Abstract
Background
Chronic kidney disease (CKD) patients have increased rates of bleeding as well as thrombosis. Fibrinogen and platelets combine to generate a mature clot, but in CKD platelets are dysfunctional. Therefore, we hypothesize that CKD patients have increased clot strength due to elevated fibrinogen levels.
Methods
Retrospective review of CKD patients (n=84) who had rTEG and fibrinogen levels measured. They were compared to healthy controls (n=134).
Results
CKD patients had statistically significant increases in ACT, angle, MA and decreases in LY30 compared to controls. Fibrinogen levels were increased in CKD patients compared to reference range. Fibrinogen levels had a positive correlation with MA (rho = 0.709, p<0.0001) in CKD patients.
Conclusions
Patients with CKD manifest a coagulopathy consisting of delayed clot formation, but increased final clot strength and decreased clot breakdown. Furthermore, the elevated clot strength is mediated by increased fibrinogen levels in CKD patients.
Keywords: Kidney Failure, Chronic, Hypercoagulability, TEG, Fibrinogen
Introduction
Chronic Kidney Disease (CKD) patients have a paradoxical hemostatic potential, with increased rates of bleeding, but are prone to thrombosis.2, 3 Increased bleeding is thought to be driven principally by platelet dysfunction, with additional contributions from alterations in the coagulation cascade with deranged vWF and platelet interactions, increased nitric oxide and impaired fibrinolysis, as well as the sequelae of anemia.2, 4, 5 Clinically relevant bleeding has been reported in 24–50% of patients on HD, with a >2-fold increased prevalence compared to patients without ESRD.2, 6
Increased rates of thrombosis, on the other hand, manifest as deep venous thrombosis (DVT), pulmonary embolus (PE), thrombosis of arteriovenous fistula or hemodialysis access catheter, or acute coronary syndromes.2, 6 VTE event rates are doubled in the presence of CKD.6 The mechanisms driving this pro-thrombotic state are unclear.3
Thrombelastography (TEG) is a viscoelastic hemostatic assay that has been widely used in clinical settings as it more closely approximates the in vivo clotting characteristics compared to standard coagulation assays.1 Standard coagulation assays use platelet poor plasma (PPP), while TEG used whole blood and therefore represents contributions from red blood cells, platelets, white blood cells in addition to clotting factors found in PPP.1 TEG measures different hemostatic variables: the rate of clot formation (activated clotting time (ACT)), rate of clot propagation (angle), clot strength (maximal amplitude(MA)) and clot breakdown 30 minutes after achieving maximum clot strength (LY30). TEG has been shown to predict thrombotic complications in a wide variety of patient populations, with an elevated MA as the best predictor.7–9 As TEG provides detailed descriptions of the different phases of clot formation and predicts clinical outcomes, it provides a good tool to characterize the hemostatic potential in CKD.
MA is composed of contributions from platelets as well as fibrinogen that combine to generate a mature clot.10, 11 CKD has been associated with both platelet dysfunction as well as hyperfibrinogenemia.2, 5, 12 Thrombocytopenia has been successfully compensated for by the addition of fibrinogen concentrate.13 Additionally, fibrinogen concentrates have been used clinically used in European centers for the treatment of traumatic coagulopathy.13–15 Therefore, it seems reasonable that despite the presence of platelet dysfunction in renal failure, the elevated fibrinogen levels could compensate for this to drive increased clot strength. We hypothesize that increased fibrinogen levels in patients with CKD will correlate with increased clot strength and an increased MA on TEG.
Material and methods
Study Design
This was a single center, retrospective review of patients undergoing dialysis access surgery by a single surgeon at Denver Health Medical Center from 4/2013–10/2016. This group of CKD patients was compared to healthy controls.
Blood Samples from Healthy Volunteers
Nursing staff collected samples from volunteers at an outpatient clinic after approval by the Colorado Multi-institutional Review Board. The study was open to patients and hospital staff not taking antiplatelet or anticoagulant medication. Volunteers with any medical comorbidities, including diabetes, renal disease, hypertension or liver disease, were excluded. While reference ranges are available through the manufacturer, we elected to perform analysis of healthy volunteers to control for the effect of altitude in Denver and exclude patients with comorbid conditions.16 Blood samples were obtained via venous puncture, and collected in tubes containing no anticoagulants.
Blood Samples from Dialysis Access Patients
Clinical data was collected from the medical record after approval was obtained by the Colorado Multi-institutional Review Board. Patients undergoing dialysis access by a single surgeon at Denver Health Medical Center were included in the study. Clinical data included age, sex, and whether or not the patient was on dialysis prior to surgery and type of dialysis (hemodialysis (HD) or peritoneal dialysis (PD). Patients who had multiple surgeries within the study period only had one surgical event included in a random fashion. Laboratory data collected includes preoperative Rapid Thrombelastography (rTEG), fibrinogen levels and glomerular filtration rate (GFR). Rapid thrombelastography and fibrinogen levels were obtained routinely on all patients undergoing dialysis access surgery in the preoperative holding area. Blood samples were collected and run in accordance with clinical guidelines at DHMC.
Rapid Thrombelastography
Blood was collected from healthy volunteers and dialysis access patients in sample tubes without any anticoagulant (Vacutainer; Becton-Dickinson, Franklin Lakes, NJ). For healthy controls, samples were run in a research lab within 10 minutes of collection by trained research staff. For dialysis access patients, samples were run in the DHMC clinical lab within 10 minutes of collection by trained laboratory personnel. In both cases, rTEG assays were run according to the manufacturer’s instructions on a TEG 5000 Thrombelastograph Hemostasis Analyzer System (Haemonetics, Niles, IL, USA), with all supplies and reagents supplied by the same manufacturer. Machines were calibrated according to manufacturer suggestions before use. All samples were run with the Rapid TEG Reagent which adds Tissue Factor and Kaolin to blood prior to the assay being performed. The following parameters were recorded from the tracings of the rTEG: Activated Clotting Time (ACT, seconds), angle (α, degrees), maximal amplitude (MA, mm), and lysis 30 minutes after MA (LY30, %).
Statistical Analysis
GraphPad Prism version 7.0a (GraphPad Software, Inc; La Jolla, CA) and SPSS software (SPSS Institute, Cary, NC) were used for statistical analysis. The proportion of patients with an elevated ACT in different groups was compared with Fishers Exact Test. TEG variables were compared between groups using Mann-Whitney U Test. In the CKD subgroup, correlation between fibrinogen and rTEG parameters was analyzed using the Spearman Rho test.
Results
Patient Population
134 healthy volunteers and 84 CKD patients were included in the study. Healthy volunteers had a median age of 31 (27–38) and were 47% male. CKD patients had a median age of 57 years (52.5–62), were 60.7% male, and 66% of patients were on dialysis (65% on HD, 1.1% on PD). Healthy patients and controls differed in age (p<0.0001) but not in proportion of patients that were male or female. Of patients not on dialysis (n=28), 25 had a GFR available preoperatively. These patients were stratified based on the National Kidney Foundation Kidney Disease Outcomes Quality Initiative (NKF KDOQI) chronic kidney disease (CKD) staging. One patient was in CKD Stage 3 (GFR 30–59), 7 patients were in CKD Stage 4 (GFR 15–29) and 17 patients in CKD Stage 5 (GFR <15).
Comparison of Healthy Controls and CKD Patients
All TEG values demonstrated statistically significant differences from healthy controls (Table 1). ACT was prolonged (>128 seconds) in 18% of CKD patients compared to 4.5% of healthy controls (p=0.0025). Angle was increased in CKD (79.8 IQR: 76.4–81.9 vs. 73.85 IQR: 70.85–76.3 degrees in controls) (p<0.0001). MA was increased in CKD (71.6 IQR: 66.95–77.18 vs. 65 IQR: 61.5–68 mm in controls) (p<0.0001). LY30 was decreased in CKD (0.9 IQR: 0.1–2.5 vs. 2.7 IQR: 1.975–3.7 % in controls) (p<0.0001). Fibrinogen levels were increased in CKD patients (median 406.5, IQR 333–513 mg/dL) compared to the clinical reference range of 150–400 mg/dL.
Table 1.
TEG parameters of healthy controls and CKD patients
| Control (n=134) | CKD (n=84) | P | |
|---|---|---|---|
| ACT>128 seconds (% of population) | 4.5% | 18% | 0.0025 |
| Angle (degrees) | 73.85 (70.85–76.3) | 79.8 (76.4–81.9) | <0.0001 |
| MA (mm) | 65 (61.5–68) | 71.6 (66.95–77.18) | <0.0001 |
| LY30 (percent) | 2.7 (1.975–3.7) | 0.9 (0.1–2.5) | <0.0001 |
Comparisons between continuous variables was done with Mann-Whitney U Test. Discrete variables were analyzed using the Fishers Exact Test.
Effect of Dialysis
Patients undergoing HD (n=55) were compared to patients not on dialysis (n=28). Patients on peritoneal dialysis were excluded from this analysis due to the low sample size. There were no significant differences in ACT or angle between groups (Table 2). Patients on HD, however, had a decreased MA compared to those not on dialysis. Additionally, patients on HD had an increased clot breakdown compared to those not on dialysis. There was a trend towards decreased fibrinogen levels in the HD group, although this did not reach significance (p<0.1). In both groups (patients receiving HD or not on dialysis) all rTEG variables still showed statistically significant differences from healthy controls (p<0.05 for all). The differences between these groups and healthy controls mirrored the changes between healthy controls and the aggregate CKD patient cohort (increased ACT, angle, MA and decreased LY30).
Table 2.
Hemostatic variables of CKD patients stratified by those receiving dialysis.
| HD (n=55) | No Dialysis (n=22) | P | |
|---|---|---|---|
| ACT>128 seconds (% of population) | 11.5% | 26.9% | 0.11 |
| Angle (degrees) | 79.45 (76.38–81.9) | 80.45 (76.6–82.35) | 0.49 |
| MA (mm) | 69.8 (64.65–75.1) | 73.75(69.23–78.4) | 0.0165 |
| LY30 (percent) | 1.3 (0.2–2.85) | 0.4 (0.025–1.525) | 0.0355 |
| Fibrinogen (mg/dL) | 394 (317.5–498) | 483 (386–541.5) | 0.0671 |
Comparisons between continuous variables was done with Mann-Whitney U Test. Discrete variables were analyzed using the Fishers Exact Test.
Correlation with TEG values
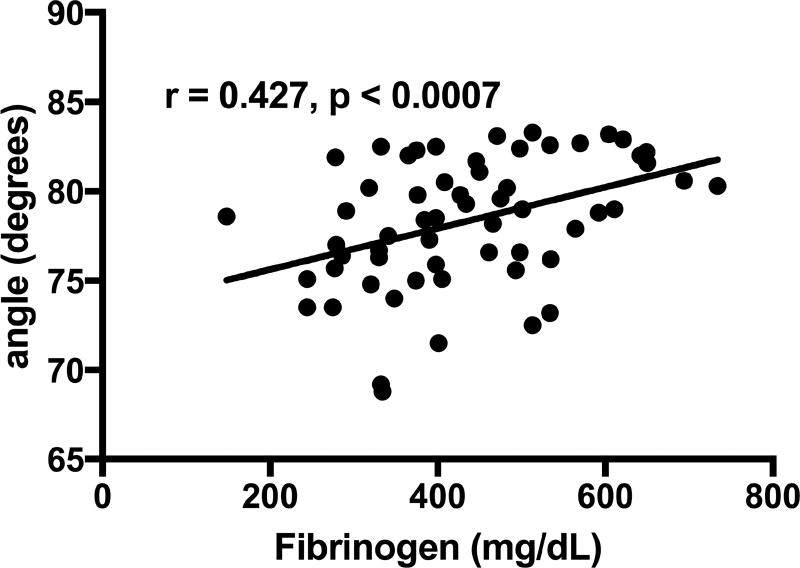
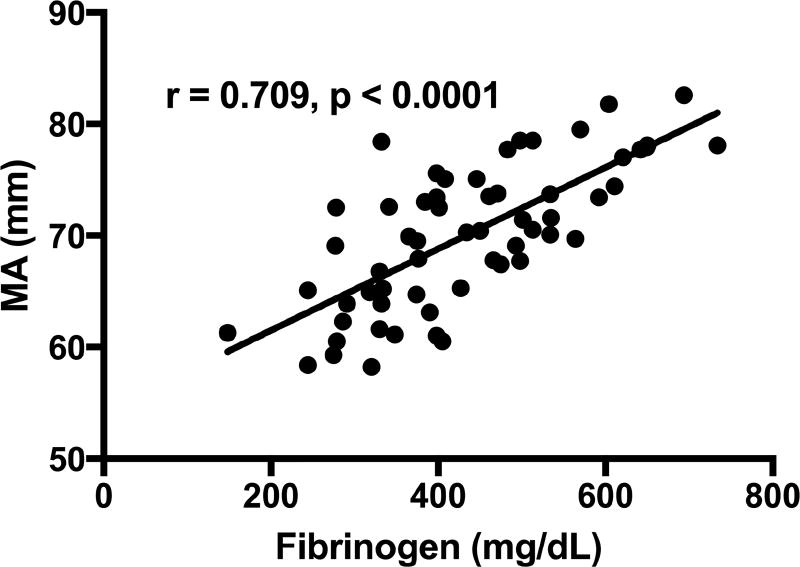
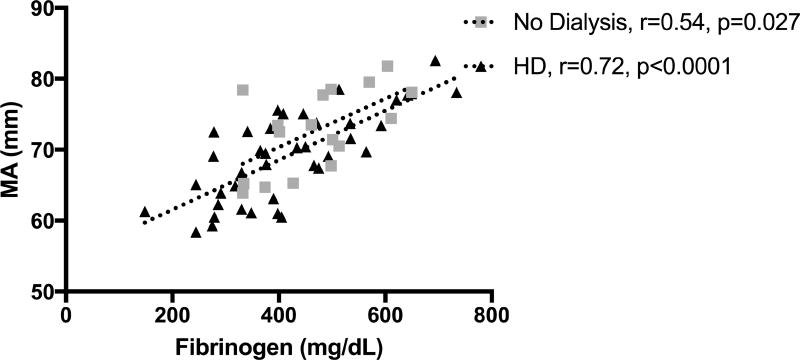
ACT did not correlate with fibrinogen levels. Angle and MA had a positive correlation with fibrinogen (rho = 0.427, p=0.0007 and rho = 0.709, p<0.0001), respectively (Figures 1 and 2). Correlation still existed between MA and fibrinogen for subgroups of patients on HD and not on dialysis (Figure 3). There was no correlation between fibrinogen and LY30.
Figure 1.
Correlation of angle with fibrinogen levels
Figure 2.
Correlation of MA with fibrinogen levels
Figure 3.
Correlation of MA with fibrinogen levels in groups receiving HD or not receiving dialysis.
Discussion
Chronic kidney disease generates a distinct hemostatic potential as compared to normal controls. CKD patients manifest a coagulopathy consisting of delayed clot formation with increased final clot strength and decreased clot breakdown when compared to healthy patients. The increased clot strength (MA) that is seen in this population is mediated by supra-normal fibrinogen levels. The increased clot strength and decreased clot breakdown is attenuated in patients receiving HD.
Our data aligns with prior work showing increased clot strength in CKD.12, 17, 18 Additionally, the findings of a delayed clot formation, decreased lysis and increased fibrinogen levels have been seen in some prior studies.12, 17, 18 The delayed clot formation seen may predispose to bleeding complications. Furthermore, the increased clot strength and decreased breakdown in this group may account for the increased thrombotic complications in this group.2, 6
This increased clot strength is mediated by increasing levels of fibrinogen. Given the known platelet dysfunction in CKD, increased fibrinogen appears to be responsible for the observed hypercoagulability as a compensatory mechanism to normalize hemostasis in the presence of platelet dysfunction.2, 12, 13 Elevated fibrinogen levels have been shown to correlate with myocardial complications in patients with Stage 5 CKD.19 This supports the theory that hyperfibrinogenemia and associated increased clot strength as assessed by increased MA, is mechanistic in the physiologic coagulopathy and the complications with thrombosis in CKD patients clinically.
HD patients have moderation of the hypercoagulable profile, with a decreased MA and increased clot breakdown compared to CKD patients not on dialysis. While their coagulation indices do not reach healthy controls, the moderation may have implications for hypercoagulable events in this group. The mechanisms for this remain to be fully explained, although some studies show decreased levels of fibrinogen and vWF after dialysis, other suggest changes arise from increased tPA levels following dialysis.20, 21
Several limitations in this study warrant discussion. While our control group was chosen to exclude any patients with comorbid conditions, they are significantly younger than the CKD group. Some of the differences between groups may represent additional comorbid conditions that coexist in the CKD population as well as the effect of increasing age. The endotheliopathy seen in CKD is not reproduced in the TEG cup.2 This may lead to overestimation of the tendency for thrombosis, as the platelet vessel wall binding may be decreased due to decreased vWF-platelet binding, or alternatively underestimation if endothelial damage and increased platelet activation is present.2 The small population size, short term follow up and a heterogeneous patient population limit our ability to directly evaluate hyperfibrinogenemia and a hypercoagulable state seen with increased MA on TEG against clinical thrombotic events. CKD patients have variable histories of upper extremity percutaneous dialysis access, different AV fistula sites / sizes, different conduits and AV graft materials which all add confounding variables to consider when assessing the impact of fibrinogen or clot strength on clinical thrombotic potential. However, increased MA and fibrinogen have been shown to correlate well with increased thrombotic complications in CKD patients and other populations and taken with evidence from this study strongly suggest that hyperfibrinogenemia contributes to the increased clot strength and associated thrombotic risk.7, 9, 19 These important clinical outcomes could be improved upon with a larger, multicenter, prospective evaluation.
These data suggest that a distinct hemostatic potential exists in CKD, consisting of a decreased speed of clot formation, but a stronger clot that is resistant to breakdown compared to healthy patients. The increased clot strength and therefore hypercoagulability seen in the CKD population appears to be mediated by fibrinogen. Thus, methods to treat hypercoagulability in this population should target fibrinogen. Additionally, the hypercoagulable state in CKD may improve with dialysis through an unknown mechanism. Prospective studies are needed to evaluate the ability of TEG and fibrinogen to predict clinical postoperative complications including thrombosis.
Acknowledgments
This study was supported in part by US Army Medical Research Acquisition Act of the Department of Defense under Contract Award Number W81XWH1220028, National Institute of General Medical Sciences grants: T32-GM008315 and P50-GM49222. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Department of Defense or the National Institutes of Health. We receive research support from Haemonetics LLC and TEM GmbH, but have no financial interests in these companies. Drs. Chapman, E.E. Moore, H.B. Moore report their patent on “Identification of Novel Disease States Using Viscoelastic Analysis in the Presence of a Thrombolytic Agent”. Drs. Chapman and E.E. Moore report their patent on “Methodologies and Reagents for Detecting Fibrinolysis and Hyperfibrinolysis”.
Thank you,
Angela Sauaia, MD PhD
University of Colorado Denver
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No authors have any other relevant financial relationships or any sources of support in the form of grants, equipment, or drugs.
References
- 1.Hans GA, Besser MW. The place of viscoelastic testing in clinical practice. Br J Haematol. 2016;173:37–48. doi: 10.1111/bjh.13930. [DOI] [PubMed] [Google Scholar]
- 2.Lutz J, Menke J, Sollinger D, et al. Haemostasis in chronic kidney disease. Nephrol Dial Transplant. 2014;29:29–40. doi: 10.1093/ndt/gft209. [DOI] [PubMed] [Google Scholar]
- 3.Eberst ME, Berkowitz LR. Hemostasis in renal disease: pathophysiology and management. Am J Med. 1994;96:168–79. doi: 10.1016/0002-9343(94)90138-4. [DOI] [PubMed] [Google Scholar]
- 4.Wardle EN, Taylor G. Fibrin breakdown products and fibrinolysis in renal disease. J Clin Pathol. 1968;21:140–6. doi: 10.1136/jcp.21.2.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaw D, Malhotra D. Platelet dysfunction and end-stage renal disease. Semin Dial. 2006;19:317–22. doi: 10.1111/j.1525-139X.2006.00179.x. [DOI] [PubMed] [Google Scholar]
- 6.Parikh AM, Spencer FA, Lessard D, et al. Venous thromboembolism in patients with reduced estimated GFR: a population-based perspective. Am J Kidney Dis. 2011;58:746–55. doi: 10.1053/j.ajkd.2011.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dai Y, Lee A, Critchley LA, White PF. Does thromboelastography predict postoperative thromboembolic events? A systematic review of the literature. Anesth Analg. 2009;108:734–42. doi: 10.1213/ane.0b013e31818f8907. [DOI] [PubMed] [Google Scholar]
- 8.Kashuk JL, Moore EE, Sabel A, et al. Rapid thrombelastography (r-TEG) identifies hypercoagulability and predicts thromboembolic events in surgical patients. Surgery. 2009;146:764–72. doi: 10.1016/j.surg.2009.06.054. discussion 72–4. [DOI] [PubMed] [Google Scholar]
- 9.Park MS, Martini WZ, Dubick MA, et al. Thromboelastography as a better indicator of hypercoagulable state after injury than prothrombin time or activated partial thromboplastin time. J Trauma. 2009;67:266–75. doi: 10.1097/TA.0b013e3181ae6f1c. discussion 75–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harr JN, Moore EE, Ghasabyan A, et al. Functional fibrinogen assay indicates that fibrinogen is critical in correcting abnormal clot strength following trauma. Shock. 2013;39:45–9. doi: 10.1097/SHK.0b013e3182787122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kornblith LZ, Kutcher ME, Redick BJ, et al. Fibrinogen and platelet contributions to clot formation: implications for trauma resuscitation and thromboprophylaxis. J Trauma Acute Care Surg. 2014;76:255–6. doi: 10.1097/TA.0000000000000108. discussion 62–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holloway DS, Vagher JP, Caprini JA, et al. Thrombelastography of blood from subjects with chronic renal failure. Thromb Res. 1987;45:817–25. doi: 10.1016/0049-3848(87)90091-0. [DOI] [PubMed] [Google Scholar]
- 13.Velik-Salchner C, Haas T, Innerhofer P, et al. The effect of fibrinogen concentrate on thrombocytopenia. J Thromb Haemost. 2007;5:1019–25. doi: 10.1111/j.1538-7836.2007.02481.x. [DOI] [PubMed] [Google Scholar]
- 14.Schochl H, Nienaber U, Hofer G, et al. Goal-directed coagulation management of major trauma patients using thromboelastometry (ROTEM)-guided administration of fibrinogen concentrate and prothrombin complex concentrate. Crit Care. 2010;14:R55. doi: 10.1186/cc8948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schlimp CJ, Voelckel W, Inaba K, et al. Impact of fibrinogen concentrate alone or with prothrombin complex concentrate (+/− fresh frozen plasma) on plasma fibrinogen level and fibrin-based clot strength (FIBTEM) in major trauma: a retrospective study. Scand J Trauma Resusc Emerg Med. 2013;21:74. doi: 10.1186/1757-7241-21-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anonymous. TEG 5000 Thrombelastograph Hemostasis System: User Manual. Niles, IL: Haemonetics; 2007. [Google Scholar]
- 17.Pivalizza EG, Abramson DC, Harvey A. Perioperative hypercoagulability in uremic patients: a viscoelastic study. J Clin Anesth. 1997;9:442–5. doi: 10.1016/s0952-8180(97)00097-4. [DOI] [PubMed] [Google Scholar]
- 18.Chapman MP, Moore EE, Burneikis D, et al. Thrombelastographic pattern recognition in renal disease and trauma. J Surg Res. 2015;194:1–7. doi: 10.1016/j.jss.2014.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zoccali C, Mallamaci F, Tripepi G, et al. Fibrinogen, mortality and incident cardiovascular complications in end-stage renal failure. J Intern Med. 2003;254:132–9. doi: 10.1046/j.1365-2796.2003.01180.x. [DOI] [PubMed] [Google Scholar]
- 20.Sabovic M, Salobir B, Preloznik Zupan I, et al. The influence of the haemodialysis procedure on platelets, coagulation and fibrinolysis. Pathophysiol Haemost Thromb. 2005;34:274–8. doi: 10.1159/000093107. [DOI] [PubMed] [Google Scholar]
- 21.Vaziri ND, Gonzales EC, Wang J, Said S. Blood coagulation, fibrinolytic, and inhibitory proteins in end-stage renal disease: effect of hemodialysis. Am J Kidney Dis. 1994;23:828–35. doi: 10.1016/s0272-6386(12)80136-3. [DOI] [PubMed] [Google Scholar]