Abstract
Subjects at risk of dementia benefit from participation in mentally stimulating activities but no prior studies have investigated similar associations in Parkinson’s disease (PD). The aim of this study was to investigate the relationship between times spent engaging in mentally stimulating activities and cognitive functions in PD while accounting for the degree of primary neurodegenerations. PD patients (N=41, 33 males; age 68.5±7.2; Hoehn & Yahr stage 2.6±0.6) completed the Community Health Activities Model Program for Seniors questionnaire, mini-mental state examination (MMSE), and [11C]dihydrotetrabenazine dopaminergic and [11C]piperidinyl propionate (PMP) acetylcholinesterase PET imaging. The subset of mentally stimulating activity items of the Community Health Activities Model Program for Seniors questionnaire was used to develop a rating scale as primary outcome variable in this study. Findings showed that mean rating scale score of time spent in mentally stimulating activities over a 4-week timespan was 20.0±8.3 hours and mean MMSE score was 28.4±1.9. Regression analysis showed that duration of participation in mentally stimulating activities was a significant predictor of MMSE scores (standardized β=0.39, t=2.8, p=0.009; total model: F(6,34)=3.5, P=0.005) independent from significant effects for cortical cholinergic activity (β=0.35, t=2.4, p=0.024). Caudate nucleus dopaminergic activity, age, education or duration of disease were not significant regressors. Post hoc analysis did not show significant effects of motor disease severity or level of physical activities. We conclude that engagement in mentally stimulating activities is associated with better cognitive abilities in PD, independent of education, severity of motor disease, nigrostriatal dopaminergic and cortical cholinergic degenerations.
Keywords: Acetylcholine, cognitive stimulation, cognition, dopamine, Parkinson disease, PET
Introduction
There is accumulating evidence that frequent engagement in mentally stimulating activities is beneficial to brain health for subjects at risk of mild cognitive impairment (Geda et al. 2011; Verghese et al. 2006) or dementia (Verghese et al. 2003). To our knowledge, no prior studies have investigated similar associations involving engagement in mentally stimulating activities in Parkinson disease (PD) patients.
It is possible that a higher degree of participation in mentally stimulating activities may simply reflect lower biological disease severity rather than mental stimulation exerting an intrinsic beneficial effect on the brain. This issue can, at least partially, be addressed by conducting studies that utilize biomarkers of biological disease severity, such as in vivo PET imaging assessments of biological substrates of disease severity and cognitive impairment.
The aim of this study was to investigate the relationship between time spent engaging in mentally stimulating activities and cognitive performance scores, while accounting for the degree of primary PD-related neurodegenerations. We hypothesized that more time participating in mentally stimulating activities is associated with less severe cognitive symptoms, independent of the degree of nigrostriatal dopaminergic and cortical cholinergic denervations as assessed by in vivo PET.
Subjects and Methods
Subjects and clinical test battery
This cross-sectional study involved 41 PD subjects (33 males, 8 females), mean age 68.5±7.2 years (range 56–84) and mean duration of disease of 8.1±3.9 years (2.5–20), who participated in an imaging biomarker study of mobility impairments in PD and who completed the CHAMPS activity questionnaire and Mini-Mental State Examination (MMSE) (Folstein et al. 1975) at time of study enrollment and imaging procedures. Subjects met the UK PD Society Brain Bank clinical diagnostic criteria. None of the subjects had evidence of dementia as defined by cognitive impairment and impaired instrumental activities of daily living. Abnormal striatal dopaminergic ([11C]DTBZ) PET findings were consistent with the diagnosis of PD in all subjects. No subjects had a history of a large artery stroke or other significant intracranial disease. Most subjects had moderate severity of disease: one patient in modified Hoehn & Yahr (HY) stage 1, 1 in stage 1.5, 6 in stage 2, 19 in stage 2.5, 12 in stage 3, 1 in stage 4, and 1 in stage 5. The mean HY stage was 2.6±0.6. The mean UPDRS motor score was 30.0±10.6 (range 7–53) (Fahn and Elton 1987). All PD subjects were treated with dopaminergic agents; 28 subjects were taking a combination of dopamine agonist and carbidopa-levodopa medications, of whom 12 were using carbidopa-levodopa alone, and one was taking a dopamine agonist alone. Mean levodopa equivalent dose (LED) was 962.5±521.3 mg (Tomlinson et al. 2010). Subjects on dopaminergic drugs were examined in the morning after withholding dopaminergic drugs overnight.
The CHAMPS activity questionnaire has items that assess physical activity also items that assess engagement in mentally stimulating activities (Stewart et al. 2001). In order to develop a rating scale of the sub-set of the CHAMPS questions assessing levels of engagement in mentally stimulating activities (i.e., items 1–6, 8, 11–13, & 17–18) a factor analysis was performed from a larger CHAMPS questionnaire database at our center. We had data available from a total of 48 subjects with PD (39 males, 9 females; mean age 68.8±7.3 years (range 56–84) and mean HY stage 2.7±0.6 (range 1–5); note that 41 of these PD subjects who had completed additional dopaminergic and cholinergic brain PET imaging are the source of the primary data analysis in this study, and 13 normal elderly subjects (6 males, 7 females; mean age 70.3±9.5 years (range 55–84). The factor analysis showed the presence of a single factor with an eigenvalue > 2 (λ=2.7). 9 out of 12 items had high loading weights (>.35): items 1–6, 8, 11, & 18. The Cronbach α was 0.68, which is in the working range for internal consistency of the scale as high (>0.9) or low values (like <0.5) should be avoided (Tavakol and Dennick 2011). Consequently, the 9 high loading items were summed and used as the primary variable in our analysis. The new rating scale included activities such as volunteering, visiting friends, computer use, crafts, attending church or reading yielding a total duration of these mentally stimulating activities over a timespan of 4 weeks as the primary outcome measure. Findings of engagement in physical activity and motor performance from this cohort of patients have been previously reported (Snider et al. 2015).
The study was approved by the Institutional Review Boards of the University of Michigan and VA Ann Arbor Health Care System. Written informed consent was obtained from all subjects prior to any research procedures.
Imaging techniques
All subjects underwent brain MRI and [11C]PMP acetylcholinesterase (AChE) and [11C]DTBZ vesicular monoamine transporter type 2 (VMAT2) PET. [11C]DTBZ PET imaging was performed in the morning after withholding dopaminergic medications overnight. After completing the [11C]DTBZ PET scan, subjects on dopaminergic medications took their medications and proceeded with the [11C]PMP PET. MRI was performed on a 3 Tesla Philips Achieva system (Philips, Best, The Netherlands) and PET imaging was performed in 3D imaging mode with an ECAT Exact HR+ tomograph (Siemens Molecular Imaging, Inc., Knoxville, TN) (Bohnen et al. 2012). The imaging studies were generally completed within 1–2 days of the clinical and neuropsychological testing sessions.
[11C]DTBZ and [11C]PMP were prepared as described previously (Shao et al. 2011). Dynamic PET scanning was performed for 70 minutes using a bolus dose of 15 mCi [11C]PMP dose. A bolus/infusion protocol was used for [11C]DTBZ (15 mCi) in 60 minutes (Koeppe et al. 1999).
Analysis
All image frames were spatially coregistered within subjects with a rigid-body transformation to reduce the effects of subject motion during the imaging session (Minoshima et al. 1993). Interactive Data Language image analysis software (Harris Geospatial Solutions, Broomfield, CO) was used to manually trace volumes of interest (VOI) on MRI images to include the caudate nucleus and putamen of each hemisphere. VOIs were bilaterally averaged. Total neocortical VOI were defined using semi-automated threshold delineation of the cortical gray matter signal on the MRI scan (Bohnen et al. 2012).
[11C]DTBZ distribution volume ratios (DVR) were estimated using the Logan plot graphical analysis method with the striatal time activity curves as the input function and the total neocortex as reference tissue (a region low in VMAT2 binding sites), with the assumption that the non-displaceable distribution is uniform across the brain at equilibrium (Koeppe et al. 1999).
AChE [11C]PMP hydrolysis rates (k3) were estimated using the striatal volume of interest (defined by manual tracing on the MRI scan of the putamen and caudate nucleus) as the reference tissue for the integral of the precursor delivery (Nagatsuka et al. 2001). AChE assessment has been recognized as a reliable marker for cholinergic pathways in the human brain (Selden et al. 1998; Shute and Lewis 1966). AChE PET imaging assesses cholinergic terminal integrity with cortical uptake reflecting basal forebrain neuron integrity.
Multiple regression analysis was performed to determine the relationship between engagement in mentally stimulating activities and neuropsychological MMSE scores while controlling for nigrostriatal VMAT2 DVR, cortical AChE hydrolysis rate (k3), HY motor disease stage, age, and duration of disease covariates in the PD subjects. The CHAMPS activity questionnaire total time spent in physical activity was also used as a covariate for confounder analysis (Stewart et al. 2001). Analyses were performed using SAS version 9.3 (SAS institute, Cary, North Carolina).
Results
Table 1 provides a summary of the clinical, cognitive, duration of mentally stimulating activities, and PET imaging findings.
Table 1.
Summary of the clinical, cognitive, duration of mentally stimulating activities, motor and PET imaging findings in the study population (n=41). Abbreviations: dihydrotetrabenazine, DTBZ; Mini-mental state examination, MMSE; Movement Disorders Society Revised Unified Parkinson’s Disease Rating Scale, MDS-UPDRS; piperidinyl propionate, PMP; VMAT2, vesicular monoamine transporter type 2.
| Mean value (standard deviation/range) | |
|---|---|
| Age (yr) | 68.5±7.2 (56–84) |
| Gender (male/female) | 33/8 |
| Duration of motor disease (yr) | 8.1±3.9 (2.5–20) |
| Time spent in mentally stimulating activities over 4-week period (hr) | 20.0±8.3 (2–39) |
| MMSE | 28.4±1.9 (22–30) |
| Education (yr) | 15.4±2.6 (12–20) |
| Hoehn & Yahr stage | 2.6±0.6 (1–5) |
| MDS-UPDRS part III (motor) score | 30.0±10.6 (7–53) |
| Dorsal caudate nucleus [11C]DTBZ VMAT2 distribution volume ratio | 1.83±0.41 (range 1.30–3.4) |
| Cortical [11C]PMP acetylcholinesterase hydrolysis rate | 0.0228±0.0034 (range 0.0175–0.0300) min−1 |
Multivariate regression analysis to account for possible confounders
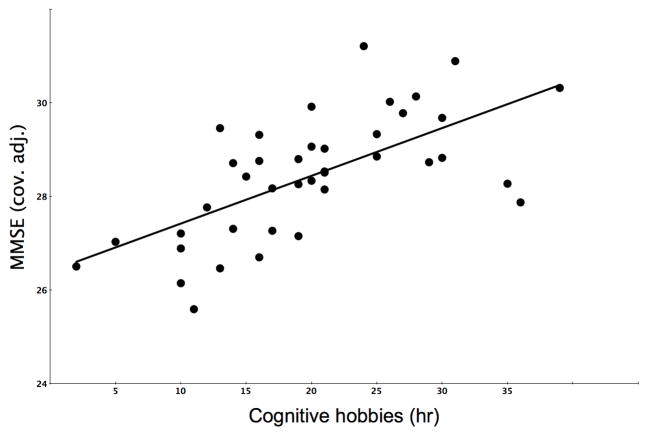
Multiple regression analysis using the MMSE score as the outcome variable demonstrated a significant overall model (total model: F(6,34)=3.8, P=0.005) with significant regressor effect for the duration of participation in mentally stimulating activities (standardized β=0.39, t=2.8, p =0.009) while accounting for effects of caudate nucleus dopaminergic activity (β=−0.07, t=−0.5, p=0.6), cortical cholinergic activity (β=0.35, t=2.4, p=0.024), education (β= −0.07, t=−0.5, p=0.6), age (β= −0.2, t=−1.6, p=0.11), and duration of disease (β=0.21, t=1.5, p=0.14; table 2;figure 1).
Table 2.
Summary findings of multiple regression model using the MMSE score as the outcome variable demonstrating a significant regressor effect for the duration of weekly participation in mentally stimulating activities while accounting for confounder variables. Standardized β coefficients are presented. Abbreviations: acetylcholinesterase, AChE; vesicular monoamine transporter type 2, VMAT2.
| Duration mentally stimulating activities | Caudate nucleus VMAT2 PET | Cortical AChE PET | Education | Duration | Overall model |
|---|---|---|---|---|---|
| β=0.39, t=2.8, p =0.009 | β= −0.07, t=−0.5, p=0.6 | β=0.35, t=2.4, p=0.024 | β= −0.07, t=−0.5, p=0.6 | β=0.21, t=1.5, p=0.14 | F(6,34)=3.8, P=0.005 |
Figure 1.
Scatter plot of the distribution of total duration of participation in mentally stimulating activities over 4 weeks and MMSE scores (cov. adj.: adjusted for co-variates of dorsal caudate nucleus dopamine binding, cortical acetylcholinesterase hydrolysis rate, duration of physical activity, duration of disease, age and Hoehn and Yahr Parkinson disease severity stage).
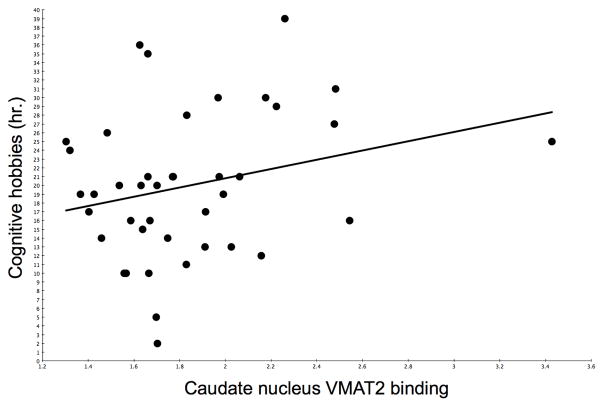
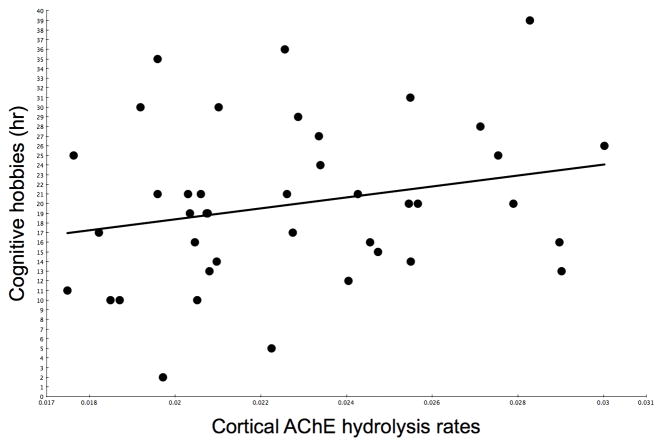
Scatter plots showing the (non-significant) relationships between caudate nucleus VMAT2 (R=0.24, P=0.14) and cortical AChE (R=0.24, P=0.13) activities and duration of participation in mentally stimulating activities are shown in figure 2 and 3, respectively.
Figure 2.
Scatter plot showing the (non-significant) relationship between dorsal caudate nucleus VMAT2 distribution volume ratios and duration of participation in mentally stimulating activities.
Figure 3.
Scatter plot showing the (non-significant) relationship between cortical AChE hydrolysis rates and duration of participation in mentally stimulating.
Exploratory post hoc analysis: accounting for effects of physical activity or parkinsonian motor impairments
An exploratory post hoc analysis was performed to account for physical activity levels that potentially may be associated with behavioral patterns associated with engagement in mentally stimulating activities. For this purpose, the CHAMPS physical activity score (as time spent in physical activity in hours per 4 weeks (Snider et al. 2015)) was entered as an additional regressor in the model. Multiple regression analysis using the MMSE score as the outcome variable demonstrated a significant overall model (F(7,33)=4.4, P=0.0016) with significant regressor effect for the duration of weekly participation in mentally stimulating activities (standardized β=0.31, t=2.1, p=0.04) while accounting for effects of caudate nucleus dopaminergic activity (β= −0.04, t=−0.31, p=0.76), cortical cholinergic activity (β=0.36, t=2.6, p=0.014), HY stage (β= −0.28, t=−2.0, p=0.058), age (β= −0.11, t=−0.8, p=0.42), duration of disease (β=0.27, t=2.0, p=0.056), and 4-week duration of physical activity (β=0.13, t=0.9, p=0.39).
A similar post hoc regression analysis entering the UPDRS motor severity score in the model confirmed the significant regressor effect for time spent in mentally stimulating activities (standardized β=0.31, t=2.2, p=0.033; F(6,36)=4.0, P=0.0036) independent from cortical cholinergic activity (β=0.30, t=2.1, p=0.041), UPDRS motor scores (β= −0.28, t=−1.8, p=0.082) and the other variables.
Discussion
Our findings show that more frequent engagement in mentally stimulating activities is associated with better cognitive performance scores independent of primary dopaminergic and cholinergic neurodegenations in PD. Our results add to a growing body of literature indicating that engagement in mental stimulation is related to preservation of cognitive performance. Among elderly adults, mentally stimulating activities have been associated with a reduced risk of mild cognitive impairment (Geda et al. 2011; Verghese et al. 2006) and dementia (Verghese et al. 2003). Our data provides additional support for the possibility that engagement in mentally stimulating activities may preserve cognitive performance, even among patients with PD.
Several mechanisms have been proposed that may promote neuroplasticity and may provide a possible explanation for this finding. Mental stimulation may promote cerebral blood flow (Silvestrini et al. 1994), which has been found to activate neurotrophic functions, synaptogenesis, and neurogenesis [see for review: (Paillard et al. 2015)]. Existing neural networks may also be reinforced, buffering against cognitive decline (La Rue 2010). Furthermore, frequent engagement in mentally stimulating activities may be indicative of an overall healthy lifestyle (Geda et al. 2011). In this case, the benefits of a healthy lifestyle may extend to preservation of brain function. However, our analysis was adjusted for time spent in physical activity (Snider et al. 2015), suggesting an intrinsic benefit of mentally stimulating activities on cognition in PD.
Many of the activities selected to operationalize mental stimulation involved social interaction. Activities such as volunteering, visiting friends, attending concerts or church may all involve some degree of social interaction. Therefore, it is feasible that social interaction may also play a role in the preservation of cognitive performance. Geda et al. reported that some social activities, such as traveling and going out with friends, were associated with decreased risk of mild cognitive impairment (Geda et al. 2011). Further research is necessary to clarify the role that social interaction plays in the preservation of cognitive capabilities. Higher degree of engagement in mentally stimulating activities may reflect lifetime activities. However, our findings were independent from the level of education.
Cortical cholinergic denervation is a major neurodegeneration associated with progressive declines across the spectrum of cognitive impairment in PD and typically occurs in the context of significant caudate nucleus dopaminergic denervation (Bohnen et al. 2015). Our findings confirm that cortical cholinergic activity is an important determinant of cognition in PD and that more engagement in mentally stimulating activities may have incremental effects.
One potential limitation of the study is that the cross-sectional study design and lack of prospective assessment does not provide etiological information regarding whether engagement in mentally stimulating activities may lower the risk of dementia. Given that only a correlational relationship has been indicated, it is also possible that there is not a direct causality but a reverse causality. In this case, a lack of engagement in mentally stimulating activities may be a symptom of cognitive decline, rather than a risk factor. If this were the case, we would expect to observe more severe dopaminergic or cholinergic degenerations in subjects reporting lower levels of engagement in mentally stimulating activities. However, our findings remained statistically significant when controlling for possible confounding effects involving differences in dopaminergic and cholinergic neurobiology as well as motor severity of disease among the study participants. Therefore, these results are not consistent with the explanation that level of engagement in mentally stimulating activities is a function of disease severity, indicating that engagement in mentally stimulating activities may have an intrinsic beneficial effect on the brain. A different bias may be the fact that subjects with more frequent engagement with mentally or mentally stimulating activity also engage more with physical activity and exercise. However, our analysis was adjusted for duration of participation with physical activity suggesting an independent effect. Furthermore, our findings were independent from PD motor disease severity and parkinsonian motor scores.
The association between duration of time spent in mentally stimulating activities and cognitive performance may be mediated by other factors, such as education, time spent in physical activities or socio-economic status. For this reason, we have adjusted our analysis for the effects of education and time spent in physical activities (Snider et al. 2015). Our main study population was a veteran population, which may reflect a more homogenous cross-section of PD patients and who may have a comparable socioeconomic status and lifestyle interests in common.
There are several limitations to this study, including the use of the MMSE as the cognitive outcome scale. For example, the MMSE has been shown to be less sensitive to detect executive cognitive function deficits in PD compared to other scales, like the Montreal Cognitive Assessment (Chou et al. 2014). Our patient population was predominantly male and therefore not adequately powered to asses for possible gender effects. Another limitation of this study is its reliance on data obtained from a self-reported activity questionnaire. Subjective reporting of self-assessed activity engagement through questionnaires may be biased and overestimate (or underestimate) the amount of time spent in certain activities to be viewed as more (or less) desirable by the respondent. As this study does not allow for independent measuring of the accuracy of self-reported engagement in mentally stimulating activities, one direction for future research could be more objective measurement or monitoring of mental activity engagements.
Another consideration is that the survey questions themselves, particularly those involving engagement in mentally stimulating activities, may not encompass the full range of activities subjects may participate in during the span of several weeks and future studies may aim to sample a wider breadth of cognitive or social activities.
Our study is based on a within-group analysis of subjects with PD. We did not compare findings to a non-PD control group. Therefore, we cannot make any inferences about disease-specific findings in our study.
We conclude that engagement in mentally stimulating activities is associated with better cognitive abilities in PD independent of nigrostriatal dopaminergic and cortical cholinergic degenerations, level of physical activity or severity of motor disease. Findings suggest that participation in mentally stimulating activities may help to promote cognitive preservation in PD. Future research involving longitudinal studies are needed to investigate whether more frequent engagement in mentally stimulating activities may lower risk of conversion to dementia in PD, which may provide a framework for new cognitive training therapies in PD (Dodge et al. 2015).
Acknowledgments
The authors thank all patients for their time commitment and research assistants Cyrus Sarosh and Christine Minderovic, PET technologists, cyclotron operators, and chemists for their assistance with the study. This work was supported by the Department of Veterans Affairs [grant number I01 RX000317]; the Michael J. Fox Foundation; and the NIH [grant numbers P01 NS015655, RO1 NS070856 with additional support from P50 NS091856].
Abbreviations
- AChE
acetylcholinesterase
- CHAMPS
Community Health Activities Model Program for Seniors questionnaire
- LED
Levodopa Equivalent Dose
- MMSE
Mini-Mental State Examination
- PD
Parkinson disease
- UPDRS
Unified Parkinson’s Disease Rating Scale
- VMAT2
vesicular monoamine transporter type 2
Footnotes
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- Bohnen NI, et al. Frequency of cholinergic and caudate nucleus dopaminergic deficits across the predemented cognitive spectrum of Parkinson disease and evidence of interaction effects. JAMA Neurol. 2015;72:194–200. doi: 10.1001/jamaneurol.2014.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnen NI, et al. Heterogeneity of cholinergic denervation in Parkinson disease. J Cereb Blood Flow Metab. 2012;32:1609–1617. doi: 10.1038/jcbfm.2012.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou KL, Lenhart A, Koeppe RA, Bohnen NI. Abnormal MoCA and normal range MMSE scores in Parkinson disease without dementia: Cognitive and neurochemical correlates. Parkinsonism Relat Disord. 2014 doi: 10.1016/j.parkreldis.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge HH, et al. Web-enabled Conversational Interactions as a Means to Improve Cognitive Functions: Results of a 6-Week Randomized Controlled Trial. Alzheimer’s & dementia. 2015;1:1–12. doi: 10.1016/j.trci.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahn S, Elton R. Members of the UPDRS development committee. Unified Parkinson’s disease rating scale. In: Fahn S, Marsden C, Calne D, Goldstein M, editors. Recent developments in Parkinson’s disease. Macmillan Healthcare Information; Florham Park, NJ: 1987. pp. 153–164. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state: a practical method for grading the cognitive state of patients for the clinician. J Psychiatry Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Geda YE, et al. Engaging in cognitive activities, aging, and mild cognitive impairment: a population-based study. J Neuropsychiatry Clin Neurosci. 2011;23:149–154. doi: 10.1176/appi.neuropsych.23.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeppe RA, Frey KA, Kuhl DE, Kilbourn MR. Assessment of extrastriatal vesicular monoamine transporter binding site density using stereoisomers of [11C]dihydrotetrabenazine. J Cereb Blood Flow Metab. 1999;19:1376–1384. doi: 10.1097/00004647-199912000-00011. [DOI] [PubMed] [Google Scholar]
- La Rue A. Healthy brain aging: role of cognitive reserve, cognitive stimulation, and cognitive exercises. Clin Geriatr Med. 2010;26:99–111. doi: 10.1016/j.cger.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Minoshima S, Koeppe RA, Fessler JA, Mintun MA, Berger KL, Taylor SF, Kuhl DE. Integrated and automated data analysis method for neuronal activation studying using O15 water PET. In: Uemura K, Lassen NA, Jones T, Kanno I, editors. Quantification of brain function to tracer kinetics and image analysis in brain PET., vol International Congress Series 1030. Quantification of brain function to tracer kinetics and image analysis in brain PET. Excerpta Medica; Tokyo: 1993. pp. 409–418. [Google Scholar]
- Nagatsuka S, et al. Kinetic analysis of [(11)C]MP4A using a high-radioactivity brain region that represents an integrated input function for measurement of cerebral acetylcholinesterase activity without arterial blood sampling. J Cereb Blood Flow Metab. 2001;21:1354–1366. doi: 10.1097/00004647-200111000-00011. [DOI] [PubMed] [Google Scholar]
- Paillard T, Rolland Y, de Souto Barreto P. Protective Effects of Physical Exercise in Alzheimer’s Disease and Parkinson’s Disease: A Narrative Review. Journal of clinical neurology. 2015;11:212–219. doi: 10.3988/jcn.2015.11.3.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selden NR, Gitelman DR, Salamon-Murayama N, Parrish TB, Mesulam MM. Trajectories of cholinergic pathways within the cerebral hemispheres of the human brain. Brain. 1998;121:2249–2257. doi: 10.1093/brain/121.12.2249. [DOI] [PubMed] [Google Scholar]
- Shao X, Hoareau R, Hockley BG, Tluczek LJ, Henderson BD, Padgett HC, Scott PJ. Highlighting the Versatility of the Tracerlab Synthesis Modules. Part 1: Fully Automated Production of [18F]Labelled Radiopharmaceuticals using a Tracerlab FXFN. J Labelled Comp Radiopharm. 2011;54:292–307. doi: 10.1002/jlcr.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shute CC, Lewis PR. Electron microscopy of cholinergic terminals and acetylcholinesterase-containing neurones in the hippocampal formation of the rat. Z Zellforsch Mikrosk Anat. 1966;69:334–343. doi: 10.1007/BF00406286. [DOI] [PubMed] [Google Scholar]
- Silvestrini M, Cupini LM, Matteis M, Troisi E, Caltagirone C. Bilateral simultaneous assessment of cerebral flow velocity during mental activity. J Cereb Blood Flow Metab. 1994;14:643–648. doi: 10.1038/jcbfm.1994.80. [DOI] [PubMed] [Google Scholar]
- Snider J, et al. Non-exercise physical activity attenuates motor symptoms in Parkinson disease independent from nigrostriatal degeneration. Parkinsonism Relat Disord. 2015;21:1227–1231. doi: 10.1016/j.parkreldis.2015.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart AL, Mills KM, King AC, Haskell WL, Gillis D, Ritter PL. CHAMPS physical activity questionnaire for older adults: outcomes for interventions. Med Sci Sports Exerc. 2001;33:1126–1141. doi: 10.1097/00005768-200107000-00010. [DOI] [PubMed] [Google Scholar]
- Tavakol M, Dennick R. Making sense of Cronbach’s alpha. International journal of medical education. 2011;2:53–55. doi: 10.5116/ijme.4dfb.8dfd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disord. 2010;25:2649–2653. doi: 10.1002/mds.23429. [DOI] [PubMed] [Google Scholar]
- Verghese J, et al. Leisure activities and the risk of amnestic mild cognitive impairment in the elderly. Neurology. 2006;66:821–827. doi: 10.1212/01.wnl.0000202520.68987.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verghese J, et al. Leisure activities and the risk of dementia in the elderly. N Engl J Med. 2003;348:2508–2516. doi: 10.1056/NEJMoa022252. [DOI] [PubMed] [Google Scholar]