Abstract
Purpose
The National Comprehensive Cancer Network (NCCN) has instituted treatment guidelines for stage IIA and stage IIB/III extremity and superficial trunk soft tissue sarcomas (ETSTS). We examined adherence to NCCN guidelines and factors associated with nonadherent treatment and survival outcomes.
Methods
Patients with stage IIA and IIB/III ETSTS (n = 15,957) were categorized as adherent or nonadherent treatment groups based on 2014 NCCN guidelines. Multivariate logistic regression models were used to determine factors associated with nonadherent treatment. Overall survival (OS) and disease-specific survival (DSS) were calculated and Cox models were used to generate adjusted survival curves and hazard ratios (HR).
Results
We found that 87.2% of patients with stage IIA disease and 58.3% of patients with stage IIB/III disease received adherent treatment. Community treatment facilities and uninsured or unknown insurance status were associated with nonadherent treatment for both stage groups. Adherent treatment was associated with higher 5-year adjusted OS and DSS in stage IIA and IIB/III patients. In Cox models, nonadherent treatment was associated with worse survival both for stage IIA (HR = 2.31, 95% confidence interval [CI] = 2.02-2.63) and stage IIB/III disease (HR = 1.63, 95% CI = 1.53-1.73). Increasing age and non-private insurance were associated with poorer outcomes. For stage IIB/III, treatment at a community center and African American race were associated with worse survival.
Conclusions
Adherence to NCCN guidelines is excellent for stage IIA and poor for stage IIB/III ETSTS. Adherent treatment was associated with improved survival outcomes, highlighting the importance of adherence to NCCN guidelines.
Keywords: sarcoma, treatment adherence, NCCN, survival, outcomes
Introduction
Consensus-based treatment guidelines were developed by the National Comprehensive Cancer Network (NCCN) to provide guidance for delivering high-quality, standardized treatment for extremity and superficial trunk soft tissue sarcoma (ETSTS).1 Although the guidelines appear ambiguous regarding the sequence and necessity of all therapeutic modalities, there remains a minimum threshold of treatment for patients with high-grade tumors ≤5cm (stage IIA) or high-grade tumors >5cm (stage IIB/III).2 According to NCCN guidelines, adherent treatment for stage IIA disease is either R0 (microscopically negative) resection alone or surgical resection plus radiation therapy (RT). Adherent treatment for patients with stage IIB/III disease is defined as surgical resection and RT, with or without chemotherapy.
Previous studies evaluating RT utilization in stage II/III extremity sarcoma demonstrated wide variability in administration, based on geographic region, age and sex.3-7 A recent study by Sherman et al8 found that 65% of patients with stage III disease were treated with surgery and RT, indicating a discrepancy between consensus recommendations and RT utilization. However, treatment for stage II and III disease was not stratified by stage IIA and IIB/III, as in the NCCN guidelines, making it difficult to ascertain the true percentage of patients receiving guideline-adherent therapy and, by extension, what risk factors are associated with nonadherent treatment.
Recently, the association between survival and adherence to NCCN guidelines has been demonstrated in multiple cancer types, including colon,9 esophageal,10 and ovarian cancer.11 However, the impact of adherence to NCCN guidelines in patients with ETSTS is unknown.
In the current study, we assessed treatment adherence to NCCN guidelines and risk factors for receiving nonadherent treatment in patients with stage IIA and IIB/III ETSTS to assess treatment quality nationally, using data from National Cancer Database (NCDB). In addition, we evaluated the impact of adherence to NCCN guidelines on stage-specific survival outcomes. We hypothesized that patients receiving guideline-adherent treatment would have higher 5-year OS and a lower risk of death than those receiving nonadherent treatment.
Materials and Methods
Data Source
The NCDB is a prospective, hospital-based cancer registry sponsored by the American College of Surgeons and the American Cancer Society.12 It captures approximately 70% of all new cancer cases in the United States and includes clinicopathologic, treatment, and outcome variables.13 As the data are de-identified, this study was considered exempt by The University of Texas MD Anderson Cancer Center Institutional Review Board.
Analytic cohort
Patients diagnosed with ETSTS (2003-2011) were identified from the NCDB Sarcoma Participant Use File (n = 99,876) using International Classification of Diseases for Oncology (3rd ed.) topography codes C471, C472, C476, C491, C492, and C496 (Supp. Table 1). All histologic subtypes included in the data were individually vetted to exclude non-sarcomatous or mixed histologies. The following subgroups were also excluded: pediatric patients, central nervous system tumors, osteosarcomas, patients not treated at the reporting hospital, stage I and IV disease, and those with incomplete information (Supp. Fig. 1). The remaining patients were grouped into stage IIA and IIB/III, according to the American Joint Committee on Cancer (7th ed) soft tissue sarcoma staging guidelines.2 The definition of adherence was based on NCCN treatment recommendations for ETSTS.1 “Adherent,” patients with stage IIA disease must have received (1) margin-negative (R0) surgery alone or (2) surgery with any margin status plus radiation therapy (either preoperatively or postoperatively). Patients with stage IIB/III disease must have undergone surgical resection and radiation therapy, with or without chemotherapy (Supp. Fig 2).
Variables
In addition to demographic and clinical data provided in the NCDB, Charlson comorbidity scores, insurance status, geographic region of the treatment facility, travel distance from the patient's ZIP code to the treatment facility, and type of treatment facility (academic, comprehensive, or community) were examined. Insurance status was categorized as private, government plan (Medicare, Medicaid, or other government policy), or uninsured/unknown. Additionally, treatment facility location was re-categorized as Northeast, Midwest, South, and West from the nine regional designations used in NCDB.
Statistical analyses
A multivariable logistic regression model was constructed to determine independent risk factors associated with receiving nonadherent treatment. Stepwise selection was used for model building. Covariates deemed essential to clinical outcomes, such as age and gender, were forced into the model. Odds ratios and 95% confidence intervals (CIs) were calculated, and forest plots generated.
Survival analyses for adherent vs. nonadherent treatment groups were conducted using three distinct statistical methods. The Kaplan-Meier estimator was used to calculate unadjusted OS curves. Disease-specific survival was estimated using relative survival, as has been previously described.9,14 In brief, relative survival was determined using the Kaplan-Meier method by calculating the ratio of observed survival in the analytic cohort to the expected survival of a comparable sex- and age-matched population, obtained from the Human Mortality Database.15 Adjusted survival curves were also estimated from Cox regression models. The models were used to estimate hazard ratios (HRs) and 95% CIs for factors associated with increased risk of death. These three distinct statistical techniques provided a range of outcomes (similar to a sensitivity analysis) to confirm the robustness of the survival estimates for adherent and nonadherent groups. P < 0.05 was considered statistically significant. SAS version 9.4 was used to conduct all analyses.
Results
Patient characteristics
In the final analytic cohort, 5,734 patients had stage IIA and 10,223 had stage IIB/III disease. Demographic information as well as disease and treatment information are summarized in Supp. Table 2. Among patients with stage IIA disease, 97.2% underwent surgery, and 91.3% of patients with stage IIB/III disease underwent surgery. Few patients with stage III disease had positive nodal disease (2.1%), and 8% underwent amputation.
Adherent and nonadherent treatment
Among those with stage IIA disease, 5,002 patients (87.2%) received adherent treatment (Supp. Fig. 2). Most patients receiving nonadherent treatment underwent inadequate surgical treatment.
Only 58.3% (n = 5,960) of stage IIB/III patients received the appropriate regimen. Nearly two-thirds of all nonadherent-treatment patients with stage IIB/III disease had surgery alone. Few patients in the nonadherent treatment group received no treatment (2.4%).
Factors associated with nonadherent treatment
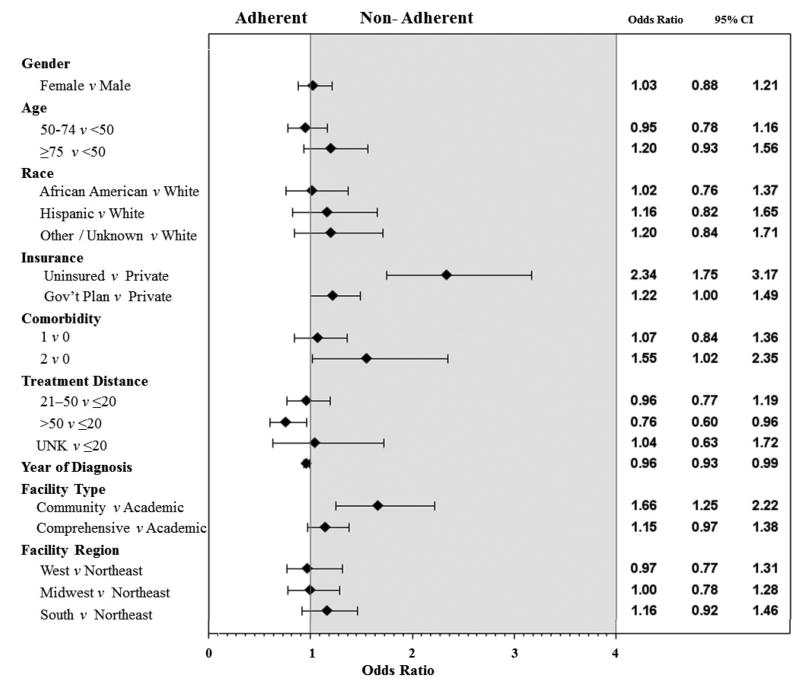
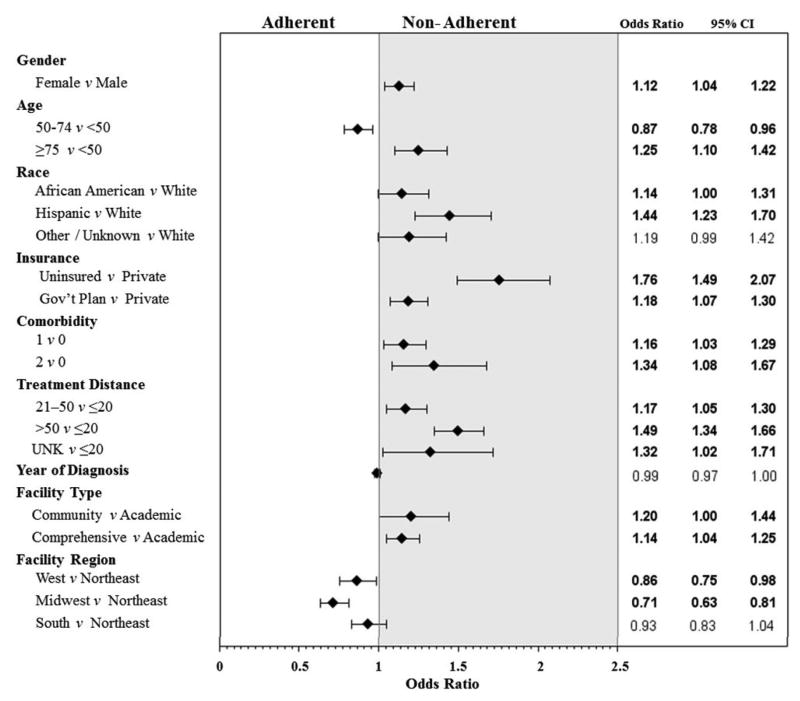
A logistic regression model was used to investigate factors associated with receiving nonadherent treatment (Figure 1). In multivariate analysis, a Charlson score ≥2 and uninsured/unknown insurance status were significantly associated with nonadherent treatment for both stage groups (P < 0.05). For stage IIA, patients treated at a community facility were less likely to receive adherent treatment (Figure 1A), and for stage IIB/III, patients treated at comprehensive cancer centers were less likely to receive adherent treatment compared to those treated at an academic facility (Figure 1B).
Figure 1.
Forest plots of factors associated with nonadherent treatment for A) stage IIA and B) stage IIB/III extremity and superficial trunk soft tissue sarcoma.
Patients with stage IIA disease who lived >50 miles from the treatment facility were more likely to receive adherent treatment, whereas for patients with stage IIB/III disease, those who lived farther away (>20 miles) were more likely to receive nonadherent treatment. Female patients, Hispanic patients, and those with government insurance or a Charlson comorbidity score >0 who had stage IIB/III disease were also more likely to receive nonadherent treatment.
Survival analyses
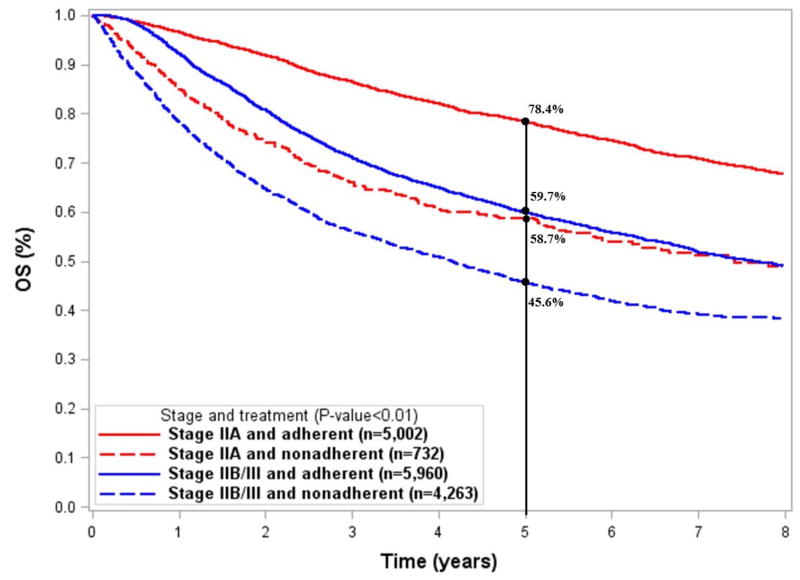
The median follow-up time for the entire cohort was 3.18 years. The 5-year unadjusted OS for all patients was 61.7%. Patients receiving adherent treatment had higher survival, regardless of stage (P < 0.01; Figure 2A). Adherence to NCCN guidelines was associated with 19.7% improvement in 5-year OS for stage IIA (78.4% vs.58.7%) and 14.1% improvement in 5-year OS (59.7% vs. 45.6%) for stage IIB/III (P < 0.01).
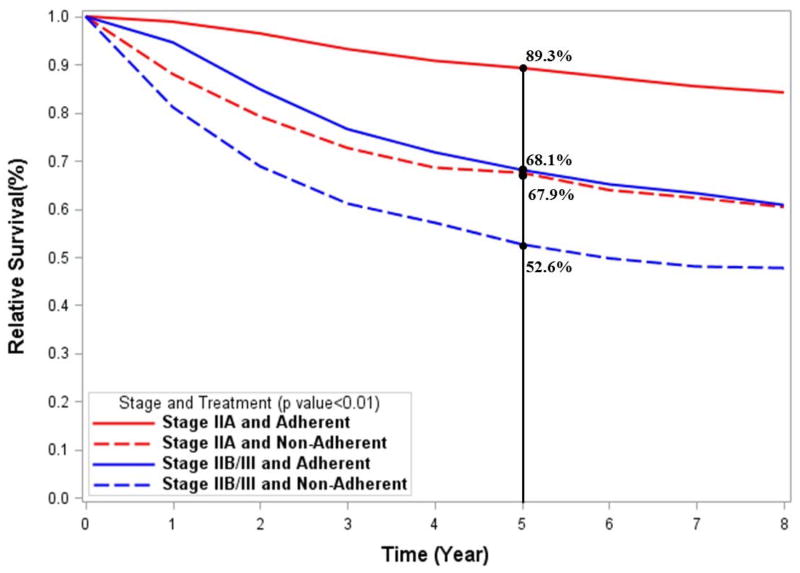
Figure 2.
Kaplan Meier curves showing the unadjusted survival by stage and treatment group for A) overall survival and B) disease-specific survival analyses.
The relative survival curves (representative of disease-specific survival) are shown in Fig. 2B. Similar to unadjusted OS, the 5-year relative survival was lower (P < 0.01) for patients receiving nonadherent treatment for both stage IIA (89.3% vs. 67.9%) and stage IIB/III(68.1% vs. 52.6%).
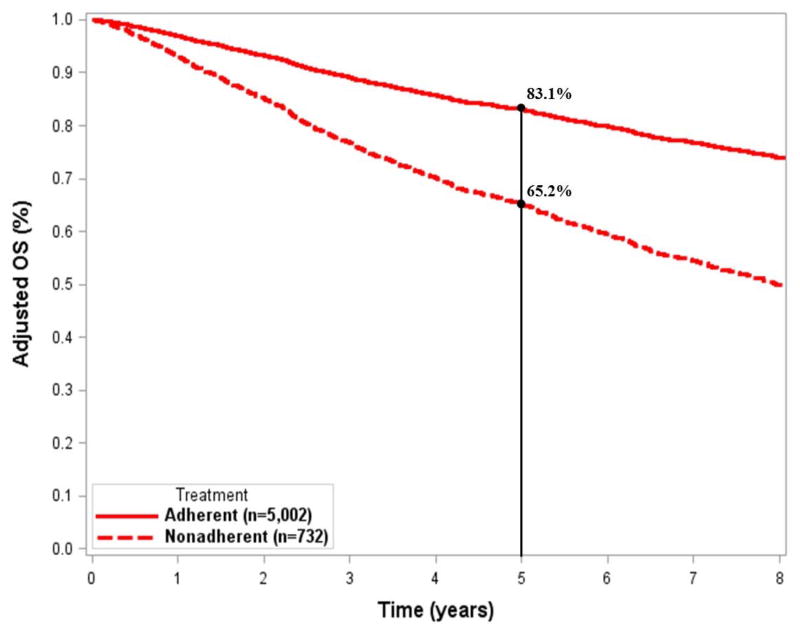
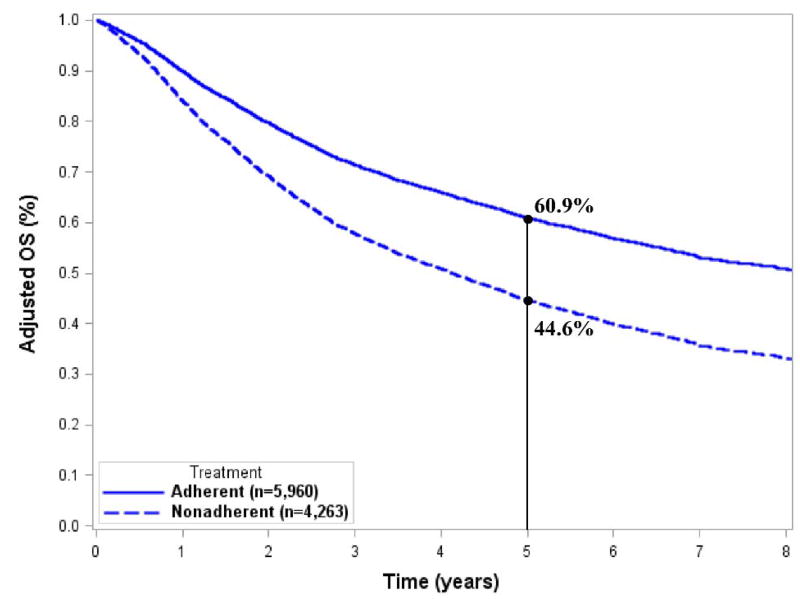
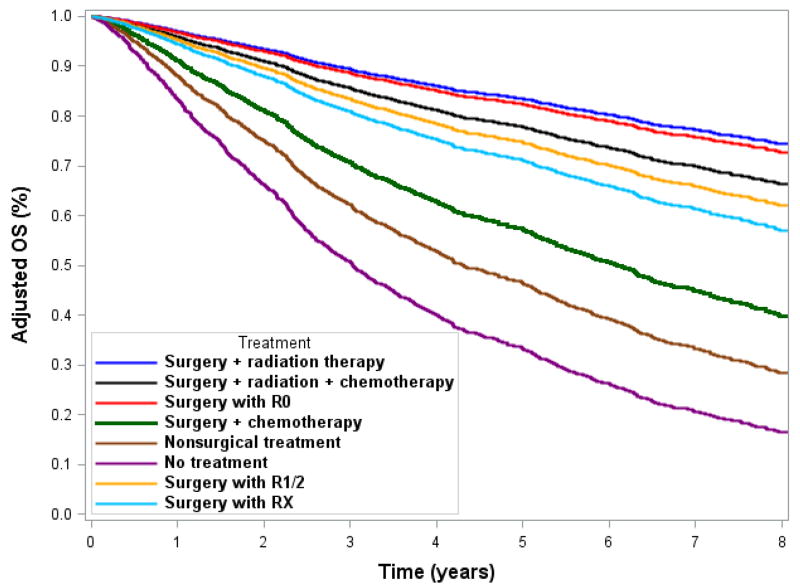
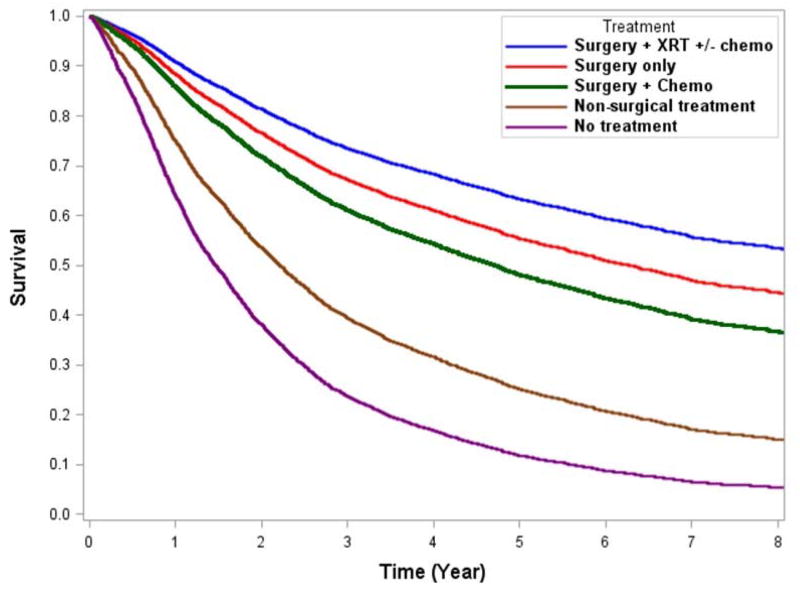
After adjusting for significant covariates, we found that for adherent treatment patients, the 5-year OS was 17.9% better for stage IIA disease (83.1% vs. 65.2%) and 16.3% better for stage IIB/III disease (60.9% vs. 44.6%) (Figs. 3A and 3B). The adjusted OS curves stratified by individual treatment regimens are shown in Fig. 4. Adherent regimens were associated with improved survival.
Figure 3.
Adjusted survival curves for A) stage IIA and B) stage IIB/III extremity and superficial trunk sarcomas.
Figure 4.
Adjusted survival curves of individual treatment regimens for A) stage IIA and B) stage IIB/III extremity and trunk soft tissue sarcoma.
Adjusted HRs for mortality are shown in Table 1. In stage IIA disease, a two-fold increased mortality risk (HR = 2.31[2.02-2.63]) was found for those receiving nonadherent treatment. In stage IIB/III disease, we found a 63% increased mortality risk (HR = 1.63[1.53-1.73]) for those receiving nonadherent treatment. For both stage groups, non-private insurance was associated with significantly worse survival outcomes compared with private insurance.
Table 1.
Cox model showing hazard ratios (HR) and 95% confidence intervals (CI) for risk of death in the period studied for our cohort of patients with extremity and superficial trunk soft tissue sarcoma (n = 15,957).
| Characteristic | Stage IIA* | Stage IIB/III* | ||||
|---|---|---|---|---|---|---|
| n = 5,734 | n = 10,223 | |||||
| HR | 95% CI | P | HR | 95% CI | P | |
| Treatment (ref. adherent) | <0.01 | <0.01 | ||||
| Nonadherent | 2.31 | 2.02-2.63 | 1.63 | 1.53-1.73 | ||
| Gender (ref. male) | 0.11 | <0.01 | ||||
| Female | 0.91 | 0.82-1.02 | 0.83 | 0.78-0.88 | ||
| Age at Diagnosis (ref. <50 years) | <0.01 | <0.01 | ||||
| 50-74 years | 1.56 | 1.31-1.85 | 1.39 | 1.27-1.52 | ||
| ≥75 years | 3.43 | 2.81-4.18 | 2.5 | 2.25-2.77 | ||
| Race (ref. white) | 0.64 | <0.01 | ||||
| African American | 0.95 | 0.77-1.18 | 1.29 | 1.16-1.42 | ||
| Hispanic | 0.97 | 0.92-1.30 | 0.86 | 0.75-0.99 | ||
| Other/unknown | 0.84 | 0.63-1.11 | 0.91 | 0.79-1.05 | ||
| Charlson Comorbidity Score (ref. 0) | <0.01 | <0.01 | ||||
| 1 | 1.24 | 1.06-1.44 | 1.29 | 1.20-1.40 | ||
| 2+ | 2.49 | 1.97-3.16 | 1.62 | 1.41-1.86 | ||
| Insurance (ref. private) | <0.01 | <0.01 | ||||
| Uninsured/unknown | 1.55 | 1.19-2.00 | 1.27 | 1.11-1.46 | ||
| Government plan | 1.64 | 1.42-1.90 | 1.48 | 1.37-1.60 | ||
| Travel Distance (ref. ≤20 miles) | 0.04 | <0.01 | ||||
| 21 to 50 miles | 0.89 | 0.76-1.04 | 1.01 | 0.93-1.09 | ||
| >50 miles | 0.95 | 0.81-1.11 | 1.02 | 0.94-1.10 | ||
| Unknown | 1.46 | 1.05-2.03 | 1.74 | 1.46-2.07 | ||
| Year of Diagnosis | 0.99 | 0.96-1.01 | 0.35 | 1.01 | 0.99-1.02 | 0.12 |
| Facility Type (ref. academic) | 0.67 | <0.01 | ||||
| Community | 1.02 | 0.83-1.26 | 1.26 | 1.11-1.43 | ||
| Comprehensive | 1.07 | 0.95-1.21 | 1.05 | 0.98-1.13 | ||
| Unknown | 1.47 | 0.47-4.61 | 1.08 | 0.54-2.17 | ||
| Facility Region (ref. Northeast) | 0.12 | 0.02 | ||||
| West | 0.91 | 0.76-1.10 | 0.87 | 0.79-0.96 | ||
| Midwest | 0.99 | 0.84-1.17 | 0.97 | 0.88-1.06 | ||
| South | 1.10 | 0.93-1.29 | 0.9 | 0.83-0.98 | ||
Discussion
This analysis of treatment adherence to NCCN guidelines revealed both encouraging and worrisome patterns of treatment for ETSTS nationally. Our data showed for stage IIA soft tissue sarcoma, treatment adherence was good, with 87.2% of patients with stage IIA disease receiving adherent treatment. However, the overall treatment adherence for stage IIB/III was poor, with only 58.3% of patients receiving adherent treatment, largely because their treatment lacked RT. Patients treated at community facilities, with increasing comorbidities and uninsured were more likely to not receive adherent therapy. Treatment adherent to NCCN guidelines was associated with 18% or 16% increases in 5-year OS in patients with stage IIA or IIB/III ETSTS, respectively.
Adherence to NCCN guidelines varies widely across cancer types (e.g. 34% in pancreas cancer vs. 94% in thyroid cancer)16,17 and even within disease types depending on stage (e.g. colon cancer ranges from 36% to 96%).18 Prior studies in ETSTS reported adherence to RT for stage II or III disease ranged from about 60% to 80% in patients who underwent surgery.4-8 However, treatment adherence to RT does not fully describe adherence to NCCN guidelines. The guidelines separate stage IIA and stage IIB/III disease with distinct treatment algorithms by stage group. We found that for stage IIA disease, when margin-negative resection is considered adherent treatment, treatment adherence is much better than shown in previous reports, approaching 90%.
Several potential explanations exist for the high nonadherence rates in stage IIB/III. First, RT is not necessary in cases where amputation was performed with an adequate margin, which represented 8% of stage IIB/III patients. A second explanation is the evolution of stage definitions over the past decade. In 2010, the seventh edition of the AJCC staging system was published and substantial changes were made to soft tissue sarcoma staging. In previous editions, lymph node involvement (N1 disease) was considered stage IV disease. In the seventh edition, N1 disease was downgraded to stage III, with lymph node dissection offered as a surgical treatment for nodal disease, along with surgery and RT to the primary tumor site. Previously, node-positive disease would have been considered stage IV (thus excluded from our analysis), and the treatment algorithm would have included chemotherapy. However, only 2.1% of patients in our cohort with stage IIB/III disease were found to have node-positive disease. In summary, even if we assume that patients who underwent amputation did receive adherent treatment and exclude patients with node-positive disease, adherence would only increase to approximately 67%. Therefore, nodal status and changing stage definitions over time—although factors to consider—do not account for most cases of nonadherence in stage IIB/III ETSTS. Progression to stage IV disease is the most likely explanation for the 6.3% of patients with stage IIB/III disease who underwent nonsurgical treatment, but we were unable to validate our hypothesis with the available data. Similarly, it is possible that some patients that underwent surgical resection without RT developed distant metastases prior to the initiation of RT. However, in the randomized trial of preoperative vs. postoperative RT in patients with soft tissue sarcoma19, only 5/96 (5.2%) developed distant metastases prior to the initiation of postoperative RT, thus it is unlikely to explain the 2,722 (33%) patients with stage IIB/III disease treated with surgery alone or surgery + chemotherapy. It may not be possible to radically decrease the number of patients with stage IIB/III disease with poor tumor biology who progress to develop nonoperative disease, but it should be feasible to substantially increase the number of patients with stage IIB/III who receive neoadjuvant or adjuvant RT along with surgery. Our data suggest that if all patients with stage IIB/III disease who undergo surgery were to receive RT, the treatment adherence would rise from 58% to 91.3%—approximating the adherence for stage IIA.
Currently, there are no data examining the impact of adherence to NCCN guideline-recommended therapy on survival. The use of RT along with margin-negative surgical resection is well established as the standard of care.20,21 However, the impact of surgery and RT on survival outcomes has been largely debated.20,21 In the current study, treatment of stage IIB/III disease with RT was associated with ∼12% improvement in 5-year OS. At least four studies analyzing SEER data have reported an OS benefit in patients with high-grade or advanced-stage sarcoma receiving RT.3,6,7,22 An additional report from the NCDB supports the link between RT and survival.5 Internationally, registry data from 922 patients in Denmark also showed that RT was associated with ∼5% improved disease-specific survival.23 If all stage IIB/III patients in our study were to have received appropriate surgical resection and RT, extrapolation of our results suggests that the 5-year OS would have increased from about 45% to around 60%.
Independent of adherence to NCCN guideline recommended treatment, for patients with stage IIB/III disease, treatment at community facilities were associated with a 26% increased risk of death compared with academic facilities. More research is needed to determine which factors are affecting treatment adherence and subsequent mortality for these patients.24Although the uninsured group was small, we did note a statistically significantly increased risk of death, which is consistent with previous studies.5
The strengths of the current study include the large sample size with detailed pathologic data available to stratify patients according into sarcoma-specific substages for which treatment recommendations are defined. Patients who did not receive surgery were also included for comparison.
This is a retrospective observational, database study which has some inherent limitations. EXTSARC-4 of the NCCN guidelines allow for non-surgical treatment in cases where surgical resection would lead to adverse functional outcomes.1 Potentially, then, some of those who were classified as nonadherent actually received adherent treatment, if the intent of the non-surgical therapy was to preserve functional outcome. However, for stage IIA, only 1.7% of patients and for stage IIB/III only 6.3% received non-surgical treatment. Therefore, the number of patients utilizing non-surgical treatment is relatively small and would not explain the large deficit in adherence seen for stage IIB/III. Additionally, registry data are subject to error during the abstraction and coding process. Other unmeasured confounders may remain that are not included in the model, leading to residual confounding.25 Another potential limitation is that only the principal surgical event is coded into the NCDB. Therefore, those with positive margins during the primary surgery may have had re-excisions which were not included in the database. As a result, there may be some misclassification with respect to margin status. Very few patients were uninsured in the analytic cohort, and they may not be representative of the 16% of adults <65 years who remain uninsured nationally.26 Similarly, minorities were proportionally less than the general US population.
Conclusion
There is considerable variability in the treatment patterns of patients with stage II & III ETSTS, and the current study provides an overall snapshot of adherence to NCCN guidelines. Adherent treatment was received by 87.2% of patients with stage IIA disease and 58.3% of patients with stage IIB/III disease. Receiving NCCN-guideline adherent treatment was associated with improved 5-year OS and a decreased risk of death. These results support the use of treatment adherent to NCCN guidelines in stage IIA and IIB/III ETSTS.
Supplementary Material
Supplementary Figure 1: Flow chart demonstrating how the final cohort of 15,957 patients with extremity and superficial trunk soft tissue sarcoma was obtained.
Supp. Figure 2: Distribution of all treatment regimens for adherent and nonadherent treatment for stage IIA and stage IIB/III extremity and superficial trunk soft tissue sarcoma. RT = radiation therapy; chemo = chemotherapy
Acknowledgments
This work was supported in part by National Institutes of Health grant T32 CA009599 (RV) and K12 Paul Calabresi Career Development Award for Clinical Oncology (CLR), as well as a cancer center support grant for The University of Texas MD Anderson Cancer Center (P30CA016672).
Footnotes
Disclaimers: None
References
- 1.von Mehren M, Randall RL, Benjamin RS, et al. NCCN Clinical Practice Guidelines in Oncology: Soft Tissue Sarcoma. 2015 [Google Scholar]
- 2.Edge SB. American Joint Committee on Cancer: AJCC cancer staging manual. 7th. New York: Springer; 2010. [DOI] [PubMed] [Google Scholar]
- 3.Bagaria SP, Ashman JB, Daugherty LC, et al. Compliance with National Comprehensive Cancer Network guidelines in the use of radiation therapy for extremity and superficial trunk soft tissue sarcoma in the United States. J Surg Oncol. 2014;109:633–8. doi: 10.1002/jso.23569. [DOI] [PubMed] [Google Scholar]
- 4.Horton JK, Gleason JF, Jr, Klepin HD, et al. Age-related disparities in the use of radiotherapy for treatment of localized soft tissue sarcoma. Cancer. 2011;117:4033–40. doi: 10.1002/cncr.25996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hou CH, Lazarides AL, Speicher PJ, et al. The use of radiation therapy in localized high-grade soft tissue sarcoma and potential impact on survival. Ann Surg Oncol. 2015;22:2831–8. doi: 10.1245/s10434-015-4639-4. [DOI] [PubMed] [Google Scholar]
- 6.Kachare SD, Brinkley J, Vohra NA, et al. Radiotherapy associated with improved survival for high-grade sarcoma of the extremity. J Surg Oncol. 2015;112:338–43. doi: 10.1002/jso.23989. [DOI] [PubMed] [Google Scholar]
- 7.Schreiber D, Rineer J, Katsoulakis E, et al. Impact of postoperative radiation on survival for high-grade soft tissue sarcoma of the extremities after limb sparing radical resection. Am J Clin Oncol. 2012;35:13–7. doi: 10.1097/COC.0b013e3181fe46d4. [DOI] [PubMed] [Google Scholar]
- 8.Sherman KL, Wayne JD, Chung J, et al. Assessment of multimodality therapy use for extremity sarcoma in the United States. J Surg Oncol. 2014;109:395–404. doi: 10.1002/jso.23520. [DOI] [PubMed] [Google Scholar]
- 9.Boland GM, Chang GJ, Haynes AB, et al. Association between adherence to National Comprehensive Cancer Network treatment guidelines and improved survival in patients with colon cancer. Cancer. 2013;119:1593–601. doi: 10.1002/cncr.27935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Molena D, Mungo B, Stem M, et al. Does Quality of Care Matter? A Study of Adherence to National Comprehensive Cancer Network Guidelines for Patients with Locally Advanced Esophageal Cancer. J Gastrointest Surg. 2015;19:1739–47. doi: 10.1007/s11605-015-2899-8. [DOI] [PubMed] [Google Scholar]
- 11.Bristow RE, Chang J, Ziogas A, et al. Adherence to treatment guidelines for ovarian cancer as a measure of quality care. Obstet Gynecol. 2013;121:1226–34. doi: 10.1097/AOG.0b013e3182922a17. [DOI] [PubMed] [Google Scholar]
- 12.American College of Surgeons: National Cancer Data Base. 2016 [Google Scholar]
- 13.American College of Surgeons: Cancer Program Categories. 2016 [Google Scholar]
- 14.Dickman PW, Sloggett A, Hills M, et al. Regression models for relative survival. Stat Med. 2004;23:51–64. doi: 10.1002/sim.1597. [DOI] [PubMed] [Google Scholar]
- 15.Shkolnikov V, Barbieri M, Wilmoth J. The Human Mortality Database. doi: 10.1093/ije/dyv105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Visser BC, Ma Y, Zak Y, et al. Failure to comply with NCCN guidelines for the management of pancreatic cancer compromises outcomes. HPB (Oxford) 2012;14:539–47. doi: 10.1111/j.1477-2574.2012.00496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adam MA, Goffredo P, Youngwirth L, et al. Same thyroid cancer, different national practice guidelines: When discordant American Thyroid Association and National Comprehensive Cancer Network surgery recommendations are associated with compromised patient outcome. Surgery. 2016;159:41–51. doi: 10.1016/j.surg.2015.04.056. [DOI] [PubMed] [Google Scholar]
- 18.Chagpar R, Xing Y, Chiang YJ, et al. Adherence to stage-specific treatment guidelines for patients with colon cancer. J Clin Oncol. 2012;30:972–9. doi: 10.1200/JCO.2011.39.6937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Sullivan B, Davis AM, Turcotte R, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. The Lancet. 2002;359:2235–2241. doi: 10.1016/S0140-6736(02)09292-9. [DOI] [PubMed] [Google Scholar]
- 20.Yang JC, Chang AE, Baker AR, et al. Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J Clin Oncol. 1998;16:197–203. doi: 10.1200/JCO.1998.16.1.197. [DOI] [PubMed] [Google Scholar]
- 21.Beane JD, Yang JC, White D, et al. Efficacy of adjuvant radiation therapy in the treatment of soft tissue sarcoma of the extremity: 20-year follow-up of a randomized prospective trial. Ann Surg Oncol. 2014;21:2484–9. doi: 10.1245/s10434-014-3732-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koshy M, Rich SE, Mohiuddin MM. Improved survival with radiation therapy in high-grade soft tissue sarcomas of the extremities: a SEER analysis. Int J Radiat Oncol Biol Phys. 2010;77:203–9. doi: 10.1016/j.ijrobp.2009.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maretty-Nielsen K, Aggerholm-Pedersen N, Safwat A, et al. Prognostic factors for local recurrence and mortality in adult soft tissue sarcoma of the extremities and trunk wall: a cohort study of 922 consecutive patients. Acta Orthop. 2014;85:323–32. doi: 10.3109/17453674.2014.908341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maretty-Nielsen K, Aggerholm-Pedersen N, Safwat A, et al. Prevalence and prognostic impact of comorbidity in soft tissue sarcoma: a population-based cohort study. Acta Oncol. 2014;53:1188–96. doi: 10.3109/0284186X.2014.888494. [DOI] [PubMed] [Google Scholar]
- 25.Szklo M, Nieto FJ. Epidemiology : beyond the basics. 3rd. Burlington, Mass: Jones & Bartlett Learning; 2014. [Google Scholar]
- 26.Ward BWC, T C, Freeman G, Schiller JS. Early Release of Selected Estimates Based on Data From the 2014 National Health Interview Survey. 2015 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Flow chart demonstrating how the final cohort of 15,957 patients with extremity and superficial trunk soft tissue sarcoma was obtained.
Supp. Figure 2: Distribution of all treatment regimens for adherent and nonadherent treatment for stage IIA and stage IIB/III extremity and superficial trunk soft tissue sarcoma. RT = radiation therapy; chemo = chemotherapy