Abstract
Hepatocellular carcinoma (HCC) is the third leading cause of cancer mortality worldwide and the fastest growing malignancy in the United States. With a 5-year survival rate below 12%, effective therapies for HCC are needed. Current treatments for HCC include microwave and radiofrequency ablation, high intensity focused ultrasound, liver transplant, surgical resection, and localized embolizations. However, each of these approaches has some limitation, making it imperative to develop improved methods for sensitizing tumors prior to therapy. We hypothesized that the use of ultrasound-triggered microbubble destruction (UTMD), which sensitizes tumors to radiotherapy by inducing vascular endothelial cell apoptosis, will selectively sensitize malignant tissue to radiotherapy and improve outcomes. To test this, 18 nude rats were inoculated in the right liver lobe with Hu7.5 HCC cells and after tumor formation, received 5 Gy radiotherapy, UTMD, or UTMD prior to radiotherapy. Compared to radiotherapy alone, there was a 170% reduction in tumor growth 7 days post treatment and a 3.2X improvement in median survival time when radiotherapy was combined with UTMD. These results indicate that UTMD is an effective adjunct when combined with radiotherapy to treat HCC.
Keywords: Hepatocellular Carcinoma, Ultrasound Contrast Agent, Ultrasound Triggered Microbubble Destruction, Antivascular Therapy, Radiotherapy
1. Introduction
Each year, more than half a million people are diagnosed with hepatocellular carcinoma (HCC) worldwide [1]. It is most common in areas where the incidence of Hepatitis B virus is prevalent such as in sub-Saharan Africa and Eastern Asia where 20 per 100,000 individuals are affected [2]. Current treatment methods, including microwave and radiofrequency ablation, high intensity focused ultrasound, liver transplant, surgical resection, and systemic chemotherapeutics are plagued by limitations, toxicity, and poor outcomes. Liver transplant has a reported 4-year overall survival rate of 85% and a recurrence free survival rate of 92% [1]. However, transplantation is limited due to strict criteria (disease must be limited to a solitary nodule less than 5 cm in diameter or up to three nodules, each measuring less than 3 cm), surgical candidacy, tumor burden and the availability of donors [1]. Surgical resection, with 5-year survival rates as high as 70%, is also limited as it is most effective for HCC patients without cirrhosis (since this underlying chronic disease results in a high risk of recurrence), which comprises only about 20–30% of patients with HCC [1]. Ablations or high intensity focused ultrasound have demonstrated tremendous results in patients who are not eligible for surgery, but limited in many cases by access to the lesion and primarily used only for early stage disease [1]. For patients with numerous or larger lesions, chemo- or radioembolization is the recommended treatment method. Radioembolization of HCC involves the local delivery of Theraspheres, 20–30 µm glass beads containing the radioactive compound Yttrium-90 into the hepatic artery, which is the tumor’s main blood supply [3]. This technique provides localized and sustained release of radiation with response rates between 25–60% based on response criteria in solid tumors (RECIST) [3].
It has been demonstrated through the use of angiogenesis inhibitors that disrupting the tumor vasculature sensitizes the neoplastic tissue to radiation treatment, increases tumor cell death, and significantly improves survival times [4]. However, vascular disrupting agents generally only cause the central regions of the tumor to become necrotic, leaving viable cells on the periphery that can regrow as a more aggressive tumor [4,5].
Disruption of the tumor vasculature has also proven to be possible using cavitation of ultrasound contrast agents, which are gas filled bubbles with diameters ranging from 1 to 8 µm [6]. Microbubble cavitation can induce increased vascular and cellular permeability and has been shown to decrease tumor vascularization through endothelial cell apoptosis [7]. This endothelial lining disruption has been shown to encompass the entire tumor vasculature, rather than just the central region of vasculature that is damaged with other known tumor vascular disrupting agents [4,5]. The tumor vasculature is incomplete and lacks neuromuscular elements compared to vasculature in normal tissue [5,7]. Therefore, damaging the endothelial lining of the vasculature using ultrasound-triggered microbubble destruction (UTMD) is limited mainly to the tumor vasculature, with little effect on normal tissue [5,7]. UTMD has been shown to increase vascular perfusion in normal tissue due to cavitation-related increases in shear forces, activation of endothelial nitric oxide synthase and increased production of nitric oxide, a potent vasodilator [8].
A variety of commercially available and clinically approved ultrasound contrast agents have been used in preclinical tumor models to alter tumor vascularity. In one study, Optison (GE Healthcare, Princeton, NJ) was destroyed by ultrasound pulses in a murine melanoma model, with treated tumors exhibiting a significant increase in avascular area after treatment [9]. Other studies in breast, bladder, and prostate subcutaneous tumor models have demonstrated synergistic effects of combining UTMD with radiation therapy [7,10–13]. Both mechanistic and parameter optimization studies have been performed in these models to sufficiently sensitize tumors to radiation therapy using UTMD.
While studies to date using UTMD and radiation are highly encouraging, this sensitization has yet to be shown in an HCC model. Additionally, because of the complex environment within the liver tissue, establishing the efficacy of UTMD and radiation treatment in a xenograft model may not translate to significant clinical results [14]. Therefore, this study explores the effects of combining microbubble destruction with radiation in a human HCC orthotopic tumor implanted into the right lobe of the liver of a nude rat to determine whether this would be an effective method to selectively cause tumor necrosis and improve survival outcomes without harming healthy liver tissue.
2. Materials and Methods
2.1 Cell line and reagents
The Human HCC cell line Hu7.5 was purchased from Apath (NY, USA) and maintained in DMEM (Thermo Fisher Scientific, Waltham, MA). Culture medium was supplemented with 10% Fetal Bovine Serum (FBS) (Thermo Fisher), 1% penicillin streptomycin, and 1% Non-Essential amino acid mixture (Thermo Fisher). Cell lines were cultured in a humidified 37°C incubator with 5% CO2.
2.2 Implantation and Tumor Growth
The animal protocol was approved by IACUC following NIH guidelines for animal care and experiments. Hepatocellular carcinoma orthotopic tumors were grown in 18 immunodeficient, nude rats (Taconic Labs) by injecting 1 × 106 Hu7.5 HCC cells in 100µL matrigel (ThermoFisher) into the right lobe of each animal’s liver under ultrasound guidance. Following inoculation, the right liver lobe of each animal was imaged twice per week using a high frequency ultrasound scanner with 32 MHz (Vevo 2100, VisualSonics, Toronto, Canada) to monitor tumor growth. During inoculation and all imaging, the animals were anesthetized with 1.0–1.5% isoflurane and oxygen.
2.3 Validation of Acoustic Parameters
Acoustic transmit parameters of the destructive pulses were quantified in a water bath using a 0.5 mm needle hydrophone (Precision Acoustics Ltd, Dorset, United Kingdom) calibrated by The National Physics Laboratory (Middlesex, United Kingdom) in April 2016 and placed at the beam focus using an x-y-z positioning system. When imaging at a depth of 3 cm and focal distance of 1 cm (limits of the scanner and parameters used for in vivo experiments) transmit parameters were found to be 4.2 MHz 1.6 µs pulses transmitted at a derated peak negative pressure of 2.5 MPa at a pulse repetition frequency of approximately 38 Hz.
2.4 Tumor Treatment and Response Evaluation
Once the tumors reached a diameter greater than 5 mm, the animals were randomized into one of three groups receiving either microbubble cavitation (UTMD) alone (0.1 mL Optison, GE Healthcare), radiation alone (5 Gy), or microbubble cavitation (UTMD) 3 hr prior to radiotherapy. Clinically, detection of HCC is limited to nodules larger than 1 cm [15]. However, because the HCC tumors in our study were grown in a significantly smaller rat model, treatment was initiated once they reached a size of 5 mm (rather than 1 cm). Prior to any treatment, tumor volume and vascularity were quantified using the Vevo 2100 and 3D stepper motor (VisualSonics). The animals in both UTMD groups received a gradual 0.1 mL injection of Optison followed by 0.3 mL saline flush over a 10–20 sec period through a 24 G angiocatheter placed in the tail vein.
After confirmation of contrast-enhancement within the mass, a series of 4 sec destructive pulses (Mechanical Index (MI) = 1.35) were generated using a Siemens S3000 scanner with 9L4 probe (Siemens Healthineers, Mountain View, CA) to cavitate microbubbles within the selected region followed by 10 sec of nonlinear imaging at a lower intensity (Cadence Pulse Sequencing, MI = 0.06) between destructive pulses to allow and measure microbubble reperfusion through the vasculature. The imaging plane was maintained at the midline of the tumor for four destructive pulses and then swept through the tumor for the remainder of UTMD. Treatment with UTMD lasted two to three minutes in each animal, until microbubble enhancement was no longer observed in the hepatic vasculature.
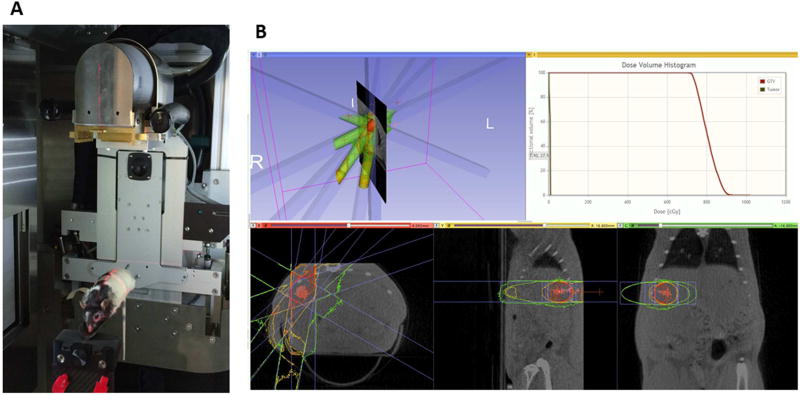
Immediately following UTMD, tumors were marked with a 2 mm metal wire (made from a segment of a 25 G spinal needle stylus) which was introduced through a 23 G spinal needle under ultrasound guidance. The groups receiving radiotherapy were given a single 5 Gy dose of radiation after being anesthetized with a combination of ketamine and xylazine (3 hr after microbubble cavitation for the group receiving both UTMD and radiation) using Thomas Jefferson University’s Small Animal Radiation Research Platform (SARRP) core facility. This unit (Xstrahl, Camberley, UK) enables full treatment planning to solid, orthotopic tumors and prevents systemic toxicity as it uses 3D conformal radiotherapy with cone beam CT guidance as shown in Figure 1. Tumors were irradiated using 4 confocal beams fractionated at 1.25 Gy per approach at a dose rate of 245 cGy/min.
Figure 1.
Small Animal Radiation Research Platform (SARRP) with animal on platform stage (A), selected region of interest and treatment planning for 5 Gy irradiation (B).
Tumor response to treatment was evaluated by monitoring tumor vascularity and tumor growth twice weekly using ultrasound with the Vevo 2100 and 32 MHz probe until the mass reached a size greater than 1.5 cm or until the animal showed a 20% loss in body weight (IACUC sacrifice criteria). Tumor volumes were calculated for each time point using 3D B-mode imaging, while fractional vascularity before, during, and after UTMD was computed using 3D power Doppler imaging.
2.5 Photoacoustic Imaging
Two rats received photoacoustic imaging of both the tumor and normal liver tissue immediately prior to and 3 hr post UTMD using a Vevo 2100 LAZR system with LZ250 Probe (VisualSonics, Toronto, Canada). Briefly, this technique transmits light at a specific wavelength through the tissue, which is then absorbed by tissue chromophores like hemoglobin, causing thermo-elastic expansion and generation of a pressure wave that was detected by the ultrasound transducer. In regards to tissue oxygenation, hemoglobin absorption spectra changes in relation to its oxygenation state, and the ratio of photoacoustic signal generated at different specific wavelengths can be used to quantify oxygen saturation (%SO2) within the tissues [16,17].
2.6 Serum Collection
Prior to tumor inoculation, immediately prior to therapy, two days after treatment, and one week post treatment, 0.5 ml of blood was collected from each animal’s tail vein. Samples were allowed to clot for 30 min, then centrifuged at 5000 RPM for 5 minutes. 75–150 µL of serum was collected from each sample and stored at −18°C. Alanine transaminase (ALT) and bilirubin (liver function markers) were measured to determine the effect of treatment on liver function.
2.7 Liver Function Assays
Liver function in all animals was measured using ALT and bilirubin assays on serum samples collected immediately prior to therapy, two days after treatment, and one week post treatment. The Bilirubin Assay Kit (Sigma-Aldrich, USA) determines serum bilirubin levels using the Jendrassik-Grof method, which measures the colorimetric product of the reaction between bilirubin with diazotized sulfanilic acid. The assay was performed according to the manufacturer instructions and bilirubin concentration in each sample was determined using the following formula (where 5 mg/dL is the bilirubin concentration of the calibrator):
The Alanine Aminotransferase Activity Assay Kit (Sigma-Aldrich, USA) determines the amount of ALT present in serum using a coupled enzyme assay which results in a colorimetric product proportional to the amount of pyruvate generated. One unit of ALT is equivalent to the amount of enzyme that generates 1.0 µmole of pyruvate per minute at 37°C. All standards and serum samples were prepared according to the manufacturer instructions and were measured in duplicate. Each sample’s absorbance (A570) value was compared to the standard curve to determine the amount of pyruvate that was generated. Then the ALT activity within each sample was determined using the following equation where B is the amount of pyruvate determined using standard curve values, Tinitial is the time of the first reading in minutes, Tfinal is the time of the last reading in minutes, and V is the sample volume (mL):
2.8 Statistical Analysis
Tumor volumes for animals in each group were averaged immediately before treatment, and 2, 7, and 10 days post-treatment. Tumor volumes were normalized to the average pre-treatment volume for each group and plotted with their standard deviation. Survival was calculated from the day of treatment until the day each animal was sacrificed using Kaplan-Meier curves. Tumor vascularity in animals receiving either UTMD alone or UTMD together with radiotherapy was averaged before UTMD treatment and after 1, 2, and 3 destructive pulses. Values for each time point were normalized to the average vascularity pre-treatment and plotted as the normalized mean ± standard deviation and analyzed using the Pearson test for correlation. Statistical difference between groups was determined using one-way ANOVAs. Statistical significance between individual groups was determined using paired t-tests. All statistics were performed in GraphPad Prism (GraphPad Software, San Diego, CA) with significance determined by α ≤ 0.05.
3. Results
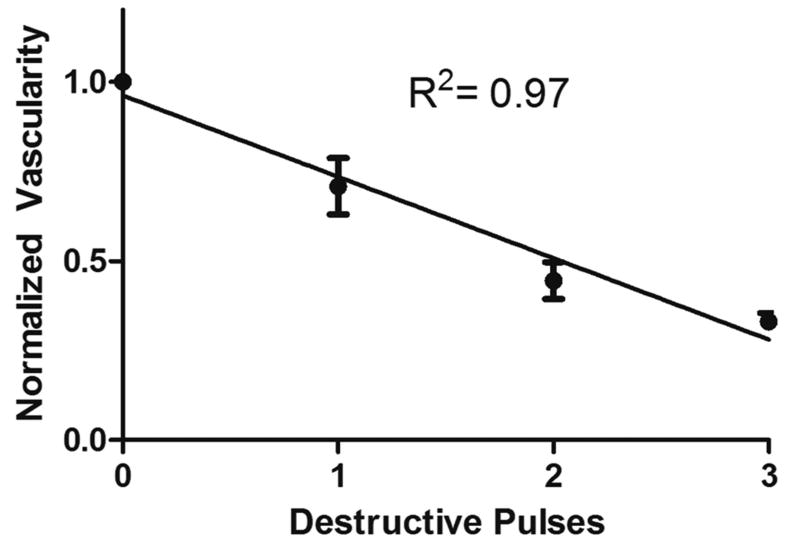
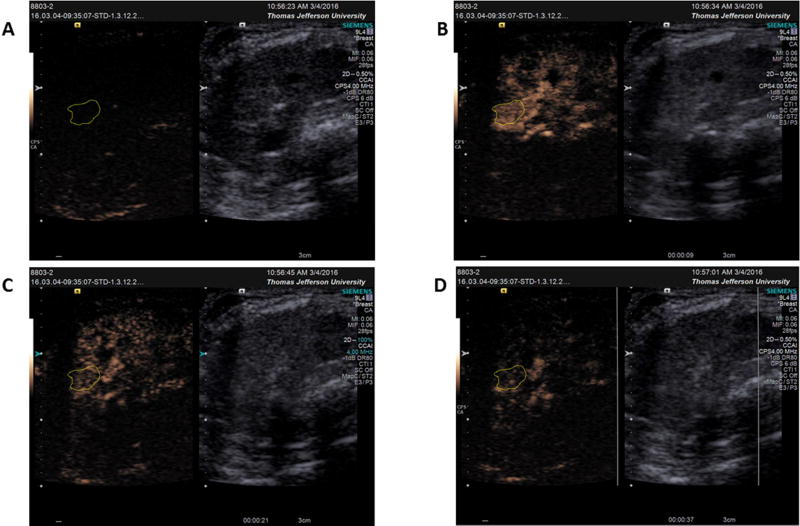
Ultrasound-triggered microbubble destruction resulted in a decrease in tumor vascularity from 100% at baseline to 71% ± 28% vascularity after one destruction sequence, 44% ± 19% after two destruction sequences, and 33% ± 8% after three destructive pulses (Fig. 2; R2 = 0.97, p = 0.016). This decrease is illustrated using contrast enhanced ultrasound (CEUS) in Figure 3A–D.
Figure 2.
Reduction in tumor vascularity (based on Optison reperfusion following destructive pulses) for animals in both UTMD groups normalized to tumor vascularity before UTMD treatment. A 67% decrease in tumor vascularity after 3 destructive pulses was observed with a significant correlation between destructive pulses and reduced vascularity (R2=0.97, p = 0.016).
Figure 3.
Example of tumor vascularity based on contrast-enhanced ultrasound A) at baseline before contrast administration B) after maximum contrast arrival but before UTMD destruction (9 sec) C) after 1 destructive pulse (21 sec) and D) after 3 destructive pulses (37 sec).
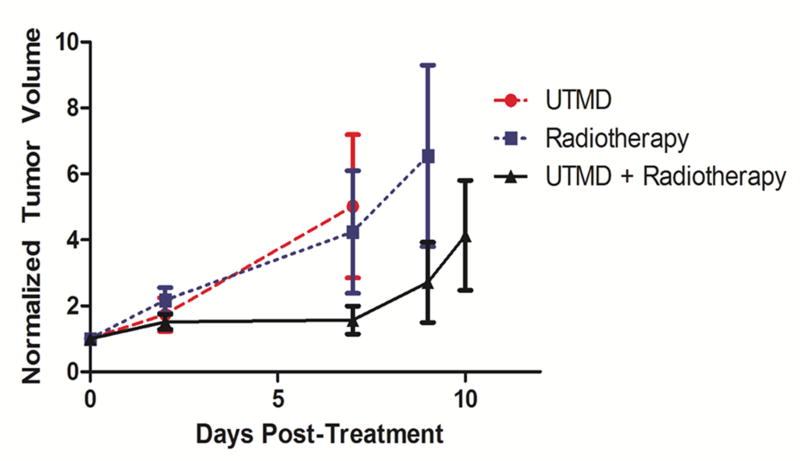
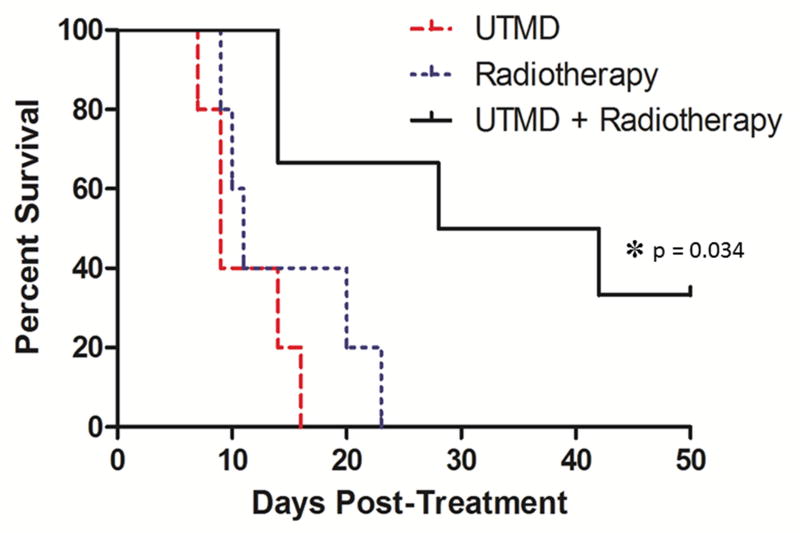
Regarding tumor control, an overall reduction of tumor growth was observed when UTMD was combined with radiotherapy relative to the control groups (Fig. 4). By day 7, the normalized volume of tumors treated by UTMD + radiation was reduced to 31% of tumors treated by UTMD alone and 59% of tumors treated by radiation alone. However, there was a great deal of variability within groups and no statistically significant difference was observed between these three groupings (p=0.61). Animal survival (sacrifice criteria listed in the methods section above) is shown in Figure 5. A significant improvement in survival was observed when radiotherapy was combined with UTMD (p=0.034). Median survival for each group was 9 d for animals treated by UTMD alone, 11 d for animals treated with radiation alone, and 35 d for animals treated with UTMD + radiation. Importantly, 2 animals receiving UTMD + radiation showed complete tumor control with tumor reduction or stability observed 50 d post treatment. There were no differences in liver function (measured using ALT and bilirubin assays) between groups receiving radiotherapy (p > 0.31).
Figure 4.
Tumor growth curves for the three treatment groups (UTMD, Radiotherapy, and UTMD + Radiotherapy) as a function of days post-treatment (Day 0 = day of treatment). The tumor volumes (mm3) recorded after treatment were normalized to the volume of the tumor before treatment. Each point on the curve represents the normalized average volume of all animals in the group. Bars represent the standard error of the mean. The difference between groups, p=0.61, difference between UTMD + radiotherapy and radiotherapy alone (p = 0.14), difference between UTMD + radiotherapy and UTMD alone (p = 0.39), and difference between UTMD alone and radiotherapy alone (p = 0.77) were not statistically significant.
Figure 5.
Survival curves for the three treatment groups (UTMD, Radiotherapy, and UTMD + Radiotherapy) with the x-axis representing days post treatment (Day 0 = day of treatment) and the y-axis representing the survival rate. A significant improvement in survival was observed when radiotherapy was combined with UTMD (p=0.034).
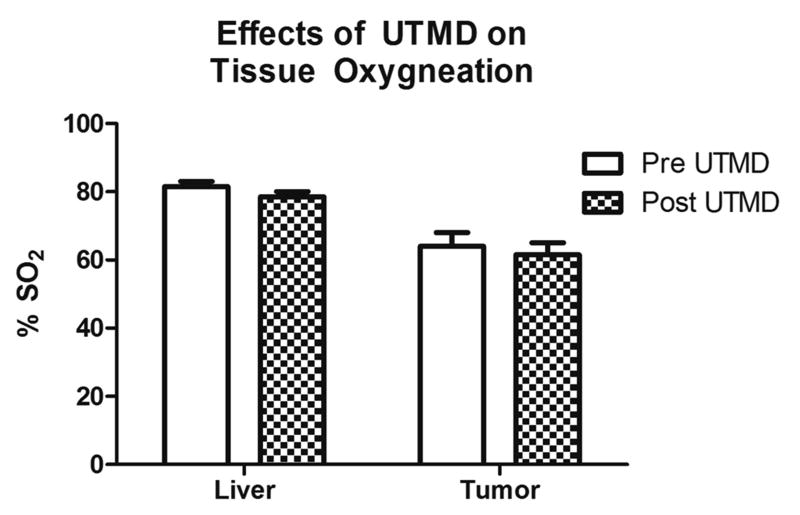
Photoacoustic imaging of each animal on the Vevo 2100 system was performed both prior to and after 3 hr treatment with UTMD in order to monitor tissue oxygenation. There were no significant differences between normal liver tissue oxygenation (p = 0.29) or tumor oxygenation levels (p = 0.68) after treatment with UTMD, showing that UTMD does not induce tumor hypoxia and therefore, should not limit the sensitivity of tumor tissue to radiation (Fig. 6).
Figure 6.
Effects of UTMD on tissue oxygenation measured using photoacoustic imaging (Vevo 2100). The differences between oxygenation levels in HCC tumor (p=0.68) and normal liver tissue (p=0.29) pre vs. post UTMD treatment were not statistically significant.
4. Discussion
The contrast agent used in our study, Optison, is a microbubble of perflutren (octofluoropropane) gas surrounded by a shell made of albumin, the most prevalent protein naturally found in human blood. It has a diameter ranging from 2 to 4.5 µm, has been used extensively in echocardiography applications, and has an exceptional safety profile [6,18]. In this study, UTMD with Optison resulted in a 67% decrease in tumor vascularity in both groups treated with UTMD (UTMD alone and UTMD + Radiotherapy). Additionally, there was a significant improvement in survival time in animals receiving combination therapy (UTMD and radiotherapy), with median survival 24 d longer than the group receiving radiation alone (a 318% improvement) and 26 d longer than the group receiving UTMD alone (a 388% improvement). Additionally, two animals showed complete response, indicating 5 Gy combined with UTMD may provide local tumor control in some cases. While there was not a statistically significant difference in tumor size between groups, the volume of tumors treated with UTMD + radiation was 31% of those treated with UTMD alone and 59% of tumors treated with radiation alone reflecting a potential sensitization of the tumor by UTMD to radiotherapy. The lack of statistical significance in this particular case is attributed to the high degree of variability in the orthotopic model and limited time for observation (with some animals requiring sacrifice as early as 7 d in the control UTMD alone group).
Interestingly, the generation of vascular disruption via UTMD was shown to not cause increased tumor hypoxia 3 hr post treatment (the time of radiation treatment). The abnormal structure of tumor vasculature and decreased perfusion often creates areas of hypoxia within the tumor microenvironment and is a significant contributing factor to radiotherapy resistance that develops within tumors [19]. The oxygen saturation both within healthy liver tissue and within the HCC tumors in each animal undergoing treatment with UTMD was measured using photoacoustics both prior to and after treatment in order to determine whether UTMD affects the tissue’s oxygenation levels, and therefore, sensitivity to radiation. As expected, tumors at baseline showed lower oxygenation saturation levels compared to the surrounding liver tissues. However, no alterations were observed 3 hr following UTMD, indicating this approach does not immediately alter tumor oxygenation (which would subsequently inhibit radiosensitivity). Photoacoustic imaging has previously been applied to UTMD radiosensitization as a means for tracking treatment response [20]. However, to our knowledge oxygenation at the time of radiotherapy application has not been previously described and our results demonstrate that the subsequent vascular disruption 3 hr post UTMD apparently does not increase tumor hypoxia.
The lack of differences in liver function assays between treatment groups demonstrates the safety of microbubble-based antivascular therapy prior to radiotherapy for the treatment of HCC. Liver function was evaluated through ALT and bilirubin assays run on serum samples collected prior to therapy, 2 d, and 7 d post therapy from animals in each group (UTMD, radiation, and UTMD + radiation). Bilirubin is a product of hemoglobin degradation which occurs within liver tissue, with increased levels associated with liver disease (normal range: 0–1 mg/dL). ALT is an enzyme found almost exclusively within hepatocytes and is another marker used to detect liver dysfunction. Increased levels within the blood (normal range: 10–55 U/L) indicates damage to, or necrosis of, hepatocytes specifically [21]. ALT and bilirubin levels within serum samples for each group fell within normal limits (0.31 – 0.93 mg/dL bilirubin and 13.4–32.5 U/L ALT), and there were no significant differences in measurement levels between groups (p > 0.31). This indicates an improvement in therapeutic gain in that the therapeutic efficacy of UTMD and radiotherapy in combination is selective for HCC tumor tissue and spares normal liver tissue.
Our results demonstrate that ultrasound triggered microbubble destruction prior to radiotherapy sensitizes tumors to treatment, improving tumor responses and survival rates in a HCC orthotopic tumor model without compromising liver function. To our knowledge this is the first work to demonstrate an improvement in therapeutic gain in HCC and the first to validate its influence in an orthotopic model. Future work will explore the use of UTMD as an adjunct in patients undergoing catheter-based Y90 radioembolization for unresectable HCC.
Highlights.
Microbubble cavitation results in a reduction in HCC tumor vascularity
UTMD before radiation therapy improves tumor control in an orthotopic model of HCC
UTMD showed no effects on overall liver function tests compared to control groups
Vascular disruption does not increase tumor hypoxia within the treatment window
Acknowledgments
This work was supported in part by the National Institutes of Health R21 CA190926 and S10 OD018171. Equipment was provided by Siemens Healthineers (Mountview, CA). We thank Susan Shamimi-Noori in the Radiology Department at Thomas Jefferson University, Voichita Bar-Ad in the Radiation Oncology Department, and Jan Hoek and Anil Noronha in the Pathology, Anatomy and Cell Biology Department at Thomas Jefferson University for their expert assistance. We also thank Dr. Terri Swanson from Pfizer for helpful discussions on development of an appropriate HCC tumor model.
Abbreviations
- CEUS
Contrast Enhanced Ultrasound
- HCC
Hepatocellular Carcinoma
- UTMD
Ultrasound Triggered Microbubble Destruction
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.El-Serag HB. Hepatocellular Carcinoma. NEJM. 2011;365(12):1118–27. doi: 10.1056/NEJMra100168. [DOI] [PubMed] [Google Scholar]
- 2.Mittal S, El-Serag HB. Epidemiology of HCC: Consider the Population. Journal of clinical gastroenterology. 2013;47(0):S2–S6. doi: 10.1097/MCG.0b013e3182872f29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andreana L, Isgrò G, Marelli L, Davies N, Yu D, Navalkissoor S, Burroughs AK. Treatment of hepatocellular carcinoma (HCC) by intra-arterial infusion of radio-emitter compounds: Transarterial radio-embolisation of HCC. Cancer Treatment Reviews. 2012;38:641–649. doi: 10.1016/j.ctrv.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 4.Hinnen P, Eskens FALM. Vascular disrupting agents in clinical development. British Journal of Cancer. 2007;96(8):1159–1165. doi: 10.1038/sj.bjc.6603694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wood AKW, Schultz SM, Lee W, Bunte RM, Sehgal CM. Antivascular Ultrasound Therapy lengthens survival of mice with implanted melanomas. Ultrasound Med Biol. 2010;36(5):853–857. doi: 10.1016/j.ultrasmedbio.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldberg BB, Raichlen JS, Forsberg F. Ultrasound Contrast Agents: Basic Principles and Clinical Applications. 2. Martin Dunitz Ltd; London: 2001. [Google Scholar]
- 7.Czarnota GJ, Karshafian R, Burns PN, Wong S, Al-Mahrouki A, Lee JW, Caissie A, Tran W, Kim C, Furukawa M, Wong E, Giles A. Tumor radiation response enhancement by acoustical stimulation of the vasculature. PNAS. 2012;109(30):E2033–E2041. doi: 10.1073/pnas.1200053109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belcik JT, Hott BH, Xie A, Zhao Y, Kim S, Lindner NJ, Ammi A, Linden JM, Lindner JR. Augmentation of limb perfusion and reversal of tissue ischemia produced by ultrasound-mediated microbubble cavitation. Circ Cardiovasc Imaging. 2015;8(4):e002979. doi: 10.1161/CIRCIMAGING.114.002979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wood AKW, Ansaloni S, Ziemer LS, Lee WM-F, Feldman MD, Sehgal CM. The Antivascular Action of Physiotherapy Ultrasound on Murine Tumors. Ultrasound in medicine & biology. 2005;31(10):1403–1410. doi: 10.1016/j.ultrasmedbio.2005.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lai P, Tarapacki C, Tran WT, Ahmed EK, Lee J, Hupple C, Iradji S, Giles A, Al-Mahrouki A, Czarnota GJ. Breast tumor response to ultrasound mediated excitation of microbubbles and radiation therapy in vivo. Oncoscience. 2016;3(3–4):98–108. doi: 10.18632/oncoscience.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Mahrouki AA, Wong E, Czarnota GJ. Ultrasound-stimulated microbubble enhancement of radiation treatments: endothelial cell function and mechanism. Oncoscience. 2015;2(12):944–957. doi: 10.18632/oncoscience.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim HC, Al-Mahrouki A, Gorjizadeh A, Sadeghi-Naini A, Karshafian R, Czarnota GJ. Quantitative Ultrasound Characterization of Tumor Cell Death: Ultrasound-Stimulated Microbubbles for Radiation Enhancement. Chen X, ed. PLoS ONE. 2014;9(7):e102343. doi: 10.1371/journal.pone.0102343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al-Mahrouki AA, Iradji S, Tran WT, Czarnota GJ. Cellular characterization of ultrasound-stimulated microbubble radiation enhancement in a prostate cancer xenograft model. Disease Models & Mechanisms. 2014;7(3):363–372. doi: 10.1242/dmm.012922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bagi CM, Andresen CJ. Models of Hepatocellular Carcinoma and Biomarker Strategy. Cancers. 2010;2(3):1441–1452. doi: 10.3390/cancers2031441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hennedige T, Kundapur Venkatesh S. Imaging of hepatocellular carcinoma: diagnosis, staging and treatment monitoring. Cancer Imaging. 2012;12(3):530–547. doi: 10.1102/1470-7330.2012.0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eisenbrey JR, Merton DA, Marshall A, LiU JB, Fox TB, Sridharan A, Forsberg F. Comparison of photoacoustically derived hemoglobin and oxygenation measurements with contrast-enhanced ultrasound estimated vascularity and immunohistochemical staining in a breast cancer model. Ultrason Imaging. 2015;37(1):42–52. doi: 10.1177/0161734614527435. [DOI] [PubMed] [Google Scholar]
- 17.Rich LJ, Seshadri M. Photoacoustic monitoring of tumor and normal tissue response to radiation. Scientific Reports. 2016;6:21237. doi: 10.1038/srep21237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller AP, Nanda NC. Contrast echocardiography: new agents. Ultrasound Med Biol. 2004;30(4):425–434. doi: 10.1016/j.ultrasmedbio.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 19.Barker HE, Paget JTE, Khan AA, Harrington KJ. The Tumour Microenvironment after Radiotherapy: Mechanisms of Resistance and Recurrence. Nature reviews Cancer. 2015;15(7):409–425. doi: 10.1038/nrc3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Briggs K, Al Mahrouki A, Nofiele J, El-Falou A, Stanisz M, Kim HC, Kolios MC, Czarnota GJ. Non-invasive Monitoring of Ultrasound-Stimulated Microbubble Radiation Enhancement Using Photoacoustic Imaging. Technology in Cancer Research & Treatment. 2014;13(5):435–444. doi: 10.7785/tcrtexpress.2013.600266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giannini EG, Testa R, Savarino V. Liver enzyme alteration: a guide for clinicians. CMAJ: Canadian Medical Association Journal. 2005;172(3):367–379. doi: 10.1503/cmaj.1040752. [DOI] [PMC free article] [PubMed] [Google Scholar]