted therapies. Continued genomic research understanding the dysregulated and co-activated pathways will improve patient selection and future EGFR-targeted strategies.
Acknowledgments
This work was supported by National Institutes of Health grants R01 DE24728 (DEJ), P50CA097190 (DEJ and JRG), and R01 DE023685 (JRG), and American Cancer Society grant CRP-13-308-06-COUN (JRG).
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1.Cohen S. Purification of a nerve-growth promoting protein from the mouse salivary gland and its neuro-cytotoxic antiserum. Proceedings of the National Academy of Sciences of the United States of America. 1960;46(3):302–11. doi: 10.1073/pnas.46.3.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wong AJ, Bigner SH, Bigner DD, Kinzler KW, Hamilton SR, Vogelstein B. Increased expression of the epidermal growth factor receptor gene in malignant gliomas is invariably associated with gene amplification. Proceedings of the National Academy of Sciences of the United States of America. 1987;84(19):6899–903. doi: 10.1073/pnas.84.19.6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nicholson RI, Gee JM, Harper ME. EGFR and cancer prognosis. European Journal of Cancer. 2001;37(Suppl 4):S9–15. doi: 10.1016/s0959-8049(01)00231-3. [DOI] [PubMed] [Google Scholar]
- 4.Herbst RS. Review of epidermal growth factor receptor biology. International. Journal of Radiation Oncology, Biology, Physics. 2004;59(2 Suppl):21–6. doi: 10.1016/j.ijrobp.2003.11.041. [DOI] [PubMed] [Google Scholar]
- 5.Cohen S. Isolation of a mouse submaxillary gland protein accelerating incisor eruption and eyelid opening in the new-born animal. The Journal of Biological Chemistry. 1962;237:1555–62. [PubMed] [Google Scholar]
- 6.Ward CW, Garrett TP. The relationship between the L1 and L2 domains of the insulin and epidermal growth factor receptors and leucine-rich repeat modules. BMC Bioinformatics. 2001;2:4. doi: 10.1186/1471-2105-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van der Veeken J, Oliveira S, Schiffelers RM, Storm G, van Bergen En Henegouwen PM, Roovers RC. Crosstalk between epidermal growth factor receptor- and insulin-like growth factor-1 receptor signaling: implications for cancer therapy. Current Cancer Drug Targets. 2009;9(6):748–60. doi: 10.2174/156800909789271495. [DOI] [PubMed] [Google Scholar]
- 8.Roudabush FL, Pierce KL, Maudsley S, Khan KD, Luttrell LM. Transactivation of the EGF receptor mediates IGF-1-stimulated shc phosphorylation and ERK1/2 activation in COS-7 cells. Journal of Biological Chemistry. 2000;275(29):22583–9. doi: 10.1074/jbc.M002915200. [DOI] [PubMed] [Google Scholar]
- 9.Ward CW, Hoyne PA, Flegg RH. Insulin and epidermal growth factor receptors contain the cysteine repeat motif found in the tumor necrosis factor receptor. Proteins. 1995;22(2):141–53. doi: 10.1002/prot.340220207. [DOI] [PubMed] [Google Scholar]
- 10.Ferguson KM, Berger MB, Mendrola JM, Cho HS, Leahy DJ, Lemmon MA. EGF activates its receptor by removing interactions that autoinhibit ectodomain dimerization. Molecular Cell. 2003;11(2):507–17. doi: 10.1016/s1097-2765(03)00047-9. [DOI] [PubMed] [Google Scholar]
- 11.Cummings RD, Soderquist AM, Carpenter G. The oligosaccharide moieties of the epidermal growth factor receptor in A-431 cells. Presence of complex- type N-linked chains that contain terminal N-acetylgalactosamine residues. Journal Biological Chemistry. 1985;260(22):11944–52. [PubMed] [Google Scholar]
- 12.Whitson KB, Whitson SR, Red-Brewer ML, McCoy AJ, Vitali AA, Walker F, et al. Functional effects of glycosylation at Asn-579 of the epidermal growth factor receptor. Biochemistry. 2005;44(45):14920–31. doi: 10.1021/bi050751j. [DOI] [PubMed] [Google Scholar]
- 13.Yewale C, Baradia D, Vhora I, Patil S, Misra A. Epidermal growth factor receptor targeting in cancer: a review of trends and strategies. Biomaterials. 2013;34(34):8690–707. doi: 10.1016/j.biomaterials.2013.07.100. [DOI] [PubMed] [Google Scholar]
- 14.Chong CR, Jänne PA. The quest to overcome resistance to EGFR-targeted therapies in cancer. Nature Medicine. 2013;19(11):1389–400. doi: 10.1038/nm.3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldman CK, Kim J, Wong WL, King V, Brock T, Gillespie GY. Epidermal growth factor stimulates vascular endothelial growth factor production by human malignant glioma cells: a model of glioblastoma multiforme pathophysiology. Molecular Biology of the Cell. 1993;4(1):121–33. doi: 10.1091/mbc.4.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sibilia M, Kroismayr R, Lichtenberger BM, Natarajan A, Hecking M, Holcmann M. The epidermal growth factor receptor: from development to tumorigenesis. Differentiation. 2007;75(9):770–87. doi: 10.1111/j.1432-0436.2007.00238.x. [DOI] [PubMed] [Google Scholar]
- 17.Rubin Grandis J, Melhem MF, Gooding WE, Day R, Holst VA, Wagener MM, et al. Levels of TGF-alpha and EGFR protein in head and neck squamous cell carcinoma and patient survival. Journal of the National Cancer Institute. 1998;90(11):824–32. doi: 10.1093/jnci/90.11.824. [DOI] [PubMed] [Google Scholar]
- 18.Grandis JR, Tweardy DJ. Elevated levels of transforming growth factor alpha and epidermal growth factor receptor messenger RNA are early markers of carcinogenesis in head and neck cancer. Cancer Research. 1993;53(15):3579–84. [PubMed] [Google Scholar]
- 19.Ang KK, Berkey BA, Tu X, Zhang HZ, Katz R, Hammond EH, et al. Impact of epidermal growth factor receptor expression on survival and pattern of relapse in patients with advanced head and neck carcinoma. Cancer Research. 2002;62(24):7350–7356. [PubMed] [Google Scholar]
- 20.Temam S, Kawaguchi H, El-Naggar AK, Jelinek J, Tang H, Liu DD, et al. Epidermal growth factor receptor copy number alterations correlate with poor clinical outcome in patients with head and neck squamous cancer. Journal of Clinical Oncology. 2007;25:2164–2170. doi: 10.1200/JCO.2006.06.6605. [DOI] [PubMed] [Google Scholar]
- 21.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177–82. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 22.Franklin WA, Veve R, Hirsch FR, Helfrich BA, Bunn PA., Jr Epidermal growth factor receptor family in lung cancer and premalignancy. Seminars in Oncology. 2002;29(1 Suppl 4):3–14. doi: 10.1053/sonc.2002.31520. [DOI] [PubMed] [Google Scholar]
- 23.Wen Y, Grandis JR. Emerging drugs for head and neck cancer. Expert Opinion on Emerging Drugs. 2015;20(2):313–29. doi: 10.1517/14728214.2015.1031653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen DJ, Nirodi CS. The epidermal growth factor receptor: a role in repair of radiation-induced DNA damage. Clinical Cancer Research. 2007;13(22 Pt 1):6555–60. doi: 10.1158/1078-0432.CCR-07-1610. [DOI] [PubMed] [Google Scholar]
- 25.Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. The New England Journal of Medicine. 2006;354(6):567–78. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 26.Bonner JA, Harari PM, Giralt J, Cohen RB, Jones CU, Sur RK, et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncology. 2010;11(1):21–8. doi: 10.1016/S1470-2045(09)70311-0. [DOI] [PubMed] [Google Scholar]
- 27.Vermorken JB, Trigo J, Hitt R, Koralewski P, Diaz-Rubio E, Rolland F, et al. Open-label, uncontrolled, multicenter phase II study to evaluate the efficacy and toxicity of cetuximab as a single agent in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck who failed to respond to platinum-based therapy. Journal of Clinical Oncology. 2007;25(16):2171–7. doi: 10.1200/JCO.2006.06.7447. [DOI] [PubMed] [Google Scholar]
- 28.Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. The New England Journal of Medicine. 2008;359(11):1116–27. doi: 10.1056/NEJMoa0802656. [DOI] [PubMed] [Google Scholar]
- 29.Ang KK, Zhang Q, Rosenthal DI, Nguyen-Tan PF, Sherman EJ, Weber RS, et al. Randomized phase III trial of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III to IV head and neck carcinoma: RTOG 0522. Journal of Clinical Oncology. 2014;32(27):2940–50. doi: 10.1200/JCO.2013.53.5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vermorken JB, Stöhlmacher-Williams J, Davidenko I, Licitra L, Winquist E, Villanueva C, et al. Cisplatin and fluorouracil with or without panitumumab in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck (SPECTRUM): an open-label phase 3 randomised trial. Lancet Oncology. 2013;14(8):697–710. doi: 10.1016/S1470-2045(13)70181-5. [DOI] [PubMed] [Google Scholar]
- 31.Mesía R, Henke M, Fortin A, Minn H, Yunes Ancona AC, Cmelak A, et al. Chemoradiotherapy with or without panitumumab in patients with unresected, locally advanced squamous-cell carcinoma of the head and neck (CONCERT-1): a randomised, controlled, open-label phase 2 trial. Lancet Oncology. 2015;16(2):208–20. doi: 10.1016/S1470-2045(14)71198-2. [DOI] [PubMed] [Google Scholar]
- 32.Machiels JP, Subramanian S, Ruzsa A, Repassy G, Lifirenko I, Flygare A, et al. Zalutumumab plus best supportive care versus best supportive care alone in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck after failure of platinum-based chemotherapy: an open-label, randomised phase 3 trial. Lancet Oncology. 2011;12(4):333–43. doi: 10.1016/S1470-2045(11)70034-1. [DOI] [PubMed] [Google Scholar]
- 33.Rodríguez MO, Rivero TC, del Castillo Bahi R, Muchuli CR, Bilbao MA, Vinageras EN, et al. Nimotuzumab plus radiotherapy for unresectable squamous-cell carcinoma of the head and neck. Cancer Biology & Therapy. 2010;9(5):343–9. doi: 10.4161/cbt.9.5.10981. [DOI] [PubMed] [Google Scholar]
- 34.Basavaraj C, Sierra P, Shivu J, Melarkode R, Montero E, Nair P. Nimotuzumab with chemoradiation confers a survival advantage in treatment- naive head and neck tumors over expressing EGFR. Cancer Biology & Therapy. 2010;10(7):673–81. doi: 10.4161/cbt.10.7.12793. [DOI] [PubMed] [Google Scholar]
- 35.Fayette J, Wirth L, Oprean C, Udrea A, Jimeno A, Rischin D, et al. Randomized Phase II Study of Duligotuzumab (MEHD7945A) vs. Cetuximab in Squamous Cell Carcinoma of the Head and Neck(MEHGAN Study) Frontiers in Oncology. 2016;6:232. doi: 10.3389/fonc.2016.00232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martins RG, Parvathaneni U, Bauman JE, Sharma AK, Raez LE, Papagikos MA, et al. Cisplatin and radiotherapy with or without erlotinib in locally advanced squamous cell carcinoma of the head and neck: A randomized phase II trial. Journal of Clinical Oncology. 2013;31(11):1415–21. doi: 10.1200/JCO.2012.46.3299. [DOI] [PubMed] [Google Scholar]
- 37.Argiris A, Ghebremichael M, Gilbert J, Lee JW, Sachidanandam K, Kolesar JM, et al. Phase III randomized, placebo-controlled trial of docetaxel with or without gefitinib in recurrent or metastatic head and neck cancer: An Eastern Cooperative Oncology Group trial. Journal of Clinical Oncology. 2013;31(11):1405–14. doi: 10.1200/JCO.2012.45.4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paez JG, Jänne PA, Lee JC, Tracy S, Greulich H, Gabriel S, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304(5676):1497–500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 39.Afghahi A, Sledge GW., Jr Targeted Therapy for Cancer in the Genomic Era. Cancer Journal. 2015;21(4):294–8. doi: 10.1097/PPO.0000000000000135. [DOI] [PubMed] [Google Scholar]
- 40.Seiwert TY, Fayette J, Cupissol D, Del Campo JM, Clement PM, Hitt R, et al. A randomized, phase II study of afatinib versus cetuximab in metastatic or recurrent squamous cell carcinoma of the head and neck. Annals of Oncology. 2014;25(9):1813–20. doi: 10.1093/annonc/mdu216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Machiels JP, Haddad RI, Fayette J, Licitra LF, Tahara M, Vermorken JB, et al. Afatinib versus methotrexate as second-line treatment in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck progressing on or after platinum-based therapy (LUX-Head & Neck 1): an open-label, randomised phase 3 trial. Lancet Oncology. 2015;16(5):583–94. doi: 10.1016/S1470-2045(15)70124-5. [DOI] [PubMed] [Google Scholar]
- 42.Harrington K, Berrier A, Robinson M, Remenar E, Housset M, de Mendoza FH, et al. Randomised Phase II study of oral lapatinib combined with chemoradiotherapy in patients with advanced squamous cell carcinoma of the head and neck: rationale for future randomised trials in human papilloma virus-negative disease. European Journal of Cancer. 2013;49(7):1609–18. doi: 10.1016/j.ejca.2012.11.023. [DOI] [PubMed] [Google Scholar]
- 43.Harrington K, Temam S, Mehanna H, D'Cruz A, Jain M, D'Onofrio I, et al. Postoperative Adjuvant Lapatinib and Concurrent Chemoradiotherapy Followed by Maintenance Lapatinib Monotherapy in High-Risk Patients With Resected Squamous Cell Carcinoma of the Head and Neck: A Phase III, Randomized, Double-Blind, Placebo-Controlled Study. Journal of Clinical Oncology. 2015;33(35):4202–9. doi: 10.1200/JCO.2015.61.4370. 10. [DOI] [PubMed] [Google Scholar]
- 44.Del Campo JM, Hitt R, Sebastian P, Carracedo C, Lokanatha D, Bourhis J, et al. Effects of lapatinib monotherapy: results of a randomised phase II study in therapy-naive patients with locally advanced squamous cell carcinoma of the head and neck. British Journal of Cancer. 2011;105(5):618–27. doi: 10.1038/bjc.2011.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Souza JA, Davis DW, Zhang Y, Khattri A, Seiwert TY, Aktolga S, et al. A phase II study of lapatinib in recurrent/metastatic squamous cell carcinoma of the head and neck. Clinical Cancer Research. 2012;18(8):2336–43. doi: 10.1158/1078-0432.CCR-11-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abdul Razak AR, Soulières D, Laurie SA, Hotte SJ, Singh S, Winquist E, et al. A phase II trial of dacomitinib, an oral pan-human EGF receptor (HER) inhibitor, as first-line treatment in recurrent and/or metastatic squamous-cell carcinoma of the head and neck. Annals of Oncology. 2013;24(3):761–9. doi: 10.1093/annonc/mds503. [DOI] [PubMed] [Google Scholar]
- 47.Cancer Genome Atlas Network. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 2015;517(7536):576–82. doi: 10.1038/nature14129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lui VW, Hedberg ML, Li H, Vangara BS, Pendleton K, Zeng Y, et al. Frequent mutation of the PI3K pathway in head and neck cancer defines predictive biomarkers. Cancer Discovery. 2013;3(7):761–9. doi: 10.1158/2159-8290.CD-13-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Soulières D, Faivre S, Mesía R, Remenár É, Li SH, Karpenko A, et al. Buparlisib and paclitaxel in patients with platinum-pretreatment recurrent or metastatic squamous cell carcinoma of the head and neck (BERIL-1): a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Oncology. 2017;18(3):323–335. doi: 10.1016/S1470-2045(17)30064-5. [DOI] [PubMed] [Google Scholar]
- 50.Gleave ME, Monia BP. Antisense therapy for cancer. Nature Reviews Cancer. 2005;5(6):468–79. doi: 10.1038/nrc1631. [DOI] [PubMed] [Google Scholar]
- 51.Lai SY, Koppikar P, Thomas SM, Childs EE, Egloff AM, Seethala RR, et al. Intratumoral epidermal growth factor receptor antisense DNA therapy in head and neck cancer: first human application and potential antitumor mechanisms. Journal of Clinical Oncology. 2009;27(8):1235–42. doi: 10.1200/JCO.2008.17.8251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hammerman PS, Hayes DN, Grandis JR. Therapeutic insights from genomic studies of head and neck squamous cell carcinomas. Cancer Discovery. 2015;5(3):239–44. doi: 10.1158/2159-8290.CD-14-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stransky N, Egloff AM, Tward AD, Kostic AD, Cibulskis K, Sivachenko A, et al. The mutational landscape of head and neck squamous cell carcinoma. Science. 2011;333(6046):1157–60. doi: 10.1126/science.1208130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Agrawal N, Frederick MJ, Pickering CR, Bettegowda C, Chang K, Li RJ, et al. Exome sequencing of head and neck squamous cell carcinoma reveals inactivating mutations in NOTCH1. Science. 2011;333(6046):1154–7. doi: 10.1126/science.1206923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lawrence MS, Stojanov P, Mermel CH, Robinson JT, Garraway LA, Golub TR, et al. Discovery and saturation analysis of cancer genes across 21 tumour types. Nature. 2014;505(7484):495–501. doi: 10.1038/nature12912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chang MT, Asthana S, Gao SP, Lee BH, Chapman JS, Kandoth C, et al. Identifying recurrent mutations in cancer reveals widespread lineage diversity and mutational specificity. Nature Biotechnology. 2016;34(2):155–63. doi: 10.1038/nbt.3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weidhaas JB, Harris J, Schaue D, Chen AM, Chin R, Axelrod R, et al. The KRAS-Variant and Cetuximab Response in Head and Neck Squamous Cell Cancer: A Secondary Analysis of a Randomized Clinical Trial. JAMA Oncology. 2016;3(4):483–491. doi: 10.1001/jamaoncol.2016.5478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zdanov S, Mandapathil M, Abu Eid R, Adamson-Fadeyi S, Wilson W, Qian J, et al. Mutant KRAS Conversion of Conventional T Cells into Regulatory T Cells. Cancer Immunology Research. 2016;4(4):354–65. doi: 10.1158/2326-6066.CIR-15-0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Van Allen EM, Lui VW, Egloff AM, Goetz EM, Li H, Johnson JT, et al. Genomic Correlate of Exceptional Erlotinib Response in Head and Neck Squamous Cell Carcinoma. JAMA Oncology. 2015;1(2):238–44. doi: 10.1001/jamaoncol.2015.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wen Y, Li H, Zeng Y, Wen W, Pendleton KP, Lui VW, et al. MAPK1E322K mutation increases head and neck squamous cell carcinoma sensitivity to erlotinib through enhanced secretion of amphiregulin. Oncotarget. 2016;7(17):23300–11. doi: 10.18632/oncotarget.8188. [DOI] [PMC free article] [PubMed] [Google Scholar]